Abstract
Objectives
Obstructive sleep apnea (OSA) is a common sleep disorder that has several health hazards, including cognitive dysfunction. Studies have thus far primarily focussed on the prevalence of cognitive impairment in patients diagnosed with OSA at sleep clinics. The present study aims to investigate the prevalence of OSA at an outpatient memory clinic.
Methods
A dataset of patients who visited our memory clinic in the period from June 2015 to September 2019 was retrospectively examined for the presence of OSA. The primary outcome measure was the prevalence of OSA, subdivided into three cognitive syndrome diagnosis groups: subjective cognitive complaints (SCC), mild cognitive impairment and dementia. Secondary outcome measures included age, education level, body mass index, substance use, depression and OSA criteria.
Results
Of the 885 patients included in this study, 153 patients had already been or were diagnosed with OSA (17.3%). The percentage of OSA in the SCC group was significantly higher compared with the dementia group (26.7% vs 8.0%; OR 3.83 [95%CI 2.43‐5.99]). Age differed significantly between the SCC group and the dementia group: 63.5 vs 71.5 years (7.6 ± 1.810; P < .001). Higher education level was associated with a lower prevalence of dementia compared to SCC (OR 0.068[95%CI 0.008‐0.588]). Severity parameters of OSA did not show significant differences across the various cognitive syndrome diagnosis groups.
Conclusions
Prevalence of OSA at our outpatient memory clinic is generally high. Especially in patients with SCC. We would therefore advocate screening for OSA at memory clinics.
Keywords: cognition, OSA, prevalence, subjective cognitive complaints
1. INTRODUCTION
Obstructive sleep apnea (OSA) is a common sleep disorder. The prevalence varies from 9.0 to 38.0% in the general population and up to 90.0% in elderly males. 1 OSA is a condition in which the upper airway partly or completely collapses, thereby impeding effective respiration. These upper airway collapses occur multiple times during sleep and last for several seconds, with marked oxygen desaturations and arousals from sleep as a consequence. 2 , 3 OSA is a worldwide disease whose prevalence is increasing as obesity rates increase. 4 The role of obesity varies in different ethnic groups, with Chinese being particularly sensitive to increases in weight.
There are many health hazards related to OSA, such as hypertension, cardiovascular diseases and diabetes. 5 , 6 Previous research also correlates OSA with cognitive impairment but to varying degrees. 7 The interrelation between sleep and neurodegenerative disorders causing dementia is complex 8 : sleep apnea is regarded as a precursor or risk factor for cognitive impairment as well as an accelerator of cognitive impairment involved in disease progression and postulated to play a role in the pathogenesis of neurodegenerative disorders. Many studies show that in patients with OSA, the primarily affected cognitive domains are attention, memory and executive functioning. 3 , 9 , 10 , 11 , 12 Other studies show only slight interference in vigilance and executive functioning, or even no difference in cognitive function at all, between OSA patients and healthy controls. 13 , 14 A recent meta‐analysis found interference in various domains including nonverbal memory, concept formation, psychomotor speed, construction, executive functioning, perception, motor control and performance, attention, speed of processing, working and verbal memory, verbal functioning and verbal reasoning. 2 And, a recent review states that cognitive complaints are not necessarily due to actual cognitive impairment, but can also be a result of OSA. 15 Taken together, current research shows that in OSA patients cognitive function may be affected and to various degrees.
All previously mentioned studies have investigated cognitive impairment in patients primarily seeking medical attention because of sleep disorders. The present study, however, evaluates the prevalence of OSA in patients with cognitive complaints being referred to a memory clinic. This is a distinctly different population. To the best of our knowledge, there has only been one study that evaluated the prevalence of sleep disturbances in patients with mild cognitive impairment (MCI) and dementia. 16 Herein, it was shown that in the MCI group 65.2% of patients had some kind of sleep disturbance (eg, insomnia, sleep disordered breathing, rapid eye movement sleep behavior disorder, restless legs syndrome or excessive daytime sleepiness), whereas in the dementia group the prevalence was in the range of 65.7% up until 90.0% (depending on the nosological diagnosis of dementia). However, this publication did not include patients with subjective cognitive complaints (SCC), a patient group that generally represents a substantial number of the total population at our outpatient memory clinic. 17
Therefore, the present study aims to evaluate the prevalence of OSA in patients that received one of the following three cognitive syndrome diagnoses after visiting our outpatient memory clinic: SCC, MCI or dementia. We hypothesize that OSA is most prevalent in the group of SCC.
2. METHODS
2.1. Participant selection
In this retrospective cohort study, all patients who visited the outpatient memory clinic of Zuyderland Medical Centre Heerlen/Brunssum, The Netherlands in the period from July 2015 to September 2019 were evaluated. Patients were included when they had visited the outpatient memory clinic with either a previous OSA diagnosis or a subsequent OSA diagnosis. An OSA diagnosis was primarily made on the basis of ancillary polysomnography when during history taking either patient or caregiver reported sleep problems suspect of OSA (ie, snoring, apneas, sore throat or headache on awaking and excessive daytime sleepiness). The patients in whom the diagnosis OSA was made due to a visit unrelated to the memory clinic assessment were excluded while doing so could have led to a false correlation. Furthermore, when no cognitive syndrome diagnosis could be made to insufficient test results, patients were also excluded. The study protocol was approved by Medical Ethical Review Committee Zuyderland (GCP‐OSAS, Z201960). The ethics committee approved conduct of the research without explicit consent from the participants.
2.2. Setting
The population at the outpatient memory clinic consists of patients with memory complaints or patients whose relatives have concerns about their cognition. In general, patients are referred by their general practitioner. The evaluation consists of a multidisciplinary clinical evaluation by a neurologist, a geriatrician and a medical psychologist followed by neuropsychological examination and neuroimaging. In conclusion, a cognitive syndrome diagnosis is given and, when possible, an explanation of the likely etiology of the cognitive syndrome (ie, nosological diagnosis). The syndrome diagnoses are; (a) SCC for patients with no objectifiable cognitive deficit; (b) MCI for patients with one of the multiple cognitive deficits but without inference in daily living and (c) dementia for patients with multiple cognitive deficits and a decline relative to the previous level of functioning. 18
When OSA was suspected at the memory clinic, patients were referred to the outpatient sleep clinic of Zuyderland. There, the diagnosis of OSA was made by a physician with specific expertise in sleep medicine (neurologist, pulmonologist or ENT doctor) based on the AASM criteria of 2013 and a polysomnography. This means that the severity of OSA was defined by the apnea/hypopnea index (AHI) using the following cut‐offs; AHI <5/hour is normal, AHI 5‐15/hour is mild OSA, AHI 15‐30/hour is moderate OSA and an AHI > 30/hour is severe OSA. 19 In addition, data about oxygen desaturation index of at least 3% and 4% per hour (ODI3% and ODI4%) and the percentage of sleep with saturation below 90% have been collected for this study.
2.3. Data collection
In the Netherlands, all medical diagnoses are listed in a standardized manner. Using this database, we compiled a list of patients with a diagnosis of both OSA and a cognitive disorder. In addition, all electronic patient files of patients who had visited the memory clinic in the study period were searched manually and data were collected about the syndrome diagnosis given. Of these patients, only age and gender were recorded as baseline characteristics. Next, patients were selected with a combination of OSA and a cognitive disorder. In these patients, the following baseline characteristics were collected: age, gender, education level and body mass index (BMI). Furthermore, data about the consumption of alcohol and use of benzodiazepines, the syndrome diagnosis and the presence of a mood disorder were collected. As mentioned earlier, OSA severity using the AHI, the ODI 3%, the ODI 4% and the percentage of sleep with saturation below 90% were recorded. Finally, data have been collected about the therapy patients used for OSA, by either a mandibular repositioning device, continuous positive airway pressure, position therapy or no therapy. As well as their therapy compliance. All previously mentioned characteristics were collected from electronic patient records of the physician visits and the polysomnography.
2.4. Data analysis
Data analysis was performed using SPSS version 25 (SPSS Inc., Chicago, Illinois). The cohort was divided based on the three recorded cognitive diagnoses (SCC, MCI and dementia). To detect group differences for the accumulated variables in between the three cognitive syndrome diagnoses, the Chi‐square test or the Fisher's exact test were applied for categorical variables where appropriate. For continuous variables, the Kruskal‐Wallis test was used since the data had a nonnormal distribution (tests of normality were performed using the Kolmogorov‐Smirnov and the Shapiro‐Wilk test). When a significant difference was found, the Games‐Howell post hoc test was performed to determine which of the cognitive syndrome diagnoses groups differed significantly. The main analyses (ie, the prevalence of OSA in the various cognitive diagnoses groups) were corrected for age and gender with the one‐way analysis of covariance (ANCOVA) test. Statistical difference was considered significant at P < .05.
3. RESULTS
3.1. Patient characteristics
In total, 897 patient records were evaluated over a 4‐year period. Of these 897 patients, eight patients could not be given a cognitive syndrome diagnosis due to invalid or incomplete test results and in four other patients the diagnosis was uncertain. In the remaining 885 patients, 378 patients were diagnosed with SCC (42.7%) while 181 patients were diagnosed with MCI (20.5%) and 326 patients received the diagnosis of dementia (36.8%).
In total, 153 out of the 885 patients were diagnosed with OSA (17.3%). Around half of the patients already had an OSA diagnosis prior to the memory clinic visit, whereas the other half were diagnosed as a result of diagnostic evaluation at the memory clinic (respectively 73/153 patients [47.7%] vs 80/153 patients [52.3%]). Not all of the 73 patients that received the diagnosis of OSA prior to the outpatient clinic were treated adequately: only 37 patients used therapy daily and in the right manner (50.7%) whereas the other 36 patients did not use their prescribed therapy daily or had stopped completely (49.3%).
Figure 1 describes the percentage of OSA in the three different cognitive diagnosis groups. In patients with SCC 102 patients had OSA (26.7%), in patients with MCI 25 had OSA (13.8%) and in patients with dementia 26 patients had OSA (8.0%). Conversely, in patients diagnosed with OSA, SCC was present in 66.7% (102/153), MCI in 16.3% (25/153) and dementia in 17.0% (26/153). Of the 153 patients with OSA and a cognitive syndrome diagnosis, six patients could not be used for the comparison of the patient characteristics in the three syndrome diagnosis because of the lack of necessary data for analysis. We found no differences in cognitive syndrome diagnoses between the patients diagnosed with OSA prior to memory clinic consultation (with or without adequate treatment) and the ones diagnosed with OSA subsequently.
FIGURE 1.
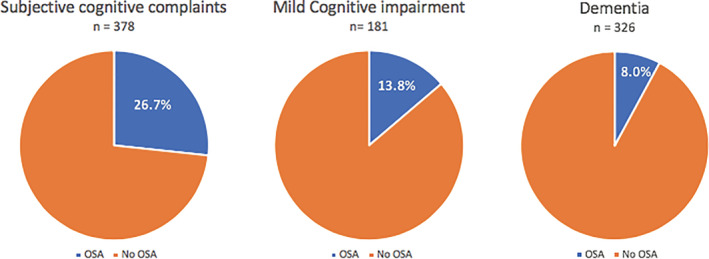
Percentage of obstructive sleep apnea (OSA) by cognitive syndrome diagnoses. This figure shows the prevalence of obstructive sleep apnea in the total population of the memory clinic subdivided into the three cognitive syndrome diagnoses groups: subjective cognitive complaints (26.7%), mild cognitive impairment (13.8%) and dementia (8.0%)
3.2. Comparison of baseline characteristics of the total cohort of the memory clinic population
The total cohort patient characteristics are presented in Table 1. Age differed significantly between the cognitive syndrome groups. On average, the youngest patients were found to have SCC while the oldest patients suffered from dementia (P < .001 for all analyses). Gender did not differ significantly between groups. Using Chi‐square test, the presence of OSA was also significantly different between groups (P < .001). Different subanalyses were performed to determine the difference in the prevalence of OSA between groups with SCC as the reference group. The prevalence of OSA was significantly higher in patients with SCC compared to MCI (OR 2.05[95%CI 1.28‐3.28]). The prevalence of OSA in patients with SCC was also significantly higher compared to dementia (OR 3.83 [95%CI 2.43‐5.99]).
TABLE 1.
Baseline characteristics of total patient cohort by syndrome diagnosis
| Subjective cognitive complaints (n = 378) | Mild cognitive impairment (n = 181) | Dementia (n = 326) | P‐value | ||||
|---|---|---|---|---|---|---|---|
| Gender | |||||||
| Male (%) | 225 | (59.5) | 98 | (54.1) | 168 | (51.5) | .096 |
| Median age, years (interquartile range) | 64 (58‐72) | 74 (65‐79) | 77 (72‐82) | <.001* | |||
| Presence of OSA | |||||||
| Yes (%) | 102 | (27.0) | 25 | (13.8) | 26 | (8.0) | <.001* |
| OSA severity (%) a | |||||||
| Mild | 28 | (7.4) | 7 | (3.8) | 10 | (3.1) | |
| Moderate | 29 | (7.7) | 10 | (5.5) | 4 | (1.2) | |
| Severe | 45 | (11.9) | 8 | (4.4) | 12 | (3.7) | |
Abbreviation: OSA, obstructive sleep apnea.
No statistical analysis has been made for this variable.
Statistically significant at P = .05.
3.3. Characteristics of patients with both OSA and cognitive impairment
Patient characteristics for the patients with both OSA and a cognitive diagnosis are presented in Table 2. For gender, BMI, the consumption of alcohol, use of benzodiazepines and the presence of a mood disorder no statistically significant differences were observed. Age differed significantly between the groups (P < .001): the Games‐Howell test showed that age in the SCC group was 7.6 years younger compared to the dementia group (±1.8; P < .001). The level of education also demonstrated a significant difference (P = .007). Between the groups of SCC and MCI, there was a benefit for patients with higher education, for bachelor's/master's degree compared to primary school (OR 0.167 [95%CI 0.031‐0.887]) and for secondary education compared to primary school (OR 0.306 [95%CI 0.107‐0.871]). For SCC compared to dementia, there was also a benefit when patients had higher education. For bachelor's/master's degree compared to primary school (OR 0.068 [95%CI 0.008‐0.588]) and for secondary education compared to primary school (OR 0.318 [95%CI 0.120‐0.841]). When these patients had a higher education level, the chance of having dementia is smaller than when patients had a lower education level.
TABLE 2.
Patient characteristics of OSA patients by syndrome diagnosis
| Subjective cognitive complaints (n = 98) | Mild cognitive impairment (n = 23) | Dementia (n = 26) | P‐value | ||||
|---|---|---|---|---|---|---|---|
| Gender | |||||||
| Male (%) | 76 | (77.6) | 18 | (78.3) | 23 | (88.4) | .464 |
| Median age, years (interquartile range) | 64 (60‐69) | 67.00 (64‐73) | 72 (68‐75) | <.001* | |||
| Median BMI, kg/m2 (interquartile range) | 30.2 (27.9‐33.7) | 30.70 (26.2‐33.8) | 31.0 (28.2‐33.7) | .803 | |||
| Education level a (%) | |||||||
| No | 0 | (0.00) | 0 | (0.00) | 0 | (0.00) | .007* |
| Primary school | 15 | (15.3) | 9 | (39.1) | 11 | (42.3) | |
| Secondary education | 60 | (61.2) | 11 | (47.8) | 14 | (53.8) | |
| Bachelor/Master's degree | 20 | (20.4) | 1 | (8.7) | 2 | (3.8) | |
| Alcohol use (%) | 61 | (62.2) | 12 | (52.2) | 12 | (46.2) | .281 |
| Benzodiazepine use (%) | 11 | (11.2) | 0 | (0.0) | 2 | (7.7) | .307 |
| Presence of a mood disorder (%) | 21 | (21.4) | 3 | (13.0) | 4 | (15.4) | .668 |
| OSA severity (%) | |||||||
| Mild | 28 | (28.6) | 7 | (30.4) | 10 | (38.5) | .408 |
| Moderate | 29 | (29.6) | 9 | (39.2) | 4 | (15.4) | |
| Severe | 41 | (41.8) | 7 | (30.4) | 12 | (46.1) | |
| Median AHI, per hour (interquartile range) | 25.1 (13.8‐40.0) | 23.6 (13.9‐40.0) | 24.5 (10.4‐42.3) | .981 | |||
| Lowest saturation percentage (interquartile range) | 84 (80‐87) | 84 (80‐88) | 85.00 (83‐88) | .601 | |||
| ODI 3%, per hour (interquartile range) | 21.2 (10.9‐34.7) | 20.4 (12.4‐36.9) | 20.5 (9.4‐38.0) | .814 | |||
| ODI 4%, per hour (interquartile range) b | 8.5 (3.0‐22.8) | 5.0 (3.9‐36.8) | 8.5 (3.8‐22.8) | .993 | |||
| Treatment (%) | |||||||
| None | 17 | (17.4) | 5 | (21.7) | 5 | (19.2) | .168 |
| Ceased treatment | 7 | (7.1) | 3 | (13.0) | 6 | (23.0) | |
| MRD | 21 | (21.4) | 2 | (8.7) | 4 | (15.4) | |
| CPAP | 53 | (54.0) | 12 | (52.2) | 11 | (42.3) | |
| Position therapy | 0 | (0.0) | 1 | (4.4) | 0 | (0.0) | |
Abbreviations: AHI, apnea/hypopnea index; BMI, body mass index; CPAP, continuous positive airway pressure; MRD, mandibular reposition device; ODI, oxygen desaturation index; OSA, obstructive sleep apnea; PSG, polysomnography.
Due to missing data the number of patients may not add up.
To be interpreted with caution because of impaired statistical power (small sample size).
Statistically significant at P = .05.
For the association between OSA severity and type of cognitive disorders, no significant correlation was observed. The specific criteria for OSA diagnosis were evaluated as well. For AHI no differences were found. A one‐way ANCOVA was conducted to determine a difference between the three syndrome diagnoses corrected for age and gender; this demonstrated no significant effect (P = .787). This result should be taken with caution because the data did not show a normal distribution and the group sizes were small (Table 2). The lowest oxygen saturation and the ODI3% were not significantly different in the three groups. For the ODI4%, data were missing due to a change in diagnostic criteria for OSA, which has been integrated at Zuyderland at the end of the year 2017. The data analysis included only 28 patients; the total sample size was therefore too small to make any conclusions. For treatment options, there was no significant correlation between the groups.
4. DISCUSSION
Globally, insufficient sleep is prevalent across various age groups, considered to be a public health epidemic that is often unrecognized, underreported, and that has rather high economic costs. 20 Insufficient sleep leads to the derailment of body systems, leading to increased incidences of cardiovascular morbidity, increased chances of diabetes mellitus, obesity and derailment of cognitive functions.
The present study aimed to estimate the prevalence of OSA at our outpatient memory clinic. Previous studies have shown a 9% to 38% OSA prevalence range in the general population. 1 To the best of our knowledge, there are no prevalence numbers published on OSA at memory clinics. According to the Dutch National Statistics Agency, OSA was expected to be prevalent within the region of our hospital since 52.2% of the population is overweight and 40.3% of the population consumes alcohol daily. 21 , 22 , 23 In the 885 patients examined in this study, we found an OSA prevalence of 17.3%. The percentage of OSA was highest in the patient group with SCC (26.7%), followed by MCI (13.8%) whereas the lowest percentage was found in the dementia group (8.0%). Conversely, we found that the majority of the 153 patients diagnosed with OSA had a cognitive syndrome diagnosis of SCC (66.7%), followed by dementia (17.0%) and MCI (16.3%).
These findings corroborate the correlation between OSA and SCC (see for review Vaessen et al 15 ). Our data did not demonstrate that more severe cases of OSA were present more frequently in the SCC group. Although this result must be interpreted with caution due to relatively small numbers, a possible explanation could be that the severity of OSA in this retrospective cohort study was based only on the AHI score. In the past years, the criteria for OSA have been under dispute. For an adequate severity classification of OSA, the ODI4% and percentage of sleep with saturation under 90% may be better parameters to determine the severity of OSA. At our hospital, such polysomnographic data are being collected since late 2017, and therefore, these parameters were not known for most of our patients. We were only able to collect the ODI4% for a small group of 28 patients, which was not enough for a proper statistical analysis. Studies have shown that the sleep of patients with dementia changes relative to elderly people without dementia. They experience more fragmented sleep which can lead to napping during the daytime. And during the time they spent asleep, the stages of sleep are lighter. 24 , 25 We evaluated several patient characteristics for an increased risk of OSA in patients with cognitive impairment. The only statistically significant differences we found were age and education level. Age is likely explained by the fact that dementia is more prevalent in the group of elderly patients (mostly above the age of 60). 26 A lower education level is associated with a higher chance of developing dementia than in people with a higher education level. 27
The present study does have several limitations. Because of the retrospective nature we had to exclude several patients with missing data, for instance when OSA diagnosis or polysomnography were performed elsewhere. Furthermore, data of OSA patients showed a skewed distribution, resulting in two groups with smaller number of patients (MCI and dementia). This resulted in the mandatory use of a statistical test with a lower proof of evidence. Finally, we collected baseline characteristics for the total cohort (age, gender and the presence of OSA with severity) where we should have collected more detailed characteristics like BMI and education level. This would have given better insight into the risk factors for the total population of our outpatient memory clinic, instead of just the OSA patients. Underreporting of symptoms in patients with cognitive disorders compared to patients with SCC may be liable to bias in ascertaining OSA. But this is unlikely given the fact that snoring and apneas are primarily reported by the bed partner. There could also have been differences in diagnostic work‐up for OSA. Physicians may be less inclined to request ancillary polysomnography in patients with dementia compared to patients with SCC. In our study, however, half of the patients had already been diagnosed with OSAS prior to the memory clinic visit. We found no differences in syndrome diagnoses between these patients and the ones diagnosed with OSA subsequently.
It can be questioned whether patients at memory clinics get a proper sleep evaluation. Because OSA is a relatively prevalent condition and a contributing factor in disorders of cognition, there is a need for a screening instrument. According to the Dutch OSA guideline, the most frequently used screening test in The Netherlands is the Epworth Sleepiness Scale. Another test is the STOP‐BANG questionnaire, which is validated for both diagnostic screening and therapeutic evaluation. 28 A recent cross‐sectional study evaluating four screening methods for OSA showed that the STOP‐BANG test had the highest sensitivity for OSA. 29 This test makes an estimation based on the following factors: snoring, tiredness, observed apnea, high blood pressure, BMI, age, neck circumference and male gender. 30 Moreover, it would be of interest to know the neuropsychological test characteristics of OSA patients at memory clinics, to further elucidate the complex relationship between OSA and cognition. Although for our study population cognitive test results are available, the current study did not have the neuropsychological assessment as a primary aim and as mentioned in the Introduction studies have produced conflicting results. We, therefore, abstained from analyzing our neuropsychological data and plan to properly investigate this prospectively, making use of a standardized OSA screening instrument for inclusion.
In conclusion, we found a relatively high prevalence of OSA at our outpatient memory clinic, especially in patients with SCC. We would therefore advocate screening for OSA at memory clinics, preferably by the use of an instrument such as the STOP‐BANG questionnaire. This would allow for prospective analysis and hence a more accurate estimation of OSA prevalence at memory clinics, although local differences are likely to remain. Future research should use not only AHI to evaluate the severity of OSA but also include other parameters such as the ODI4% and percentage sleep with saturation under 90%.
FUNDING
The authors did not receive specific funding for this work.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Conceptualization: Britt Linssen, Esther Bergman, Pim Klarenbeek, Erik Hoff
Data curation: Britt Linssen, Esther Bergman, Erik Hoff
Formal analysis: Britt Linssen
Investigation: Britt Linssen
Methodology: Esther Bergman
Resources: Erik Hoff
Supervision: Pim Klarenbeek, Erik Hoff
Writing ‐ original draft preparation: Britt Linssen
Writing ‐ review and editing: Esther Bergman, Pim Klarenbeek, Erik Hoff
All authors have read and approved the final version of the manuscript.
Erik Hoff had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.
TRANSPARENCY STATEMENT
The corresponding author (Erik Hoff) affirms that this manuscript is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
ACKNOWLEDGMENT
We wish to thank Dr Audrey Merry from the Zuyderland Academy of Zuyderland Medical Centre for her expertise in and assistance with the data analysis.
Linssen B, Bergman E, Klarenbeek P, Hoff E. Prevalence of obstructive sleep apnea at an outpatient memory clinic. Health Sci Rep. 2021;4:e228 10.1002/hsr2.228
DATA AVAILABILITY STATEMENT
Data that support the findings of our study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Ferini‐Strambi L, Lombardi GE, Marelli S, Galbiati A. Neurological deficits in obstructive sleep apnea. Curr Treat Options Neurol. 2017;19(4):16. [DOI] [PubMed] [Google Scholar]
- 2. Stranks EK, Crowe SF. The cognitive effects of obstructive sleep apnea: an updated meta‐analysis. Arch Clin Neuropsychol. 2016;31(2):186‐193. [DOI] [PubMed] [Google Scholar]
- 3. Aloia MS, Arnedt J, Davis JD, Riggs RL, Byrd D. Neuropsychological sequelae of obstructive sleep apnea‐hypopnea syndrome: a critical review. J Int Neuropsychol Soc. 2004;10(5):772‐785. [DOI] [PubMed] [Google Scholar]
- 4. Lim DC, Pack AI. Obstructive sleep apnea: update and future. Annu Rev Med. 2017;68:99‐112. [DOI] [PubMed] [Google Scholar]
- 5. Xu H, Wang H, Guan J, et al. Effects of continuous positive airway pressure on neurocognitive architecture and function in patients with obstructive sleep apnoea: study protocol for a multicentre randomised controlled trial. BMJ Open. 2017;7(5):e014932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Silverberg DS, Oksenberg A, Iaina A. Sleep related breathing disorders are common contributing factors to the production of essential hypertension but are neglected, underdiagnosed and undertreated. Am J Hypertens. 1997;10(12 Pt 1):1319‐1325. [DOI] [PubMed] [Google Scholar]
- 7. Cohen‐Zion M, Stepnowsky C, Marler ST, Kripke DF, ANcoli‐Israel S. Changes in cognitive function associated with sleep disordered breathing in older people. J Am Geriatr Soc. 2001;49(12):1622‐1627. [DOI] [PubMed] [Google Scholar]
- 8. Malhotra RK. Neurodegenerative disorders and sleep. Sleep Med Clin. 2018;13(1):63‐70. [DOI] [PubMed] [Google Scholar]
- 9. Gagnon K, Baril AA, Gagnon JF, et al. Cognitive impairment in obstructive sleep apnea. Pathol Biol (Paris). 2014;62(5):233‐240. [DOI] [PubMed] [Google Scholar]
- 10. Davies CR, Harrington JJ. Impact of obstructive sleep apnea on neurocognitive function and impact of continuous positive air pressure. Sleep Med Clin. 2016;11(3):287‐298. [DOI] [PubMed] [Google Scholar]
- 11. Bawden FC, Oliveira CA, Caramelli P. Impact of obstructive sleep apnea on cognitive performance. Arq Neuropsiquiatr. 2011;69:585‐589. [DOI] [PubMed] [Google Scholar]
- 12. Quan SF, Chan CS, Dement WC, et al. The association between obstructive sleep apnea and neurocognitive performance – the apnea positive pressure long‐term efficacy study (APPLES). Sleep. 2011;34(3):303‐314B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beebe DW, Groesz L, Welles C, Nichols A, McGee K. The neuropsychological effects of obstructive sleep apnea: a meta‐analysis of norm‐referenced and case controlled data. Sleep. 2003;26(3):298‐307. [DOI] [PubMed] [Google Scholar]
- 14. Quan SF, Wright R, Baldwin CM, et al. Obstructive sleep apnea‐hypoapnea and neurocognitive functioning in the sleep heart health study. Sleep Med. 2006;7(6):498‐507. [DOI] [PubMed] [Google Scholar]
- 15. Vaessen TJ, Overeem S, Sitskoorn MM. Cognitive complaints in obstructive sleep apnea. Sleep Med Rev. 2015;19:51‐58. [DOI] [PubMed] [Google Scholar]
- 16. Guarnieri B, Adorni F, Musicco M, et al. Prevalence of sleep disturbances in mild cognitive impairment and dementing disorders: a multicenter Italian clinical cross‐sectional study on 431 patients. Dement Geriatr Cogn Disord. 2012;33(1):50‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Van Kempen‐Senden MW, Hoff EI. Memory clinic in a shrinking region. Int J Geriatr Psychiatry. 2014;29(3):328–330. [DOI] [PubMed] [Google Scholar]
- 18. Nederlandse Vereniging voor Klinische Geriatrie , Richtlijn Diagnostiek en Behandeling van dementie, 2014. https://www.google.nl/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=2ahUKEwim1tmfocblAhVSbFAKHTtrAxMQFjAAegQIARAC&url=https%3A%2F%2Fwww.nvvp.net%2Fstream%2Frichtlijn‐diagnostiek‐en‐behandeling‐van‐dementie‐2014&usg=AOvVaw0uZ1uPJPhy9UEGZAC8jXHs
- 19. Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chattu VK, Manzar MD, Kumary S, Burman D, Spence DW, Pandi‐Perumal SR. The global problem of insufficient sleep and its serious public health implications. Healthcare (Basel). 2018;7(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gezondheidsmonitor Volwassenen en Ouderen, GGD'en, CBS en RIVM. Obesitas volwassenen, cijfers en context 2019, Volksgezondheidenzorg.info https://www.volksgezondheidenzorg.info/onderwerp/overgewicht/regionaal‐internationaal/regionaal#node‐overgewicht‐ggd‐regio. Accessed July 26, 2019.
- 22. Gezondheidsmonitor Volwassenen en Ouderen, GGD'en, CBS en RIVM. Alcoholgebruik volgens richtlijn per GGD‐regio, cijfers en context 2019, Volksgezondheidenzorg.info. https://www.volksgezondheidenzorg.info/onderwerp/alcoholgebruik/regionaal‐internationaal/regionaal#node‐alcoholgebruik‐volgens‐richtlijn‐ggd‐regio. Accessed July 26, 2019.
- 23. Senaratna CV, Perret JL, Lodge CJ, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med. 2017;34:70‐81. [DOI] [PubMed] [Google Scholar]
- 24. Miller MA. The role of sleep and sleep disorders in the development, diagnosis, and management of neurocognitive disorders. Front Neurol. 2015;6:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16(1):40‐81. [DOI] [PubMed] [Google Scholar]
- 26. Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;8(1):63‐75. [DOI] [PubMed] [Google Scholar]
- 27. Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population‐based perspective. Alzheimers Dement. 2015;11(6):718‐726. [DOI] [PubMed] [Google Scholar]
- 28. Federatie Medisch Specialisten, Richtlijnendatabase: Klachten en co‐morbiditeiten bij obstructief slaapapneu (OSA). https://www.nvvc.nl/Richtlijnen/Obstructief_slaapapneu_OSA_bij_volwassenen.pdf. Accessed February 10, 2019.
- 29. Duarte RLM, Magalhães‐da‐Silveira O‐E‐STS, Rabahi MF, Mello FCQ, Gozal D. Predicting obstructive sleep apnea in patients with insomnia: a comparative study with four screening instruments. Lung. 2019;197(4):451‐458. [DOI] [PubMed] [Google Scholar]
- 30. Chung F, Abdullah HR, Liao P. STOP‐Bang questionnaire: a practical approach to screen for obstructive sleep apnea. Chest. 2016;149(3):631‐638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data that support the findings of our study are available from the corresponding author upon reasonable request.


