Case presentation
A 38-year-old woman with MS receiving natalizumab presented to the neurology clinic with the complaint of a new neurologic symptom.
Clinical course
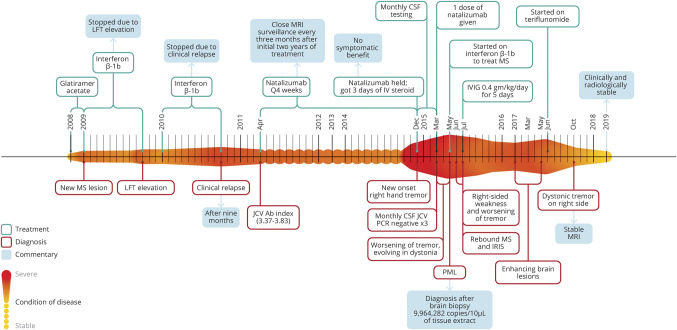
The patient had a 6-year history of clinically stable MS, albeit exhibiting radiographic progression despite strict adherence to daily subcutaneous glatiramer acetate (GA). Furthermore, on switching first to weekly IM interferon beta (IFN-β)1a, she experienced clinical relapses and subsequently developed recalcitrant transaminitis while using three times weekly, subcutaneous IFN-β 1b (figure 1).
Figure 1. Chronological heat map.
In this figure, we detail the condition of the patient over time. The longitudinal axis (left to right) depicts the condition of disease, where the smaller amplitude and lighter color indicates greater stability of MS. Alternately, the expanded amplitude of the colored heat map (above and below the horizontal linear axis over time) designates increased disease activity (whether on a clinical or paraclinical basis) or complications of the treatment of disease (e.g., PML). Four other fields of information are added either above or below the heat map and include information about treatments, diagnoses, commentaries adding contextual perspectives, and results from specific test assessments from each most relevant period of clinical decision-making. Each field is consistently color coded throughout as defined in the figure legend. IRIS = immune reconstitution inflammatory syndrome; IVIG = IV immunoglobulin; JCV Ab = John Cunningham Polyomavirus antibody; LFT = liver function test; PML = progressive multifocal leukoencephalopathy.
Owing to continued disease activity and side effects from GA and IFN, she was switched to monthly IV natalizumab despite John Cunningham Polyomavirus antibody positivity (JCV Ab+) with an Ab index 3.37–3.83. She remained clinically and radiographically stable from the inception of natalizumab with surveillance MRIs performed quarterly. However, after her 47th natalizumab infusion, she developed a coarse action and position tremor involving her right distal upper extremity. Brain MRI revealed a nonenhancing left thalamic T2 hyperintensity (figure 2, A and B).
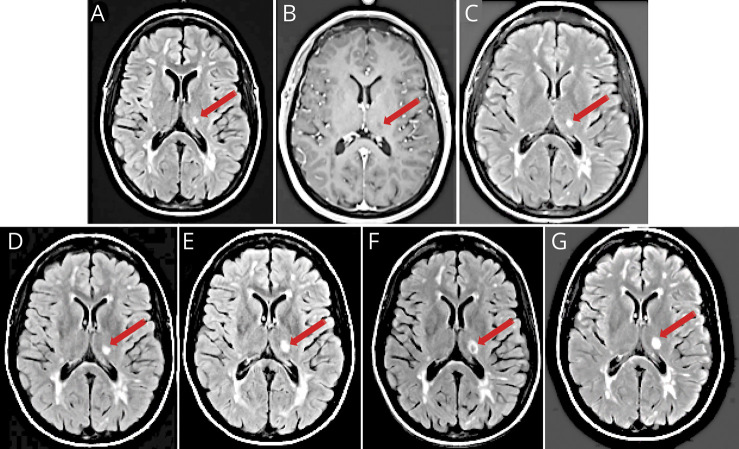
Figure 2. Evolution of the left thalamic lesion.
In (A), an axial T2 fluid-attenuated inversion recovery (FLAIR) image demonstrates a new hyperintense lesion localized to the left thalamus (red arrows), with periventricular and juxtacortical lesions typical of MS. In (B), an axial T1 postcontrast scan shows hypointensity of the left thalamic lesion without contrast enhancement. In (C–G), we present axial FLAIR images performed serially at 3, 7, 11, 16, and 20 weeks, respectively, after the inception of the right upper extremity tremor. Over this period, the lesion has slightly increased in size, and in (F), the lesion takes on a ring configuration with central hypointensity (red arrows). This lesion failed to exhibit any evidence of contrast enhancement over the period of surveillance imaging.
Natalizumab was suspended while her physicians investigated the underlying etiology for her new clinical symptom and corresponding imaging abnormality. CSF analyses was noninflammatory and JCV DNA was undetectable by PCR. The initial diagnostic supposition was that the lesion was potentially on the basis of inflammatory demyelination, even in the absence of gadolinium (Gd) enhancement. The patient was treated empirically with 1 g IV methylprednisolone daily for 3 doses without benefit.
Given concerns of her history of protracted natalizumab treatment and a high JCV antibody index, monthly serial MRIs were obtained (figure 2, C–G), which showed lesion progression, although JCV DNA was not detectable on serial CSF PCR analyses (4 in total).
Over time, the patient's tremor gradually worsened despite treatment with clonazepam and carbamazepine. Botulinum injection in combination with levetiracetam did mitigate the amplitude and frequency characteristics of the tremor such that the patient was able to recognize functional improvements. Notwithstanding such benefits on her tremor, she later exhibited features of dystonia that further interfered with her use of the right arm. Despite the unremarkable CSF analyses, clinical suspicion for progressive multifocal leukoencephalopathy (PML) remained high, although natalizumab was redosed once at 3 months to reduce the risk for potential rebound disease activity.1
Differential diagnosis
The patient's right upper extremity tremor corresponded with a new lesion that localized to the left ventral lateral posterior aspect of the thalamic nuclear complex—the target for the projections emanating from the right dentate nucleus of the cerebellum—which then projects to the left precentral gyrus, thereby providing right cerebellar influence on limb movements in the right arm.
At the time of presentation, the possibility of a new MS demyelinating lesion was considered high in the differential diagnosis. Demyelinating lesions due to MS are commonly localized in white matter (especially when using conventional MRI sequences), but focal lesions in the thalamus are also well documented.2 Typically, however, new demyelinating symptomatic lesions exhibit enhancement, and the absence of such in our patient compelled us to broaden the diagnostic differential.
Clinical manifestations of PML in MS are diverse and depend on the areas of the brain involved. Typically, brain MRI shows a T2 hyperintense/T1 hypointense lesion(s) with sharp margins, commonly involving subcortical white matter including extension into the U-fibers, although cortical involvement is often reported. Contrast enhancement is exceptional in HIV-associated PML, yet it is seen in about 40% of MS PML cases.3 Despite high sensitivity (>95%) of CSF PCR for JCV in PML, JCV has infrequently remained undetectable in the CSF in cases where PML was confirmed by brain biopsy.4
Final diagnosis
As a precise diagnosis could not be made from the imaging and laboratory data, a biopsy of the lesion was performed 5 months after presentation. Histology demonstrated bizarre astrocytes, abundant CD68-positive macrophages, and nuclear changes suggestive of viral cytopathic effects. JCV DNA was detected at 9,964,282 copies/10 μL of extract confirming the diagnosis of PML.
Discussion
PML is a known complication of treatment with natalizumab in patients with MS with a history of previous exposure to JCV.5 Furthermore, risk of PML in patients who test positive for serum JCV Abs exponentially increases with a history of previous immunosuppression (e.g., cyclophosphamide, mitoxantrone, etc) and longer duration of continuous natalizumab therapy (e.g., >24 months).6 This does not include pulse steroids for treating relapses. This risk is further stratified by the quantitative index where a JCV Ab index >1.5 in patients with MS treated with natalizumab at 4-week intervals is associated with high risk of PML,7 although data suggest that extended interval dosing confers a lower risk of PML without compromising efficacy.8–10
Diagnostic confirmation of PML can often represent a formidable challenge, particularly given the heterogeneity in imaging characteristics. The conspicuity of PML lesion morphology in the context of HIV/AIDS can be distinctive from lesions evolving as a consequence of protracted immunosuppression in patients with autoimmune disorders.3 In HIV, PML lesions are typically hypointense and, on occasion, can exhibit enhancement, whereas such lesions in the context of patients with MS treated with natalizumab exhibit enhancement in 30%–40% of cases.3 Together, clinical presentation, radiologic findings, and presence of CSF JCV PCR DNA play important roles in PML diagnosis, although tissue biopsy also has a prominent and necessary role when other assessments fails to confirm the diagnosis.
Our case report presented herein illustrates that in spite of highly sensitive CSF assays for JCV DNA, such findings cannot adequately counter against the diagnosis of PML, particularly when a clinical syndrome in the setting of an atypical CNS lesion remains suspicious. In situations such as this, tissue biopsy is imperative and represents the gold standard.
Treatment of natalizumab-associated PML is complex. Plasmapheresis is an option, but one needs to consider the effect that the rapid removal of natalizumab may have in accelerating the development of the immune reconstitution inflammatory syndrome (IRIS). This is especially important because the clinical outcome can be worse with early PML-IRIS compared with late PML-IRIS.11 Corticosteroids may be used for PML-IRIS and have demonstrated benefit when used judiciously, although no randomized control studies are available.
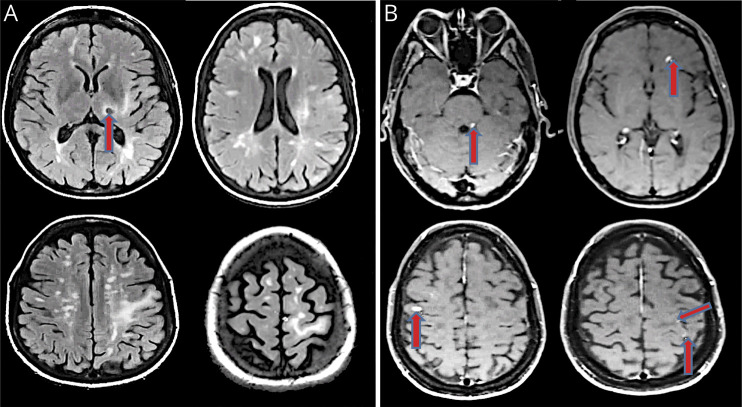
The patient experienced clinical worsening with increased right sided weakness and dystonic tremor a few weeks after the biopsy. Postcontrast MRI performed at this time (27 weeks after symptom onset) showed nodular enhancement in the right frontal and parietal subcortical white matter, left frontal periventricular and precentral subcortical white matter, and in the left dorsolateral pons in the region of the left superior cerebellar peduncle (figure 3, small arrow heads). Steroids were not used at this time because she had not tolerated them well on earlier administration. Instead, the patient was treated with 0.4 g/kg/d IV immunoglobulin for a total of 5 days, after which she exhibited further improvement in her symptoms.
Figure 3. Interval neuroradiographic progression.
In (A), we present axial fluid-attenuated inversion recovery images revealing disseminated lesions characteristic for inflammatory demyelination in the periventribular zones, in the centrum semiovale, in the corona radiata, and in the cortex and juxtacortical zones, in addition to the previously identified left thalamic lesion, here exhibiting a ring configuration with central hypointensity (red arrow). In (B), we present axial T1 postcontrast images showing various nodular enhancing lesions in left dorsolateral pons and bilateral frontal lobes and patchy punctate enhancements in left frontal and parietal white matter (red arrows).
Unfortunately, there are limited data in the literature regarding disease-modifying therapy (DMT) options in such patients who have been diagnosed with natalizumab-associated PML. However, one small case series suggests that INF-β and GA, as well as dimethyl fumarate and fingolimod, seem to be safe in post-PML patients, although long-term safety data are lacking.12,13 There are a few case reports that describe utilization of rituximab in post-PML patients.14
In the case reported herein, there was significant concern that use of an immunosuppressive DMT might provoke recrudescence of PML, thereby compromising neurologic recovery. As such, the patient was started on immunomodulatory therapy, in spite of its previous incomplete effectiveness for treating her MS. Specifically, IFN-β 1b was selected over daily subcutaneous GA because of presumed more rapid onset of action with IFN therapy and the patient preference regarding tolerability with fewer injections.
The patient tolerated IFN-β 1b without side effects or radiologic activity for 19 months. However, she then developed a left parietal lobe T2 hyperintensity that demonstrated incomplete peripheral Gd-enhancement, most compatible with an active MS lesion. Repeat MRIs after 2 and 4 months showed additional new enhancing lesions, although the previously identified left thalamic lesion and the subcortical white matter changes extending from the left thalamus to prefrontal cortex seemed less conspicuous. IFN-β neutralizing antibodies were not detected.
Once clinical stabilization was achieved, attention then shifted to the intensification of the patient's MS DMT, with the goal to use an agent with properties capable of achieving a durable remission while also conferring a low risk of PML recrudescence. For these reasons, once-daily oral teriflunomide was identified as an acceptable next step in the patient's treatment course (figure 1). This agent targets the biosynthetic enzyme, dihydro-orotate dehydrogenase, thereby inhibiting the de novo pyrimidine synthesis pathway (principally used by rapidly dividing cells such as T and B lymphocytes and for virion replication).
To date, our patient has remained clinically and radiologically stable for more than 2 years after the treatment transition to teriflunomide. Despite the achievement of this period of remission in our patient's disease activity, careful and systematic clinical and paraclinical surveillance investigations will be imperative. Until evidence-based data sufficient to provide treatment guidelines become available, the management of MS after the development and survival of PML must be formulated on a case-by-case basis.
Acknowledgment
The authors thank medical illustrators Mr. Jason Ooi and Dr. Matthew Parsons for their creation of the chronological heat map (figure 1).
Appendix. Authors
Footnotes
See page e930
Study funding
The National Multiple Sclerosis Society Case Conference Proceedings is a peer review publishing initiative from the monthly “Diagnostic and Treatment Challenges in MS and Neuroimmunology Webinars”; sponsored by the National Multiple Sclerosis Society Fellowship Training Program.
Disclosure
N. Anadani: clinical fellowship funded by National MS Society. M. Hyland: research support from Biogen, PCORI. R.A. Cruz: clinical fellowship funded by National MS Society. R. Lisak serves as a member of the Editorial Board of Clinical Neuropharmacology; he serves on the Medical Advisory Committee of the Myasthenia Gravis Foundation of America and on its Grants Review Committee, on the Clinical Advisory Committee of the GBS/CIDP Foundation, International and its Research/Grants Committee and its Committee on Centers of Excellence, the Board of Trustees of the Michigan Chapter of the National Multiple Sclerosis Society and is Health Care Advisory Committee and on the Board of Trustees of the DMC Foundation; he has served as a consultant for Argenx, Novartis, Janssen, GLG Consulting, Guidepoint Consulting, Third Bridge Consulting, and Globe Life Sciences; he chaired the adjudication committee for a medDay Pharmaceutical clinical trial; he receives research funding (with salary support paid directly to Wayne State University) from NIH, Argnex, UCB Ra Pharmaceuticals and Alexion; and he has received royalties from Wiley-Blackwell (International Neurology) and Oxford University Press (Neuroimmunology). K. Costello has nothing to disclose. E.O. Major: PML Adjudication Committees for Takeda/Millennium Pharma; Roche/Genentech, GSK, and Shire; consult PolpharmaBiologics, S.A. Y. Jassam: past member of the board of governors of the consortium of multiple sclerosis center (CMSC): 2015–2019. BIOGEN post-ECTRIMS advisory board (no consultation fees accepted); current contracted research sponsorship relations: present support: co-investigator: Roche Protocol #WA21493/ACT44 (2017–2021), Roche Protocol #WA21093 (2017–2022), Roche Protocol #WA25046 (2017–2021), Roche Protocol #NB40900 (formerly Chugai Pharmaceutical Protocol #SA-309JG) (2017–2021), MedImmune LLC Protocol #CDIA-MEDI-551-1155 (2017–2020), Novartic Protocol #CFTY720D2403 (2017–2020), Genzyme Protocol #LPS13649 (2017–2020), MedDay Protocol #MD1003CT2016-01MS-SPI2 (2017–2020), Mallinckrodt Protocol #MNK14274069 (2017–2020), Actelion Protocol #AC-058B302 (2017–2020), Alexion Pharmaceuticals Protocol #ECU-NMO-302 (2017–2020), Novartis Protocol #COMB157G2399 (2018–2025), TG Therapeutics Protocol #TG1101-RMS303 (2020–2023), Alexion Pharmaceuticals Protocol #ALXN1210-NMO-307 (2020–2024), Roche Protocol #WA40404 (2020–2028); past support: primary investigator: FLUENT study, Novartis pharmaceuticals; co-investigator: Teva Protocol #MS-LAQ-301E (May 2010–2018); Teva Pharmaceuticals USA Protocol #TV5600-CNS-20006015-2017, Novartis Protocol #CFTY720D2312 (2017–2018), Genentech Protocol #MN30035 (2017–2019), Novartis Protocol #COMB157G2301 (2017–2019), Alexion Pharmaceuticals Protocol #ECU-NMO-301 (2017–2020), TG Therapeutics Protocol #TG1101-RMS301 (2017–2020). E. Meltzer served as a consultant for Genzyme and received honoraria from Novartis. T.C. Varkey and M.S. Parsons have nothing to disclose. A.D. Goodman received personal compensation for consulting from Teva and Adamas; received research support from Biogen, Genentech-Roche, Sanofi-Genzyme, and Teva. Unrelated to the current work, J.S. Graves over the past year has grant/contract research support from the National MS Society, Biogen, and Octave Biosciences; she serves on a steering committee for a trial supported by Novartis; she has received honoraria for a non-promotional, educational activity for Sanofi-Genzyme; she has received speaker fees from Alexion and BMS and served on an advisory board for Genentech. S. Newsome has received consultant fees for scientific advisory boards from Biogen, Genentech, Celgene, EMD Serono, Novartis, Greenwich Biosciences, is an advisor for Autobahn Therapeutics and BioIncept, a clinical adjudication committee member for a MedDay Pharmaceuticals clinical trial and has received research funding (paid directly to institution) from Biogen, Novartis, Genentech, National MS Society, Department of Defense, and Patient Centered Outcomes Institute. S.S. Zamvil is Deputy Editor of Neurology: Neuroimmunology & Neuroinflammation and is an Associate Editor for Frontiers in Immunology and Frontiers in Neurology; he serves on the Advisory Committee for the American Congress on Treatment and Research in Multiple Sclerosis (ACTRIMS) and is a standing member of the research grant review committee for the National Multiple Sclerosis Society (NMSS); he has served on the Editorial Board of the Journal of Clinical Investigation, The Journal of Immunology, and The Journal of Neurological Sciences, and has been a charter member of the grant review committee for the NIH Clinical Neuroimmunology and Brain Tumors (CNBT); he has served, or serves, as a consultant and received honoraria from Alexion, Biogen-Idec, EMD-Serono, Genzyme, Novartis, Roche/Genentech, and Teva Pharmaceuticals Inc., and has served on Data Safety Monitoring Boards for Lilly, BioMS, Teva and Opexa Therapeutics; currently, S.S. Zamvil receives research grant support from the NIH, NMSS, Weill Institute, Race to Erase MS and the Maisin Foundation. E.M. Frohman has received speaker and consulting fees from Novartis, Genzyme, Biogen and Janssen. T.C. Frohman has received consulting honorarium from Genzyme. Go to Neurology.org/NN for full disclosures.
References
- 1.Prosperini L, Kinkel RP, Miravalle AA, Iaffaldano P, Fantaccini S. Post-natalizumab disease reactivation in multiple sclerosis: systematic review and meta-analysis. Ther Adv Neurol Disord Epub 2019 Mar 29. [DOI] [PMC free article] [PubMed]
- 2.Minagar A, Barnett MH, Benedict R, et al. The thalamus and multiple sclerosis Modern views on pathologic, imaging, and clinical aspects. Neurology 2013;80:210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yousry TA, Pelletier D, Cadavid D, et al. Magnetic resonance imaging pattern in natalizumab‐associated progressive multifocal leucoencephalopathy, Ann Neurol 2012;72:779–787. [DOI] [PubMed] [Google Scholar]
- 4.Berger J, Aksamit AJ, Clifford DB, et al. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology 2013;80:1430–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloomgren G, Richman S, Hotermans C, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med 2012;366:1870–1880. [DOI] [PubMed] [Google Scholar]
- 6.Zhovtis Ryerson L, Foley J, Chang IH, et al. Risk of natalizumab-associated PML in patients with MS is reduced with extended interval dosing. Neurology 2019;93;e1452–e1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho PR, Koendgen H, Campbell N, Haddock B, Richman S, Chang I. Risk of natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: a retrospective analysis of data from four clinical studies. Lancet Neurol 2017;16:925–933. [DOI] [PubMed] [Google Scholar]
- 8.Zhovtis Ryerson L, Frohman TC, Foley J, et al. Extended interval dosing of natalizumab in multiple sclerosis. J Neurol Neurosurg Psychiatry 2016;87:885–889. 10.1136/jnnp-2015-312940. [DOI] [PubMed] [Google Scholar]
- 9.Clerico M, De Mercanti SF, Signori A, et al. Extending the interval of natalizumab dosing: is efficacy preserved? Neurotherapeutics 2020;17:200–207. 10.1007/s13311-019-00776-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryerson LZ, Foley J, Chang I, et al. Risk of natalizumab-associated PML in patients with MS is reduced with extended interval dosing. Neurology 2019;93:e1452–e1462. 10.1212/WNL.0000000000008243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan K, Roda R, Ostrow L, McArthur J, Nath A. PML-IRIS in patients with HIV infection. Clinical manifestations and treatment with steroids. Neurology 2009;72:1458–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maillart E, Vidal JS, Brassat D, et al. Natalizumab-PML survivors with subsequent MS treatment: clinico-radiologic outcome. Neurol Neuroimmunol Neuroinflamm 2017;4:e346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maillart E, Louapre C, Lubetzki C, Papeix C. Fingolimod to treat severe multiple sclerosis after natalizumab-associated progressive multifocal leukoencephalopathy: a valid option? Mult Scler 2014;20:505–509. [DOI] [PubMed] [Google Scholar]
- 14.Hoepner R, Faissner S, Ellrichmann G, Schneider R, Gold R. Rituximab post progressive multifocal leukoencephalopathy: a feasible therapeutic option in selected cases. Ther Adv Neurol Disord 2014;7:289–291. [DOI] [PMC free article] [PubMed] [Google Scholar]