Dear Editor,
Patient‐derived organoids (PDOs) closely recapitulate human colorectal cancer biology and have recently emerged as preclinical models for personalized therapy design. 1 However, PDOs cultured in Matrigel (PDOsMatrigel) failed to predict outcome for treatment with the first‐line FO chemotherapeutic regimen (5‐fluorouracil plus oxaliplatin) for metastatic colorectal cancer (mCRC) patients. 2 In this study, we establish in vitro culture conditions of mCRC‐PDOs in a hydroxypropyl cellulose allyl conjugated with collagen (HA‐Coll sponge), 3 and utilized this system to examine the drug sensitivity of FO regimen (Figure 1A).
FIGURE 1.
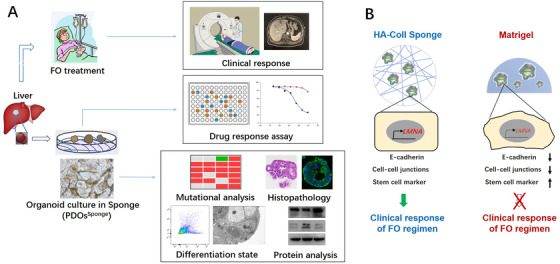
The experimental design and graphic mechanism. (A) Image‐guided biopsies were used to generate PDOs from liver metastatic colorectal cancers. PDOs were established from biopsies obtained before FO treatment in HA‐Coll sponge. Histopathology, molecular profiling, differentiation state, and protein analysis of PDOs and their parental tissues were characterized and compared. PDOs were used for FO drug screening, and ex vivo response to FO regimen was compared with clinical response. (B) The physical elasticity (E) of HA‐Coll sponge is close to that of the colorectal tissue, so that PDOs in HA‐Coll sponge could keep the similar expression level of lamin‐A as their parental tumor tissues. The lamin‐A protein could modulate the differentiation of colorectal epithelial cell to keep their epithelial state, which is important for their drug‐sensitivity on FO chemotherapeutic regimen. However, PDOs in Matrigel have lower expression level of lamin‐A and acquire mesenchymal characteristics, thereby they gradually form the drug resistance to FO regimen
A total of 12 tumor organoid cultures from metastatic lesion were developed from 12 patients enrolled in Renji hospital between September 2018 and October 2019 (Table S1). In the HA‐Coll sponge, the primary tumor cells organized into 3D spheroids within 3 days, although its size was smaller than that of organoid grown in Matrigel, and continues to grow until reaching similar sizes at about day 7 and its size was relative homogeneous (Figure 2A, Figure S1A and B). PDOs in HA‐Coll sponge (PDOsSponge) proliferated at the same level as PDOsMatrigel in the next generation (Figure 2B and C, Figure S1C and D). H&E staining showed notable morphological similarity among PDOsSponge and parental patient biopsies, and the “cystic versus solid” structure of the epithelium were preserved (Figure 2D, Figure S1E); the typical expression pattern of CDX‐2 (caudal type homeox‐2) and CK7 (keratin 7) in CRC was observed in PDOsSponge (Figure S1F). Collectively, the mCRC‐PDOs were successfully cultured in HA‐Coll sponge.
FIGURE 2.
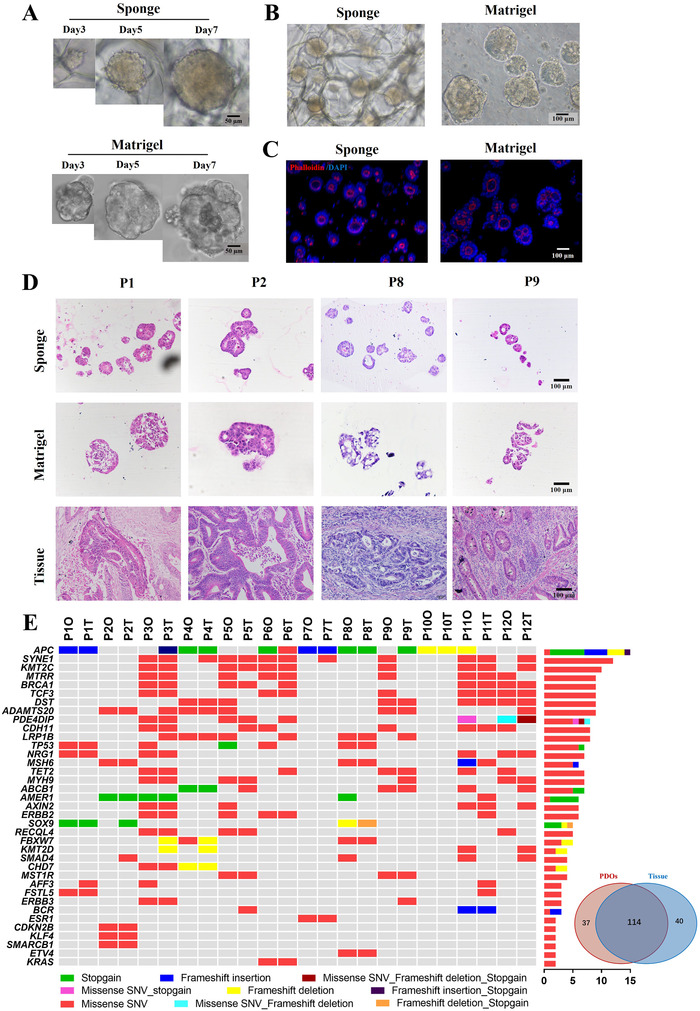
Phenotype and genotype of mCRC patient‐derived organoids (mCRC‐PDOs) in HA‐Coll sponges. Freshed isolated tumor cells derived from mCRC patients were seeded into HA‐Coll sponges or Matrigel in 48‐well plates (5 × 103/well). (A) Representative time course of the mCRC organoids from the same parental biopsy in HA‐Coll sponges (top) or Matrigel (bottom). (B) Representative phase‐contrast images of mCRC‐PDOs derived from the same parental biopsy in HA‐Coll sponges or Matrigel 14 days after implantation. (C) Representative 3D confocal fluorescence images of mCRC‐PDOs derived from the same parental biopsy in HA‐Coll sponges or Matrigel 14 days after implantation. Phalloidin for cytoskeleton(red). Counterstain is DAPI (blue). (D) PDOsSponge reconstitute the histology and morphology of parental tissue. H&E staining of PDOs from FO‐sensitive PR patients comparing to their parental tumors. (E) Molecular characterization of mCRC‐PDOs in HA‐Coll sponges. Heatmap showing the most frequently mutated genes in PDOsSponge and their parental tissues. Venn diagram demonstrating 74% mutational overlap between PDOsSponge and parental tissue biopsies
To explore whether PDOsSponge keep the genetic characteristics of the parental tumor, the next‐generation sequencing (NGS) was used to examine 561 cancer‐related genes in 12 pairs of PDOsSponge and their parental tumor tissues. The molecular landscape of the PDOsSponge largely overlapped with parental tumor and a 74% overlap in mutational spectrum was observed between these two groups (Figure 2E, Table S2), indicating that the major mCRC molecular genotypes are represented in PDOsSponge.
Next, we examined the predictive value of mCRC‐PDOsSponge on FO regime by comparisons of their ex vivo response data with clinical responses in patients. Twelve patients enrolled in our study were applied with FO therapy in clinic and the PDOsSponge were established from the biopsy before the treatment (Table S1, Figure 3A and Figure S2). The PDOsSponge were treated with different concentrations of 5‐FU and oxaliplatin, and after 6 days the growth rates were examined by Cell‐Titer Glo2.0 assay. We fitted dose–response curves (DRCs) and quantified responses to FO by calculating the IC50 and the area under the DRC (AUCDRC), both of which were significantly different between PDOsSponge from PR/SD versus PD lesions (Figure 3B–D). However, there were no significant difference on IC50 or AUCDRC in the PDOs cultured in Matrigel (Figure 3E–G). In addition to FO regimen, irinotecan‐based chemotherapy is also used for CRC patients as second‐line regimen. 4 Figure S3 showed that there were no significant differences on IC50 between PDOsSponge and PDOsMatrigel, suggesting that the distinction of these two groups mainly reflect the clinical response to FO chemotherapy but not irinotecan‐based regimen and highlighted the clinical potential of PDOsSponge for FO therapeutic selection in mCRC.
FIGURE 3.

PDOs in HA‐Coll sponges can predict drug response of metastatic colorectal patients to FO therapy. (A) Waterfall plot of 12 patients’ overall responses and best responses of the biopsied lesion in the FO‐treated PDOs cohort. Red indicates PD, green indicates SD, and blue indicates PR (PR, partial response; SD, stable disease; PD, progressive disease). (B) Fitted dose‐response curves (DRCs) of 12 PDOsSponge exposed to FO in vitro. Red lines represent PDOs from PD patients(n = 6), green lines represent PDOs derived from SD patients (n = 2), and blue lines represent PDOs derived from PR patients (n = 4). IC50 represent in vitro sensitivity of PDOs to FO. (C and D) The IC50 values were quantified and the area under the DRC (AUCDRC) was calculated by integrating the DRC of each PDO in (B). (E–G) Drug sensitivity of PDOs in Matrigel does not predict clinical response of parental colorectal patients to FO therapy. (E) Fitted dose–response curves (DRCs) of 12 PDOsMatrigel exposed to FO in vitro. (F and G) The IC50 values were quantified and the area under the DRC (AUCDRC) was calculated by integrating the DRC of each PDO in (E)
Epithelial to mesenchymal transition (EMT) has been demonstrated to play important roles in therapeutic resistance, 5 so we wondered if EMT was involved in the differential drug‐sensitivity between PDOsSponge and PDOsMatrigel. First, the expression of E‐cadherin in PDOs and the parental tumors from PR patients was examined by immunofluorescence and the results showed that PDOsSponge had similar expression level of E‐cadherin as their correspondent parental tissues, while it was dramatically decreased in PDOsMatrigel (Figure 4A, Figure S4A). These results were confirmed by the data of western blotting (Figure S5A‐H) and by the qPCR results of other EMT‐related makers (Figure S5I). In addition, activation of the EMT usually elicits changes in cell morphology 6 and the results of the transmission electron microscopy (TEM) demonstrated that cell–cell junctions were observed in both PDOsSponge and parental biopsies, while the intercellular connections in the PDOsMatrigel were much looser and minimal (Figure 4B, Figure S4B). Next, we utilized EMT inhibitors, methacycline HCl or (E)‐SIS3, to inhibit the progression of EMT (Figure S6A–C) and the ex vivo drug‐sensitivity experiments showed that, after adding methacycline HCl, the IC50 values of FO in PDOsMatrigel were significantly reduced (Figure 4C, Figure S4C). The same tendency was also observed in those treated with (E)‐SIS3 (Figure 4D, Figure S4D), suggesting that blocking EMT in PDOsMatrigel could recover its drug‐sensitivity in FO chemotherapy; however, this recovery was not observed in irinotecan‐treated groups (Figure S6D and E). Whether EMT inhibitors could be used in PDO culture for drug screening will need more experiments to confirm, particularly in different chemotherapeutic drug combination. Conclusively, our results indicated that PDOsSponge remained the original epithelial state of parental tumor tissues, which is important for the drug‐sensitivity of FO, but not for irinotecan.
FIGURE 4.
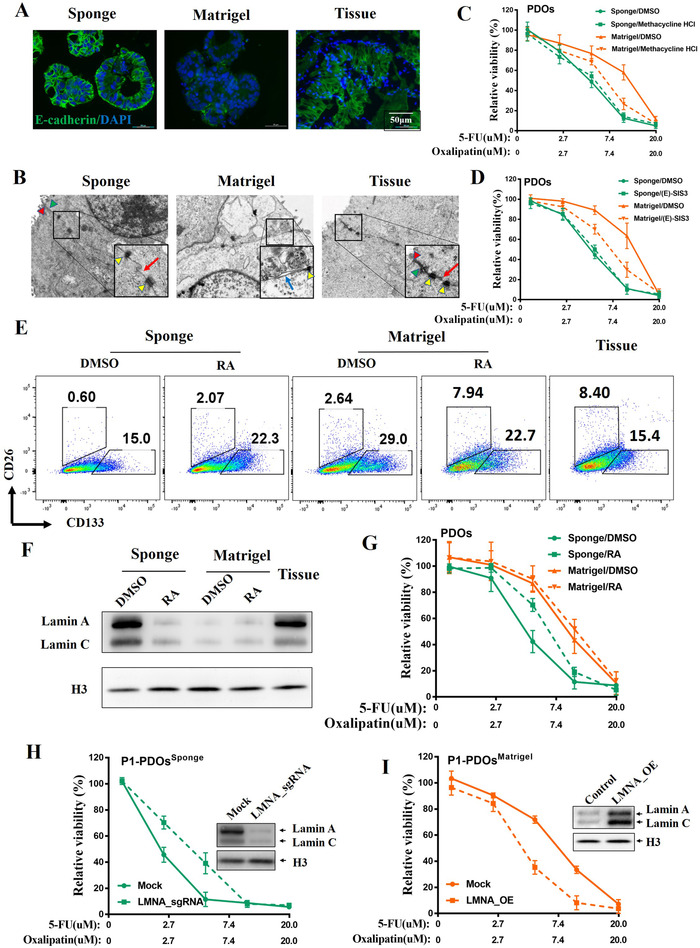
HA‐Coll sponge can keep the epithelial and differentiation state of PDOs. (A) The expression levels of epithelial marker E‐cadherin of representative P1 were examined by immunofluorescence. The epithelial cells of parental biopsy were isolated as control. (B) Cell–cell junctions in PDOsSponge and PDOsMatrigel from representative P1 were examined by transmission electron microscopy. Red arrowhead for tight junctions; Green arrowhead for adherens junctions; yellow arrowhead for desmosome; red arrow for gap junctions; blue arrow for dissolution of cell–cell junctions. (C and D) The ex vivo dose‐response curves (DRCs) of representative PDOs from P1 exposed to FO regimen after adding the EMT inhibitors: Methacycline HCl (10 µM) or (E)‐SIS3 (3 µM). (E) Lamin‐A contribute to differentiation state of PDOsSponge. The proportion of CRC‐CSCs in PDOs and parental tissues from P1 was analyzed by flow cytometry. PDOs were pretreated with 5‐FU and oxaliplatin for 6 days with or without 1 µM retinoic acid (RA). (F) The expression levels of lamin A protein in PDOs and their parental tumor tissues from P1 were examined by immunoblotting. (G) The DRCs of PDOs from P1 treated with 5‐FU and oxaliplatin in the presence of RA. (H) The DRCs of PDOsSponge from P1 treated with 5‐FU and oxaliplatin after LMNA KO. (I) The DRCs of PDOsMatrigel from P1 treated with 5‐FU and oxaliplatin after LMNA overexpressed
The epithelial state of cells usually reflected their differential states, 7 so we used CD133 and CD26 antibodies to examine the percentage of CSCs in the PDOs by flow cytometry. Figure 4E and Figure S4E demonstrated that the percentage of CD133+ cells in PDOsMatrigel was obviously higher than those in PDOsSponge and parental tumor tissue, although CD26 maybe was not proper marker for CSCs in our experiments. Mechanical tension on a cell from its environment control gene expression to modulate tissue‐specific differentiation 8 and stiff environment could increase the transcription of lamin‐A to alter nuclear rheology, thereby promoting cell differentiation. 9
To figure out if lamin‐A was involved in keeping the epithelial state in PDOsSponge, the expression level of lamin‐A in nuclear proteins was examined by western blotting. Figure 4F and Figure S4F showed that the expression of lamin‐A was dramatically decreased in PDOsMatrigel, but not in PDOsSponge, which was consistent with the previous studies that the matrix stiffness of Sponge 3 is at the similar level to that of intestinal epithelial cells. 10 Next we added retinoic acid (RA) to inhibit the expression level of lamin‐A in PDOsSponge (Figure 4F, Figure S4F), 9 and thereby enhance the percentage of CSCs in PDOsSponge (Figure 4E, Figure S4E), consequently, the IC50 value of FO in PDOsSponge was dramatically increased by RA (Figure 4G, Figure S4G). To further confirm these results, we knocked out LMNA in PDOsSponge and overexpressed LMNA in PDOsMatrigel to perform the drug response assay. The same tendency with RA results was observed (Figure 4H and I, Figure S7), suggesting that lamin‐A contributes to the epithelial state of PDOsSponge.
In summary, we reported that the mCRC‐PDOsSponge could recapitulate the phenotype and genotype of the parental tumor tissue. PDOsSponge could keep the similar expression level of lamin‐A as their parental tumor tissues, maintain the epithelial state of colorectal epithelial cell, and predict drug responses to FO chemotherapeutic regimen (Figure 1B).
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
Yan Zhang and Yanjie Xu: Conceptualization, methodology, and writing and original draft preparation. Jianjun Chen and Ming Zhong: Funding acquisition and data curation. Yizhou Huang and Yang Luo: Resources, visualization, and investigation. An‐Chih Hsieh and Jianyi Chen: Resources. Han Li: Software and validation. Xunbin Wei, Wei‐Qiang Gao, and Ming Zhong: Project administration and supervision. Yanjie Xu and Yan Zhang: Writing, reviewing, and editing.
Supporting information
Supporting Information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
The work reported here is supported by grants from the National Natural Science Foundation of China (81773250 and 81972670 to Y. Zhang; 81873555 and 81672347 to M. Zhong) and Technology Cooperation Program of Shanghai (18410741700 to J. Chen).
Contributor Information
Xunbin Wei, Email: xwei01@sjtu.edu.cn.
Wei‐Qiang Gao, Email: gao.weiqiang@sjtu.edu.cn.
Ming Zhong, Email: drzhongming1966@163.com.
Yan Zhang, Email: yanzh@sjtu.edu.cn.
REFERENCES
- 1. Vlachogiannis G, Hedayat S, Vatsiou A, et al. Patient‐derived organoids model treatment response of metastatic gastrointestinal cancers. Science. 2018;359:6378.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ooft SN, Weeber F, Dijkstra KK, et al. Patient‐derived organoids can predict response to chemotherapy in metastatic colorectal cancer patients. Sci Trans Med. 2019;11 10.1126/scitranslmed.aay2574 [DOI] [PubMed] [Google Scholar]
- 3. Nugraha B, Hong X, Mo X, et al. Galactosylated cellulosic sponge for multi‐well drug safety testing. Biomaterials. 2011;32:6982‐6994. [DOI] [PubMed] [Google Scholar]
- 4. McQuade RM, Stojanovska V, Bornstein JC, et al. Colorectal cancer chemotherapy: the evolution of treatment and new approaches. Curr Med Chem. 2017;24:1537‐1557. [DOI] [PubMed] [Google Scholar]
- 5. Nieto MA, Huang RYJ, Jackson RA, et al. EMT: 2016. Cell. 2016;166(1):21. [DOI] [PubMed] [Google Scholar]
- 6. Shibue T, Weinberg A. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14(10):611‐629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Polyak K, Weinberg A. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9(4):265‐273. [DOI] [PubMed] [Google Scholar]
- 8. Bainer R. Cell biology. Strength under tension. Science. 2013;341(6149):965‐966. [DOI] [PubMed] [Google Scholar]
- 9. Swift J, Ivanovska IL, Buxboim A, et al. Nuclear lamin‐A scales with tissue stiffness and enhances matrix‐directed differentiation. Science. 2013;341(6149). 10.1126/science.1240104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brauchle E, Kasper J, Daum R, et al. Biomechanical and biomolecular characterization of extracellular matrix structures in human colon carcinomas. Matrix Biol. 2018;180:68‐69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information


