Abstract
Objective
The objective of this study was to review the current evidence on the effects of Mg2+ deficiency on cardiovascular disease (CVD) and hypertension, since Mg2+ is a potent vasodilator and modulates vasomotor tone, blood pressure and peripheral blood flow. Several factors could contribute to its deficiency and when it occurs, is associated with an increased incidence of cardiovascular disease (CVD), hypertension, heart failure (HF), and cardiac arrhythmias.
Methods
In order to get a better to get an updated perspective of the current status of Mg2+ deficiency and its implications in CVD, hypertension, and cardiac arrhythmias, a focused Medline search of the English language literature was conducted between 2014 and 2018 and 30 pertinent papers were retrieved.
Results
The analysis of data showed that Mg2+ deficiency is difficult to occur, under normal circumstances, because it is plentiful in green leafy vegetables, cereals, nuts, and the drinking water. However, Mg2+ deficiency can occur under special circumstances such as hypertension and HF treated with large doses of diuretics, patients with chronic kidney disease (CKD) treated with hemodialysis, and patients with gastroesophageal reflux disease treated with proton pump inhibitors. When hypomagnesemia occurs, it is associated with serious cardiac arrhythmias and aggravation of hypertension.
Conclusion
The analysis of data suggests that Mg2+ deficiency does occur and it is associated with an increased incidence of CVD, HF, serious cardiac arrhythmias, and hypertension. Retaining normal Mg2+ levels will prevent the onset of these diseases.
Keywords: Magnesium, Deficiency, Cardiovascular disease, Cardiac arrhythmias, Hypertension
1. Introduction
Magnesium (Mg2+) is the 4th most common mineral in the human body after calcium (Ca2+), potassium (K+), and sodium (Na+) and is a cofactor in more than 300 enzyme systems in the cells and plays a significant role in cardiovascular homeostasis and it is essential for DNA, RNA and adenosine triphosphate (ATP) synthesis and it should be continuously replenished [1], [2], [3]. Under normal circumstances Mg2+ deficiency is difficult to occur, because it is abundant in green leafy vegetables, cereal, nuts, legumes, and the drinking water [1], [2], [3], except under special circumstances, such as in patients with hypertension treated with diuretics, patients with end stage renal disease on chronic hemodialysis, patients treated chronically with proton pump inhibitors (PPIs), and subjects drinking soft water [3], [4], [5], [6], [7], [8], [9]. Thus, significant Mg2+ deficiency is a serious problem, because it could increase the onset de novo hypertension, worsen preexisting hypertension, increase the incidence of cardiovascular disease (CVD), HF, and cause serious arrhythmias and death [3], [4], [5], [6], [7], [8], [9]. However, Mg2+ deficiency does occur even in the absence of preexisting diseases, since epidemiologic studies in Europe, North America and other countries, show that the Mg2+ content in these foods is less than 30–50% of the recommended daily allowance (RDA) of 420 mg/day of Mg2+ for men and 320 mg/day for women, due mostly to the use of fertilizers and processed foods [1], [2]. The most important reservoir of Mg2+ is the bone (60%), the intracellular tissues (39%) and only1% is located extracellularly [1], [2]. The serum Mg2+ is 55–70% ionized, 20–30% protein bound, and 5–15% is complexed with other elements [11]. Normal Mg2+ levels are important because it plays a significant role for the synthesis of DNA, RNA, and protein synthesis, muscle and nerve transmission, neuromuscular conduction, signal transduction, blood glucose control, BP regulation, and enzyme function, such as hexokinase, creatine kinase, cyclase, and adenosine triphosphate (ATP)-dependent synthesis of glutathione, an important intracellular antioxidant ( [2], [11]. Since Mg2+ deficiency is associated with several metabolic and cardiovascular conditions, a focused Medline search of the recent English language literature was conducted between 2014 and 2018 using the terms, magnesium, magnesium deficiency, causes, cardiovascular disease, cardiac arrhythmias, hypertension, death, and 30 pertinent papers were retrieved. These papers together with collateral literature will be discussed in this review.
1.1. Magnesium functions in the body
Magnesium is mainly absorbed from the small intestine and some from the large intestine [11], [12]. Of the total dietary Mg intake about 24%–76% is absorbed from the gut and the rest is eliminated in the feces. Also, the Mg2+ absorption varies with the amount of intake. The lower the intake, the higher is the absorption. The Mg2+ homeostasis in the plasma is controlled by the gut and kidneys, which excrete about 2400 mg/day, of which 2300 mg is immediately reabsorbed from the thick ascending limb of Henle, and only 3%–5% is eliminated in the urine [13]. The magnesium absorption from the kidneys is mediated through the action of Transient Receptor Potential Melastatin (TRPM), a subfamily of the transient receptor potential proteins superfamily involved in transporting other cellular cations. Recently, TRPM6 and TRPM7 have been reported as unique transporters of Mg2+ [2]. However, Mg2+ status is difficult to estimate, because most of it is intracellular or in bone. Magnesium is an important element in the body since it is involved in over 300 intracellular functions and its deficiency is serious associated with several clinical and metabolic conditions, such as atherosclerosis, CVD, HF, cardiac arrhythmias, T2DM, and hypertension through several mechanisms [2], [11], [12]. The most important include: a) energy production, through generation of ATP by break down and energetic utilization of carbohydrates, proteins and fats. b) Enzyme activation, such as mitochondrial ATP synthase, hexokinase, creatine kinase, adenylate cyclase, phosphofructokinase, and tyrosine kinase, c) Calcium antagonism, by interfering with Ca2+ influx at the cell membrane and regulating vascular muscle tone, muscle contraction/relaxation, neurotransmitter release, neuromuscular impulse conduction through inhibition of calcium-dependent acetylcholine release at the motor end plate, and maintenance and stabilization of cell membrane physiology, and d) through regulation of potassium movement in myocardial cells, protection against stress, vasodilation of the coronary and peripheral arteries, reduction of platelet activation, and economization of cardiac pump function [2].
1.2. Clinical and metabolic effects of magnesium deficiency
Under normal circumstances, Mg2+ deficiency is infrequent and only occurs under special circumstances, like deficient intake, the action of certain conditions, such as alcoholism, diabetes, malabsorption Crohn's disease, ulcerative colitis, celiac disease, short bowel syndrome, hyperaldosteronism, hyperparathyroidism, hyperthyroidism, hemodialysis for renal failure, and drugs like diuretics [2], [4], [11], [12], [13], [14], as well as many other drugs and conditions listed in Table 1. However, when Mg2+ deficiency occurs, it is associated with increased incidence of CVD, HF, cardiac arrhythmias and hypertension, as well as aggravation of their preexisting status [2], [3], [4], [5], [6], [14], [15], [16]. Several observational and clinical studies have shown that Mg2+ is involved in many essential physiological, biochemical, and cellular processes regulating cardiovascular function. Among these, is the role of Mg2+ in modulating vascular smooth muscle tone, endothelial cell function, and myocardial excitability, which play a significant role in the pathophysiology of several disorders including atherosclerosis, CVD, hypertension, and heart failure [16], [17], [18]. Its beneficial effects on the cardiovascular system are mediated through enhancement of endothelium-dependent vasodilation, improvement in lipid metabolism, reduction of inflammation, and inhibition of platelet aggregability [19], [20]. As a key intracellular electrolyte, it is also involved in cardiac electrophysiology through regulation of cation flux (Ca2+, K+) across the cardiomyocytes and plays an important role in the prevention of cardiac arrhythmias [10], [20].
Table 1.
Potential causes of magnesium deficincy.
| Diseases | Drugs |
|---|---|
| Alcoholism | Antacids |
| Aldosteronism | Caffeine |
| Coeliac disease | Cisplatin |
| Colectomy | Cyclosporin |
| Diarrhea | Digoxin |
| Heart failure | Diuretics |
| Hemodialysis | Estrogens |
| Hyperpathyroidism or hypoparathyroidism | Laxatives |
| Hyperthyroidism | Proton pump inhibitors |
| Kidney disease | Tacrolimus |
| Liver disease | High dose vitamin D |
| Metabolic acidosis | Soft water |
| Pancreatitis | |
| Pregnancy | |
1.2.1. Cardiovascular Disease
Based on these properties, Mg2+ has been shown by several studies to prevent reperfusion myocardial ischemia by inhibiting the cytoplasmic Ca2+ overload, conserving cellular ATP, reducing myocardial oxygen consumption, and protecting the post-ischemic myocardium from oxidative damage [21], [22]. These actions of Mg2+ resulted in performance of the second Leicester Intravenous Magnesium Intervention Trial (LIMITS-2) to investigate the intravenous Mg2+ therapy in reducing early mortality after acute MI [23]. In this study, 2,316 patients with suspected acute MI were randomized to intravenous magnesium sulfate (MgSO4) or placebo for 28 days. There was a 24% relative reduction of mortality and a 25% reduction in left ventricular failure in the MgSO4 treated group compared with the placebo treated group. However, two subsequent trials, Fourth International Study of Infarct Survival (ISIS-4) and the Magnesium in Coronaries (MAGIC) failed to demonstrate a benefit of Mg2+ therapy in acute MI [24], [25]. In the ISIS-4 trial, 58,050 patients with suspected acute MI were randomized to oral captopril, oral controlled-release isosorbide mononitrate, or intravenous MgSO4. The study showed a 35-day increased mortality, increased incidence of cardiogenic shock, and HF in the MgSO4 group, but no benefits were observed with the other treatments [24]. The MAGIC trial compared the short-term effects of intravenous MgSO4 versus placebo in 6,213 patients with ST elevation acute MI. At 30 days, 15.3% of patients in the MgSO4 group and 15.2% of patients in the placebo group died [25]. No benefit or harm with MgSO4 was noted in subgroup analysis. Potential explanations for the conflicting results of these trials could be due to the time of randomization from symptom onset regarding attainment of significant Mg2+ blood levels to prevent reperfusion injury, and the concomitant administration of aspirin, b-blockers, and angiotensin-converting enzyme inhibitors. Also, a review and meta-analysis by Li et al. of 26 randomized controlled trials involving 73,363 patients from 1/1966 to 6/2006 that compared intravenous Mg2+ treatment with placebo, after careful analysis and eliminating heterogeneity, did not demonstrate any significant benefit of treatment with intravenous MgSO4 in patients with acute myocardial infarction (MI) or HF compared to placebo with the exception of prevention of serious cardiac arrhythmias odds ratio (OR) 0.72 (95% CI 0.19–0.84) by fixed effect and OR 0.51 (95% CI 0.33–0.79) by random effect compared to placebo [26]. It is speculated that the high dose of MgSO4 used (≥75 mmol or ≥ 182 mg) could be responsible for the adverse effects, especially the severe hypotension and cardiogenic shock. Perhaps a lower dose of MgSO4 (<75 mmol or < 182 mg) could have been safer. On the other hand, observational studies have shown a cardioprotective effect of normal Mg2+ levels. Also, a recent review and meta-analysis by Del Gobbo et al. [19], of 16 studies involving 313,041 patients showed an inverse association between serum Mg2+ levels and the incidence of CVD and ischemic heart disease (IHD). The results of this review suggest the need for new clinical trials to evaluate the potential role of Mg2+ in the prevention of CVD and IHD.
1.2.2. Cardiac Arrhythmias
The prevention and treatment of cardiac arrhythmias is the most accepted use of Mg2+ by physicians. The antiarrhythmic effects of Mg2+ are being mediated through its effects on myocardial excitability by modulating the voltage-dependent Na+, Ca2+, and K+ channels in the generation of cardiac action potential and the pathogenesis of cardiac arrhythmias. In vitro studies in cardiac myocytes by Mubagwa et al. [27], showed that Mg2+ had no effect on inward channels, but it decreased the outward current amplitude in concentration and voltage-dependent manner. The cardiac membrane stabilizing action of Mg+ was primarily due to its modulation of the voltage-dependent L-type Ca2+ channels. L-type Ca2+ channel amplitude is decreased with high and increased with low intracellular Mg2+ levels [28], [29]. The physiological effects of Mg2+ on cardiac ion channels suggest that Mg2+ influences the cardiac impulse formation and conduction thereby plays a critical role in the pathogenesis and treatment of cardiac arrhythmias. The recent Framingham Offspring Study of 3,530 participants, showed that low serum Mg2+ was associated with the development of atrial fibrillation (AF) in patients without CVD [30]. Magnesium has been reported to have several diverse electrophysiological actions on the conduction system of the heart that include: antagonism of the L and T-type calcium channels, prolongation of the sinus node recovery time, and reductions of the automaticity of the heart, atrioventricular nodal conduction time, antegrade and retrograde conduction over an accessory pathway, and His-ventricular conduction time [31]. With respect to ventricular conduction pathway, it suppresses early and delayed after depolarization and homogenizes transmural ventricular repolarization. Several other reviews and meta-analyses have shown that intravenous Mg2+ administration is indeed an effective and safe strategy for the prevention of postcardiac AF or its treatment [32], [33]. Also, intravenous Mg2+ can be given safely, in combination with other antiarrhythmic drugs for the postcardiac prevention of AF in critically ill patients [34], [35]. In addition to atrial arrhythmias, Mg2+ is also effective for the treatment of ventricular arrhythmias associated with the long QT syndrome (polymorphic ventricular tachycardia/torsade de pointes) and digoxin toxicity [36], [37]. Ventricular arrhythmias are also, common in patients with HF and Mg2+ supplementation is very helpful in preventing episodes of ventricular tachycardia in such patients [38], [39]. Also, data from the Magnesium in Cardiac Arrhythmias (MAGICA) trial showed a significant reduction in ventricular premature beats in patients with frequent ventricular arrhythmias (>720 beats/24 h) following a 50% increase in the minimum daily intake of Mg2+ and K+ for 3 weeks [40].
1.2.3. Hypertension
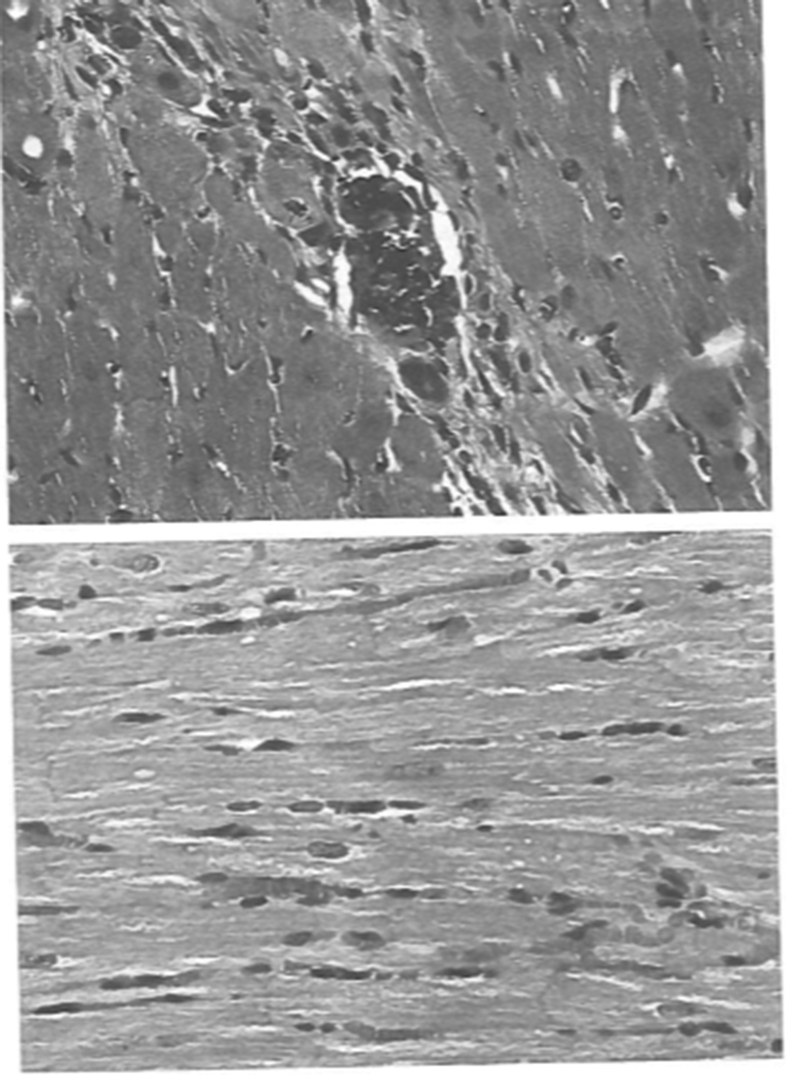
Clinical as well as animal studies have shown that Mg2+ deficiency is associated with de novo incidence or aggravation of preexisting hypertension [3], [4], [41], [42]. In a population-based study of 4,272 Mexican subjects ages 20–65 years of age, hypomagnesemia (serum Mg2+ 1.46 mg/dl) was associated with a significant increase in the prevalence of prehypertension (BP 120–139/80-89 mmHg) compared to normomagnesemic subjects, OR, 1.78 (95% CI 1.5–4.0, p < 0.0005) after adjustments for age, sex, smoking, body mass index (BMI), waist circumference (WC), glucose and lipid levels [41]. In another major clinical trial of 1000 ambulatory hypertensive patients from our center, diuretic-induced hypomagnesemia (serum Mg2+ ≤ 1.25 mEq/L) and hypokalemia (serum K+ ≤ 3.4 mEq/L) were associated with aggravation of their hypertension, since these patients required more medicines for the same control of BP as the normomagnesemic and normokalemic patients [4]. Their laboratory data are listed in Table 2. Several other older studies have also shown an association of Mg2+ deficiency with the incidence of hypertension with a preponderance in African Americans [43], [44], [45], [46]. The higher Mg2+ deficiency in African Americans is possibly due to lower Mg2+ intake compared to Whites. The effects of Mg2+ deficiency on BP, on hemodynamic, renal, and cardiorenal tissue changes were examined in our laboratory in spontaneously hypertensive rats (SHR) fed free Mg2+ diet for 2 months, compared to control SHR fed a normal chow diet [47]. The diet-induced hypomagnesemia resulted in significant aggravation of their BP and severe tissue disorganization with metastatic myocardial tissue calcification (Fig. 1). The metabolic effects of hypomagnesemia in these SHR are summarized in Table 3. Hemodynamically, there was a significant increase in mean arterial pressure (MAP), total peripheral vascular resistance index (TPRI), and renal vascular resistance (RVR), (all < 0.05). Also, there was a significant decrease in renal blood flow (RBF) and hematocrit (p < 0.05) and no change in cardiac index (CI) and glomerular filtration rate (GFR). The myocardial metastatic tissue calcification has been attributed to increased intracellular Ca2+ transfer due the lack of inhibition by Mg2+ [48] and has been previously demonstrated by other investigators as well [49]. The Ca2+ and Mg2+ flux across the external membrane is regulated by a Ca2+ pump (Ca2∗-Mg2+ ATPase), Ca2+ channels, and binding to the membrane. In cell membranes of hypertensive patients several investigators have shown an increase in Ca2+/Mg2+ ratio of greater than 2.0 [50], [51], [52] (see Table 4).
Table 2.
Blood chemistries of hypertensive patients.
| Group 1 | Group 2 | Group 3 | Group 4 | |
|---|---|---|---|---|
| Magnesium | Low | Low | Normal | Normal |
| Potassium | Low | Normal | Low | Normal |
| Patients (No) | 20 (2%) | 25 (2.5%) | 150 (15%) | 805 (80.5%) |
| Sodium (mEq/L) | 139 ± 1 | 140 ± 0.7 | 140 ± 0.3 | 143 ± 0.9 |
| Potassium (mEq/L) | 3.1 ± .05 | 4.0 ± .06*** | 3.2 ± .02 | 4.4 ± .07*** |
| Magnesium (mEq/L) | 1.14 ± .03 | 1.16 ± .02 | 1.54 ± .02* | 1.65 ± .04* |
| Calcium (mg/dl) | 9.0 ± 0.3 | 10.1 ± 0.2** | 9.8 ± 0.1** | 9.8 ± 0.1** |
| Phosphorus (mg/dl) | 3.2 ± 0.2 | 3.1 ± 0.1 | 2.9 ± 0.1 | 3.1 ± 0.1 |
| BUN 9 mg/dl) | 17.0 ± 2.0 | 15.0 ± 2.0 | 18.0 ± 0.4 | 16.5 ± 1.1 |
| Creatinine (mg/dl) | 1.4 ± 0.1 | 1.2 ± 0.5 | 1.3 ± .02 | 1.3 ± .07 |
| Uric acid (mg/dl) | 7.1 ± 0.8 | 7.4 ± 0.3 | 8.1 ± 0.2* | 7.3 ± 0.3 |
Fig. 1.
Metastatic Myocardial Calcification Due to Hypomagnesemia. Upper. Light microscopy of myocardial tissue from a hypomagnesemic SHR shows tissue necrosis with granular calcification of degenerated and disorganized myocardial fibers HE x 250. Lower. Light microscopy from control SHR shows that myocardial tissue architecture is well preserved and there are no areas of tissue necrosis or calcification. HE x 250.
Table 3.
Metabolic and tissue effects of hypomagnesemia in SHR (mean ± SEM).
| Control | Hypomagnesemic | P | |
|---|---|---|---|
| SHR (No) | 12 | 12 | NS |
| SBP (mmHg) | 200 ± 4 | 212 ± 6 | <0.05 |
| Weight (grams) | 299 ± 13 | 292 ± 9 | NS |
| Fluid Intake (ml/24 h) | 25 ± 2 | 28 ± 2 | NS |
| Urine Output (ml/24 h) | 14 ± 1 | 15 ± 1 | NS |
| UNaV (mEq/24 h) | 2.05 ± 0.11 | 2.46 ± 0.11 | <0.01 |
| UKV (mEq/24 h) | 0.33 ± 0.01 | 0.42 ± 0.01 | <0.01 |
| Serum Na+ (mEq/l) | 139 ± 0.6 | 141 ± 0.5 | <0.05 |
| Serum K+ (mEq/l) | 4.49 ± 0.06 | 4.05 ± 0.1 | <0.01 |
| Serum Mg2+ (mEq/l) | 1.98 ± 0.1 | 0.46 ± 0.05 | <0.01 |
| Serum Ca@+ (mg/dl) | 9.5 ± 0.2 | 9.5 ± 0.2 | NS |
| BUN (mg/dl) | 35.7 ± 3.3 | 40.7 ± 0.9 | NS |
| Tissue Mg2+ (mEq/100 g dry weight | |||
| Heart | 6.86 ± 0.74 | 5.93 ± 0.43 | NS |
| Kidney | 4.78 ± 0.21 | 4.54 ± 0.22 | NS |
SBP = systolic blood pressure through a tail cuff, UNaV = urinary sodium excretion, UkV = urinary potassium excretion, BUN = blood urea nitrogen, SHR = spontaneously hypertensive rats.
Table constructed from data by Chrysant et al. [47].
Table 4.
Beneficial cardiometabolic effects of magnesium.
Magnesium is involved in several essential physiological, biochemical, and cellular processes regulating cardiovascular function. These functions are mostly mediated through calcium antagonism and inhibition of its intracellular transfer. These include:
|
1.2.4. Preeclampsia and eclampsia
Hypertensive complications of pregnancy like preeclampsia and eclampsia are important causes of maternal mortality and account for nearly 18% of all maternal deaths worldwide of about 6200–77000 deaths/year [53] In a recent large review on the incidence of preeclampsia and eclampsia by Abalos et al. [54] of 44 countries including 75,658,998 women, the crude incidence of preeclampsia of 37,652,006 women was 2.3% (1.2%–4.2%) and the crude incidence of eclampsia of 38,006,998 women was 1.1% (0.1%–2.7%. Regarding the prevention and treatment of preeclampsia and eclampsia, the use of MSO4 has been practiced for more than a century and it is still considered the treatment of choice for the prevention or control of eclamptic seizures [55], [56], [57]. The mechanism of action of MSO4 in the profilaxis and treatment of eclampsia has not been clearly defined nor has been any rigorous evaluation of therapeutic serum magnesium levels for the prevention or treatment of eclamptic seizures. The minimum accepted therapeutic level of 2 mmol/L is based on clinical and laboratory observations from earlier studies, rather than standard exposure-response studies [58], [59]. The two regimens currently recommended and accepted internationally for the use of MSO4 for the prevention and Treatment of preeclampsia and Eclampsia are those by the Collaborative eclampsia Trial (CET) for intravenous (IV) MSO4 administration [55], and by the Magpie Trial [56] for the intramuscular (IM) administration of MSO4. Recently, a renewed interest has been raised regarding the minimum effective dose of MSO4 for the prevention and treatment of eclampsia. In a recent systematic review, Okusanya et al. [57], analyzed the available data on the clinical pharmacokinetic properties of MSO4 when used for the treatment of preeclampsia and eclampsia in 1466 women from 27 studies. They analyzed different regimens used. Seven studies reported data on the standard regimen of the CET [55] of 4 g IV loading dose and 1 g/hour continuous IV maintenance infusion. The baseline magnesium levels were 0.74–0.85 mmol/l. Following the loading dose, the magnesium levels rose sharply by ½ hour to 1.48–1.70 mmol/l and reached a steady state of 1.64 mmol/l and at no point reached the 2.00 mmol/l. The volume of distribution varied considerably between 15.60 l and 32.20 l. But the estimated plasma clearance was more consistent, ranging between 4.81 and 4.28 l/hour, respectively. Similar results were obtained by studies using the Magpie trial of IM administration of 10 g loading dose and 5 g IM maintenance dose every 4 h [56]. Other investigators used different IV loading doses ranging from 4 to 6 g and maintenance doses of 1–2 g/h. Regarding the IM maintenance administration of MSO4 doses, the regimens used, were similar with the standard Prichard regimen. The data from this review suggest that MGSO4 can be protective even with serum concentrations of <2 mmol/l. Therefore, titrating MGSO4 injections to achieve a pre-set therapeutic range of 2–3.5 mmol/l maybe toxic without necessarily protecting against seizures. With respect to pharmacokinetic profiles of the two currently recommended regimens [55], [56], their comparability and clinical efficacy are reassuring and do not justify further increases in the total dose of MGSO4 for the prophylaxis and treatment of eclampsia.
2. Discussion
Magnesium is an important mineral, because it is a major cofactor for the function of over 300 intracellular enzyme systems and has several beneficial cardiometabolic effects (Table 3) and it should be continuously replenished. Although Mg2+ deficiency is difficult to occur under normal circumstances, since it is plentiful in foods and water, many diseases and other factors could contribute to its deficiency (Table 1). Regarding the Mg2+ homeostasis in the body, this is maintained through the gut and kidneys by regulating its absorption and excretion [11], [12], [13], because in contrast to other ions, Mg2+ is neither regulated nor protected by a hormone and it is considered an ion orphan [58]. However, when Mg2+ deficiency does occur, this is associated with an increased incidence of CVD, cardiac arrhythmias, hypertension and mortality [3], [4], [5], [6], [7], [8], [9], [10]. Diuretic-induced hypomagnesemia and hypokalemia is associated with worsening of preexisting coronary heart disease (CHD) and hypertension [4], [6]. Also, with de novo incidence of CHD and hypertension [3], [9], [14], [15], [16], [17], [18], [19], [41], [42], [43]. In a large study of 1000 ambulatory hypertensive patients from our center treated with high doses of hydrochlorotiazide (HCTZ) 50–100 mg/day, there was a 4.5% incidence of hypomagnesemia using very strict criteria of serum Mg2+ levels of ≤1.25 mEq/L. Had we used higher levels of serum Mg2+ the incidence of hypomagnesemic patients could have been higher. The Mg2+ deficiency might have been the reason for the resistance to treatment of BP, since these patients required more drugs for the same control of their BP compared with the normomagnesemic patients (2.5 vs 1.6 drugs), respectively [4]. Also, in an experimental study, dietary induced hypomagnesemia in SHR was associated with aggravation of their hypertension with an increase in PVR and RVR and a decrease in RBF [47]. In these rats, the hypomagnesemia was associated with increased intracellular Ca2+ concentration and metastatic tissue necrosis and calcification of the heart (Fig. 1). Similar findings have also, been reported by other investigators [49]. Magnesium plays a significant role in the membrane transport of K+ and Ca2+. Hypomagnesemia, besides intracellular hypercalcemia, is also associated with intracellular hypokalemia, which is difficult to correct if hypomagnesemia is not previously corrected [59]. Magnesium itself is a potent vasodilator, whereas Ca2+ is a vasoconstrictor and a major determinant of peripheral vascular tone and it may be involved in the pathoetiology of hypertension [60]. Another major problem with hypomagnesemia is its association with the chronic use of PPIs. Several recent studies have reported that patients treated on a chronic basis with PPIs, develop a PPI-induced hypomagnesemia with serious cardiac arrhythmias [8], [61], [62]. Serious ventricular arrhythmia (torsades de pointes) in patients on PPI treatment has been reported by several investigators [62], [63], [64], [65], [66], [67]. Regarding the mechanism of their hypomagnesemic effect, hypochlorhydria and an increase of the gut PH could play a role in Mg2+ absorption. In addition, the PPI-induced hypomagnesemia could also, be due to increased gastro-renal losses, via a dysfunction of the transient receptor potential mechanism 6/7 (TRPM6/7) located in the intestine as well as the distant convoluted tubule [66]. Interestingly, recent data suggest that carriers of TRPM6 polymorphisms are at increased risk of PPI-induced hypomagnesemia [67]. However, the evidence is not unique since other studies have not demonstrated PPI-induced hypomagnesemia, even in persons receiving concomitant drugs that induce hypomagnesemia, like diuretics, cisplatin or carboplatin [68], [69], [70]. Regardless of the evidence pro or against, it will be prudent for persons on long-term use of PPIs to periodically check their serum Mg2+ levels and correct a possible deficiency. Another major clinical use of Mg2+ is its effectiveness in the treatment of preeclampsia and eclampsia seizures [55], [56], [57]. Preeclampsia is a gestational disorder associated with increase in BP, proteinuria and peripheral edema. If not treated it will progress to eclampsia and generalized seizures endangering the life of pregnant woman and the fetus. Therefore, Mg2+ depletion should be avoided in pregnant women. A debatable aspect regarding Mg2+ deficiency is the drinking of softened water. Although there are studies demonstrating that persons living in areas of soft water, have an increased incidence of CHD [9], there are no studies available so far to demonstrate the harmful effects of drinking water from water softeners.
Conflict of interest
The authors declare no conflict of interest and that no funds were received for the preparation of the manuscript.
References
- 1.Ismail A.A. Magnesium: a mineral essential for health yet generally underestimated or even ignored. J. Nutr. Food Sci. 2016;6:2. [Google Scholar]
- 2.Grober U., Schmidt J., Kisters K. Magnesium in prevention and therapy. Nutrients. 2015;7:8199–8226. doi: 10.3390/nu7095388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi M.K., Bae Y.J. Association of magnesium intake with high blood pressure in Korean adults. Korean national health and nutrition examination survey 2007-2009. PLoS One. 2015;10:e0130405. doi: 10.1371/journal.pone.0130405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whang R., Chrysant S., Dillard B. Hypomagnesemia and hypokalemia in 1,000 treated ambulatory hypertensive patients. J. Am. Coll. Nutr. 1982;1:317–322. doi: 10.1080/07315724.1982.10719001. [DOI] [PubMed] [Google Scholar]
- 5.Lutsey P.L., Alonso A., Michos E.D. Serum magnesium, phosphorus, and calcium are associated with risk of incident heart failure: the Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Clin. Nutr. 2014;100:756–764. doi: 10.3945/ajcn.114.085167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dyckner T., Wester P.O. Potassium/magnesium depletion in patients with cardiovascular disease. Am. J. Med. 1987;82(Suppl 3A):11–17. doi: 10.1016/0002-9343(87)90127-6. [DOI] [PubMed] [Google Scholar]
- 7.Selim G.N., Spaspvski G., Tasija L. Hypomagnesemia and cause-specific mortality in hemodialysis patients: 5-year follow-up analysis. Int. J. Artif. Organs. 2017;40:542–549. doi: 10.5301/ijao.5000611. [DOI] [PubMed] [Google Scholar]
- 8.William J.H., Danziger J. Proton-pump inhibitor-induced hypomagnesemia: current research and proposed mechanisms. World J. Nephrol. 2016;5:152–157. doi: 10.5527/wjn.v5.i2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Momeni M., Gharedaghi Z., Mehadi Amin M. Does water hardness have preventive effect on Cardiovascular disease? Int. J. Prev. Med. 2014;5:159–163. [PMC free article] [PubMed] [Google Scholar]
- 10.Lip G.Y.H., Coca A., Kahan T. Hypertension and cardiac arrhythmias: a consensus document from the european heart rhythm association (EHRA) and ESC council on hypertension, endorsed by the heart rhythm society (HRS), asia-pacific heart rhythm society (APHRS) and sociedad latinoamericana de Estimulacion cardiaca y electrofisilogia (SOLEACE) Europace. 2017;19:891–911. doi: 10.1093/europace/eux091. [DOI] [PubMed] [Google Scholar]
- 11.Jahnen-Dechent J., Ketteler M. Magnesium basics. Clin Kidney J. 2012;5:i3–i14. doi: 10.1093/ndtplus/sfr163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kostov K., Halacheva L. Role of magnesium deficiency in promoting atherosclerosis, endothelial dysfunction, and arterial stiffening as risk factors for hypertension. Int. J. Mol. Sci. 2018;19:1724. doi: 10.3390/ijms19061724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saris N.E., Mervaala E., Karppanen H. Magnesium: an update on physiological, clinical and analytical aspects. Clin. Chim. Acta. 2000;294:1–26. doi: 10.1016/s0009-8981(99)00258-2. [DOI] [PubMed] [Google Scholar]
- 14.Di Nicolantonio J.J., O'Keefe J.H., Wilson W. Subclinical magnesium deficiency: a principal driver of cardiovascular disease and public health crisis. BMJ Open. 2018;5:e000668. doi: 10.1136/openhrt-2017-000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kieboom B.C., Niemeijer M.N., Leening M.J.G. Serum magnesium and the risk of death from coronary heart disease and sudden cardiac death. J Am Heart Assoc. 2016;5:e002707. doi: 10.1161/JAHA.115.002707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolte D., Vijayaraghavan K., Khera S. Role of magnesium in cardiovascular diseases. Cardiol. Rev. 2014;22:182–192. doi: 10.1097/CRD.0000000000000003. [DOI] [PubMed] [Google Scholar]
- 17.Laurant P., Touyz R.M. Physiological and pathophysiological role of magnesium in the cardiovascular system: implications in hypertension. J. Hypertens. 2000;18:1177–1191. doi: 10.1097/00004872-200018090-00003. [DOI] [PubMed] [Google Scholar]
- 18.Shechter M. Magnesium and cardiovascular system. Magnes. Res. 2010;23:60–72. doi: 10.1684/mrh.2010.0202. [DOI] [PubMed] [Google Scholar]
- 19.Del Gobbo L.C., Imamura F., Wu J.H.Y. Circulating and dietary magnesium and risk of cardiovascular disease: a systematic review and meta-analysis of prospective studies. Am. J. Clin. Nutr. 2013;98:160–173. doi: 10.3945/ajcn.112.053132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panchal A.R., Berg K.M., Kudechuk P.J. 2018 American Heart Association focused update on advanced cardiovascular life support use of antiarrhythmic drugs during and immediately after cardiac arrest: an update to the American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2018;138:e740–e749. doi: 10.1161/CIR.0000000000000613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silverman H.S., Stern M.D. Ionic basis of ischaemic cardiac injury: insights from cellular studies. Cardiovasc. Res. 1994;28:581–597. doi: 10.1093/cvr/28.5.581. [DOI] [PubMed] [Google Scholar]
- 22.Antman E.M. Magnesium in acute myocardial infarction: overview of available evidence. Am. Heart J. 1996;132:487–495. doi: 10.1016/s0002-8703(96)90341-5. [DOI] [PubMed] [Google Scholar]
- 23.Woods K.L., Fletcher S., Roffe C. Intravenous magnesium sulfate in suspected acute myocardial infarction: results of the second Leicester Intravenous Magnesium Intervention Trial (LIMITS-2) Lancet. 1992;339:1553–1558. doi: 10.1016/0140-6736(92)91828-v. [DOI] [PubMed] [Google Scholar]
- 24.ISIS-4 A randomised factorial trial assessing early oral captopril, oral mononitrate, and intravenous magnesium sulfate in 58,050 patients with suspected acute myocardial infarction. ISIS-4 (Fourth International Study of Infarct Survival) Collaborative Group. Lancet. 1995;345:669–685. [PubMed] [Google Scholar]
- 25.Early administration of intravenous magnesium to high-risk patients with acute myocardial infarction in the Magnesium in Coronaries (MAGIC) Trial: a randomised controlled trial. Lancet. 2002;360:1189–1196. doi: 10.1016/s0140-6736(02)11278-5. [DOI] [PubMed] [Google Scholar]
- 26.Li J., Zhang Q., Zhang M. Intravenous magnesium for acute myocardial infarction. Cochrane Database Syst. Rev. 2007:CD002755. doi: 10.1002/14651858.CD002755.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mubagwa K., Gwanyanya A., Zakharov S. Regulation of cation channels in cardiac and smooth muscle cells by intracellular magnesium. Arch. Biochem. Biophys. 2007;458:73–89. doi: 10.1016/j.abb.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 28.Wang M., Tashiro M., Berlin J.R. L-type calcium current by intracellular magnesium in rat cardiac myocytes. J. Physiol. 2004;555(pt 2):383–396. doi: 10.1113/jphysiol.2003.048538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White R.E., Hartzell H.C. Effects of intracellular free magnesium on calcium current in isolated cardiac myocytes. Science. 1988;239(484 pt 1):778–780. doi: 10.1126/science.2448878. [DOI] [PubMed] [Google Scholar]
- 30.Khan A.M., Lubitz S.A., Sullivan L.M. Low serum magnesium and the development of atrial fibrillation in the community: the Framingham Heart Study. Circulation. 2013;127:33–38. doi: 10.1161/CIRCULATIONAHA.111.082511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho K.M. Intravenous magnesium for cardiac arrhythmias: jack of all trades. Magnes. Res. 2008;21:65–68. [PubMed] [Google Scholar]
- 32.Gu W.J., Wu Z.J., Wang P.F. Intravenous magnesium prevents atrial fibrillation after coronary artery bypass grafting: a meta-analysis of 7 double-blind, placebo-controlled, randomized clinical trials. Trials. 2012;13:41. doi: 10.1186/1745-6215-13-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Onalan O., Crystal E., Daoulah A. Meta-analysis of magnesium therapy for the acute management of rapid atrial fibrillation. Am. J. Cardiol. 2007;99:1726–1732. doi: 10.1016/j.amjcard.2007.01.057. [DOI] [PubMed] [Google Scholar]
- 34.Cagli K., Ozeke O., Ergun K. Effect of low-dose amiodarone and magnesium combination on atrial fibrillation after coronary artery surgery. J. Card. Surg. 2006;21:458–464. doi: 10.1111/j.1540-8191.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- 35.Tercius A.J., Kluger J., Coleman C.I. Intravenous magnesium sulfate enhances the ability of intravenous ibutilide to successfully convert atrial fibrillation or flutter. Pacing Clin. Electrophysiol. 2007;30:1331–1335. doi: 10.1111/j.1540-8159.2007.00866.x. [DOI] [PubMed] [Google Scholar]
- 36.Kinlay S., Buckley N.A. Magnesium sulfate in the treatment of ventricular arrhythmias due to digoxin toxicity. J. Toxicol. Clin. Toxicol. 1995;33:55–59. doi: 10.3109/15563659509020216. [DOI] [PubMed] [Google Scholar]
- 37.Tzivoni D., Banai S., Schuger C. Treatment of torsade de pointes with magnesium sulfate. Circulation. 1988;77:392–397. doi: 10.1161/01.cir.77.2.392. [DOI] [PubMed] [Google Scholar]
- 38.Gottlieb S.S., Fisher M.I., Pressel M.D. Effects of intravenous magnesium sulfate on arrhythmias in patients with congestive heart failure. Am. Heart J. 1993;125:1645–1650. doi: 10.1016/0002-8703(93)90754-w. [DOI] [PubMed] [Google Scholar]
- 39.Ceremuzynski L., Gebalska J., Wolk R. Hypomagnesemia in heart failure with ventricular arrhythmias: beneficial effects of magnesium supplementation. J. Intern. Med. 2000;247:78–86. doi: 10.1046/j.1365-2796.2000.00585.x. [DOI] [PubMed] [Google Scholar]
- 40.Zehender M., Meinertz T., Faber T. Antiarrhythmic effects of increasing the daily intake of magnesium and potassium in patients with frequent ventricular arrhythmias. Magnesium in Cardiac Arrhythmias (MAGICA) Investigators. J. Am. Coll. Cardiol. 1997;29:1028–1034. doi: 10.1016/s0735-1097(97)00053-3. [DOI] [PubMed] [Google Scholar]
- 41.Rodrigqez-Ramirez M., Simental-Mendia L.E., Gonzales-Ortiz M. Prevalence of prehypertension in Mexico and its association with hypomagnesemia. Am. J. Hypertens. 2015;28:1024–1030. doi: 10.1093/ajh/hpu293. [DOI] [PubMed] [Google Scholar]
- 42.Durlach J., Drlach V., Rayssiguier Y. Magnesium and blood pressure. II. Clinical studies. Magnes. Res. 1992;5:147–153. [PubMed] [Google Scholar]
- 43.Rsnick K.M., Bardicef O., Altura B.T. Serum ionized magnesium: relation to blood pressure and racial factors. Am. J. Hypertens. 1997;10(12 Pt 1):1420–1424. doi: 10.1016/s0895-7061(97)00364-6. [DOI] [PubMed] [Google Scholar]
- 44.Ford E.S. Race, education, and dietary cations: findings from the third national health and nutrition examination survey. Ethn. Dis. 1998;8:10–20. [PubMed] [Google Scholar]
- 45.Fox C.H., Ramsoomair D., Mahoney M.C. An investigation of hypomagnesemia among ambulatory urban African Americans. J. Fam. Pract. 1999;48:636–639. [PubMed] [Google Scholar]
- 46.Fox C.H., Mahoney M.C., Ramsoomair D. Magnesium deficiency in African- Americans: does it contribute to increased cardiovascular risk factors? J. Natl. Med. Assoc. 2003;95:257–262. [PMC free article] [PubMed] [Google Scholar]
- 47.Chrysant S.G., Ganousis L., Chrysant C. Hemodynamic and metabolic effects of hypomagnesemia in spontaneously hypertensive rats. Cardiology. 1988;75:81–89. doi: 10.1159/000174354. [DOI] [PubMed] [Google Scholar]
- 48.Sontia B., Touyz R.M. Magnesium transport in hypertension. Pathophysiology. 2007;14:205–211. doi: 10.1016/j.pathophys.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 49.Heggtveit H.A., Herman L., Mishra R.K. Cardiac necrosis and calcification in experimental magnesium deficiency. Light and electron microscopic study. Am. J. Pathol. 1964;45:757–782. [PMC free article] [PubMed] [Google Scholar]
- 50.Kisters K., Tepel M., Spieker C. Decreased membrane Mg2+ concentrations in a subgroup of hypertensives: membrane model for the pathogenesis of primary hypertension. Am. J. Hypertens. 1998;11:1390–1393. doi: 10.1016/s0895-7061(98)00169-1. [DOI] [PubMed] [Google Scholar]
- 51.Kosch M., Hausberg M., Westermann G. Alterations in calcium and magnesium content of red cell membranes in patients with primary hypertension. Am. J. Hypertens. 2001;14:254–258. doi: 10.1016/s0895-7061(00)01271-1. [DOI] [PubMed] [Google Scholar]
- 52.Kisters K., Wessels F., Tokmak F. Early-onset increased calcium and decreased magnesium concentrations and an increased calcium/magnesium ratio in SHR versus WKY. Magnesium Res. 2004;17:264–269. [PubMed] [Google Scholar]
- 53.Khan K.S., Wojdyla D., Say L. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066–1074. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 54.Abalos E., Cuesta C., Grosso A.L. Global and regional estimates of preeclampsia and Eclampsia: a systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013;170:1–7. doi: 10.1016/j.ejogrb.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 55.The Eclampsia Trial Collaborative Group Which anticonvulsant for women with eclampsia? Evidence from the collaborative eclampsia trial. Lancet. 1995;354:1455–1463. [PubMed] [Google Scholar]
- 56.The Magpie Trial Collaborative Group Do women with preeclampsia, and their babies, benefit from magnesium sulfate? The Magpie Trial: a randomised placebo controlled trial. Lancet. 2002;359:1877–1890. doi: 10.1016/s0140-6736(02)08778-0. [DOI] [PubMed] [Google Scholar]
- 57.Okusanya B., Oladapo O.T., Long Q. Clinical pharmacokinetic properties of magnesium sulfate in women with preeclampsia and Eclampsia. BJOG. 2016;123:356–366. doi: 10.1111/1471-0528.13753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Agus Z.S. Mechanisms and causes of hypomagnesemia. Curr. Opin. Nephrol. Hypertens. 2016;25:301–307. doi: 10.1097/MNH.0000000000000238. [DOI] [PubMed] [Google Scholar]
- 59.Whang R., Aikawa J.K. Magnesium deficiency and refractoriness to potassium repletion. J. Chronic Dis. 1977;30:65–68. doi: 10.1016/0021-9681(77)90075-3. [DOI] [PubMed] [Google Scholar]
- 60.Touyz R.M., Milne F.J., Reinach S.G. Platelet and erythrocyte Mg2+, Ca2+, Na+, K+ and cell membrane adenosine triphosphatase activity in essential hypertension in blacks. J. Hypertens. 1992;10 doi: 10.1097/00004872-199206000-00010. 671- 578. [DOI] [PubMed] [Google Scholar]
- 61.Janett S., Camozzi P., Peeters G.G.A.M. Hypomagnesemia induced by long-term treatment with proton-pump inhibitors. Gastroenterol Res Pract. 2015;2015:951768. doi: 10.1155/2015/951768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lazzerini P.E., Bertolozzi I., Finizola F. Proton pump inhibitors and serum magnesium levels in patients with torsades de pointes. Front. Pharmacol. 2018;9:363. doi: 10.3389/fphar.2018.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Asajima H., Saito N., Ohmura Y., Ohmura K. Lanoprazole precipitated QT prolongation and torsade de pointes associated with disopyramide. Eur. J. Clin. Pharmacol. 2012;68:331. doi: 10.1007/s00228-011-1119-z. [DOI] [PubMed] [Google Scholar]
- 64.Bibawy J.N., Parikh V., Wahha J. Pantoprazole (proton pump inhibitor) contributing to torsades de pointes storm. Circ Arrhythm Electrophysiol. 2013;6:e17. doi: 10.1161/CIRCEP.112.000101. [DOI] [PubMed] [Google Scholar]
- 65.Hansen B.A., Bruserud O. Hypomagnesemia as a potentially life-threatening adverse effect of omeprazole. Oxf Med Case Rep. 2016;2016:147–149. doi: 10.1093/omcr/omw062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Famularo G., Gasbarrone L., Minisola G. Hypomagnesemia and proton pump inhibitors. Expert Opin. Drug Saf. 2013;12:709–716. doi: 10.1517/14740338.2013.809062. [DOI] [PubMed] [Google Scholar]
- 67.Hess M.W., de Baaij J.H., Brockman M.M. Common single nucleotide polymorphisms in transient receptor potential melastatin type 6 increase risk the risk for proton pump inhibitor-induced hypomagnesemia: a case control study. Pharmacogenetics Genom. 2017;27:83–88. doi: 10.1097/FPC.0000000000000259. [DOI] [PubMed] [Google Scholar]
- 68.Park C.H., Kim E.H., Roh Y.H. The association between the use of proton pump inhibitors and the risk of hypomagnesemia: a systematic review and meta-analysis. PLoS One. 2014;9:e112558. doi: 10.1371/journal.pone.0112558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chowdhry M., Shah K., Kemper S. Proton pump inhibitors not associated with hypomagnesemia, regardless of dose or concomitant diuretic use. J. Gastroenterol. Hepatol. 2018;33:1717–1721. doi: 10.1111/jgh.14141. [DOI] [PubMed] [Google Scholar]
- 70.Biyik M., Solak Y., Ucar R. Hypomagnesemia among outpatient long-term proton pump inhibitor users. Am. J. Therapeut. 2017;24:e52–e55. doi: 10.1097/MJT.0000000000000154. [DOI] [PubMed] [Google Scholar]