Abstract
Background and aims:
The transition of macrophage to foam cells is a major hallmark of early stage atherosclerotic lesions. This process is characterized by the accumulation of large cytoplasmic lipid droplets containing large quantities of cholesterol esters (CE), triacylglycerol (TAG) and phospholipid (PL). Although cholesterol and CE metabolism during foam cell formation has been broadly studied, little is known about the role of the glycerolipids (TAG and PL) in this context. Here we studied the contribution of glycerolipid synthesis to lipid accumulation, focusing specifically on the first and rate-limiting enzyme of the pathway: glycerol-3-phosphate acyltransferase (GPAT).
Methods:
We used RAW 264.7 cells and bone marrow derived macrophages (BMDM) treated with oxidized LDL (oxLDL)
Results:
We showed that TAG synthesis is induced during the macrophage to foam cell transition. The expression and activity of GPAT3 and GPAT4 also increased during this process, and these two isoforms were required for the accumulation of cell TAG and PL. Compared to cells from wildtype mice after macrophage to foam cell transition, Gpat4−/− BMDM released more pro-inflammatory cytokines and chemokines, suggesting that the activity of GPAT4 could be associated with a decrease in the inflammatory response, probably by sequestering signaling precursors into lipid droplets.
Conclusions:
Our results provide evidence that TAG synthesis directed by GPAT3 and GPAT4 is required for lipid droplet formation and the modulation of the inflammatory response during the macrophage-foam cell transition.
Keywords: Oxidized LDL, lipid droplets, glycerolipids
Graphical Abstract
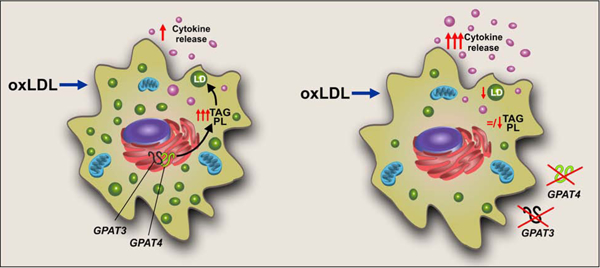
1. Introduction
Atherosclerosis is a leading cause of vascular disease worldwide [1]. It is a chronic disease characterized by the deposition of excessive lipids in the arterial intima. Early stage atherosclerotic lesions are characterized by a massive transition of macrophage to foam cells [2]. The generation of these cells has been associated with the uncontrolled incorporation of oxidized low density lipoproteins (oxLDL), lipoproteins with a high content of cholesterol esters (CE), an increase in cholesterol esterification, and impaired cholesterol efflux. These features result in the accumulation of large cytoplasmic lipid droplets (LD) consisting of a core of triacylglycerol (TAG) and CE surrounded by a monolayer of phospholipids (PL), free cholesterol (Chol), and LD-associated proteins [3–5]. Although for many years LDs had been considered to be inert organelles that stored neutral lipids, more recently they have attracted great interest as dynamic structures at the hub of lipid and energy metabolism, playing a central role in the development of multiple disorders [6]. In this context, LDs serve, in part, to buffer the cells against toxicity from excessive amounts of unesterified sterols. When macrophages are overwhelmed by excessive amounts of lipids, they undergo apoptotic and necrotic cell death, contributing to lesion instability and, ultimately, to stroke and heart attack [7].
Chol and CE metabolism during foam cell formation has been broadly studied [8–13], but little is known about the role of TAG and PL in this context. The de novo synthesis of these glycerolipids in mammalian cells begins with the acylation of glycerol-3-phosphate, catalyzed by glycerol-3-phosphate acyltransferase (GPAT, EC 2.3.1.15). This step is believed to be rate limiting [14]. As occurs in many other lipid metabolic reactions, this activity is catalyzed by several GPAT isoforms. Four different genes encode GPAT isoforms 1–4, which differ in tissue expression pattern, subcellular localization, fatty acyl-CoA substrate preference, and sensitivity to sulfhydryl reagents such as N-ethylmaleimide (NEM) [15]. GPAT1 (the product of the Gpam gene) and GPAT2 are mitochondrial isoforms, whereas GPAT3 (also known as AGPAT9/10) and GPAT4 (also known as AGPAT6) are located primarily in the ER [16]. Specific GPAT isoforms are thought to be responsible for glycerolipid synthesis in different cells; GPAT1 and GPAT4 are predominant in hepatocytes [16], and GPAT3 is the major isoform in differentiated adipocytes [17]. It is unknown which GPAT isoform accounts for the increase in TAG and PL content during macrophage to foam cell transition.
The aim of the present study was to determine the contribution of glycerolipid synthesis to the increased lipid content during the macrophage to foam cell transition. In order to elucidate the relevance of the ER isoforms for the cytokine and chemokine release profile observed during foam cell formation, we focused on the role of the GPAT isoforms.
2. Materials and methods
RAW 264.7 cells and BMDM were incubated with 100 µg/ml oxLDL and the accumulation of lipid droplets and different lipid species was analyzed. Metabolic radiolabeling assays were performed to analyze the de novo lipid synthesis after treatment. The expression and enzymatic activity of GPAT isoforms were assessed using qPCR and radiolabeling assays, respectively. Stable RAW264.7 cell lines in which Gpat3 was silenced (shGpat3), or BMDM obtained from knock out mice for Gpat3 or Gpat4 were subjected to similar lipid synthesis and composition analysis after oxLDL treatment. Finally, we analyzed the effect of these depletions in GPAT enzymatic activity and cytokines and chemokines release after oxLDL treatment.
Animal protocols were approved by the University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee.
Additional details of experimental procedures are included in Supplementary Materials.
3. Results
3.1. Lipid content, triacylglycerol synthesis and GPAT activity increase during macrophage to foam cell transition.
To study the glycerolipid dynamics during the macrophage to foam cell transition, we used two macrophage models treated with oxLDL: the murine RAW 264.7 cell line and mouse bone-marrow-derived macrophages (BMDM). LD growth was clearly observed after 24h oxLDL treatment in both cell types (Fig. 1A and B). RAW 264.7 cells showed a 25-fold increase in LD area while BMDM exhibited a 2.5-fold increment during their transition to foam cells (Fig. 1C and D left panel). We also analyzed changes in lipid content. As expected, CE and PL content increased during this process, consistent with the increase in LDs (Supplementary Fig. 3). In addition, TAG content also increased 2-fold and 11-fold after 24h oxLDL treatment of RAW 264.7 cells and BMDM, respectively (Fig. 1C and D right panel). These results show that the increment of LD in foam cells correlates with an increase, not only of CE and PL, but also of TAG.
FIGURE 1.
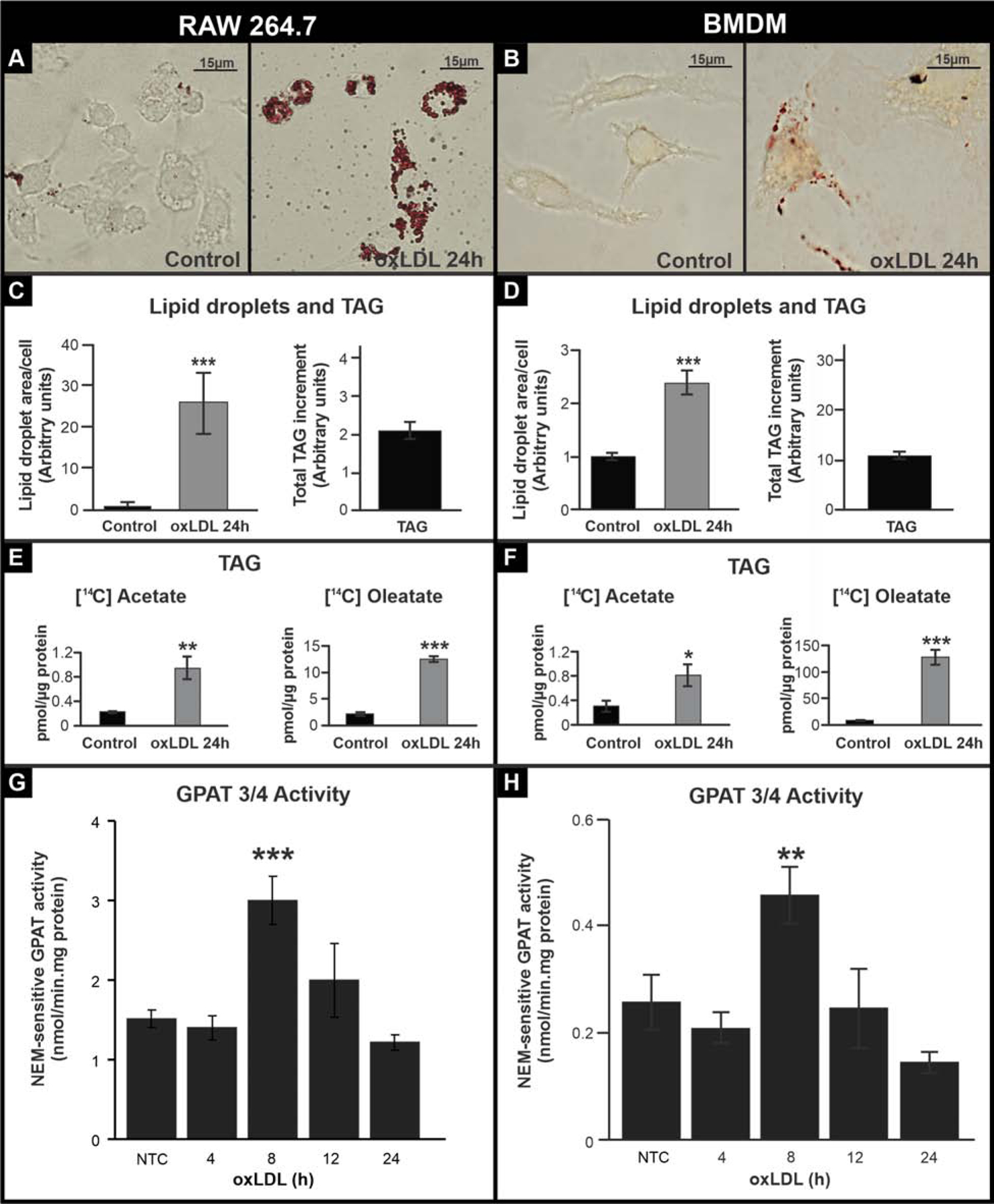
TAG content and de novo synthesis during macrophage to foam cell transition.
(A and B) oxLDL treatment of RAW 264.7 cells (left panel) and BMDM (right panel). Cells were treated for 24 h with oxLDL and fixed and stained with Oil red-O to visualize lipid droplets. (C and D) LD area per cell (average of 100 cells per treatment) and increase in TAG content in macrophages after 24 h oxLDL treatment compared to the control. (G-H) Cells were treated for 24 h with oxLDL and 2.2 mM [14C]acetate or 0.1 mM [14C]oleate (OA). Lipids were extracted, and lipid classes were analyzed by TLC. Radiolabeled lipids were quantified with a Bioscan scanner. (E and F) Cells were treated with oxLDL, and GPAT activity was assayed in total membranes in the presence or absence of NEM. NEM-sensitive GPAT activity (corresponding to GPAT3/4 activity) was calculated by subtracting NEM-resistant GPAT activity from the total. NTC: non-oxLDL treated control. Data are from three independent experiments. *** p<0.001, ** p<0.01, * p<0.05.
The greater glycerolipid content observed after foam cell formation could be the consequence of either an increase in lipid uptake, a decrease in catabolism, and/or the triggering of pathways of de novo synthesis. The increase in TAG was 50% of the CE increase in RAW cells and 37% in BMDM. Considering that the average of LDL TAG/CE content is only 10%, it seemed unlikely that the higher TAG content had occurred exclusively from a greater uptake of lipid, so we considered the possibility that TAG synthesis was up-regulated. We analyzed the incorporation of [14C]acetate and [14C]oleate into total lipids. The incorporation of [14C]acetate represents the use of de novo synthetized FA whereas the incorporation of [14C]oleate represents the use of exogenous pre-formed FA. Incorporation of both substrates into total lipids was either lower or did not change after foam cell development (Supplementary Fig. 4). However, when analyzing the distribution of radioactivity in different lipid fractions in both cell types, we observed that the incorporation of both substrates was diminished by about 50% in PL (Supplementary Fig. 4), whereas [14C]oleate incorporation into TAG increased 6-fold and 13-fold and [14C]acetate incorporation into TAG increased 5-fold and 3-fold during foam cell formation in RAW 264.7 cells and BMDM, respectively. These results indicate that TAG synthesis is induced during the macrophage to foam cell transition.
We then hypothesized that GPAT, the first and committed step in the de novo TAG synthesis pathway, would be up-regulated after foam cell transition. NEM-resistant activity (GPAT1) did not change (Supplementary Fig. 4), however after 8 h, NEM-sensitive GPAT activity (i.e. GPAT3 plus GPAT4) increased ~2-fold in both RAW 264.7 and BMDM (Fig. 1G and H). To determine which GPAT isoforms were responsible for the induction of TAG synthesis, we analyzed mRNA expression. In both RAW 264.7 cells and BMDM, Gpat1 and Gpat4 expression did not change after oxLDL treatment (Supplementary Fig. 5), and Gpat2 expression was very low and did not increase (data not shown). In contrast, Gpat3 was up-regulated in both cellular models. After the 8 h-oxLDL treatment, RAW 264.7 cell Gpat3 mRNA increased 35 fold and after 24h-oxLDL treatment, BMDM Gpat3 mRNA increased 10 fold (Supplementary Fig. 5). These results suggested that GPAT3 might be the GPAT isoform that contributes to the initiation of TAG synthesis during macrophage to foam cell transition.
3.2. GPAT3 and GPAT4 contribute to the increase in lipid droplet and TAG during foam cell formation.
Based on GPAT activity and expression results, and to determine the contribution of GPAT3 activity to the increase in LDs, TAG, and PL after foam cell formation, we silenced Gpat3 in RAW 264.7 cells (shGpat3 cell line) (Supplementary Fig. 1) and isolated BMDM from Gpat3−/− and Gpat4−/− mice [18]. Scrambled (SCR) RAW 264.7 cells and wt BMDM were used as controls. The increase in LD area observed in control cells after foam cell formation (Fig. 1A and B) was diminished by ~70%, ~40% and ~50% in shGpat3 cells, Gpat3−/− macrophages, and Gpat4−/− macrophages, respectively (Fig. 2). ShGpat3 cells accumulated 40% less TAG after their transition to foam cells compared to SCR controls, whereas in Gpat3−/− and Gpat4−/− BMDM, the increment in TAG content was 25% and 50% lower than in wt cells, respectively (Fig 2 C and D). PL accumulation was also diminished in the three models after 24h-oxLDL treatment, but no significant changes were observed in the amount of CE (Supplementary Fig. 6). These results demonstrate that not only GPAT3, but also GPAT4 is required for the observed increases in LDs, TAG, and PL during the macrophage to foam cell transition.
Figure 2.
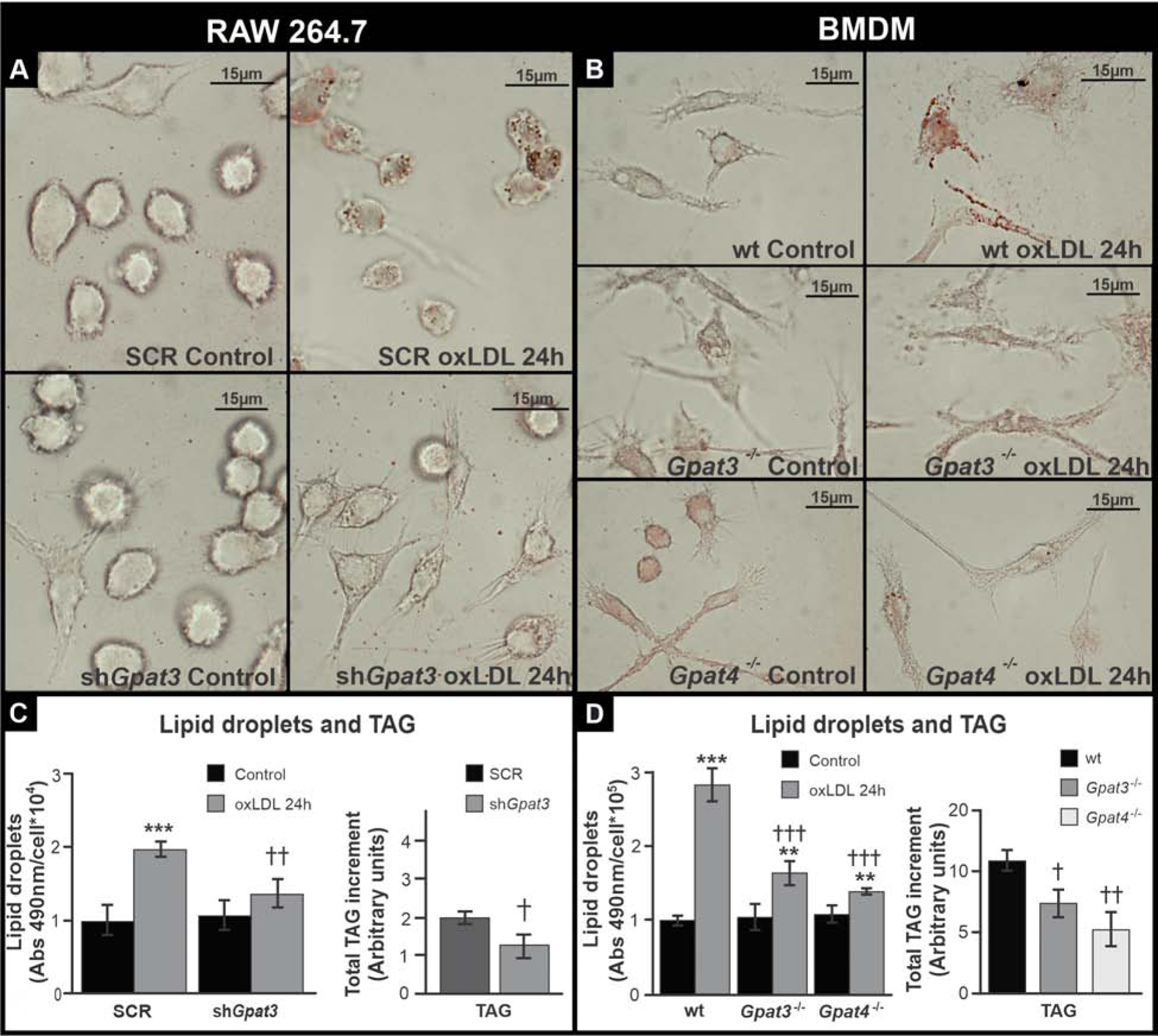
Effect of GPAT3 and GPAT4 silencing or knock-out on lipid accumulation after foam cell formation.
(A and C) RAW 264.7 SCR and shGpat3 cells. (B and D) Wt, Gpat3−/− and Gpat4−/− BMDM. (A and B) Cells were treated for 24 h with oxLDL, fixed, and stained with Oil red-O to visualize LDs. (C and D) LD and TAG content was analyzed colorimetrically after 24 h oxLDL treatment. Data are from three independent experiments. *** p<0.001, ** p<0.01 with respect to controls. ††† p<0.001, †† p<0.01 and † p<0.05 with respect to wt or SCR + oxLDL.
GPAT3 and GPAT4 are required for enhanced glycerolipid synthesis during macrophage to foam cell transition
To determine the contribution of GPAT3 versus GPAT4 to NEM-sensitive GPAT activity, specific activity was measured in shGpat3, and in Gpat3−/− and Gpat4−/− BMDM. In both shGpat3 RAW cells and in KO-BMDM treated for 8 h with oxLDL, NEM-sensitive GPAT activity did not increase (Fig. 3A and B), abrogating the peak in activity observed in wt and SCR cells (Fig. 1G). Interestingly, in Gpat4−/− BMDM, activity was ~50% lower than in wt even under basal conditions, without exposure to oxLDL, and remained stable throughout foam cell formation, suggesting that GPAT3 is involved in the changes observed in GPAT activity at 8 h. Nevertheless, LDs and TAG were lower also in Gpat4−/− BMDM (Fig. 2D).
FIGURE 3.
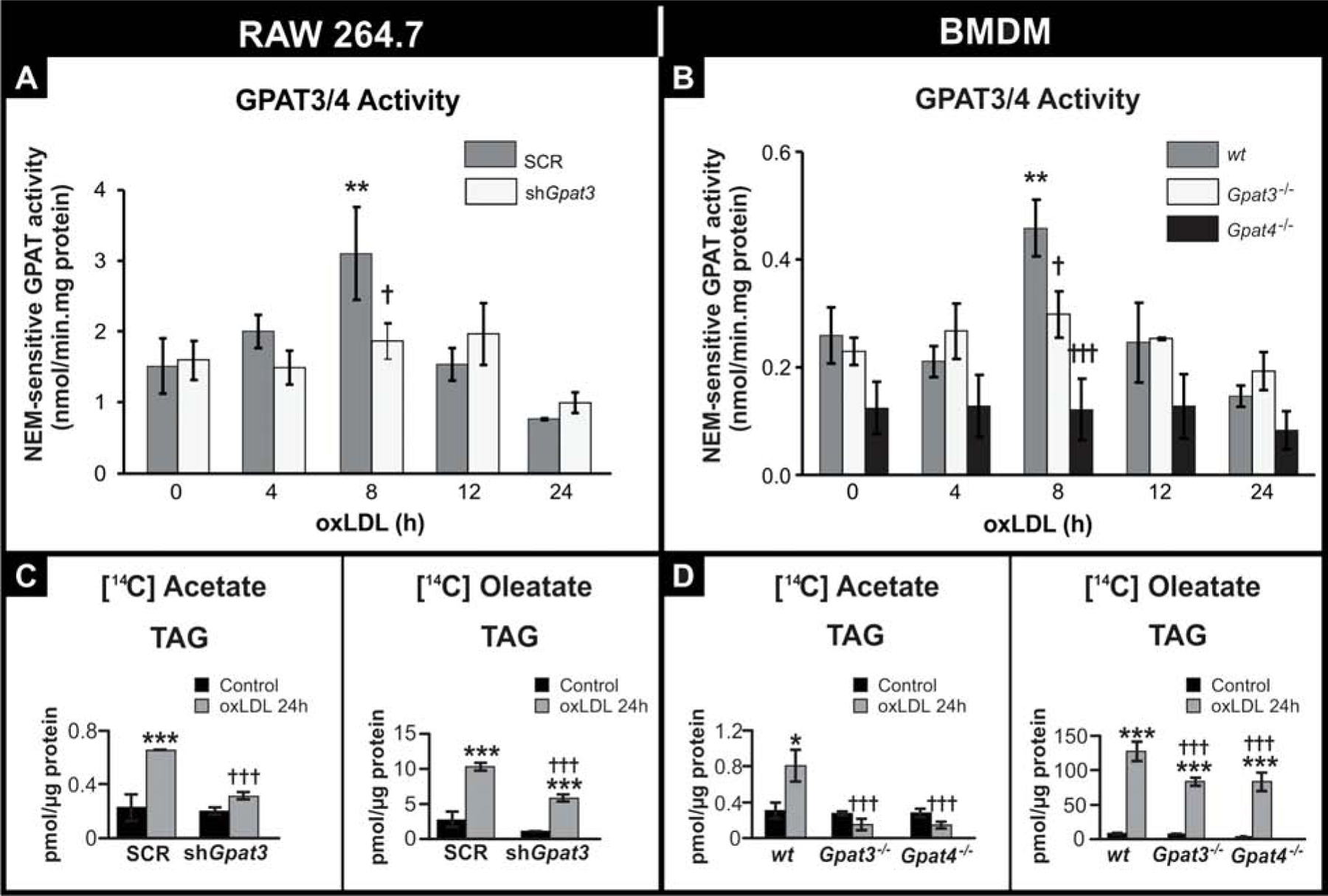
Effect of GPAT3 and GPAT4 silencing or knockout on GPAT activity and TAG synthesis after oxLDL treatment.
(A and C) RAW 264.7 SCR and shGpat3 cells. (B and D) wt, Gpat3−/− and Gpat4−/− BMDM. (A and B) Cells were treated for the indicated times with oxLDL, and GPAT activity was assayed in total membranes in the presence or absence of NEM. NEM-sensitive GPAT activity (corresponding to GPAT3/4 activity) was calculated by subtracting NEM-resistant GPAT activity from the total. (C and D) Cells were treated for 24 h with oxLDL and 2.2 mM [14C]acetate or 0.1 mM [14C]oleate. Lipids were extracted and separated by TLC. Radiolabeled TAG were quantified with a Bioscan scanner. Data for all figures are from three independent experiments. *** p<0.001, ** p<0.01, with respect to controls. ††† p<0.001 with respect to wt or SCR + oxLDL.
To establish the roles of GPAT3 and GPAT4 in the increase in TAG observed during the macrophage to foam cell transition, we next assessed glycerolipid synthesis after incubating shGpat3, and Gpat3−/− and Gpat4−/− macrophages with oxLDL and [14C]acetate or [14C]oleate. After foam cell transition, the amount of incorporated radioactivity from both radioactive precursors into total lipids decreased in the three types of GPAT-deficient cells compared to their controls (Supplementary Fig. 7). The reductions were primarily due to a decrease in incorporation into TAG (Fig. 3C and D). Supplementary Fig. 7 shows the radiolabeled substrate incorporation into the different lipid fractions. These results indicate that deficiency of either GPAT3 or GPAT4 in macrophages results in decreased GPAT activity and reduced TAG synthesis during macrophage to foam cell transition.
3.3. The lack of GPAT4 increases pro-inflammatory cytokine and chemokine release during macrophage to foam cell transition.
An increase in the release of pro-inflammatory cytokines and chemokines occurs during macrophage to foam cell transition during the formation of the arteriosclerotic plaque [19,20]. It has been proposed that cytokines are stored in LDs and that some phospholipids such as phosphatidylcholine might be essential for cytokine release [18,21–23]. Thus, we studied the effect of the absence of GPAT3 or GPAT4 on cytokine and chemokine release during the macrophage to foam cell transition. The absence of GPAT3 did not consistently modify the cytokine and chemokine release profile; in Gpat3−/− BMDM, TNF-α secretion increased significantly and MCP-1 release decreased, while IL-1, IL-6 and IL-10 showed no significant changes with respect to wt cells (Fig. 4). On the other hand, with respect to wt BMDM treated with oxLDL, Gpat4−/− BMDM increased their secretion of all the cytokines and chemokines analyzed. These results suggest that the activity of GPAT4 has an effect on the release of pro-inflammatory signaling molecules during the macrophage to foam cell transition.
FIGURE 4.
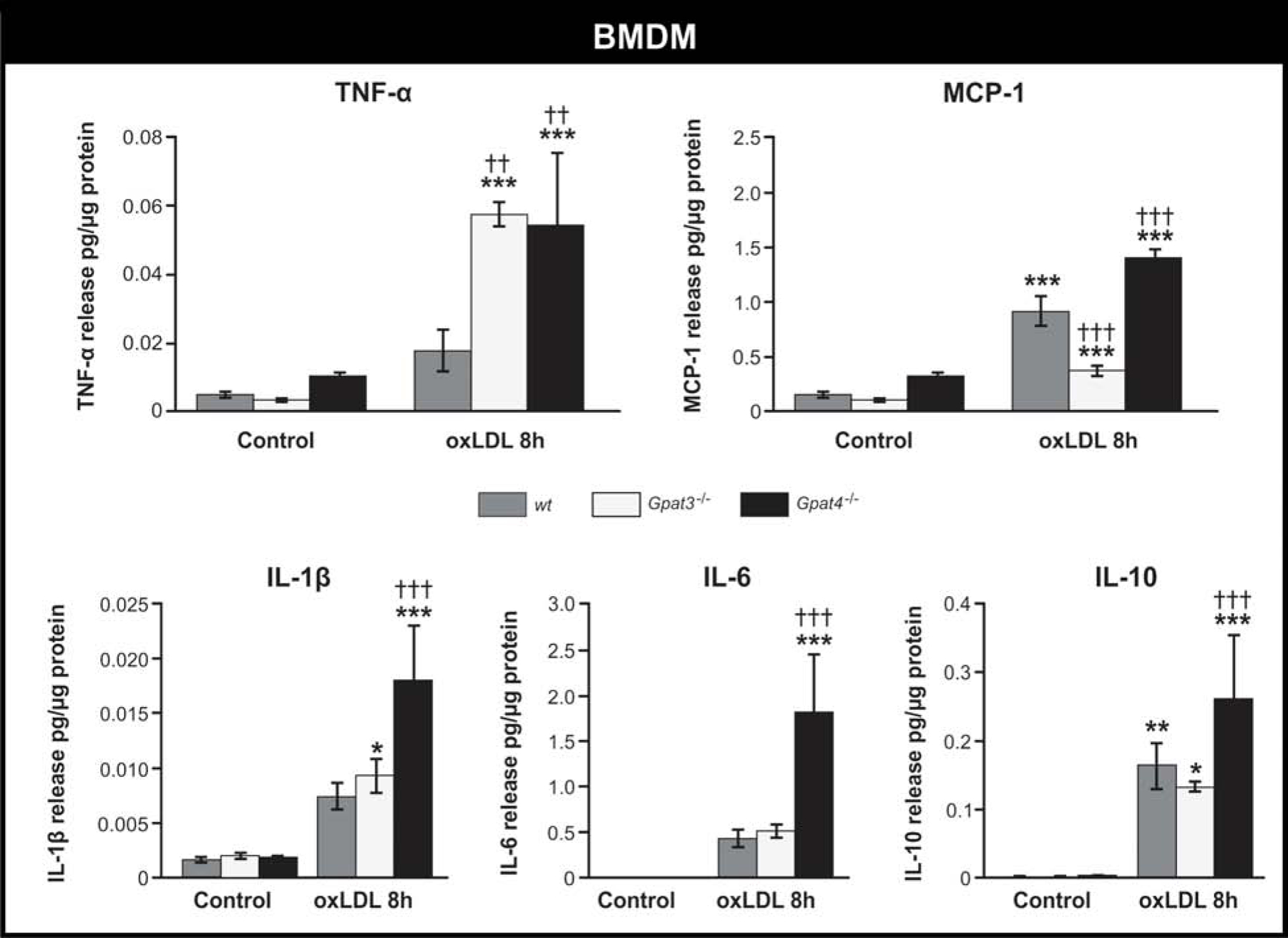
Effect of the lack of GPAT3 or GPAT4 on cytokine and chemokine release during macrophage to foam cell transition.
Wt, Gpat3−/−, and Gpat4−/− BMDM were treated with oxLDL for 8h, and the media was collected. Cytokine and chemokine concentrations were measured using LUMINEX MAGPIX technology. Data represent the average ± SD of 3 independent experiments. *** p<0.001, ** p<0.01, * p<0.05 with respect to the control. ††† p<0.001 and †† p<0.01, with respect to wt + oxLDL.
4. Discussion
Previous studies have shown that during foam cell formation, in addition to CE, TAG and PL also accumulate in LDs [24–26]. The primary purpose of the present study was to elucidate the contribution of glycerolipid synthesis to this lipid accumulation, to determine which of the 4 GPAT isoforms is activated during the macrophage to foam cell transition, and to learn whether this biosynthetic pathway influences macrophage function. Our data show that glycerolipid synthesis is induced during macrophage to foam cell transition, that macrophages incorporate both exogenous and de novo synthetized FAs, and that FA are preferentially incorporated into the TAG fraction. Enhanced TAG synthesis is consistent with the higher TAG content of the cores of the characteristic large droplets found in foam cells. Our data show that during foam cell transition both Gpat3 mRNA and GPAT3/4 activity are induced, and that both of these isoforms are required to initiate glycerolipid synthesis and the accumulation of TAG and PL in LDs. Our laboratory and others [18,27] have previously shown that Gpat3 and Gpat4 are also induced during the classical activation of macrophage, and that these enzymes are required for the increment in LDs observed during activation. Although the scenarios of classical activation and foam cell formation differ in many aspects, both are linked to processes of inflammation, which play a fundamental role in mediating all stages of atherosclerosis [28,29]. In this context, oxLDL are recognized and incorporated by macrophage scavenger receptors such as CD36 and scavenger receptor A (SRA) [29,30]. oxLDL are also ligands for TLR4 receptors that recognize bacteria and initiate the macrophage inflammatory response [31]. Further study is warranted to determine the relevance of signaling cascades activated after TLR4 or CD36/SRA stimulation, the possible crosstalk between them, and the effect of each one on lipid metabolism in the context of foam cell formation.
Foam cell formation involves an induction of lipid uptake, primarily CE, which is the main lipid component of the incorporated oxLDLs [32]. Within cells, CE is hydrolyzed into free FA and cholesterol. Subsequently, the induction of the acyl:cholesterol acyltransferase 1 (ACAT1) captures the newly released FAs and re-esterifies them into CE [33,34]. This activity, together with the induction of GPATs which also incorporate FAs into glycerolipids, might function as a strategy to avoid an increase in potentially toxic intracellular FAs [35].
Previous results have suggested that a certain amount of TAG is required in order to incorporate CE into the large LDs present in foam cells, because a reduction in droplet TAG content has been linked to a change in the physical state of the CE and a consequent altered structure of the TAG-depleted CE droplets [25]. The observed reduction in the total LD area and multiple small LDs in Gpat3−/− and Gpat4−/− is consistent with this hypothesis.
We next analyzed the functional role of the glycerolipids that accumulate during macrophage to foam cell transition. Since it is known that inflammation plays a central role in atherosclerosis [32], we analyzed the impact of lipid composition changes on the release of pro-inflammatory cytokines and chemokines. Even though in our model we use oxLDL preparations that might contain some intrinsic pro-inflammatory lipids that could account for part of the inflammatory response observed, the same oxLDL preparation was used in each genotype, allowing us to study the specific effect of Gpat4 and Gpat3 during this inflammatory process. Our results demonstrate that after foam cell formation, the lack of GPAT4, and the consequent reduction in stored TAG, results in an increased release of several pro-inflammatory cytokines and chemokines. We had observed a similar pattern in Gpat4−/− macrophages after classical activation [18]. These results suggest that GPAT4 might act by suppressing the inflammatory process not only during M1 macrophage activation, but also the transition of macrophage to foam cells. Even though the importance of lipid metabolism in macrophage funtion is clear, the molecular mechanisms linking GPAT4 activity and cytokine/chemokine release remain unknown. As we previously proposed [18], a possible mechanism by which GPAT4 could affect the release of pro-inflammatory molecules may involve the capacity of macrophages to incorporate FAs into TAG via GPAT4, and in that way, reduce the pool of specific FA precursors of pro-inflammatory signals, such as ceramides, eicosanoids, diacylglycerol and lysophosphatidic acid. Thus, sequestration of FA by GPAT4 activity could reduce inflammation. In agreement with this idea, a similar mechanism has been proposed for diacylglycerol acyltransferase 1 (DGAT1), the terminal enzyme of the same pathway, which esterifies acyl-CoAs to diacylglycerol to form TAG [36] and for Lipin 1, another enzyme upstream in the same glycerolipid synthesis pathway [37,38]. In addition, further connections between the glycerolipid synthesis pathway and the inflammatory process during atherogenesis have been established. Phosphatidylcholine, another product of this pathway, is essential for proper Golgi remodeling and vesicle liberation, and is thus required for the release of TNF-α and IL-6 [23]. TAG accumulation in LDs triggers apoptosis in foam cells, a striking aspect of atherogenesis [39], whereas phospholipase A2 regulates LD biogenesis [40] and macrophage intracellular lipids (such as FA, acyl-CoAs, and glycerolipids, among others) regulate a wide variety of signaling pathways [41–44]. Finally, mice lacking macrophage acyl:cholesterol acyltransferase 1 (ACAT1), which also uses FA to synthesize CE, develop increased atherosclerosis and exacerbated inflammation [45,46].
In summary, the present study demonstrates that TAG synthesis is induced during the transition of macrophage to foam cells and that GPAT3 and GPAT4 are both required for the increase in LDs, TAG and PL that occurs during this process. In addition, our results show that the lack of GPAT4 increases the release of inflammatory molecules, providing evidence that glycerolipid synthesis is critical for macrophage function during foam cell formation.
Although the present study has the limitation of being done in vitro, it suggests that inducing GPAT4 with the consequent sequestration of pro-inflammatory precursor molecules into TAG might be a therapeutic approach to reduce inflammation associated with atherosclerosis. Additional physiological assays using mouse models for atherosclerosis combined with an overexpression of GPAT4 would be necessary to test this hypothesis.
Supplementary Material
Highlights.
Lipid droplet triacylglycerol contents increase after macrophage to foam-cell transition
Triacylglycerol biosynthesis contributes to the increase in lipid droplets
GPAT3 and 4 are key enzymes required for the development of the foam-cell phenotype
GPAT4 modulates cytokine release during foam-cell formation
Acknowledgements
We thank Mario Raul Ramos for his assistance with the illustrations, Marianela Santana and Guillermina Mangione for their technical assistance and Dr. Pamela Young for her technical advice. We also thank Pfizer for providing the Gpat3−/− mice.
Financial support
This work was supported by ANPCyT PICT-2014 3214 and PICT-2017 3877, UNLP M202 (MRGB) and, NIH DK56598 (RAC).
ABBREVIATIONS:
- BMDM
Bone Marrow-derived macrophages
- CE
Cholesterol esters
- Chol
Cholesterol
- FA
Fatty acid
- GPAT
Glycerol-3-phosphate acyltransferase
- LD
Lipid droplet
- NEM
N-ethyl maleimide
- oxLDL
Oxidized low-density lipoprotein
- PL
Phospholipid
- SCR
Scrambled (negative control)
- TAG
triacylglycerol
- TLC
thin-layer chromatography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Herrington W, Lacey B, Sherliker P, Armitage J, Lewington S, Epidemiology of Atherosclerosis and the Potential to Reduce the Global Burden of Atherothrombotic Disease., Circ. Res 118 (2016) 535–46. 10.1161/CIRCRESAHA.115.307611. [DOI] [PubMed] [Google Scholar]
- [2].Ruparelia N, Chai JT, Fisher EA, Choudhury RP, Inflammatory processes in cardiovascular disease: A route to targeted therapies, Nat. Rev. Cardiol 14 (2017) 133–144. 10.1038/nrcardio.2016.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rader DJ, Pure E, Lipoproteins, macrophage function, and atherosclerosis: beyond the foam cell?, Cell Metab 1 (2005) 223–230. [DOI] [PubMed] [Google Scholar]
- [4].Yu XH, Fu YC, Zhang DW, Yin K, Tang CK, Foam cells in atherosclerosis, Clin.Chim.Acta 424:245–52 (2013) 245–252. [DOI] [PubMed] [Google Scholar]
- [5].Martin S, Parton RG, Lipid droplets: a unified view of a dynamic organelle, Nat Rev Mol Cell Biol 7 (2006) 373–378. 10.1038/nrm1912. [DOI] [PubMed] [Google Scholar]
- [6].Guo Y, Cordes KR, V Farese R Jr., T.C. Walther, Lipid droplets at a glance, J.Cell Sci 122 (2009) 749–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Maxfield FR, Tabas I, Role of cholesterol and lipid organization in disease, Nature 438 (2005) 612–621. [DOI] [PubMed] [Google Scholar]
- [8].Sekiya M, Osuga J, Igarashi M, Okazaki H, Ishibashi S, The role of neutral cholesterol ester hydrolysis in macrophage foam cells, J.Atheroscler.Thromb 18 (2011) 359–364. [DOI] [PubMed] [Google Scholar]
- [9].Ouimet M, Marcel YL, Regulation of lipid droplet cholesterol efflux from macrophage foam cells, Arterioscler.Thromb.Vasc.Biol 32 (2012) 575–581. [DOI] [PubMed] [Google Scholar]
- [10].Brown MS, Ho YK, Goldstein JL, The cholesteryl ester cycle in macrophage foam cells. Continual hydrolysis and re-esterification of cytoplasmic cholesteryl esters, J. Biol. Chem 255 (1980) 9344–9352. [PubMed] [Google Scholar]
- [11].Ghosh S, Macrophage cholesterol homeostasis and metabolic diseases: critical role of cholesteryl ester mobilization, Expert.Rev.Cardiovasc.Ther 9 (2011) 329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mahlberg FH, Glick JM, Jerome WG, Rothblat GH, Metabolism of cholesteryl ester lipid droplets in a J774 macrophage foam cell model, Biochim.Biophys.Acta 1045 (1990) 291–298. [DOI] [PubMed] [Google Scholar]
- [13].Chistiakov DA, V Bobryshev Y, Orekhov AN, Macrophage-mediated cholesterol handling in atherosclerosis, J.Cell Mol.Med 20 (2016) 17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bell RM, Coleman RA, Enzymes of glycerolipid synthesis in eukaryotes, Annu.Rev.Biochem 49 (1980) 459–487. [DOI] [PubMed] [Google Scholar]
- [15].Gonzalez-Baró MR, Lewin TM, Coleman RA, Regulation of Triglyceride Metabolism II. Function of mitochondrial GPAT1 in the regulation of triacylglycerol biosynthesis and insulin action, Am. J. Physiol. - Gastrointest. Liver Physiol. 292 (2007). 10.1152/ajpgi.00553.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wendel AA, Lewin TM, Coleman RA, Glycerol-3-phosphate acyltransferases: rate limiting enzymes of triacylglycerol biosynthesis, Biochim.Biophys.Acta 1791 (2009) 501–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cao J, Perez S, Goodwin B, Lin Q, Peng H, Qadri A, Zhou Y, Clark RW, Perreault M, Tobin JF, Gimeno RE, Mice deleted for GPAT3 have reduced GPATactivity in white adipose tissue and altered energy and cholesterol homeostasis in diet-induced obesity, Am. J. Physiol. - Endocrinol. Metab 306 (2014) E1176 http://ajpendo.physiology.org/content/306/10/E1176.abstract. [DOI] [PubMed] [Google Scholar]
- [18].Quiroga IY, Pellon-Maison M, Suchanek AL, Coleman RA, Gonzalez-Baro MR, Glycerol-3-phosphate acyltransferases 3 and 4 direct glycerolipid synthesis and affect functionality in activated macrophages., Biochem. J 476 (2019) 85–99. 10.1042/BCJ20180381. [DOI] [PubMed] [Google Scholar]
- [19].Bjorkbacka H, V Kunjathoor V, Moore KJ, Koehn S, Ordija CM, Lee MA, Means T, Halmen K, Luster AD, Golenbock DT, Freeman MW, Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways, Nat.Med 10 (2004) 416–421. [DOI] [PubMed] [Google Scholar]
- [20].Michelsen KS, Doherty TM, Shah PK, Arditi M, Role of Toll-like receptors in atherosclerosis, Circ.Res 95 (2004) e96–e97. [PubMed] [Google Scholar]
- [21].Bozza PT, Magalhães KG, Weller PF, Leukocyte lipid bodies - Biogenesis and functions in inflammation., Biochim. Biophys. Acta 1791 (2009) 540–51. 10.1016/j.bbalip.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Silva AR, Pacheco P, Vieira-de-Abreu A, Maya-Monteiro CM, D’Alegria B, Magalhaes KG, de Assis EF, Bandeira-Melo C, Castro-Faria-Neto HC, Bozza PT, Lipid bodies in oxidized LDL-induced foam cells are leukotriene-synthesizing organelles: a MCP-1/CCL2 regulated phenomenon, Biochim. Biophys. Acta - Mol. Cell Biol. Lipids 1791 (2009) 1066–1075. http://www.sciencedirect.com/science/article/pii/S1388198109001528. [DOI] [PubMed] [Google Scholar]
- [23].Tian Y, Pate C, Andreolotti A, Wang L, Tuomanen E, Boyd K, Claro E, Jackowski S, Cytokine secretion requires phosphatidylcholine synthesis, J. Cell Biol 181 (2008) 945 http://jcb.rupress.org/content/181/6/945.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Funk JL, Feingold KR, Moser AH, Grunfeld C, Lipopolysaccharide stimulation of RAW 264.7 macrophages induces lipid accumulation and foam cell formation, Atherosclerosis 98 (1993) 67–82. http://www.sciencedirect.com/science/article/pii/002191509390224I. [DOI] [PubMed] [Google Scholar]
- [25].Lada AT, Willingham MC, St Clair RW, Triglyceride depletion in THP-1 cells alters cholesteryl ester physical state and cholesterol efflux, J. Lipid Res 43 (2002) 618–628. [PubMed] [Google Scholar]
- [26].Rajamaki K, Lappalainen J, Oorni K, Valimaki E, Matikainen S, Kovanen PT, Eklund KK, Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation, PLoS.One 5 (2010) e11765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Feingold KR, Shigenaga JK, Kazemi MR, McDonald CM, Patzek SM, Cross AS, Moser A, Grunfeld C, Mechanisms of triglyceride accumulation in activated macrophages, J.Leukoc.Biol 92 (2012) 829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Libby P, Ridker PM, Maseri A, Inflammation and atherosclerosis, Circulation 105 (2002) 1135–1143. [DOI] [PubMed] [Google Scholar]
- [29].Prieur X, Roszer T, Ricote M, Lipotoxicity in macrophages: evidence from diseases associated with the metabolic syndrome, Biochim.Biophys.Acta 1801 (2010) 327–337. [DOI] [PubMed] [Google Scholar]
- [30].Endemann G, Stanton LW, Madden KS, Bryant CM, White RT, Protter AA, CD36 is a receptor for oxidized low density lipoprotein, J. Biol. Chem 268 (1993) 11811–11816. [PubMed] [Google Scholar]
- [31].Rhoads JP, Major AS, How oxidized low-density lipoprotein activates inflammatory responses, Crit. Rev. Immunol 38 (2018) 333–342. 10.1615/CritRevImmunol.2018026483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dushkin MI, Macrophage/foam cell is an attribute of inflammation: mechanisms of formation and functional role, Biochem. (Mosc.) 77 (2012) 327–338. [DOI] [PubMed] [Google Scholar]
- [33].Li AC, Glass CK, The macrophage foam cell as a target for therapeutic intervention, Nat.Med 8 (2002) 1235–1242. [DOI] [PubMed] [Google Scholar]
- [34].McLaren JE, Ramji DP, Interferon gamma: a master regulator of atherosclerosis, Cytokine Growth Factor Rev 20 (2009) 125–135. [DOI] [PubMed] [Google Scholar]
- [35].Unger RH, Orci L, Lipoapoptosis: its mechanism and its diseases, Lipids in Apoptosis 1585 (2002) 202–212. http://www.sciencedirect.com/science/article/pii/S1388198102003426. [DOI] [PubMed] [Google Scholar]
- [36].Koliwad SK, Streeper RS, Monetti M, Cornelissen I, Chan L, Terayama K, Naylor S, Rao M, Hubbard B, V Farese R Jr., DGAT1-dependent triacylglycerol storage by macrophages protects mice from diet-induced insulin resistance and inflammation, J. Clin. Invest 120 (2010) 756–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Vozenilek AE, Navratil AR, Green JM, Coleman DT, Blackburn CMR, Finney AC, Pearson BH, Chrast R, Finck BN, Klein RL, Orr AW, Woolard MD, Macrophage-Associated Lipin-1 Enzymatic Activity Contributes to Modified Low-Density Lipoprotein-Induced Proinflammatory Signaling and Atherosclerosis, Arterioscler. Thromb. Vasc. Biol 38 (2018) 324–334. 10.1161/ATVBAHA.117.310455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Meana C, Pena L, Lorden G, Esquinas E, Guijas C, Valdearcos M, Balsinde J, Balboa MA, Lipin-1 integrates lipid synthesis with proinflammatory responses during TLR activation in macrophages, J. Immunol 193 (2014) 4614–4622. [DOI] [PubMed] [Google Scholar]
- [39].Aflaki E, Radović B, Chandak PG, Kolb D, Eisenberg T, Ring J, Fertschai I, Uellen A, Wolinski H, Kohlwein SD, Zechner R, Levak-Frank S, Sattler W, Graier WF, Malli R, Madeo F, Kratky D, Triacylglycerol accumulation activates the mitochondrial apoptosis pathway in macrophages, J. Biol. Chem 286 (2011) 7418–7428. 10.1074/jbc.M110.175703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Guijas C, Rodríguez JP, Rubio JM, Balboa MA, Balsinde J, Phospholipase A2 regulation of lipid droplet formation., Biochim. Biophys. Acta 1841 (2014) 1661–71. 10.1016/j.bbalip.2014.10.004. [DOI] [PubMed] [Google Scholar]
- [41].Faergeman NJ, Knudsen J, Role of long-chain fatty acyl-CoA esters in the regulation of metabolism and in cell signalling, Biochem.J 323 (1997) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Freigang S, Ampenberger F, Weiss A, Kanneganti TD, Iwakura Y, Hersberger M, Kopf M, Fatty acid-induced mitochondrial uncoupling elicits inflammasome-independent IL-1alpha and sterile vascular inflammation in atherosclerosis, Nat.Immunol 14 (2013) 1045–1053. [DOI] [PubMed] [Google Scholar]
- [43].Yung YC, Stoddard NC, Chun J, LPA receptor signaling: pharmacology, physiology, and pathophysiology, J. Lipid Res 55 (2014) 1192–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kritikou E, van Puijvelde GH, van der Heijden T, van Santbrink PJ, Swart M, Schaftenaar FH, Kroner MJ, Kuiper J, Bot I, Inhibition of lysophosphatidic acid receptors 1 and 3 attenuates atherosclerosis development in LDL-receptor deficient mice, Sci.Rep 6:37585 d (2016) 37585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Fazio S, Major AS, Swift LL, Gleaves LA, Accad M, Linton MF, V Farese R Jr., Increased atherosclerosis in LDL receptor-null mice lacking ACAT1 in macrophages, J.Clin.Invest 107 (2001) 163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wakabayashi T, Takahashi M, Yamamuro D, Karasawa T, Takei A, Takei S, Yamazaki H, Nagashima S, Ebihara K, Takahashi M, Ishibashi S, Inflammasome activation aggravates cutaneous xanthomatosis and atherosclerosis in ACAT1 (acyl-CoA cholesterol acyltransferase 1) deficiency in bone marrow, Arterioscler. Thromb. Vasc. Biol 38 (2018) 2576–2589. 10.1161/ATVBAHA.118.311648. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


