Abstract
Age-related macular degeneration (AMD) is the commonest cause of severe visual loss and blindness in developed countries among individuals aged 60 and older. AMD slowly progresses from early AMD to intermediate AMD (iAMD) and ultimately late-stage AMD. Late AMD encompasses either neovascular AMD (nAMD) or geographic atrophy (GA). nAMD is defined by choroidal neovascularization (CNV) and hemorrhage in the subretinal space at the level of the macula. This induces a rapid visual impairment caused by the death of photoreceptor cells. Intravitreal injection of anti-vascular endothelial growth factor (VEGF) antibodies is the standard treatment of nAMD but adds to the burden of patient care. GA is characterized by slowly expanding photoreceptor, and retinal pigment epithelium (RPE) degeneration patches progressively leading to blindness. There is currently no therapy to cure GA. Late AMD continues to be an unmet medical need representing a major health problem with millions of patients worldwide. Oxidative stress and inflammation are recognized as some of the main risk factors to developing late AMD. The antioxidant formulation AREDS (Age-Related Eye Disease Studies), contains β-carotene, which has been replaced by lutein and zeaxanthin in AREDS2, are given to patients with iAMD but have a limited effect on the incidence of nAMD and GA. Thus, to avoid or slowdown the development of late stages of AMD (nAMD or GA), new therapies targeting iAMD are needed such as crocetin obtained through hydrolysis of crocin, an important component of saffron (Crocus sativus L.), and norbixin derived from bixin extracted from Bixa orellana seeds. We have shown that these apocarotenoids preserved more effectively RPE cells against apoptosis following blue light exposure in the presence of A2E than lutein and zeaxanthin. In this review, we will discuss the potential use of apocarotenoids to slowdown the progression of iAMD, to reduce the incidence of both forms of late AMD.
1. Introduction
Age-related macular degeneration (AMD) is the main cause of blindness in the industrialized world with over 30 million people suffering from this disease [1]. In the US, the number of patients is expected to increase from 9.1 million in 2010 to up to 17.8 million in 2050 [1, 2]. The situation is even more critical in Europe, as it is estimated that by the end of 2020 almost 59 million Europeans will develop at least one form of the disease [3]. Novel therapeutic strategies are needed to reduce disease prevalence [1, 2, 4]. AMD is a chronic disease that may progress slowly from early AMD to intermediate AMD (iAMD) and ultimately late-stage AMD, either neovascular (nAMD) or geographic atrophy (GA). Although visual acuity under photopic conditions remains good in the early stages of AMD, disease impact on patients with iAMD is severe with a loss in quality of life owing to poor visual acuity under low luminance conditions that affects many aspects of normal life activities [5–8]. Moreover, the economic burden of AMD on society is very high and will increase as the population ages [9, 10].
Neovascular AMD is defined by choroidal neovascularization (CNV) and hemorrhage in the subretinal space at the level of the macula. This induces the rapid death of photoreceptor cells and then rapid loss of vision [11]. Currently, three drugs targeting vascular endothelial growth factor (anti-VEGF) are in use for nAMD. Two of them, ranibizumab (Lucentis®, Genentech) and aflibercept (Eylea®, Regeneron), are approved for this indication. The third one, bevacizumab (Avastin®, Genentech), is used off-label for nAMD and is a cheaper and then a more cost-effective treatment [12]. The efficacy of the three treatments administered via monthly or as-needed intravitreous injections has been shown in numerous studies [13]. However, undetected occult CNV and/or incomplete compliance of patients omitting some injections lead to suboptimal treatment efficacy [12]. Moreover, development of macular atrophy or fibrosis secondary to nAMD jeopardizes long-term visual acuity in these patients [14, 15]. To improve treatment efficacy, quality of life, and compliance of patients with wet AMD, long-term antiangiogenic therapies or combination therapies are currently in development by several companies [13]. In addition, based on the results of phase III clinical trials (HAWK and HARRIER), brolucizumab (Novartis) has received market authorization in the US and Europe under the name Beovu® in the fall of 2019. Beovu® offers both greater fluid resolution versus Eylea® and the ability to maintain eligible nAMD patients on a three-month dosing interval immediately after a three-month loading phase [16].
GA is characterized by slowly expanding lesions of photoreceptors and retinal pigment epithelium (RPE) leading to progressive retinal degeneration and dysfunction [17–19]. Severe and irreversible loss of central vision may result from GA when the macula is involved. The recent results of several clinical trials testing anticomplement strategies showed a reduction of GA growth rate. The FILLY, NCT02503332 testing Pegcetacoplan® (APL-2) from Apellis Pharmaceuticals Inc., [20] and NCT02686658 using Zimura® from Iveric Bio reported approximately a reduction by 30% of the growth of GA. Another clinical trial (BEACON, NCT02087085) testing brimonidine (Brimo DDS®), a neuroprotective molecule developed by Allergan and now owned by AbbVie [21], reduced GA growth by approximately 12%. These results await confirmations by phase III clinical trials, and new therapeutic options will not be available to patients until the respective drug candidates are registered by US and European authorities. It should also be noted that the extension of areas of GA was not completely halted, and no visual acuity (VA) gain was reported. Thus, even if these drugs become commercially available, it is expected that GA will remain a major unmet clinical need [17, 22]. Therefore, the development of new drugs or alternative strategies able to entirely stop GA progression is still required. Multiple factors have been implicated in the evolution of iAMD and the development of both late forms of AMD. These include age [23, 24] and environmental factors (mainly smoking) [25, 26]. Genetic factors are also involved in the pathology, the main one being polymorphism in the factor H of complement (CfH), which increases by 3- to 6-fold the risk of developing AMD [27–30]. Other genetic polymorphisms have been associated with increased AMD risk [2], including polymorphisms in the ARMS2/HTRA1 loci [31, 32]. ARMS2 polymorphism affects the function of retinal mitochondria, while HTRA1 regulates transforming growth factor-β expression involved in angiogenesis and extracellular matrix deposition. A pivotal role for inflammation in iAMD and both forms of late AMD has also been reported [33, 34]. The initial cause of inflammation and the subsequent retinal destruction observed during AMD remain a subject of debate [35]. It is most probably caused by oxidative stress, which is recognized as a major risk factor in AMD development [36–43]. Therefore, antioxidant and immunosuppressive therapies are likely to be beneficial for patients with iAMD and may reduce the incidence of GA and nAMD. Here, we review existing knowledge on iAMD physiopathology and treatment modalities and propose that apocarotenoids, thanks to their very high antioxidant activity and anti-inflammatory properties, could benefit patients with iAMD and potentially reduce the incidence of late AMD.
2. Material and Methods
Although not a systematic review, relevant studies published and available on PubMed up to the 7th of July 2020 were searched for. Using Boolean operators (e.g., AND, OR), the applied search terms included combinations of the following key words: “AREDS”, “AREDS2”, “lutein”, “zeaxanthin”, “saffron”, “crocetin”, “crocin”, “bixin”, “norbixin”, “annatto”, “ocular”, “retina”, “macula”, “macular”, “age-related macular degeneration”, “AMD”, “intermediate AMD”, “(anti)-inflammatory”, and “(anti)-oxidant”. To minimise the risk of omitting relevant studies, the reference lists of all eligible papers were also manually checked. Only publications in the English language were included. In addition, although nonexhaustive, we searched for formulations of supplements containing carotenoids and available for purchase on the web.
3. Results and Discussion
3.1. Intermediate AMD
IAMD can evolve towards the fast-developing exudative form or the atrophic form of AMD or some combination of these two endpoints (Figure 1) [44]. IAMD is characterized by the progressive accumulations of lipid and protein waste between the Bruch's membrane and the basal side of the RPE and called drusen. In many patients with iAMD, waste materials also accumulate and form Reticular Pseudo-Drusen (RPD) between the apical side of the RPE and the photoreceptor outer segment [45–48]. Rod photoreceptors mediate “night vision” (scotopic visual process), whereas diurnal (photopic) vision is mediated by cones [45]. The first visual signs of iAMD are poor night vision associated with the disappearance of rod photoreceptors [45, 46, 48]. Visual tests used to follow the declining visual functions during iAMD include dark adaptation (DA), scotopic and/or mesopic microperimetry, and low-luminance visual acuity (LLVA) [49]. In addition, changes in multifocal electroretinogram (mfERG) response density and latency [50] and retinal flicker sensitivity [51] are also used to follow loss of vision in patients with mild to moderate AMD [52]. Rod-related visual function depends upon unimpaired transportation of nutrients and in particular vitamin A (the precursor of retinal, a key component of the visual pigment rhodopsin) from the choroidal vasculature through RPE cells up to the rod photoreceptors. It has been proposed that drusen impairs this transport. In addition, RPD might also disturb the close interaction between RPE cells and rods that is necessary for the visual cycle to occur. It is therefore not surprising that drusen and RPD are a major risk factor for apparition of early visual deficits [53] and for evolution of iAMD to late stage AMD (nAMD or GA) [54–58]. Strategies attempting to slowdown the evolution of iAMD towards late stages of the disease appear to be an interesting option. At present, most medications for patients with intermediate stage of AMD rely on dietary supplements based on the Age-Related Eye Disease Studies: AREDS and AREDS2 (Table 1).
Figure 1.
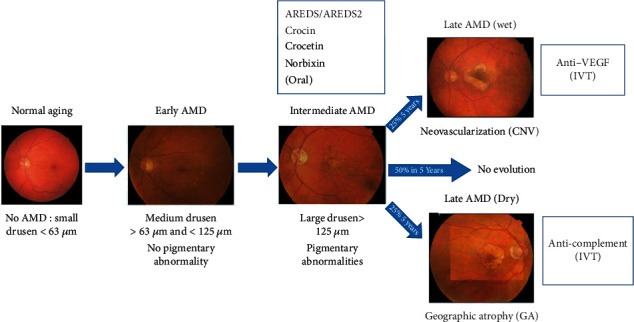
Summary of AMD clinical evolution adapted from the Beckmann classification system [42] and treatment modalities. AMD: age-related macular degeneration; CNV: choroidal neovascularization; GA: geographic atrophy; IVT: intravitreal injections; VEGF: vascular endothelial growth factor.
Table 1.
Carotenoid content of various preparations as announced in the commercial advertisement (but not verified).
| Product | Supplier | Carotenoid amount per day |
|---|---|---|
| AREDS1 original formula | — | B15 |
| AREDS2 original formula | — | L 10, Z 2 |
| AREDS2 Preser vision | Bausch & Lomb | L 10, Z 2 |
| AREDS2 plus Zn free | Eyepromise | L 10, Z 10 |
| AREDS2 with resveratrol | Fortifeye vitamins | L 10, Z 2, A 2 |
| Advanced AREDS2 formula | Vitalux | L 10, Z 2 |
| AREDS2 | VitOptics | L 10, Z 2 |
| Macula-Z | Horus Pharma | L 10, Z 2 |
| Nutrof | Thea Pharma | L 10, Z 2 |
| Vitalux plus | Alcon/Novartis | L 6, Z |
| Lutein+zeaxanthin | Piping rock | L 20, Z 1 |
| Senior vision care complex | Piping rock | L 5, Z 8 μg |
| Ocuvite | Bausch & Lomb | L 5, Z 1 |
| Suveal duo caps | Densmore Laboratoire | L 10, Z 2 |
| Premium MariLut® | Time Sheet | L 10, M 10, Z 2 |
| MacuGuard | Life Extension | L 10, M/Z 4, C |
| True vision | Nature City | L 10, M/Z 2, C 0.6 |
| Eye protector | Pure Synergy | L 10, M/Z 5, A 2, C 3 |
| Luteine crocine 20 mg | Essence pure | L 20, Z 2, C 0.6 |
| AffronEye® | Pharmactive | C 0.6 |
A: astaxanthin; B: beta-carotene; C: crocin; L: lutein; M: meso-zeaxanthin; Z: zeaxanthin. Unless otherwise indicated, amounts are in mg.
3.2. AREDS/AREDS2 Formulations
The AREDS formulation was developed empirically [59]. The effects of the antioxidant AREDS formulation were analyzed in a blinded, randomized, and controlled study on several thousand patients. The original AREDS formulation (described in Table 1) contained β-carotene (Figure 2(a)) and vitamin C, vitamin E, and zinc among other components. Supplementation with AREDS reduced the risk of developing late AMD by an estimated 25% (5 years incidence of late AMD decreased from 28% to 20%) [59]. However, treatment with AREDS over 8 years did not entirely block iAMD progression, and a loss of vision still occurred in patients. Moreover, β-carotene was reported to increase the risk of developing lung cancer in cigarette smokers [60]. Since then, β-carotene has been replaced by the macular xanthophyll lutein (Figure 2(b)) and zeaxanthin (Figure 2(c)) in the AREDS2 formulation (Table 1) [61]. AREDS2 supplementation appeared superior to the AREDS formulation to reduce the risk of developing late AMD [61]. Nevertheless, subsequent meta-analysis showed that the benefits of antioxidants and of the AREDS/AREDS2 antioxidative formulations were limited [4, 60]. Thus, the development of an improved oral and safe treatment with better efficacy on iAMD evolution is still needed and is the focus of intensive current research.
Figure 2.
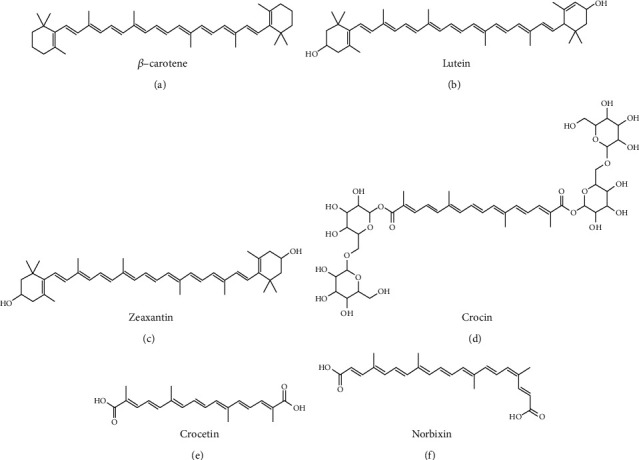
The chemical structures of antioxidant molecules that are used or could be used for the treatment of the intermediate form of AMD.
Targeting oxidative stress is the rationale behind the AREDS/AREDS2 protocols. β-carotene, lutein, and zeaxanthin (L/Z) are known for their antioxidant properties. In the organism of mammals, carotenoids originate exclusively from the diet [62, 63]. β-Carotene is rapidly cleaved into retinol/vitamin A in the liver. Thus, ingested β-carotene is not found in the eye [62, 63]. However, β-carotene exerts some protective effects in AREDS supplemented patients, suggesting that these effects are systemic. By contrast to β-carotene, oxidized carotenoids including xanthophylls are not cleaved in the liver. The xanthophylls (L/Z), which can be extracted from marigold flowers (Tagetes erecta L.), are naturally present in the mammalian retina and most particularly at the level of the macula and fovea [62, 63]. As a result, L/Z are macular pigments. The protective effects of L/Z appear to be both systemic and local. The local action of L/Z, partly depending on their ability to filter phototoxic blue light radiation due to their maximum absorption around 460 nm and via their antioxidant activity, has been demonstrated [64]. Supplementation with L/Z augments their intraocular concentrations in animals [65]. Epidemiological evidence has shown that patients with lower concentrations of macular pigment optical density (MPOD) measurements are at a higher risk of developing AMD. It was suggested that the MPOD concentrations depend on the amount of these products in the diet and that ocular concentrations of L/Z would be increased in a small cohort of patients from an AREDS2 ancillary study receiving L/Z supplementation [66]. However, MPOD was not modified after 6 months of lutein and zeaxanthin dietary supplementation [67]. By contrast and similarly to β-carotene, L/Z protective effects are also linked to their systemic antioxidant properties. Indeed, daily supplementation of L/Z in rats for 42 days significantly increased the serum levels of catalase, an antioxidant enzyme, compared to serum concentrations in the nonsupplemented rats. Simultaneously, the total antioxidant capacity was increased significantly by L/Z supplementation over placebo, indicating that L/Z supplementation has a profound action on the systemic antioxidant defense system [64]. Accordingly, the serum concentration of L/Z was doubled in patients receiving the AREDS2 formulation for several years compared to the normal population without supplementation [61]. Additional components such as zinc, a cofactor of superoxide dismutase (SOD; a key antioxidant enzyme), most probably amplify the systemic antioxidant effects of the AREDS2 formulation (Table 1). At the systemic and local levels, L/Z act on several types of cells important for AMD physiopathology. In vitro, lutein alone reduces the VEGF expression in RPE cells [68] and also reduced expression of interleukin- (IL-) 6, VEGF, and matrix metalloproteinase- (MMP-) 9 in macrophages, which have been implicated in AMD [68, 69]. Similarly, production of the chemokine (C-C motif) ligand 2 (CCL2) by microvascular endothelial cells and RPE was downregulated by lutein in vitro [68]. Lutein also activates nuclear factor erythroid 2-related factor 2 (Nrf2), the master gene of antioxidant response, in ARPE-19 cells, a RPE cell line, in vitro [70]. Moreover, antiangiogenic and anti-inflammatory effects were also observed in vivo in the model of choroidal angiogenesis following laser-induced CNV in mice treated with lutein showing reduced infiltration by macrophages, reduced production of inflammatory cytokines (IL-1-β, IL-12, and TNF-α) and chemokines (including CCL2, CCL3, and CCL5), and limited neovascularization at the site of laser impact [68, 71]. These effects are linked to reduced NF-κB activation, due to inhibition of IκB-α degradation [68]. Because oxidative stress and inflammation have been implicated in AMD pathophysiology, these observations probably explain the reduced risk of AREDS2-treated patients to develop late AMD and particularly nAMD [61]. In addition, in vivo supplementation of L/Z is also protective in two animal models of retinal degeneration, which develop a phenotype similar to the atrophy observed in patients' eyes with GA. In aged CCL2/CX3CR1-deficient mice on a Crb1rd8 genetic background, L/Z supplementation reduced ocular accumulation of N-retinylidene-N-retinylethanolamine (A2E, a major toxic component of drusen) [72]. L/Z also inhibited retinal IL-1β, TNF-α, Cox2, iNOS, and VEGF expression in vivo and preserved the retinal architecture [72]. In the light-challenged albino Balb/c mice model of retinopathy, supplementation with L/Z reduced the expression of several endoplasmic reticulum and oxidative stress markers and a lower number of apoptotic photoreceptors [73]. These effects correlated with preserved retinal structures and functions measured by electroretinography (ERG) [73]. Finally, in an in vivo model of oxidative stress following consumption of a high fat diet, oxidative damage, and inflammation cascade was partially reversed by supplementation with L/Z, and this effect involved Nrf2 regulation [74]. However, as said above, despite these convincing proofs of efficacy in vitro and in vivo, interest in AREDS supplementation for humans appears limited. Indeed, despite a reduction of the incidence of formation of large drusen (>125 mm) and pigmentary abnormalities has been reported, no effects were observed on overall incidence of iAMD following intakes of pro-vitamin A carotenoids and dietary vitamin E [75] or L/Z intake [76]. In another study, an effect on early AMD incidence was only observed in women younger than 75 years old only [77], but nonsignificant effects on incidence of late AMD were reported [76, 77]. Thus, the use of other compounds with more potent antioxidative properties could improve the management of iAMD.
3.3. Crocins and Crocetin
Powder of saffron (Crocus sativus L., Iridaceae) has been used in traditional medicine since antiquity. Between 100,000 and 200,000 saffron flowers are required to produce 1 kg of dry powder. Saffron contains safranal and a mix of several antioxidant molecules derived from β-carotene including crocin and crocetin. This review focuses on the last two compounds. Crocin and crocetin are also found in larger amounts in the fruits of gardenia (Gardenia jasminoides Ellis). Crocetin is a dicarboxylic 9,9′-diapo-carotenoid (C20H24O4) (Figure 2(d)) derived from the naturally occurring crocin, its digentiobioside (Figure 2(e)) [78]. Crocetin and the various isoforms of crocin (crocins) are bioavailable following oral ingestion and are present in the blood plasma in the form of native crocetin and mono- and di-glucuronide crocetin conjugates [79], all having antioxidative properties. Crocetin and crocins also have the capacity to significantly absorb light at 256, 315, 423, and 449 nm and at 235, 324, 432, and 457 nm, respectively [80]. Based on these properties, saffron components, crocins, and crocetin could be beneficial for iAMD patients. Several in vitro and in vivo studies testing the efficacy of saffron, crocins, or crocetin in models reproducing pathophysiological process of iAMD have been performed and are summarized hereafter. In vitro, saffron extracts and its major components display neuroprotective actions through several mechanisms. For instance, crocin protects bovine and nonhuman primate photoreceptors against cell death induced through strong intensity illumination with blue or white light [81]. Preventive protection by crocin is dose-dependent (EC50 = 30 μM) and is associated with inhibition of caspase activity [82]. Similarly, it has been shown that saffron partially preserves the viability of mouse primary retinal cells and a photoreceptor cell line (661 W cells) exposed to toxic doses of ATP. In this model, neuroprotection by saffron was associated with a reduction of intracellular calcium increase induced by ATP and was mediated through saffron action on the purinergic P2X7 receptor [83]. In parallel, it has been shown in vitro as well that crocetin reduces the effects of oxidative stress induced by tert-butyl-hydroperoxide in the ARPE-19 cell line [84]. Pretreatment of RPE cells with crocetin prevented apoptosis evidenced by lactate dehydrogenase release, intracellular ATP depletion, and nuclear condensation [84]. Crocetin preserved junctional and cytoskeleton integrity that are essential for RPE functionality [84]. The neuroprotective effect of saffron components could also be related to their known anti-inflammatory properties. Retinal microglial cells play critical roles in maintaining retinal homeostasis and ocular immune and inflammatory responses. During AMD, chronic microglial activation has been implicated in neuronal degeneration through the release of proinflammatory cytokines and neurotoxic factors. The in vitro effects of crocin and crocetin on proinflammatory gene expression in activated BV-2 microglia cell line and primary microglia were examined [85]. Both crocin and crocetin were shown to inhibit lipo-polysaccharides- (LPS-) induced NF-κB activation, tumor necrosis factor-α (TNF-α), IL-1β, nitric oxide (NO), and reactive oxygen species (ROS) production by rat microglial cells [85]. Interestingly, amyloid-β accumulates in drusen in the eyes of patients with AMD, and crocin has been shown to reduce NO release from microglia stimulated with interferon-γ and amyloid-β [85]. In a similar study, crocins stimulated microglial phagocytosis, which is important for retinal homeostasis, and significantly reduced gene expression of IL-6 and CCL2 [86]. These authors also reported that crocin inhibited iNOS gene expression and NO production in LPS-challenged BV-2 microglia [86]. These results suggest that crocins and crocetin may provide neuroprotection by reducing the production of various neurotoxic molecules by activated microglia. Accordingly, in vivo microglial activation in retinas of albino rats exposed to light damage was reduced by saffron treatment [87]. In the same experiments, Di Marco and coworkers reported that saffron inhibited the MMP3 expression and activity, which was associated with improved retinal structure [87]. These animal studies, as well as others, confirm the neuroprotective effects observed following saffron treatment. Indeed, oral supplementation for 20 weeks with saffron in the model of apoE−/− mice fed with a high-fat diet resulted in preservation of retinal thickness when compared with non-supplemented mice [88]. The outcomes of the study suggest the potential neuroprotective role of saffron against retinal damage induced by oxidative stress. Moreover, supplementation for 6 weeks with 1 mg/kg/day of β-carotene or saffron preserved retinal histology of 2-month-old albino rats exposed during 24 h to intense light (1000 lux) [89]. Interestingly, saffron was more effective than β-carotene to preserve photoreceptor functionality tested through electroretinography (ERG). This suggested that saffron administration could preserve visual function in iAMD patients and perhaps more efficiently than the β-carotene-containing AREDS formulation. The effects of saffron supplementation in humans with various ocular disorders, including iAMD, have been reviewed recently [90, 91]. In 2010, Falsini and coworkers reported a significant improvement of visual function determined through measure of multifocal ERG (mfERG) in patients with iAMD taking orally 20 mg daily of saffron (representing 0.6 mg of crocetin) per day for three months [92]. A subsequent study of the effects of long-term oral administration of saffron over a period of 12 months by the same authors [93] reported sustained improvement of mean fERG sensitivity and mean visual acuity in patients with early AMD. More recently, the efficacy and safety of three-month oral saffron supplementation was assessed in a randomized, double-blinded, and placebo-controlled crossover trial on 100 adults with mild/moderate AMD. Unfortunately, saffron supplementation only moderately improved mean visual acuity and mfERG, including in participants with AMD using AREDS supplements concomitantly [50]. Nevertheless, a comparison of long-term (29 months) supplementation with saffron versus L/Z has shown that visual function (measured by mfERG) remained stable in the saffron-treated group while it had deteriorated in the L/Z-treated group [87] supporting the benefit of saffron compared to the AREDS2 formulation. This also suggests that other compounds with a higher antioxidative potential than crocin and crocetin could potentially be even more beneficial to visual function in the early stages of AMD. In recent years, some dietary supplements containing crocins/crocetin in addition to L/Z have appeared on the market (Table 1), but their use is limited by the lack of convincing clinical trials data and the preponderant use of AREDS/AREDS2 formulations.
3.4. Norbixin
9′-cis-Norbixin is a 6,6′-di-apo-carotenoid extracted from annatto (Bixa orellana) seeds. Norbixin (Figure 2(f)) structure is close to that of crocetin. Norbixin is used as a food colouring agent (E160b) [94]. Tolerability of norbixin is well known, based on both animal and human studies, and the safety data support the use of norbixin as a food additive (with an acceptable daily intake of 0.3 mg norbixin/kg of body weight per day). It has been demonstrated in cellular and animal models that norbixin limits the appearance of symptoms similar to those observed during iAMD in humans [95]. In vitro, norbixin preserved the survival of primary cultures of porcine RPE cells challenged with A2E in the presence of blue light illumination [95]. Interestingly, it was shown that the effectiveness of norbixin in this in vitro test was superior to the photo-protective effects of L/Z and crocetin [95]. However, norbixin and crocetin effectiveness in vitro and in vivo against endoplasmic reticulum stress is similar [96]. In vivo, acute norbixin treatments of albino Wistar rats and ABCA4−/−/Rdh8−/− double-knockout mice exposed to intense light (Blue Light Damage (BLD) model) protect retinal tissues and photoreceptor cells [95]. Similar results were observed in albino Balb/c mice exposed to BLD [96]. In addition, supplementation of ABCA4−/−/Rdh8−/− mice with a diet containing norbixin also prevented the reduction of rod and cone photoreceptor electrical activity measured by scotopic and photopic ERG amplitude, respectively [95, 92]. This indicates that norbixin could potentially preserve “night” and “day” visual acuity in humans. Interestingly, norbixin reduced the uptake of A2E by porcine RPE cells in vitro [95]. A2E accumulation is observed during the early stages of AMD. Accordingly, long-term in vivo administration of norbixin reduces the ocular accumulation of A2E in ABCA4−/− and Rdh8−/− mice [97], suggesting that norbixin could slowdown the subretinal accumulation of A2E that is observed during iAMD. It was further demonstrated in vitro that norbixin significantly reduced the production of VEGF and of several inflammatory cytokines, such as IL-6 and IL-8, in porcine RPE cells cultivated in the presence of A2E (V. Fontaine, M. Fournié, E. Monteiro, T. Boumedine, C. Balducci, L. Guibout, M. Latil, PJ. Dilda, J.-A. Sahel, S. Veillet, R. Lafont, S. Camelo, manuscript posted on bioRxiv) [98]. These in vitro data confirm the anti-inflammatory effects of norbixin observed in human studies in vivo [99, 100]. In vivo, consumption of a high-fat meal induces the production of the proinflammatory cytokines IL-1, TNF-α, and IL-6 in human plasma, which can be reduced by norbixin treatment [94]. Interestingly, norbixin also reduces ROS production by ARPE-19 following exposure to antimycin A-induced oxidative stress [101]. Conversely, in human plasma, norbixin elevated the levels of the antioxidant glutathione and of glutathione peroxidase in vivo [100]. In addition, oral norbixin administration to humans following a high-fat meal reduced the plasma concentration of malondialdehyde (MDA) [100]. These observations are promising, since MDA level increases have been observed during early stages of AMD [102]. Altogether, this suggests that norbixin, through its antioxidative and anti-inflammatory activity, may be beneficial to treat patients with iAMD. Moreover, norbixin through inhibition of A2E accumulation could slow or even stop the progression from iAMD to late dry AMD and could preserve normal rod-mediated “night vision” in iAMD patients. The safety and efficacy of norbixin or related molecules should be evaluated in future clinical trials in patients with iAMD. However, drug development using norbixin and apocarotenoids in general, as active principles, requires a better understanding of their exact mode of action. We recently started to explore the molecular clues explaining the antioxidant and anti-inflammatory properties of norbixin and found out that it modulates very precisely the activity of certain nuclear receptors [98, 101]. Due to the pleiotropic effects of nuclear receptors that have been implicated in AMD [103], this observation supports the broad beneficial effects of norbixin.
4. Conclusion
There is no effective therapeutic strategy for the late form of dry AMD, and intraocular treatments of nAMD are costly and do not prevent long-term loss of vision. As both GA and nAMD originate from iAMD, treating patients at this early stage of the disease could potentially prevent the development of late AMD. Since oxidative stress and inflammation appear to play an essential role in iAMD, the use of therapeutic strategies is aimed at reducing oxidative stress, and inflammation is potentially attractive. At present, prescribing the antioxidant AREDS/AREDS formulations to iAMD patients is the only available therapeutic strategy to reduce late AMD incidence, but its effectiveness appears limited. Here, we described the potential use of apocarotenoids such as crocin, crocetin, and norbixin, for long-term therapy to slowdown the progression of iAMD towards late stages of the disease. Developing such new and more effective treatments for patients with iAMD could drastically reduce the incidence of late forms of AMD in the general population and then could reduce the burden for society as a whole. Indeed, limiting the development of GA and nAMD will not only benefit patients by improving their quality of life but also provide “peace of mind” to the healthy and caregivers. It is also expected that development of such oral drugs will reduce costs to healthcare providers if it becomes the preferred treatment of patients with the intermediate form of AMD.
Acknowledgments
The contribution of Dr. L.N. Dinan (Biophytis) for critical reading of the manuscript and language improvement is acknowledged. We thank L. Guibout for his help with the presentation of the chemical structures. This work was financed by the Biophytis.
Conflicts of Interest
Biophytis declare that their potential commercial interests had no impact on the scientific conduct of the study or the analysis/interpretation of the literature reviewed. ML, PJD, RL, SC, and SV are employees of Biophytis.
References
- 1.Rein D. B., Wittenborn J. S., Zhang X., et al. Forecasting age-related macular degeneration through the year 2050: the potential impact of new treatments. Archives of Ophthalmology. 2009;127(4):533–540. doi: 10.1001/archophthalmol.2009.58. [DOI] [PubMed] [Google Scholar]
- 2.van Lookeren Campagne M., LeCouter J., Yaspan B. L., Ye W. Mechanisms of age-related macular degeneration and therapeutic opportunities. The Journal of Pathology. 2014;232(2):151–164. doi: 10.1002/path.4266. [DOI] [PubMed] [Google Scholar]
- 3.Wong W. L., Su X., Li X., et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. The Lancet Global Health. 2014;2(2):e106–e116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 4.Evans J. B., Syed B. A. New hope for dry AMD? Nature Reviews Drug Discovery. 2013;12(7):501–502. doi: 10.1038/nrd4038. [DOI] [PubMed] [Google Scholar]
- 5.Kosnik W., Winslow L., Kline D., Rasinski K., Sekuler R. Visual changes in daily life throughout adulthood. Journal of Gerontology. 1988;43(3):P63–P70. doi: 10.1093/geronj/43.3.P63. [DOI] [PubMed] [Google Scholar]
- 6.Scilley K., Jackson G. R., Cideciyan A. V., Maguire M. G., Jacobson S. G., Owsley C. Early age-related maculopathy and self-reported visual difficulty in daily life. Ophthalmology. 2002;109(7):1235–1242. doi: 10.1016/S0161-6420(02)01060-6. [DOI] [PubMed] [Google Scholar]
- 7.Berdeaux G. H., Nordmann J. P., Colin E., Arnould B. Vision-related quality of life in patients suffering from age-related macular degeneration. American Journal of Ophthalmology. 2005;139(2):271–279. doi: 10.1016/j.ajo.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 8.Wood J. M., Black A. A., Mallon K., Kwan A. S., Owsley C. Effects of age-related macular degeneration on driving performance. Investigative Ophthalmology & Visual Science. 2018;59(1):273–279. doi: 10.1167/iovs.17-22751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown M. M., Brown G. C., Stein J. D., Roth Z., Campanella J., Beauchamp G. R. Age-related macular degeneration: economic burden and value-based medicine analysis. Canadian Journal of Ophthalmology. 2005;40(3):277–287. doi: 10.1016/S0008-4182(05)80070-5. [DOI] [PubMed] [Google Scholar]
- 10.Rein D. B., Zhang P., Wirth K. E., et al. The economic burden of major adult visual disorders in the United States. Archives of Ophthalmology. 2006;124(12):1754–1760. doi: 10.1001/archopht.124.12.1754. [DOI] [PubMed] [Google Scholar]
- 11.Holz F. G., Pauleikhoff D., Klein R., Bird A. C. Pathogenesis of lesions in late age-related macular disease. American Journal of Ophthalmology. 2004;137(3):504–510. doi: 10.1016/j.ajo.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 12.Ciulla T. A., Huang F., Westby K., Williams D. F., Zaveri S., Patel S. C. Real-world outcomes of anti-vascular endothelial growth factor therapy in neovascular age-related macular degeneration in the United States. Ophthalmology Retina. 2018;2(7):645–653. doi: 10.1016/j.oret.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Shao J., Choudhary M. M., Schachat A. P. Neovascular age-related macular degeneration. Developments in Ophthalmology. 2016;55:125–136. doi: 10.1159/000438969. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen C. L., Gillies M. C., Nguyen V., et al. Characterization of poor visual outcomes of neovascular age-related macular degeneration treated with anti-vascular endothelial growth factor agents. Ophthalmology. 2019;126(5):735–742. doi: 10.1016/j.ophtha.2018.11.036. [DOI] [PubMed] [Google Scholar]
- 15.Mantel I., Dirani A., Zola M., Parvin P., De Massougnes S., Bergin C. Macular atrophy incidence in anti-vascular endothelial growth factor-treated neovascular age-related macular degeneration: risk factor evaluation for individualized treatment need of ranibizumab or aflibercept according to an observe-and-plan regimen. Retina. 2019;39(5):906–917. doi: 10.1097/IAE.0000000000002054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dugel P. U., Koh A., Ogura Y., et al. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127(1):72–84. doi: 10.1016/j.ophtha.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 17.Girmens J. F., Sahel J. A., Marazova K. Dry age-related macular degeneration: a currently unmet clinical need. Intractable & Rare Diseases Research. 2012;1(3):103–114. doi: 10.5582/irdr.2012.v1.3.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleckenstein M., Mitchell P., Freund K. B., et al. The progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology. 2018;125(3):369–390. doi: 10.1016/j.ophtha.2017.08.038. [DOI] [PubMed] [Google Scholar]
- 19.Sadda S. R., Guymer R., Holz F. G., et al. Consensus definition for atrophy associated with age-related macular degeneration on OCT. Ophthalmology. 2018;125(4):537–548. doi: 10.1016/j.ophtha.2017.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao D. S., Grossi F. V., El Mehdi D., et al. Complement C3 inhibitor pegcetacoplan for geographic atrophy secondary to age-related macular degeneration: a randomized phase 2 trial. Ophthalmology. 2020;127(2):186–195. doi: 10.1016/j.ophtha.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Kuppermann B. D., Patel S. S., Boyer D. S., et al. Phase 2 study of the safety and efficacy of brimonidine drug delivery system (Brimo Dds) generation 1 in patients with geographic atrophy secondary to age-related macular degeneration. Retina. 2020;Publish Ahead of Print doi: 10.1097/IAE.0000000000002789. [DOI] [PubMed] [Google Scholar]
- 22.Kassa E., Ciulla T. A., Hussain R. M., Dugel P. U. Complement inhibition as a therapeutic strategy in retinal disorders. Expert Opinion on Biological Therapy. 2019;19(4):335–342. doi: 10.1080/14712598.2019.1575358. [DOI] [PubMed] [Google Scholar]
- 23.Bonilha V. L. Age and disease-related structural changes in the retinal pigment epithelium. Clinical Ophthalmology. 2008;2(2):413–424. doi: 10.2147/opth.s2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinnunen K., Petrovski G., Moe M. C., Berta A., Kaarniranta K. Molecular mechanisms of retinal pigment epithelium damage and development of age-related macular degeneration. Acta Ophthalmologica. 2012;90(4):299–309. doi: 10.1111/j.1755-3768.2011.02179.x. [DOI] [PubMed] [Google Scholar]
- 25.Neuner B., Komm A., Wellmann J., et al. Smoking history and the incidence of age-related macular degeneration--results from the Muenster Aging and Retina Study (MARS) cohort and systematic review and meta-analysis of observational longitudinal studies. Addictive Behaviors. 2009;34(11):938–947. doi: 10.1016/j.addbeh.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 26.La Torre G., Pacella E., Saulle R., et al. The synergistic effect of exposure to alcohol, tobacco smoke and other risk factors for age-related macular degeneration. European Journal of Epidemiology. 2013;28(5):445–446. doi: 10.1007/s10654-013-9798-7. [DOI] [PubMed] [Google Scholar]
- 27.Edwards A. O., Ritter R., 3rd, Abel K. J., Manning A., Panhuysen C., Farrer L. A. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308(5720):421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 28.Haines J. L., Hauser M. A., Schmidt S., et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308(5720):419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 29.Klein R. J., Zeiss C., Chew E. Y., et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308(5720):385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maller J., George S., Purcell S., et al. Common variation in three genes, including a noncoding variant in CFH, strongly influences risk of age-related macular degeneration. Nature Genetics. 2006;38(9):1055–1059. doi: 10.1038/ng1873. [DOI] [PubMed] [Google Scholar]
- 31.Dewan A., Liu M., Hartman S., et al. HTRA1 promoter polymorphism in wet age-related macular degeneration. Science. 2006;314(5801):989–992. doi: 10.1126/science.1133807. [DOI] [PubMed] [Google Scholar]
- 32.Friedrich U., Datta S., Schubert T., et al. Synonymous variants in HTRA1 implicated in AMD susceptibility impair its capacity to regulate TGF-β signaling. Human Molecular Genetics. 2015;24(22):6361–6373. doi: 10.1093/hmg/ddv346. [DOI] [PubMed] [Google Scholar]
- 33.Sennlaub F., Auvynet C., Calippe B., et al. CCR2(+) monocytes infiltrate atrophic lesions in age-related macular disease and mediate photoreceptor degeneration in experimental subretinal inflammation in Cx3cr1 deficient mice. EMBO Molecular Medicine. 2013;5(11):1775–1793. doi: 10.1002/emmm.201302692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Copland D. A., Theodoropoulou S., Liu J., Dick A. D. A perspective of AMD through the eyes of immunology. Investigative Ophthalmology & Visual Science. 2018;59(4):AMD83–AMD92. doi: 10.1167/iovs.18-23893. [DOI] [PubMed] [Google Scholar]
- 35.Bianchi E., Scarinci F., Ripandelli G., et al. Retinal pigment epithelium, age-related macular degeneration and neurotrophic keratouveitis. International Journal of Molecular Medicine. 2013;31(1):232–242. doi: 10.3892/ijmm.2012.1164. [DOI] [PubMed] [Google Scholar]
- 36.Shen J. K., Dong A., Hackett S. F., Bell W. R., Green W. R., Campochiaro P. A. Oxidative damage in age-related macular degeneration. Histology and Histopathology. 2007;22(12):1301–1308. doi: 10.14670/HH-22.1301. [DOI] [PubMed] [Google Scholar]
- 37.Hollyfield J. G., Bonilha V. L., Rayborn M. E., et al. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nature Medicine. 2008;14(2):194–198. doi: 10.1038/nm1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hollyfield J. G. Age-related macular degeneration: the molecular link between oxidative damage, tissue-specific inflammation and outer retinal disease: the proctor lecture. Investigative Ophthalmology & Visual Science. 2010;51(3):1275–1281. doi: 10.1167/iovs.09-4478. [DOI] [PubMed] [Google Scholar]
- 39.Cano M., Thimmalappula R., Fujihara M., et al. Cigarette smoking, oxidative stress, the anti-oxidant response through Nrf2 signaling, and age-related macular degeneration. Vision Research. 2010;50(7):652–664. doi: 10.1016/j.visres.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buschini E., Piras A., Nuzzi R., Vercelli A. Age related macular degeneration and drusen: neuroinflammation in the retina. Progress in Neurobiology. 2011;95(1):14–25. doi: 10.1016/j.pneurobio.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 41.Camelo S. Potential sources and roles of adaptive immunity in age-related macular degeneration: shall we rename AMD into autoimmune macular disease? Autoimmune Diseases. 2014;2014:11. doi: 10.1155/2014/532487.532487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Datta S., Cano M., Ebrahimi K., Wang L., Handa J. T. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Progress in Retinal and Eye Research. 2017;60:201–218. doi: 10.1016/j.preteyeres.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen M., Xu H. Parainflammation, chronic inflammation, and age-related macular degeneration. Journal of Leukocyte Biology. 2015;98(5):713–725. doi: 10.1189/jlb.3RI0615-239R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferris F. L., 3rd, Wilkinson C. P., Bird A., et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120(4):844–851. doi: 10.1016/j.ophtha.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Curcio C. A., Medeiros N. E., Millican C. L. Photoreceptor loss in age-related macular degeneration. Investigative Ophthalmology & Visual Science. 1996;37(7):1236–1249. [PubMed] [Google Scholar]
- 46.Jackson G. R., Owsley C., Curcio C. A. Photoreceptor degeneration and dysfunction in aging and age-related maculopathy. Ageing Research Reviews. 2002;1(3):381–396. doi: 10.1016/S1568-1637(02)00007-7. [DOI] [PubMed] [Google Scholar]
- 47.Flamendorf J., Agron E., Wong W. T., et al. Impairments in dark adaptation are associated with age-related macular degeneration severity and reticular pseudodrusen. Ophthalmology. 2015;122(10):2053–2062. doi: 10.1016/j.ophtha.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spaide R. F., Ooto S., Curcio C. A. Subretinal drusenoid deposits AKA pseudodrusen. Survey of Ophthalmology. 2018;63(6):782–815. doi: 10.1016/j.survophthal.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 49.Cocce K. J., Stinnett S. S., Luhmann U. F. O., et al. Visual function metrics in early and intermediate dry age-related macular degeneration for use as clinical trial endpoints. American Journal of Ophthalmology. 2018;189:127–138. doi: 10.1016/j.ajo.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Broadhead G. K., Grigg J. R., McCluskey P., Hong T., Schlub T. E., Chang A. A. Saffron therapy for the treatment of mild/moderate age-related macular degeneration: a randomised clinical trial. Graefe's Archive for Clinical and Experimental Ophthalmology. 2019;257(1):31–40. doi: 10.1007/s00417-018-4163-x. [DOI] [PubMed] [Google Scholar]
- 51.Falsini B., Fadda A., Iarossi G., et al. Retinal sensitivity to flicker modulation: reduced by early age-related maculopathy. Investigative Ophthalmology & Visual Science. 2000;41(6):1498–1506. [PubMed] [Google Scholar]
- 52.Finger R. P., Schmitz-Valckenberg S., Schmid M., et al. MACUSTAR: development and clinical validation of functional, structural, and patient-reported endpoints in intermediate age-related macular degeneration. Ophthalmologica. 2019;241(2):61–72. doi: 10.1159/000491402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y., Wang X., Rivero E. B., et al. Photoreceptor perturbation around subretinal drusenoid deposits as revealed by adaptive optics scanning laser ophthalmoscopy. American Journal of Ophthalmology. 2014;158(3):584–596.e1. doi: 10.1016/j.ajo.2014.05.038. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Sisternes L., Simon N., Tibshirani R., Leng T., Rubin D. L. Quantitative SD-OCT imaging biomarkers as indicators of age-related macular degeneration progression. Investigative Ophthalmology & Visual Science. 2014;55(11):7093–7103. doi: 10.1167/iovs.14-14918. [DOI] [PubMed] [Google Scholar]
- 55.Wu Z., Ayton L. N., Luu C. D., Guymer R. H. Longitudinal changes in microperimetry and low luminance visual acuity in age-related macular degeneration. JAMA Ophthalmology. 2015;133(4):442–448. doi: 10.1001/jamaophthalmol.2014.5963. [DOI] [PubMed] [Google Scholar]
- 56.Abdelfattah N. S., Zhang H., Boyer D. S., et al. Drusen volume as a predictor of disease progression in patients with late age-related macular degeneration in the fellow eye. Investigative Ophthalmology & Visual Science. 2016;57(4):1839–1846. doi: 10.1167/iovs.15-18572. [DOI] [PubMed] [Google Scholar]
- 57.Lains I., Miller J. B., Park D. H., et al. Structural changes associated with delayed dark adaptation in age-related macular degeneration. Ophthalmology. 2017;124(9):1340–1352. doi: 10.1016/j.ophtha.2017.03.061. [DOI] [PubMed] [Google Scholar]
- 58.Sakurada Y., Parikh R., Gal-Or O., et al. CUTICULAR DRUSEN: risk of geographic atrophy and macular neovascularization. Retina. 2020;40(2):257–265. doi: 10.1097/iae.0000000000002399. [DOI] [PubMed] [Google Scholar]
- 59.Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Archives of Ophthalmology. 2001;119(10):1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Evans J. Antioxidant supplements to prevent or slow down the progression of AMD: a systematic review and meta-analysis. Eye. 2008;22(6):751–760. doi: 10.1038/eye.2008.100. [DOI] [PubMed] [Google Scholar]
- 61.The Age-Related Eye Disease Study 2 (AREDS2) Research Group. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report no. 3. JAMA Ophthalmology. 2014;132(2):142–149. doi: 10.1001/jamaophthalmol.2013.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arunkumar R., Calvo C. M., Conrady C. D., Bernstein P. S. What do we know about the macular pigment in AMD: the past, the present, and the future. Eye. 2018;32(5):992–1004. doi: 10.1038/s41433-018-0044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bernstein P. S., Li B., Vachali P. P., et al. Lutein, zeaxanthin, and meso-zeaxanthin: the basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Progress in Retinal and Eye Research. 2016;50:34–66. doi: 10.1016/j.preteyeres.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Madhavan J., Chandrasekharan S., Priya M. K., Godavarthi A. Modulatory effect of carotenoid supplement constituting lutein and zeaxanthin (10:1) on anti-oxidant enzymes and macular pigments level in rats. Pharmacognosy Magazine. 2018;14(54):268–274. doi: 10.4103/pm.pm_340_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li B., Rognon G. T., Mattinson T., et al. Supplementation with macular carotenoids improves visual performance of transgenic mice. Archives of Biochemistry and Biophysics. 2018;649:22–28. doi: 10.1016/j.abb.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bernstein P. S., Ahmed F., Liu A., et al. Macular pigment imaging in AREDS2 participants: an ancillary study of AREDS2 subjects enrolled at the Moran Eye Center. Investigative Ophthalmology & Visual Science. 2012;53(10):6178–6186. doi: 10.1167/iovs.12-10275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Korobelnik J. F., Rougier M. B., Delyfer M. N., et al. Effect of dietary supplementation with lutein, zeaxanthin, and ω-3 on macular pigment. JAMA Ophthalmology. 2017;135(11):1259–1266. doi: 10.1001/jamaophthalmol.2017.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Izumi-Nagai K., Nagai N., Ohgami K., et al. Macular pigment lutein is antiinflammatory in preventing choroidal neovascularization. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27(12):2555–2562. doi: 10.1161/ATVBAHA.107.151431. [DOI] [PubMed] [Google Scholar]
- 69.Oh J., Kim J. H., Park J. G., et al. Radical scavenging activity-based and AP-1-targeted anti-inflammatory effects of lutein in macrophage-like and skin keratinocytic cells. Mediators of Inflammation. 2013;2013:8. doi: 10.1155/2013/787042.787042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Frede K., Ebert F., Kipp A. P., Schwerdtle T., Baldermann S. Lutein activates the transcription factor Nrf2 in human retinal pigment epithelial cells. Journal of Agricultural and Food Chemistry. 2017;65(29):5944–5952. doi: 10.1021/acs.jafc.7b01929. [DOI] [PubMed] [Google Scholar]
- 71.Yanai R., Chen S., Uchi S. H., Nanri T., Connor K. M., Kimura K. Attenuation of choroidal neovascularization by dietary intake of ω-3 long-chain polyunsaturated fatty acids and lutein in mice. PLoS One. 2018;13(4, article e0196037) doi: 10.1371/journal.pone.0196037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ramkumar H. L., Tuo J., Shen D. F., et al. Nutrient supplementation with n3 polyunsaturated fatty acids, lutein, and zeaxanthin decrease A2E accumulation and VEGF expression in the retinas of Ccl2/Cx3cr1-deficient mice on Crb1rd8 background. The Journal of Nutrition. 2013;143(7):1129–1135. doi: 10.3945/jn.112.169649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yu M., Yan W., Beight C. Lutein and zeaxanthin isomers protect against light-induced retinopathy via decreasing oxidative and endoplasmic reticulum stress in BALB/cJ mice. Nutrients. 2018;10(7):p. 842. doi: 10.3390/nu10070842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tuzcu M., Orhan C., Muz O. E., Sahin N., Juturu V., Sahin K. Lutein and zeaxanthin isomers modulates lipid metabolism and the inflammatory state of retina in obesity-induced high-fat diet rodent model. BMC Ophthalmology. 2017;17(1):p. 129. doi: 10.1186/s12886-017-0524-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.VandenLangenberg G. M., Mares-Perlman J. A., Klein R., Klein B. E., Brady W. E., Palta M. Associations between antioxidant and zinc intake and the 5-year incidence of early age-related maculopathy in the Beaver Dam Eye Study. American Journal of Epidemiology. 1998;148(2):204–214. doi: 10.1093/oxfordjournals.aje.a009625. [DOI] [PubMed] [Google Scholar]
- 76.Cho E., Hankinson S. E., Rosner B., Willett W. C., Colditz G. A. Prospective study of lutein/zeaxanthin intake and risk of age-related macular degeneration. The American Journal of Clinical Nutrition. 2008;87(6):1837–1843. doi: 10.1093/ajcn/87.6.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moeller S. M., Parekh N., Tinker L., et al. Associations between intermediate age-related macular degeneration and lutein and zeaxanthin in the Carotenoids in Age-related Eye Disease Study (CAREDS): ancillary study of the Women’s Health Initiative. Archives of Ophthalmology. 2006;124(8):1151–1162. doi: 10.1001/archopht.124.8.1151. [DOI] [PubMed] [Google Scholar]
- 78.Lautenschlager M., Lechtenberg M., Sendker J., Hensel A. Effective isolation protocol for secondary metabolites from saffron: semi-preparative scale preparation of crocin-1 and trans-crocetin. Fitoterapia. 2014;92:290–295. doi: 10.1016/j.fitote.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 79.Asai A., Nakano T., Takahashi M., Nagao A. Orally administered crocetin and crocins are absorbed into blood plasma as crocetin and its glucuronide conjugates in mice. Journal of Agricultural and Food Chemistry. 2005;53(18):7302–7306. doi: 10.1021/jf0509355. [DOI] [PubMed] [Google Scholar]
- 80.Lech K., Witowska-Jarosz J., Jarosz M. Saffron yellow: characterization of carotenoids by high performance liquid chromatography with electrospray mass spectrometric detection. Journal of Mass Spectrometry. 2009;44(12):1661–1667. doi: 10.1002/jms.1631. [DOI] [PubMed] [Google Scholar]
- 81.Laabich A., Vissvesvaran G. P., Lieu K. L., et al. Protective effect of crocin against blue light- and white light-mediated photoreceptor cell death in bovine and primate retinal primary cell culture. Investigative Ophthalmology & Visual Science. 2006;47(7):3156–3163. doi: 10.1167/iovs.05-1621. [DOI] [PubMed] [Google Scholar]
- 82.Yamauchi M., Tsuruma K., Imai S., et al. Crocetin prevents retinal degeneration induced by oxidative and endoplasmic reticulum stresses via inhibition of caspase activity. European Journal of Pharmacology. 2011;650(1):110–119. doi: 10.1016/j.ejphar.2010.09.081. [DOI] [PubMed] [Google Scholar]
- 83.Corso L., Cavallero A., Baroni D., et al. Saffron reduces ATP-induced retinal cytotoxicity by targeting P2X7 receptors. Purinergic Signal. 2016;12(1):161–174. doi: 10.1007/s11302-015-9490-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Karimi P., Gheisari A., Gasparini S. J., et al. Crocetin prevents RPE cells from oxidative stress through protection of cellular metabolic function and activation of ERK1/2. International Journal of Molecular Sciences. 2020;21(8, article 2949) doi: 10.3390/ijms21082949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nam K. N., Park Y. M., Jung H. J., et al. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. European Journal of Pharmacology. 2010;648(1-3):110–116. doi: 10.1016/j.ejphar.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 86.Yorgun M. A., Rashid K., Aslanidis A., Bresgen C., Dannhausen K., Langmann T. Crocin, a plant-derived carotenoid, modulates microglial reactivity. Biochemistry and Biophysics Reports. 2017;12:245–250. doi: 10.1016/j.bbrep.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Di Marco S., Carnicelli V., Franceschini N., et al. A multitask neuroprotective agent for retinal degenerative diseases. Antioxidants. 2019;8(7):p. 224. doi: 10.3390/antiox8070224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Doumouchtsis E. K., Tzani A., Doulamis I. P., et al. Effect of saffron on metabolic profile and retina in apolipoprotein E-knockout mice fed a high-fat diet. Journal of Dietary Supplements. 2017;15(4):471–481. doi: 10.1080/19390211.2017.1356417. [DOI] [PubMed] [Google Scholar]
- 89.Maccarone R., Di Marco S., Bisti S. Saffron supplement maintains morphology and function after exposure to damaging light in mammalian retina. Investigative Ophthalmology & Visual Science. 2008;49(3):1254–1261. doi: 10.1167/iovs.07-0438. [DOI] [PubMed] [Google Scholar]
- 90.Fernandez-Albarral J. A., de Hoz R., Ramirez A. I., et al. Beneficial effects of saffron (Crocus sativus L.) in ocular pathologies, particularly neurodegenerative retinal diseases. Neural Regeneration Research. 2020;15(8):1408–1416. doi: 10.4103/1673-5374.274325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sepahi S., Ghorani-Azam A., Hossieni S. M., Mohajeri S. A., Khodavrdi E. Pharmacological effects of saffron and its constituents in ocular disorders from in vitro studies to clinical trials; a systematic review. Current Neuropharmacology. 2020;18 doi: 10.2174/1570159X18666200507083346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Falsini B., Piccardi M., Minnella A., et al. Influence of saffron supplementation on retinal flicker sensitivity in early age-related macular degeneration. Investigative Ophthalmology & Visual Science. 2010;51(12):6118–6124. doi: 10.1167/iovs.09-4995. [DOI] [PubMed] [Google Scholar]
- 93.Piccardi M., Marangoni D., Minnella A. M., et al. A longitudinal follow-up study of saffron supplementation in early age-related macular degeneration: sustained benefits to central retinal function. Evidence-Based Complementary and Alternative Medicine. 2012;2012:9. doi: 10.1155/2012/429124.429124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Satyanarayana A., Prabhakara Rao P. G., Rao D. G. Chemistry processing and toxicology of Anatto (Bixa orellana L) Journal of Food Science and Technology. 2003;40(2):131–141. [Google Scholar]
- 95.Fontaine V., Monteiro E., Brazhnikova E., et al. Norbixin protects retinal pigmented epithelium cells and photoreceptors against A2E-mediated phototoxicity in vitro and in vivo. PLoS One. 2016;11(12, article e0167793) doi: 10.1371/journal.pone.0167793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tsuruma K., Shimazaki H., Nakashima K., et al. Annatto prevents retinal degeneration induced by endoplasmic reticulum stress in vitro and in vivo. Molecular Nutrition & Food Research. 2012;56(5):713–724. doi: 10.1002/mnfr.201100607. [DOI] [PubMed] [Google Scholar]
- 97.Fontaine V., Monteiro E., Fournie M., et al. Systemic administration of the di-apocarotenoid norbixin (BIO201) is neuroprotective, preserves photoreceptor function and inhibits A2E and lipofuscin accumulation in animal models of age-related macular degeneration and Stargardt disease. Aging. 2020;12(7):6151–6171. doi: 10.18632/aging.103014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fontaine V., Fournié M., Monteiro E., et al. BioRxiv; 2020. A2E induces the transactivation of RARs, PPARs and RXRs and its effects are counteracted by norbixin in retinal pigment epithelium cells in vitro. [Google Scholar]
- 99.Roehrs M., Figueiredo C. G., Zanchi M. M., et al. Bixin and norbixin have opposite effects on glycemia, lipidemia, and oxidative stress in streptozotocin-induced diabetic rats. International Journal of Endocrinology. 2014;2014:10. doi: 10.1155/2014/839095.839095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Roehrs M., Conte L., da Silva D. T., et al. Annatto carotenoids attenuate oxidative stress and inflammatory response after high-calorie meal in healthy subjects. Food Research International. 2017;100:771–779. doi: 10.1016/j.foodres.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 101.Fontaine V., Monteiro E., Fournie M., et al. Involvement of peroxisome proliferator activator receptors (PPARs) in the photoprotective activity of BIO201. Acta Ophthalmologica. 2017;95(Supplement 259) doi: 10.1111/j.1755-3768.2017.0F070. [DOI] [Google Scholar]
- 102.Schutt F., Bergmann M., Holz F. G., Kopitz J. Proteins modified by malondialdehyde, 4-hydroxynonenal, or advanced glycation end products in lipofuscin of human retinal pigment epithelium. Investigative Ophthalmology & Visual Science. 2003;44(8):3663–3668. doi: 10.1167/iovs.03-0172. [DOI] [PubMed] [Google Scholar]
- 103.Malek G., Lad E. M. Emerging roles for nuclear receptors in the pathogenesis of age-related macular degeneration. Cellular and Molecular Life Sciences. 2014;71(23):4617–4636. doi: 10.1007/s00018-014-1709-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


