Abstract
Metallic compounds contribute to the oxidative stress of ambient particulate matter (PM) exposure. The toxicity of redox inert ions of cadmium, mercury, lead and zinc, as well as redox-active ions of vanadium and chromium is underlain by dysregulation of mitochondrial function and loss of signaling quiescence. Central to the initiation of these effects is the interaction of metal ions with cysteinyl thiols on glutathione and key regulatory proteins, which leads to impaired mitochondrial electron transport and persistent pan-activation of signal transduction pathways. The mitochondrial and signaling effects are linked by the production of H2O2, generated from mitochondrial superoxide anion or through the activation of NADPH oxidase, which extends the range and amplifies the magnitude of the oxidative effects of the metals. This oxidative burden can be further potentiated by inhibitory effects of the metals on the enzymes of the glutathione and thioredoxin systems. Along with the better-known Fenton-based mechanisms, the non-redox cycling mechanisms of oxidative stress induced by metals constitute significant pathways for cellular injury induced by PM inhalation.
1. Introduction
Observational and experimental studies have long associated adverse health effects with metallic constituents found on particulate matter (PM) [1–3]. Closely reflecting the contribution of specific emission sources, the list of metals reported in analyses of PM varies considerably with the sampling location and seasonality. Surveys conducted around the world have generally found that metals of crustal origin, such as Al, Fe, Cu, Sn, Mo and Mn are found in the coarse PM fraction (PM10, defined as particles with a mean aerodynamic diameter of 10 μm or less), while the fine PM fraction (PM2.5, particles with a mean aerodynamic diameter of 2.5 μm or less) that is more closely associated with anthropogenic sources such as fuel combustion, brake and tire wear, and metallurgy, is reported to contain As, Co, Pb, V, Ni and Zn. However, in some locations, Cd, Cr and Cu have also been associated with PM10 and, in others, Zn and Ni have been found to span both coarse and fine fractions [2,4,5].
Oxidative stress is a recognized feature of the toxicology of metals that is most readily understood through the capacity of transition metals with adjacent valence states (e.g., Fe(III)/Fe(II), Cu(I)/Cu(II), V(IV)/V (V)) to support single electron reduction of peroxides, H2O2 in the case of the Fenton reaction, to generate the highly reactive oxidant hydroxyl radical. In the presence of a suitable reductant, such as superoxide anion in the Haber-Weiss reaction, the metal ion can be reduced to its starting valence state to complete a redox cycle that can result in oxidative damage to biomolecules [6]. The reduction of the known carcinogen hexavalent chromium (Cr(VI)) by cellular reductants to Cr (III) is reversed by oxidation of Cr(III) to Cr(IV), Cr(V) and Cr(VI) by cellular peroxides, making chromium a toxicologically relevant redox active metal [7].
Less well appreciated is that the toxicity of metals that are incapable of participating in redox cycling such Zn2+, Cd2+, Hg2+ and Pb2+ also involves oxidative stress that includes the generation of reactive oxygen species from cellular sources. Adding complexity, some redox-active metals such as V ion induce oxidative stress through non-redox mechanisms in addition to redox cycling [6], and ligands of non-redox cycling metals (e.g., mercuric ion (Hg2+)) can be redox active when complexed with thiols [8]. This article will review mechanisms of oxidative stress induced by PM metals, focusing on the interaction of metals with cysteinyl thiols as well as those mediated by H2O2 produced by cellular processes dysregulated by metal exposure. The focus will thus be on changes in bioenergetics and aberrant activation of cellular signaling that ultimately underlie adverse responses, such as inflammatory gene expression and cell death, in cells exposed to metals commonly found in PM.
Most, if not all, of the studies cited in this review represent proof-of-principle experimental work aimed at elucidating mechanisms of action hypothesized to underlie the toxicological effects of exposure to metal. In keeping with this objective and as a matter of practical necessity, mechanistic studies of this type use concentrations of metal that often exceed those to which the tissue or cell would be exposed to in vivo in environmental and occupational settings. Furthermore, cultured cell models may lack structural elements such as cilia and secretory cells, as well as mucin-containing airway surface liquid that overlies the airway epithelium in vivo. Similarly, soluble components of the epithelial lining fluid, such as uric acid, glutathione and ascorbate, represent protective mechanisms that may be absent in cell culture media. Subcellular preparations, such as isolated mitochondria, are still further removed from physiological conditions. In addition, the studies reviewed here typically utilize acute or subchronic exposures that often do not replicate chronic exposure scenarios. Together these factors present an inherent challenge to approximating the dose of metal that would be ultimately delivered to a toxicological target in vivo and thus limit the translational utility of these mechanistic studies. Therefore, extrapolations to the public health implications of their findings must be made with caution. Nonetheless, the primary role of such mechanistic studies is to provide biological plausibility that informs the risk assessment process at a fundamental level. Furthermore, computational models based on mechanistic data have great potential to reduce uncertainties in low-dose extrapolations, thus affording a rational foundation on which regulatory approaches to mitigating adverse health effects of PM exposure may be based.
1.1. Direct interaction of PM metals with thiols
While effects on cellular lipids and nucleic acids have also been described [9,10], the interaction between metals and proteins are most pertinent to our current understanding of non-redox oxidative stress caused by PM metals. Metal-protein interactions can be seen as indirectly involving virtually any amino acid that promotes or stabilizes direct binding by cysteine or histidine [11,12]. However, the toxicology of PM metals is largely driven by their effects on the function of cysteinyl thiols [13,14]. The affinity of metals like As, Hg, and Pb for thiols has long been known to be central to their toxicity, involving both adverse effects as well as the binding, transport and disposition of the metal [13,15,16]. In fact, metal thiol binding is the principle behind the development of the reagent British anti-Lewisite (2,3-dimercaptopropanol) in the 1940s for the treatment of intoxication with As, which was subsequently expanded to the treatment of Hg and Pb poisoning. While some metal ions associated with PM such as Zn2+ and Cu2+ are essential nutrients with multiple physiological functions (see Ref. [17] for an excellent recent review of Zn biology by W. Maret), the toxicology of inhalational exposure to supraphysiological concentrations of essential metals mimics that of toxicological exposure to xenobiotic metals.
Heavy metal ions have also been shown to displace redox active metals from coordination sites making them available for redox cycling. For example, the displacement of iron from binding sites by Cd2+ was reported to be responsible for the increased lipid peroxidation induced by exposure of rats to this heavy metal [18]. While Cd2+ (like Zn2+) is redox inert, the free iron it displaces catalyzes single-electron reduction of peroxides to form hydroxyl and carbon-centered radicals that initiate damaging free radical reactions throughout the cell by reacting with unsaturated fatty acids to produce a variety of electrophiles such as aldehydes, including 4-hydroxynonenal, as well as reactive lipid hydroperoxides [19]. In addition, as reviewed by Ghio in this series, the displacement of iron bound to ferritin, transferrin or lactoferrin by a variety of environmental metals dysregulates iron intracellular homeostasis and initiates an oxidative response. Iron displacement by exposure of human bronchial epithelial cells to V ions activates a response pathway that culminates in the extracellular release of superoxide anion, as the cell attempts to correct a perceived iron deficiency by reducing extracellular iron for the purpose of importing it via divalent metal transporter 1 [20].
The consequences of toxicological interaction of metals with thiols have functional similarities with physiological oxidative modification of cysteinyl thiols, a posttranslational protein modification that is pivotal in the regulation of many cellular processes, including energy metabolism and signal transduction [21–27]. In the past few years, the modification of cellular cysteinyl thiols by the addition of one or more SH residues to generate highly nucleophilic sulfanes (e.g., persulfide, polysulfides) has been recognized and its toxicological implications explored [28–32]. The interactions of these sulfanes with metals are beginning to be studied [33]. The mechanism that regulates cysteinyl redox switching exploits the broad range of valence states that the sulfur atom can attain (from −2 to +6), which enables the cysteinyl thiol to assume a spectrum of redox states, from the reduced thiol (-SH) and thiolate (-S−) forms, to the oxidized sulfenic (-SOH), disulfide (–SS–), sulfinic (-SO2H) and sulfonic (-SO3H) species. The thiolate anion is a much stronger nucleophile than the thiol and is much more readily oxidized [27,34]. The pKa of the cysteinyl thiol, the deprotonation of which forms the thiolate anion, correlates with its susceptibility to sulfenylation. However, thiol pKa is not the sole determinant of whether a protein is subject to redox regulation. Indeed, physiological sulfenylation is a process restricted to specific regulatory proteins [35–38].
The toxicity of several environmentally relevant metals is driven by a high affinity for thiols [39]. While the thiolate anion is a natural high affinity target for interaction with metal cations, the electron pair on the sulfur of the protonated thiol is also capable of coordinating with metal ions [13,40]. Coordination with a metal ion can also lower the pKa of a thiol to promote its ionization [41] and its subsequent oxidation to the sulfenyl form. It bears repeating that the reactivity of a thiol is dependent on structural features such as the availability of vicinal positively charged amino acid residues and hydrogen bonding that can stabilize the thiolate form [27,35,36]. Within a biological context, a thiol that is sequestered by interaction with a metal ion may be effectively as unavailable to serve its physiological role as one that is oxidized.
1.2. The effect of PM metals on the intracellular glutathione redox potential
Total concentrations of reduced and oxidized glutathione (GSH and GSSG, respectively) are 1–5 mM in most cell types and thus constitute a dominant intracellular redox pair that serves to buffer the cell against oxidative stress and prevents further irreversible oxidation [42]. Numerous studies have reported that exposure to PM metals like Hg, Pb, Ni, V and Zn, as well as the metalloid As, reduces levels of glutathione in tissues or cultured cells [18]. Exposure to arsenic has also been associated with increased consumption of GSH, through direct coordination, as well as via its reduction of As (V) to As (III) [43]. While there is consensus that metal exposure leads to lowered glutathione concentrations, much of the evidence relies on assay of total glutathione, GSH or GSSG, but typically not all three measurements. Since the glutathione redox potential is established by the ratio of GSH to GSSG and calculated by the Nernst equation (as ([GSH]2/[GSSG])), a complete data set that includes the total glutathione content is needed to establish a mechanism of action for metal-induced effects on glutathione. As detailed by Go and Jones [44], different enzymatic reactions in the glutathione system are dependent on the concentration of GSH or GSSG, while others respond to EGSH. Therefore, the lack of complete information presents a limitation in interpreting the consequences of metal effects on glutathione. A further restriction is that the mechanism responsible for metal-induced decreases in glutathione content reported in these studies cannot be determined without knowledge of the concentration of reactive species (e.g., H2O2) produced secondarily by the metal, which may also contribute to GSH oxidation through reactions catalyzed by enzymes such as glutathione peroxidase.
Live cell imaging studies using roGFP, a genetically encoded ratiometric sensor of EGSH [45] have proved to be insightful in the study of the oxidative effects of Zn2+ exposure [46–48]. Zn2+ -induced elevations in cytosolic and mitochondrial EGSH have been shown in cultured human skin [46] and airway [49] epithelial cell lines expressing roGFP. A major advantage of the live cell imaging monitoring of EGSH is that it is non-destructive, avoiding potential artifacts that may arise during sample processing. When combined with other fluorophores such as the H2O2-specific sensor HyPer [48–50], non-invasive monitoring of EGSH with roGFP affords high temporal resolution that is well-suited to examining the role of H2O2 in metal-induced glutathione oxidation. Extending earlier findings by Cheng et al. showing that increased H2O2 levels following exposure to Zn2+ are dependent on mitochondrial respiration [46], Wages et al. used ectopic mitochondrial expression of catalase to show that the Zn2+ -induced increase in cytosolic EGSH is linked to mitochondrial H2O2 production in human bronchial epithelial cells expressing HyPer or roGFP [49].
1.3. The effect of PM metals on the glutathione and thioredoxin systems
The importance of glutathione as a dominant redox buffer is owed not only to its millimolar intracellular concentration but also to the wide array of enzymes and enzyme families that are dedicated to its synthesis, maintenance and utilization that, together, comprise the glutathione system [42,51]. These include rate-limiting synthetic enzymes such γ-glutamylcysteine synthetase [52], the NADPH-dependent glutathione reductases [53], the xenobiotic metabolizing superfamily of glutathione-S-transferases [54], the selenocysteine-containing glutathione peroxidases [55], and thiol/disulfide oxidoreductases such as the glutaredoxins [56] and the protein disulfide isomerases [57].
Considering that many of these enzymes interact with glutathione using redox-active cysteinyl (or selenyl) groups by forming mixed disulfide (or selenide-sulfide) intermediates with GSH, it is not surprising that they too are subject to electrophilic attack by metals [58]. Indeed, exposure to Zn2+ inhibits glutathione reductase in astrocytes [59]. The effect of Zn2+ and Cd2+ on glutathione reductase is reported to be non-competitive for GSSG and NADPH, while the effect of Ni2+ is non-competitive for GSSG but uncompetitive for NADPH, suggesting metal-dependent specificities in this effect [60]. The activity of glutathione synthase can reportedly promote the reduction of As(V) to As(III) [61]. Sodium arsenite has also been reported to decrease the activities of glutathione peroxidase as well as that of the heme-containing H2O2 scavenging enzyme catalase in cultured human fibroblasts [62]. Similarly, Pb2+ exposure reduces catalase activity by inhibiting heme synthesis in rats [63] and is associated with decreased glutathione reductase activity in humans [64]. Superoxide dismutase and catalase activities in erythrocytes have been shown to be reduced in workers occupationally exposed to lead [65]. Hg2+ reportedly inhibits glutathione synthetase in addition to glutathione reductase, catalase and superoxide dismutase [16,66]. Intriguingly, there is also recent evidence that glutaredoxin, an enzyme that mediates thiol disulfide exchanges and has an important function repairing glutathionylated proteins [56], has a physiological role in maintaining homeostatic concentrations of iron and copper in the cell [14,67].
The thioredoxin system represents an independent antioxidant defense system that stands mostly, but not entirely [68], apart from the glutathione system [69–71]. In its reduced form, the low molecular mass protein thioredoxin is a dithiol that reduces protein disulfides directly via a thiol exchange mechanism. The resulting oxidized thioredoxin is reduced by the selenocysteine-containing thioredoxin reductases in an NADPH-dependent reaction that is similar to the reduction of oxidized glutathione by glutathione reductase. Thioredoxin reductase activity is inhibited by As ions [72,73] as well as Hg and Cr ions [14,74]. A very important family of proteins reduced by thioredoxins, the peroxiredoxins are abundant and highly efficient peroxidases that are increasingly recognized as pivotal regulators of intracellular peroxide levels [69–71]. As an alternative mechanism to reduction by thioredoxin, peroxiredoxins can also be glutathionylated and recycled by glutaredoxin [75]. Hexavalent chromium (CrVI) is a potent inhibitor of cytosolic and mitochondrial thioredoxin system components thioredoxin, thioredoxin reductase and peroxiredoxin in human bronchial epithelial cells [76].
Peroxiredoxins appear to also be subject to a more lasting oxidative inactivation through sulfinylation (-SO2H) of the catalytic cysteine, a process that can be reversed by the ATP-dependent sulfiredoxin [77]. This feature of peroxiredoxins serves as a key component of the “floodgate hypothesis” of peroxide-based signaling. According to this model the extremely fast catalytic activity of the peroxiredoxins is reconciled with the far slower rate of sulfenylation of regulatory thiol switches on protein targets by invoking the inactivation of peroxiredoxin by sulfinylation caused by a rapid, localized and transient accumulation of high levels of H2O2 [78]. However, unresolved issues, such as the high intracellular concentrations of GSH and GAPDH thiols, pose challenges to this hypothesis [70]. One of the most startling recent findings in the redox biology field is that hydrogen peroxide induced formation of protein disulfides is actually enabled by peroxiredoxins [71,79], implying that these enzymes have pro-oxidative as well as antioxidant activities, the mechanistic specificity for which remains unclear at this time [71].
In addition to these metal-induced impairments of the redox homeostasis defense mechanisms, there are numerous reports of increased levels of glutathione and glutathione system enzymes. Exposure to Pb increases glutathione levels in the liver, kidney and red blood cells [18]. Cadmium exposure has been shown to increase glutathione peroxidase in Leydig cells in a rat model of testicular carcinogenesis [80], and increases expression of thioredoxin reductase in bovine vascular endothelial cells through an Nrf2 signaling process [81]. Epidemiological studies have shown that mRNA expression of glutathione peroxidase, thioredoxin reductase, thioredoxin and peroxiredoxins is elevated in peripheral blood leukocytes of workers occupationally exposed to elemental mercury (Hg0) [82]. Similarly, the expression levels of thioredoxin reductase, glutathione-S-transferase and peroxiredoxin were significantly increased in circulating leukocytes of metal workers exposed to the metalloid arsenic III, compared to an unexposed control group [83]. These effects have been ascribed to adaptive responses to the oxidative stress induced by the metal exposure [18]. However, there is also evidence that suggests a higher level of control can be involved in these antioxidant responses. Nishimoto and colleagues demonstrated that knockdown of thioredoxin reductase in HeLa cells sensitized cells to a low dose of Cd2+ but decreased cytotoxicity induced by a higher dose of Cd2+, suggesting that thioredoxin reductase has a role in promoting cell death under certain conditions [84].
Given the multifaceted role and essentiality of glutathione and thioredoxin in maintaining intracellular redox homeostasis, any impairment in these systems, be it direct or indirect, is relevant to the oxidative stress of exposure to PM metals. The toxicological significance of the disarming of cellular defenses against oxidative stress induced by environmental oxidants is profound whether it occurs directly or is caused by the reactive oxidant species that these oxidants may produce, or (as addressed below) oxidants that are formed secondary to the dysregulation of cellular processes. Broadly, the impairment of glutathione or thioredoxin system components potentially also represents a diminished capacity to maintain a reductive tone to oppose the baseline oxidative stress incurred by mitochondrial generation of O2.− during respiration. Moreover, it also reduces the cell’s capacity to return to the resting redox homeostatic setpoint following physiological departures from redox homeostasis as they occur normally in signaling, a process that has been referred to as “eustress” [85].
1.4. Effects of PM metals on cellular bioenergetics
The requirement of NADPH as the source of reducing equivalents for the activity of both glutathione reductase and thioredoxin reductase links the glutathione and thioredoxin systems to glucose utilization as the ultimate source of reducing power available to the cell to defend against oxidative stress. The principal source of NADPH in mammalian cells is the pentose phosphate pathway (PPP), one of the secondary glucose metabolism pathways that is particularly active in tissues with a high metabolic requirement for reducing equivalents, such as fatty acid synthesis activity in the liver or mitotic activity. A dynamic mechanism exists to divert glucose-6-phosphate from glycolysis into the pentose phosphate pathway as a rapid metabolic adaptation to increase NADPH synthesis under conditions of oxidative stress. The pivotal event in this process is the reversible oxidative inactivation of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a rate limiting enzyme in glycolysis [86].
As elegantly elucidated by Tobias Dick’s group, the catalytic cysteine in GAPDH (Cys-152) is inactivated by H2O2-mediated sulfenylation through a process that is dependent on a second, neighboring cysteine (Cys-156) that does not participate in the catalytic activity of the enzyme. These investigators showed that Cys-156 in GAPDH does not form a disulfide with the catalytic cysteine but rather has an indirect but crucial role in enabling the oxidative inactivation of GAPDH by acting as an essential component of a proton relay that permits its oxidation by physiological concentrations of H2O2 [87]. The dual reactivity of the nucleophilic thiol of Cys-152 towards its substrate (glyceraldehyde-3-phosphate) and H2O2 provides a clear example of the importance of considering kinetic factors involved in driving enzymatic reactions to completion and not only those that initiate them [87].
Because GAPDH is highly expressed in the cytosol of most cell types, its reactive thiols constitute a significant redox buffer, the concentration of which rivals that of glutathione on a mass basis [87] and, therefore, is also a potential site of metal coordination. Zn2+ ions potently inhibit GAPDH activity with a reported IC50 of 150 nM, suggesting a potential regulatory role for this specific metal ion in controlling cellular bioenergetics under physiological conditions [17]. There is evidence that Zn2+ also decreases the availability of nicotinamide adenine dinucleotide (NAD+), which can inhibit GAPDH activity, and that metabolic correction of the NAD+ deficit by the addition of pyruvate or oxaloacetate to bypass glycolysis or by preventing the degradation of NAD+ can protect neurons from Zn2+-induced cytotoxicity at levels of 40 μM [88]. Thus, careful consideration of the concentration of Zn2+ is needed in evaluating the significance of reported physiological or toxicological effects on energy metabolism. This point is reinforced by a report that exposure of bovine pulmonary artery endothelial cells to 3–5 μM Cd2+ or Hg2+ results in a profound inhibition of GAPDH, while slightly lower doses in the range of 1–2 μM appeared to stimulate an adaptive effect that includes an increase in GSH levels [89].
Extrapolating from the effect induced by oxidative inactivation of GAPDH by H2O2, metal-induced inhibition of GAPDH would seem to also incur a built-in antioxidant compensatory mechanism in the form of increased production of NADPH through the pentose phosphate pathway, which would serve to mitigate the oxidative effects of metal exposure. However, unlike the oxidation of the catalytic cysteine by H2O2, metal-induced inhibition of GAPDH may not be reversible, implying a sustained shunting of glucose-6-phosphate away from glycolysis, the citric acid cycle and electron transport. Furthermore, exposure to Cd2+ and Hg2+ has also been shown to inhibit the rate limiting enzyme in the pentose phosphate pathway, glucose-6-phosphate dehydrogenase (G-6-PD) [89], making predictions about the effectiveness of the pentose pathway as a compensatory mechanism for metal-induced oxidative stress tenuous at best. This case illustrates the point that the general reactivity of metals with protein thiols introduces considerable unpredictability in the assessment of the toxicological outcome of exposure to PM metals. The net effect of metal-induced dysregulation of multiple serial, opposing or interdependent cellular processes that are normally closely regulated may be too chaotic to predict or interpret using a systems approach, and therefore require empirical determination using an experimental approach that monitors specific readouts in real time.
1.5. PM metal effects on mitochondrial respiration as a source of reactive oxygen species
Mitochondria are a major intracellular source of reactive oxygen species [90]. It is estimated that approximately 0.1% of the molecular oxygen used in mitochondrial respiration is released prematurely as the partially reduced oxygen species superoxide anion (O2.−) [91]. The sites of generation of mitochondrial superoxide have been identified as Complexes I and III [90,92–94], with complex II implicated as a regulator of O2.− production by the electron transport chain [95]. Physiologically, mitochondrial respiration is subject to close regulation by processes that involve redox modifications of cysteinyl thiols and glutathione [96].
Virtually all aspects of mitochondrial function, including oxidative phosphorylation, control of cell death through apoptosis, and mitochondrial biogenesis and autophagy, is subject to redox regulation that is mediated by oxidation of cysteinyl thiols in mitochondrial proteins (see Ref. [97] for review). Metals found as components of ambient PM have been shown to impair mitochondrial function, including Hg, Pb, Cd, As, V and Zn [98,99]. Exposure to Zn2+ causes a loss of mitochondrial membrane potential in intact cells [46] as well as in isolated mitochondria [100]. A recent study showed that exposure of mouse and human primary hepatocytes to arsenite induces increased production of mitochondrial reactive oxygen species that appeared to be derived from altered Complex I activity and expression following a non-linear dose response relationship referred to as “mitohormesis” [101]. In mitochondria of bovine cardiac myocytes Zn2+ targets a proton channel in the hydroquinone oxidation center of complex III, leading to inhibition of electron transport [102]. Sequential inhibition of FoF1 ATPase, followed by uncoupling of oxidative phosphorylation, inhibition of electron transport, oxidation of mitochondrial GSH and lipid peroxidation have all been reported in isolated rat renal cortex mitochondria exposed to Hg (II) [103]. Similarly, mitochondrial dysfunction including changes in membrane permeability was accompanied by inhibition of ATPase, superoxide dismutase and glutathione peroxidase activities in human embryonic kidney cells exposed to Cd2+ [104]. Despite these similarities, a study comparing the effects of Hg and Cd in rat liver mitochondria found clear evidence of specificity in the toxicity of these metals on individual parameters of mitochondrial function [105].
Increased mitochondrial Ca2+ levels are known to increase the rate of ATP production, as the Ca2+ -sensitive dehydrogenases of the TCA cycle are stimulated by elevated Ca2+. The increased ATP production in turn leads to increased leakage of free electrons to generate ROS [106]. Exposure to Zn, Cd, and Hg ions has been shown to elevate the intracellular Ca2+ concentration from the intracellular calcium storage on the endoplasmic/sarcoplasmic reticulum (ER/SR) that functions to maintain low cytoplasmic Ca2+ levels. These metals promote Ca2+ release from the ER or inhibit Ca2+ efflux to the inter membrane space by inhibiting the activities of the ER/SR Ca2+ ATPase (SERCA) or the Ca2+-ATPase (SPCA) [107–109]. The elevation of the Ca2+ level within mitochondria is controlled by several ion channels, including the mitochondrial calcium uniporter (MCU), that can be disrupted by ions of Hg, Cd, and Cu, allowing increased calcium influx into the mitochondria [105].
Excessive influx of Ca2+ induces persistent depolarization and the opening of the mitochondrial permeability transition pore (MPTP) to compensate for the calcium homeostasis. Although much about the MPTP, including its composition, remains poorly understood, it is clear that opening of the MPTP is a determining event in cell death [110]. Loss of mitochondrial calcium homeostasis and increased concentrations of reactive oxygen species in the mitochondrial matrix are triggering events in MPTP activation [111] that leads to mitochondrial swelling, loss of mitochondrial membrane potential, uncoupling of oxidative phosphorylation and increased permeability to mitochondrial proteins such as cytochrome C [112]. In rat liver mitochondria, exposure to Hg2+ induces mitochondrial swelling, collapse of the mitochondrial membrane potential and release of cytochrome C [113]. The metalloid arsenic has been shown to induce release of cytochrome C through activation of the MPTP [114]. Electron flow through mitochondrial complexes I and III has been implicated as sources of the reactive oxygen species that lead to MPTP activation [110,112]. Belyaeva and colleagues showed that impairment of the electron transport chain by exposure of rat PC12 neurocytes to Cd2+ or Hg2+ led to permeabilization of the mitochondrial membrane induced by MPTP activation [105]. Specifically, these workers identified the P-site and S-site in Complexes I and III as likely sites of metal binding leading to ROS formation that causes oxidation of thiols and MPTP opening [105,115]. Inhibition of complexes I, II and III and ensuing generation of reactive oxygen species and ATP depletion have also been shown to lead to MPTP opening and cytochrome C release induced by exposure of isolated rat liver mitochondria to pentavalent vanadium (V(V)) [116].
The B-cell leukemia 2 (Bcl-2) family of pro- and anti-apoptotic proteins are a major part of the intrinsic (non-receptor mediated) mitochondrial pathway that leads to programmed cell death [117]. The activation of apoptosis in the intrinsic pathway is effected by the oligomerization and insertion of BAX/BAK into the outer mitochondrial membrane to form the mitochondrial apoptosis-induced channel (MAC). MAC is a pore through which high-molecular weight solutes such as cytochrome C can pass into the cytosol, and event that commits the cell to undergo apoptosis by triggering the activation of caspases that lead to cell death. Bcl-2, the protein after which the family is named, is anti-apoptotic in that it exerts a suppressive effect on MAC formation [118]. Decreased expression of Bcl-2 has been linked to apoptosis induced by exposure of human lung fibroblasts to arsenic trioxide [119]. An elevated BAX/Bcl-2 ratio has been reported in apoptosis induced by exposure of PC-12 rat adrenal medullary cells to lead ions [120,121], while expression of Bcl-2 suppressed cadmium-induced cell death in Rat-1 fibroblasts [122]. The effect of zinc on Bcl-2-induced apoptosis appears to be more complex. Zinc ions have been reported to suppress apoptosis in U937 cells exposed to H2O2 by increasing the Bcl-2/BAX ratio [123]. However, a subsequent report showed that Zn ions induce apoptosis in cultured human prostate epithelial cells through degradation of Bcl-2 protein [124]. A recent study demonstrated an increase in the BAX/Bcl-2 ratio in human aortic endothelial cells exposed to zinc oxide nanoparticles [125]. The effect of zinc on the apoptotic protease caspase-3 also appears controversial, with studies reporting that zinc ions potently inhibit caspase-3 activity [126,127], while another showed that Zn-induced inhibition of caspase3 does not prevent apoptosis in HeLa cells [128].
Given its function as the site of 4-electron oxygen reduction to H2O, metal-induced inhibition of cytochrome C oxidase activity (Complex IV) is also relevant to oxidative stress induced by exposure to PM metals. Particularly well worked out is the effect of Zn2+ on cytochrome C oxidase, as the strong affinity of this metal ion with specific targets has been used to elucidate bioenergetic pathways in the mitochondrion [129]. Multiple binding sites for Zn2+ on cytochrome C oxidase have been identified on the negatively charged mitochondrial membrane [130], in proximity to the proton channel entrance [131,132]. In addition, high affinity binding sites for Zn2+ have been reported on the external (P side) of the mitochondrial membrane [133,134]. The net effect of supraphysiological concentrations of Zn2+ on the mitochondrion appears to be uncoupling of mitochondrial respiration caused by inhibition of two of the four proton pumping steps required for full reduction of oxygen to water by cytochrome C oxidase [135]. The loss of cytochrome C oxidase activity can be expected to lead to impaired electron and proton flow and increased production of superoxide from complexes I and III [96].
1.6. Dysregulation of signaling by metal exposure
Reversible protein phosphorylation is a pivotal regulatory event that transduces extracellular signals into cellular responses that determine virtually every aspect of the life of the cell, from division to differentiation, migration, adhesion, gene expression, energy utilization, and cell survival. Protein phosphorylation is mediated by kinases at the expense of ATP and occurs on specific serine/threonine or tyrosine amino acid residues, with the regulation of some proteins involving dual phosphorylation at a specific serine/threonine as well as a tyrosine [136,137]. The activity of many kinases is itself regulated by phosphorylation, establishing signaling cascades or pathways that may be stratified by an alternating pattern of phosphorylation on serine/threonine, tyrosine phosphorylation or both [137]. Although examples of deactivation are also known, phosphorylation of specific protein domains usually leads to structural changes that modulate a protein function, typically an activation or gain of function. This view is supported by the fact that levels of phosphoproteins in resting cells are typically very low, with an appreciable but transient increase evident in cells that are stimulated physiologically [136].
Dysregulation of protein phosphorylation, fundamentally manifested as sustained levels of protein phosphorylation, is a well characterized feature associated with pathologic cellular behavior seen in cancer, particularly the unrestrained growth that drives neoplasia and the invasiveness leading to metastasis. Acute inflammatory responses and adaptive changes, such as fibroblast growth that gives rise to fibrosis, are similarly understood to be the result of the activation of specific phosphorylation-dependent signaling pathways [138]. As discussed in this section, aberrant signaling caused by the loss of signaling homeostasis induced by metals contributes to the adverse health effects associated with exposure to ambient PM.
Phosphatases are hydrolases that catalyze the removal of a phosphate from a phosphoric acid monoester in a reaction involving nucleophilic attack on the phosphorus atom in the presence of water [139]. While far less studied, the role of phosphatases in signaling is equally vital to that of kinases in intracellular signaling. By opposing the activity of the kinases, the constitutive dephosphorylating activity of phosphatases enables two fundamental features of phosphorylation-dependent signaling: homeostasis and reversibility [140]. The relatively low intracellular levels of phosphoproteins in unstimulated cells is the result of phosphatase activity that greatly exceeds the basal activity of kinases. In effect, the constitutive phosphatase activity represents the primary homeostatic mechanism that maintains signaling quiescence in the absence of an extracellular signal. Control of phosphorylation-dependent signaling in stimulated cells is the result of activation of kinase activity and concurrent inhibition of phosphatases.
Phosphatases involved in signaling regulation are classified as the protein phosphatases (PP), with specificity for phosphoserine and phosphothreonine, and the protein tyrosine phosphatases (PTP). The dysregulation of PPs is associated with a wide spectrum of morbidities ranging from cancer to diabetes, Alzheimer’s disease, autoimmune disorders and diabetes [141–145]. It is estimated that over 90 % of the protein phosphatase activity towards phosphothreonine and phosphoserine is the action of PP1 and PP2A enzymes [146]. Exposure to Zn2+ has been shown to inhibit PP activity directed at GABAA receptors present in isolated rat pyramidal cells [147]. In contrast, exposure to Pb appears to induce the expression of PP1 and PP2A, reducing Tau phosphorylation at specific serine residues, in a process that has been associated with learning and memory deficits in young rats [148].
In contrast to the PP, the PTP superfamily is highly diverse, with over 100 members spread over multiple classes of enzymes based on their structure and substrate specificities [149,150]. The PTP encompass the receptor-like PTPS, some of which have dual catalytic domains (e.g., PTPα) [151], and the well-known nonreceptor PTP such as PTP1B, which dephosphorylate the epidermal growth factor and platelet derived growth factor receptors. PTP1B also dephosphorylates the insulin and leptin receptors and is thus involved in the regulation of energy metabolism [152–155]. The superfamily also includes the srchomology domain-bearing PTP that include SHP1 and SHP2 and are themselves regulated by phosphorylation, and SHP-PEST, which is involved in the regulation of c-src kinase [156]. The low-molecular weight PTP are involved in the dephosphorylation of growth factor receptors, transcription factors and src-family members [157]. PTPs in the Cdc25 group are involved in regulating cell-cycle progression and are regulated by phosphorylation by Chk1 and Chk2 kinases, which targets them for ubiquitination and proteolytic degradation. Dual specificity phosphatases that dephosphorylate phosphoserine and phosphothreonine in addition to phosphotyrosine residues are also in the PTP superfamily [158]. Included in this group is phosphatase and tensin homolog deleted on chromosome 10 (PTEN), whose activities include the dephosphorylation of phosphatidylinositol-3,4,5-trisphosphate in opposition to the phosphorylating activity of PI3kinase [159]. The enzymes that dephosphorylate the mitogen activated protein kinases (MAPK) P38, JNK and ERK1/2, and thus oppose the activity of the dual specificity kinases MAPKK, are also dual specificity phosphatases.
The PTP were among the first proteins to be demonstrated to be regulated by redox modification [160]. As diverse as they are, all members of the PTP superfamily of phosphatases have a conserved CX5R motif that includes the catalytic cysteine responsible for the nucleophilic attack on the phosphorus atom of phosphotyrosine. Due to the amino acids surrounding the catalytic pocket in PTP, the pKa of the catalytic cysteine in PTP is low (approximately 4.5) and it therefore exists intracellularly as a thiolate, which makes it a superior nucleophile. However, as mentioned earlier, thiolates are also highly susceptible to oxidation to the sulfenic form [149,161].
Work done in the laboratory of Sue Goo Rhee in the 1990s demonstrated that signaling induced by stimulation of A431 skin carcinoma cells with epidermal growth factor was accompanied by an increase in H2O2 and transient, oxidation-dependent loss of PTP1B activity [160]. Physiological inhibition of PTP during signaling activation was subsequently shown in members of every class of PTP and has been demonstrated to be mediated by the sulfenylation of the invariant catalytic cysteine in these enzymes. Until recently, the oxidation of the catalytic cysteine in PTP was thought to involve a direct reaction with H2O2. However, as alluded to in an earlier section, the rate of H2O2 reaction with PTP appears to be too slow to be competitive with the reactivity of the much more abundant peroxiredoxins [70]. It has been proposed and there is a growing body of evidence to support that protein sulfenylation, including that of the PTP catalytic cysteine, is mediated by the action of the peroxiredoxins [71]. Whether it is direct or indirect, the sulfenylation of the catalytic cysteine leads to formation of a sulfenamide through a nucleophilic attack of a backbone amide on the sulfenic group. Sulfenamide formation is thought to be a mechanism that protects the catalytic cysteine in PTP from undergoing further oxidation to the sulfinic or sulfonic forms, which are not known to be reversible [161].
The vanadate ion (VO43−) is a phosphate analog that is a well-characterized competitive inhibitor of PTP [149,162]. In fact, inhibition of PTP activity with vanadate is standard experimental practice in signal transduction studies to preserve the state of protein phosphorylation during the preparation of protein extracts for analysis [163]. Pharmacological strategies to potentiate or prolong phosphorylation-dependent signaling using vanadium-based drugs to target PTP have also been devised in the treatment of diseases such as diabetes i.e., insulin mimesis [164], with varying degrees of success [149]. Peroxovanadium compounds formed from the reaction of vanadium ions with H2O2 are potent and irreversible inhibitors of PTP [164,165]. Toxicologically, exposure of a human airway epithelial cell line to residual oil fly ash, a vanadium-rich constituent of ambient PM derived from the combustion of oil [166], inhibited PTP activity and induced a time-dependent accumulation of protein phosphotyrosines. These effects were shown to be mimicked by solutions containing vanadium salts but not by other major metallic compounds present in residual oil fly ash [167], and were subsequently found to include inhibition of PTP1B in primary human airway epithelial cells exposed to vanadium ions [168]. Cr(V) and Cr(VI) are isoelectronic with V(IV) and V(V), respectively, and some studies have shown that Cr ions are PTP inhibitors [7].
The divalent zinc ion is also known to be potent inhibitor of PTP activity [169]. Picomolar fluctuations in the concentration of intracellular Zn2+ have been shown to modulate growth factor signaling [170]. Primary human airway epithelial cells exposed to Zn2+ had marked reduction in the activity of PTP of a broad range of molecular weights, including PTP1B, with accompanying increase in levels of protein phosphotyrosines [171]. As recently reviewed [17], the mechanism of Zn2+-induced inhibition of PTP has been characterized extensively by Maret and colleagues [172–175]. Their work has shown that Zn2+ binds to the catalytic aspartate in the PTP and the intermediate formed by the attack of the catalytic cysteine on the phosphate group of the phosphorylated tyrosine. Interestingly, the inhibitory binding of the Zn2+ ion renders the catalytic cysteine in PTP unavailable for oxidation and is, therefore, a mechanism that is distinct from the coordination of thiols that Zn2+ shares with other heavy metals [17]. In addition to the inhibition of PTP activity, exposure of airway epithelial cells to Zn2+ has also been shown to decrease the activity of PTEN, a PTP that opposes PI3K kinase activity. This was shown to occur through a mechanism that involves proteolytic degradation of PTEN as well as decreased levels of PTEN mRNA, suggesting that Zn2+ exposure can trigger a signaling-dependent regulatory response that modulates the expression of PTP [176]. Perhaps not surprisingly, given its chemical similarities to Zn2+, Cd2+ has also been found to inhibit PTP activity [177].
While the phosphate analog arsenate has been reported to inhibit PTP1B in a cell-free preparation [178], Karin and colleagues reported that exposure to trivalent, but not pentavalent, arsenic ions resulted in a loss of PTPase activity in HeLa cells [179]. More recently, the mechanism of PTPase inhibition by mono- and di-methylated forms of As III was investigated in HePG2 cells and recombinant PTP1B and CD45 [180]. This work showed that PTPase inhibition is mediated by binding of methylated As, specifically dimethylarsinous acid, to the catalytic cysteine in PTP1B (Cys215), while inorganic As III does not inhibit PTPase directly. Consistent with this finding, treatment with sodium arsenite (AsO33−) did not induce changes in total PTP or PTP1B activity in primary human airway epithelial cells under conditions in which vanadate and Zn2+ induced profound inhibition [168]. In a study using recombinant PTP to evaluate the effects of various metal ions, divalent copper (Cu2+) was distinguished from Fe3+ and Zn2+ by the irreversibility of its inhibitory effect using the metal chelator EDTA, suggesting that the effect of Cu2+ is secondary to its generation of reactive oxygen species [177]. However, a more recent study reported that Cu(II)-amino acid complexes are potent inhibitors of PTP activity, appearing to rule out the involvement of ROS [181].
1.7. Signaling activation induced by PM metal exposure
Work cited above showing that treatment with metals that inhibit PTP result in a time-dependent accumulation of protein phosphotyrosines [167,168] illustrates the essential role that dephosphorylating activity plays in maintaining intracellular signaling quiescence in resting cells and in limiting the duration of signaling. Further, studies have also shown that metal-induced loss of PTP activity is a mechanism that is sufficient to initiate signaling through a permissive process wherein basal activity of kinases is unopposed, thus leading to an autoactivation that is distinct from physiological signaling in that it does not require ligand-mediated receptor activation. Specifically, loss of dephosphorylating activity directed at specific signaling intermediates has been linked to metal-induced activation of signaling in human lung cells exposed to PM metals. Exposure of a human bronchial epithelial cell line to Zn2+ or VO2+ caused a marked inhibition in phosphatase activity directed at phosphorylated forms of the MAP kinases JNK and ERK [182]. Similarly, Tal et al. [183] showed that exposure of primary human airway epithelial cell cultures (HAEC) to Zn2+ or VO2+ ions induced phosphorylation of EGFR that was accompanied by a profound loss of total PTP activity and impairment of the rate of dephosphorylation of phosphotyrosines 1068 and 845. Notably, inhibition of P-EGFR dephosphorylation was shown in cell lysates prepared from HAEC treated with Zn2+ using a recombinant substrate, as well as in intact HAEC that had been pre-treated with EGF to induce phosphorylation of endogenous EGFR [183].
Exposure to PM metals has been associated with a broad array of specific signaling pathways known to lead to cellular responses such as inflammatory gene expression thought to contribute to the health effects of PM inhalation [184]. In a human airway epithelial cell line, exposure to As, Cr, Cu, V and Zn compounds was shown to activate ERK1/2, JNK and P38, MAP kinases [185]. This was subsequently shown for As, V and Zn to occur via Raf-1- [171] and Ras-dependent signaling [186] initiated by the EGF receptor [171]. The induction of EGFR activation in human airway epithelial cells exposed to Zn ions has been extensively characterized [183,187,188], including the role of PI3Kinase [189]. Similarly, exposure to As [190], Cu [191] and V ions [192] has been associated with activation of NFkB, while Cr [193] and Zn [188] ions have been shown to activate Src family kinases. Cadmium-induced expression of the cytokine IL-6 and the CXC chemokine IL-8 in human astrocytes has been shown to involve MAPK and NFkB pathway activation [194]. Recent work in zebrafish embryos suggests that the effects of Pb and Cd ions can be distinguished by their patterns of MAPK activation and expression of the nuclear factor erythroid-2 related factor 2 (Nrf2) gene [195].
Nrf2 is a Cap’n’Collar basic-region leucine zipper transcription factor that controls the expression of genes regulated by an antioxidant response element (ARE, also referred to as an electrophile response element EpRE) [196,197]. These genes include those encoding a range of critical modulators of the cellular antioxidant defense mechanism, such as glutathione-S-transferase (GSTP1), peroxiredoxin (PRDX1), hemeoxygenase (HMOX1), sulfiredoxin (SRXN1), thioredoxin reductase (TXNRD1), and NADPH quinone oxidase (NQO1) [83]. Nrf2 is constitutively suppressed by ubiquitination and proteasomal degradation by a process that is facilitated by the binding of its repressor, Kelch-like ECH-associated protein 1 (Keap1) [197]. Modification of regulatory thiols on Keap1 by oxidants and by electrophiles decreases its efficiency in promoting the degradation of Nrf2, leading to NrF2 stabilization and enabling ARE transcriptional activity [196]. Specifically, S-nitrosation of Cys-151 by NO and alkylation of Cys-273 and Cys-288 have been shown to act as switches controlling the activity of Keap1. The mechanism responsible for the activation of Keap1 by Zn2+ has been well worked out by Hayes and colleagues, who showed that it involves a conformational switch initiated by Zn2+ binding to His225, Cys-226 and Cys-613 [198]. Similarly, the activation of Nrf2 by As3+ and Cd2+ ions in a reconstituted system is dependent on Cys-151 [199]. Nrf2 activation has been demonstrated to protect against oxidative stress and apoptosis induced by exposure of mouse liver cells to hexavalent chromium ions [200].
1.8. NOX activation induced by PM metal exposure
NADPH oxidase (NOX) activity is recognized as the primary regulated cellular sources of reactive oxygen species [201]. In the classic neutrophil model, NOX activity is the result of the coordinated activation of a complex of proteins recruited to the cell membrane leading to the reduction of molecular oxygen by NADPH to produce superoxide anion by a flavocytochrome b558 comprised of gp91phox and p22phox. Translocation of a cytosolic regulatory subcomplex, formed by p47phox, p67phox and p40phox, and the small GTPase Rac in its GTP bound form completes the NOX complex [202]. The signaling pathway leading to GTP-Rac formation from the inactive GDP-bound state is dependent on protein kinase C (PKC) activity. NOX complexes exist in a variety of cell types in the lung, including immunocytes (neutrophils, lymphocytes, eosinophils, dendritic cells), fibroblasts, smooth muscle cells and the airway epithelium [42].
Exposure of vascular smooth muscle cells to mercury ions (Hg2+) has been shown to induce the activation of ERK1/2 and P38 MAP kinases and is accompanied by activation of NADPH oxidase activity [203]. Activation of NOX has been implicated in cell death induced by exposure of cortical neurons to Zn2+ [204]. Similarly, arsenite has been shown to stimulate NOX activity in porcine aortic endothelial cells through a mechanism that involves the translocation of Rac1 [205]. Exposure of human promyelocytic leukemia cells to the metalloid arsenic, or the metals cadmium, mercury or nickel stimulates cell growth in a manner dependent on NOX activation [206].
As stated above, PKC activation is involved in the signaling leading to NOX activation. A number of metals have been shown to induce PKC activation. Microglial cells exposed to Zn2+ show PKC activation that is linked to NOX activation [207]. Vanadate-induced activation of protein kinase B (PKB, more commonly known as Akt) requires PKC activation in a mouse epidermal cell line [208]. A more recent study reported that Cd2+ exposure induces PKC activation and PKB/Akt activation in a signaling process that modulates autophagic and apoptotic death in kidney cells [209]. A recent field study found an association between T-cell immunosuppression and PKC expression and activation in a population exposed to arsenic from coal-burning [210]. The effect of Pb exposure on PKC, has proven more difficult to elucidate, with some studies showing activation while others report profound inhibition (Reviewed in Ref. [211]). As discussed earlier, metal exposure can result in intracellular calcium overload, which act as a cofactor to activate PKC through a calpain-dependent proteolytic activation mechanism [212].
2. Conclusions
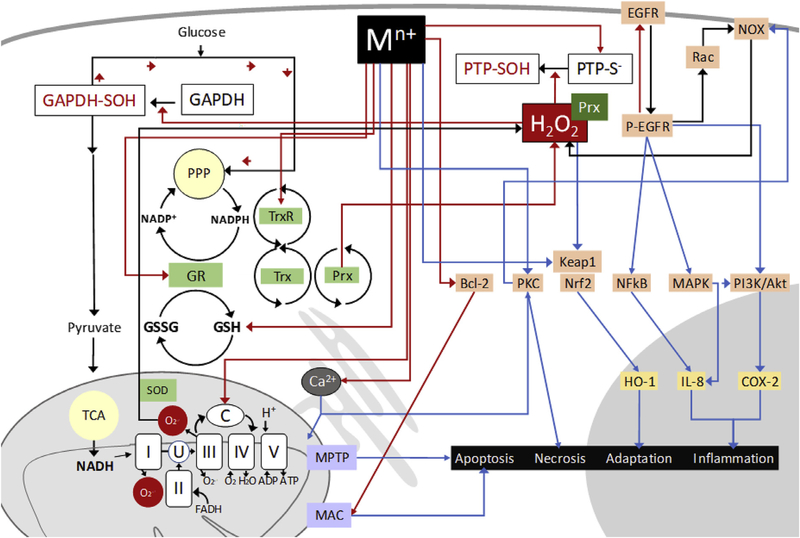
Metals commonly found in ambient particulate matter present an oxidative challenge to cells through multiple mechanisms. Non-redox cycling ions of zinc, cadmium, lead and chromium, as well as those of the redox-active metals mercury and vanadium interact with thiols to promote their oxidation or otherwise render them unavailable for function. When the thiol reactivity involves glutathione or the cysteinyl residues of antioxidant enzymes, the result is a diminished cellular capacity to maintain redox homeostasis in the face of oxidative pressure incurred by intrinsic metabolic functions such as energy metabolism, as well as that posed by external oxidative pressure. Coordination or oxidation of thiols essential to electron transport in the mitochondria increases the rate of superoxide generation, thereby increasing intracellular H2O2 levels. The inactivation of phosphatases, either directly by metal ions or by H2O2, results in unopposed basal or stimulated kinase activity leading to a sustained and disordered pan-activation of signaling processes, with the potential to alter gene expression, cell growth or survival (Fig. 1). Given the pivotal physiological roles of H2O2 in regulating intracellular signaling and bioenergetics, increased production of H2O2 as an outcome of signaling dysregulation has profound implications for cells exposed to PM metal ions, whether the metal ions are redox active or not.
Fig. 1.
Non-cycling mechanisms of oxidative stress induced by metals in ambient air particulate matter. Redox inert PM metal ions of Pb, Cr, Cd, Zn and As, as well as those of some redox active metals like V and Hg, can interact with cysteinyl thiols in glutathione (GSH) and regulatory proteins. Direct or H2O2-mediated inhibition of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) increases levels of reduced nicotinamide adenine dinucleotide phosphate (NADPH) through the pentose phosphate pathway (PPP), while inhibition of protein tyrosine phosphatases (PTP) allows unopposed basal kinase activity in the epidermal growth factor receptor (EGFR) and downstream signaling through mitogen activating kinase (MAPK), nuclear factor kappa B (NFkB) and phosphatidyl-inositol-3 kinase (PI3K/Akt), leading to downstream signaling, inflammatory gene expression and activation of NADPH oxidase (NOX), a source of H2O2. Metal effects on mitochondrial proteins impairs electron flow, increasing the production of superoxide anion (O2.−) which is dismutated to H2O2 by superoxide dismutase (SOD). Dysregulation of Ca2+ transport increases its levels in the mitochondria and the cytosol, promoting the opening of the membrane permeability transition pore (MPTP) and the activation of NOX by kinases such as protein kinase C (PKC). Oxidative effects are potentiated by direct metal interaction with thiol-containing antioxidant enzymes glutathione reductase (GR), thioredoxin (Trx) and Trx-system proteins thioredoxin reductase (TrxR) and peroxiredoxin (Prx). Evidence is mounting for a pro-oxidant role of Prx in mediating intracellular reactions of H2O2. Binding to the metal sensing module in Keap1 leads to the activation of nuclear erythroid factor 2 kinase, hemeoxygenase-1 expression and adaptation. Metal-induced activation of B-cell lymphoma 2 (Bcl-2) can lead to apoptosis through the activation of the mitochondrial apoptosis-induced channel (MAC). Activating processes are depicted by blue arrows, inhibitory effects are shown in red arrows.
Acknowledgments
Disclaimer
The research described in this article has been reviewed by the National Health and Environmental Effects Research Laboratory, U.S. Environmental Protection Agency, and approved for publication. The contents of this article should not be construed to represent agency policy nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
Abbreviations
- Bcl-2
B-cell lymphoma 2
- EGFR
epidermal growth factor receptor
- GSH
glutathione
- GR
glutathione reductase
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HO-1
hemeoxygenase-1
- H2O2
hydrogen peroxide
- MPTP
membrane permeability transition pore
- MAC
mitochondrial apoptosis-induced channel
- MAPK
mitogen activated protein kinase
- NOX
NADPH oxidase
- NADPH
nicotinamide adenine dinucleotide phosphate
- Nrf2
nuclear erythroid factor 2 kinase
- NFkB
nuclear factor kappa B
- PM
particulate matter
- PPP
pentose phosphate pathway
- Prx
peroxiredoxin
- PI3K/Akt
phosphatidyl-inositol-3 kinase
- PKC
protein kinase C
- PTP
protein tyrosine phosphatases
- O2.−
superoxide anion
- SOD
superoxide dismutase
- Trx
thioredoxin
- TrxR
thioredoxin reductase
Footnotes
Declaration of competing interest
None.
References
- [1].Strak M, Janssen NA, Godri KJ, Gosens I, Mudway IS, Cassee FR, Lebret E, Kelly FJ, Harrison RM, Brunekreef B, Steenhof M, Hoek G, Respiratory health effects of airborne particulate matter: the role of particle size, composition, and oxidative potential-the RAPTES project, Environ. Health Perspect. 120 (2012) 1183–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chen LC, Lippmann M, Effects of metals within ambient air particulate matter (PM) on human health, Inhal. Toxicol. 21 (2009) 1–31. [DOI] [PubMed] [Google Scholar]
- [3].Kelly FJ, Fussell JC, Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter, Atmos. Environ. 60 (2012) 504–526. [Google Scholar]
- [4].Malandrino M, Casazza M, Abollino O, Minero C, Maurino V, Size resolved metal distribution in the PM matter of the city of Turin (Italy), Chemosphere 147 (2016) 477–489. [DOI] [PubMed] [Google Scholar]
- [5].Song F, Gao Y, Size Distributions of Trace Elements Associated with Ambient Particular Matter in the Affinity of a Major Highway in the New Jersey-New York Metropolitan Area Atmospheric Environment vol. 45, (2011), pp. 6714–6723. [Google Scholar]
- [6].Valko M, Jomova K, Rhodes CJ, Kuca K, Musilek K, Redox- and non-redox-metal-induced formation of free radicals and their role in human disease, Arch. Toxicol. 90 (2016) 1–37. [DOI] [PubMed] [Google Scholar]
- [7].Levina A, Lay PA, Redox chemistry and biological activities of chromium (III) complexes, in: Vincent JB (Ed.), The Nutritional Biochemistry of Chromium (III), Elsevier, 2019, pp. 281–321. [Google Scholar]
- [8].Aliaga ME, Lopez-Alarcon C, Barriga G, Olea-Azar C, Speisky H, Redox-active complexes formed during the interaction between glutathione and mercury and/or copper ions, J. Inorg. Biochem. 104 (2010) 1084–1090. [DOI] [PubMed] [Google Scholar]
- [9].Mattson MP, Metal-catalyzed disruption of membrane protein and lipid signaling in the pathogenesis of neurodegenerative disorders, Ann. N. Y. Acad. Sci. 1012 (2004) 37–50. [DOI] [PubMed] [Google Scholar]
- [10].Sergent O, Morel I, Cillard J, Involvement of metal ions in lipid peroxidation: biological implications, Met. Ions Biol. Syst. 36 (1999) 251–287. [PubMed] [Google Scholar]
- [11].Stadtman ER, Oxidation of free amino acids and amino acid residues in proteins by radiolysis and by metal-catalyzed reactions, Annu. Rev. Biochem. 62 (1993) 797–821. [DOI] [PubMed] [Google Scholar]
- [12].Kluska K, Adamczyk J, Krezel A, Metal binding properties of zinc fingers with a naturally altered metal binding site, Metallomics 10 (2018) 248–263. [DOI] [PubMed] [Google Scholar]
- [13].Giles NM, Watts AB, Giles GI, Fry FH, Littlechild JA, Jacob C, Metal and redox modulation of cysteine protein function, Chem. Biol. 10 (2003) 677–693. [DOI] [PubMed] [Google Scholar]
- [14].Ouyang Y, Peng Y, Li J, Holmgren A, Lu J, Modulation of thiol-dependent redox system by metal ions via thioredoxin and glutaredoxin systems, Metallomics 10 (2018) 218–228. [DOI] [PubMed] [Google Scholar]
- [15].English AM, Wilcox DE, Effects of metal ions on the oxidation and nitrosation of cysteine residues in proteins and enzymes, Met. Ions Biol. Syst. 38 (2001) 313–350. [PubMed] [Google Scholar]
- [16].Quig D, Cysteine metabolism and metal toxicity, Altern. Med. Rev. 3 (1998) 262–270. [PubMed] [Google Scholar]
- [17].Maret W, The redox biology of redox-inert zinc ions, Free Radic. Biol. Med. 134 (2019) 311–326. [DOI] [PubMed] [Google Scholar]
- [18].Stohs SJ, Bagchi D, Oxidative mechanisms in the toxicity of metal ions, Free Radic. Biol. Med. 18 (1995) 321–336. [DOI] [PubMed] [Google Scholar]
- [19].Cuypers A, Plusquin M, Remans T, Jozefczak M, Keunen E, Gielen H, Opdenakker K, Nair AR, Munters E, Artois TJ, Nawrot T, Vangronsveld J, Smeets K, Cadmium stress: an oxidative challenge, Biometals 23 (2010) 927–940. [DOI] [PubMed] [Google Scholar]
- [20].Ghio AJ, Stonehuerner J, Soukup JM, Dailey LA, Kesic MJ, Cohen MD, Iron diminishes the in vitro biological effect of vanadium, J. Inorg. Biochem. 147 (2015) 126–133. [DOI] [PubMed] [Google Scholar]
- [21].Emanuele S, D’Anneo A, Calvaruso G, Cernigliaro C, Giuliano M, Lauricella M, The double-edged sword profile of redox signaling: oxidative events as molecular switches in the balance between cell physiology and cancer, Chem. Res. Toxicol. 31 (2018) 201–210. [DOI] [PubMed] [Google Scholar]
- [22].Fra A, Yoboue ED, Sitia R, Cysteines as redox molecular switches and targets of disease, Front. Mol. Neurosci. 10 (2017) 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Groitl B, Jakob U, Thiol-based redox switches, Biochim. Biophys. Acta (2014) 1335–1343 1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Herrmann JM, Becker K, Dick TP, Highlight: dynamics of thiol-based redox switches, Biol. Chem. 396 (2015) 385–387. [DOI] [PubMed] [Google Scholar]
- [25].Nietzel T, Mostertz J, Hochgrafe F, Schwarzlander M, Redox regulation of mitochondrial proteins and proteomes by cysteine thiol switches, Mitochondrion 33 (2017) 72–83. [DOI] [PubMed] [Google Scholar]
- [26].Paulsen CE, Carroll KS, Orchestrating redox signaling networks through regulatory cysteine switches, ACS Chem. Biol. 5 (2010) 47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Paulsen CE, Carroll KS, Cysteine-mediated redox signaling: chemistry, biology, and tools for discovery, Chem. Rev. 113 (2013) 4633–4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Abiko Y, Nakai Y, Luong NC, Bianco CL, Fukuto JM, Kumagai Y, Interaction of quinone-related electron acceptors with hydropersulfide Na2S2: evidence for one-electron reduction reaction, Chem. Res. Toxicol. 32 (2019) 551–556. [DOI] [PubMed] [Google Scholar]
- [29].Abiko Y, Shinkai Y, Unoki T, Hirose R, Uehara T, Kumagai Y, Polysulfide Na2S4 regulates the activation of PTEN/Akt/CREB signaling and cytotoxicity mediated by 1,4-naphthoquinone through formation of sulfur adducts, Sci. Rep. 7 (2017) 4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ezerina D, Takano Y, Hanaoka K, Urano Y, Dick TP, N-acetyl cysteine functions as a fast-acting antioxidant by triggering intracellular H2S and sulfane sulfur production, Cell. Chem. Biol. 25 (2018) 447–459 e444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kumagai Y, Abiko Y, Environmental electrophiles: protein adducts, modulation of redox signaling, and interaction with persulfides/polysulfides, Chem. Res. Toxicol. 30 (2017) 203–219. [DOI] [PubMed] [Google Scholar]
- [32].Shinkai Y, Kumagai Y, Sulfane sulfur in toxicology: a novel defense system Against electrophilic stress, Toxicol. Sci. 170 (2019) 3–9. [DOI] [PubMed] [Google Scholar]
- [33].Abiko Y, Yoshida E, Ishii I, Fukuto JM, Akaike T, Kumagai Y, Involvement of reactive persulfides in biological bismethylmercury sulfide formation, Chem. Res. Toxicol. 28 (2015) 1301–1306. [DOI] [PubMed] [Google Scholar]
- [34].Marino SM, Gladyshev VN, Redox biology: computational approaches to the investigation of functional cysteine residues, Antioxidants Redox Signal. 15 (2011) 135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ferrer-Sueta G, Manta B, Botti H, Radi R, Trujillo M, Denicola A, Factors affecting protein thiol reactivity and specificity in peroxide reduction, Chem. Res. Toxicol. 24 (2011) 434–450. [DOI] [PubMed] [Google Scholar]
- [36].Roos G, Foloppe N, Messens J, Understanding the pK(a) of redox cysteines: the key role of hydrogen bonding, Antioxidants Redox Signal. 18 (2013) 94–127. [DOI] [PubMed] [Google Scholar]
- [37].Bonanata J, Turell L, Antmann L, Ferrer-Sueta G, Botasini S, Mendez E, Alvarez B, Coitino EL, The thiol of human serum albumin: acidity, microenvironment and mechanistic insights on its oxidation to sulfenic acid, Free Radic. Biol. Med. 108 (2017) 952–962. [DOI] [PubMed] [Google Scholar]
- [38].Poole LB, The basics of thiols and cysteines in redox biology and chemistry, Free Radic. Biol. Med. 80 (2015) 148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Valko M, Morris H, Cronin MT, Metals, toxicity and oxidative stress, Curr. Med. Chem. 12 (2005) 1161–1208. [DOI] [PubMed] [Google Scholar]
- [40].Krezel A, Maret W, The biological inorganic chemistry of zinc ions, Arch. Biochem. Biophys. 611 (2016) 3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sauer K, Thauer RK, Methyl-coenzyme M formation in methanogenic archaea. Involvement of zinc in coenzyme M activation, Eur. J. Biochem. 267 (2000) 2498–2504. [DOI] [PubMed] [Google Scholar]
- [42].Halliwell B, Gutteridge JMC, Free Radicals in Biology and Medicine, Oxfords University Press, New York, 2015. [Google Scholar]
- [43].Radabaugh TR, Aposhian HV, Enzymatic reduction of arsenic compounds in mammalian systems: reduction of arsenate to arsenite by human liver arsenate reductase, Chem. Res. Toxicol. 13 (2000) 26–30. [DOI] [PubMed] [Google Scholar]
- [44].Go YM, Jones DP, Thiol/disulfide redox states in signaling and sensing, Crit. Rev. Biochem. Mol. Biol. 48 (2013) 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Meyer AJ, Dick TP, Fluorescent protein-based redox probes, Antioxidants Redox Signal. 13 (2010) 621–650. [DOI] [PubMed] [Google Scholar]
- [46].Cheng WY, Tong H, Miller EW, Chang CJ, Remington J, Zucker RM, Bromberg PA, Samet JM, Hofer TP, An integrated imaging approach to the study of oxidative stress generation by mitochondrial dysfunction in living cells, Environ. Health Perspect. 118 (2010) 902–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Corteselli EM, Samet JM, Gibbs-Flournoy EA, Imaging approaches to assessments of toxicological oxidative stress using genetically-encoded fluorogenic sensors, J. Vis. Exp. 32 (2018) e56945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wages PA, Cheng WY, Gibbs-Flournoy E, Samet JM, Live-cell imaging approaches for the investigation of xenobiotic-induced oxidant stress, Biochim. Biophys. Acta 1860 (2016) 2802–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wages PA, Silbajoris R, Speen A, Brighton L, Henriquez A, Tong H, Bromberg PA, Simmons SO, Samet JM, Role of H2O2 in the oxidative effects of zinc exposure in human airway epithelial cells, Redox Biol. 3 (2014) 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Cheng WY, Larson JM, Samet JM, Monitoring intracellular oxidative events using dynamic spectral unmixing microscopy, Methods 66 (2014) 345–352. [DOI] [PubMed] [Google Scholar]
- [51].Filomeni G, Rotilio G, Ciriolo MR, Cell signalling and the glutathione redox system, Biochem. Pharmacol. 64 (2002) 1057–1064. [DOI] [PubMed] [Google Scholar]
- [52].Griffith OW, Mulcahy RT, The enzymes of glutathione synthesis: gamma-glutamylcysteine synthetase, Adv. Enzymol. Relat. Area Mol. Biol. 73 (1999) 209–267 xii. [DOI] [PubMed] [Google Scholar]
- [53].Couto N, Wood J, Barber J, The role of glutathione reductase and related enzymes on cellular redox homoeostasis network, Free Radic. Biol. Med. 95 (2016) 27–42. [DOI] [PubMed] [Google Scholar]
- [54].Mannervik B, Five decades with glutathione and the GSTome, J. Biol. Chem. 287 (2012) 6072–6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Brigelius-Flohe R, Maiorino M, Glutathione peroxidases, Biochim. Biophys. Acta 1830 (2013) 3289–3303. [DOI] [PubMed] [Google Scholar]
- [56].Fernandes AP, Glutaredoxins Holmgren A, glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system, Antioxidants Redox Signal. 6 (2004) 63–74. [DOI] [PubMed] [Google Scholar]
- [57].Hatahet F, Ruddock LW, Protein disulfide isomerase: a critical evaluation of its function in disulfide bond formation, Antioxidants Redox Signal. 11 (2009) 2807–2850. [DOI] [PubMed] [Google Scholar]
- [58].Farina M, Aschner M, Glutathione antioxidant system and methylmercury-induced neurotoxicity: an intriguing interplay, Biochim. Biophys. Acta Gen. Subj. (2019) 129285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Bishop GM, Dringen R, Robinson SR, Zinc stimulates the production of toxic reactive oxygen species (ROS) and inhibits glutathione reductase in astrocytes, Free Radic. Biol. Med. 42 (2007) 1222–1230. [DOI] [PubMed] [Google Scholar]
- [60].Tandogan B, Ulusu NN, Inhibition of purified bovine liver glutathione reductase with some metal ions, J. Enzym. Inhib. Med. Chem. 25 (2010) 68–73. [DOI] [PubMed] [Google Scholar]
- [61].Nemeti B, Anderson ME, Gregus Z, Glutathione synthetase promotes the reduction of arsenate via arsenolysis of glutathione, Biochimie 94 (2012) 1327–1333. [DOI] [PubMed] [Google Scholar]
- [62].Lee TC, Ho IC, Modulation of cellular antioxidant defense activities by sodium arsenite in human fibroblasts, Arch. Toxicol. 69 (1995) 498–504. [DOI] [PubMed] [Google Scholar]
- [63].Mylroie AA, Umbles C, Kyle J, Effect of dietary copper supplementation on eythrocyte superoxide dismutase activity, ceruloplasmin and related parameters in rats ingesting lead acetate, in: Hemphill (Ed.), Trace Substances in Environmental Health, University of Missouri Press, Columbia, 1984, pp. 497–504. [Google Scholar]
- [64].Howard JK, Human erythrocyte glutathione reductase and glucose 6-phosphate dehydrogenase activities in normal subjects and in persons exposed to lead, Clin. Sci. Mol. Med. 47 (1974) 515–520. [DOI] [PubMed] [Google Scholar]
- [65].Patil AJ, Bhagwat VR, Patil JA, Dongre NN, Ambekar JG, Jailkhani R, Das KK, Effect of lead (Pb) exposure on the activity of superoxide dismutase and catalase in battery manufacturing workers (BMW) of Western Maharashtra (India) with reference to heme biosynthesis, Int. J. Environ. Res. Public Health 3 (2006) 329–337. [DOI] [PubMed] [Google Scholar]
- [66].Mahboob M, Shireen KF, Atkinson A, Khan AT, Lipid peroxidation and antioxidant enzyme activity in different organs of mice exposed to low level of mercury, J. Environ. Sci. Health B 36 (2001) 687–697. [DOI] [PubMed] [Google Scholar]
- [67].Xiao Z, La Fontaine S, Bush AI, Wedd AG, Molecular mechanisms of glutaredoxin enzymes: versatile hubs for thiol-disulfide exchange between protein thiols and glutathione, J. Mol. Biol. 431 (2019) 158–177. [DOI] [PubMed] [Google Scholar]
- [68].Zhou S, Sorokina EM, Harper S, Li H, Ralat L, Dodia C, Speicher DW, Feinstein SI, Fisher AB, Peroxiredoxin 6 homodimerization and heterodimerization with glutathione S-transferase pi are required for its peroxidase but not phospholipase A2 activity, Free Radic. Biol. Med. 94 (2016) 145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Lu J, Holmgren A, The thioredoxin antioxidant system, Free Radic. Biol. Med. 66 (2014) 75–87. [DOI] [PubMed] [Google Scholar]
- [70].Netto LE, Antunes F, The roles of peroxiredoxin and thioredoxin in hydrogen peroxide sensing and in signal transduction, Mol. Cells 39 (2016) 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Stocker S, Van Laer K, Mijuskovic A, Dick TP, The conundrum of hydrogen peroxide signaling and the emerging role of peroxiredoxins as redox relay hubs, Antioxidants Redox Signal. 28 (2018) 558–573. [DOI] [PubMed] [Google Scholar]
- [72].Huang C, Ke Q, Costa M, Shi X, Molecular mechanisms of arsenic carcinogenesis, Mol. Cell. Biochem. 255 (2004) 57–66. [DOI] [PubMed] [Google Scholar]
- [73].Shi H, Shi X, Liu KJ, Oxidative mechanism of arsenic toxicity and carcinogenesis, Mol. Cell. Biochem. 255 (2004) 67–78. [DOI] [PubMed] [Google Scholar]
- [74].Branco V, Godinho-Santos A, Goncalves J, Lu J, Holmgren A, Carvalho C, Mitochondrial thioredoxin reductase inhibition, selenium status, and Nrf-2 activation are determinant factors modulating the toxicity of mercury compounds, Free Radic. Biol. Med. 73 (2014) 95–105. [DOI] [PubMed] [Google Scholar]
- [75].Peskin AV, Pace PE, Behring JB, Paton LN, Soethoudt M, Bachschmid MM, Winterbourn CC, Glutathionylation of the active site cysteines of peroxiredoxin 2 and recycling by glutaredoxin, J. Biol. Chem. 291 (2016) 3053–3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Myers CR, Antholine WE, Myers JM, The pro-oxidant chromium(VI) inhibits mitochondrial complex I, complex II, and aconitase in the bronchial epithelium: EPR markers for Fe-S proteins, Free Radic. Biol. Med. 49 (2010) 1903–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Rhee SG, Jeong W, Chang TS, Woo HA, Sulfiredoxin, the cysteine sulfinic acid reductase specific to 2-Cys peroxiredoxin: its discovery, mechanism of action, and biological significance, Kidney Int. Suppl. (2007) S3–8. [DOI] [PubMed] [Google Scholar]
- [78].Rhee SG, Woo HA, Multiple functions of peroxiredoxins: peroxidases, sensors and regulators of the intracellular messenger H(2)O(2), and protein chaperones, Antioxidants Redox Signal. 15 (2011) 781–794. [DOI] [PubMed] [Google Scholar]
- [79].Winterbourn CC, Hampton MB, Redox biology: signaling via a peroxiredoxin sensor, Nat. Chem. Biol. 11 (2015) 5–6. [DOI] [PubMed] [Google Scholar]
- [80].Koizumi T, Li ZG, Tatsumoto H, DNA damaging activity of cadmium in Leydig cells, a target cell population for cadmium carcinogenesis in the rat testis, Toxicol. Lett. 63 (1992) 211–220. [DOI] [PubMed] [Google Scholar]
- [81].Sakurai A, Nishimoto M, Himeno S, Imura N, Tsujimoto M, Kunimoto M, Hara S, Transcriptional regulation of thioredoxin reductase 1 expression by cadmium in vascular endothelial cells: role of NF-E2-related factor-2, J. Cell. Physiol. 203 (2005) 529–537. [DOI] [PubMed] [Google Scholar]
- [82].Kuras R, Reszka E, Wieczorek E, Jablonska E, Gromadzinska J, Malachowska B, Kozlowska L, Stanislawska M, Janasik B, Wasowicz W, Biomarkers of selenium status and antioxidant effect in workers occupationally exposed to mercury, J. Trace Elem. Med. Biol. 49 (2018) 43–50. [DOI] [PubMed] [Google Scholar]
- [83].Janasik B, Reszka E, Stanislawska M, Jablonska E, Kuras R, Wieczorek E, Malachowska B, Fendler W, Wasowicz W, Effect of arsenic exposure on NRF2-KEAP1 pathway and epigenetic modification, Biol. Trace Elem. Res. 185 (2018) 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Nishimoto M, Sakaue M, Hara S, Short-interfering RNA-mediated silencing of thioredoxin reductase 1 alters the sensitivity of HeLa cells toward cadmium, Biol. Pharm. Bull. 29 (2006) 543–546. [DOI] [PubMed] [Google Scholar]
- [85].Sies H, Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress, Redox Biol. 11 (2017) 613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Schuppe-Koistinen I, Moldeus P, Bergman T, Cotgreave IA, S-thiolation of human endothelial cell glyceraldehyde-3-phosphate dehydrogenase after hydrogen peroxide treatment, Eur. J. Biochem. 221 (1994) 1033–1037. [DOI] [PubMed] [Google Scholar]
- [87].Peralta D, Bronowska AK, Morgan B, Doka E, Van Laer K, Nagy P, Grater F, Dick TP, A proton relay enhances H2O2 sensitivity of GAPDH to facilitate metabolic adaptation, Nat. Chem. Biol. 11 (2015) 156–163. [DOI] [PubMed] [Google Scholar]
- [88].Sheline CT, Behrens MM, Choi DW, Zinc-induced cortical neuronal death: contribution of energy failure attributable to loss of NAD(+) and inhibition of glycolysis, J. Neurosci. 20 (2000) 3139–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Wolf MB, Baynes JW, Cadmium and mercury cause an oxidative stress-induced endothelial dysfunction, Biometals 20 (2007) 73–81. [DOI] [PubMed] [Google Scholar]
- [90].Murphy MP, How mitochondria produce reactive oxygen species, Biochem. J. 417 (2009) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Brand MD, The sites and topology of mitochondrial superoxide production, Exp. Gerontol. 45 (2010) 466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Fato R, Bergamini C, Leoni S, Lenaz G, Mitochondrial production of reactive oxygen species: role of complex I and quinone analogues, Biofactors 32 (2008) 31–39. [DOI] [PubMed] [Google Scholar]
- [93].Koopman WJ, Nijtmans LG, Dieteren CE, Roestenberg P, Valsecchi F, Smeitink JA, Willems PH, Mammalian mitochondrial complex I: biogenesis, regulation, and reactive oxygen species generation, Antioxidants Redox Signal. 12 (2010) 1431–1470. [DOI] [PubMed] [Google Scholar]
- [94].Orr AL, Vargas L, Turk CN, Baaten JE, Matzen JT, Dardov VJ, Attle SJ, Li J, Quackenbush DC, Goncalves RL, Perevoshchikova IV, Petrassi HM, Meeusen SL, Ainscow EK, Brand MD, Suppressors of superoxide production from mitochondrial complex III, Nat. Chem. Biol. 11 (2015) 834–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Ralph SJ, Moreno-Sanchez R, Neuzil J, Rodriguez-Enriquez S, Inhibitors of succinate: quinone reductase/Complex II regulate production of mitochondrial reactive oxygen species and protect normal cells from ischemic damage but induce specific cancer cell death, Pharm. Res. 28 (2011) 2695–2730. [DOI] [PubMed] [Google Scholar]
- [96].Handy DE, Loscalzo J, Redox regulation of mitochondrial function, Antioxidants Redox Signal. 16 (2012) 1323–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Mailloux RJ, Jin X, Willmore WG, Redox regulation of mitochondrial function with emphasis on cysteine oxidation reactions, Redox Biol. 2 (2014) 123–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Meyer JN, Leung MC, Rooney JP, Sendoel A, Hengartner MO, Kisby GE, Bess AS, Mitochondria as a target of environmental toxicants, Toxicol. Sci. 134 (2013) 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Meyer JN, Hartman JH, Mello DF, Mitochondrial toxicity, Toxicol. Sci. 162 (2018) 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Dineley KE, Richards LL, Votyakova TV, Reynolds IJ, Zinc causes loss of membrane potential and elevates reactive oxygen species in rat brain mitochondria, Mitochondrion 5 (2005) 55–65. [DOI] [PubMed] [Google Scholar]
- [101].Chavan H, Christudoss P, Mickey K, Tessman R, Ni HM, Swerdlow R, Krishnamurthy P, Arsenite effects on mitochondrial bioenergetics in human and mouse primary hepatocytes follow a nonlinear dose response, Oxid. Med. Cell. Longev. (2017) 92513032017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Link TA, von Jagow G, Zinc ions inhibit the QP center of bovine heart mitochondrial bc1 complex by blocking a protonatable group, J. Biol. Chem. 270 (1995) 25001–25006. [DOI] [PubMed] [Google Scholar]
- [103].Santos AC, Uyemura SA, Santos NA, Mingatto FE, Curti C, Hg(II)-induced renal cytotoxicity: in vitro and in vivo implications for the bioenergetic and oxidative status of mitochondria, Mol. Cell. Biochem. 177 (1997) 53–59. [DOI] [PubMed] [Google Scholar]
- [104].Mao WP, Zhang NN, Zhou FY, Li WX, Liu HY, Feng J, Zhou L, Wei CJ, Pan YB, He ZJ, Cadmium directly induced mitochondrial dysfunction of human embryonic kidney cells, Hum. Exp. Toxicol. 30 (2011) 920–929. [DOI] [PubMed] [Google Scholar]
- [105].Belyaeva EA, Korotkov SM, Saris NE, In vitro modulation of heavy metal-induced rat liver mitochondria dysfunction: a comparison of copper and mercury with cadmium, J. Trace Elem. Med. Biol. 25 (Suppl 1) (2011) S63–S73. [DOI] [PubMed] [Google Scholar]
- [106].McCormack JG, Halestrap AP, Denton RM, Role of calcium ions in regulation of mammalian intramitochondrial metabolism, Physiol. Rev. 70 (1990) 391–425. [DOI] [PubMed] [Google Scholar]
- [107].Shen AN, Cummings C, Hoffman D, Pope D, Arnold M, Newland MC, Aging, motor function, and sensitivity to calcium channel blockers: an investigation using chronic methylmercury exposure, Behav. Brain Res. 315 (2016) 103–114. [DOI] [PubMed] [Google Scholar]
- [108].Hirano T, Murakami M, Fukada T, Nishida K, Yamasaki S, Suzuki T, Roles of zinc and zinc signaling in immunity: zinc as an intracellular signaling molecule, Adv. Immunol. 97 (2008) 149–176. [DOI] [PubMed] [Google Scholar]
- [109].Biagioli M, Pifferi S, Ragghianti M, Bucci S, Rizzuto R, Pinton P, Endoplasmic reticulum stress and alteration in calcium homeostasis are involved in cadmium-induced apoptosis, Cell Calcium 43 (2008) 184–195. [DOI] [PubMed] [Google Scholar]
- [110].Briston T, Roberts M, Lewis S, Powney B, M.S. J, Szabadkai G, Duchen MR, Mitochondrial permeability transition pore: sensitivity to opening and mechanistic dependence on substrate availability, Sci. Rep. 7 (2017) 10492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Boyman L, Coleman AK, Zhao G, Wescott AP, Joca HC, Greiser BM, Karbowski M, Ward CW, Lederer WJ, Dynamics of the mitochondrial permeability transition pore: transient and permanent opening events, Arch. Biochem. Biophys. 666 (2019) 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Belyaeva EA, Sokolova TV, Emelyanova LV, Zakharova IO, Mitochondrial electron transport chain in heavy metal-induced neurotoxicity: effects of cadmium, mercury, and copper, ScientificWorldJournal 2012 (2012) 136063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Ma L, Bi KD, Fan YM, Jiang ZY, Zhang XY, Zhang JW, Zhao J, Jiang FL, Dong JX, In vitro modulation of mercury-induced rat liver mitochondria dysfunction, Toxicol. Res. 7 (2018) 1135–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Bustamante J, Nutt L, Orrenius S, Gogvadze V, Arsenic stimulates release of cytochrome c from isolated mitochondria via induction of mitochondrial permeability transition, Toxicol. Appl. Pharmacol. 207 (2005) 110–116. [DOI] [PubMed] [Google Scholar]
- [115].Belyaeva EA, Glazunov VV, Korotkov SM, Cd2+ versus Ca2+-produced mitochondrial membrane permeabilization: a proposed direct participation of respiratory complexes I and III, Chem. Biol. Interact. 150 (2004) 253–270. [DOI] [PubMed] [Google Scholar]
- [116].Hosseini MJ, Shaki F, Ghazi-Khansari M, Pourahmad J, Toxicity of vanadium on isolated rat liver mitochondria: a new mechanistic approach, Metallomics 5 (2013) 152–166. [DOI] [PubMed] [Google Scholar]
- [117].Knight T, Luedtke D, Edwards H, Taub JW, Ge Y, A delicate balance - the BCL-2 family and its role in apoptosis, oncogenesis, and cancer therapeutics, Biochem. Pharmacol. 162 (2019) 250–261. [DOI] [PubMed] [Google Scholar]
- [118].Kinnally KW, Antonsson B, A tale of two mitochondrial channels, MAC and PTP, in apoptosis, Apoptosis 12 (2007) 857–868. [DOI] [PubMed] [Google Scholar]
- [119].Park WH, Kim SH, Arsenic trioxide induces human pulmonary fibroblast cell death via the regulation of Bcl-2 family and caspase-8, Mol. Biol. Rep. 39 (2012) 4311–4318. [DOI] [PubMed] [Google Scholar]
- [120].Xu J, Ji LD, Xu LH, Lead-induced apoptosis in PC 12 cells: involvement of p53, Bcl-2 family and caspase-3, Toxicol. Lett. 166 (2006) 160–167. [DOI] [PubMed] [Google Scholar]
- [121].Xu J, Lian LJ, Wu C, Wang XF, Fu WY, Xu LH, Lead induces oxidative stress, DNA damage and alteration of p53, Bax and Bcl-2 expressions in mice, Food Chem. Toxicol. 46 (2008) 1488–1494. [DOI] [PubMed] [Google Scholar]
- [122].Kim MS, Kim BJ, Woo HN, Kim KW, Kim KB, Kim IK, Jung YK, Cadmium induces caspase-mediated cell death: suppression by Bcl-2, Toxicology 145 (2000) 27–37. [DOI] [PubMed] [Google Scholar]
- [123].Fukamachi Y, Karasaki Y, Sugiura T, Itoh H, Abe T, Yamamura K, Higashi K, Zinc suppresses apoptosis of U937 cells induced by hydrogen peroxide through an increase of the Bcl-2/Bax ratio, Biochem. Biophys. Res. Commun. 246 (1998) 364–369. [DOI] [PubMed] [Google Scholar]
- [124].Untergasser G, Rumpold H, Plas E, Witkowski M, Pfister G, Berger P, High levels of zinc ions induce loss of mitochondrial potential and degradation of antiapoptotic Bcl-2 protein in in vitro cultivated human prostate epithelial cells, Biochem. Biophys. Res. Commun. 279 (2000) 607–614. [DOI] [PubMed] [Google Scholar]
- [125].Liang S, Sun K, Wang Y, Dong S, Wang C, Liu L, Wu Y, Role of Cyt-C/caspases-9,3, Bax/Bcl-2 and the FAS death receptor pathway in apoptosis induced by zinc oxide nanoparticles in human aortic endothelial cells and the protective effect by alpha-lipoic acid, Chem. Biol. Interact. 258 (2016) 40–51. [DOI] [PubMed] [Google Scholar]
- [126].Perry DK, Smyth MJ, Stennicke HR, Salvesen GS, Duriez P, Poirier GG, Hannun YA, Zinc is a potent inhibitor of the apoptotic protease, caspase-3. A novel target for zinc in the inhibition of apoptosis, J. Biol. Chem. 272 (1997) 18530–18533. [DOI] [PubMed] [Google Scholar]
- [127].Bodiga VL, Thokala S, Vemuri PK, Bodiga S, Zinc pyrithione inhibits caspase-3 activity, promotes ErbB1-ErbB2 heterodimerization and suppresses ErbB2 downregulation in cardiomyocytes subjected to ischemia/reperfusion, J. Inorg. Biochem. 153 (2015) 49–59. [DOI] [PubMed] [Google Scholar]
- [128].Lambert JC, Wang GW, Kang YJ, Zinc inhibition of caspase-3 activation does not protect HeLa cells from apoptotic cell death, Toxicol. Appl. Pharmacol. 175 (2001) 89–93. [DOI] [PubMed] [Google Scholar]
- [129].Lee DW, El Khoury Y, Francia F, Zambelli B, Ciurli S, Venturoli G, Hellwig P, Daldal F, Zinc inhibition of bacterial cytochrome bc(1) reveals the role of cytochrome b E295 in proton release at the Q(o) site, Biochemistry 50 (2011) 4263–4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Kuznetsova SS, Azarkina NV, Vygodina TV, Siletsky SA, Konstantinov AA, Zinc ions as cytochrome C oxidase inhibitors: two sites of action, Biochemistry (Mosc.) 70 (2005) 128–136. [DOI] [PubMed] [Google Scholar]
- [131].Muramoto K, Hirata K, Shinzawa-Itoh K, Yoko-o S, Yamashita E, Aoyama H, Tsukihara T, Yoshikawa S, A histidine residue acting as a controlling site for dioxygen reduction and proton pumping by cytochrome c oxidase, Proc. Natl. Acad. Sci. U. S. A. 104 (2007) 7881–7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Qin L, Mills DA, Hiser C, Murphree A, Garavito RM, Ferguson-Miller S, Hosler J, Crystallographic location and mutational analysis of Zn and Cd inhibitory sites and role of lipidic carboxylates in rescuing proton path mutants in cytochrome c oxidase, Biochemistry 46 (2007) 6239–6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Vygodina TV, Zakirzianova W, Konstantinov AA, Inhibition of membrane-bound cytochrome c oxidase by zinc ions: high-affinity Zn2+-binding site at the P-side of the membrane, FEBS Lett. 582 (2008) 4158–4162. [DOI] [PubMed] [Google Scholar]
- [134].Mills DA, Schmidt B, Hiser C, Westley E, Ferguson-Miller S, Membrane potential-controlled inhibition of cytochrome c oxidase by zinc, J. Biol. Chem. 277 (2002) 14894–14901. [DOI] [PubMed] [Google Scholar]
- [135].Martino PL, Capitanio G, Capitanio N, Papa S, Inhibition of proton pumping in membrane reconstituted bovine heart cytochrome c oxidase by zinc binding at the inner matrix side, Biochim. Biophys. Acta 1807 (2011) 1075–1082. [DOI] [PubMed] [Google Scholar]
- [136].Graves JD, Krebs EG, Protein phosphorylation and signal transduction, Pharmacol. Ther. 82 (1999) 111–121. [DOI] [PubMed] [Google Scholar]
- [137].Weiel JE, Ahn NG, Seger R, Krebs EG, Communication between protein tyrosine and protein serine/threonine phosphorylation, Adv. Second Messenger Phosphoprotein Res. 24 (1990) 182–195. [PubMed] [Google Scholar]
- [138].Finkel T, Gutkind JS, Signal Transduction and Human Disease, John Wiley and Sons, Inc., Hoboken, NJ, 2003. [Google Scholar]
- [139].Lee EY, Silberman SR, Ganapathi MK, Petrovic S, Paris H, The phosphoprotein phosphatases: properties of the enzymes involved in the regulation of glycogen metabolism, Adv. Cyclic Nucl. Res. 13 (1980) 95–131. [PubMed] [Google Scholar]
- [140].Hardie DG, Roles of protein kinases and phosphatases in signal transduction, Symp. Soc. Exp. Biol. 44 (1990) 241–255. [PubMed] [Google Scholar]
- [141].Sontag JM, Sontag E, Protein phosphatase 2A dysfunction in Alzheimer’s disease, Front. Mol. Neurosci. 7 (2014) 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Houge G, Haesen D, Vissers LE, Mehta S, Parker MJ, Wright M, Vogt J, McKee S, Tolmie JL, Cordeiro N, Kleefstra T, Willemsen MH, Reijnders MR, Berland S, Hayman E, Lahat E, Brilstra EH, van Gassen KL, Zonneveld-Huijssoon E, de Bie CI, Hoischen A, Eichler EE, Holdhus R, Steen VM, Doskeland SO, Hurles ME, FitzPatrick DR, Janssens V, B56delta-related protein phosphatase 2A dysfunction identified in patients with intellectual disability, J. Clin. Investig. 125 (2015) 3051–3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Crispin JC, Hedrich CM, Tsokos GC, Gene-function studies in systemic lupus erythematosus, Nat. Rev. Rheumatol. 9 (2013) 476–484. [DOI] [PubMed] [Google Scholar]
- [144].Kowluru A, Matti A, Hyperactivation of protein phosphatase 2A in models of glucolipotoxicity and diabetes: potential mechanisms and functional consequences, Biochem. Pharmacol. 84 (2012) 591–597. [DOI] [PubMed] [Google Scholar]
- [145].Ruvolo PP, The broken “Off” switch in cancer signaling: PP2A as a regulator of tumorigenesis, drug resistance, and immune surveillance, BBA Clin. 6 (2016) 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Reynhout S, Janssens V, Physiologic functions of PP2A: lessons from genetically modified mice, Biochim. Biophys. Acta Mol. Cell Res. 1866 (2019) 31–50. [DOI] [PubMed] [Google Scholar]
- [147].SidAhmed-Mezi M, Kurcewicz I, Rose C, Louvel J, Sokoloff P, Pumain R, Laschet JJ, Mass spectrometric detection and characterization of atypical membrane-bound zinc-sensitive phosphatases modulating GABAA receptors, PLoS One 9 (2014) e100612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Rahman A, Khan KM, Al-Khaledi G, Khan I, Al-Shemary T, Over activation of hippocampal serine/threonine protein phosphatases PP1 and PP2A is involved in lead-induced deficits in learning and memory in young rats, Neurotoxicology (Little Rock) 33 (2012) 370–383. [DOI] [PubMed] [Google Scholar]
- [149].Tonks NK, Protein tyrosine phosphatases–from housekeeping enzymes to master regulators of signal transduction, FEBS J. 280 (2013) 346–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Samet JM, Tal TL, Toxicological disruption of signaling homeostasis: tyrosine phosphatases as targets, Annu. Rev. Pharmacol. Toxicol. 50 (2010) 215–235. [DOI] [PubMed] [Google Scholar]
- [151].Hunter T, Lindberg RA, Middlemas DS, Tracy S, van der Geer P, Receptor protein tyrosine kinases and phosphatases, Cold Spring Harbor Symp. Quant. Biol. 57 (1992) 25–41. [DOI] [PubMed] [Google Scholar]
- [152].Cromlish WA, Tang M, Kyskan R, Tran L, Kennedy BP, PTP1B-dependent insulin receptor phosphorylation/residency in the endocytic recycling compartment of CHO-IR cells, Biochem. Pharmacol. 72 (2006) 1279–1292. [DOI] [PubMed] [Google Scholar]
- [153].Ravichandran LV, Chen H, Li Y, Quon MJ, Phosphorylation of PTP1B at Ser (50) by Akt impairs its ability to dephosphorylate the insulin receptor, Mol. Endocrinol. 15 (2001) 1768–1780. [DOI] [PubMed] [Google Scholar]
- [154].Tsou RC, Rak KS, Zimmer DJ, Bence KK, Improved metabolic phenotype of hypothalamic PTP1B-deficiency is dependent upon the leptin receptor, Mol. Metab. 3 (2014) 301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Tsou RC, Zimmer DJ, De Jonghe BC, Bence KK, Deficiency of PTP1B in leptin receptor-expressing neurons leads to decreased body weight and adiposity in mice, Endocrinology 153 (2012) 4227–4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Zhan XL, Wishart MJ, Guan KL, Nonreceptor tyrosine phosphatases in cellular signaling: regulation of mitogen-activated protein kinases, Chem. Rev. 101 (2001) 2477–2496. [DOI] [PubMed] [Google Scholar]
- [157].Raugei G, Ramponi G, Chiarugi P, Low molecular weight protein tyrosine phosphatases: small, but smart, Cell. Mol. Life Sci. 59 (2002) 941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Lindberg RA, Quinn AM, Hunter T, Dual-specificity protein kinases: will any hydroxyl do? Trends Biochem. Sci. 17 (1992) 114–119. [DOI] [PubMed] [Google Scholar]
- [159].Planchon SM, Waite KA, Eng C, The nuclear affairs of PTEN, J. Cell Sci. 121 (2008) 249–253. [DOI] [PubMed] [Google Scholar]
- [160].Lee SR, Kwon KS, Kim SR, Rhee SG, Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor, J. Biol. Chem. 273 (1998) 15366–15372. [DOI] [PubMed] [Google Scholar]
- [161].Tanner JJ, Parsons ZD, Cummings AH, Zhou H, Gates KS, Redox regulation of protein tyrosine phosphatases: structural and chemical aspects, Antioxidants Redox Signal. 15 (2011) 77–97. [DOI] [PubMed] [Google Scholar]
- [162].Swarup G, Cohen S, Garbers DL, Inhibition of membrane phosphotyrosyl-protein phosphatase activity by vanadate, Biochem. Biophys. Res. Commun. 107 (1982) 1104–1109. [DOI] [PubMed] [Google Scholar]
- [163].Gordon JA, Use of vanadate as protein-phosphotyrosine phosphatase inhibitor, Methods Enzymol. 201 (1991) 477–482. [DOI] [PubMed] [Google Scholar]
- [164].Bevan AP, Drake PG, Yale JF, Shaver A, Posner BI, Peroxovanadium compounds: biological actions and mechanism of insulin-mimesis, Mol. Cell. Biochem. 153 (1995) 49–58. [DOI] [PubMed] [Google Scholar]
- [165].Huyer G, Liu S, Kelly J, Moffat J, Payette P, Kennedy B, Tsaprailis G, Gresser MJ, Ramachandran C, Mechanism of inhibition of protein-tyrosine phosphatases by vanadate and pervanadate, J. Biol. Chem. 272 (1997) 843–851. [DOI] [PubMed] [Google Scholar]
- [166].Ghio AJ, Silbajoris R, Carson JL, Samet JM, Biologic effects of oil fly ash, Environ. Health Perspect. 110 (Suppl 1) (2002) 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Samet JM, Stonehuerner J, Reed W, Devlin RB, Dailey LA, Kennedy TP, Bromberg PA, Ghio AJ, Disruption of protein tyrosine phosphate homeostasis in bronchial epithelial cells exposed to oil fly ash, Am. J. Physiol. 272 (1997) L426–L432. [DOI] [PubMed] [Google Scholar]
- [168].Samet JM, Silbajoris R, Wu W, Graves LM, Tyrosine phosphatases as targets in metal-induced signaling in human airway epithelial cells, Am. J. Respir. Cell Mol. Biol. 21 (1999) 357–364. [DOI] [PubMed] [Google Scholar]
- [169].Haase H, Maret W, Intracellular zinc fluctuations modulate protein tyrosine phosphatase activity in insulin/insulin-like growth factor-1 signaling, Exp. Cell Res. 291 (2003) 289–298. [DOI] [PubMed] [Google Scholar]
- [170].Wilson M, Hogstrand C, Maret W, Picomolar concentrations of free zinc(II) ions regulate receptor protein-tyrosine phosphatase beta activity, J. Biol. Chem. 287 (2012) 9322–9326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Wu W, Graves LM, Jaspers I, Devlin RB, Reed W, Samet JM, Activation of the EGF receptor signaling pathway in human airway epithelial cells exposed to metals, Am. J. Physiol. 277 (1999) L924–L931. [DOI] [PubMed] [Google Scholar]
- [172].Bellomo E, Hogstrand C, Maret W, Redox and zinc signalling pathways converging on protein tyrosine phosphatases, Free Radic. Biol. Med. 75 (Suppl 1) (2014) S9. [DOI] [PubMed] [Google Scholar]
- [173].Bellomo E, Massarotti A, Hogstrand C, Maret W, Zinc ions modulate protein tyrosine phosphatase 1B activity, Metallomics 6 (2014) 1229–1239. [DOI] [PubMed] [Google Scholar]
- [174].Bellomo E, Birla Singh K, Massarotti A, Hogstrand C, Maret W, The metal face of protein tyrosine phosphatase 1B, Coord. Chem. Rev. 327–328 (2016) 70–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Bellomo E, Abro A, Hogstrand C, Maret W, Domene C, Role of zinc and magnesium ions in the modulation of phosphoryl transfer in protein tyrosine phosphatase 1B, J. Am. Chem. Soc. 140 (2018) 4446–4454. [DOI] [PubMed] [Google Scholar]
- [176].Wu W, Wang X, Zhang W, Reed W, Samet JM, Whang YE, Ghio AJ, Zinc-induced PTEN protein degradation through the proteasome pathway in human airway epithelial cells, J. Biol. Chem. 278 (2003) 28258–28263. [DOI] [PubMed] [Google Scholar]
- [177].Kim JH, Cho H, Ryu SE, Choi MU, Effects of metal ions on the activity of protein tyrosine phosphatase VHR: highly potent and reversible oxidative inactivation by Cu2+ ion, Arch. Biochem. Biophys. 382 (2000) 72–80. [DOI] [PubMed] [Google Scholar]
- [178].Zhang YL, Zhang ZY, Low-affinity binding determined by titration calorimetry using a high-affinity coupling ligand: a thermodynamic study of ligand binding to protein tyrosine phosphatase 1B, Anal. Biochem. 261 (1998) 139–148. [DOI] [PubMed] [Google Scholar]
- [179].Cavigelli M, Li WW, Lin A, Su B, Yoshioka K, Karin M, The tumor promoter arsenite stimulates AP-1 activity by inhibiting a JNK phosphatase, EMBO J. 15 (1996) 6269–6279. [PMC free article] [PubMed] [Google Scholar]
- [180].Rehman K, Chen Z, Wang WW, Wang YW, Sakamoto A, Zhang YF, Naranmandura H, Suzuki N, Mechanisms underlying the inhibitory effects of arsenic compounds on protein tyrosine phosphatase (PTP), Toxicol. Appl. Pharmacol. 263 (2012) 273–280. [DOI] [PubMed] [Google Scholar]
- [181].Wang Q, Lu L, Yuan C, Pei K, Liu Z, Guo M, Zhu M, Potent inhibition of protein tyrosine phosphatase 1B by copper complexes: implications for copper toxicity in biological systems, Chem. Commun. 46 (2010) 3547–3549. [DOI] [PubMed] [Google Scholar]
- [182].Kim YM, Reed W, Wu W, Bromberg PA, Graves LM, Samet JM, Zn2+-induced IL-8 expression involves AP-1, JNK, and ERK activities in human airway epithelial cells, Am. J. Physiol. Lung Cell Mol. Physiol. 290 (2006) L1028–L1035. [DOI] [PubMed] [Google Scholar]
- [183].Tal TL, Graves LM, Silbajoris R, Bromberg PA, Wu W, Samet JM, Inhibition of protein tyrosine phosphatase activity mediates epidermal growth factor receptor signaling in human airway epithelial cells exposed to Zn2+, Toxicol. Appl. Pharmacol. 214 (2006) 16–23. [DOI] [PubMed] [Google Scholar]
- [184].Samet JM, Ghio AJ, Particle associated metals and oxidative stress in signaling, in: Borm K.D.a.P. (Ed.), Particle Toxicology, Taylor and Francis Group, LLC, Boca Raton, FL, 2007, pp. 161–175. [Google Scholar]
- [185].Samet JM, Graves LM, Quay J, Dailey LA, Devlin RB, Ghio AJ, Wu W, Bromberg PA, Reed W, Activation of MAPKs in human bronchial epithelial cells exposed to metals, Am. J. Physiol. 275 (1998) L551–L558. [DOI] [PubMed] [Google Scholar]
- [186].Wu W, Jaspers I, Zhang W, Graves LM, Samet JM, Role of Ras in metal-induced EGF receptor signaling and NF-kappaB activation in human airway epithelial cells, Am. J. Physiol. Lung Cell Mol. Physiol. 282 (2002) L1040–L1048. [DOI] [PubMed] [Google Scholar]
- [187].Wu W, Graves LM, Gill GN, Parsons SJ, Samet JM, Src-dependent phosphorylation of the epidermal growth factor receptor on tyrosine 845 is required for zinc-induced Ras activation, J. Biol. Chem. 277 (2002) 24252–24257. [DOI] [PubMed] [Google Scholar]
- [188].Samet JM, Dewar BJ, Wu W, Graves LM, Mechanisms of Zn(2+)-induced signal initiation through the epidermal growth factor receptor, Toxicol. Appl. Pharmacol. 191 (2003) 86–93. [DOI] [PubMed] [Google Scholar]
- [189].Wu W, Silbajoris RA, Whang YE, Graves LM, Bromberg PA, Samet JM, p38 and EGF receptor kinase-mediated activation of the phosphatidylinositol 3-kinase/Akt pathway is required for Zn2+-induced cyclooxygenase-2 expression, Am. J. Physiol. Lung Cell Mol. Physiol. 289 (2005) L883–L889. [DOI] [PubMed] [Google Scholar]
- [190].Zhang C, Ferrari R, Beezhold K, Stearns-Reider K, D’Amore A, Haschak M, Stolz D, Robbins PD, Barchowsky A, Ambrosio F, Arsenic promotes NF-Kappab-Mediated fibroblast dysfunction and matrix remodeling to impair muscle stem cell function, Stem Cells 34 (2016) 732–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Kennedy T, Ghio AJ, Reed W, Samet J, Zagorski J, Quay J, Carter J, Dailey L, Hoidal JR, Devlin RB, Copper-dependent inflammation and nuclear factor-kappaB activation by particulate air pollution, Am. J. Respir. Cell Mol. Biol. 19 (1998) 366–378. [DOI] [PubMed] [Google Scholar]
- [192].Jaspers I, Samet JM, Erzurum S, Reed W, Vanadium-induced kappaB-dependent transcription depends upon peroxide-induced activation of the p38 mitogen-activated protein kinase, Am. J. Respir. Cell Mol. Biol. 23 (2000) 95–102. [DOI] [PubMed] [Google Scholar]
- [193].O’Hara KA, Klei LR, Barchowsky A, Selective activation of Src family kinases and JNK by low levels of chromium(VI), Toxicol. Appl. Pharmacol. 190 (2003) 214–223. [DOI] [PubMed] [Google Scholar]
- [194].Phuagkhaopong S, Ospondpant D, Kasemsuk T, Sibmooh N, Soodvilai S, Power C, Vivithanaporn P, Cadmium-induced IL-6 and IL-8 expression and release from astrocytes are mediated by MAPK and NF-kappaB pathways, Neurotoxicology (Little Rock) 60 (2017) 82–91. [DOI] [PubMed] [Google Scholar]
- [195].Yin J, Wang AP, Li WF, Shi R, Jin HT, Wei JF, Sensitive biomarkers identification for differentiating Cd and Pb induced toxicity on zebrafish embryos, Environ. Toxicol. Pharmacol. 56 (2017) 340–349. [DOI] [PubMed] [Google Scholar]
- [196].Yamamoto M, Kensler TW, Motohashi H, The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis, Physiol. Rev. 98 (2018) 1169–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].McMahon M, Lamont DJ, Beattie KA, Hayes JD, Keap1 perceives stress via three sensors for the endogenous signaling molecules nitric oxide, zinc, and alkenals, Proc. Natl. Acad. Sci. U. S. A. 107 (2010) 18838–18843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].McMahon M, Swift SR, Hayes JD, Zinc-binding triggers a conformationalswitch in the cullin-3 substrate adaptor protein KEAP1 that controls transcription factor NRF2, Toxicol. Appl. Pharmacol. 360 (2018) 45–57. [DOI] [PubMed] [Google Scholar]
- [199].He X, Ma Q, Critical cysteine residues of Kelch-like ECH-associated protein 1 in arsenic sensing and suppression of nuclear factor erythroid 2-related factor 2, J. Pharmacol. Exp. Ther. 332 (2010) 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].He X, Lin GX, Chen MG, Zhang JX, Ma Q, Protection against chromium (VI)-induced oxidative stress and apoptosis by Nrf2. Recruiting Nrf2 into the nucleus and disrupting the nuclear Nrf2/Keap1 association, Toxicol. Sci. 98 (2007) 298–309. [DOI] [PubMed] [Google Scholar]
- [201].Sirokmany G, Donko A, Geiszt M, Nox/duox family of NADPH oxidases: lessons from knockout mouse models, Trends Pharmacol. Sci. 37 (2016) 318–327. [DOI] [PubMed] [Google Scholar]
- [202].Bedard K, Krause KH, The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology, Physiol. Rev. 87 (2007) 245–313. [DOI] [PubMed] [Google Scholar]
- [203].Aguado A, Galan M, Zhenyukh O, Wiggers GA, Roque FR, Redondo S, Pecanha F, Martin A, Fortuno A, Cachofeiro V, Tejerina T, Salaices M, Briones AM, Mercury induces proliferation and reduces cell size in vascular smooth muscle cells through MAPK, oxidative stress and cyclooxygenase-2 pathways, Toxicol. Appl. Pharmacol. 268 (2013) 188–200. [DOI] [PubMed] [Google Scholar]
- [204].Kim YH, Koh JY, The role of NADPH oxidase and neuronal nitric oxide synthase in zinc-induced poly(ADP-ribose) polymerase activation and cell death in cortical culture, Exp. Neurol. 177 (2002) 407–418. [DOI] [PubMed] [Google Scholar]
- [205].Smith KR, Klei LR, Barchowsky A, Arsenite stimulates plasma membrane NADPH oxidase in vascular endothelial cells, Am. J. Physiol. Lung Cell Mol. Physiol. 280 (2001) L442–L449. [DOI] [PubMed] [Google Scholar]
- [206].Mohammadi-Bardbori A, Rannug A, Arsenic, cadmium, mercury and nickel stimulate cell growth via NADPH oxidase activation, Chem. Biol. Interact. 224 (2014) 183–188. [DOI] [PubMed] [Google Scholar]
- [207].Mortadza SS, Sim JA, Stacey M, Jiang LH, Signalling mechanisms mediating Zn(2+)-induced TRPM2 channel activation and cell death in microglial cells, Sci. Rep. 7 (2017) 45032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Li J, Dokka S, Wang L, Shi X, Castranova V, Yan Y, Costa M, Huang C, Activation of aPKC is required for vanadate-induced phosphorylation of protein kinase B (Akt), but not p70S6k in mouse epidermal JB6 cells, Mol. Cell. Biochem. 255 (2004) 217–225. [DOI] [PubMed] [Google Scholar]
- [209].So KY, Oh SH, Cadmium-induced heme-oxygenase-1 expression plays dual roles in autophagy and apoptosis and is regulated by both PKC-delta and PKB/Akt activation in NRK52E kidney cells, Toxicology 370 (2016) 49–59. [DOI] [PubMed] [Google Scholar]
- [210].Zeng Q, Luo P, Gu J, Liang B, Liu Q, Zhang A, PKC theta-mediated Ca(2+)/NFAT signalling pathway may be involved in T-cell immunosuppression in coal-burning arsenic-poisoned population, Environ. Toxicol. Pharmacol. 55 (2017) 44–50. [DOI] [PubMed] [Google Scholar]
- [211].Kasten-Jolly J, Lawrence DA, The cationic (calcium and lead) and enzyme conundrum, J. Toxicol. Environ. Health B Crit. Rev. 21 (2018) 400–413. [DOI] [PubMed] [Google Scholar]
- [212].Steinberg SF, Mechanisms for redox-regulation of protein kinase C, Front. Pharmacol. 6 (2015) 128. [DOI] [PMC free article] [PubMed] [Google Scholar]