Abstract
Objective
Antibodies to myelin oligodendrocyte glycoprotein (MOG) are associated with CNS demyelination inclusive of optic neuritis (ON) and transverse myelitis (TM). To examine whether peripheral nervous system (PNS) involvement is associated with MOG antibody–associated disorders (MOGAD), we performed detailed characterization of an Australasian MOGAD cohort.
Methods
Using a live cell–based assay, we diagnosed 271 adults with MOGAD (2013–2018) and performed detailed clinical and immunologic characterization on those with likely PNS involvement.
Results
We identified 19 adults with MOGAD and PNS involvement without prior TM. All patients had CNS involvement including ON (bilateral [n = 3], unilateral [n = 3], and recurrent [n = 7]), a cortical lesion (n = 1), meningoencephalitis (n = 1), and subsequent TM (n = 4). Clinical phenotyping and neurophysiology were consistent with acute inflammatory demyelinating polyneuropathy (n = 1), myeloradiculitis (n = 3), multifocal motor neuropathy (n = 1), brachial neuritis (n = 2), migrant sensory neuritis (n = 3), and paresthesia and/or radicular limb pain (n = 10). Onset MRI spine was consistent with myeloradiculitis with nerve root enhancement in 3/19 and normal in 16/19. Immunotherapy resulted in partial/complete PNS symptom resolution in 12/15 (80%) (steroids and/or IV immunoglobulin n = 9, rituximab n = 2, and plasmapheresis n = 1). We identified serum antibodies targeting neurofascin 155, contactin-associated protein 2, or GM1 in 4/16 patients with MOGAD PNS compared with 0/30 controls (p = 0.01). There was no binding to novel cell surface antigens using an in vitro myelinating sensory neuronal coculture model.
Conclusions
Myeloradiculitis, combined central and peripheral demyelination syndromes, and inflammatory neuropathies may be associated with MOGAD and may be immunotherapy responsive. We identified a subgroup who may have pathology mediated by coexistent autoantibodies.
Myelin oligodendrocyte glycoprotein (MOG) is a component of myelin expressed in the CNS on the outer lamellae of myelin.1,2 The refinement of live cell–based assays that preserve the conformational structure of full-length human MOG has enabled the accurate detection of serum and CSF MOG antibodies to diagnose patients with MOG antibody–associated disorders (MOGAD).3–6 The classic associations of MOGAD include CNS demyelination manifesting as acute disseminated encephalomyelitis in children and optic neuritis (ON) and transverse myelitis (TM) in children and adults in the absence of the typical radiologic white matter changes seen in MS. However, the clinical spectrum is expanding with increasing clinical experience.7–9
Herein, we identified 19 patients with symptoms suggestive of peripheral nervous system (PNS) involvement from a large Australasian cohort of adult patients with MOGAD. We describe the clinical associations of these patients and further identify a subgroup that have coexistent surface-targeting autoantibodies with pathogenic potential.
Methods
Study design, patients, and control recruitment
From 2013 to 2018, our laboratory was referred 4,820 adult serum samples for testing for MOG antibodies and identified 271 adults as being seropositive using a live cell–based assay as we have previously described.5,7 We undertook systematic questioning of 62 clinicians from 35 tertiary referral centers within the Australian and New Zealand MOG Study Group regarding these 271 seropositive adult patients to identify those who had prominent sensory and motor symptoms consistent with PNS involvement. Patients presenting with symptoms attributed to PNS involvement who had prior TM, which was either radiologically confirmed or clinically suspected (due to the presence of sphincter dysfunction, a sensory level, Lhermitte phenomenon, spasticity, hyperreflexia, or a positive Babinski response on neurologic examination), were excluded, as preexisting myelitis may confound interpretation of pain and sensory changes.
We identified 19 patients (15 female [79%], median age at onset 34 years, range 9–68 years) from 271 adults with MOGAD who fulfilled our inclusion criteria and undertook detailed clinical characterization from clinical records and review of neurophysiologic, radiologic, and serologic results in collaboration with their treating clinicians. All patients were adults at the time of first detecting MOG antibody seropositivity, but 2/19 patients had clinical onset of demyelination in childhood, with clinical phenotypes consistent with MOGAD. None of these patients fulfilled the revised 2017 McDonald criteria for MS.10 All patients' clinical notes and correspondence were available for review, and documentation of physical examination findings was used to calculate the Sensory Functions System Score (SFSS) as a component of the Expanded Disability Status Scale to quantify sensory dysfunction before and following therapy.
We additionally selected the following age- and sex-matched control groups (23 female [77%], median age 38, range 20–69 years): patients with MOGAD without any PNS involvement (n = 10), other neurologic diseases (n = 10), and healthy controls (n = 10).
Standard protocol approvals, registrations, and patient consents
Ethics approval for this study was granted by the Sydney Children's Hospitals Network Human Ethics Committee and affiliated sites (12/SCHN/395, SSA/13/WMEAD/53, SSA/13/CRGH/257, and SSA/13/RPAH/599) and the University of Oxford (14/SC/0280, REC16/YH/0013). Informed consent was obtained from all patients and controls.
Assays for the detection of serum antibodies implicated in peripheral neuropathies
Antigen-specific live cell–based assays were performed on patient and control sera for the following antigens as previously described6,11 and as used in the diagnostic laboratory service provided by the Inflammatory Neuropathy and Autoimmune Neurology Groups in Oxford: neurofascin (NF) 155, NF186, contactin 1 (CNTN1), contactin-associated protein-like 1 (CASPR1), CASPR2, and leucine-rich glioma inactivated 1 (LGI1). Samples that were identified as seropositive were titrated to identify end-point dilutions. ELISAs were performed for the detection of ganglioside antibodies GM1, GQ1b, and sulfatide antibodies, as previously described.12
Detection of human sera immunoglobulin G binding to human induced pluripotent stem cell (iPSC)-derived sensory neuronal cultures myelinated with rat Schwann cells
Myelinating cocultures of human iPSC-derived sensory neurons and rat Schwann cells were generated using a previously published method13,14 to identify binding to potential cell surface myelin antigens.
Statistical analysis
Statistics and figures were generated using Prism software version 8.0 (GraphPad Software, La Jolla, CA).
Data availability
Qualified investigators may request access to anonymized data relevant to this study, pending appropriate institutional review board approvals.
Results
Clinical phenotyping
Sixteen of 19 patients in the MOGAD PNS cohort had a relapsing clinical course when taking into account both central and peripheral manifestations of their neurologic disease. Three of 19 had a monophasic presentation with concurrent CNS and PNS involvement, 11/19 had CNS demyelination as the initial presentation, and 5/19 had PNS involvement as the initial presentation. The clinical and immunologic characterization of the MOGAD PNS cohort is summarized in table 1 and detailed in supplementary e-table 1, links.lww.com/NXI/A352.
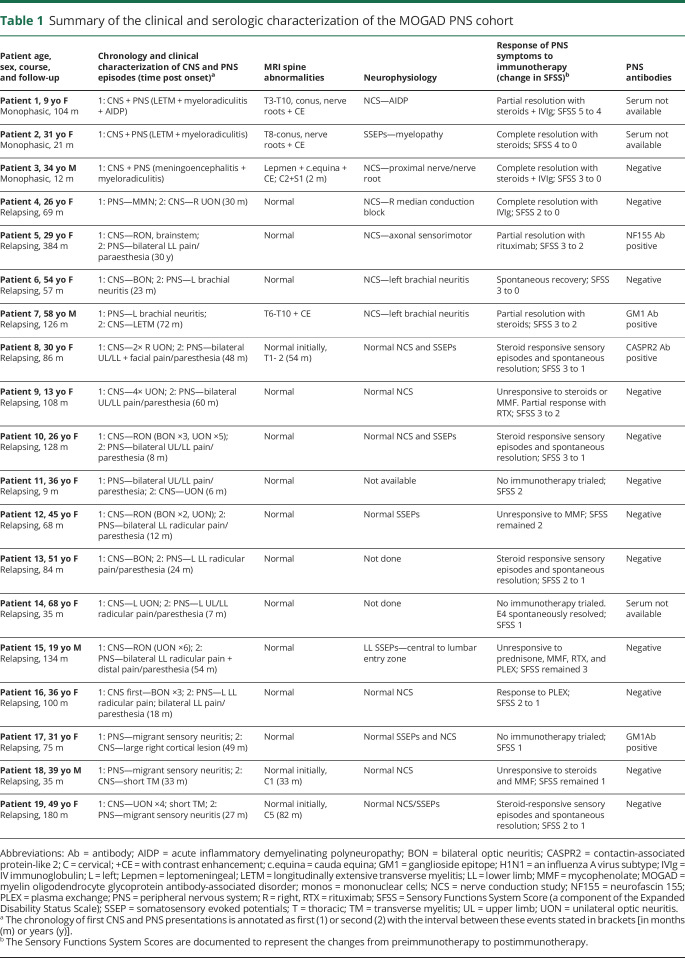
Table 1.
Summary of the clinical and serologic characterization of the MOGAD PNS cohort
All patients with MOGAD had CNS involvement during their disease course. This included monophasic bilateral ON (BON, n = 3), monophasic unilateral ON (UON, n = 3), recurrent ON (either BON or UON or a combination of these phenotypes, n = 7); a large cortical lesion (n = 1), and a meningoencephalitic presentation (n = 1). Two patients had longitudinally extensive transverse myelitis (LETM) concurrent with neurophysiologically confirmed PNS involvement. Two patients developed TM some years following the onset of their PNS manifestations. None of the patients had identified cranial neuropathies. These 19 patients had a total of 47 episodes consistent with CNS demyelination (figure 1A). Overall, ON was the most frequent CNS phenotype, making up 39/47 (83%) episodes.
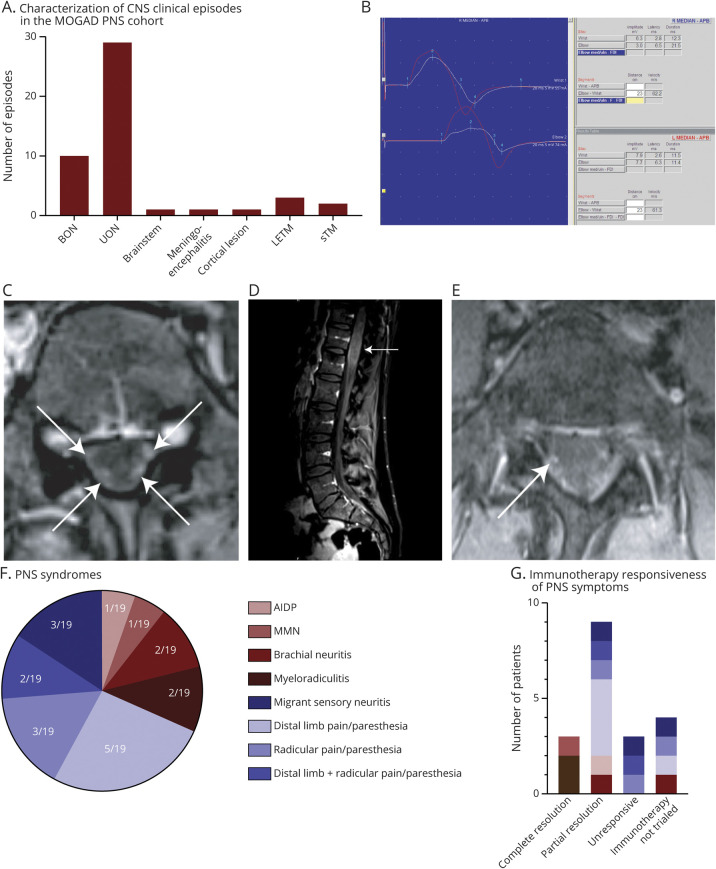
Figure 1. The clinical, neurophysiological, and radiological characterization of PNS involvement in MOGAD.
(A) Clinical phenotyping of the number of CNS episodes in patients in this cohort. ON, inclusive of both BON and UON presentations, was the most frequent clinical phenotype, making up 39/47 (83%) episodes. (B) The right median nerve motor response (white) is smaller than the left (red) with a further reduction of amplitude at the elbow and with reduced persistence of median nerve F wave responses. These findings are consistent with right median neuropathy with conduction block at the elbow in a patient diagnosed with MMN. (C) Axial T1-weighted MRI with gadolinium showing enhancement of dorsal and ventral nerve roots (white arrows). (D) Sagittal T1-weighted MRI with gadolinium showing expansion of the conus (white arrow). (E) Axial fat-suppressed T1 with gadolinium showing enhancement of intradural right S1 nerve root (white arrow). (F) Proportion of the PNS syndromes identified in this cohort of patients with MOGAD. (G) A summary of the immunotherapy responsiveness of particular PNS syndromes—complete resolution occurred in 3/15 patients in whom immunotherapy was trialed (2 with myeloradiculitis and 1 with multifocal motor neuropathy), with 9/15 having only partial resolution and 3/15 being unresponsive to immunotherapy. AIDP = acute inflammatory demyelinating polyneuropathy; BON = bilateral optic neuritis; CIDP = chronic inflammatory demyelinating polyneuropathy; LETM = longitudinally extensive transverse myelitis; MMN = multifocal motor neuropathy; MOG = myelin oligodendrocyte glycoprotein; ON = optic neuritis; PNS = peripheral nervous system; sTM = short transverse myelitis; UON = unilateral optic neuritis.
Serologic and CSF analyses
Serum and/or CSF antibodies targeting aquaporin 4 antibodies were tested in 13/19 patients and were negative. Screening for other autoimmune, infectious, and granulomatous etiologies was performed in the majority of patients (supplementary e-table 1, links.lww.com/NXI/A352). CSF analyses was undertaken in 13/19 patients and revealed a mononuclear lymphocytic pleocytosis in 4/13, an elevated protein level in 4/13, and intrathecal oligoclonal bands in 3/13. The highest CSF protein levels were present in the 3 patients with LETM + acute inflammatory demyelinating polyneuropathy (AIDP) (n = 1, 3.95 g/L) and myeloradiculitis (n = 2–0.68 g/L and 2 g/L). Six of 13 patients had unremarkable CSF analyses.
Neurophysiologic and radiologic characterization
Neurophysiologic investigations were available in 16/19 patients including nerve conduction studies (NCSs) and EMG alone (7/16), somatosensory evoked potentials (SSEPs) alone (4/16), and both NCS/EMG and evoked potentials (5/16) (table 1, supplementary e-table 1, links.lww.com/NXI/A352). In 7 patients, neurophysiology was consistent with a final diagnosis of AIDP (n = 1), multifocal motor neuropathy (MMN, n = 1; figure 1B), an axonal sensorimotor neuropathy (n = 1), myeloradiculitis (n = 2), and brachial neuritis (n = 2). In 1 patient with distal limb and radicular pain/paresthesia (patient 15), despite repeated MRI not indicative of TM and no clinical features of myelitis, the lower limb SSEPs 2 years after PNS symptom onset were consistent with a spinal cord lesion with follow-up SSEPs being within normal limits.
MR spinal imaging was available in all patients and was normal at onset of PNS symptoms in 16/19 patients and abnormal in 3/19. Of the 3 with abnormal MRIs, patient 1 had concurrent LETM with diffusely enhancing nerve roots and neurophysiologically confirmed AIDP. Patient 2 had a swollen thoracolumbar spinal cord with involvement of the conus and diffusely enhancing spinal nerve roots (figure 1, C and D). Patient 3 had enhancement of the cauda equina nerve roots only (figure 1E) and no spinal involvement on onset MRI, but developed an asymptomatic C2 lesion on follow-up, which subsequently resolved. In 3 patients (patients 8, 18, and 19), spinal MRI at PNS symptom onset and follow-up was repeatedly negative, except for a single time point (54, 33, and 82 months postonset of PNS symptoms, respectively), which revealed a spinal lesion consistent with short TM that resolved on subsequent imaging.
Characterization of PNS syndrome
The classification of PNS syndromes in this cohort is summarized in figure 1F. The PNS manifestations of 6/19 patients were diagnosed as AIDP (n = 1), myeloradiculitis (n = 2), MMN (n = 1), and brachial neuritis (n = 2). The remaining 13/19 patients had PNS manifestations, which were more difficult to characterize. These included prominent distal limb pain and paresthesia in 5 patients, limb pain and paresthesia in a radicular pattern in 3, a combination of distal pain/paresthesia and radicular involvement in 2, and a possible migrant sensory neuritis (Wartenberg neuritis) in 3 patients. One of the patients with distal lower limb pain/paresthesia had neurophysiology consistent with an axonal sensorimotor neuropathy.
There was no significant difference in mean age at onset between the patients who had confirmed diagnoses of AIDP, myeloradiculitis, MMN, or brachial neuritis (35 years) compared with those with distal limb pain/paresthesia and/or radicular pain (36 years). None of the patients in this cohort had concomitant diabetes mellitus.
Immunotherapy responsiveness and outcomes
The treatment of the CNS manifestations of MOGAD is detailed in supplementary e-table 1, links.lww.com/NXI/A352. Immunotherapy was trialed for the treatment of the PNS syndromes in 15/19 patients with documented symptomatic improvement in 12/15 (80%) treated with steroids plus IV immunoglobulin (IVIg) (n = 2), steroids alone (n = 6), IVIg (n = 1), rituximab (n = 2), and plasmapheresis (n = 1). Patient responses to immunotherapy ranged from complete resolution (3/15) and partial resolution (9/15) to unresponsive (3/15) according to patient and clinician reports (figure 1G). Sensory Functions System Scores in the 15 patients in whom immunotherapy was trialed were significantly higher preimmunotherapy (mean 2.7, median 3, range 1–5) compared with postimmunotherapy (mean 1.4, median 1, range 0–4) (p = 0.0004) (table 1, supplementary e-table 1, links.lww.com/NXI/A352).
Five patients reported recurrence of PNS symptoms with tapering off prednisone (n = 3), 6 months after rituximab infusion (n = 1), and repeatedly at 3 weeks following scheduled monthly plasmapheresis (n = 1). Complete resolution following immunotherapy occurred in 3 patients who had either myeloradiculitis (n = 2) or MMN (n = 1), with the patients with paresthesia and/or radicular pain either demonstrating a partial response or no response following immunotherapy. Targeted treatment for neuropathic pain was documented as being trialed and partially successful in 1 patient and unsuccessful in 4/19 patients (supplementary e-table 1, links.lww.com/NXI/A352).
Evaluation of antibody-associated peripheral neuropathies
Serum collected within 6 months of the onset of PNS symptoms was available in 16/19 patients, with 7 patients having serial samples available. In patients who had a first presentation with PNS manifestations, serum samples collected at the onset of peripheral symptoms for other diagnostic tests were available to test retrospectively. A total of 26 serum samples (all of which were MOG antibody seropositive) from this cohort were tested for antibodies associated with peripheral neuropathies (table 1, supplementary e-table 1, links.lww.com/NXI/A352 appendix e-1, links.lww.com/NXI/A353). Antibodies associated with peripheral autoantibody-mediated neuropathies were identified in 4/16 patients with MOGAD PNS and 0/30 controls (p = 0.01).
Patient 5 had a nerve conduction study consistent with an axonal sensorimotor neuropathy 1 year after the onset of her bilateral ascending foot pain/paresthesia and was seropositive for NF155 antibodies to an end-point dilution of 1:6,400 (figure 2A). Live cell–based assays analyzed by microscopy identified serum binding for total immunoglobulin G (IgG) and IgG1, IgG2, and IgG4 subclasses. This patient had initial improvement in her neuropathic symptoms following the institution of rituximab; however, her clinical response subsequently plateaued despite maintaining B-cell depletion with rituximab. Patient 8 was positive for CASPR2 antibodies in 3 serial samples taken during relapsing sensory episodes, up to end-point dilutions of 1:3,200, 1:3,200, and 1:12,800, respectively (figure 2B). She experienced pain and paresthesia starting in the feet, initially intermittent, followed by similar symptoms in the hands, in combination with additional radicular limb pain and atypical facial pain in the absence of MRI changes in the spine at onset and for over 4 years of radiologic follow-up with recurrent symptoms of pain. During exacerbations of pain on 3 occasions, she reported a marked reduction in symptoms following commencement of steroids. At 54 months postonset, and while asymptomatic, she was noted to have increased T1/2 signal on her MRI, which resolved on subsequent imaging. Two patients had low titer GM1 IgM antibodies. Patient 7 (brachial neuritis) was positive at a dilution of 1:100; however, sample exhaustion did not permit further end-point titrations. This patient had partial resolution of his symptoms with steroids, although residual deficits remain. Patient 17 (migrant sensory neuritis) was positive for GM1 IgM antibodies to an end-point dilution of 1:200. This patient was never trialed on immunotherapy.
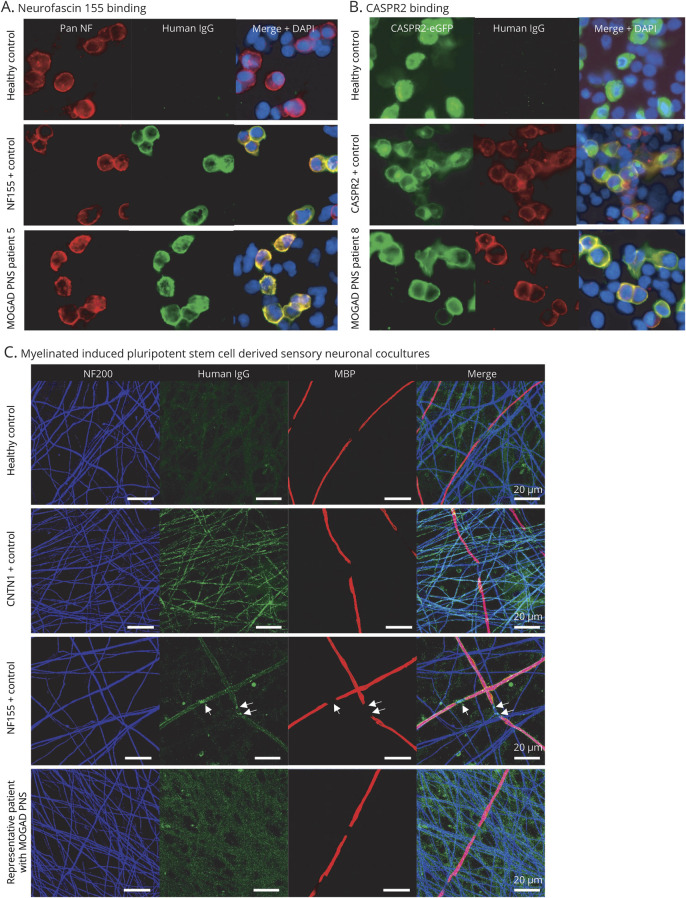
Figure 2. Antibody-associated peripheral nerve involvement.
(A) Human IgG binding (green) colocalizes on the cell surface of HEK293 cells to binding of a commercial pan-neurofascin antibody (red) when cells were incubated with sera from an NF155 positive control and MOGAD PNS patient 5, but not in a healthy control. (B) Human IgG binding (red) colocalizes on the cell surface of HEK293 cells transfected with CASPR2-eGFP (green) when cells were incubated with sera from a CASPR2-positive control and MOGAD PNS patient 8, but not in a healthy control. (C) Images of serum-treated myelinating cocultures labeled with anti-human IgG antibodies (green). Anti-NF200 (blue) and anti-MBP (red) primary antibodies were used to visualize axonal processes and myelin internodes, respectively. This coculture, treated with serum from a healthy control, anti-CNTN1 antibody or anti-NF155 antibody positive controls, and a representative MOGAD PNS patient are shown. Arrows indicate the nodal/paranodal deposition of human IgG. Note lack of specific labeling in healthy control and MOGAD PNS patient serum. CASPR2 = contactin-associated protein-like 2; CNTN1 = contactin 1; DAPI 4′,6-diamidino-2- phenylindole; eGFP = enhanced green fluorescent protein; IgG = immunoglobulin G; MBP = myelin basic protein; MOGAD = myelin oligodendrocyte glycoprotein antibody–associated disorder; NF = neurofascin; NF155 = neurofascin 155; NF200 = neurofilament 200; PNS = peripheral nervous system.
None of the 16 patients with MOGAD PNS or 30 controls were positive for NF186, CNTN1, CASPR1, LGI1, GQ1b, or sulfatide antibodies. In addition, none of the patient or control sera revealed discernible IgG binding in myelinated sensory neuron cocultures in comparison to positive controls (figure 2C).
Discussion
In our collective clinical experience, we have noted challenging sensory symptoms in a subgroup of patients with MOGAD suggestive of PNS involvement, prompting a structured evaluation of this phenomenon. Herein, we highlight the association of myeloradiculitis and combined central and peripheral demyelination (CCPD) in MOGAD, the immunotherapy responsiveness of many patients, and identify a subgroup who may have a coexisting antibody-mediated neuropathy.
We previously identified the presence of prominent ventral and dorsal nerve root enhancement in a series of children with MOGAD,15 which has been corroborated in 2 recent case reports.16,17 The first 3 patients in this MOGAD PNS cohort demonstrated diffusely swollen and enhancing nerve roots. Although a noncompressive myeloradiculopathy would normally raise suspicion for inflammatory, infectious, and infiltrative etiologies, we propose that myeloradiculitis is increasingly identified as being associated with MOGAD and should be an early diagnostic consideration. It has long been recognized that CCPD occurs usually in the context of MS, although this is rare and biologically enigmatic.18 A case report of a patient with MOGAD with CCPD who presented with multifocal demyelination and an acquired demyelinating sensory and motor neuropathy has been described.17 Our case series identifies a second patient with MOGAD with CCPD who presented with concurrent LETM and AIDP.
Animal studies have identified MOG in the PNS of rodents and primates at a cytoplasmic rather than cell surface localization and have implicated contributions of MOG to neuropathic pain.19,20 The significant differences in binding epitopes of MOG antibodies in humans compared with rodents5,21 and the lack of surface PNS expression of MOG in humans suggest that it is less likely that MOG antibodies are driving immune-mediated PNS presentations in these patients.
A potential explanation for some patients having immunotherapy-responsive peripherally referred symptoms may be attributed to radiologically negative TM, and the patients in our cohort underwent repeated spinal imaging to exclude this possibility. A case series of 73 patients with MOGAD at first presentation of clinical myelitis highlighted an initially normal spinal MRI in 10% of patients, with half going on to develop radiologically detectable cord involvement with follow-up imaging.22 Apart from the 3 patients with concurrent TM and either AIDP or myeloradiculitis, the remaining 16/19 patients in the present cohort did not have clinical or radiologic evidence suggestive of a coexistent or prior myelopathy at PNS symptom onset, and 13/16 did not have cord lesions on follow-up imaging. Although this suggests a peripheral etiology in this cohort, we acknowledge that radiologically negative cord involvement and central pain may yet be a contributing factor in some of the patients we describe with normal neurophysiology and radiology.
Another explanation for immune-mediated PNS involvement in a small group of patients with MOGAD is the presence of 2 overlapping autoantibody-mediated syndromes. Albeit rare, the coexistence of multiple autoantibodies implicated in the pathogenesis of autoimmune encephalitis and antibody-associated demyelination is now well established.23–25 In this cohort, we describe 4 patients, all of whom exhibited CNS manifestations consistent with MOGAD, who were additionally positive for PNS autoantibodies.
NF155 antibodies targeting cell adhesion molecules at the paranode have been described in patients with CIDP and CCPD.26,27 A previous case report demonstrates an overlap of aquaporin 4 (AQP4) antibody–positive neuromyelitis optica spectrum disorder and NF155 antibody–associated CIDP. Our cohort highlights the novel observation of a patient with MOGAD and coexisting NF155 antibodies. Antibodies to CASPR2 are identified in autoimmune encephalitis, peripheral nerve hyperexcitability, and neuromyotonia11,28 with neuropathic pain in up to 46% of patients.29,30 There are reports of CASPR2 and LGI1 antibodies associated with neuropathic pain in the absence of associated CNS manifestations (29,31 and Irani and Ramanathan, unpublished observations). The patient in this MOGAD PNS cohort with strongly positive CASPR2 antibodies experienced neuropathic pain with rapid amelioration of symptoms following steroid initiation. GM1 antibodies are typically associated with acute motor axonal neuropathy and MMN, although they have been described in motor neurone disease and chronic neuropathies.26,32 Two patients in our cohort were positive for GM1 antibodies at low titers, with diagnoses of brachial neuritis and migrant sensory neuritis, respectively. The clinical relevance of these results requires conservative consideration.
Close to a third of patients in this cohort had distal limb pain and paresthesia either in isolation or in combination with radicular symptoms with normal neurophysiology, suggestive of a small fiber neuropathy (SFN). SFN is a painful disease characterized by the loss of unmyelinated and thinly myelinated sensory nerve fibers and often normal NCS and requires quantitative sensory testing and evaluation of intraepidermal nerve fiber density for definitive diagnosis.33 The patients in this cohort who may be considered as having a SFN had the same median age at symptom onset as the patients with neurophysiologically confirmed AIDP, MMN, brachial neuritis, and myeloradiculitis; and none of the patients in this cohort had diabetes mellitus, excluding an age related or metabolic confounder. Although some of the patients with pain/paresthesia had intermittent symptoms, which were steroid responsive early in the clinical course with asymptomatic intervals, many went on to develop more long-term persistent neuropathic pain and loss of pinprick and temperature sensation in a glove and stocking distribution, suggesting that the long-term functional sequelae and pain experience of these patients are considerable.
An alternate explanation for PNS presentations identified in patients with MOGAD includes the presence of binding to as yet unidentified target antigens in the peripheral nerve sheath. However, the absence of prominent binding of patient sera IgG in a myelinating sensory neuronal coculture system13,14 does not support this assertion. Alternative etiologies include as yet unidentified immune processes that may be T cell–, cytokine-, or complement-mediated or coexisting toxic, ischemic, or metabolic processes. The partial or complete immunotherapy responsiveness of 80% of the patients with MOGAD PNS who were trialed on immunotherapy suggests an inflammatory phenomenon. Our clinical experience recognizes that PNS involvement is less common in pediatric patients with MOGAD compared with adults.
Our study is limited by the retrospective nature of case identification and characterization. Patients presenting with symptoms attributed to PNS involvement who had prior TM, which was either radiologically confirmed or clinically suspected, were excluded from this study to mitigate the confounding effect of prior TM on pain and sensory symptoms, which may have resulted in an underestimation of PNS involvement in MOGAD in this cohort. The subjective nature of how pain is experienced, the lack of SSEPs to exclude radiologically negative myelitis in some of the patients, and the lack of definitive histopathology including skin and nerve biopsies or more definitive investigations including quantitative sensory testing or autonomic function testing in the patients we describe pose additional limitations. Prospective recruitment of patients with MOGAD with PNS involvement and systematic neurophysiologic evaluation and targeted biopsies would be of value to distinguish potentially treatable inflammatory neuropathies from other etiologies including fibromyalgia.
Nevertheless, this cohort enables discussion of an emerging theme of PNS involvement in patients with MOGAD. Specifically, MOG antibodies should be considered as part of the diagnostic workup of patients who present with peripheral nerve root enhancement or enlargement, myeloradiculitis, and CCPD. In patients with known MOGAD, we now recognize challenging sensory symptoms that are attributable to the PNS, which are often immunotherapy responsive, and may be associated in a subgroup with PNS specific autoantibodies as part of an overlap syndrome. A wider awareness of autoimmune pain, detailed investigation with neurophysiology, consideration of skin or nerve biopsy, and a trial of immunotherapy in selected patients may translate to improved symptom control and quality of life in these patients and add to the literature on causes of autoimmune pain.
Acknowledgment
The authors thank Professor Steve Vucic for allowing them to consent and recruit a number of patients from his clinic to act as controls for this study.
Glossary
- AIDP
acute inflammatory demyelinating polyneuropathy
- AQP4
aquaporin 4
- BON
bilateral optic neuritis
- CNTN1
contactin 1
- CASPR1
contactin-associated protein-like 1
- CASPR2
contactin-associated protein-like 2
- CCPD
combined central and peripheral demyelination
- CIDP
chronic inflammatory demyelinating polyneuropathy
- HEK293
human embryonic kidney 293
- IgG
immunoglobulin G
- iPSC
induced pluripotent stem cell
- IVIg
IV immunoglobulin
- LETM
longitudinally extensive transverse myelitis
- LGI1
leucine-rich glioma inactivated 1
- MMN
multifocal motor neuropathy
- MOG
myelin oligodendrocyte glycoprotein
- MOGAD
MOG antibody–associated disorder
- NCS
nerve conduction study
- NF155
neurofascin 155
- NF186
neurofascin 186
- OCB
oligoclonal band
- ON
optic neuritis
- PMN
polymorphonuclear cell
- PNS
peripheral nervous system
- SFN
single fiber neuropathy
- SSEP
somatosensory evoked potential
- TM
transverse myelitis
- UON
unilateral optic neuritis
Appendix 1. Authors
Appendix 2. Australian and New Zealand MOG Study Group

Study funding
This work was supported by the National Health and Medical Research Council (NHMRC) (Australia), the Petre Foundation (Australia), Multiple Sclerosis Research Australia (Australia), the Brain Foundation, the Sydney Research Excellence Initiative 2020 Neuroimmunology Group (University of Sydney, Australia), the Wellcome Trust (UK), Medical Research Council (UK), and the GBS/CIDP Foundation International.
Disclosure
S. Rinaldi is supported by Medical Research Council (UK), grant number MR/P008399/1 and has received speaker's honoraria from Fresenius, Alnylam, and Excemed and payments to provide expert medicolegal advice on inflammatory neuropathies and their treatment. He has received complimentary registration and prize money from the Peripheral Nerve Society and a travel bursary from the European School of Neuroimmunology. He is a member of the GBS and Associated Inflammatory Neuropathies (GAIN) patient charity medical advisory board. He runs a not-for-profit nodal/paranodal antibody testing service at the Nuffield Department of Clinical Neurosciences, John Radcliffe Hospital, Oxford, UK, in partnership with Clinical Laboratory Immunology of Oxford University Hospitals. A. Davies receives funding from the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC) and is a named inventor on a patent application (WO/2020/009437) entitled “Immune cell treatment of nerve damage.” He has received speaker honorarium from the Korean Association of Immunologists and travel grants from BioLegend and the International Association for the Study of Pain. J. Fehmi reports no disclosures. H.N. Beadnall has received honoraria for presentations and advisory boards and has received educational travel support from Biogen, Sanofi-Genzyme, Merck, Novartis, and/or Roche. J. Wang reports no disclosures. T.A. Hardy has received honoraria or travel sponsorship from Bayer-Schering, Novartis, Biogen Idec, Merck Serono, Roche, Teva, Alexion, and Sanofi-Genzyme. He has received travel grant funding from MS Research Australia. He is Co-Editor of “Advances in Clinical Neuroscience and Rehabilitation.” M.H. Barnett has received institutional support for research, speaking, and/or participation in advisory boards for Biogen, Merck, Novartis, Roche, Sanofi-Genzyme, and Alexion. He is a consulting neurologist for RxMx and research director for the Sydney Neuroimaging Analysis Centre. S.A. Broadley has received honoraria for attendance at advisory boards and travel sponsorship from Bayer-Schering, Biogen Idec, Merck Serono, Novartis, and Sanofi-Genzyme; has received speaker honoraria from Biogen Idec and Genzyme; and is a principal investigator for clinical trials sponsored by Biogen Idec, Novartis, ATARA, Alexion, and Genzyme. P. Waters is a coapplicant and receives royalties on patent application WO/2010/046716 (U.K. patent no. PCT/GB2009/051441) entitled “Neurological Autoimmune Disorders.” The patent has been licensed to Euroimmun AG for the development of assays for LGI1 and other VGKC-complex antibodies. He is codirector of the Oxford Autoimmune Neurology Diagnostic Laboratory where live MOG antibody tests are run. He has received honoraria from Biogen Idec, Mereo Biopharma, Retrogenix, UBC, and Euroimmun AG; travel grants from the Guthy-Jackson Charitable Foundation; and research funding from Euroimmun AG. S.W. Reddel reports grants and personal fees from Genzyme Sanofi, personal fees and departmental support from the Government of Australia, Baxter, Biogen, CSL, and Merck; and departmental support from Novartis, outside the subject of the submitted work. S.R. Irani is supported by the Wellcome Trust (104079/Z/14/Z), the UCB- Oxford University Alliance, BMA Research Grants-Vera Down grant (2013) and Margaret Temple (2017), Fulbright UK-US commission (MS-SOCIETY research award), and Epilepsy Research UK (P1201). The research was funded/supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC; the views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health). S.R. Irani is a coapplicant and receives royalties on patent application WO/2010/046716 (U.K. patent no. PCT/GB2009/051441) entitled “Neurological Autoimmune Disorders.” The patent has been licensed for the development of assays for LGI1 and other VGKC-complex antibodies. He has received honoraria from UCB, MedImmune, ADC Therapeutics, and MedLink Neurology. F. Brilot has received research funding from the Star Scientific Foundation, The Trish Multiple Sclerosis Research Foundation, Multiple Sclerosis Research Australia, and the National Health Medical Research Council (Australia). R.C. Dale has received research funding from the Star Scientific Foundation, The Trish Multiple Sclerosis Research Foundation, Multiple Sclerosis Research Australia, the Petre Foundation, and the National Health Medical Research Council (Australia). Professor Dale has received honoraria from Biogen Idec as an invited speaker. S. Ramanathan has received research funding from the National Health and Medical Research Council (Australia), the Petre Foundation (Australia), the Brain Foundation (Australia), and the University of Sydney. She serves as a consultant on an advisory board for UCB and has been an invited speaker for Biogen and Excemed. H. Butzkueven has received an NHMRC Practitioner Fellowship; serves on advisory boards for Novartis, Biogen, and Merck; is a consultant for the MS Brain Health Steering Committee (Oxford Health Policy Forum); has received research grants from Biogen, Novartis, and Roche; and has received lecture fees from Biogen and Merck. C.L. Fraser reports no disclosures. V.S.C. Fung receives a salary from NSW Health; has received unrestricted research grants from the Michael J. Fox Foundation, AbbVie, and Merz; is on advisory boards and/or has received travel grants from AbbVie, Allergan, Cavion, Ipsen, Merz, Praxis, Seqirus, Stada, Teva, and UCB; and receives royalties from Health Press Ltd. A.P.D. Henderson and M.C. Kiernan report no disclosures. J. Lechner-Scott has accepted travel compensation from Novartis, Biogen, Roche, and Merck. Her institution receives the honoraria for talks and advisory board commitment and research grants from Biogen, Merck, Roche, Teva, and Novartis. M.P. Marriott has received travel sponsorship, honoraria, trial payments, and research and clinical support from Bayer-Schering Pharma, Biogen Idec, BioCSL, Genzyme, Novartis, and Sanofi-Aventis Genzyme. P. McCombe, M. Monif, J.D.E. Parratt, P.W. Rowe, N. Shuey, M. Slee, I. Sutton, E. Tantsis, and B. Trewin report no disclosures. A.V.d. Walt has received travel support and served on advisory boards for Novartis, Biogen, Merck Serono, Roche, and Teva. She receives grant support from the National Health and Medical Research Council of Australia and MS Research Australia. O. White and C. Yiannikas report no disclosures. Go to Neurology.org/NN for full disclosures.
References
- 1.Schluesener HJ, Sobel RA, Linington C, Weiner HL. A monoclonal antibody against a myelin oligodendrocyte glycoprotein induces relapses and demyelination in central nervous system autoimmune disease. J Immunol 1987;139:4016–4021. [PubMed] [Google Scholar]
- 2.Brunner C, Lassmann H, Waehneldt TV, Matthieu JM, Linington C. Differential ultrastructural localization of myelin basic protein, myelin/oligodendroglial glycoprotein, and 2',3'-cyclic nucleotide 3'-phosphodiesterase in the CNS of adult rats. J Neurochem 1989;52:296–304. [DOI] [PubMed] [Google Scholar]
- 3.Ramanathan S, Dale RC, Brilot F. Anti-MOG antibody: the history, clinical phenotype, and pathogenicity of a serum biomarker for demyelination. Autoimmun Rev 2016;15:307–324. [DOI] [PubMed] [Google Scholar]
- 4.Reindl M, Waters P. Myelin oligodendrocyte glycoprotein antibodies in neurological disease. Nat Rev Neurol 2019;15:89–102. [DOI] [PubMed] [Google Scholar]
- 5.Tea F, Lopez JA, Ramanathan S, et al. Characterization of the human myelin oligodendrocyte glycoprotein antibody response in demyelination. Acta Neuropathol Commun 2019;7:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reindl M, Schanda K, Woodhall M, et al. International multicenter examination of MOG antibody assays. Neurol Neuroimmunol Neuroinflamm 2020;7:e674. doi: 10.1212/NXI.0000000000000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramanathan S, Reddel SW, Henderson A, et al. Antibodies to myelin oligodendrocyte glycoprotein in bilateral and recurrent optic neuritis. Neurol Neuroimmunol Neuroinflamm 2014;1:e40. doi: 10.1212/NXI.0000000000000640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jurynczyk M, Messina S, Woodhall MR, et al. Clinical presentation and prognosis in MOG- antibody disease: a UK study. Brain 2017;140:3128–3138. [DOI] [PubMed] [Google Scholar]
- 9.Ramanathan S, Mohammad S, Tantsis E, et al. Clinical course, therapeutic responses and outcomes in relapsing MOG antibody-associated demyelination. J Neurol Neurosurg Psychiatry 2018;89:127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018;17:162–173. [DOI] [PubMed] [Google Scholar]
- 11.Irani SR, Alexander S, Waters P, et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan's syndrome and acquired neuromyotonia. Brain 2010;133:2734–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willison HJ, Veitch J, Swan AV, et al. Inter-laboratory validation of an ELISA for the determination of serum anti-ganglioside antibodies. Eur J Neurol 1999;6:71–77. [DOI] [PubMed] [Google Scholar]
- 13.Clark AJ, Kaller MS, Galino J, Willison HJ, Rinaldi S, Bennett DLH. Co-cultures with stem cell-derived human sensory neurons reveal regulators of peripheral myelination. Brain 2017;140:898–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson R, Menassa DA, Davies AJ, et al. Seronegative antibody-mediated neurology after immune checkpoint inhibitors. Ann Clin Transl Neurol 2018;5:640–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tantsis EM, Prelog K, Alper G, et al. Magnetic resonance imaging in enterovirus-71, myelin oligodendrocyte glycoprotein antibody, aquaporin-4 antibody, and multiple sclerosis- associated myelitis in children. Dev Med Child Neurol 2019;61:1108–1116. [DOI] [PubMed] [Google Scholar]
- 16.Sundaram S, Nair SS, Jaganmohan D, Unnikrishnan G, Nair M. Relapsing lumbosacral myeloradiculitis: an unusual presentation of MOG antibody disease. Mult Scler 2020;26:509–511. [DOI] [PubMed] [Google Scholar]
- 17.Vazquez Do Campo R, Stephens A, Marin Collazo IV, Rubin DI. MOG antibodies in combined central and peripheral demyelination syndromes. Neurol Neuroimmunol Neuroinflamm 2018;5:e503. doi: 10.1212/NXI.0000000000000503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamm C, Zettl UK. Autoimmune disorders affecting both the central and peripheral nervous system. Autoimmun Rev 2012;11:196–202. [DOI] [PubMed] [Google Scholar]
- 19.Pagany M, Jagodic M, Schubart A, et al. Myelin oligodendrocyte glycoprotein is expressed in the peripheral nervous system of rodents and primates. Neurosci Lett 2003;350:165–168. [DOI] [PubMed] [Google Scholar]
- 20.von Budingen HC, Mei F, Greenfield A, et al. The myelin oligodendrocyte glycoprotein directly binds nerve growth factor to modulate central axon circuitry. J Cell Biol 2015;210:891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayer MC, Breithaupt C, Reindl M, et al. Distinction and temporal stability of conformational epitopes on myelin oligodendrocyte glycoprotein recognized by patients with different inflammatory central nervous system diseases. J Immunol 2013;191:3594–3604. [DOI] [PubMed] [Google Scholar]
- 22.Sechi E, Krecke KN, Pittock SJ, et al. Frequency and characteristics of MRI-negative myelitis associated with MOG autoantibodies. Mult Scler 2020:doi: 10.1177/1352458520907900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Titulaer MJ, Hoftberger R, Iizuka T, et al. Overlapping demyelinating syndromes and anti-N- methyl-D-aspartate receptor encephalitis. Ann Neurol 2014;75:411–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez-Hernandez E, Guasp M, Garcia-Serra A, et al. Clinical significance of anti-NMDAR concurrent with glial or neuronal surface antibodies. Neurology 2020;94:e2302–e2310. [DOI] [PubMed] [Google Scholar]
- 25.Leite MI, Coutinho E, Lana-Peixoto M, et al. Myasthenia gravis and neuromyelitis optica spectrum disorder: a multicenter study of 16 patients. Neurology 2012;78:1601–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fehmi J, Scherer SS, Willison HJ, Rinaldi S. Nodes, paranodes and neuropathies. J Neurol Neurosurg Psychiatry 2018;89:61–71. [DOI] [PubMed] [Google Scholar]
- 27.Kawamura N, Yamasaki R, Yonekawa T, et al. Anti-neurofascin antibody in patients with combined central and peripheral demyelination. Neurology 2013;81:714–722. [DOI] [PubMed] [Google Scholar]
- 28.Lang B, Makuch M, Moloney T, et al. Intracellular and non-neuronal targets of voltage-gated potassium channel complex antibodies. J Neurol Neurosurg Psychiatry 2017;88:353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu M, Bennett DLH, Querol LA, et al. Pain and the immune system: emerging concepts of IgG-mediated autoimmune pain and immunotherapies. J Neurol Neurosurg Psychiatry 2020;91:177–188. [DOI] [PubMed] [Google Scholar]
- 30.Gadoth A, Pittock SJ, Dubey D, et al. Expanded phenotypes and outcomes among 256 LGI1/CASPR2-IgG-positive patients. Ann Neurol 2017;82:79–92. [DOI] [PubMed] [Google Scholar]
- 31.Klein CJ, Lennon VA, Aston PA, McKeon A, Pittock SJ. Chronic pain as a manifestation of potassium channel-complex autoimmunity. Neurology 2012;79:1136–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willison HJ, Yuki N. Peripheral neuropathies and anti-glycolipid antibodies. Brain 2002;125:2591–2625. [DOI] [PubMed] [Google Scholar]
- 33.Devigili G, Rinaldo S, Lombardi R, et al. Diagnostic criteria for small fibre neuropathy in clinical practice and research. Brain 2019;142:3728–3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Qualified investigators may request access to anonymized data relevant to this study, pending appropriate institutional review board approvals.