Abstract
Objective
To evaluate to which extent serum neurofilament light chain (NfL) increase is related to diffusion tensor imaging–MRI measurable diffuse normal-appearing white matter (NAWM) damage in MS.
Methods
Seventy-nine patients with MS and 10 healthy controls underwent MRI including diffusion tensor sequences and serum NfL determination by single molecule array (Simoa). Fractional anisotropy and mean, axial, and radial diffusivities were calculated within the whole and segmented (frontal, parietal, temporal, occipital, cingulate, and deep) NAWM. Spearman correlations and multiple regression models were used to assess the associations between diffusion tensor imaging, volumetric MRI data, and NfL.
Results
Elevated NfL correlated with decreased fractional anisotropy and increased mean, axial, and radial diffusivities in the entire and segmented NAWM (for entire NAWM ρ = −0.49, p = 0.005; ρ = 0.49, p = 0.005; ρ = 0.43, p = 0.018; and ρ = 0.48, p = 0.006, respectively). A multiple regression model examining the effect of diffusion tensor indices on NfL showed significant associations when adjusted for sex, age, disease type, the expanded disability status scale, treatment, and presence of relapses. In the same model, T2 lesion volume was similarly associated with NfL.
Conclusions
Our findings suggest that elevated serum NfL in MS results from neuroaxonal damage both within the NAWM and focal T2 lesions. This pathologic heterogeneity ought to be taken into account when interpreting NfL findings at the individual patient level.
MS is an inflammatory autoimmune disease of the CNS, in which both acute and chronic inflammation lead to demyelination and neuronal damage.1 Neurofilament light chain (NfL) is one of the most promising soluble biomarkers for assessing disease activity in MS.2 The cause and underlying pathology of the increased NfL concentration indicative of neuroaxonal injury may, however, vary greatly both inter- and intraindividually. Significant CSF or blood NfL elevations have been shown in association with acute focal inflammation and gadolinium-enhancing lesions in relapsing-remitting MS (RRMS).3–8 On the other hand, moderate elevations in NfL concentrations have been measured in patients with chronic progressive MS.5,6,8–10
We hypothesize that the NfL elevation in the context of more advanced MS disease where no signs of acute inflammation are present is caused by the diffuse pathologic process in the normal-appearing white matter (NAWM), which leads to a diffuse neuroaxonal damage not visible in conventional MRI. This diffuse axonal injury in the NAWM can, however, be sensitively measured using diffusion tensor imaging (DTI).11 Of the DTI scalars, fractional anisotropy is highly sensitive for microstructural changes overall, whereas axial and radial diffusivities are more specific to axonal and myelin damage, respectively.12 It is not known whether DTI scalars associate with NfL in MS.
The aim of this study was to measure to which extent serum NfL increase is related to the diffuse damage in the NAWM, including the type and spatial distribution of these changes. To address this, we performed correlation analyses of NAWM DTI and serum NfL levels and showed significant associations between these measures.
Methods
Standard protocol approvals, registrations, and patient consents
The study was approved by the Ethical Committee of the Hospital District of Southwest Finland. Written informed consent was obtained from all participants according to the Declaration of Helsinki.
Study cohort
Seventy-nine patients with MS from the Neurology Outpatient Clinic of the Division of Clinical Neurosciences at the Turku University Hospital, Turku, Finland, and 10 healthy age-matched controls were included in the study. MRI and serum sampling were performed ≥30 days after a clinical relapse. Clinical disease course, disease duration, and patient age were reviewed, and the Expanded Disability Status Scale (EDSS)13 score was assessed by the investigating neurologist.
Serum samples
Blood samples were collected, and serum was stored at −40°C within 4 hours of sampling. Concentration of serum NfL was measured by single molecule array (Simoa) assay technology as described previously.6,14
MRI and DTI
Brain MRI was performed in Turku PET center with a 3 T MRI Phillips Ingenuity scanner (Philips Healthcare, Cleveland, OH). Conventional MRI (3-dimensional T1-weighted MRI, T2, and fluid-attenuated inversion recovery [FLAIR] with spatial resolution of 1 × 1 × 1 mm) and DTI sequences were included in the protocol. The details of the imaging protocol have been described previously.15 For DTI sequences, the following parameters were used: b value = 1,000 s/mm−2, repetition time/time to echo = 9,500/120 milliseconds, field of view = 256 × 256 mm, spatial resolution 2 × 2 × 2 mm, acquisition matrix 128 × 128 mm, flip angle = 90°, and acceleration factor 2 with 33 (n = 15), 64 (n = 48), or 67 gradient directions (n = 16). The number of gradient directions did not have a remarkable impact on fractional anisotropy, mean diffusivity, radial diffusivity, and axial diffusivity.15
NAWM region of interest was created by excluding all lesions and the cerebellar white matter from the white matter region of interest (appendix e-1, links.lww.com/NXI/A355). The lesions were identified from FLAIR images using Lesion Segmentation Toolbox.16 The NAWM region of interest was further segmented to 6 subregions (frontal, parietal, temporal, occipital, cingulate, and deep white matter, which includes left and right unsegmented white matter and insula) using FreeSurfer software.17 The areas included in subregions are shown in detail in appendix e-2. The FreeSurfer software was also used to define the volumes of cortical gray matter, whole cerebral white matter, NAWM, and total T1 and T2 lesion volumes according to our previously reported methodology.18
The DTI data were preprocessed and analyzed with ExploreDTI software.19 T1 and raw diffusion-weighted image files were first flip permuted after which the T1 file was masked. The files were then converted to diffusion tensor maps using robust diffusion tensor estimation after which diffusion tensor maps were corrected for motion, eddy current, and echo planar imaging/susceptibility induced distortions using the robust estimation of tensors by outlier rejection tensor estimation method. Fractional anisotropy, mean diffusivity, radial diffusivity, and axial diffusivity maps were extracted from the corrected DTI maps. Coregistering of maps to corresponding T1-weighted images was performed using SPM8 (The Wellcome Centre for Human Neuroimaging, University College London) running on MATLAB (The MathWorks, Natick, MA). Finally, the mean values for fractional anisotropy, mean diffusivity, radial diffusivity, and axial diffusivity within the whole NAWM and in the segmented regions of NAWM were calculated in MATLAB.
Statistical analysis
The statistical analysis was performed using R statistical software (version 4.0.0). The differences between different MS groups and between patients and healthy controls were assessed using the Wilcoxon rank-sum (Mann-Whitney U) test. Holm multiple comparison adjustment was used in RRMS vs secondary progressive MS (SPMS) vs healthy control multiple comparisons. The division into NfL(low) and NfL(high) subgroups was based on the median NfL value of the healthy controls.
To test our main hypothesis, Spearman correlations were calculated to assess the relationships between NfL and DTI indices (fractional anisotropy, mean diffusivity, radial diffusivity, and axial diffusivity) in the whole NAWM and in NAWM subregions. Spearman correlation was used instead of Pearson correlation to avoid outlier-induced overestimation of correlations. The p values of correlation analyses were further adjusted with the false discovery rate method (Benjamini-Hochberg procedure) for the number of investigated parameters (n = 28).20 The NfL values were further modeled by the DTI indices and volumetric MRI data using multiple regression models. The logarithm of NfL was used as the response because nontransformed values led to non-normality of residuals. The models were adjusted by sex, age, disease type (relapsing-remitting/secondary progressive), the EDSS score, disease-modifying treatment (no treatment, first line [dimethyl fumarate, glatiramer acetate, interferon-beta, and teriflunomide], or second line [fingolimod, natalizumab, and rituximab] treatment), and the presence of relapses within 1 year before sampling (yes/no). The normality of the residuals was checked using the Shapiro-Wilk test. Variance inflation factor values were used to check that independent variables were not highly correlated with each other. Regression coefficients were standardized to make them directly comparable to each other. For standardization, the regression results were multiplied by twice the SD of the DTI or volume variables.21 The p values of the multiple regression model were adjusted with the false discovery rate method for the number of investigated variables (n = 32).
In addition, to verify the quality of the data and increase the generalizability and comparability of the results, we used Spearman correlation to assess the relationships between following parameters: NfL with age, disease duration, the EDSS score, parenchymal fractions of NAWM, cortical gray matter, and T1 and T2 lesion volumes; DTI with the EDSS score and parenchymal fractions of NAWM, cortical gray matter, and T1 and T2 lesion volumes (results are shown in appendix e-3, links.lww.com/NXI/A355). Spearman correlation was used instead of Pearson correlation to avoid outlier-induced overestimation of correlations and because Spearman correlation is preferred in case of ordinal variables, i.e., EDSS score. All statistical tests were 2 tailed, and p = 0.05 was used as the threshold for statistical significance.
Data availability
The anonymized raw data will be shared over the next 3 years on request from a qualified investigator.
Results
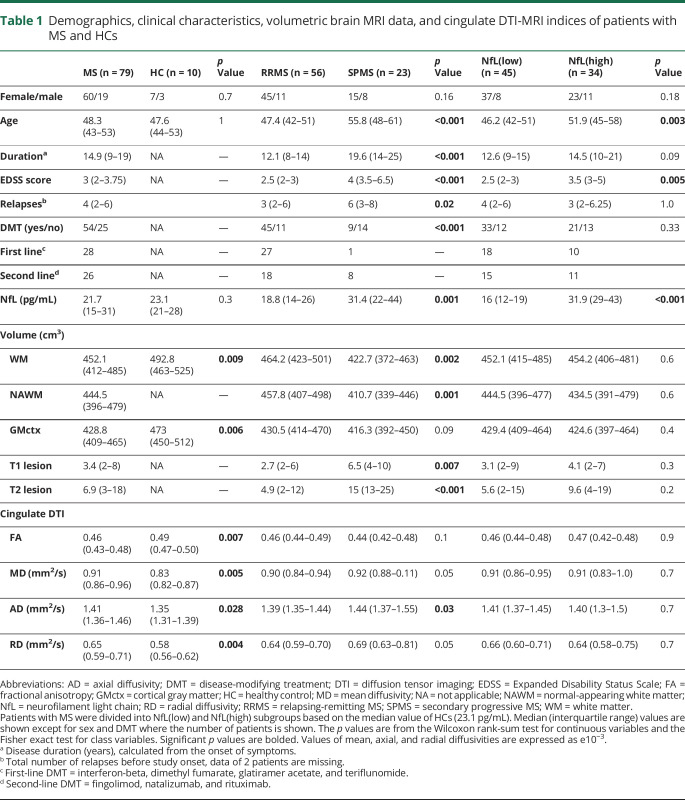
The demographics, clinical characteristics, and conventional imaging data of the 79 patients with MS included in the study are shown in table 1. The median (interquartile range [IQR]) age of this cohort was 48.3 (43–53) years, which is similar to the age of the healthy individuals in the control group (47.6 [44–53], p = 1). Overall, the patient cohort was represented with a quite stable disease, as only 13% of the patients (9 RRMS and 1 SPMS) had had a relapse within 1 year before sampling. Sixty-eight percent of the patients (45 RRMS and 9 SPMS) were on disease-modifying therapy (dimethyl fumarate n = 3, fingolimod n = 14, glatiramer acetate n = 5, interferon beta-1a n = 6, natalizumab n = 8, rituximab n = 4, and teriflunomide n = 14). In MRI, white matter and cortical gray matter volumes were decreased in patients compared with healthy controls. DTI measures were obtained from both the entire cerebral NAWM and from 6 different NAWM subregions. In the cingulate area, there were significant differences in all 4 DTI parameters in patients with MS compared with healthy controls: fractional anisotropy was decreased, whereas mean diffusivity, radial diffusivity, and axial diffusivity were increased (table 1).
Table 1.
Demographics, clinical characteristics, volumetric brain MRI data, and cingulate DTI-MRI indices of patients with MS and HCs
Serum NfL levels in patients with MS and healthy controls
The median (IQR) NfL level was higher in patients with SPMS compared with patients with RRMS (31.4 [22–44] vs 18.8 [14–26] pg/mL, p = 0.001, table 1). NfL levels in the whole MS group were not different from healthy controls (21.7 [15–31] vs 23.1 [21–28] pg/mL, p = 0.3) regardless whether the analysis was performed with age correction. Acute inflammation did not appear to be a factor to affect the NfL level in this cohort with relatively modest acute inflammatory activity, as the median NfL value among the patients with or without relapse within the previous year was comparable (22.3 [16–32] vs 18.6 [15–25] pg/mL, p = 0.3, Wilcoxon rank-sum, data not shown).
Characterization of patients with high NfL levels
To explore associations between increased NfL levels and DTI-measurable diffuse neuroaxonal damage, patients with MS were divided into NfL(high) and NfL(low) subgroups. The division was based on the median NfL value measured among healthy controls (23.1 [21–28] pg/mL). In the NfL(high) subgroup, the NfL concentration was significantly elevated compared with healthy controls (p = 0.018, table 1), and in the further NfL vs DTI correlation analyses, we focused on the NfL(high) subgroup. Based on the demographic and clinical data, the patients in the NfL(high) subgroup were at a more advanced stage of the disease. The NfL(high) subgroup included more patients with SPMS (p = 0.003), and the patients in the subgroup were also older (p = 0.003) and had a higher EDSS score (p = 0.005; table 1). In evaluation using conventional MRI, the NAWM, white matter, or cortical gray matter volumes and T1 and T2 lesion loads were not different between NfL(low) and NfL(high) subgroups (table 1).
Associations between NfL and DTI
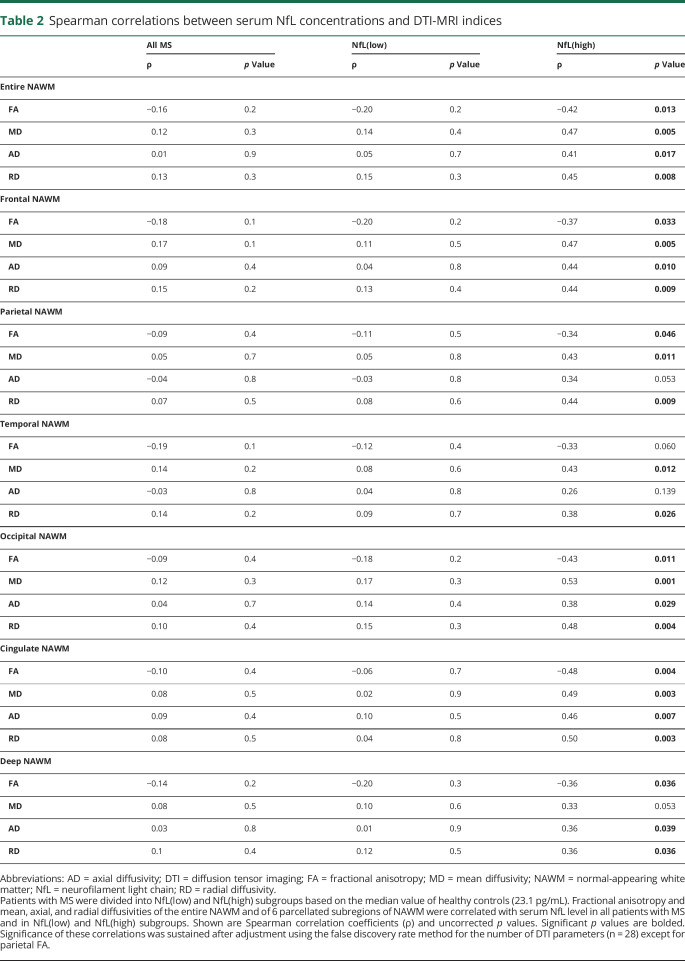
Our results show that DTI metrics of diffuse neuroaxonal damage within the NAWM associate with high serum NfL levels in MS. As a demonstration of this, in the NfL(high) subgroup, several (n = 24) significant correlations were found between serum NfL levels and NAWM DTI indices both in the entire NAWM and in various brain subregions: higher NfL levels were associated with lower fractional anisotropy and higher diffusivity (mean, axial, and radial) in the whole NAWM (figure 1) and in all its subregions, except for fractional anisotropy in temporal NAWM, axial diffusivity in parietal and temporal NAWM, and mean diffusivity in remaining NAWM (table 2). Significance of these correlations was sustained after adjustment using the false discovery rate method for the number of DTI parameters (n = 28) except for parietal fractional anisotropy. Results remained similar when the data were analyzed without patients who had had a relapse within the previous year before sampling (n = 3, data not shown). No correlations between serum NfL levels and DTI metrics were observed in the NfL(low) subgroup or in the overall MS cohort (table 2).
Figure 1. Correlation of serum NfL and DTI indices of the whole NAWM in the NfL(high) subgroup.
The serum level of NfL is moderately associated with fractional anisotropy (A), mean diffusivity (B), axial diffusivity (C), and radial diffusivity (D). The NfL(high) subgroup is comprised of patients with serum NfL above the median value of healthy controls (23.1 pg/mL). Shown are Spearman correlation coefficients (ρ) and p values. The black lines indicate the level and direction of the relationship. AD = axial diffusivity; DTI = diffusion tensor imaging; FA = fractional anisotropy; MD = mean diffusivity; NAWM = normal-appearing white matter; NfL = neurofilament light chain; RD = radial diffusivity.
Table 2.
Spearman correlations between serum NfL concentrations and DTI-MRI indices
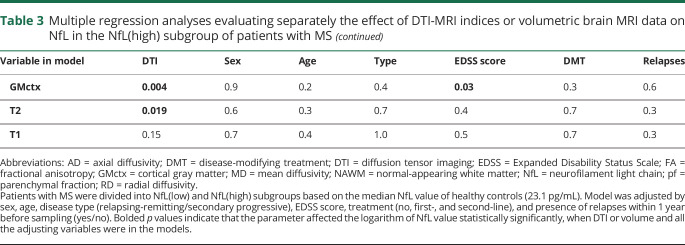
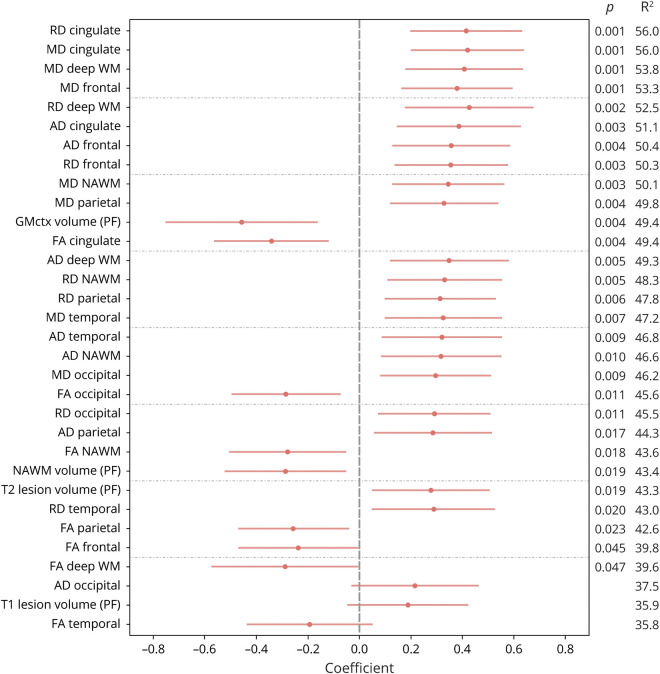
Multiple regression modeling was performed to further evaluate the effect of DTI on NfL. In the NfL(high) subgroup, the DTI indices were more significantly associated with NfL than with the clinical parameters included in the model (sex, age, disease type, EDSS score, treatment, and the presence of relapses within 1 year before sampling) (table 3). On average, 48% of the variance in NfL (SD 4.4) could be explained by DTI indices and the clinical parameters mentioned above (figure 2). There were multiple (n = 26) significant associations between NfL and DTI in the whole NAWM and in all its 6 subregions. All except 2 of the associations remained significant after adjustment with the false discovery rate method for the number of investigated variables (n = 32). The strongest associations were observed in the cingulate area between higher NfL and higher mean and radial diffusivity and in deep and frontal NAWM between higher NfL and higher mean diffusivity (figure 2). Analyzing the data without the patients with a relapse (n = 3) within the previous year did not alter the associations (data not shown).
Table 3.
Multiple regression analyses evaluating separately the effect of DTI-MRI indices or volumetric brain MRI data on NfL in the NfL(high) subgroup of patients with MS
Figure 2. The effect of DTI-MRI indices or volumetric MRI data on NfL in the NfL(high) subgroup.
The NfL(high) subgroup is comprised of patients with serum NfL above the median value of healthy controls (23.1 pg/mL). Logarithmic serum NfL was modeled separately by DTI indices of the entire and parcellated NAWM and volumetric brain MRI data using multiple regression analyses. Models were adjusted by sex, age, disease type (RRMS/SPMS), the EDSS score, treatment (no, first, and second line), and presence of relapses within 1 year before sampling (yes/no). The results are illustrated using dot and whisker plots in which red dots represent standardized regression coefficients and red lines represent the CIs of the estimates. Significant p values of the DTI parameters and the percentage of variance in the response that can be explained by the independent variables (R2) are also shown. The results were ordered according to the R2 value. All except 2 of the p values (fractional anisotropy of the frontal and deep NAWM) shown in the figure remained significant after adjustment using the false discovery rate method for the number of investigated variables (n = 32). AD = axial diffusivity; DTI = diffusion tensor imaging; EDSS = Expanded Disability Status Scale; FA = fractional anisotropy; GMctx = cortical gray matter; MD = mean diffusivity; NAWM = normal-appearing white matter; NfL = neurofilament light chain; PF = parenchymal fraction; RD = radial diffusivity; RRMS = relapsing-remitting MS; SPMS = secondary progressive MS; WM = white matter.
Higher NfL was also associated with lower NAWM and cortical gray matter and higher T2 lesion volumes (figure 2). The volumes and clinical adjustments explained 43.4%, 49.4%, and 43.3% of the variance in NfL, respectively.
Discussion
Present results demonstrate that the DTI-MRI measures of NAWM correlate with serum NfL in MS. We found that among patients with more advanced disease, increased serum NfL levels associate with DTI measures reflecting diffuse microstructural damage, i.e., decreased fractional anisotropy and increased mean, axial, and radial diffusivities in the NAWM. In a multiple regression model, which was adjusted with sex, age, disease type, EDSS score, treatment and the presence of relapses, the DTI indices were more significantly associated with NfL than the above-mentioned demographic and clinical parameters.
The pathophysiology of MS involves acute and chronic mechanisms that lead to gradual axonal and myelin damage that eventually manifest by brain atrophy and clinically by steady worsening of disability in almost all patients over time. This diffuse pathology, which in the majority of cases leads to progressive worsening of the MS-related symptoms, is now the focus of interest for patients and physicians, as modern disease-modifying therapies have led to an almost complete suppression of relapse activity, while their impact on progression is at best modest. Our incomplete understanding of the molecular mechanisms that lead to progression is one of the main reasons for the failure in development of more efficacious therapies for secondary progressive form of MS. Another impediment is the relative insensitivity of current measurement tools to capture features of progression at subclinical stages and hence to quantitate the effects of antineurodegenerative treatments at a time point where the overall brain structure is still largely intact. Specifically, conventional MRI can only quantitate brain volume loss as an end result of the disease but does not provide insights into the underlying microstructural changes. DTI is a nonconventional MRI technique that provides a measure of such diffuse changes, beyond volumetry.
NfL is a highly sensitive biomarker to detect neuronal damage and is the first of its kind applicable in blood-derived probes.22 However, the cause and underlying pathology of the increased NfL concentration may vary greatly both inter- and intraindividually. In clinically isolated syndrome and RRMS, acute NfL elevation is particularly associated with focal inflammation, i.e., relapses and focal lesion formation.4–8,23 However, the indication for the contribution of diffuse pathology to higher NfL levels has so far been only incidental and consisted of associations of increased NfL with more advanced clinical disability and brain atrophy.8,24 Novel findings presented here provide more direct evidence for the effect of diffuse pathologic process in the NAWM on increased serum NfL. We observed that among a subgroup of older and more disabled patients, NfL associates both with radial and axial diffusivities. In the context of MS, these DTI scalars are thought to reflect myelin loss and axonal damage, respectively.11 NfL also associated with mean diffusivity that can also indicate on axonal and myelin loss.11 The associations were most pronounced in the cingulate, deep, and frontal NAWM.
NfL differs in 2 important aspects from MRI: first, as a signal of neurodegeneration, it reflects ongoing pathologic processes in real time, while imaging captures morphologic features that are inherently retrospective. Second, NfL levels reflect neuronal damage in the CNS comprehensively, i.e., in addition to brain pathology, it also captures spinal cord pathology that is not routinely evaluated by imaging in the workup of MS for individual patients. As a downside, NfL levels cannot differentiate between acute inflammatory and chronic neurodegenerative disease activity. Our results suggest that DTI-MRI allows us to categorize the pathogenic source of NfL at least semiquantitatively. We demonstrate that both diffuse axonal damage within the NAWM measured using DTI and focal inflammatory white matter lesion load measured using conventional MRI contributed to the elevated NfL serum levels. This highlights that both focal and diffuse pathologic changes must be taken into account when levels of NfL in individual patients are interpreted. A somewhat larger proportion of NfL variance was explained by DTI indices in average than by T2 lesion load. This implies that in nonrelapsing patients, the ongoing diffuse microstructural damage in the NAWM outside lesions might predominate over focal axonal damage that has occurred within lesions, as a source of NfL in serum. Accordingly, in other neurologic disease with brain diffuse pathology such as amyotrophic lateral sclerosis, Alzheimer disease, traumatic brain injury, and age-related white matter pathology, elevated serum NfL levels have been associated with DTI-measurable diffuse damage within the CNS.25–28 These studies are further evidence for the concept that brain diffuse, chronic neuropathologic mechanisms contribute to NfL release and that nonconventional MRI techniques are able to identify the CNS areas of its morphologic source.
Current results demonstrate that the cingulate area is the brain area with the most prominent white matter tract damage outside focal lesions. Periventricular regions are characteristically the brain area with a particularly heavy lesion load in MS.29 We addressed the question whether the cingulum-NAWM DTI abnormalities would arise from the dirty-appearing white matter due to close proximity to a heavy lesion load, but the relative lesion volume in the cingulate area was not found to be significantly larger compared with lesion load in other regions or in the whole NAWM. Therefore, it seems unlikely that differences in the DTI indices would be solely due to dirty-appearing white matter adjacent to lesions, whereas it is more likely that remote lesions lead to DTI changes in the cingulate NAWM through Wallerian degeneration.30 Also, other studies have observed diffusion changes in the cingulate area of patients with MS.31–33 Because the cingulum bundle of fibers projects from the cingulate gyrus to the entorhinal cortex in the brain, allowing for communication between components of the limbic system, the DTI abnormalities may be the morphologic substrate for impaired cognitive function and fatigue, symptoms that are typical in progressive MS.34,35
Present results are in line with previous observations on the relation between NfL concentration and MS disease subtype, with progressive patients having greater NfL levels compared with relapsing-remitting patients.5,6,8–10,23,24 However, the median NfL value of the entire patient cohort was relatively low compared with healthy controls, which is the main limitation of our study. Because of this, we were not able to use the previously defined cutoff values3 for NfL(low) and NfL(high) subgroups. Instead, we used the median value of healthy controls. Although the divider used in our study was based on a small group of individuals, similar values have been observed in previous, larger studies.6,8 One reason for the low median NfL level could be that the patients with RRMS are represented with a relatively benign disease course. In addition, our patients with RRMS were sampled during a relapse-free time and had no signs of ongoing focal inflammatory activity in the MRI. Moreover, most of the patients (68%) were using a disease-modifying treatment at the time of evaluation. Hence, similar as observed in other studies,3,5,23,36,37 this resulted in low serum NfL concentration. Following the similarity in the NfL levels between healthy controls and patients with MS, the main results of our study are based on a relatively low number of patients. In addition, the patient numbers under different immunomodulatory treatments varied substantially. However, the heterogeneity of the cohort was taken account in the multiple regression model where both EDSS score and disease-modifying treatment were used as adjustments.
Our findings suggest that elevated serum NfL in MS results from neuroaxonal damage both within the NAWM and in focal T2 lesions. The association between DTI-measurable diffuse microstructural white matter damage and serum NfL is further conceptual evidence for the latter being a useful monitoring tool in assessing the degree of ongoing neurodegenerative processes in MS.
Acknowledgment
The authors thank all patients with MS and healthy controls who participated in this study. They also thank the personnel of Turku PET Centre for their excellent technical assistance.
Glossary
- DTI
diffusion tensor imaging
- EDSS
Expanded Disability Status Scale
- FLAIR
fluid-attenuated inversion recovery
- IQR
interquartile range
- NAWM
normal-appearing white matter
- NfL
neurofilament light chain
- RRMS
relapsing-remitting MS
- SPMS
secondary progressive MS
Appendix. Authors
Study funding
This work was supported by the Finnish Academy, the Sigrid Juselius Foundation.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/NN for full disclosures.
References
- 1.Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med 2018;378:169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varhaug KN, Torkildsen Ø, Myhr KM, Vedeler CA. Neurofilament light chain as a biomarker in multiple sclerosis. Front Neurol 2019;10:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuhle J, Kropshofer H, Haering DA, et al. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology 2019;92:e1007–e1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varhaug KN, Barro C, Bjørnevik K, et al. Neurofilament light chain predicts disease activity in relapsing-remitting MS. Neurol Neuroimmunol Neuroinflamm 2018;5:e422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Novakova L, Zetterberg H, Sundström P, et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology 2017;89:2230–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Disanto G, Barro C, Benkert P, et al. Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol 2017;81:857–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siller N, Kuhle J, Muthuraman M, et al. Serum neurofilament light chain is a biomarker of acute and chronic neuronal damage in early multiple sclerosis. Mult Scler 2019;25:678–686. [DOI] [PubMed] [Google Scholar]
- 8.Barro C, Benkert P, Disanto G, et al. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain 2018;141:2382–2391. [DOI] [PubMed] [Google Scholar]
- 9.Ferraro D, Guicciardi C, De Biasi S, et al. Plasma neurofilaments correlate with disability in progressive multiple sclerosis patients. Acta Neurol Scand 2020;141:16–21. [DOI] [PubMed] [Google Scholar]
- 10.Jakimovski D, Zivadinov R, Ramanthan M, et al. Serum neurofilament light chain level associations with clinical and cognitive performance in multiple sclerosis: a longitudinal retrospective 5-year study. Mult Scler 2020;26:1670–1681. [DOI] [PubMed] [Google Scholar]
- 11.Sbardella E, Tona F, Petsas N, Pantano P. DTI measurements in multiple sclerosis: evaluation of brain damage and clinical implications. Mult Scler Int 2013;2013:671730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tae WS, Ham BJ, Pyun SB, Kang SH, Kim BJ. Current clinical applications of diffusion-tensor imaging in neurological disorders. J Clin Neurol 2018;14:129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444–1452. [DOI] [PubMed] [Google Scholar]
- 14.Kuhle J, Barro C, Andreasson U, et al. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin Chem Lab Med 2016;54:1655–1661. [DOI] [PubMed] [Google Scholar]
- 15.Bezukladova S, Tuisku J, Matilainen M, et al. Insights into disseminated MS brain pathology with multimodal diffusion tensor and PET imaging. Neurol Neuroimmunol Neuroinflamm 2020;7:e691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt P, Gaser C, Arsic M, et al. An automated tool for detection of FLAIR-hyperintense white-matter lesions in Multiple Sclerosis. Neuroimage 2012;59:3774–3783. [DOI] [PubMed] [Google Scholar]
- 17.Salat DH, Greve DN, Pacheco JL, et al. Regional white matter volume differences in nondemented aging and Alzheimer's disease. Neuroimage 2009;44:1247–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rissanen E, Tuisku J, Vahlberg T, et al. Microglial activation, white matter tract damage, and disability in MS. Neurol Neuroimmunol Neuroinflamm 2018;5:e443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leemans A, Jeurissen B, Sijbers J, et al. ExploreDTI: a graphical toolbox for processing, analysing, and visualizing diffusion MR data. Presented at the 17th Annual Meeting of International Society for Magnetic Resonance in Medicine; Honolulu, HI; 2009, p. 3537.
- 20.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 1995;57:289–300. [Google Scholar]
- 21.Gelman A. Scaling regression inputs by dividing by two standard deviations. Stat Med 2008;27:2865–2873. [DOI] [PubMed] [Google Scholar]
- 22.Khalil M, Teunissen CE, Otto M, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol 2018;14:577–589. [DOI] [PubMed] [Google Scholar]
- 23.Cantó E, Barro C, Zhao C, et al. Association between serum neurofilament light chain levels and long-term disease course among patients with multiple sclerosis followed up for 12 years. JAMA Neurol 2019;76:1359–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Högel H, Rissanen E, Barro C, et al. Serum glial fibrillary acidic protein correlates with multiple sclerosis disease severity. Mult Scler 2020;26:210–219. [DOI] [PubMed] [Google Scholar]
- 25.Menke RA, Gray E, Lu CH, et al. CSF neurofilament light chain reflects corticospinal tract degeneration in ALS. Ann Clin Transl Neurol 2015;2:748–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore EE, Hohman TJ, Badami FS, et al. Neurofilament relates to white matter microstructure in older adults. Neurobiol Aging 2018;70:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ljungqvist J, Zetterberg H, Mitsis M, Blennow K, Skoglund T. Serum neurofilament light protein as a marker for diffuse axonal injury: results from a case series study. J Neurotrauma 2017;34:1124–1127. [DOI] [PubMed] [Google Scholar]
- 28.Schultz SA, Strain JF, Adedokun A, et al. Serum neurofilament light chain levels are associated with white matter integrity in autosomal dominant Alzheimer's disease. Neurobiol Dis 2020;142:104960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narayanan S, Fu L, Pioro E, et al. Imaging of axonal damage in multiple sclerosis: spatial distribution of magnetic resonance imaging lesions. Ann Neurol 1997;41:385–391. [DOI] [PubMed] [Google Scholar]
- 30.Ciccarelli O, Werring DJ, Barker GJ, et al. A study of the mechanisms of normal-appearing white matter damage in multiple sclerosis using diffusion tensor imaging: evidence of Wallerian degeneration. J Neurol 2003;250:287–292. [DOI] [PubMed] [Google Scholar]
- 31.Pokryszko-Dragan A, Banaszek A, Nowakowska-Kotas M, et al. Diffusion tensor imaging findings in the multiple sclerosis patients and their relationships to various aspects of disability. J Neurol Sci 2018;391:127–133. [DOI] [PubMed] [Google Scholar]
- 32.Kolasa M, Hakulinen U, Brander A, et al. Diffusion tensor imaging and disability progression in multiple sclerosis: a 4-year follow-up study. Brain Behav 2019;9:e01194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andersen O, Hildeman A, Longfils M, et al. Diffusion tensor imaging in multiple sclerosis at different final outcomes. Acta Neurol Scand 2018;137:165–173. [DOI] [PubMed] [Google Scholar]
- 34.Koenig KA, Sakaie KE, Lowe MJ, et al. The relationship between cognitive function and high-resolution diffusion tensor MRI of the cingulum bundle in multiple sclerosis. Mult Scler 2015;21:1794–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pardini M, Bonzano L, Bergamino M, et al. Cingulum bundle alterations underlie subjective fatigue in multiple sclerosis. Mult Scler 2015;21:442–447. [DOI] [PubMed] [Google Scholar]
- 36.Sejbaek T, Nielsen HH, Penner N, et al. Dimethyl fumarate decreases neurofilament light chain in CSF and blood of treatment naïve relapsing MS patients. J Neurol Neurosurg Psychiatry 2019;90:1324–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piehl F, Kockum I, Khademi M, et al. Plasma neurofilament light chain levels in patients with MS switching from injectable therapies to fingolimod. Mult Scler 2018;24:1046–1054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The anonymized raw data will be shared over the next 3 years on request from a qualified investigator.