Abstract
Context
Weight loss is known to improve health, however the influence of variability in body weight around the overall trajectory on these outcomes is unknown. Few studies have measured body weight frequently enough to accurately estimate the variability component.
Objective
To investigate the association of 12-month weight variability and concurrent weight change with changes in health markers and body composition.
Methods
This study was a secondary analysis of the NoHoW trial, a 2 × 2 factorial randomised controlled trial promoting evidence-based behaviour change for weight loss maintenance. Outcome measurements related to cardiometabolic health and body composition were taken at 0, 6 and 12 months. Participants were provided with Wi-Fi connected smart scales (Fitbit Aria 2) and asked to self-weigh regularly over this period. Associations of weight variability and weight change with change in outcomes were investigated using multiple linear regression with multiple levels of adjustment in 955 participants.
Results
Twelve models were generated for each health marker. Associations between weight variability and changes in health markers were inconsistent between models and showed no evidence of a consistent relationship, with all effects explaining <1% of the outcome, and most 0%. Weight loss was consistently associated with improvements in health and body composition, with the greatest effects seen in percent body fat (R2 = 10.4–11.1%) followed by changes in diastolic (4.2–4.7%) and systolic (3–4%) blood pressure.
Conclusion
Over 12-months, weight variability was not consistently associated with any measure of cardiometabolic health or body composition, however weight loss consistently improved all outcomes.
Trial registration number
ISRCTN88405328.
Keywords: Weight variability, Weight cycling, Cardiometabolic disease
Highlights
-
•
Previous longitudinal investigations suggest that variability is a risk factor for obesity-related diseases.
-
•
We tested whether 12-month variability impacted traditional markers of disease.
-
•
No association between weight variability and any risk factor was found.
-
•
Weight loss consistently improved health independent of the degree of variability.
-
•
The mechanism linking long-term variability to risk of disease remains unclear.
1. Introduction
Cardiometabolic health is closely associated with obesity, with increased BMI increasing the risk of co-morbidities such as type 2 diabetes and cardiovascular disease [1]. Increasing obesity prevalence worldwide has coincided with quadrupled type 2 diabetes diagnoses in the past 30 years, which is expected to rise over 10% of the world's total population by 2045 [2]. As little as 5% weight loss can significantly decrease the risk of obesity-related disease through reductions in blood pressure and improved blood lipid levels and glucoregulation [3].
An individual's body weight can be defined longitudinally by both the overall trend and associated variability around that trend (e.g. an individual gaining weight may do so in a very stable or variable manner). In the past few years, a series of prospective studies have reported direct associations between high body weight variability (BWV) and increased risk of cardiovascular disease [4,5], type 2 diabetes incidence [6,7], and mortality [8,9] including results from meta-analyses [10,11] and samples of over 6 million individuals [12]. However, some studies have shown null effects or even beneficial effects of BWV on disease and mortality risk [[13], [14], [15]]. The mechanisms linking BWV to detrimental health outcomes are unclear, though cross-sectional studies suggest associations between greater BWV and elevated blood pressure [16], haemoglobin A1c (HbA1c) [13] or reduced high density lipoprotein cholesterol (HDL-C) [17]. Furthermore, large rebounds in cardiometabolic health markers following weight regain have also been reported [18]. The physiological pathways relating BWV to these potential cardiometabolic responses are largely unexplored.
Increased frequency in the measurement of body weight may facilitate more valid estimation of BWV. When aligned with repeated measures of cardiometabolic health these estimates may enable more appropriate investigation of the relationship between BWV and health. However, until recently such data has not been available in research environments. In a recent pan-European multi-centre weight loss maintenance intervention (the NoHoW trial [19]) we collected frequent body weight measurements over 12 months from a large sample of individuals provided with smart scales, and took measures of cardiometabolic health and body composition at 0, 6 and 12 months. The aim of the present study was to investigate the combined associations of 12-month weight variability with concurrent changes in health markers and body composition over 12 months, adjusted for weight change. We hypothesised that greater BWV would be associated with adverse concurrent changes to health and body composition.
2. Methods
2.1. Study design
The NoHoW trial was a 2 × 2 factorial randomised controlled trial testing the efficacy of a digital toolkit for promoting evidence-based behaviour change for weight loss maintenance. It was delivered in three centres located in the United Kingdom (Leeds), Denmark (Copenhagen), and Portugal (Lisbon). A detailed description of the trial can be found elsewhere [19]. Participants were randomised into 4 arms upon entry to the trial ((1) active control, (2) self-regulation and motivation, (3) contextual behavioural emotion regulation and (4) self-regulation, motivation and emotion regulation (i.e. arms 2 and 3 combined)). For the present analysis we pooled trial arms.
All participants were provided with a Fitbit Aria body weight smart scale (Fitbit Inc, San Francisco, CA, USA) and a Fitbit Charge 2 activity monitor (Fitbit Inc, San Francisco, CA, USA). Participants were instructed to weigh themselves at least twice per week. Outcome measures were made at 0, 6 and 12 months. The trial is registered with the ISRCTN registry (ISRCTN88405328). The NoHoW study received funding from the European Union's Horizon 2020 research and innovation programme (grant agreement number: 643309). Ethical approval was granted by local institutional ethics committees at the Universities of Leeds (17–0082; 27-Feb-2017), Lisbon (17/2016; 20-Feb-2017) and the Capital Region of Denmark (H-16030495; 8-Mar-2017).
2.2. Participants
Details of enrolled participants can be found in full elsewhere [19]. Participants provided informed consent before participation. Briefly, individuals were eligible if they were aged 18 years or older, had verification of ≥5% weight loss in the 12 months prior to recruitment (excluding surgical weight loss) and had a BMI of ≥25 kg/m2 prior to weight loss. For inclusion in the present analysis, participants had to have ≥20 weight measurements over 12 months to generate estimates of weight variability, as determined by our recent validation study [20]. 955 individuals had available data for all outcome variables, minimum physical activity (PA) data and covariates. A sub-sample was generated which had available DXA measurements (n = 439). A participant flow diagram is shown in Supplementary Fig. 1.
2.3. Body weight
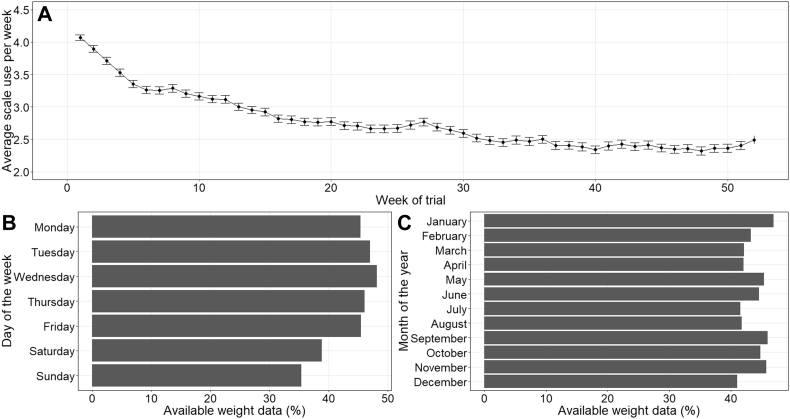
All participants were provided with a Fitbit Aria scale which shows excellent agreement with a calibrated research grade SECA 704s [21]. Data collected from the device was synchronised to a personal Fitbit account which participants could access on their electronic device and data from each personal account was regularly updated to the NoHoW data hub. Data was collected from the scales for up to 2 years, though only the first 12 months were analysed due to temporal proximity to health markers (measured at 0,6 and 12 months). Scale use was described in Fig. 1; 1A shows average scale use per week and frequency of data availability by day of the week and month of the year is summarized in Fig. 1B and C respectively.
Fig. 1.
A. Average scale use in all participants (n = 955) for each week of the trial with standard error bars. B. completeness of data per day of the week relative to the total amount of data possible for the given day. C. shows completeness of data per month of the year relative the amount of data possible for the given year.
2.4. Cardiometabolic health markers
Systolic and diastolic BP and resting heart rate (RHR) were recorded every 6 months by a Microlife BP A2 blood pressure monitor after resting in a sitting position for 10 min. Three readings were taken and the average values were used. Blood lipids (total cholesterol, low-density lipoprotein cholesterol (LDL-C), HDL-C and triglycerides) were measured at 0 and 12 months by a fasting capillary blood sample using an Alere AfinionTM AS100 Analyser. Similarly, fasting blood samples for the analysis of HbA1c were taken at 0 and 12 months and analysed using the Alere AfinionTM AS100 Analyser.
2.5. Body composition
Body composition was estimated at 0, 6 and 12 months by bio-impedance analysis (BIA) using the ImpediMed SFB7 multifrequency bio-impedance analyser in all three centres following the manufacturer's instructions and by dual-energy X-ray absorptiometry (DXA) at two centres: Portugal (Hologic Explorer-W, Waltham, USA) and Denmark (Norland XR-800, Swissray, USA). Estimates of body composition bio-electrical impedance were transformed using Moissl equations [22]. Percent body fat was calculated by dividing fat (kg) by body weight (kg) and multiplying by 100. A tape measure was used to record the hip (at the maximum circumference over the buttocks) and waist (under the midline of the participant's armpit) circumference to the nearest centimetre. The waist–hip ratio (WHR) was calculated by dividing hip and waist circumference.
2.6. Physical activity
The Fitbit Charge 2 is a wrist-worn activity monitor which estimates PA metrics based on data obtained from incorporated sensors. Minute-level data was synced via the Fitbit mobile application to Fitbit servers and to the NoHoW data hub. Step counts were used as the primary measure of PA due to greater reliability than other measures such as energy expenditure [23,24]. The first four weeks of physical activity data were removed as the novelty of receiving a new self-monitoring device (and initial problems with set up) is shown to produce sporadic increases in physical activity. Steps were aggregated to two-week averages. Participants were required to have valid data for at least the first 9 months, during which at least 12 valid weeks (6 two-week blocks) were required. This was deemed enough data to estimate initial and change in PA. Initial and change in PA were estimated by generating a regression between time and average steps, whereby the intercept acted as initial PA, and the beta coefficient as the change in PA. Full explanation of data processing for PA data is given in Supplementary material 1.
2.7. Body weight variability
Body weight variability was estimated using three previously used methods: root mean square error (RMSE) and coefficient of variation (CV) derived from linear trends [14,[25], [26], [27], [28], [29]] and mean absolute successive weight variability (MASWV) [4,7,30]. We devised a method to overcome the limitations of these approaches (including assumption of linearity in weight trajectory) which we termed the non-linear mean deviation (NLMD) method. All BWV measures are described and illustrated in full in Supplementary material 2. RMSE was estimated by taking the mean square of the relative residual error in the linear relationship between weight and time. The CV was calculated by dividing the mean weight by the standard deviation of the weight. MASWV was calculated by taking the absolute mean of the relative change in successive body weights. The NLMD was calculated by first fitting a locally estimated scatterplot smoothing (LOESS) regression to the body weight data which acts as a smoother. The fit of the regression was then subtracted from the observed body weight and the relative mean deviation from the non-linear trend was calculated.
2.8. Statistical analysis
Body weight data from scales was screened for outliers based on limits of physiological plausibile weight change (Supplementary Table 2). All key variables were assessed for normality via visual inspection of QQ plots and histograms and any variable deemed non-parametric were log transformed. Characteristics of the population at baseline were described by mean and standard deviation in the whole group and by sex due to known differences in physiological variables (particularly body composition) between sexes. Differences between sexes were tested using student t-tests and chi-squared tests. Correlations between key variables (including baseline and change scores) were evaluated and reported in full (supplementary file 2). To test the main hypotheses (that greater BWV would be associated with adverse concurrent changes to health and body composition), a pre-post approach was used employing a multiple linear regression with the post-score as the outcome and pre-score as a covariate, a method which is generally preferred to regression against the change-score [31]. All continuous variables were standardised by taking the mean and standard deviation of all variables, subtracting the mean and dividing by the standard deviation. Weight change (%) was calculated as the difference between weight at baseline and 12-months and converted to percent.
Three regression models were generated to test the primary hypotheses. First, model 1 included only the baseline outcome value, weight change (%) and BWV as covariates; second, model 2 included the same variables as model 1 and in addition age, sex, BMI and lastly model 3 included the same variables as model 2 plus initial and change in PA (steps) (due to the known confounding effect on PA on the relationship between weight, health and body composition). Each model was run for all four BWV metrics. We ran the models in a separate sub-sample for those with data available for body composition measured by DXA (n = 439) and full details are provided in Supplementary material 3. We probed for interactions between weight change and BWV estimates but found no significant associations therefore left these out of all models. All p-values within models were adjusted for multiple comparisons using the Bonferroni-Holt method. Model results are given in Supplementary Tables 2 and 3 which summarise the associations of weight change and BWV on outcome variables using standardised β-coefficients, standard errors and p-values. In order to compare the effect size of BWV estimates and weight change on health outcomes, we calculated the change in the adjusted R2 value of the model when the variable of interest (BWV estimate or weight change) was added to the model (which was complete except this variable); these values are summarized in Supplementary Figs. 4–5. As a sensitivity analysis, we separated the analysis into exposure and follow-up periods, full detailed can be viewed in Supplementary material 4. Significant associations were observed at p < 0.05. All analyses were conducted in R (version 3.5.1).
3. Results
Baseline characteristics are presented in Table 1. A total of 955 (653 women) met the criteria for inclusion. The group had a mean weight loss of 11.8 (±5.1) % in the 12-months prior to recruitment. On average, participants were aged 45.3 (±11.5) years, overweight (BMI = 29.4 (±5.0) kg/m2) and achieved above number of recommended steps per day [32] (mean steps = 10,833 (±3469)) around baseline (after removing the first 4 weeks). Average values for all health measures were within normal range (i.e. not hypertensive, hyperglycaemic or hyperlipidaemic [33]). Over 12 months, weight change was on average +0.56 (6.6) % (ranging from −30.8% to +36.3%); SBP and DBP decreased by 1.7 (10.6) and 0.3 (6.8) mmHg respectively and RHR increased by 1.3 (8.5) bpm. Total cholesterol increased by 0.19 (0.66) mmol/L; LDL-C decreased by a 0.05 (0.66) mmol/L; HDL-C increased by 0.15 (0.30) mmol/L; triglycerides increased by 0.21 (0.81) mmol/L and HbA1c increased by 0.09 (0.20) %. Body fat measured by BIA decreased by 0.50 (5.0) %. Waist and hip circumferences were 93.9 (13.7) and 109.1 (10.8) cm respectively, resulting in an average WHR of 0.86 (0.09). Over 12 months, participants weighed themselves on average 159 (89) times, the frequency of which decreased over the 12-month period (Fig. 1).
Table 1.
Participant characteristics.
| Variable | All (n = 955) | Male (n = 302) | Female (n = 653) | P-value |
|---|---|---|---|---|
| Centre (%) | <0.001 | |||
| Denmark | 354 (37.1) | 63 (20.9) | 291 (44.6) | |
| Portugal | 310 (32.5) | 175 (57.9) | 135 (20.7) | |
| UK | 291 (30.5) | 64 (21.2) | 227 (34.8) | |
| Age (years) | 45.29 (11.5) | 43.54 (10.81) | 46.10 (11.81) | 0.001 |
| BMI (kg/m2) | 29.43 (5.08) | 29.10 (4.44) | 29.57 (5.35) | 0.184 |
| Previous weight loss (%) | 11.8 (5.5) | 11.2 (5.1) | 12.1 (5.6) | <0.001 |
| Weight (kg) | 84.2 (16.5) | 91.27 (15.7) | 80.88 (15.8) | <0.001 |
| Initial steps | 10,816 (3493.7) | 11,584 (3842.0) | 10,461 (3262.8) | <0.001 |
| Number of body weight measurements | 158 (89) | 160 (90) | 157 (89) | <0.001 |
| SBP (mmHg) | 122.17 (14.76) | 127.39 (13.64) | 119.75 (14.64) | <0.001 |
| DBP (mmHg) | 76.54 (8.86) | 80.08 (8.61) | 74.91 (8.50) | <0.001 |
| HR (bpm) | 65.43 (10.30) | 62.28 (10.20) | 66.88 (10.03) | <0.001 |
| Cholesterol (mmol/L) | 4.90 (1.01) | 4.78 (0.91) | 4.96 (1.06) | 0.01 |
| LDL-C (mmol/L) | 2.77 (0.85) | 2.80 (0.75) | 2.75 (0.90) | 0.416 |
| HDL-C (mmol/L) | 1.58 (0.41) | 1.42 (0.33) | 1.65 (0.41) | <0.001 |
| Triglycerides (mmol/L) | 1.21 (0.68) | 1.22 (0.71) | 1.21 (0.67) | 0.806 |
| HbA1c (%) | 5.17 (0.34) | 5.20 (0.31) | 5.15 (0.35) | 0.039 |
| Fat free mass (kg) | 51.8 (9.6) | 61.83 (7.80) | 47.11 (6.22) | <0.001 |
| Fat mass (kg) | 51.77 (9.62) | 29.44 (10.94) | 33.76 (12.04) | <0.001 |
| Body fat (%) | 37.79 (8.62) | 31.45 (7.28) | 40.73 (7.55) | <0.001 |
| Hip (cm) | 109.13 (10.76) | 106.09 (8.21) | 110.53 (11.49) | <0.001 |
| Waist (cm) | 93.91 (13.71) | 99.22 (13.07) | 91.45 (13.30) | <0.001 |
| WHR | 0.86 (0.09) | 0.93 (0.08) | 0.83 (0.07) | <0.001 |
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol.
Baseline characteristics reported as mean and standard deviation unless stated otherwise. P-values denote results of student t-tests for continuous variables and chi-squared tests for categorical variables between sexes.
Tables 2–3 provides results from regression models 1–3. In all models and after inclusion of BWV by all methods, 12 months percent weight loss was consistently changes in the direction of improved health, with direct associations observed between weight change and changes in SBP (p < 0.001 for all), DBP (p < 0.001 for all), RHR (p < 0.001 for all), total cholesterol (p < 0.05 for all); LDL-C (p < 0.001 for all); triglycerides (p < 0.001 for all), HbA1c (p < 0.001 for all) and an inverse association with changes in HDL-C (p < 0.05 for all). Weight loss was also associated with reduced percent body fat (p < 0.001 for all) and WHR (p < 0.001 for all). The variance explained (R2 change) by addition of weight change to multivariate models was greatest for changes in percent body fat (10.4–11.1%) followed by changes in DBP (4.2–4.7%), SBP (3–4%), RHR (2–2.4%), triglycerides (1.8–2.4%), HbA1c (1.4–1.6%), WHR (1.6–1.9%), HDL-C (0.3–0.4%), total cholesterol (0.2–0.3%), and lastly LDL-C (0.1–0.2%).
Associations between BWV and changes in health markers varied by the method used (summary illustrations in Supplementary Figs. 4–5). No significant associations were observed between any measure of BWV and DBP, RHR, HDL-C or percent body fat. A significant inverse association was seen between NLMD and SBP for model 1 (β = −3.4 (1.5), p = 0.026) but for no other methods or models. Significant, direct associations were observed for LDL-C between some methods of BWV and in some models, though results were generally inconsistent. Similarly, some analyses showed significance for triglycerides and HbA1c, though results varied between methods and models and in direction and magnitude. One significant association was observed between BWV (by NLMD) and WHR, though this association was not present for any other models. The greatest variance was explained in the relationship between RMSE and change in LDL-C (1%), with all other relationships explaining <0.9% of the variance in outcomes and most at approximately 0%.
Baseline characteristics of the DXA sub-sample and associated model results are provided in Supplementary material 3. Results of the linear models can be viewed in Supplementary Table 3. The 439 individuals in this sub sample gained on average 0.9 (6.2) % body weight, accompanied by a 0.06 (4.7) % increase in body fat. Weight change was directly associated with change in body fat (p < 0.001 for all analyses) which explained between 9 and 11% of the variance. Significant, inverse associations between BWV (by CV and RMSE) and body fat (by DXA) were observed in all models (p < 0.05 for all), though these explained <0.5% of the change in body fat. No associations were observed for other measures of BWV.
4. Discussion
Despite the conclusions of a series of recent observational studies implicating BWV as a potential risk factor for cardiometabolic disease and mortality, the physiological mechanisms remain unclear. This was the first study to examine short-term associations of combined high-precision BWV estimates and weight change with concurrent changes in markers of cardiometabolic disease and body composition. We found that weight loss across 12 months was consistently associated with improvements in all indices of health and reduced percent body fat (as measured by both BIA and DXA). Associations between 12-month BWV and changes in cardiometabolic health markers were weak and inconsistent between models. We observed associations between BWV (by RMSE and CV) and change in percent body fat (by DXA) which were significant in all models but explained no more than 0.5% of the observed effect. In our sensitivity analysis (see Supplementary material 4) which employed a longitudinal structure by temporal separation of exposure and outcome, the results of the primary analysis were supported.
The associations between weight loss and improvements in health markers are well-supported by results from observational studies [34,35], clinical trials [3,36] and meta-analyses [37]. By standardising regression coefficients, we were able to directly compare the slope of each relationship (in addition to variance explained). Following adjustment, the strongest associations with 12-month weight change were seen for changes in SBP and percent body fat, followed by DBP and heart rate. Associations with changes in blood lipids and HbA1c were minor, consistent with previous research showing that body weight is more closely related to blood pressure than lipids [3,38], potentially because blood lipids are more strongly influenced by diet or exercise [39]. To adjust for the potentially confounding effect of PA we added initial and change in steps recorded from the Fitbit Charge 2.
We found that the associations between BWV and health outcomes were inconsistent between models and did not explain greater than 1% of the variance in responses. This is inconsistent with previous evidence suggested a detrimental role of BWV. For example, a previous study showed significant associations between greater self-reported history of weight cycling history and lower HDL-C in 485 women, however observed no associations on blood pressure, glucose and other blood lipids [17]. In a similar study, self-reported weight cycling history in 121 women was associated with increased waist circumference, resting metabolic rate (per kg) and adiponectin, however no impact on the metabolic risk factors measured in the present study [40]. In another study, self-reported weight cycling increased the risk of hypertension after 2 years [41], similar results reported by a recent study which reported this effect was mediated by increased visceral adipose tissue [16]. We found no associations between any measure of BWV and body composition or WHR, as proposed suggested previously [42,43]. Results from an analysis of 3632 Framingham health study participants showed increased in risk of becoming metabolically unhealthy (67%); getting type 2 diabetes (58%) and getting hypertension (74%) in those defined as having high BWV compared to stable body weight [44]. However, individuals with high BWV were also 163% more likely to have obesity. Further physiological evidence comes from a string of recent animal studies exposing mice to weight cycling which have shown detrimental effects on glucose [45] and insulin [46] levels, inflammatory markers [47] and hepatic steatosis [48]. These studies have the advantage of being able to accurately manipulate body weight, though physiological effects cannot necessarily be extrapolated to humans.
This study has several strengths. We estimated BWV using frequent measures of body weight (~3 times per week) over 12 months which attenuates the potential error associated with infrequent measurements used to estimate BWV in previous studies. Multiple methods of calculating BWV were employed due to heterogeneity in the statistical approaches used in previous studies. We devised a new method of calculating BWV based on critical evaluation of present methods, termed NLMD, which aimed to overcome the assumption of linearity associated with previous methods. We collected weight data using Wi-Fi-connected devices, overcoming the biases associated with self-reported data.
The present study has limitations. First, the sample were recent weight losers (mean = 11.8%) and therefore had experienced recent health improvements which may limit subsequent responses. This is supported by the observation that our sample had a mean BMI of 29.4 kg/m2 at baseline yet all health measures were, on average, within a healthy range. It has been hypothesised that BWV is a risk factor for disease specifically in clinically unhealthy populations [34,49] and therefore effects may be limited in this group. The BWV observed in the present group aiming to maintain weight loss may not be representative of the general population. Next, the sample was comprised mostly of individuals with overweight and obesity, though it is hypothesised that BWV has greater effect on health and body composition in lean individuals [42]. The exposures and outcomes were measured concurrently (over the same time period) and therefore causality cannot be inferred. To address this, we added a sensitivity analysis with a longitudinal structure (investigating the effect of 0–6 month BWV and weight change on subsequent changes in outcome variables), though results did not differ. Lastly, measurements were only made over 12 months, though many longitudinal studies showing detrimental effects of BWV occur over several years.
To conclude, we found little evidence to support the hypothesis that BWV has any substantial association with changes in risk factors for cardiometabolic disease or body composition over a 12-month measurement period in a sample who had recently lost ~11% body weight, though weight loss was consistently associated with health benefits as expected. With the recent uptake of smart scales in research trials, long-term studies must be conducted which track body weight frequently and examine effects on both hard outcomes and their associated physiological mechanisms. Together, these steps may help increase our understanding of the relationships between BWV and health.
Credit author statement
JT was involved in conceptualization of the secondary analysis conducted; JT, ROD and GH were responsible for the statistical methodology; JT was responsible for the primary analysis code; JT, ROD, CD, IS, JE, ALP, SCL, JO, BLH, RJS were responsible for conducting the research and collection of data; JT wrote the original manuscript; JS was responsible for supervision of the secondary analysis; BLH and JS were responsible for funding acquisition and project management; all authors were involved in review and editing of the final manuscript.
Declaration of competing interest
All named authors have nothing to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijchy.2020.100045.
Contributor Information
Jake Turicchi, Email: psjt@leeds.ac.uk.
Ruairi O'Driscoll, Email: psrod@leeds.ac.uk.
Graham Horgan, Email: g.horgan@abdn.ac.uk.
Cristiana Duarte, Email: c.duarte@leeds.ac.uk.
Inês Santos, Email: inescrsantos@gmail.com.
Jorge Encantado, Email: jencantado@fmh.ulisboa.pt.
Antonio L. Palmeira, Email: p126@ulusofona.pt.
Sofus C. Larsen, Email: sofus.larsen@regionh.dk.
Jack K. Olsen, Email: jack.kvistgaard.olsen@regionh.dk.
Berit L. Heitmann, Email: Berit.Lilienthal.Heitmann@regionh.dk.
R. James Stubbs, Email: r.j.stubbs@leeds.ac.uk.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Kivimäki M., Kuosma E., Ferrie J.E., Luukkonen R., Nyberg S.T., Alfredsson L. Overweight, obesity, and risk of cardiometabolic multimorbidity: pooled analysis of individual-level data for 120 813 adults from 16 cohort studies from the USA and Europe. Lancet Public Health. 2017 Jun 1;2(6):e277–e285. doi: 10.1016/S2468-2667(17)30074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forouhi N.G., Wareham N.J. Epidemiology of diabetes. Medicine. 2019;47:22–27. doi: 10.1016/j.mpmed.2014.09.007. (United Kingdom). Elsevier Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wing R.R., Lang W., Wadden T.A., Safford M., Knowler W.C., Bertoni A.G. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011 Jul;34(7):1481–1486. doi: 10.2337/dc10-2415. http://www.ncbi.nlm.nih.gov/pubmed/21593294 [Internet] [cited 2018 Jun 10];Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bangalore S., Fayyad R., Laskey R., DeMicco D.A., Messerli F.H., Waters D.D. Body-weight fluctuations and outcomes in coronary disease. N. Engl. J. Med. 2017 Apr 6;376(14):1332–1340. doi: 10.1056/NEJMoa1606148. http://www.nejm.org/doi/10.1056/NEJMoa1606148 [Internet] [cited 2017 Dec 22];Available from: [DOI] [PubMed] [Google Scholar]
- 5.Cologne J., Takahashi I., French B., Nanri A., Misumi M., Sadakane A. Association of weight fluctuation with mortality in Japanese adults. JAMA Netw. Open. 2019 Mar 15;2(3) doi: 10.1001/jamanetworkopen.2019.0731. http://www.ncbi.nlm.nih.gov/pubmed/30874785 [Internet] [cited 2019 May 15];Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park K.-Y., Hwang H.-S., Cho K.-H., Han K., Nam G.E., Kim Y.H. Body weight fluctuation as a risk factor for type 2 diabetes: results from a nationwide cohort study. J. Clin. Med. 2019 Jun 30;8(7):950. doi: 10.3390/jcm8070950. https://www.mdpi.com/2077-0383/8/7/950 [Internet] [cited 2019 Nov 13];Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhee E.-J., Cho J.H., Kwon H., Park S.E., Park C.-Y., Oh K.-W. Increased risk of diabetes development in individuals with weight cycling over 4 years: the Kangbuk Samsung Health study. Diabetes Res. Clin. Pract. 2018 May;139:230–238. doi: 10.1016/j.diabres.2018.03.018. http://www.ncbi.nlm.nih.gov/pubmed/29574105 [Internet] [cited 2019 Jan 7] Available from: [DOI] [PubMed] [Google Scholar]
- 8.Choi D., Choi S., Park M. Impact of weight variability on mortality among Korean men and women: a population based study. Sci. Rep. 2019 doi: 10.1038/s41598-019-46037-7. OPEN. [cited 2019 Nov 13]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nam G.E., Cho K.H., Han K., Han B., Cho S.J., Roh Y.K. Impact of body mass index and body weight variabilities on mortality: a nationwide cohort study. Int. J. Obes. 2018 May 17 doi: 10.1038/s41366-018-0079-0. http://www.ncbi.nlm.nih.gov/pubmed/29777238 [Internet] [cited 2019 Jan 7]; Available from: [DOI] [PubMed] [Google Scholar]
- 10.Kodama S., Fujihara K., Ishiguro H., Horikawa C., Ohara N., Yachi Y. Unstable bodyweight and incident type 2 diabetes mellitus: a meta-analysis. J Diabetes Investig. 2017 Jul;8(4):501–509. doi: 10.1111/jdi.12623. http://www.ncbi.nlm.nih.gov/pubmed/28083921 [Internet] [cited 2018 Jun 10] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kilpeläinen T., Kellow N.J., Teasdale S.B., Yu X., Zou H., Yin P. 2019. Body-Weight Fluctuation Was Associated with Increased Risk for Cardiovascular Disease, All-Cause and Cardiovascular Mortality: A Systematic Review and Meta-Analysis.www.frontiersin.org [cited 2019 Dec 9]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim M.K., Han K., Park Y.-M., Kwon H.-S., Kang G., Yoon K.-H. Associations of variability in blood pressure, glucose and cholesterol concentrations, and body mass index with mortality and cardiovascular outcomes in the general population. Circulation. 2018 Dec 4;138(23):2627–2637. doi: 10.1161/CIRCULATIONAHA.118.034978. https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.118.034978 [Internet] [cited 2019 Jan 7] Available from: [DOI] [PubMed] [Google Scholar]
- 13.Oh T.J., Moon J.H., Choi S.H., Lim S., Park K.S., Cho N.H. Body-weight fluctuation and incident diabetes mellitus, cardiovascular disease, and mortality: a 16-year prospective cohort study. J. Clin. Endocrinol. Metab. 2019 Mar 1;104(3):639–646. doi: 10.1210/jc.2018-01239. http://www.ncbi.nlm.nih.gov/pubmed/30500906 [Internet] [cited 2019 Jun 9] Available from: [DOI] [PubMed] [Google Scholar]
- 14.Saito Y., Takahashi O., Arioka H., Kobayashi D. Associations between body fat variability and later onset of cardiovascular disease risk factors. Meyre D., editor. PloS One. 2017 Apr 3;12(4) doi: 10.1371/journal.pone.0175057. http://dx.plos.org/10.1371/journal.pone.0175057 [Internet] [cited 2017 Dec 22] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yokomichi H., Ohde S., Takahashi O., Mochizuki M., Takahashi A., Yoda Y. Weight cycling and the subsequent onset of type 2 diabetes mellitus: 10-year cohort studies in urban and rural Japan. BMJ Open. 2017 Jun 8;7(5) doi: 10.1136/bmjopen-2016-014684. http://www.ncbi.nlm.nih.gov/pubmed/28596244 [Internet] [cited 2017 Dec 22] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeigler Z.S., Birchfield N., Moreno K., James D., Swan P. 2018. Fatness and Fluctuating Body Weight: Effect on Central Vasculature.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5994146/pdf/biores.2017.0044.pdf [cited 2018 Jun 21]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olson M.B., Kelsey S.F., Bittner V., Reis S.E., Reichek N., Handberg E.M. Weight cycling and high-density lipoprotein cholesterol in women: evidence of an adverse effect: a report from the NHLBI-sponsored WISE study. Women's Ischemia Syndrome Evaluation Study Group. J. Am. Coll. Cardiol. 2000 Nov 1;36(5):1565–1571. doi: 10.1016/s0735-1097(00)00901-3. http://www.ncbi.nlm.nih.gov/pubmed/11079659 [Internet] [cited 2019 Nov 13] Available from: [DOI] [PubMed] [Google Scholar]
- 18.Kroeger C.M., Hoddy K.K., Varady K.A. Impact of Weight Regain on Metabolic Disease Risk: a Review of Human Trials. J Obes. 2014;2014:614519. doi: 10.1155/2014/614519. http://www.ncbi.nlm.nih.gov/pubmed/25197563 [Internet] [cited 2018 Jun 10]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott S.E., Duarte C., Encantado J., Evans E.H., Harjumaa M., Heitmann B.L. The NoHoW protocol: a multicentre 2 × 2 factorial randomised controlled trial investigating an evidence-based digital toolkit for weight loss maintenance in European adults. BMJ Open. 2019 Sep 30;9(9) doi: 10.1136/bmjopen-2019-029425. http://www.ncbi.nlm.nih.gov/pubmed/31575569 [Internet] [cited 2019 Oct 3] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turicchi J., O'Driscoll R., Finlayson G., Duarte C., Palmeira A.L., Larsen S. Data imputation and body weight variability calculation using linear and non-linear methods in data collected from digital smart scales: a simulation and validation study. JMIR Prepr. 2020 doi: 10.2196/17977. https://preprints.jmir.org/preprint/17977 [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaffer J.A., Diaz K., Alcántara C., Edmondson D., Krupka D.J., Chaplin W.F. An inexpensive device for monitoring patients' weights via automated hovering. Int. J. Cardiol. 2014;172(2):263–264. doi: 10.1016/j.ijcard.2013.12.123. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3984000/pdf/nihms566520.pdf [Internet] [cited 2019 Apr 19] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moissl U.M., Wabel P., Chamney P.W., Bosaeus I., Levin N.W., Bosy-Westphal A. Body fluid volume determination via body composition spectroscopy in health and disease. Physiol. Meas. 2006 Sep 1;27(9):921–933. doi: 10.1088/0967-3334/27/9/012. [DOI] [PubMed] [Google Scholar]
- 23.Feehan L.M., Geldman J., Sayre E.C., Park C., Ezzat A.M., Young Yoo J. Accuracy of fitbit devices: systematic review and narrative syntheses of quantitative data. JMIR mHealth and uHealth. 2018;6 doi: 10.2196/10527. JMIR Publications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Driscoll R., Turicchi J., Beaulieu K., Scott S., Matu J., Deighton K. How well do activity monitors estimate energy expenditure? A systematic review and meta-analysis of the validity of current technologies. Br. J. Sports Med. 2018 Sep 7 doi: 10.1136/bjsports-2018-099643. http://www.ncbi.nlm.nih.gov/pubmed/30194221 [Internet] [cited 2018 Nov 20];bjsports-2018-099643. Available from: [DOI] [PubMed] [Google Scholar]
- 25.Lee J., Kawakubo K., Kobayashi Y., Mori K., Kasihara H., Tamura M. PAPER Effects of ten year body weight variability on cardiovascular risk factors in Japanese middle-aged men and women. Int. J. Obes. 2001;25:1063–1067. doi: 10.1038/sj.ijo.0801633. https://www.nature.com/articles/0801633.pdf [Internet] [cited 2017 Dec 22] Available from: [DOI] [PubMed] [Google Scholar]
- 26.French S.A., Jeffery R.W., Folsom A.R., Williamson D.F., Byers T. Relation of weight variability and intentionality of weight loss to disease history and health-related variables in a population-based sample of women aged 55-69 years. Am. J. Epidemiol. 1995;142(12) doi: 10.1093/oxfordjournals.aje.a117598. http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.1008.4671&rep=rep1&type=pdf [Internet] [cited 2017 Dec 22] Available from: [DOI] [PubMed] [Google Scholar]
- 27.Folsom A.R., French S.A., Zheng W., Baxter J.E., Jeffery R.W. Weight variability and mortality: the Iowa women's health study. Int. J. Obes. Relat. Metab. Disord. 1996 Aug;20(8):704–709. http://www.ncbi.nlm.nih.gov/pubmed/8856391 [Internet] [cited 2019 Jan 7] Available from: [PubMed] [Google Scholar]
- 28.Taylor C.B., Jatulis D.E., Fortmann S.P., Kraemer H.C. Weight variability effects: a prospective analysis from the stanford five-city project. Am. J. Epidemiol. 1995 Mar 1;141(5):461–465. doi: 10.1093/oxfordjournals.aje.a117448. http://www.ncbi.nlm.nih.gov/pubmed/7879790 [Internet] [cited 2019 Oct 15] Available from: [DOI] [PubMed] [Google Scholar]
- 29.Nakanishi N., Nakamura K., Suzuki K., Tatara K. Effects of weight variability on cardiovascular risk factors; a study of nonsmoking Japanese male office workers. Int. J. Obes. Relat. Metab. Disord. 2000 Sep;24(9):1226–1230. doi: 10.1038/sj.ijo.0801389. http://www.ncbi.nlm.nih.gov/pubmed/11033995 [Internet] [cited 2019 Oct 15] Available from: [DOI] [PubMed] [Google Scholar]
- 30.Yeboah P., Hsu F.-C., Bertoni A.G., Yeboah J. Body mass index, change in weight, body weight variability and outcomes in type 2 diabetes mellitus (from the ACCORD trial) Am. J. Cardiol. 2019 Feb 15;123(4):576–581. doi: 10.1016/j.amjcard.2018.11.016. http://www.ncbi.nlm.nih.gov/pubmed/30553512 [Internet] [cited 2019 Oct 15] Available from: [DOI] [PubMed] [Google Scholar]
- 31.Vickers A.J., Altman D.G. Analysing controlled trials with baseline and follow up measurements. Br. Med. J. 2001;323:1123–1124. doi: 10.1136/bmj.323.7321.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tudor-Locke C., Craig C.L., Brown W.J., Clemes S.A., De Cocker K., Giles-Corti B. How many steps/day are enough? for adults. Int. J. Behav. Nutr. Phys. Activ. 2011 Jul 28:8. doi: 10.1186/1479-5868-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Houston M.C., Basile J., Bestermann W.H., Egan B., Lackland D., Hawkins R.G. American Journal of the Medical Sciences. Lippincott Williams and Wilkins; 2005. Addressing the global cardiovascular risk of hypertension, dyslipidemia, and insulin resistance in the Southeastern United States; pp. 276–291. [DOI] [PubMed] [Google Scholar]
- 34.Aucott L.S., Philip S., Avenell A., Afolabi E., Sattar N., Wild S. Patterns of weight change after the diagnosis of type 2 diabetes in Scotland and their relationship with glycaemic control, mortality and cardiovascular outcomes: a retrospective cohort study. BMJ Open [Internet] 2016 Jul 1;6(7):e010836. doi: 10.1136/bmjopen-2015-010836. https://bmjopen.bmj.com/content/6/7/e010836 [cited 2020 Aug 10]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sabaka P., Dukat A., Gajdosik J., Bendzala M., Caprnda M., Simko F. The effects of body weight loss and gain on arterial hypertension control: an observational prospective study. Eur. J. Med. Res. 2017;22:43. doi: 10.1186/s40001-017-0286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamman R.F., Wing R.R., Edelstein S.L., Lachin J.M., Bray G.A., Delahanty L. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006 Sep;29(9):2102–2107. doi: 10.2337/dc06-0560. http://www.ncbi.nlm.nih.gov/pubmed/16936160 [Internet] [cited 2019 Jan 6] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma C., Avenell A., Bolland M., Hudson J., Stewart F., Robertson C. Effects of weight loss interventions for adults who are obese on mortality, cardiovascular disease, and cancer: systematic review and meta-analysis. BMJ. 2017 Nov 14;359:j4849. doi: 10.1136/bmj.j4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wadden T.A., Anderson D.A., Foster G.D. Two-year changes in lipids and lipoproteins associated with the maintenance of a 5 % to 10% reduction in initial weight: some findings and some questions. Obes. Res. 1999;7(2):170–178. doi: 10.1002/j.1550-8528.1999.tb00699.x. [DOI] [PubMed] [Google Scholar]
- 39.Clifton P.M. Diet, exercise and weight loss and dyslipidaemia. Pathology. 2019;51:222–226. doi: 10.1016/j.pathol.2018.10.013. Elsevier B.V. [DOI] [PubMed] [Google Scholar]
- 40.Strychar I., Lavoie M.-È., Messier L., Karelis A.D., Doucet É., Prud’homme D. Anthropometric, metabolic, psychosocial, and dietary characteristics of overweight/obese postmenopausal women with a history of weight cycling: a monet (Montreal Ottawa new Emerging team) study. J. Am. Diet Assoc. 2009 Apr;109(4):718–724. doi: 10.1016/j.jada.2008.12.026. http://www.ncbi.nlm.nih.gov/pubmed/19328269 [Internet] [cited 2018 Jun 21] Available from: [DOI] [PubMed] [Google Scholar]
- 41.Schulz M., Liese A.D., Boeing H., Cunningham J.E., Moore C.G., Kroke A. Associations of short-term weight changes and weight cycling with incidence of essential hypertension in the EPIC-Potsdam study. J. Hum. Hypertens. 2005 Jan;19(1):61–67. doi: 10.1038/sj.jhh.1001776. [DOI] [PubMed] [Google Scholar]
- 42.Montani J.-P., Schutz Y., Dulloo A.G. Dieting and weight cycling as risk factors for cardiometabolic diseases: who is really at risk? Obes. Rev. 2015 Feb;16:7–18. doi: 10.1111/obr.12251. http://www.ncbi.nlm.nih.gov/pubmed/25614199 [Internet] [cited 2018 Jun 10] Available from: [DOI] [PubMed] [Google Scholar]
- 43.Rodin J., Radke-Sharpe, Rebuffé-Scrive M., Greenwood M. Weight cycling and fat distribution. Int. J. Obes. 1994;14(4):303–310. [PubMed] [Google Scholar]
- 44.Sponholtz T.R., van den Heuvel E.R., Xanthakis V., Vasan R.S. Association of variability in body mass index and metabolic health with cardiometabolic disease risk. J Am Heart Assoc. 2019;8(7) doi: 10.1161/JAHA.118.010793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schofield S.E., Parkinson J.R.C., Henley A.B., Sahuri-Arisoylu M., Sanchez-Canon G.J., Bell J.D. Metabolic dysfunction following weight cycling in male mice. Int. J. Obes. 2017;41(3):402–411. doi: 10.1038/ijo.2016.193. http://www.ncbi.nlm.nih.gov/pubmed/27840414 [Internet] [cited 2018 Jun 10] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simonds S.E., Pryor J.T., Cowley M.A. Repeated weight cycling in obese mice causes increased appetite and glucose intolerance. Physiol. Behav. 2018 Oct;194:184–190. doi: 10.1016/j.physbeh.2018.05.026. http://linkinghub.elsevier.com/retrieve/pii/S0031938418302531 [Internet] [cited 2018 Jun 10]. Available from: [DOI] [PubMed] [Google Scholar]
- 47.Li X., Jiang L., Yang M., Wu Y.-W., Sun J.-Z. Impact of weight cycling on CTRP3 expression, adipose tissue inflammation and insulin sensitivity in C57BL/6J mice. Exp Ther Med. 2018 Sep;16(3):2052–2059. doi: 10.3892/etm.2018.6399. http://www.ncbi.nlm.nih.gov/pubmed/30186439 [Internet] [cited 2019 Jan 7] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barbosa-da-Silva S., Fraulob-Aquino J.C., Lopes J.R., Mandarim-de-Lacerda C.A., Aguila M.B. Weight cycling enhances adipose tissue inflammatory responses in male mice. Federici M., editor. PloS One. 2012 Jul 25;7(7) doi: 10.1371/journal.pone.0039837. http://www.ncbi.nlm.nih.gov/pubmed/22848362 [Internet] [cited 2019 Jan 7] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zoppini G., Verlato G., Targher G., Bonora E., Trombetta M., Muggeo M. Variability of body weight, pulse pressure and glycaemia strongly predict total mortality in elderly type 2 diabetic patients. The Verona Diabetes Study. Diabetes Metab Res Rev. 2008 Nov;24(8):624–628. doi: 10.1002/dmrr.897. http://www.ncbi.nlm.nih.gov/pubmed/18802932 [Internet] [cited 2018 Jun 10] Available from: [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.