Abstract
Background
Managing blood pressure reduces CVD risk, but optimal treatment thresholds remain unclear as it is a balancing act to avoid hypotension-related adverse events.
Objectives
This systematic review, meta-analysis and meta-regression evaluated the benefits of intensive BP treatment in hypertensive older adults.
Methods
We systematically searched PubMed, MEDLINE, EMBASE, and the Cochrane Library of Controlled Trials until January 31, 2020. Studies comparing different BP treatments/targets and/or active BP against placebo treatment, with a minimum 12 months follow-up, were included. Risk ratios (RR) and 95% CIs were calculated using a random effects model. The primary outcome was RR of major cardiovascular events (MCEs); secondary outcomes included myocardial infarction (MI), stroke, heart failure (HF), cardiovascular (CV) mortality, and all-cause mortality.
Results
We included 16 studies totaling 65,890 hypertensive participants (average age 69.4 years) with a follow-up period from 1.8 to 4.9 years. Intensive BP treatment significantly reduced the relative risk of MCEs by 26% (RR:0.74, 95%CI 0.64–0.86, p = 0.000; I2 = 79.71%). RR of MI significantly reduced by 13% (RR:0.87, 95%CI 0.76–1.00, p = 0.052; I2 = 0.00%), stroke by 28% (RR:0.72, 95%CI 0.64–0.82, p = 0.000; I2 = 32.45%), HF by 47% (RR:0.53, 95% CI 0.43–0.66, p = 0.000; I2 = 1.23%), and CV mortality by 24% (RR:0.76, 95%CI 0.66–0.89, p = 0.000; I2 = 39.74%). All-cause mortality reduced by 17% (RR:0.83, 95%CI 0.73–0.93, p = 0.001; I2 = 53.09%). Of the participants - 61% reached BP targets and 5% withdrew; with 1 hypotension-related event per 780 people treated.
Conclusions
Lower BP treatment targets are optimal for CV protection, effective, well-tolerated and safe, and support the latest hypertension guidelines.
Keywords: Hypertension, Blood pressure treatment targets, Cardiovascular events, Mortality
Abbreviations: BP, blood pressure; CI, confidence interval; CV, cardiovascular; CVD, cardiovascular disease; HF, heart failure; MCE, major cardiovascular event; RR, relative risk or risk ratio
Highlights
-
•
Question: What is the optimal blood pressure target in older adults with hypertension?
-
•
Findings: Intensive blood pressure treatment reduces RR of MCEs and all-cause mortality; it is well tolerated and safe.
-
•
Meaning: Systolic BP targets of <130 mmHg are optimal for cardiovascular protection & support the ACC/AHA hypertension guidelines.
1. Introduction
Hypertension remains a major global disease burden, being the primary risk factor for cardiovascular disease (CVD), the leading cause of death worldwide [1]. Hypertension prevalence rises with age [2] and the financial cost and related complications are significant [3].
The benefits of BP reduction on CVD risk are well-known, but the optimal treatment threshold remains unclear as treatment targets vary across randomized control trials [[4], [5], [6], [7], [8], [9], [10], [11], [12], [13]]. Some studies recommend a target BP of <140/90 mmHg for the management of hypertension in elderly patients but the National Institutes of Health (NIH) Systolic Blood Pressure Intervention Trial (SPRINT) showed a systolic BP target of <120 mmHg resulted in significantly lower rates of major cardiovascular events and all-cause mortality even in elderly hypertensive patients [14]. This work contributed to a re-evaluation of the guideline recommendations for management of hypertension. The current American College of Cardiology/American Heart Association (ACC/AHA) recommends a systolic BP treatment target of <130 mmHg in adults irrespective of the age [15]. However, the 2018 European Society of Cardiology/European Society of Hypertension (ESC/ESH) guidelines indicate a systolic BP target of <140 mmHg in all patients and a lower target of between 120 and 129 mmHg in adults below 65 years [14].
Previous meta-analyses have shown that a reduction in systolic BP (<120–130 mmHg) could significantly reduce CVD risk [[16], [17], [18], [19], [20]] especially in patients with chronic kidney disease, vascular diseases or diabetes. In people with diabetes, intensive BP treatment offers greater vascular protection [21], a significant reduction in all-cause mortality, albuminuria progression and CV events [19] and significant risk reductions for MCEs [17]. In mixed populations, intensive BP treatment resulted in improved cardiovascular protection [18] and decreased heart failure risk [22]. In contrast, Takami et al. [23] found no reductions in composite cardiovascular outcomes. Intensive blood pressure treatment is not devoid of adverse effects secondary to hypotension and may result in dizziness, syncope, transient cognitive impairment and possible organ failure [21].
Our primary aim was to establish if intensive BP treatment is beneficial in hypertensive patients without comorbid diseases. A second aim was to calculate the number of patients needed to treat (NNT) for intensive anti-hypertensive therapy, as well as the number needed to harm (NNH) for events that were caused by hypotension.
2. Methods
2.1. Search strategy
A systematic search of the literature was performed using PubMed, MEDLINE, EMBASE, and the Cochrane Library of Controlled Trials until January 31, 2020. Search criteria included numerous terms, both free text and MeSH including (intensive or strict or tight or low or optimal or active) AND (“blood pressure” or antihypertens∗) AND (treatment or therapy or target or goal or lowering or control) AND (“cardiovascular outcomes” or mortality or morbidity) (see Supplementary Table S1). Systematic reviews and study bibliographies were reviewed for additional studies. This study was restricted to randomized control trials (RCTs) with no language restrictions. Two reviewers (BB,GD) conducted the search and full article eligibility review.
2.2. Study selection
Studies comparing different BP treatments/targets and/or intensive BP treatment against a standard BP treatment (with or without placebo) in hypertensive adults with a minimum follow-up of 12 months were included. Sub-analyses of trials of other diseased populations that reported on a hypertensive-only subgroup were also included. We excluded studies that compared BP reduction treatments by assessing one antihypertensive agent against another or combined interventions.
2.3. Analyses
2.3.1. Data extraction, outcome measures and secondary analyses
Data extraction was conducted by two investigators (BB,GD). The primary outcome measure was relative risk of MCEs and secondary outcome measures included MI, stroke, HF, CV mortality and all-cause mortality.
Secondary analyses were performed on study follow-up duration, systolic BP treatment target effects on outcome events, NNT and NNH. Clinically the years of follow-up as well as the set BP targets are important to infer whether the systolic BP target matters in terms of intensity (aggressiveness) as recommended in the recent American hypertension guidelines. In addition, in accounting for NNT and NNH, the number of years of follow-up is clinically relevant.
2.3.2. Meta-regression for covariates
Covariates included follow-up years and upper systolic BP treatment targets; these were used to perform meta-regression analysis on all outcome events.
2.3.3. Statistical analysis
Risk ratios (RRs) and 95% confidence intervals (CIs) were calculated for each outcome of the individual included study trials. For the combined analysis, the random effects model was used; Forest plots were generated to provide visual representation of the effect of intensive BP treatment on RRs for outcome measures. Sub-analyses were performed using two follow-up durations (<3 versus ≥3 years) and systolic BP treatment targets (<160, 150, 140, 130 and 120 mmHg). To avoid the limitation of an arbitrary cut-off, meta-regression analysis was performed (BB) to investigate the heterogeneity of results using study follow-up years and systolic BP treatment targets as covariates with a 5% level of significance and 95% CI [24] as it uses a continuous data model of follow up duration. All analyses were carried out using Comprehensive Meta-Analysis (CMA) V3 (Biostat Inc., NJ, USA).
2.4. Number needed to treat and number needed to harm
We calculated NNT (BB,MP) for all outcome measures to determine the effectiveness of the treatment [25]. NNH was calculated by recording the number of events related to hypotensive episodes (e.g. dizziness, syncope or hypotension) to determine how many patients need to be treated for one to have an adverse effect. The NNT and NNH were calculated using the pooled RR, as per the formula provided by the Cochrane Handbook for Systematic Reviews [26]. As recommended, in calculating the NNT, a number of values for the control event rate (CER) were applied, with a mean CER used to calculate presented NNT values [26,27].
2.5. Heterogeneity and publication bias
The I-squared (I2) test was used to determine variation across the included studies due to heterogeneity rather than chance. I2 values of <25% are considered low risk, 50% moderate risk, whereas >75% show a high risk of heterogeneity [28]. Publication bias was evaluated by visual inspection of the funnel plot for all outcomes with Egger's regression test [29].
2.6. Quality assessment
The methodological quality of the included studies was assessed (BB,GD) using a modified JADAD scale [30]. Three domains were assessed based on study description as randomization (score: 0–2), blinding (score: 0–2), and account of all patients included (score: 0–1) for a maximum score of 5. Any study with a total score ≤2 was described as low quality and ≥3 was considered high quality.
3. Results
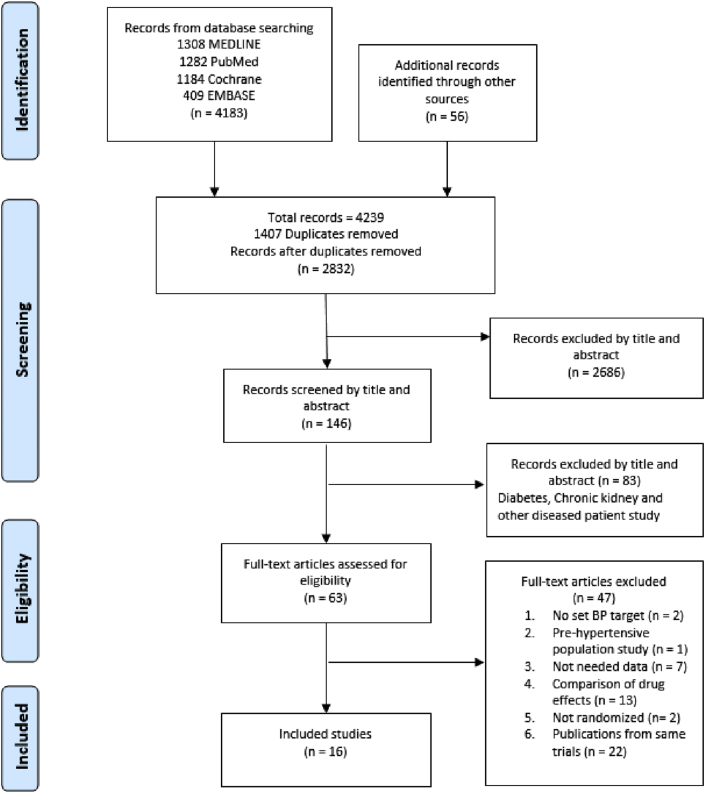
The systematic database search identified a total of 4239 records. An additional 56 records were found in reference lists from the initial search. After removal of duplicates, 2832 manuscripts were screened by title and abstract yielding 146 publications (Fig. 1). Of these, 83 publications were excluded because their study populations included participants with comorbidities. A further 47 articles were excluded for not meeting inclusion criteria. Sixteen randomized controlled trials on BP treatments were included in the final selection.
Fig. 1.
PRISMA flow diagram.
3.1. Study characteristics
Seventeen comparisons were considered from the 16 included studies [[4], [5], [6], [7], [8], [9], [10], [11], [12], [13],[31], [32], [33], [34], [35], [36]] (Table 1) totaling 65,890 participants of which 36,599 were treated ‘intensively’ and 29,291 ‘standardly’. All participants were hypertensive. Average participant age was 69.4 (±7.1) years with 52.4% female participants. Trials were conducted worldwide and had an average follow-up of 3.2 years (±8 months), ranging from 1.8 to 4.9 years. Participants in the intensive BP treatment groups (ITx) were medicated with up to 3 medications to reach the specific BP target. All control groups received a standard BP treatment (STx) with a set value for their treatment target while some studies administered a placebo to the participants. Eight studies [5,6,[8], [9], [10],13,31,33] compared intensive to standard BP treatment targets while 8 others [4,7,11,12,32,[34], [35], [36]] compared intensive BP treatment to standard treatment plus placebo. Nine studies [4,5,11,12,31,32,[34], [35], [36]] had intensive BP targets of <150–160 mmHg for systolic BP and <80–90 mmHg for diastolic BP, while seven others [[6], [7], [8], [9], [10],13,33] had systolic BP targets <120–140 mmHg. Mean baseline BP of study participants was 165.9/90.6 mmHg (range: 139.4/75–195/102 mmHg) in the intensive cohorts and 166.1/90.7 mmHg (range: 139.3/75–195/105 mmHg) in the standard BP treatment group. Overall, means of achieved BP were 140.7/79.1 and 150.1/83.5 mmHg in the intensive and standard BP treatment groups, respectively. Achieved systolic BP reduction from baseline ranged from 14 to 35.7 mmHg in intensive versus 3–27.7 mmHg in the standard cohorts while diastolic BP reductions ranged from 5 to 23.9 mmHg in intensive cohorts versus 1.9–19.8 mmHg in the standard cohorts. The characteristics of the included studies are summarized in Table 1.
Table 1.
Characteristics of included studies.
| Study/Author | Study Design/Country | Population |
Target BP for ITX and STX (mmHg) |
Follow-Up Years | Baseline BP (mmHg) |
Achieved BP (mmHg) |
Achieved BP reduction (mmHg) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | N | Female % | ITx n | STx N | ITx | STx | ITx | STx | ITx | STx | |||
| ANBP 1981 [35] | Randomized/Australia | 63.6 | 582 | 45.4 | <80 293 |
NR Placebo 289 |
3.9 | 166.3/100.7 | 163.9/100.4 | NR/87.3 | NR/93.7 | NR/13.4 | NR/6.7 |
| BBB 1994 [31] | Randomized multicentre/Sweden | 60 | 2127 | 53.9 | ≤80 1064 |
90–100 1063 |
4.9 | 155/95 | 155/94 | 141/83 | 152/91 | 14/12 | 3/3 |
| Cardio-Sis 2009 [8] | Randomized multicentre/Italy | 67 | 1111 | 59 | <130 558 |
<140 553 |
2 | 163.3/89.6 | 163.3/89.7 | 131.9/77.4 | 135.6/78.7 | 31.4/12.2 | 27.7/11 |
| COPE 2017 [13] Sub-analysis | Randomized multicentre/Japan | 63.3 | 3001 | 49.8 | <140/90 1733 |
≥140/90 1268 |
3.7 | 151.4/88.1 | 157.3/89.4 | 128/74 | 142/80 | 23.4/14.1 | 15.3/9.4 |
| FEVER 2005 [7] | Randomized multicentre/China | 61.5 | 9711 | 39.0 | <140 4841 |
<160/90 Placebo 4870 |
3.3 | 154.2/91 | 154.4/91.3 | 138.1/82.3 | 141.6/83.9 | 16.2/8.7 | 12.8/7.4 |
| HOT 1998a [5] | Randomized multicentre/Europe, Asia North/South America | 61.5 | 9394 | 47 | ≤80 6262 |
≤90 3132 |
3.8 | 170/105 | 170/105 | 140.1/81.1 | 143.8/85.2 | 29.9/23.9 | 26.2/19.8 |
| HOT 1998b [5] | Randomized multicentre/Europe, Asia North/South America | 61.5 | 9396 | 47 | ≤85 6264 |
≤90 3132 |
3.8 | 170/105 | 170/105 | 142.0/83.2 | 143.8/85.2 | 28/21.8 | 26.2/19.8 |
| HYVET 2008 [4] | Randomized multicentre/Europe, China, Australasia, Tunisia | 83.6 | 3845 | 60.5 | <150/80 1933 |
NR Placebo 1912 |
2 | 173.0/90.8 | 173.0/90.8 | 143.5/77.9 | 158.5/84.0 | 29.5/12.9 | 14.5/6.8 |
| JATOS 2008 [6] | Randomized multicentre/Japan | 73.6 | 4418 | 61.1 | <140 2212 |
140–160 2206 |
2 | 171.6/89.1 | 171.5/89.1 | 135.9/74.8 | 145.6/78.1 | 35.7/14.3 | 25.9/11 |
| SCOPE 2003 [32] | Randomized multicentre/Europe | 76.4 | 4937 | 64.5 | <160/85 2477 |
<160/90 Placebo 2460 |
3.7 | 166.0/90.3 | 166.5/90.4 | 145.2/79.9 | 148.5/81.6 | 20.8/10.4 | 18/8.8 |
| SHEP 1989 [34] | Randomized multicentre/USA | 72 | 551 | 63 | <160 443 |
NR Placebo 108 |
2.8 | 172/75 | 172/75 | 141/68 | 157/73 | 31/7 | 15/2 |
| SPRINT 2019 [10] Sub-analysis | Randomized multicentre/USA, Puerto Rico | 62.4 | 4298 | 33.5 | <120 2148 |
<140 2150 |
3.12 | 139.4/81.8 | 139.3/81.9 | 122.8/NA | 135.3/NR | 16.6 | 4/NR |
| STOP- Hypertension 1991 [11] | Randomized multicentre/Sweden | 75.7 | 1627 | 63 | <160/95 812 |
NR Placebo 815 |
2.1 | 195/102 | 195/102 | 167/87 | 186/96 | 28/15 | 9/6 |
| Syst-China 1998 [12] | Randomized multicentre/China | 66.5 | 2394 | 35.7 | <150 1253 |
NR Placebo 1141 |
3 | 170.7/86.1 | 170.2/85.9 | 150.7/81.1 | 159.3/84 | 20/5 | 10.9/1.9 |
| Syst-Eur 1997 [36] |
Randomized multicentre/Europe | 70.2 | 4695 | 66.8 | <150 2398 |
NR Placebo 2297 |
2 | 173·9/85.5 | 173·8/85.5 | 150.9/78.5 | 160.8/83.5 | 23/7 | 13/2 |
| VALISH 2010 [33] | Randomized multicentre/Japan | 76.1 | 3079 | 62.4 | <140 1545 |
≥140 -<150 1534 |
3 | 169.5/81.7 | 169.6/81.2 | 136.6/74.8 | 142.0/76.5 | 32.9/6.9 | 27.6/4.7 |
| Wei et al., 2013 [9] | Randomized single centre/China | 76.6 | 724 | 33.7 | <140/90 363 |
<150/90 361 |
4 | 158.8/83.7 | 160.3/84.8 | 135.7/76.2 | 149.7/82.1 | 23.1/7.5 | 10.6/2.7 |
ANBP: Australia National Blood Pressure, BBB: Behandla Blodtryck Battre, Cardio-Sis: Studio Italiano Sugli Effetti Cardiovascolari del Controllo della Pressione Arteriosa Sistolica, COPE: Combination Therapy of Hypertension to Prevent Cardiovascular Events, DBP: diastolic blood pressure, FEVER: Felodipine Event Reduction, HOT: Hypertension Optimal Treatment, HPT: hypertensive, HYVET: Hypertension in the Very Elderly Trial, ITx: Intensive treatment, JATOS: Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients, n: number, NR: not reported, SBP: systolic blood pressure, SCOPE: Study on Cognition and Prognosis in the Elderly, SHEP: Systolic Hypertension in the Elderly Program, SPRINT: Systolic Blood Pressure Intervention Trial, STOP-Hypertension: Swedish Trial in Old Patients with Hypertension, STx: standard treatment, Syst-China: Systolic Hypertension in China, Syst-Eur: Systolic Hypertension in Europe, VALISH: Valsartan in Elderly Isolated Systolic Hypertension.
3.2. Medication protocols used in the included studies
Five studies [5,6,9,12,36] utilized calcium channel blockers (CCBs) as first-line treatment, four studies [4,10,34,35] used diuretics and two [32,33] used angiotensin–II–receptor blockers (ARBs) exclusively, three studies employed angiotensin-converting-enzyme inhibitors (ACEIs) [[8], [9], [10]] and 4 used beta-blockers (BB) [8,9,11,13] as first-line treatment. Four studies [8,10,11,13] used a combination of 2 drug treatments as the first line treatment (see Supplementary Table S2).
3.3. Effect of intensive BP treatment on cardiovascular events and mortality
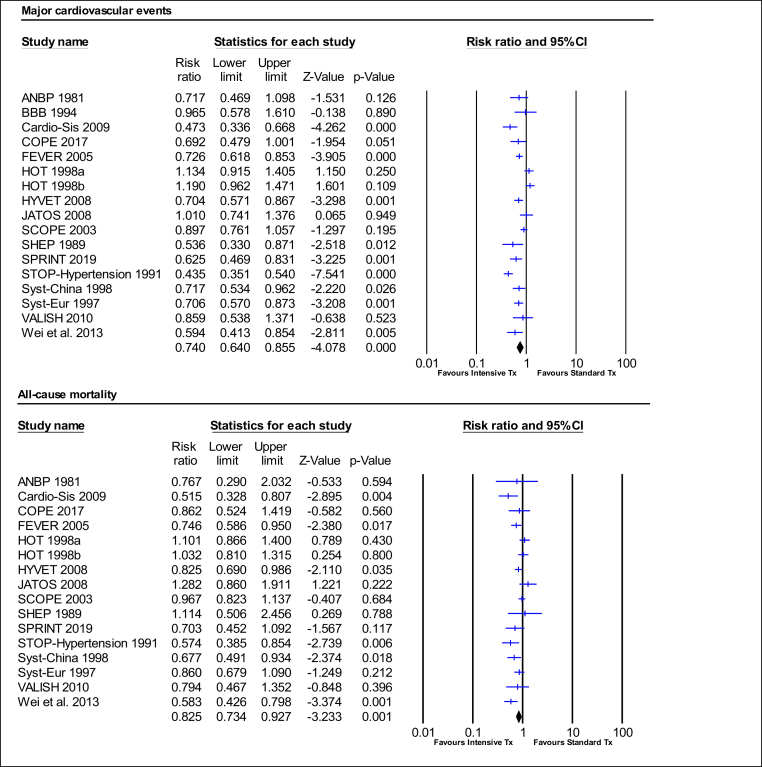
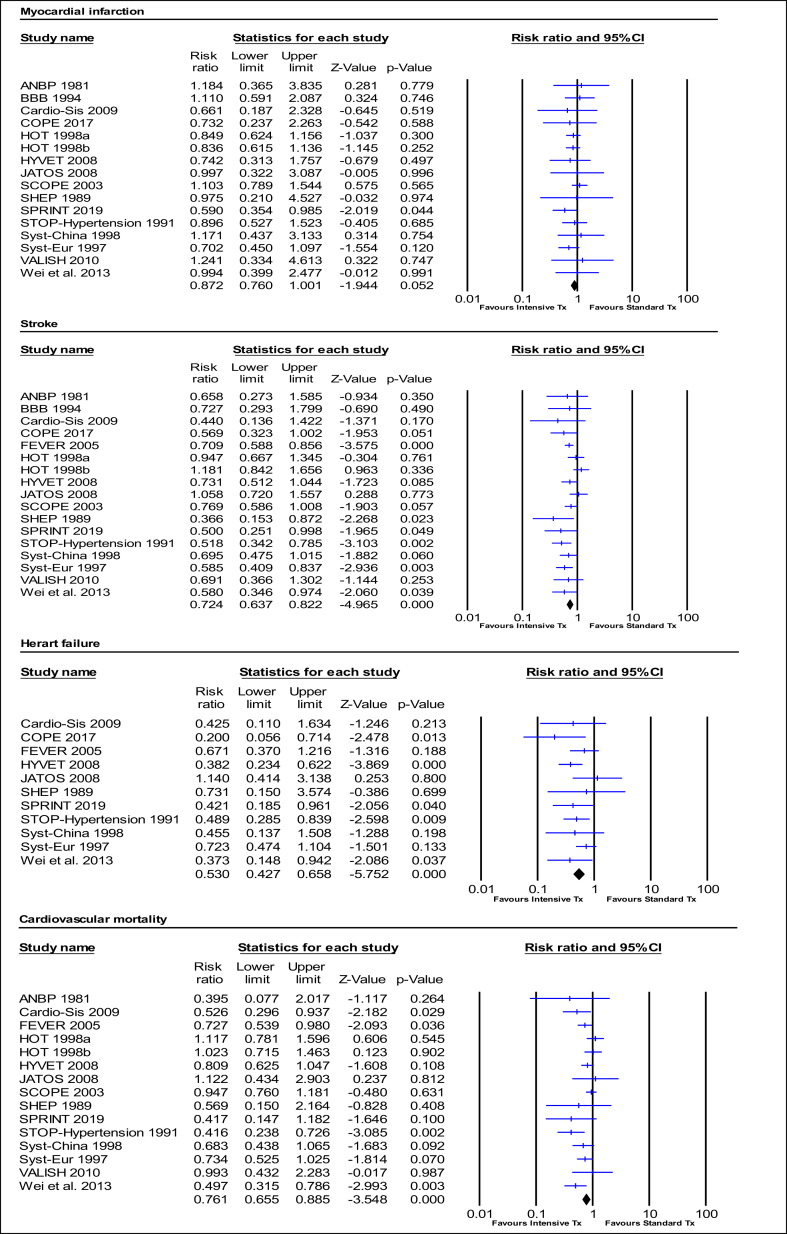
Intensive BP treatment significantly reduced the relative risk of MCEs by 26% (RR:0.74, 95%CI 0.64–0.86, p = 0.000; I2 = 79.71%). The relative risk of MI was reduced by 13% (RR:0.87, 95%CI 0.76–1.00, p = 0.052; I2 = 0.00%), stroke by 28% (RR:0.72, 95%CI 0.64–0.82, p = 0.000; I2 = 32.45%), HF by 47% (RR:0.53, 95% CI 0.43–0.66, p = 0.000; I2 = 1.23%), and cardiovascular mortality by 24% (RR:0.76, 95%CI 0.66–0.89, p = 0.000; I2 = 39.74%). Intensive BP treatment also significantly reduced the relative risk of all-cause mortality by 17% (RR:0.83, 95%CI 0.73–0.93, p = 0.001; I2 = 53.09%) (Fig. 2, Fig. 3, Table 2).
Fig. 2.
Effect of intensive BP treatment on relative risk of major cardiovascular event and all-cause mortality.
A p-value <0.05 represents a significant pooled point of estimate of risk ratio. Short vertical lines across each horizontal lines and horizontal lines represents risk ratio and 95% confidence interval (CI) for each study. The vertical line on the scale 1 interval across all horizontal lines represents the estimate of overall risk ratio. The diamond represents the 95% CI for pooled estimates of effect of risk ratio. Tx represent treatment.
Fig. 3.
Effect of intensive BP treatment on relative risk of cardiovascular outcome events.
A p-value <0.05 represents a significant pooled point of estimate of risk ratio. Short vertical lines across each horizontal lines and horizontal lines represents risk ratio and 95% confidence interval (CI) for each study. The vertical line on the scale 1 interval across all horizontal lines represents the estimate of overall risk ratio. The diamond represents the 95% CI for pooled estimates of effect of risk ratio. Tx represent treatment.
Table 2.
Summary of effects of intensive BP treatment on outcome events.
| Outcome events | Studies (Comparison) | N |
Events |
Risk ratio | 95% CI | p-value | Heterogeneity |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| ITx | STx | ITx | STx | I2 | p-value | |||||
| Major cardiovascular events | 16 (17) | 36599 | 29291 | 1892 | 2071 | 0.740 | 0.64–0.86 | 0.000 | 79.71 | 0.000 |
| Myocardial infarction | 15 (16) | 31758 | 24421 | 447 | 377 | 0.872 | 0.76–1.00 | 0.052 | 0.00 | 0.953 |
| Stroke | 16 (17) | 36599 | 29291 | 795 | 926 | 0.724 | 0.64–0.82 | 0.000 | 32.45 | 0.097 |
| Heart failure | 11 (11) | 18694 | 17681 | 134 | 242 | 0.530 | 0.43–0.66 | 0.000 | 1.23 | 0.430 |
| Cardiovascular mortality | 14 (15) | 33802 | 26960 | 688 | 744 | 0.761 | 0.66–0.89 | 0.000 | 39.74 | 0.057 |
| All-cause mortality | 15 (16) | 35535 | 28228 | 1449 | 1424 | 0.825 | 0.73–0.93 | 0.001 | 53.09 | 0.006 |
CI: confidence interval, I2: heterogeneity, ITx: Intensive treatment, N: total number of participants, STx: standard treatment.
3.3.1. Sub-analyses of outcome events
Sub-analyses of diastolic versus systolic BP treatment targets revealed that setting systolic BP treatment targets was significantly more effective in reducing the relative risk of cardiovascular events (except MI) and all-cause mortality (Supplementary Table S3, Figs. S1 and S2), with the largest difference (systolic vs diastolic BP treatment targets) in the relative risk of cardiovascular mortality at 32%. Sub-analyses to investigate the effect of follow-up duration (<3 versus ≥3 years) and different systolic BP treatment targets on cardiovascular events and all-cause mortality showed that a follow-up duration <3 years had significantly greater relative risk reduction for all outcome events, except MI and all-cause mortality, in the intensive compared to the standard BP treatment groups (Supplementary Table S4, Figs. S3 and S4). The difference in relative risk was more pronounced for HF (8%) and cardiovascular mortality (9%).
Sub-analyses of intensive systolic BP treatment targets were performed for <160, <150, <140, <130 and <120 mmHg targets. There was a tendency for lower systolic BP treatment targets to produce greater reduction in the relative risk for all outcome events; however, only one study each was available for the <130 and <120 mmHg target categories (Supplementary Table S5, Figs. S5 and S6).
3.4. Meta-regression for covariates
Follow-up years and upper systolic BP treatment targets were set as covariates to perform meta-regression analysis on all outcome events. There was a tendency for follow-up years to influence outcomes (longer follow-up times increased the relative risk). Shorter follow-up years significantly reduced relative risk of MCEs (p = 0.028) with a moderate heterogeneity across studies (I2 = 71.3%, p = 0.000) (Supplementary Fig. S7). The effect of upper systolic BP treatment targets to influence outcome events was not significant; however, there was a non-significant trend towards a relative risk reduction of MI (p = 0.070) with no heterogeneity among studies (I2 = 0.0%, p = 0.983) (Supplementary Fig. S8).
3.5. NNT and NNH
Overall NNT was calculated for all outcome events; NNH was calculated for studies [7,8,10,13,32] reporting hypotension-related events. Overall NNTs for statistically significant risk reduction of outcome events were 38 (95% CI: 27–70) patients for composite MCEs and 85 (95% CI: 55–211) for all-cause mortality. NNT for non-significant risk reduction of outcome events and NNH are reported in the Supplementary Table S6.
3.6. Study success and withdrawal
Only eight of the included studies [4,6,8,13,[33], [34], [35], [36]] reported the proportion of participants who achieved treatment targets. Of these, BP treatment targets were achieved in 61.3% of participants in the intensive versus 25.1% of participants in the standard treatment groups. Six studies [4,6,8,32,33,36] reported total withdrawals from treatment. Withdrawal rate was 5.3% in the intensive versus 7.4% in the standard treatment groups (Supplementary Table S7).
3.7. Heterogeneity and publication bias
Most analyses demonstrated low to moderate heterogeneity with relative risk of MCEs having the highest heterogeneity of 79.7%. The Egger funnel plots showed minimal evidence of publication bias with an intercept of −1.17 (95%CI −4.62–2.28, p = 0.481) for risk of MCEs, 0.14 (95%CI −0.64–0.91, p = 0.714) for MI, −0.94 (95%CI −2.36–0.48, p = 0.177) for stroke, −0.53 (95%CI −2.28–1.23, p = 0.515) for HF, −1.2 (95%CI −2.63–0.24, p = 0.096) for CV mortality, and −1.0 (95%CI −2.87–0.88, p = 0.273) for all-cause mortality (Supplementary Figs. S9–S14).
3.8. Study quality
The median JADAD score was 5 out of a maximum score of 5 (Supplementary Table S8). All trials were of high quality with only four losing points for not describing the method of randomization or blinding.
4. Discussion
This systematic review with meta-analyses and meta-regression evaluated the effect of intensive BP treatment in people with hypertension. We included a total of 65,890 hypertensive participants (50 years and older, average age 69.4 years) with a follow-up period from 1.8 to 4.9 years. We found reductions in the relative risk of CV outcome events (including MCEs, MI, stroke, HF and CV mortality) and all-cause mortality. Intensive BP treatment was well tolerated with 61.3% of participants reaching their BP targets and a withdrawal rate of 5.3%.
4.1. Effect of intensive BP treatment on cardiovascular outcome events and all-cause mortality
Our meta-analyses showed that intensive BP treatment significantly reduced the relative risk of MCEs, MI, stroke, HF, CV mortality, as well as all-cause mortality in hypertensive adults. Heterogeneity across studies was considered low to moderate for relative risk of all outcomes events with the exception of MCEs (I2 = 79.7%). Our data were in contrast to previous studies that found low BP was associated with higher death rates, due to hypotensive effects, in the older population [37,38]. In hypertensive patients with diabetes or CVD risk, BP treatment has been shown to be effective in lowering the relative risk of CV events and all-cause mortality [17,19,21]. Interestingly, more recent studies [9,10,13] showed greater relative risk reduction for CV events possibly due to more aggressive BP treatment targets, replacing the placebo with a set target in the standard treatment group and more effective medication protocols.
Sub-analyses of systolic versus diastolic BP treatment targets revealed that targeting systolic BP seemed more effective and provided a greater relative risk reduction than diastolic BP treatment targets, except for MI. The 3 trials (4 comparisons) using diastolic BP treatment targets exclusively are over 20 years old with recent trials preferring to control systolic as well as diastolic pressure. Moreover, in the early years of the mid-20th century controlling high BP was defined by controlling diastolic BP while elevated systolic BP was associated with age progression [39]. More importantly, in 1993 the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure (JNC) changed its recommendation to systolic BP control [40]. However, a statement to address the importance of systolic BP was only issued in 2000 by the JNC's Clinical Advisory Committee. Additionally, over the years evidence-based research findings have progressed the use of better medications. Hypertension was considered an untreatable condition until the late 50s when thiazide diuretics were first introduced [41] for successful anti-hypertensive treatment. A sub-analysis of follow-up duration (<3 and ≥ 3 years) showed that relative risk reduction for CV outcome events was significantly greater in those studies with shorter follow-up durations, except for MI. A possible reason for this might be a decrease in participants' compliance over the years as well as compounding factors such as aging and the associated onset of comorbidities. Sub-analyses for systolic BP treatment targets showed greater relative risk reduction for all CV outcome events, except MI, with more aggressive systolic BP treatment targets. Although the lower systolic BP target groups (<120 and 130 mmHg) included only one study each. This outcome is certainly expected and in line with latest ESC/ESH and ACC/AHA guidelines, respectively, following recommended BP targets <140/80 mmHg [14] and <130/80 mmHg [15] for people with uncomplicated hypertension.
Our meta-regression analyses for the covariates follow-up years and systolic BP treatment targets showed that an increase in follow-up years actually decreased the size of the relative risk reduction for MCEs. This confirms the results of the sub-analyses. The meta-regression analyses for systolic BP treatment targets were not significant; only MI showed a non-significant tendency towards a reduced size of the relative risk with decreasing systolic BP targets.
The current meta-analyses, sub-analyses and meta-regression analyses demonstrate the beneficial effects of lower BP treatment targets, in support of the recent changes to the ACC/AHA hypertension guidelines [15]. Despite the methodological differences in the included studies, our meta-analysis is in agreement with findings of a recent meta-analysis [18] that showed significant reductions in CV outcomes in a hypertensive population (including hypertension with comorbidities). Our results also agree with previous systematic reviews [21,42] and the most recent meta-analyses [17,19] in people with diabetes, cardiovascular disease and chronic kidney disease. In contrast, Takami et al. [23] found no reduction in the relative risk of composite CV outcomes with intensive BP lowering; however, they reported a greater relative risk reduction in CV mortality (39%) and all-cause mortality (24%). The observed difference could possibly be due to the difference in population age; Takami et al. [23] included only very elderly hypertensive patients (≥70 years) while the current study involved people with hypertension ≥50 years (average age 69.4 years). Moreover, Takami et al. [23] included a limited number of studies (n = 5) in their systematic review which might have less statistical power compared to the 16 included studies in the current review.
4.2. Overall NNT and NNH of outcome events
To place our findings in a relevant clinical context, the overall NNT for the composite MCEs was relatively low with respect to the average follow-up years.
Overall the impact of the intensive BP treatment was beneficial (NNT = 38) for composite MCEs with one outcome prevented for every 38 patients treated. Assuming an adherence of 61% for 3.3 years, the likely NNT would be 62 patients. In contrast, NNH (778 patients) for hypotension-related events was high, even though only five studies reported hypotension-related events. For a total of 778 persons treated intensively, one would experience a hypotensive related event (ranging from dizziness to possible organ failure). This would mean that for every 21(778/38) MCEs one would expect only one hypotensive event. The NNT observed suggests a meaningful real-world effect. This indicates that intensive BP treatment with lower BP treatment targets is effective and safe. To our knowledge no one has reported on this previously.
4.3. Strength and limitations
The current study included all studies to date designed to evaluate the effects of active or intensive BP treatment in people with hypertension. The major limitation of this work is that, because BP treatment targets have reduced with time, there is significant overlap between intensive and standard treatments between the included studies. Another limitation of our work is that we cannot guarantee that none of the included patients might have exhibited comorbid disease; since data was presented at group-level, we were unable to separate those individuals with and without additional chronic diseases. There was minimal publication bias and study quality was very high as 15 out of the 16 studies were registered trials. To our knowledge, this is the first systematic review to include meta-regression analyses, as well as NNT and NNH to evaluate clinical benefits.
Our systematic review has some limitations, 13 studies used systolic BP treatment targets whereas 3 used diastolic BP treatment targets. Comparison of intensive versus standard BP treatment also varied across studies, as some studies compared intensive BP treatment with standard BP treatment with and without placebo while others had set target BP for both intensive treatment and standard BP treatment. Studies used a range of different medication protocols to achieve set BP targets with calcium channel blockers being the most popular. As treatment success was only 61% overall, the results probably underestimate intensive treatment benefits, but reported medication adherence probably mirrors real-word patient experiences. Moreover, not all included studies reported on treatment success, withdrawal and adverse events especially hypotension-related events.
5. Conclusion
Our systematic review and the analyses demonstrate clear benefits of intensive BP treatment suggesting that intensive BP treatment reduces the risk of CV outcome events and all-cause mortality in hypertensive older adults without comorbidities. We provide evidence that lower BP treatment targets are optimal for CV protection and support the latest hypertension guidelines. Almost 780 patients must be treated intensively before one hypotensive event occurs.
Author contributions
All authors contributed equally to this work - conception and design (NS,GD), acquisition of data (BB,GD), analyses (BB,GD, MP) and interpretation of data (BB,MP,NS,GD), drafting the article (BB,GD) and critically revising it (NS). All authors gave final approval for the submission.
Funding
None.
Declaration of competing interest
None of the authors declare any conflicts of interest.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijchy.2020.100040.
Appendix A. Supplementary data
The following is the supplementary data to this article:
Multimedia component 1
References
- 1.GBD 2017 Causes of death collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen B., Bauman A., Ding D. Association between lifestyle risk factors and incident hypertension among middle-aged and older Australians. Prev. Med. 2019;118:73–80. doi: 10.1016/j.ypmed.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Harrap S.B., Lung T., Chalmers J. New blood pressure guidelines pose difficult choices for Australian physicians. Circ. Res. 2019;124(7):975–977. doi: 10.1161/CIRCRESAHA.118.314637. [DOI] [PubMed] [Google Scholar]
- 4.Beckett N.S., Peters R., Fletcher A.E. Treatment of hypertension in patients 80 years of age or older. N. Engl. J. Med. 2008;358(18):1887–1898. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 5.Hansson L., Zanchetti A., Carruthers S.G. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet. 1998;351(9118):1755–1762. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- 6.JATOS Study Group Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS) Hypertens. Res. 2008;31(12):2115–2127. doi: 10.1291/hypres.31.2115. [DOI] [PubMed] [Google Scholar]
- 7.Liu L., Zhang Y., Liu G., Li W., Zhang X., Zanchetti A. The felodipine event reduction (FEVER) study: a randomized long-term placebo-controlled trial in Chinese hypertensive patients. J. Hypertens. 2005;23(12):2157–2172. doi: 10.1097/01.hjh.0000194120.42722.ac. [DOI] [PubMed] [Google Scholar]
- 8.Verdecchia P., Staessen J.A., Angeli F. Usual versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): an open-label randomised trial. Lancet. 2009;374(9689):525–533. doi: 10.1016/S0140-6736(09)61340-4. [DOI] [PubMed] [Google Scholar]
- 9.Wei Y., Jin Z., Shen G. Effects of intensive antihypertensive treatment on Chinese hypertensive patients older than 70 years. J. Clin. Hypertens. 2013;15(6):420–427. doi: 10.1111/jch.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Attar A., Sayadi M., Jannati M. Effect of intensive blood pressure lowering on cardiovascular outcomes based on cardiovascular risk: a secondary analysis of the SPRINT trial. Eur J Prev Cardiol. 2019;26(3):238–245. doi: 10.1177/2047487318800741. [DOI] [PubMed] [Google Scholar]
- 11.Dahlof B., Lindholm L.H., Hansson L., Schersten B., Ekbom T., Wester P.O. Morbidity and mortality in the Swedish trial in old patients with hypertension (STOP-Hypertension) Lancet. 1991;338(8778):1281–1285. doi: 10.1016/0140-6736(91)92589-t. [DOI] [PubMed] [Google Scholar]
- 12.Liu L., Wang J.G., Gong L., Liu G., Staessen J.A. Comparison of active treatment and placebo in older Chinese patients with isolated systolic hypertension. J. Hypertens. 1998;16(12):1823–1829. doi: 10.1097/00004872-199816120-00016. [DOI] [PubMed] [Google Scholar]
- 13.Umemoto S., Ogihara T., Matsuzaki M. Effects of calcium channel blocker benidipine-based combination therapy on target blood pressure control and cardiovascular outcome: a sub-analysis of the COPE trial. Hypertens. Res. 2017;40(4):376–384. doi: 10.1038/hr.2016.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams B., Mancia G., Spiering W. 2018 ESC/ESH guidelines for the management of arterial hypertension:the task force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH) Eur. Heart J. 2018;39(33):3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 15.Whelton P.K., Carey R.M., Aronow W.S. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J. Am. Coll. Cardiol. 2018;71(19):e127–e248. doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Staessen J.A., Wang J.-G., Thijs L. Cardiovascular protection and blood pressure reduction: a meta-analysis. Lancet. 2001;358(9290):1305–1315. doi: 10.1016/S0140-6736(01)06411-X. [DOI] [PubMed] [Google Scholar]
- 17.Li L., Li L. Intensive versus usual control of hypertension in the prevention of cardiovascular and renal outcomes: a cumulative meta-analysis of randomized controlled trials. Kidney Blood Press. Res. 2019;44(3):384–395. doi: 10.1159/000499009. [DOI] [PubMed] [Google Scholar]
- 18.Sakima A., Satonaka H., Nishida N., Yatsu K., Arima H. Optimal blood pressure targets for patients with hypertension: a systematic review and meta-analysis. Hypertens. Res. 2019;42(4):483–495. doi: 10.1038/s41440-018-0123-4. [DOI] [PubMed] [Google Scholar]
- 19.Wang J., Chen Y., Xu W., Lu N., Cao J., Yu S. Effects of intensive blood pressure lowering on mortality and cardiovascular and renal outcomes in type 2 diabetic patients: a meta-analysis. PloS One. 2019;14(4) doi: 10.1371/journal.pone.0215362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blood Pressure Lowering Treatment Trialists' Collaboration Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362(9395):1527–1535. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 21.Xie X., Atkins E., Lv J. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet. 2016;387(10017):435–443. doi: 10.1016/S0140-6736(15)00805-3. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y., Liang M., Sun C. Effect of intensive lowering of systolic blood pressure treatment on heart failure events: a meta-analysis of randomized controlled studies. J. Hum. Hypertens. 2019;33(9):648–657. doi: 10.1038/s41371-019-0221-z. [DOI] [PubMed] [Google Scholar]
- 23.Takami Y., Yamamoto K., Arima H., Sakima A. Target blood pressure level for the treatment of elderly hypertensive patients: a systematic review and meta-analysis of randomized trials. Hypertens. Res. 2019;42(5):660–668. doi: 10.1038/s41440-019-0227-5. [DOI] [PubMed] [Google Scholar]
- 24.Baker W., Michael White C., Cappelleri J. Understanding heterogeneity in meta-analysis: the role of meta-regression. Int. J. Clin. Pract. 2009;63(10):1426–1434. doi: 10.1111/j.1742-1241.2009.02168.x. [DOI] [PubMed] [Google Scholar]
- 25.Sackett D.L., Richardson W.S., Rosenberg W., Haynes R.B. Churchill Livingstone; Edinburgh, UK: 1997. Evidence-based Medicine: How to Practice & Teach EBM. [Google Scholar]
- 26.Schünemann H.J., Vist G.E., Higgins J.P. Chapter 15: interpreting results and drawing conclusions. In: Higgins J., Thomas J., Chandler J., editors. Cochrane Handbook for Systematic Reviews of Interventions. second ed. John Wiley & Sons; Chichester (UK): 2019. [Google Scholar]
- 27.Veroniki A.A., Bender R., Glasziou P., Straus S.E., Tricco A.C. The number needed to treat in pairwise and network meta-analysis and its graphical representation. J. Clin. Epidemiol. 2019;111:11–22. doi: 10.1016/j.jclinepi.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. Br. Med. J. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jadad A.R., Moore R.A., Carroll D. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Contr. Clin. Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 31.Hannson L. The BBB Study: the effect of intensified antihypertensive treatment on the level of blood pressure, side-effects, morbidity and mortality in "well-treated" hypertensive patients. Blood Pres. 1994;3(4):248–254. doi: 10.3109/08037059409102265. [DOI] [PubMed] [Google Scholar]
- 32.Lithell H., Hansson L., Skoog I. The study on cognition and prognosis in the elderly (SCOPE): principal results of a randomized double-blind intervention trial. J. Hypertens. 2003;21(5):875–886. doi: 10.1097/00004872-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Ogihara T., Saruta T., Rakugi H. Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension. 2010;56(2):196–202. doi: 10.1161/HYPERTENSIONAHA.109.146035. [DOI] [PubMed] [Google Scholar]
- 34.Perry H.M., Jr., Smith W.M., McDonald R.H. Morbidity and mortality in the systolic hypertensive elderly program (SHEP) pilot study. Stroke. 1989;20:4–13. doi: 10.1161/01.str.20.1.4. [DOI] [PubMed] [Google Scholar]
- 35.Report by the Management Committee Treatment of mild hypertension in the elderly: a study initiated and administered by the National Heart Foundation of Australia. Med. J. Aust. 1981;2(8):398–402. [PubMed] [Google Scholar]
- 36.Staessen J.A., Fagard R., Thijs L. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. Lancet. 1997;350(9080):757–764. doi: 10.1016/s0140-6736(97)05381-6. [DOI] [PubMed] [Google Scholar]
- 37.Hakala S.-M., Tilvis R., Strandberg T. Blood pressure and mortality in an older population: a 5-year follow-up of the Helsinki Ageing Study. Eur. Heart J. 1997;18(6):1019–1023. doi: 10.1093/oxfordjournals.eurheartj.a015360. [DOI] [PubMed] [Google Scholar]
- 38.Satish S., Freeman D.H., Jr., Ray L., Goodwin J.S. The relationship between blood pressure and mortality in the oldest old. J. Am. Geriatr. Soc. 2001;49(4):367–374. doi: 10.1046/j.1532-5415.2001.49078.x. [DOI] [PubMed] [Google Scholar]
- 39.Psaty B.M., Manolio T.A., Smith N.L. Time trends in high blood pressure control and the use of antihypertensive medications in older adults: the cardiovascular health study. Arch. Intern. Med. 2002;162(20):2325–2332. doi: 10.1001/archinte.162.20.2325. [DOI] [PubMed] [Google Scholar]
- 40.The fifth report of the Joint National Committee on detection, evaluation, and treatment of high blood pressure (JNC V) Arch. Intern. Med. 1993;153(2):154–183. [PubMed] [Google Scholar]
- 41.Saklayen M.G., Deshpande N.V. Timeline of history of hypertension treatment. Front Cardiovasc Med. 2016;3 doi: 10.3389/fcvm.2016.00003. 3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lv J., Neal B., Ehteshami P. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: a systematic review and meta-analysis. PLoS Med. 2012;9(8) doi: 10.1371/journal.pmed.1001293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multimedia component 1