ABSTRACT
Background
It has been acknowledged that medical life-threatening experiences such as an acute myocardial infarction (MI) often lead to acute stress disorder symptoms (ASS), which in turn can result in the development of post-traumatic stress symptoms (PTSS). Previous studies have suggested an association between various traumatic experiences and alexithymia. The association of alexithymia with ASS and PTSS in patients with MI is elusive.
Objectives
The aim of this study was to examine the association of alexithymia with MI-induced ASS and PTSS in patients at high risk of developing PTSD.
Method
Patients (N = 154) were examined twice, once within 48 hours, and then again three months after acute MI. All patients completed the self-rating Acute Stress Disorder Scale (ASDS) within 48 hours after the cardiac event. Three months after hospital discharge, all patients completed the Toronto Alexithymia Scale (TAS-20) and underwent the Clinician-Administered PTSD Scale (CAPS), a structured interview to assess the severity of PTSS. Descriptive statistics, correlations, multivariate linear regressions, and moderation analysis were conducted.
Results
The linear regression model explained 23% of the variance in MI-induced PTSS-symptoms (F(6.109) = 5.58, p < 0.001, R2 = 0.23. ASS was significantly related to PTSS severity (r (152) = p < 0.001). The scores of the TAS-20 subscale difficulties identifying feelings (DIF) were found to significantly moderate this relationship (R2 = 0.03, p = 0.04). The scores of TAS-20 subscales DDF and EOT as well as the TAS-20 total score had no influence on the relationship between ASS and PTSS (p > 0.05).
Conclusion
In MI patients with high levels of DIF, ASS predicted the development of PTSS. If replicated, the finding may inform emotion-oriented interventions to investigate whether increasing the capacity to identify feelings following acute MI could be beneficial in preventing the development of PTSS.
KEYWORDS: alexithymia, posttraumatic stress symptoms, acute stress disorder symptoms, myocardial infarction
HIGHLIGHTS: The main results of this study showed a moderating effect of the alexithymia trait ‘difficulty identifying feelings’ on the association between symptoms of acute stress disorder and symptoms of PTSD in patients after acute myocardial infarction (MI).
Antecedentes: Se ha reconocido que experiencias médicas en las que se pone en peligro la vida, tal y como el infarto agudo al miocardio (MI por sus siglas en inglés) frecuentemente pueden llevar a síntomas de Trastorno de estrés agudo (ASS por sus siglas en inglés), que pueden llevar a desarrollar síntomas de estrés postraumático (PTSS por sus siglas en inglés). Estudios previos han sugerido una asociación entre numerosas experiencias traumáticas y alexitimia. La asociación entre la alexitimia con los ASS y PTSS en pacientes con infarto agudo al miocardio es evasiva.
Objetivos: Examinar la asociación entre la alexitimia y los ASS y PTSS inducidos por MI en pacientes con elevado riesgo de desarrollar un TEPT.
Método: Pacientes (N=154) fueron examinados dos veces, una vez dentro de las primeras 48 horas y en otra ocasión tres meses después del infarto agudo al miocardio. Todos los pacientes completaron la Escala Del Trastorno de Estrés Agudo, que es auto-puntuada (ASDS por sus siglas en inglés) dentro de las primeras 48 horas posteriores al evento cardíaco. Tres meses posteriores al alta hospitalaria, todos los pacientes completaron la Escala de Toronto de Alexitimia (TAS-20) y fueron sometidos a una entrevista estructurada para evaluar la gravedad de los PTSS (CAPS). Se condujeron análisis de estadística descriptiva, correlaciones, regresiones lineares multivariadas y análisis de moderación.
Resultados: El modelo linear de regresión explicó el 23% de la varianza en los PTSS inducidos por el MI. F (6.109) = 5.58, p < 0.001, R2 =0.23. Los ASS estuvieron significativamente relacionados a la severidad de los PTSS (r (152) = 0.46, p < 0.001). Los puntajes de la subescala de dificultad de identificar sentimientos (DIF por sus siglas en inglés) dentro de las TAS-20 moderaba significativamente esta relación (R2 = 0.03, p = 0.04). Los puntajes de las subescalas de TAS-20 DDF y EOT así como el puntaje total de la TAS-20 no tuvieron ninguna influencia en la relación entre ASS y PTSS (p > 0.05).
Conclusión: en pacientes con MI y con altos niveles de DIF, los ASS predijeron el desarrollo de PTSS. Si se pudiesen replicar, estos hallazgos pueden apoyar intervenciones orientadas a las emociones para investigar si es que la capacidad de identificar sentimientos posteriormente a haber sufrido un infarto agudo al miocardio pudiese ser beneficioso en prevenir el desarrollo de PTSS.
Palabras clave: alexitimia, síntomas de estrés postraumático, síntomas de trastorno de estrés agudo, infarto al miocardio
目的:人们已经认识到, 危及生命的医疗经历, 例如急性心肌梗塞 (MI), 通常会导致急性应激障碍症状 (ASS), 进而可能导致创伤后应激症状 (PTSS) 的发展。前人研究表明各种创伤经历与述情障碍之间存在关联。 MI患者中述情障碍与ASS和PTSS的关联尚不清楚。本研究旨在考查PTSD高风险患者中述情障碍与MI诱发的ASS和PTSS的关联。
方法:对154名患者进行了两次测量, 分别在急性MI48小时内和三个月后。所有患者在心脏事件发生后48小时内均完成了自评急性应激障碍量表 (ASDS) 。出院三个月后, 所有患者均完成了多伦多述情障碍量表 (TAS-20), 并完成了一项评估PTSS严重程度的结构性访谈——临床用PTSD量表 (CAPS) 。
结果:线性回归模型解释了MI诱发的PTSS症状的23%的方差 (F(6.109) = 5.58, p< 0.001, R2 =0.23)。 ASS与PTSS严重程度显著相关 (r (152) = 0.46, p< 0.001)。发现TAS-20分量表识别感觉困难 (DIF) 的得分对此关联有显著调节作用 (R2 = 0.03, p= 0.04)。TAS-20分量表DDF和EOT的得分以及TAS-20总得分对ASS和PTSS之间的关联没有影响 (p > 0.05)。
结论:在DIF高的MI患者中, ASS预测了PTSS的发展。如果可重复, 该发现可能会为情绪干预提供信息, 以考查提高急性心肌梗死后识别情绪的能力是否有助于预防PTSS的发展。
关键词: 述情障碍, 创伤后应激症状, 急性应激障碍症状, 心肌梗塞
1. Introduction
Estimated life-time prevalence rates of posttraumatic stress disorder (PTSD) in the general population range between less than 1% and up to 10% (Hepp et al., 2006). In addition to more traditional causes of a psychological trauma such as man-made and natural disaster-related traumatic experiences, it has been acknowledged that medical life-threatening experiences such as an acute myocardial infarction (MI) may induce PTSD (Gurevich, Devins, & Rodin, 2002; Schelling, 2008; Spindler & Pedersen, 2005; Tedstone & Tarrier, 2003). The abruptness of the event, the concrete danger of death, and the patients’ intense sense of loss of control and helplessness during the event (Kutz, Shabtai, Solomon, Neumann, & David, 1994) as well as the intrusive experience of the treatments, such as coronary surgery, angioplasty, angiography, pacemaker implantation, stress testing and even the side effects of medications, can also lead to the development of PTSD (Alonzo, 2000). As a result, about 18% and 23% of patients with an acute MI, who perceive the event as life-threatening and distressing, show clinically relevant acute stress symptoms (ASS) (Ginzburg et al., 2003).
DSM-IV criteria for acute stress disorder (ASD) include symptoms of dissociation, re-experiencing, avoidance, and hyperarousal that may cause substantial disruption in everyday life (American Psychiatric Association, 2000). These symptoms are often temporary, easing off in hours or sometimes days up to four weeks after an event (Bryant & Harvey, 2000). Most trauma survivors who eventually developed PTSD did not meet the criteria for ASD initially (Bryant, Creamer, O’Donnell, Silove, & McFarlane, 2008). According to one study, ASD may not be sufficiently distinct from PTSD without the duration criterion (Brewin, Andrews, & Rose, 2003). ASD may function as a tool to identify patients for whom immediate treatment may be necessary (Meiser-Stedman et al., 2017).
The prevalence rate of PTSD following an acute MI was 4%, whereas substantial posttraumatic stress symptoms (PTSS) were found in 12% of the patients (Edmondson et al., 2013). PTSS after an MI are often not recognized by health care providers and can have significant consequences on how patients convalesce (Roberge, Dupuis, & Marchand, 2010). PTSS are associated with an increased risk of hospital readmission, recurrent MI and all-cause mortality (Edmondson et al., 2012), poor quality of life, as well as adverse health behaviours and medical comorbidities (Edmondson & von Känel, 2017). In addition, PTSS are associated with poor adherence to cardiac medication (Kronish, Edmondson, Li, & Cohen, 2012). Younger age, as well as a sense of helplessness, pain and fear of dying during MI (Hari et al., 2010), lower educational level (Ginzburg, Solomon, & Bleich, 2002) and depressive symptoms (Whitehead, Strike, Perkins-Porras, & Steptoe, 2005) have all been shown to be predictive for PTSS in the first year after acute MI. The persistence of PTSS (Hari et al., 2010; Marke & Bennett, 2013; Pedersen, von Domburg, & Larsen, 2004; Wikman, Bhattacharyya, Perkins-Porras, & Steptoe, 2008) or even an increase over the first three years (Meli, Birk, Edmondson, & Bonanno, 2020) might be one explanation for their adverse health prognosis.
Alexithymia has been described as an emotional processing deficit (Edwards, Shivaji, Micek, & Wupperman, 2020) characterized by 1) problems in identifying feelings and in differentiating between feelings and bodily sensations; 2) the inability to communicate feelings to others; 3) limited imagination resulting in a lack of fantasy; and 4) a stimulus-bound, cognitive-style by external observations only (Larsen, Brand, Bermond, & Hijman, 2003; Taylor, Bagby, & Parker, 2003; Taylor et al., 1988). It is often accompanied with negative affect, social evasion and poor relationships (Tesio, Goerlich, Hosoi, & Castelli, 2019). The prevalence of alexithymia in the general population ranges from 5% to 15% (Samur, 2013) and from 13 to 30% in post-MI patients (Freyberger, 1977; Kojima, Frasure-Smith, & Lespérance, 2001; Salminen, Saarijarvi, Aarela, Toikka, & Kauhanen, 1999).
Whether alexithymia is a trait, or a state continues to be debated. In long-term studies, alexithymia has been found to be a stable, dimensional personality trait. Taylor and Bagby (2012) concluded in their review that there is a strong support for the validity, stability and dimensional nature of the construct which goes along with maladaptive defences and coping mechanisms putting a person at high risk to develop medical and psychiatric disorders. Alexithymia is now recognized as a trans-nosographic construct, which acts as a non-specific risk factor for multiple physical and mental diseases (Caretti & La Barbera, 2005; Taylor & Bagby, 2012).
A strong association between alexithymia and PTSS has been reported by both cross-sectional and longitudinal studies (Frewen, Dozois, Neufeld, & Lanius, 2008; Sondergaard & Theorell, 2004). One of the characteristics of alexithymia, namely difficulties in in identifying feelings (DIF) was found to be highly congruent with the construct of PTSD (Evren, Dalbudak, Cetin, Durkaya, & Evren, 2010; Sondergaard & Theorell, 2004). According to one study, DIF independently predicted PTSS in adult survivors of physical trauma (Zahradnik, Stewart, Marshall, Schell, & Jaycox, 2009). Several studies suggest that trait alexithymia has an influence on the development and persistence of PTSS (Cloitre, Garvert, Brewin, Bryant, & Maercker, 2013; O’Brien, Gaher, & Pope, 2008; Zahradnik et al., 2009).
Moreover, various studies have established a link between alexithymia and MI-induced PTSS (Bennett & Brooke, 2010; Chung, Dennis, Berger, Jones, & Rudd, 2012; Gao, Zhao, Li, & Cao, 2015). One previous study reported that deficits in emotional processing and lack of social attachment explained 24.2% of PTSS (Bennett & Brooke, 2010). Furthermore, in addition to age, low social support, and patients’ awareness of MI alexithymia was found to be a significant and independent predictor of PTSS between six- and 12-months post-MI (Bennett & Brooke, 2010). According to Gao et al. (2015), attachment anxiety and DIF predicted PTSS in first-time MI patients. These authors found that, except for externally oriented thinking, all sub-dimensions of alexithymia were significantly correlated with PTSS. Another study suggested that alexithymia could act as a moderator between traumatic events and PTSS (Park et al., 2015).
Moreover, DIF, as an important component of alexithymia, has been found to play a role in the severity of PTSS after stroke, which is also one kind of life-threatening disease (Wang, Chung, Hyland, & Bahkeit, 2011). Therefore, it is reasonable to explore the relationship between alexithymia and PTSS among MI patients. Taken together, alexithymia may play an important role in the development of PTSS after MI.
However, no research has addressed alexithymia as a possible moderator of the association between MI-induced ASS and later development of PTSS. Also, existing studies rarely explore the distinct influences of subdimensions of alexithymia, making it unclear whether there are specific sub-dimensions related with PTSD symptoms (Frewen et al., 2008). Consequently, it is necessary to examine the role of alexithymia following MI and to look at the relationship between alexithymia and PTSS more thoroughly. Thus, the aim of this study was to examine the role of alexithymia in the relationship between ASS and subsequent PTSS three months after the experience of an acute MI. We specifically hypothesized that alexithymia would moderate this relationship such that ASS would predict stronger PTSS independent of demographic, psychosocial and medical variables.
2. Methods
2.1. Participants
Participants in the current study were a subsample of the MI-SPRINT randomized controlled trial (RCT) that investigated the effects of early psychological counselling for the prevention of MI-induced PTSS (Meister et al., 2013; von Känel et al., 2018). Consecutive patients, aged 18 years and older, with verified acute ST elevation myocardial infarction (STEMI) or non-STEMI, referred for acute coronary care intervention to the Cardiology Department, Bern University Hospital, Switzerland, were recruited between January 2013 and December 2015. ST elevation myocardial infarction (STEMI) is characterized by symptoms of myocardial ischaemia and is associated with persistent electrocardiographic ST elevation and subsequent release of biomarkers (e.g. troponin) of myocardial necrosis. Recruitment details are described elsewhere (von Känel et al., 2018). In short, as soon as a patient was admitted, the research staff called the study centre, where an independent person randomly assigned the participants to trauma-focused counselling (intervention group) or stress-focused counselling (control group). The randomization list for group allocation was computer-generated with Research Randomizer (www.randomizer.org) and was only accessible to researchers after the end of the entire study.The sample consisted of 190 MI patients who were randomized to either the trauma-focused intervention group (n = 97) or the stress-focused active control group (n = 93). Of these, 154 (81.1%) completed the 3-month follow-up assessment and were included in our analysis. All participants were of Caucasian origin.
Eligible participants were aged 18 years and older with stable circulatory conditions according to the treating cardiologist (i.e. no cardiogenic shock signs such as paleness, restlessness, cold sweat and heart rate > 100/min and systolic blood pressure < 100 mmHg) and high levels of acute distress during MI. Acute distress during MI was based on numeric rating scale scores (range 0–10) for ‘pain intensity (during MI)’ of at least 5 plus ‘fear of dying (until admission to the coronary care unit)’ and/or ‘worrying and feeling helpless (when being informed about having MI)’ of at least 5 each. The categorization of high risk of experiencing distress such as ASS and PTSS after MIis based on our previous study of 297 patients (61 ± 10 years, 83% men) who self-rated PTSS using the posttraumatic diagnostic scale following an acute MI. Measures of subjective perception of MI (i.e. fear of dying, sense of helplessness, pain) all predicted posttraumatic stress (Guler et al., 2009) as well as CVD-related hospital readmissions, independent of other prognostic factors (von Känel et al., 2011). Participants were excluded if they had emergency coronary artery bypass grafting, comorbid diseases likely to cause death within 1 year, were not fully oriented, or had cognitive impairment. Additional exclusion criteria were current severe clinical depression in the patient´s history, suicidal ideations in the previous 2 weeks, inadequate knowledge of German, or current participation in another RCT. We assumed that depressed patients would struggle to follow a 45-minute counselling session and to comprehend its content due to concentration difficulties. Therefore, patients clinically judged to have severe depression were excluded. The study was conducted in accordance with the Declaration of Helsinki and the guidelines on Good Clinical Practice and registered under ClinicalTrials.gov (NCT01781247). The study was independently monitored by the independent Clinical Trials Unit, Faculty of Medicine, University of Bern, and approved by the ethics committee of the State of Berne (KEK No.170/12). All participants gave written informed consent for study participation. None of the subjects received financial compensation.
2.2. Measures
Participants completed validated questionnaires within 48 hours of MI (baseline measures) as well as three months after MI (3-months follow-up measures). Baseline measures included sociodemographic factors, depression history, severity of depressive symptoms, risk of post- discharge death and recurrent MI after acute coronary syndrome, symptoms of dissociation, re-experiencing, avoidance and hyperarousal and PTSD prior to MI. Assessment of MI-induced PTSS and alexithymia were included in the 3-month follow-up analyses.
2.3. Baseline measures (within 48 hours of MI)
The current study used self-report data from the baseline assessment as follows: sociodemographic factors (sex, age, marital status, living and employment status, level of education), depression history was orally assessed with standardized questions about previously diagnosed depressive episodes, severity of depressive symptoms measured with the 13-item cognitive depressive symptom subscale of the Beck Depression Inventory (BDI; total score 0–39) (Beck, 1993), risk of post-discharge death and recurrent MI after acute coronary syndrome, estimated with the Global Registry of Acute Coronary Events (GRACE) risk score (Fox et al., 2006), ASS, of dissociation, re-experiencing, avoidance and hyperarousal measured with the German version of the Acute Stress Disorder Scale (ASDS) (Helfricht et al., 2009), and PTSD prior to current MI assessed with a 3-item screener (Franklin, Sheeran, & Zimmerman, 2002).
The ASDS is a self-rated inventory (Helfricht et al., 2009) that (a) indexes ASD and (b) predicts posttraumatic stress disorder (PTSD) (Bryant, 2000). It comprises 19 items for the four subscales dissociation (5 items), re-experiencing (4 items), avoidance (4 items) and arousal (6 items) based on the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). Each item is scored on a five-point Likert scale (0 = ‘not at all,’ 4 = ‘extremely)’ with a sum score between 0 and 76. The cut-off score for the ASDS scale has been set at 56 (Bryant, 2000). Psychometric properties of the ASDS show a good sensitivity (95%) and specificity (83%) as well as a strong test-retest reliability (r = 0.94) (Bryant, 2000). All patients were asked to complete a questionnaire referring to the cardiac event as the potentially traumatic event. The ASDS showed good internal consistency in our sample (Cronbach’s alpha = 0.83).
Previous traumatic experiences that had possibly resulted in PTSS in the three months prior to hospitalization were explored using three items from the Structured Clinical Interview for DSM-IV-PTSD (SCID-PTSD). Patients were asked to self-report whether they had a) experienced a traumatic event (‘such as a major disaster, very serious accident or fire, being physically assaulted, seeing another person killed or dead, or badly hurt, or hearing about something horrible that has just happened to someone close to you’) prior to their MI-related hospitalization; b) whether the event came back ‘in nightmares, flashbacks, or thoughts you cannot get rid of’; and c) whether the experience of ‘thoughts or feelings coming back’ had happened in the past three months. These questions were recently applied to screen for PTSD cases in patients after an acute coronary syndrome. Research showed that three ‘yes’ responses to these questions correctly identified 97% of PTSD cases (Franklin et al., 2002).
2.4. 3-month follow-up measures
At 3 months after MI, the severity of MI-induced PTSS was assessed and expressed as the total score of the validated German version of the Clinician-Administered PTSD Scale (CAPS) (Schnyder & Moergeli, 2002). Counsellors (doctoral students in psychology and medicine), trained and supervised by senior clinical psychotherapists with degrees in psychology or psychiatry, carried out the CAPS interview. The interviewers were given a 2-day (16 hours) training course before they carried out the CAPS interview independently.
Interviewers rated each of the 17 PTSD symptoms according to DSM-IV in terms of frequency and intensity during the previous month between 0 (never) and 4 (almost always) to obtain a total severity score (range 0–136) in reference to the MI event. A symptom is given when frequency is at least 1 point and intensity is at least 2 points. One of 5 symptoms is required for the re-experiencing cluster, 3 of 7 symptoms for the avoidance cluster, and 2 of 5 symptoms for the hyperarousal cluster. Patients who met the criteria for all 3 symptom clusters were diagnosed to have PTSD. Participants were classified with sub-syndromal PTSD if they met criterion B (re-experiencing) and either criterion C (avoidance/numbing) or criterion D (over-arousal), but not both (Blanchard et al., 1995). All others were classified as non-PTSD cases. The German version of the CAPS showed good internal consistency for the severity score of all 17 symptom items in our sample (Cronbach’s α = 0.79). The CAPS interview and the scoring were conducted by the same person.
Deficiency in understanding, processing and describing emotions was measured with the German version of the 20-item Toronto Alexithymia scale (TAS-20) (Schafer, Schneider, Sitte, & Franz, 2002). The TAS-20 is a self-report measure assessing three components of the alexithymia construct: (1) DIF (‘I often don’t know why I am angry’); (2) difficulty describing feelings (DDF) (‘People tell me to describe my feelings more’); and (3) externally oriented thinking (EOT) (‘It is difficult for me to reveal my innermost feelings, even to close friends’). Items are rated on a 5-point Likert scale between 1 (strongly disagree) and 5 (strongly agree); 5 items are negatively keyed (items 4, 5, 10, 18, and 19). The total alexithymia score is the sum of the responses of the three subscales. The TAS-20 uses cut-off scoring equal to or less than 51 for ‘non-alexithymia’ and equal to or greater than 61 for ‘alexithymia’. Scores between 52 and 60 indicate ‘possible alexithymia’ (Bagby, Parker, & Taylor, 1994). The German version of the TAS-20 showed good convergent and clinical validity (Bach, Bach, de Zwaan, Serim, & Böhmer, 1996). The TAS-20 demonstrated good internal consistency in our sample (Cronbach’s alpha = 0.84). Cronbach’s alpha for the subscale DIF showed good internal consistency (Cronbach’s alpha = 0.70), medium internal consistency for EOT (Cronbach’s alpha = 0.53) and high internal consistency for the subscale DDF (Cronbach’s alpha = 0.85).
2.5. Intervention
The intervention consisted of either trauma-focused counselling (intervention group) or stress counselling (active control group). Counselling was given within 48 hours of admission by study therapists as a single 45-minute session. In both groups, intervention consisted of a counselling session that covered in the first five minutes topics of most immediate concern to the individual patient. The trauma-focused intervention used an educational and resource-oriented approach targeting individual resources and coping strategies, teaching cognitive restructuring to specifically prevalent MI-triggered traumatic reactions as well as explaining the concept of psychological trauma and PTSS that might possibly emerge in the aftermath of MI. In the active control intervention group, patients received information about the role of psychosocial stress in coronary heart disease, stress management and behavioural health strategies to improve everyday functioning after MI. After the counselling session, all patients received an information booklet on either coping with posttraumatic stress symptoms related to the experience of MI or coping with stress in general.
2.6. Statistical analysis
Descriptive statistics for patient characteristics and further inferential statistical analysis were conducted using SPSS 25.0 for Windows (SPSS Inc., Chicago, IL, USA). All statistical tests were two-tailed and the significance value was set at α = 0.05. As all data were approximately normally distributed, not missing in a random pattern, multiple imputations (k = 5) for education and scores of fear of dying, helplessness, ASS and cognitive depressive symptoms according to von Känel et al. (2018) were used for data input of missing data (15.7%). Kolmogorov-Smirnov tests indicated that ASDS and TAS-20 scores were normally distributed, although the CAPS sum score was positively skewed. Therefore, Spearman’s correlations were calculated to assess bivariate associations among model variables (ASDS, CAPS, TAS-20 subscales) and continuous demographic characteristics (depression, PDS, pain, fear, helplessness). Sociodemographic and clinical baseline characteristics that were significantly associated with the model variables were included as covariates in the moderation analysis. Potential moderating effects of the three TAS-20 subscales (DIF, DDF, EOT) as well as the TAS-20 total score on the association between ASS and CAPS scores were investigated through moderation analysis using the PROCESS macro for SPSS (version 3.3) (Hayes, 2017a). PROCESS is used to examine moderation and mediation, with benefits including the programme‘s ability to generate bootstrapped confidence intervals for model coefficients and interaction effects (Hayes, 2017a). In the moderation analysis using PROCESS (model 1), ASDS scores were entered as the predictor variable (X), the CAPS score (PTSS severity) was the outcome variable (Y), and TAS-20 subscales were added as the moderator variables (M) in separate models. Given their potential association with MI-induced PTSD, sociodemographic (age, sex, education) and clinical baseline characteristics (GRACE score, history of PTSD, history of depression, and pain intensity during MI) were investigated as potential covariates. Those showing statistically significant association with PTSS at 3-month follow-up were included as covariates in moderation analyses. Post-hoc analyses using simple slope analyses and the Johnson-Neyman method were used to further investigate any significant moderation effects observed. Further, regression centring was performed. Regression output showed no concern for multivariate outliers using Cook’s distance, multicollinearity or violation of homoscedasticity assumption constructing a residuals plot.
3. Results
3.1. Participant characteristics
A total of 154 patients were included in the analysis. The mean age of participants was 59.9 years (SD = 11.2), and 21.7% (n = 33) were women. Table 1 shows the participants’ baseline characteristics. Significant differences between patients without alexithymia, with possible alexithymia and with alexithymia emerged for history of depression and ASS (p < 0.001) and PTSS (F(152.2) = 4.08, p = 0.02)).
Table 1.
Descriptive statistics on alexithymia subgroups measured 3 months after acute MI.
| No alexithymia (N = 93) | Possible alexithymia (n = 24) | alexithymia (n = 37) | p-value | |
|---|---|---|---|---|
| Age (yrs) | 58.27 | 58.92 | 55.67 | 0.68 |
| University or high school degree | 20.61% | 6.73% | 7.71% | 0.09 |
| Previous MI (%) | 8.20% | 10.01% | 7.72% | 0.90 |
| Pain intensity (M± SD) | 7.86 ±.19 | 7.39 ±.33 | 7.50 ±.49 | 0.42 |
| Fear of dying (M± SD) | 5.13 ±.33 | 5.19 ±.56 | 5.83 ±.82 | 0.73 |
| Helplessness (M± SD) | 5.39 ±.30 | 5.69 ±.50 | 5.6 ±.75 | 0.84 |
| History of depression (%) | 25.80% | 10.54% | 69.21% | 0.001* |
| Acute stress disorder symptoms (M± SD) | 14.61 ± 1.11 | 18.16 ± 1.89 | 17.33 ± 2.79 | 0.001* |
| Posttraumatic stress symptoms | 9.66 ± 8.3 | 12.28 ± 12.3 | 22.5 ± 19.5 | 0.02* |
ANOVA, level of significance two-tailed p < 0.05 *
Abbreviations: MI: Myocardial infaction, yrs: year; M: mean; SD: standard deviation
The prevalence rates of alexithymia in our sample were 24%% (n = 37). Mean scores of the TAS-20 subscales were as follows: DIF (M = 12.09, SD = 3.56), DDF (M = 13.28, SD = 5.09) and EOT (M = 20.72, SD = 4.39).
No patient had ASD and one patient had PTSD (prevalence 0.5%); 17 (11.7%) patients fulfilled criteria for subsyndromal PTSD. Effects of the intervention were published elsewhere (von Känel et al., 2018) and revealed no difference in interviewer-rated PTSS between trauma-focused counselling (mean, 11.33; 95% Cl, 9.23–13.43) and stress counselling (9.88; 7.36–12.40; p = 0.40), depressive symptoms (6.01, 4.98–7.03, vs. 4.71, 3.65–5.77; p = 0.08), global psychological distress (5.15, 4.07–6.23, vs. 3.80, 2.60–5.00; p = 0.11), and the risk for cardiovascular-related hospitalization/all-cause mortality (OR, 0.67; 95% CI, 0.37–1.23).
3.2. Associations between outcome variables and sociodemographic variables
Associations between the outcome variable (CAPS) and categorical sociodemographic variables (sex (rs(152) = 0.3, p = 0.04), history of depression (rs(152) = 0.6, p = 0.02) and clinical variables (helplessness (rs(152) = 0.4, p = 0.03) were significant. These variables (sex, history of depression, helplessness) were therefore included as covariates in the model. Previous MI and pain during MI showed no significant associations with both the ASDS and CAPS scores (p > 0.05).
The TAS-20 subscale DDF was associated with pain (r(152) = −0.18, p = 0.02) and history of depression (r(152) = 0.48, p < 0.001). The TAS-20 subscale EOT was negatively correlated with education (r(152) = −0.22, p = 0.01). No significant associations emerged with the TAS-20 subscale DIF and the TAS-20 total score (p > 0.05) (see Table 2).
Table 2.
Descriptive statistics and intercorrelations.
| Variable | CAPS | ASD | TAS-DIF | TAS-DDF | TAS-EOT | DEP | HEL | GEN |
|---|---|---|---|---|---|---|---|---|
| CAPS | (-) | |||||||
| ASDS | 0.46* | (-) | ||||||
| TAS-DIF | 0.21* | 0.26* | (-) | |||||
| TAS-DDF | 0.38* | 0.39* | 0.65* | (-) | ||||
| TAS-EOT | −0.10 | 0.24* | −0.74* | 0.26* | (-) | |||
| DEP | 0.17* | 0.13 | −0.01 | −0.54* | 0.20 | (-) | ||
| HEL | 0.19* | 0.23* | 0.60 | −0.10 | 0.70 | 0.07 | (-) | |
| GEN | 0.15* | −0.02 | 0.17* | −0.08 | 0.10 | 0.2* | −0.07 | (-) |
N = 154. CAPS = Clinician-administered PTSD scale, ASDS = acute stress disorder scale, TAS-DIF: Toronto alexithymia scale – difficulties identifying feelings subscale, TAS-DDF: Toronto alexithymia scale – difficulties describing feelings subscale, TAS-EOT: Toronto alexithymia scale – externally oriented thinking, DEP: history of depression, HEL: helplessness, GEN: gender. * p < 0.05
3.3. Determinants of PTSS
The determinants of MI-induced PTSS severity were explored in a regression model explaining 23% of the total variance (F(6.109) = 5.58, p < 0.001, R2 = 0.23, (Table 3). ASS were significantly associated with PTSS (r(152) = p < 0.001). The simultaneously entered TAS-20 subscale DIF was found to significantly moderate this relationship (R2 = 0.03, p = 0.04). The interaction effect is illustrated in Figure 1. The TAS-20 subscales DDF and EOT as well as the TAS-20 total score had no influence on the relationship between ASS and PTSS (p > 0.05).
Table 3.
Parameter estimates and model prediction for determinants of post-traumatic stress symptoms measured with CAPS after MI.
| R | R2 | MSE | F | df1 | df2 | p-value |
|---|---|---|---|---|---|---|
| 0.48 | 0.23 | 90.33 | 5.58 | 6.02 | 109.00 | 0.001 |
| Model | ||||||
| β | SE | t | p-value | LLCI | ULCI | |
| constant | 2.18 | 2.52 | 0.87 | 0.39 | −2.77 | 7.12 |
| ASDS | 0.23 | 0.13 | 2.33 | 0.02* | 0.03 | 0.42 |
| TAS-DIF | −1.55 | 1.98 | −0.78 | 0.44 | −5.48 | 2.38 |
| Interaction | 0.13 | 0.07 | 2.04 | 0.04* | 0.00 | 0.26 |
| Depression | 2.53 | 2.15 | 1.18 | 0.24 | −1.72 | 6.78 |
| Helplessness | 0.61 | 0.36 | 1.71 | 0.09 | −0.12 | 1.31 |
| Gender | 0.62 | 2.47 | 0.25 | 0.81 | −4.29 | 5.52 |
The table presents the final significant model showing the significant interaction term of ASDS and TAS-DIF on determinants of post-traumatic stress symptoms measured with CAPS. ASDS = acute stress disorder scale, TAS-DIF: TAS-20 subscale difficulties identifying feelings.
Figure 1.
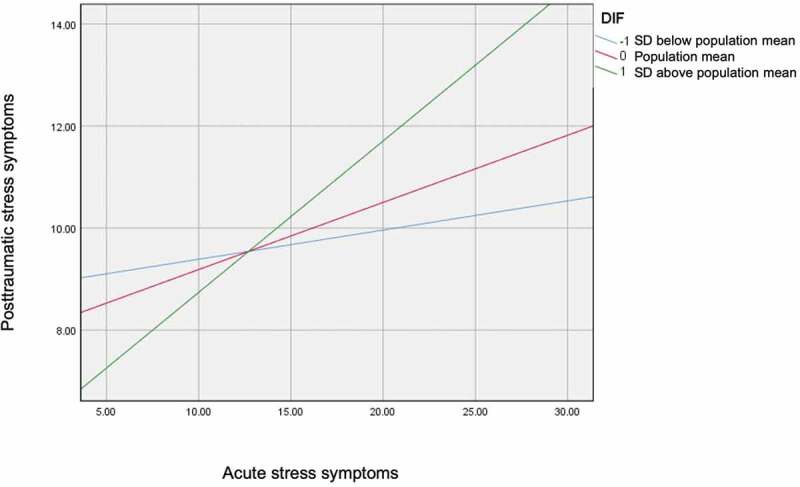
Illustration for the simple slopes of acute symptoms predicting posttraumatic stress symptoms for 1 SD below the mean difficulties identifying feelings (DIF), the mean of DIF and 1 SD above the mean of DIF.
We probed the interaction in two ways using simple slopes and the Johnson-Neyman method High (+1 SD above the mean of the TAS-20 subscale DIF), medium (mean of the TAS-20 subscale DIF), and low (−1 SD below the mean of the TAS-20 subscale DIF) were used.The Johnson-Neyman method was applied to identify the threshold(s) of the moderator (M) alexithymia where the association between the predictor X (ASDS score) and the outcome Y (CAPS score) transitions between statistical significance and non-significance (Hayes & Montoya, 2017b). Simple slope analysis revealed that when DIF was higher, the association between ASS and PTSS became stronger. In other words, the impact of ASS on PTSS were different in patients with medium (mean, β = 0.29, p < 0.001) and high (1 SD above the mean, β = 0.42, p < 0.001) levels of DIF compared to those with low levels of DIF (see Table 4).
Table 4.
TAS-DIF (at levels of low/medium/high) as a moderator between ASDS and CAPS.
| β | SE | t | p-value | LLCI | ULCI | |
|---|---|---|---|---|---|---|
| −1 SD = −0.73 | 0.13 | 0.12 | 1.07 | 0.29 | −0.11 | 0.37 |
| M =0.29 | 0.27 | 0.09 | 2.83 | 0.01* | 0.08 | 0.45 |
| +1SD = 1.3 | 0.42 | 0.11 | 3.71 | 0.001* | 0.19 | 0.62 |
The table presents the results of the simple slopes analysis. TAS-DIF was a significant moderator of the relationship between ASD and PTSD at medium and high levels of TAS-DIF.
Using the simple slopes method, at low levels, DIF was not a significant moderator of the relationship between ASS and PTSS (p > 0.2); however, at both medium (t(153) = 1.07, p = 0.01) and high levels (t(153) = 3.71, p = 0.001), DIF was a significant moderator of the association between ASS and PTSS. The moderator value defining the Johnson-Neyman significance region was a DIF value of −0.19 (% below: 26.72, % above 73.28), confirming the results of the first method used (see supplementary Tables 4 and 5 for additional results).
Adjustment for intervention
The significance of the interaction between DIF and ASS was maintained when the intervention was additionally adjusted for. Moreover, the main effect of the intervention was not significant.
4. Discussion
This study is a secondary analysis of data collected in the MI-SPRINT RCT with the aim of investigating whether alexithymia is a moderator of the association between MI-induced ASS and PTSS in patients at risk for developing PTSD. The findings of our study suggest that the association between ASS and PTSS is moderated through the alexithymia trait DIF. The alexithymia traits DDF and EOT did not influence the relationship between ASS within 48 hours of acute MI and the development of PTSS three months after acute MI.
In line with a previous systematic review (Bryant, 2011), we found ASS to be predictive of PTSS. Therefore, it seems reasonable to identify patients with ASS in medical settings so that prompt and adequate support can be offered. However, ASD may not identify all individuals who will develop PTSD. Research has shown that the majority of traumatized patients who develop PTSD do not initially meet the criteria for ASD (Bryant et al., 2008). Therefore, application of broader concepts of ASS was recommended in order to identify those trauma survivors in need of mental health care who might fail to meet the diagnostic criteria for ASD (Bryant, 2011). In a previous sample of 116 post-MI patients of which 21 (18%) were diagnosed with ASD, only one-third had developed PTSD at the 7-month follow-up (Ginzburg et al., 2003). Another study showed that 22% and 52% of patients with an acute coronary syndrome reported intense and moderate distress, respectively (i.e. perception of the cardiac event as stressful and being frightened about cardiac symptoms) and fear of dying within 3 days of hospital admission (Whitehead et al., 2005). Moreover, this type of acute stress symptoms and pain scores predicted PTSS at the 3-month follow-up (Whitehead, Perkins-Porras, Strike, & Steptoe, 2006). Indeed, acute MI is perceived by patients as highly stressful (Whitehead et al., 2005), and, moreover, as part of our study design, we included patients with a certain amount of peritraumatic distress. Acute alexithymia has been described as a transitory state that decreases once an acute stress episode has resolved (Freyberger, 1977). It is likely that three months post-MI, some of our patients were still in this transition stage. Meanwhile, chronic secondary alexithymia is seen as a more permanent state associated with adjustment to chronic conditions (Freyberger, 1977). Moreover, there is growing evidence suggesting that alexithymia may decrease across the course of trauma-focused treatments (Cloitre, Koenen, Cohen, & Han, 2002; Zorzella, Muller, Cribbie, Bambrah, & Classen, 2020). Zorzella et al. (2020) showed a positive relationship between improvements in alexithymia and improvements in all trauma-specific outcomes over the course of treatment.
It is therefore necessary to investigate other variables such as high peritraumatic distress measured, for instance, with fear of dying, helplessness and pain during acute MI or alexithymia traits to identify patients at high risk of developing PTSD following an acute MI. In our study sample, no patient met the diagnostic criteria for ASD, but nearly one-quarter of patients had alexithymia (22.6%) and about 12% of patients had subsyndromal PTSD. This finding indicates that DIF may be an important moderating factor for the prediction of clinically significant PTSS, although not full PTSD. It is likely that our early intervention substantially prevented the development of full PTSD, regardless of the group patients were assigned to. On the other hand, the results may indicate that MI-induced emotional reactions may be different than in other traumatic experiences. This assumption should be explored in future research. However, since almost 12% of our patients fulfilled criteria for subsyndromal PTSD, which is also of clinical relevance, DIF seems to be particularly associated with PTSS in MI-patients.
Consistent with previous studies showing a link between different subscales of alexithymia and PTSD (Eichhorn, Brähler, Franz, Friedrich, & Glaesmer, 2014; Gao et al., 2015; Leising, Grande, & Faber, 2009; O’Brien et al., 2008), we found that patients with elevated DIF may be at greater risk for the development of PTSS. This finding is in line with another study that applied the TAS-20 in a sample of MI-patients and showed a strong correlation between DIF and MI-induced PTSS (Gao et al., 2015).
A possible explanation for the reported association between DIF and PTSS might be that patients scoring high in alexithymia have a negative bias towards their feelings and emotions, so that psychological distress can only be insufficiently processed resulting in PTSS. Another possible explanation is that humans experience a variety of negative feelings when being confronted with life-threatening situations. These feelings can include, for instance, fear, horror and rage. Being unable to identify and regulate these peritraumatic emotions increases the risk of PTSS (Declercq, Vanheule, & Deheegher, 2010). This might explain why DIF were found to have the strongest correlation with PTSS in patients following MI. The finding that high levels of DIF are associated with PTSS are in agreement with studies conducted with groups of individuals suffering from other traumatic events (Sondergaard & Theorell, 2004; Zahradnik et al., 2009). A third possible explanation for an association between DIF and PTSS is that DIF shows strong association with emotion regulation and emotional numbing in PTSS which may suggest an overlap of symptoms (Badura, 2003; Frewen, Dozois, Neufeld, & Lanius, 2012). When patients have limited awareness of their feelings and emotions, the psychological distress may not be reduced, but instead might prevail to finally resulting into PTSS.
Especially, patients with DIF might cope with avoidance in life-threatening events. This tendency might eventually lead to enhanced stressful experiences thereby increasing the risk of developing psychological distress and PTSS (Craparo, Gori, Petruccelli, Cannella, & Simonelli, 2014). Other studies also showed the strongest associations between DIF and PTSD across different types of trauma (Edwards, 2019) and in several mental disorders (Bamonti et al., 2010; Evren et al., 2012). In this context, it is of interest to note that a meta-analysis of the association between alexithymia and PTSD concluded that alexithymia may predict neural responses to PTSS provocation and is correlated with PTSS (Frewen et al., 2008).
Whereas our study as a novelty may complement this meta-analytic data, we report here that DIF may possibly interact with ASS in determining subsequent PTSS, in post-MI patients.
In the present study, the overall prevalence of alexithymia was 22.63% (mean age = 59.9 years) in our post-MI sample, which is much greater than the 10% observed in a large general population sample (Salminen et al., 1999). However, the general population sample was considerably younger (mean age = 39.2 years), and the prevalence of alexithymia typically increases with age (Onor, Trevisiol, Spano, Aguglia, & Paradiso, 2010). A further comparison can be made with the study by Todarello, Taylor, Parker, and Fanelli (1995), who reported a 55% prevalence in a hypertensive group as against 33% in psychiatric patients (n = 113, mean age: 39.0) and 16% in normal controls (n = 130, mean age: 40.8). Although alexithymia features are likely less frequently observed among healthy people than in post-MI patients, it is unclear whether the prevalence of alexithymia is higher following MI than in other medical conditions or may change to a normal level over time. Our patients with alexithymia had marginally lower education levels and significantly more often a history of depression than patients without alexithymia. These observations are in agreement with the current literature showing negative associations between alexithymia and education (Karukivi & Saarijärvi, 2014) along with positive associations between alexithymia and measures of negative emotions (Bagby et al., 1994; Hemming, Haddock, Shaw, & Pratt, 2019). It remains unclear whether alexithymia as a defence against unpleasant emotions manifests in individuals with high levels of negative emotions, or whether different coping mechanisms, or other reasons render alexithymic people more vulnerable to experiencing negative emotions.
These findings highlight the role of addressing alexithymia in trauma therapy, and the need to accurately focus on the deficits and issues related to alexithymia at an early stage of therapy with post-MI patients in order to facilitate improvements in trauma-specific symptoms.
5. Clinical implications
The present research provides novel findings on the complex associations between MI-induced ASS and PTSS and the moderating role of DIF. This field of research is still underexplored, and to our knowledge there are no in-depth analyses on the relationship between alexithymia and PTSS in cardiac populations. The findings of the current study may have important clinical implications. In-hospital evaluation of DIF along with ASS may be useful for early detection of patients at risk of developing PTSS after hospital discharge. Moreover, it may be possible to lower the risk of future PTSS in patients with high levels of ASS through interventions targeting DIF, such as mindfulness-based strategies (Norman, Marzano, Coulson, & Oskis, 2019).
6. Limitations
There are several limitations to our study. Firstly, it is unclear how many of our patients were alexithymic prior to their MI or whether their state changed over time. Longitudinal studies would be needed to investigate the relation between ASS and PTSS and the moderating role of DIF.
Secondly, we assessed alexithymia only once in order to limit the burden imposed by the MI-SPRINT protocol on patients during the acute phase of a medical condition. Even if the common view of alexithymia is that of a stable trait, we cannot exclude the possibility that alexithymia measures were influenced by emotional and cognitive processes during recovery from and adaptation to the cardiac event.
Thirdly, the inclusion criterion of high distress during MI and the exclusion criterion of clinically relevant depressive symptoms limit the generalizability of our findings.
Moreover, it has to be noted that none of our patients fulfilled the diagnostic criteria of ASD and only one patient fulfilled all the criteria for full PTSD. Fourthly, it remains unclear whether our results would be the same with the new DSM-5 criteria for ASD and PTSD.
Additionally, alexithymia can be seen either as a state that is subject to fluctuations or a personality trait and needs to be addressed in future research with longitudinal studies. Furthermore, the small sample of those with symptoms of ASS and PTSS is a significant limitation to our study.
Moreover, the role of DIF in PTSS is still unknown and needs to be examined in process research. In conclusion, only DIF as an alexithymia trait had a moderating influence on the relationship between ASS and PTSS. A further exploration of the causal relationship between alexithymia and posttraumatic stress needs to be addressed in future studies.
7. Conclusions
This study showed a moderating effect of the alexithymia trait DIF on the association between ASS and PTSS in patients after acute MI. Patients with high levels of DIF had significantly higher levels of MI-induced PTSS at three-month follow-up than patients with smaller levels of DIF. This finding may help early identification of patients with acute MI who are at risk of developing PTSS. More research is needed with respect to the causal relation of alexithymia and cardiovascular disease.
Funding Statement
The MI-SPRINT study is funded by the Swiss National Science Foundation (project number 140960, principal investigator: RvK). Additional financial support comes from the Teaching and Research Directorate, Bern University Hospital, Switzerland. The funding bodies had no influence on the study design, in the writing of the manuscript, and in the decision to submit the manuscript for publication.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Alonzo, A. A. (2000). The experience of chronic illness and post-traumatic stress disorder: The consequences of cumulative adversity. Social Science & Medicine, 50(10), 1475–13. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . (2000). Diagnostic and statistical manual of mental disorders (4th ed text revision). Washington, DC: Author. [Google Scholar]
- Bach, M., Bach, D., de Zwaan, M., Serim, M., & Böhmer, F. (1996). [Validation of the German version of the 20-item Toronto Alexithymia scale in normal persons and psychiatric patients]. Psychotherapie, Psychosomatik, Medizinische Psychologie, 46(1), 23–28. [PubMed] [Google Scholar]
- Badura, A. S. (2003). Theoretical and empirical exploration of the similarities between emotional numbing in posttraumatic stress disorder and alexithymia. Journal of Anxiety Disorders, 17(3), 349–360. [DOI] [PubMed] [Google Scholar]
- Bagby, R. M., Parker, J. D., & Taylor, G. J. (1994). The twenty-item Toronto Alexithymia scale–I. Item selection and cross-validation of the factor structure. Journal of Psychosomatic Research, 38(1), 23–32. [DOI] [PubMed] [Google Scholar]
- Bamonti, P. M., Heisel, M. J., Topciu, R. A., Franus, N., Talbot, N. L., & Duberstein, P. R. (2010). Association of Alexithymia and depression symptom severity in adults aged 50 years and older. The American Journal of Geriatric Psychiatry, 18(1), 51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck, A. T. (1993). Manual for the beck depression inventory. Archives of General Psychiatry, 4(6), 561–571. [DOI] [PubMed] [Google Scholar]
- Bennett, P., & Brooke, S. (2010). Intrusive memories, post-traumatic stress disorder and myocardial infarction. British Journal of Clinical Psychology, 38(4), 411–416. [DOI] [PubMed] [Google Scholar]
- Blanchard, E. B., Hickling, E. J., Taylor, A. E., Forneris, C. A., Loos, W., & Jaccard, J. (1995). Effects of varying scoring rules of the clinician administered PTSD scale (CAPS) for the diagnosis of post-traumatic stress disorder in motor vehicle accident. Behaviour Research and Therapy, 33(4), 471–475. [DOI] [PubMed] [Google Scholar]
- Brewin, C. R., Andrews, B., & Rose, S. (2003). Diagnostic overlap between acute stress disorder and PTSD in victims of violent crime. American Journal of Psychiatry, 160(4), 783–785. [DOI] [PubMed] [Google Scholar]
- Bryant, R., & Harvey, A. (2000). Acute stress disorder: A handbook of theory, assessment, and treatment. APA, 109(2), 341–344. [Google Scholar]
- Bryant, R. A. (2011). Acute stress disorder as a predictor of posttraumatic stress disorder: A systematic review. The Journal of Clinical Psychiatry, 72(2), 233–239. [DOI] [PubMed] [Google Scholar]
- Bryant, R. A., Creamer, M., O’Donnell, M. L., Silove, D., & McFarlane, A. C. (2008). A multisite study of the capacity of acute stress disorder diagnosis to predict posttraumatic stress disorder. The Journal of Clinical Psychiatry, 69(6), 923–929. [DOI] [PubMed] [Google Scholar]
- Caretti, V., & La Barbera, D. (2005). Alessitimia. Valutazione ed Intervento. Roma: Astrolabio. [Google Scholar]
- Chung, M. C., Dennis, I., Berger, Z., Jones, R., & Rudd, H. (2012). Posttraumatic stress disorder following myocardial infarction: Personality, coping, and trauma exposure characteristics. The International Journal of Psychiatry in Medicine, 42(5), 393–419. [DOI] [PubMed] [Google Scholar]
- Cloitre, M., Garvert, D. W., Brewin, C. R., Bryant, R. A., & Maercker, A. (2013). Evidence for proposed ICD-11 PTSD and complex PTSD: A latent profile analysis. European Journal of Psychotraumatology, 4(1), 20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloitre, M., Koenen, K. C., Cohen, L. R., & Han, H. (2002). Skills training in affective and interpersonal regulation followed by exposure: A phase-based treatment for PTSD related to childhood abuse. Journal of Consulting and Clinical Psychology, 70(5), 1067–1074. [DOI] [PubMed] [Google Scholar]
- Craparo, G., Gori, A., Petruccelli, I., Cannella, V., & Simonelli, C. (2014). Intimate partner violence: Relationships between Alexithymia, depression, attachment styles, and coping strategies of battered women. The Journal of Sexual Medicine, 11(6), 1484–1494. [DOI] [PubMed] [Google Scholar]
- Declercq, F., Vanheule, S., & Deheegher, J. (2010). Alexithymia and posttraumatic stress: Subscales and symptom clusters. Journal of Clinical Psychology, 66(10), 1076–1089. [DOI] [PubMed] [Google Scholar]
- Edmondson, D., Richardson, S., Falzon, L., Davidson, K. W., Mills, M. A., Neria, Y., & Fontenelle, L. (2012). Posttraumatic stress disorder prevalence and risk of recurrence in acute coronary syndrome patients: A meta-analytic review. PLoS One, 7(6), 38915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson, D., Richardson, S., Fausett, J. K., Falzon, L., Howard, V. J., & Kronish, I. M. (2013). Prevalence of PTSD in survivors of stroke and transient ischemic attack: A meta-analytic review. PLoS One, 8(6), e66435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson, D., & von Känel, R. (2017). Posttraumatic stress disorder and cardiovascular disease. The Lancet Psychiatry, 4(4), 320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards, E., Shivaji, S., Micek, A., & Wupperman, P. (2020). Distinguishing alexithymia and emotion differentiation conceptualizations through linguistic analysis. Personality and Individual Differences, 157, 109801. [Google Scholar]
- Edwards, K. S., Vaca, K. C., Naderi, S., & Tremmel, J. A. (2019). Patient-Reported Psychological Distress After Spontaneous Coronary Artery Dissection: evidence for post-traumatic stress. Journal of Cardiopulmonary Rehabilitation and Prevention, 39(5), E20–E23. doi: 10.1097/HCR.0000000000000460 [DOI] [PubMed] [Google Scholar]
- Eichhorn, S., Brähler, E., Franz, M., Friedrich, M., & Glaesmer, H. (2014). Traumatic experiences, alexithymia, and posttraumatic symptomatology: A crosssectional population-based study in Germany. European Journal of Psychotraumatology, 5(4), 23870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evren, C., Cagil, D., Ulku, M., Ozcetinkaya, S., Gokalp, P., Cetin, T., & Yigiter, S. (2012). Relationship between defense styles, alexithymia, and personality in alcohol-dependent inpatients. Comprehensive Psychiatry, 53(6), 860––867.. [DOI] [PubMed] [Google Scholar]
- Evren, C., Dalbudak, E., Cetin, R., Durkaya, M., & Evren, B. (2010). Relationship of alexithymia and temperament and character dimensions with lifetime post-traumatic stress disorder in male alcohol-dependent inpatients. Psychiatry and Clinical Neurosciences, 64(2), 111–119. [DOI] [PubMed] [Google Scholar]
- Fox, K. A., Dabbous, O. H., Goldberg, R. J., Pieper, K. S., Eagle, K. A., Van de Werf, F., … Granger, C. B., Jr. (2006). Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: Prospective multinational observational study (GRACE). BMJ, 333(7578), 1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin, C. L., Sheeran, T., & Zimmerman, M. (2002). Screening for trauma histories, posttraumatic stress disorder (PTSD), and subthreshold PTSD in psychiatric outpatients. Psychological Assessment, 14(4), 467–471. [DOI] [PubMed] [Google Scholar]
- Frewen, P. A., Dozois, D., Neufeld, R., & Lanius, R. (2008). Meta‐analysis of alexithymia in posttraumatic stress disorder. Journal of Traumatic Stress, 21(2), 243–246. [DOI] [PubMed] [Google Scholar]
- Frewen, P. A., Dozois, D. A., Neufeld, R. J., & Lanius, R. A. (2012). Disturbances of emotional awareness and expression in posttraumatic stress disorder: Meta-mood, emotion regulation, mindfulness, and interference of emotional expressiveness. Psychological Trauma: Theory, Research, Practice, and Policy, 4(2), 152–161. [Google Scholar]
- Freyberger, H. (1977). Supportive psychotherapeutic techniques in primary and secondary Alexithymia. Psychotherapy and Psychosomatics, 28(1–4), 337–342. [DOI] [PubMed] [Google Scholar]
- Gao, W., Zhao, J., Li, Y., & Cao, F.-L. (2015). Post-traumatic stress disorder symptoms in first-time myocardial infarction patients: Roles of attachment and alexithymia. Journal of Advanced Nursing, 71(11), 2575–2584. [DOI] [PubMed] [Google Scholar]
- Ginzburg, K., Solomon, Z., & Bleich, A. (2002). Repressive coping style, acute stress disorder, and posttraumatic stress disorder after myocardial infarction. Psychosomatic Medicine, 64(5), 748–757. [DOI] [PubMed] [Google Scholar]
- Ginzburg, K., Solomon, Z., Koifman, B., Keren, G., Roth, A., Kriwisky, M., … Bleich, A. (2003). Trajectories of posttraumatic stress disorder following myocardial infarction: A prospective study. The Journal of Clinical Psychiatry, 64(10), 1217–1223. [DOI] [PubMed] [Google Scholar]
- Guler, E., Schmid, J. P., Wiedemar, L., Saner, H., Schnyder, U., & Känel, R. V. (2009). Clinical diagnosis of posttraumatic stress disorder after myocardial infarction. Clinical Cardiology: An International Indexed and Peer‐Reviewed Journal for Advances in the Treatment of Cardiovascular Disease, 32(3), 125––129.. doi: 10.1002/clc.20384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich, M., Devins, G. M., & Rodin, G. M. (2002). Stress response syndromes and cancer: Conceptual and assessment issues. Psychosomatics, 43(4), 259–281. [DOI] [PubMed] [Google Scholar]
- Hari, R., Begre, S., Schmid, J.-P., Saner, H., Gander, M.-L., & von Känel, R. (2010). Change over time in posttraumatic stress caused by myocardial infarction and predicting variables. Journal of Psychosomatic Research, 69(2), 143–150. [DOI] [PubMed] [Google Scholar]
- Hayes, A. (2017a). Introduction to mediation, moderation, and conditional process analysis. In: A regression-based approach (2th ed.). New York: Guilford Publications. doi:10.1080/19312458.2016.1271116 [Google Scholar]
- Hayes, A. F., & Montoya, A. K. (2017b). A tutorial on testing, visualizing, and probing an interaction involving a multicategorical variable in linear regression analysis. Communication Methods and Measures, 11(1), 1–30. [Google Scholar]
- Helfricht, S., Landolt, M. A., Moergeli, H., Hepp, U., Wegener, D., & Schnyder, U. (2009). Psychometric evaluation and validation of the German version of the acute stress disorder scale (ASDS) across two distinct trauma populations. Journal of Traumatic Stress, 22(5), 476–480. [DOI] [PubMed] [Google Scholar]
- Hemming, L., Haddock, G., Shaw, J., & Pratt, D. (2019). Alexithymia and its associations with depression, suicidality, and aggression: An overview of the literature. Frontiers in Psychiatry, 10(10), 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepp, U., Gamma, A., Milos, G., Eich, D., Ajdacic-Gross, V., Rossler, W. (2006). Prevalence of exposure to potentially traumatic events and PTSD: The Zurich cohort study. European Archives of Psychiatry and Clinical Neuroscience, 256(3), 151–158. [DOI] [PubMed] [Google Scholar]
- Karukivi, M., & Saarijärvi, S. (2014). Development of alexithymic personality features. World Journal of Psychiatry, 4(3), 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima, M., Frasure-Smith, N., & Lespérance, F. (2001). Alexithymia following myocardial infarction: Psychometric properties and correlates of the Toronto Alexithymia scale. Journal of Psychosomatic Research, 51(3), 487–495. [DOI] [PubMed] [Google Scholar]
- Kronish, I. M., Edmondson, D., Li, Y., & Cohen, B. E. (2012). Post-traumatic stress disorder and medication adherence: Results from the mind your heart study. Journal of Psychiatric Research, 46(12), 1595–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutz, I., Shabtai, H., Solomon, Z., Neumann, M., & David, D. (1994). Post-traumatic stress disorder in myocardial infarction patients: Prevalence study. The Israel Journal of Psychiatry and Related Sciences, 31(1), 48–56. [PubMed] [Google Scholar]
- Larsen, J. K., Brand, N., Bermond, B., & Hijman, R. (2003). Cognitive and emotional characteristics of alexithymia: A review of neurobiological studies. Journal of Psychosomatic Research, 54(6), 533–541. [DOI] [PubMed] [Google Scholar]
- Leising, D., Grande, T., & Faber, R. (2009). The Toronto Alexithymia scale (TAS-20): A measure of general psychological distress. Journal of Research in Personality, 43(4), 707–710 [Google Scholar]
- Marke, V., & Bennett, P. (2013). Predicting post-traumatic stress disorder following first onset acute coronary syndrome: Testing a theoretical model. British Journal of Clinical Psychology, 52(1), 70–81. [DOI] [PubMed] [Google Scholar]
- Meiser-Stedman, R., McKinnon, A., Dixon, C., Boyle, A., Smith, P., & Dalgleish, T. (2017). Acute stress disorder and the transition to posttraumatic stress disorder in children and adolescents: Prevalence, course, prognosis, diagnostic suitability, and risk markers. Depression and Anxiety, 34(4), 348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister, R., Princip, M., Schmid, J. P., Schnyder, U., Barth, J., Znoj, H., … von Känel, R. (2013). Myocardial infarction - stress prevention intervention (MI-SPRINT) to reduce the incidence of posttraumatic stress after acute myocardial infarction through trauma-focused psychological counseling: Study protocol for a randomized controlled trial. Trials, 14(1), 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meli, L., Birk, J., Edmondson, D., & Bonanno, G. A. (2020). Trajectories of posttraumatic stress in patients with confirmed and rule-out acute coronary syndrome. General Hospital Psychiatry, 62, 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman, H., Marzano, L., Coulson, M., & Oskis, A. (2019). Effects of mindfulness-based interventions on alexithymia: A systematic review. Evidence Based Mental Health, 22(1), 36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien, C., Gaher, R. M., & Pope, C. (2008). Smiley P difficulty identifying feelings predicts the persistence of trauma symptoms in a sample of veterans who experienced military sexual trauma. Journal of Nervous and Mental Disease, 196(3), 252–255. [DOI] [PubMed] [Google Scholar]
- Onor, M., Trevisiol, M., Spano, M., Aguglia, E., & Paradiso, S. (2010). Alexithymia and aging: A neuropsychological perspective. The Journal of Nervous and Mental Disease, 198(12), 891–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J., Jun, J. Y., Lee, Y. J., Kim, S., Lee, S. H., Yoo, S. Y., & Kim, S. J. (2015). The association between alexithymia and posttraumatic stress symptoms following multiple exposures to traumatic events in North Korean refugees. Journal of Psychosomatic Research, 78(1), 77–81. [DOI] [PubMed] [Google Scholar]
- Pedersen, S. S., von Domburg, R. T., & Larsen, M. L. (2004). The effect of low social support on short-term prognosis in patients following a first myocardial infarction. Scandinavian Journal of Psychology, 45(4), 313–318. [DOI] [PubMed] [Google Scholar]
- Roberge, M. A., Dupuis, G., & Marchand, A. (2010). Post-traumatic stress disorder following myocardial infarction: Prevalence and risk factors. Canadian Journal of Cardiology, 26(5), e170–e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen, J. K., Saarijarvi, S., Aarela, E., Toikka, T., & Kauhanen, J. (1999). Prevalence of alexithymia and its association with sociodemographic variables in the general population of Finland. Journal of Psychosomatic Research, 46(1), 75–82. [DOI] [PubMed] [Google Scholar]
- Samur, D. (2013). Four decades of research on alexythymia: Moving towards clinical applications. Frontiers in Psychology, 4(86a1). doi: 10.3389/fpsyg.2013.00861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer, R., Schneider, C., Sitte, W., & Franz, M. (2002). Evidence of validity of the German version of the TAS-20. Contribution to the 52nd conference of the German board of psychosomatic medicine in bad honnef. Psychotherapie, Psychosomatik, medizinische Psychologie, 52(11), 449–453. [DOI] [PubMed] [Google Scholar]
- Schelling, G. (2008). Post-traumatic stress disorder in somatic disease: Lessons from critically ill patients. Progress in Brain Research, 167, 229–237. [DOI] [PubMed] [Google Scholar]
- Schnyder, U., & Moergeli, H. (2002). German version of clinician-administered PTSD scale. Journal of Traumatic Stress, 15(6), 487–492. [DOI] [PubMed] [Google Scholar]
- Sondergaard, H. P., & Theorell, T. (2004). Alexithymia, emotions and PTSD; findings from a longitudinal study of refugees. Nordic Journal of Psychiatry, 58(3), 185–191. [DOI] [PubMed] [Google Scholar]
- Spindler, H., & Pedersen, S. S. (2005). Posttraumatic stress disorder in the wake of heart disease: Prevalence, risk factors, and future research directions. Psychosomatic Medicine, 67(5), 715–723. [DOI] [PubMed] [Google Scholar]
- Taylor, G. J., & Bagby, R. M. (2012). The alexithymia personality dimension. In Widiger T. A. (Ed.), Oxford library of psychology. The Oxford handbook of personality disorders (pp. 648–673). Oxford: Oxford University Press. [Google Scholar]
- Taylor, G. J., Bagby, R. M., & Parker, J. D. (2003). The 20-item Toronto Alexithymia scale, IV. Reliability and factorial validity in different languages and cultures. Journal of Psychosomatic Research, 55(3), 277–283. [DOI] [PubMed] [Google Scholar]
- Taylor, G. J., Bagby, R. M., Ryan, D. P., Parker, J. D. A., Doody, K. F., & Keefe, P. (1988). Criterion validity of the Toronto Alexithymia scale. Psychosomatic Medicine, 50(5), 500–509. [DOI] [PubMed] [Google Scholar]
- Tedstone, J. E., & Tarrier, N. (2003). Posttraumatic stress disorder following medical illness and treatment. Clinical Psychology Review, 23(3), 409–448. [DOI] [PubMed] [Google Scholar]
- Tesio, V., Goerlich, K. S., Hosoi, M., & Castelli, L. (2019). Alexithymia: State of the art and controversies. Clinical and neuroscientific evidence. Frontiers Media. doi: 10.3389/978-2-88963-106-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todarello, O., Taylor, G. J., Parker, J. D. A., & Fanelli, M. (1995). Alexithymia in essential hypertensive and psychiatric outpatients: A comparative study. Journal of Psychosomatic Research, 39(8), 987–994. [DOI] [PubMed] [Google Scholar]
- von Känel, R., Barth, J., Princip, M., Meister-Langraf, R. E., Schmid, J. P., Znoj, H., … Schnyder, U. (2018). Early psychological counseling for the prevention of posttraumatic stress induced by acute coronary syndrome: The MI-SPRINT randomized controlled trial. Psychotherapy and Psychosomatics, 87(2), 75–84. [DOI] [PubMed] [Google Scholar]
- Von Känel, R., Hari, R., Schmid, J. P., Wiedemar, L., Guler, E., Barth, ... Begré S. (2011). Non-fatal cardiovascular outcome in patients with post traumatic stress symptoms caused by myocardial infarction. Journal of Cardiology, 58(1), 61––68.. doi: 10.1016/j.jjcc.2011.02.007 [DOI] [PubMed] [Google Scholar]
- Wang, X., Chung, M. C., Hyland, M. E., & Bahkeit, M. (2011). Posttraumatic stress disorder and psychiatric co-morbidity following stroke: The role of alexithymia. Psychiatry Research, 188(1), 51–57. [DOI] [PubMed] [Google Scholar]
- Whitehead, D. L., Perkins-Porras, L., Strike, P. C., & Steptoe, A. (2006). Post-traumatic stress disorder in patients with cardiac disease: Predicting vulnerability from emotional responses during admission for acute coronary syndromes. Heart, 92(9), 1225–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead, D. L., Strike, P., Perkins-Porras, L., & Steptoe, A. (2005). Frequency of distress and fear of dying during acute coronary syndromes and consequences for adaptation. The American Journal of Cardiology, 96(11), 1512–1516. [DOI] [PubMed] [Google Scholar]
- Wikman, A., Bhattacharyya, M., Perkins-Porras, L., & Steptoe, A. (2008). Persistence of posttraumatic stress symptoms 12 and 36 months after acute coronary syndrome. Psychosomatic Medicine, 70, 764–772. [DOI] [PubMed] [Google Scholar]
- Zahradnik, M., Stewart, S. H., Marshall, G. N., Schell, T. L., & Jaycox, L. H. (2009). Anxiety sensitivity and aspects of alexithymia are independently and uniquely associated with posttraumatic distress. Journal of Traumatic Stress, 22(2), 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorzella, K. P. M., Muller, R. T., Cribbie, R. A., Bambrah, V., & Classen, C. C. (2020). The role of alexithymia in trauma therapy outcomes: Examining improvements in PTSD, dissociation, and interpersonal problems. Psychological trauma Theory, Research, Practice, and Policy, 12(1), 20–28. [DOI] [PubMed] [Google Scholar]


