Abstract
Background: Radical prostatectomy for de novo prostate cancer (PCa) among kidney transplant (KT) recipients (KTRs) can be challenging because of the location of the renal allograft, which may make robot-assisted radical prostatectomy (RARP) difficult to perform. In this study, we present the first case of RARP in a patient with two renal allografts in both iliac fossae.
Case Presentation: A 72-year-old KTR was found to have organ-confined PCa. He had a first KT (in the right iliac fossa) 20 years ago, which he lost because of chronic allograft nephropathy, followed by a second KT (in the left iliac fossa) 8 years ago, which is now functioning well. We performed RARP with a right-nerve sparing technique. The surgical duration was 208 minutes, with an estimated blood loss of 50 mL and no intraoperative complications. The postoperative course was unremarkable. During the 21-month follow-up period, there was no incontinence or biochemical recurrence and the allograft function remained normal.
Conclusion: RARP is feasible and can be performed safely in KT patients with two renal allografts in the pelvis.
Keywords: prostate cancer, kidney transplant recipients, robot-assisted radical prostatectomy, second renal allograft
Introduction and Background
Renal transplantation provides the best therapy for patients with end-stage organ failure. The number of the kidney transplant (KT) recipients (KTRs) is increasing and patients can live longer with advanced immunosuppressive therapy. Thus, after the transplant, the diagnosis of de novo malignancies has become problematic for long-term graft survivors. As in the general population, prostate cancer (PCa) is the most common malignancy seen in KTRs. Among the post-transplant malignancies, PCa presents as a therapeutic dilemma. Performing robot-assisted laparoscopic prostatectomy (RARP) on these patients may be technically treacherous especially when there are two renal allografts within the pelvis. In this study, we present the first case of RARP in a recipient with two kidney grafts in both iliac fossae.
Presentation of Case
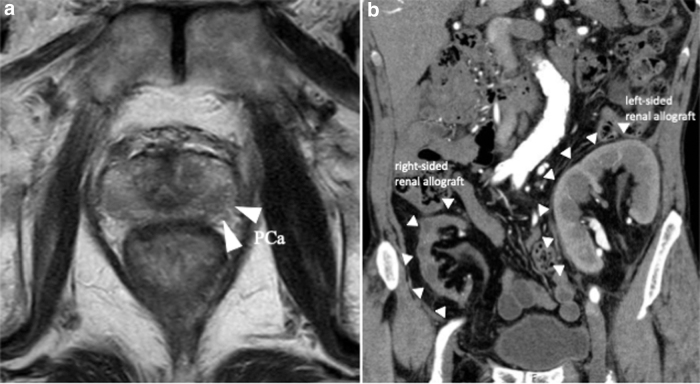
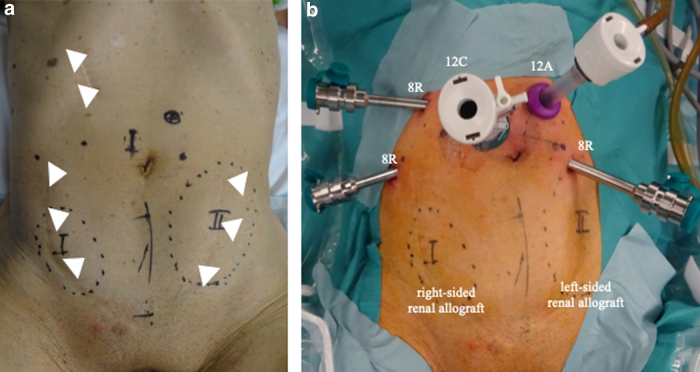
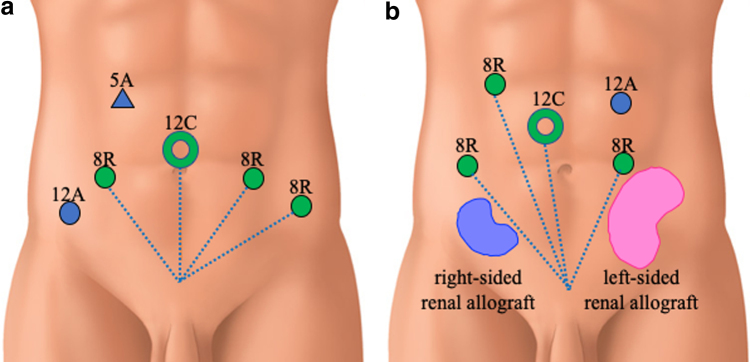
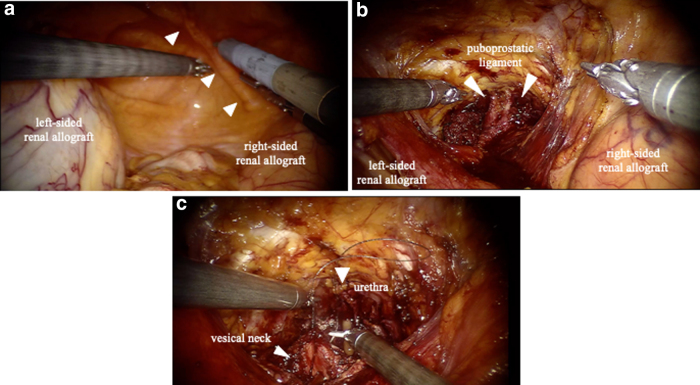
A 72-year-old KTR presented with an elevated prostate-specific antigen of 4.97 ng/mL during cancer screening. The digital rectal examination was negative, but the prostate MRI demonstrated that there were prostatic nodules in the left lobe apex (Fig. 1a). The prostate biopsy revealed prostatic adenocarcinoma in 2 of the 14 cores, with a Gleason score of 3 + 3. The bone scintigraphy and CT did not indicate any metastasis. The first renal allograft was in the right iliac fossa and was for end-stage chronic renal failure, but was lost because of chronic allograft nephropathy after 12 years. He underwent a second kidney transplantation at 64 years in the left iliac fossa (Fig. 1b). He also previously underwent open cholecystectomy at 39 years and retroperitoneoscopic left nephrectomy because of renal cell carcinoma at 68 years (Fig. 2a). The patient elected to undergo RARP with a right nerve sparing technique and was placed in the Trendelenburg position. Our standard six-port RARP was modified to a five-port approach to save the left kidney graft (second graft) and ureter from operative injury (Fig. 2b). In our standard port placement, the fourth robotic arm is placed on the left side with 5 and 12 mm assistant ports on the right (Fig. 3a). In this case, the right-sided transplant kidney had become atrophic and a larger space could be obtained, thus we placed the fourth robotic arm on the right side (ipsilateral to the left graft) and to keep the port-to-port length, the right robotic port moved cranially (Fig. 3b). The left robotic port was moved to just medial to the left graft (Fig. 3b). This reduced the risk of left graft injury from the passage of instruments by the assistant and the left robotic instrument. After the transection of the right umbilical ligament (Fig. 4a), the Retzius space was separated. The space was developed in a right-to-left manner to free the bladder from the abdominal wall and we did not dissect the left umbilical ligament to prevent injury to the left transplanted ureter. The bilateral endopelvic fascia was incised. Then the deep dorsal vein plexus was ligated (Fig. 4b). The bladder neck was dissected from the prostate, followed by the dissection of the seminal vesicles and the vasa deferentia. The right neurovascular bundle was preserved through an antegrade approach. The vascular pedicles were transected using large Hem-o-lok clips (Teleflex, Morrisville, NC, USA), and the urethra was separated. The rhabdosphincter was reconstructed using a V-Loc suture (Medtronic, Minneapolis, MN, USA) with tension-free vesicourethral anastomosis (Fig. 4c). Pelvic lymph node dissection was not performed to prevent any damage to the grafts. The specimen was then removed, the pelvic drainage tube was placed, and the incisions were closed. This procedure was completed within 208 minutes, with no intraoperative complications. The estimated blood loss was 50 mL. Pathology analysis revealed negative surgical margins and the tumor stage was classified as T2aNxM0, the Gleason score was 4 + 3. There were no clinical complications throughout the perioperative period. The catheter was removed after 6 days, and the patient was discharged on postoperative day 7. The mean glomerular filtration rates preoperatively, postoperatively, and at 3 months postoperatively were 45, 44, and 53 mL/min, respectively. At the 21-month follow-up appointment, there was unobstructed urination, no urinary incontinence, no biochemical recurrence, and his kidney graft had been functioning well.
FIG. 1.
Preoperative imaging. (a) Magnetic resonance image shows prostate cancer. (b) CT image shows the transplanted kidneys in the both iliac fossae.
FIG. 2.
(a) The surface locations of the renal allografts. The arrows indicate scars from previous surgery. (b) The port configuration before the docking of the robotic system.
FIG. 3.
Port positions. (a) Standard port positions. (b) Port positions in this case. 12C: camera port (12 mm), 8R: da Vinci arm port (8 mm), 12A: assistant port (12 mm).
FIG. 4.
(a) The transplanted kidneys in both iliac fossae. The arrows indicate the right umbilical ligament. (b) The Retzius space is developed and the deep dorsal vein complex have been ligated. (c) Vesicourethral anastomosis.
Discussion and Literature Review
De novo cancer development among KTRs has become one of the main causes of death in this population. PCa is one of the most common malignancies seen in KTRs. In Japan, the national survey reported 6.5% patients (17 out of 2822 cases) were found to have PCa after kidney transplantation between 2001 and 2010.1 There are several treatment options for PCa, including radical prostatectomy, external beam radiotherapy (EBR), brachytherapy, and active surveillance. A recent systematic review from Europe demonstrated that the majority of patients with PCa in KTRs are treated with radical prostatectomy (82%) instead of EBR (12%) or brachytherapy (6%).2 EBR and brachytherapy tend to be avoided as they carry the possibility of radiation-induced ureteral or nephrotic damages. According to this systematic review, the most common surgical technique was open retropubic prostatectomy (58%); RARP was performed only in 14% of the cases. Pelvic lymphadenectomy was unilateral and on the opposite side of the graft, bilateral, and not performed in 26%, 6.1%, and 67.6% of the patients, respectively. The mean estimated blood loss was around 400 mL, and the mean operative time was 180 minutes. The surgical margins were positive in 26% of the patients. In this study, the operative time was 208 minutes and the estimated blood loss was 50 mL, with negative surgical margins despite bilateral occupation of the iliac spaces by the kidney grafts. The systematic review also concluded that the oncologic outcomes are comparable with those in the nontransplanted population.2
There are few reports with a small number of patients. Jhaveri et al.3 reported the first RARP in KTRs in 2008. According to most of the authors, they needed change robotic ports configuration and move the assistant port on the contralateral side to the renal graft to avoid the graft injury. In our case, the Retzius space was so narrow that the modification of the port sites was necessary to avoid renal allograft injury during the use of instruments. Although the working space was considerably limited when compared with the single kidney graft cases, the robotic EndoWrist has range of motion greater than the human wrist and we could overcome this limitation by using of the robotic endo-wrist and different port-site arrangements. The robotic transperineal approach has also been performed effectively.4 This approach poses minimal risks to the renal graft or transplant ureter. The robotic-perineal approach although is only mastered by a few surgeons. Because the allografts were transplanted into both iliac fossae, we omitted pelvic lymphadenectomy. Even in single KT cases, we do not recommend contralateral iliac lymphadenectomy because the consequent tissue adhesion would be harmful for possible second kidney transplantations in the patient's future. RARP for KTRs has a minor limitation in this way.
Conclusion
We completed RARP in a patient who possessed double sequential kidney grafts in both iliac fossae. RARP is feasible even in second renal allograft recipients and can be accomplished safely with port modifications to protect the precious kidney grafts from an operative injury.
Acknowledgment
We thank Editage for the English language editing.
Abbreviations Used
- CT
computed tomography
- EBR
external beam radiotherapy
- KTR
kidney transplant recipient
- MRI
magnetic resonance imaging
- PCa
prostate cancer
- PSA
prostate-specific antigen
- RARP
robot-assisted radical prostatectomy
Disclosure Statement
No competing financial interests exist.
Funding Information
No funding was received.
Cite this article as: Minami K, Harada H, Sasaki H, Higuchi H, Tanaka H (2020) Robot-assisted radical prostatectomy in a second kidney transplant recipient, Journal of Endourology Case Reports 6:4, 540–543, DOI: 10.1089/cren.2020.0146.
References
- 1. Takuro M, Shuntaro S, Takashi K, et al. National survey of de novo malignancy after solid organ transplantation in Japan. Surg Today 2018;48:618–624 [DOI] [PubMed] [Google Scholar]
- 2. Vital H, Romain B, Albert B, et al. Management of localized prostate cancer in kidney transplant patients: A systematic review from the EAU guidelines on renal transplantation panel. Eur Urol Focus 2018;4:153–162 [DOI] [PubMed] [Google Scholar]
- 3. Jhaveri JK, Tan GY, Scherr DS, et al. Robot-assisted laparoscopic prostatectomy in the renal allograft transplant recipient. J Endourol 2008;22:2475–2479 [DOI] [PubMed] [Google Scholar]
- 4. Tugcu V, Simsek A, Yigitbasi I, et al. Robot-assisted perineal radical prostatectomy in a post-kidney transplant recipient. J Endourol Case Rep 2018;4:21–24 [DOI] [PMC free article] [PubMed] [Google Scholar]