Abstract
Cochlear implants (CIs) and hearing aids (HAs) are advanced assistive hearing devices that perform sound processing to achieve acoustic to acoustic/electrical stimulation, thus enabling the prospects for hearing restoration and rehabilitation. Since commercial CIs/HAs are typically constrained by manufacturer design/production constraints, it is necessary for researchers to use research platforms (RPs) to advance algorithms and conduct investigational studies with CI/HA subjects. While previous CI/HA research platforms exist, no study has explored establishing a formal evaluation protocol for the operational safety and reliability of RPs. This study proposes a two-phase analysis and evaluation paradigm for RPs. In the acoustic phase 1 step, a signal processing acoustic space is explored in order to present a sampled set of audio input content to explore the safety of the resulting output electric/acoustic stimulation. In the parameter phase 2 step, the configurable space for realizable electrical stimulation pulses is determined, and overall stimulation reliability and safety are evaluated. The proposed protocol is applied and demonstrated using Costakis Cochlear Implant Mobile. Assessment protocol observations, results, and additional best practices for subsampling of the acoustic and parameter test spaces are discussed. The proposed analysis-evaluation protocol establishes a viable framework for assessing RP operational safety and reliability. Guidelines for adapting the proposed protocol to address variability in RP configuration due to experimental factors such as custom algorithms, stimulation techniques, and/or individualization are also considered.
I. INTRODUCTION
Nearly a billion people worldwide, including approximately 50% of young adults between the ages of 12 and 35 in middle- and high-income countries, are at risk of developing hearing loss arising from noise exposure or use of personal audio devices (World Health Organization, 2015). Hearing aids (HAs) and cochlear implants (CIs), commonly referred to as assistive hearing devices (AHDs), are effective choices to restore auditory function for individuals with sensorineural hearing loss (Loizou, 1999). Clinical sound processors (SPs) have played a major role in enabling speech and auditory perception among CI patients. Modern SPs are designed with an emphasis on capturing both the slowly varying spectro-temporal envelope and rapidly varying fine structure effectively (Dudley, 1940; Flanagan and Golden, 1966; Fant, 1970; Tyler and Kelsay, 1990; Knutson et al., 1991; Zeng, 2008). All modern multi-electrode implants follow a tonotopic organization in the cochlea, namely, the apical part of the cochlea encodes low frequencies while the basal end encodes high frequencies. Filter banks are used to extract a frequency partition and to extract audio features to electrically stimulate corresponding electrodes in the cochlea (Wilson et al., 1991; Kiefer et al., 2001; Koch et al., 2004; Wilson and Dorman, 2008, Kąkol and Kostek, 2016). However, subjective studies using CI subjects with clinical SPs have reported high variability in audiological outcomes. This high degree of variability can be attributed to numerous factors, such as engineering limitations in the clinical SPs, individual subject implantation characteristics, individualistic preferences, and biological factors involved in electrical stimulation (Litovsky et al., 2017).
Researchers and scientists have taken to using hearing-based research platforms (RPs) to assess scientific speech/hearing theories of technology advancements. RPs are designed to enable flexible CI functionality, allowing researchers to develop and evaluate algorithms by carrying out psychophysical studies with CI subjects. These experimental interfaces allow researchers to customize the elements in the signal path on a trial-by-trial basis. Hence, most constraints and limitations that pose challenges with clinical SPs are eliminated. Modern RPs can be controlled using either computational devices, such as personal computers (PCs) or laptop/tablets or mobile/smartphone devices, allowing researchers the flexibility of developing algorithms in higher level programming languages and/or using open source algorithms/software. Additionally, researchers use patient information to personalize RP settings to address the idiosyncrasies of a CI subject. Typically, the process of RP personalization is also termed as mapping (MAP), implying the programming of RP to meet the specific needs of CI subject. A MAP is a memory unit that allows the researcher to specify characteristics related to the signal processing operations and electrical stimulation, such as pre-processing filters, electrode and filter bank characteristics, loudness growth function, electrical stimulation parameters, and other factors. Further, the electrical stimulation strategy encapsulates the stimulation sequence, spatial and temporal activity of the implanted electrode array. RPs have conventionally facilitated the development of electrical stimulation strategies (Backus et al., 2015). Interleaved activation of electrodes on a frame-by-frame basis in continuous-interleaved-sampling (CIS) strategy (Eddington et al., 1978; Wilson et al., 1991) and “n-of-m” electrode selection (Wilson et al., 1988) in Spectral Peak (SPEAK) and Advanced Combination Encoder (ACE) strategies (McDermott et al., 1992; Patrick et al., 2006) have significantly advanced speech intelligibility and auditory perception among CI subjects. Emerging techniques such as virtual channel and hybrid stimulation are being employed to develop advanced strategies such as HiRes120 (Koch et al., 2004) and four-electrode current steering schemes, respectively. As RPs continue to evolve in flexibility for benchtop/laboratory testing, it is critical for researchers to ensure that the platform achieves safe stimulation (Ali et al., 2013). RP flexibility should allow researchers to advance speech and other audiological outcomes among CI subjects without causing detrimental effects on the physiological function of the ear (e.g., either short-term or long-term effects).
Many studies, both human and animal, have focused on the impact of electrical stimulation of implanted electrodes on the cochlea (Walsh and Leake-Jones, 1982; Shepherd et al., 1983). There have been reportedly significant electrochemical differences between theoretical, in vitro, and in vivo studies (Green et al., 2014; Leung et al., 2014). Foundational principles established in cortical stimulation research have conventionally been used to establish limits on stimulation and on various stimulation parameters, such as charge per phase, charge density, pulse rate, and electrode geometry (Agnew et al., 1986; McCreery et al., 1986; Normann et al., 1999). Among CI subjects, comfort levels and loudness perception are directly dependent upon several electrical stimulation factors, such as stimulation mode, pulse rate, inter-phase gap (IPG), temporal envelope, stimulation current level, and number of channels (Shannon, 1983; Chatterjee and Zwislocki, 1998; Chatterjee et al., 2000; McKay et al., 2001). Depending upon higher [>300 pulses per second (pps)] or lower (100 pps) stimulation rates, loudness can be modeled as an exponential or power function of the pulse amplitude, respectively (Zeng and Shannon, 1994). IPG influences an increase in the magnitude of loudness (Prado-Guitierrez et al., 2006; Ramekers et al., 2014). The effect of IPG on loudness and neural excitation has also been found to be a significant factor when compared to stimulation rates (McKay and Henshall, 2003). Although existing research on stimulation-induced tissue damage remains unclear (i.e., whether due to physiological or toxic by-products), it is hypothesized to be dependent on the electrode and stimulation parameters (Huang and Shepherd, 1999). Stimulation-induced damage during CI stimulation can be caused due to biphasic charge imbalance and/or generation of electrochemical by-products. In biphasic pulses, the charge balance between anodic and cathodic phases must be established to prevent irreversible corrosion of electrodes and potential deposit of metal oxides at the electrode-tissue interface (McCreery et al., 1992; Fu et al., 2000). Concerns regarding clinical application of CIs were primarily addressed by ensuring biological/neural damage prevention (McCreery et al., 1990). Hence, many stimulation parameters are typically restricted to prescribing target-ranges to ensure safe and reliable performance of CIs (McCreery et al., 1995). However, unlike a clinical SP, the safety of a RP is not always guaranteed, and researchers may have to take additional steps and apply established ethical principles to ensure safe experimentation with CI subjects.
One of the earliest research interfaces, entitled the “Boys Town Nucleus Cochlear Implant,” was assessed for risk (Shannon et al., 1990). Many protective measures were undertaken in the design to prevent damage to the internal receiver, electrical imbalance, and neural aspects of RP. Based on these findings and several other stimulation safety-based studies, the US Food and Drug and Administration (FDA) prescribed guidelines and established safety limits on the maximum permissible charge density that can be applied on intracochlear electrodes. The FDA set an upper limit of up to 216 mC/cm2 for CIs with clinical SPs and a conservative upper limit of 100 mC/cm2 for RPs (Shannon, 1992). Along with stimulation parameters, some other factors involved in designing RPs are cost, real-time/offline processing, benchtop vs portability, unilateral-bilateral and/or bimodal, electric and/or acoustic simulation, ease of programmability (high level languages such as matlab and java), suitability for complex algorithms, and suitability for unsupervised take-home trials. In addition to these factors, RPs need to comply with regulatory guidelines established by the FDA. CI manufacturers, including Advanced Bionics, Cochlear Corp., MED-EL, and others, are key players involved in developing popular RPs, such as Clarion (Shannon et al., 1999; Shannon et al., 2002; Cochlear Corp., 2020; MED-EL, 2020; Advanced Bionics, 2020), SPAIDE (Van Immerseel et al., 2005), Nucleus Implant Communicator (CRC and HearWorks, 2003a, 2003b; Cochlear Corp., 2006; Stohl et al., 2008), Apex (Francart et al., 2008), and Research Interface Box (University of Innsbruck, 2001). The design of the Spectral Maxima Sound Processor allowed researchers to use built-in test functionality that generated a continuous pulse train at a comfortable level to verify the safety of the system function (McDermott et al., 1993). SPAIDE has a built-in stimulation check that continuously monitors for configuration inconsistencies, large and/or unbalanced intracochlear current. Upon detection, SPAIDE is designed to halt stimulation, ensuring patient safety (Van Immerseel et al., 2005). Safety evaluation of high rate stimulation and acute, chronic, physiologic, and histologic evaluation of NIC-CI24RE were carried out with both humans and animals (Patrick et al., 2006). In our previous CI-PDA-based RP, the interface board was completely isolated and inaccessible to researchers, thereby preventing any user hardware modifications that could jeopardize safety issues (Ali et al., 2013). Error checking routines that monitor stimulation parameters were also built into the software on our previous CI-PDA, and safety was primarily ensured by constraining the stimulation parameters to comply with safe limits established by the FDA and Cochlear Corp. In summary, many stimulation safety-based studies focused on RPs discuss some aspects relating to their test evaluation for continuous monitoring, software checks, and testing, without actually formulating and publishing the details of a safety evaluation. Modern CI research has expanded upon traditional speech-based areas, where the focus has shifted toward naturalistic data, music, and environmental sound categories in recent years (Spitzer et al., 1992; Tye-Murray et al., 1992; Tyler and Lowder, 1992; Zhao et al., 1997; Gfeller et al., 1997; Deller et al., 2000; Hinderink et al., 2000; Vandali et al., 2005; Damen et al., 2007; Looi et al., 2008a, 2008b; Cooper et al., 2008; Crew and Galvin, 2012). Many subjective studies consider RPs to be safe, and the possible impact of introducing custom SP algorithm or stimulation strategies is not examined (Skinner et al., 1994; Mo et al., 2005; Reed and Delhorne, 2005; Loizou, 2006; Chasin, 2012; Shokouhi et al., 2015; Shokouhi and Hansen, 2017). The responsibility of safe stimulation also rests on the ethical approaches adopted by researchers, which include CI subject instructions to abort overstimulation by quickly removing their transmission coil (Litovsky et al., 2017).
Recently, a total of 20 consensus statements on the use of unilateral cochlear implantation in adults with Sensorineural Hearing Loss (SNHL) were identified and assessed in several areas, such as level of awareness of cochlear implantation. These include best practice of the clinical pathway from diagnosis to surgery; best practice guidelines for surgery; clinical effectiveness of cochlear implantation; factors associated with post-implantation outcomes; the association between hearing loss and depression, cognition, and dementia; and cost implications of cochlear implantation (Buchman et al., 2020). To our knowledge, this current proposed analysis and evaluation protocol is the first study that is devoted specifically to identifying steps required to assess the safety of RP in a systematic manner. The remaining structure of this study is as follows. The proposed approach aimed at establishing a standard test protocol that can be easily adopted to assess RP safety is presented in Sec. II. The proposed protocol is applied in two phases: (i) the acoustic phase 1 step and (ii) the parameter phase 2 step, addressing both acoustic sound exposure a CI user might experience in daily naturalistic settings and the potential range of parameter configurations a researcher may explore for a given CI system. To evaluate the proposed protocol, the Costakis Cochlear Implant Mobile (CCi-MOBILE) RP is used (discussed in Sec. III). Sections IV and V describe the specific makeup of the RP test protocol, including the acoustic sound test battery, methodology, observation, and results with CCi-MOBILE. Section VI illustrates the development of the robust burn-in protocol with a reduced data/parameter set of conditions in the acoustic test battery. Guidelines presented in Sec. VI can be applied to develop a synoptic protocol to assess RP under minor adjustments in a subject's MAP, custom algorithms, and/or CI strategy.
NOTE: In this study, the focus is on establishing a viable test/analysis protocol (e.g., burn-in protocol) that can be applied to assess the functionality of any RP. This protocol is then applied to the current CCi-MOBILE platform to demonstrate the viability of this proposed burn-in solution.
II. PROPOSED ANALYSIS AND EVALUATION PROTOCOL
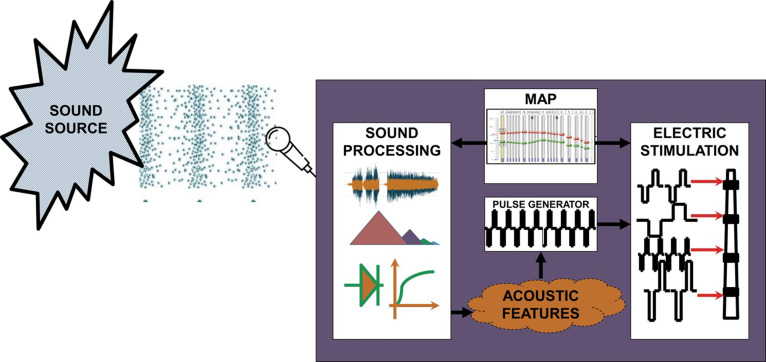
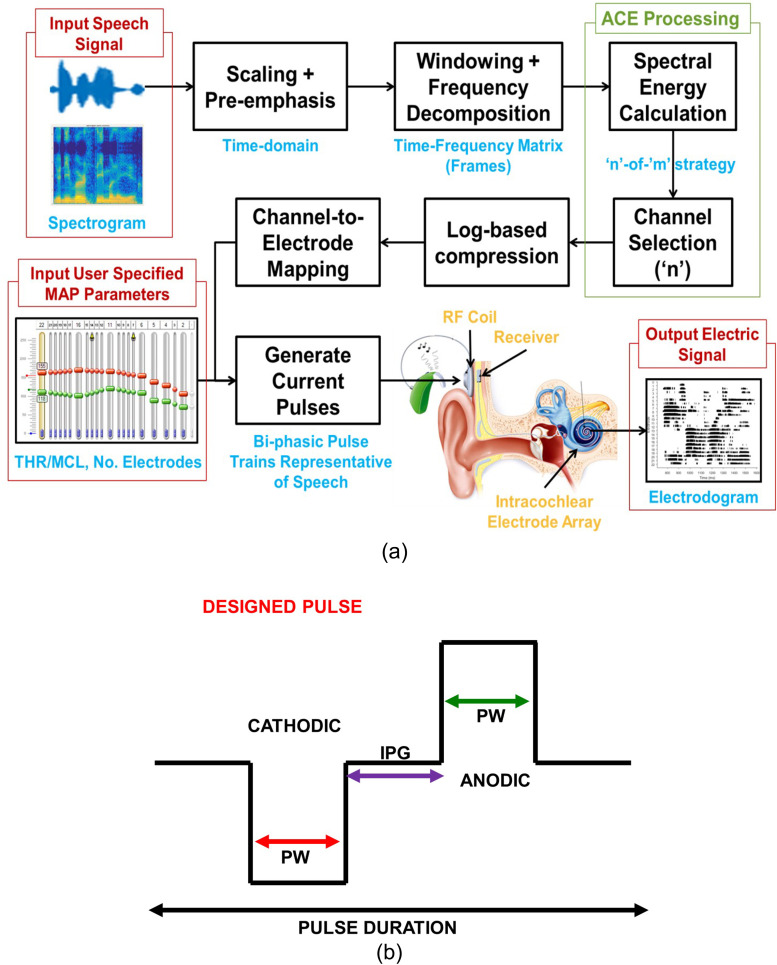
RPs are fundamentally comprised of sound processing and electrical stimulation parameters, as shown in Fig. 1. The proposed analysis and evaluation may involve analysis of SP components that influence the signal path, electrical stimulation strategy, and resulting stimuli. The MAP is typically used to configure both SP and electrical stimulation elements. Typical SP components may involve subband filtering, envelope detection, logarithmic growth function, noise or reverberation processing, etc. The processed acoustic features are used to generate stimulation, and an electrical pulse generator is used to generate the electrical stimulus. A MAP contains CI subject specific information that can be used to personalize and configure the SP settings and the electrical stimulation parameters. The physical realizability of the pulse is determined by several factors, and therefore it is possible that the RP may not generate the electrical stimulation with researcher specified settings under certain conditions. Additionally, RPs may allow researchers to customize any of the hardware, software, and/or firmware subsystems. Hence, the proposed testing framework should ensure that (i) RP produces only safe stimulation under any acoustic condition and (ii) RP permits only valid customization that results in safe stimulation. Therefore, the testing protocol requires RP to be assessed for all the possible combinations of electrical stimulation parameters and acoustical conditions.
FIG. 1.
(Color online) Function level description of a typical RP that models CIs and HAs.
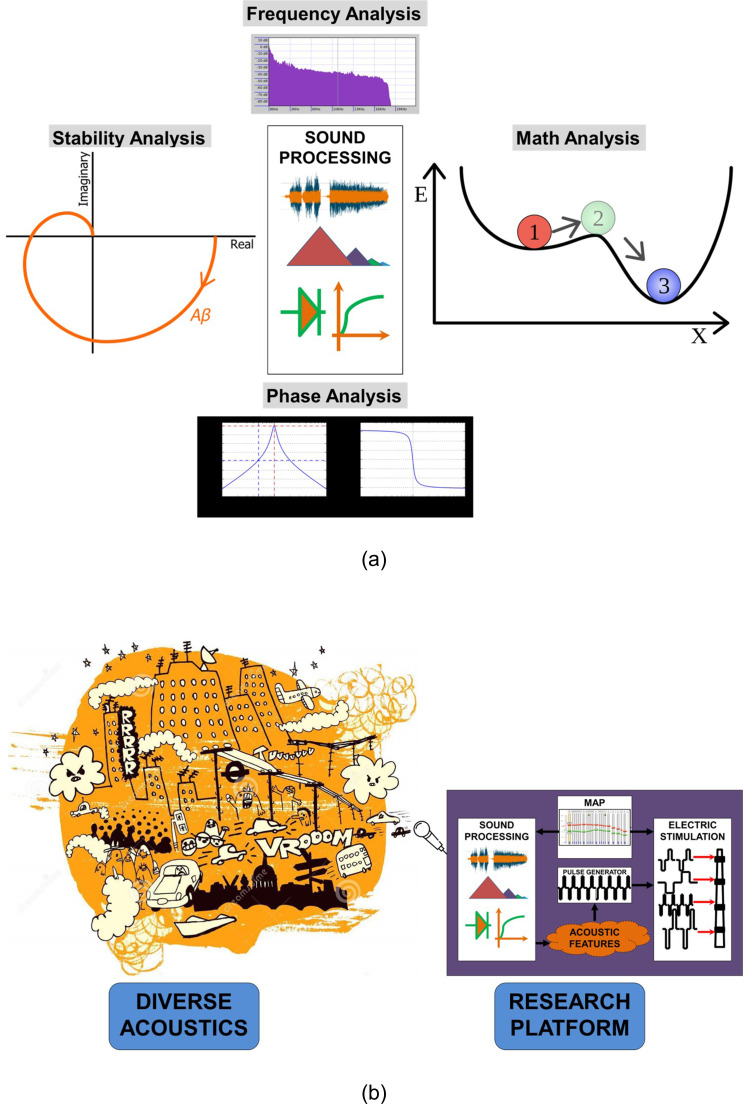
The proposed analysis and evaluation framework is divided into two distinct and independent phases. In phase one, SP is assessed under diverse acoustical conditions, whereas in phase two, the electrical stimulation is evaluated. The test battery for acoustical assessment should include and simulate all the possible ranges of sounds to which a CI subject could be exposed. Electrical stimulation assessment should include all the possible stimulation configurations that a researcher could enter into the RP. Verification of stimulation configuration validates the safety of electrical stimulation under the specified electrical stimulation strategy that is built into the RP. Additional guidelines and recommendations for testing are provided to address custom algorithms. The proposed testing protocol for RPs that emulate CI functionality can be extended to HA, since the core SP component remains common among both.
A. SP analysis and acoustic-based evaluation phase
The goal here is to probe and simulate all the possible acoustical conditions a CI subject may encounter and ensure that RP operates as expected in a safe manner. The testing protocol also stipulates tests at a system level assessing each component for stable operation. The RP may allow modification of certain customizable properties of the SP either through MAP entry or customized programmability, as shown in Fig. 2(a). Every SP component can be assessed using properties such as frequency/phase characteristics, pole zero plots, bounded-input/bounded-output (BIBO) stability, and others. Primary component level assessment is followed by a comprehensive system level verification. The test battery with all the acoustical conditions is applied, and the electrical stimulation is assessed for safety, as shown in Fig. 2(b). As a general guideline, the test battery can be expanded based on the possible experimental conditions. It is recommended to use a wider diversity of acoustic sound conditions to best represent potential real-world acoustic scenarios, and hence that would indicate a testing protocol with a longer duration (e.g., testing in this phase requires that the actual sound be played/submitted as input to the microphone and RP system).
FIG. 2.
(Color online) (a) Proposed analysis and assessment of SP characteristics of RP. (b) Proposed acoustic-based evaluation of RP by applying diverse acoustical conditions.
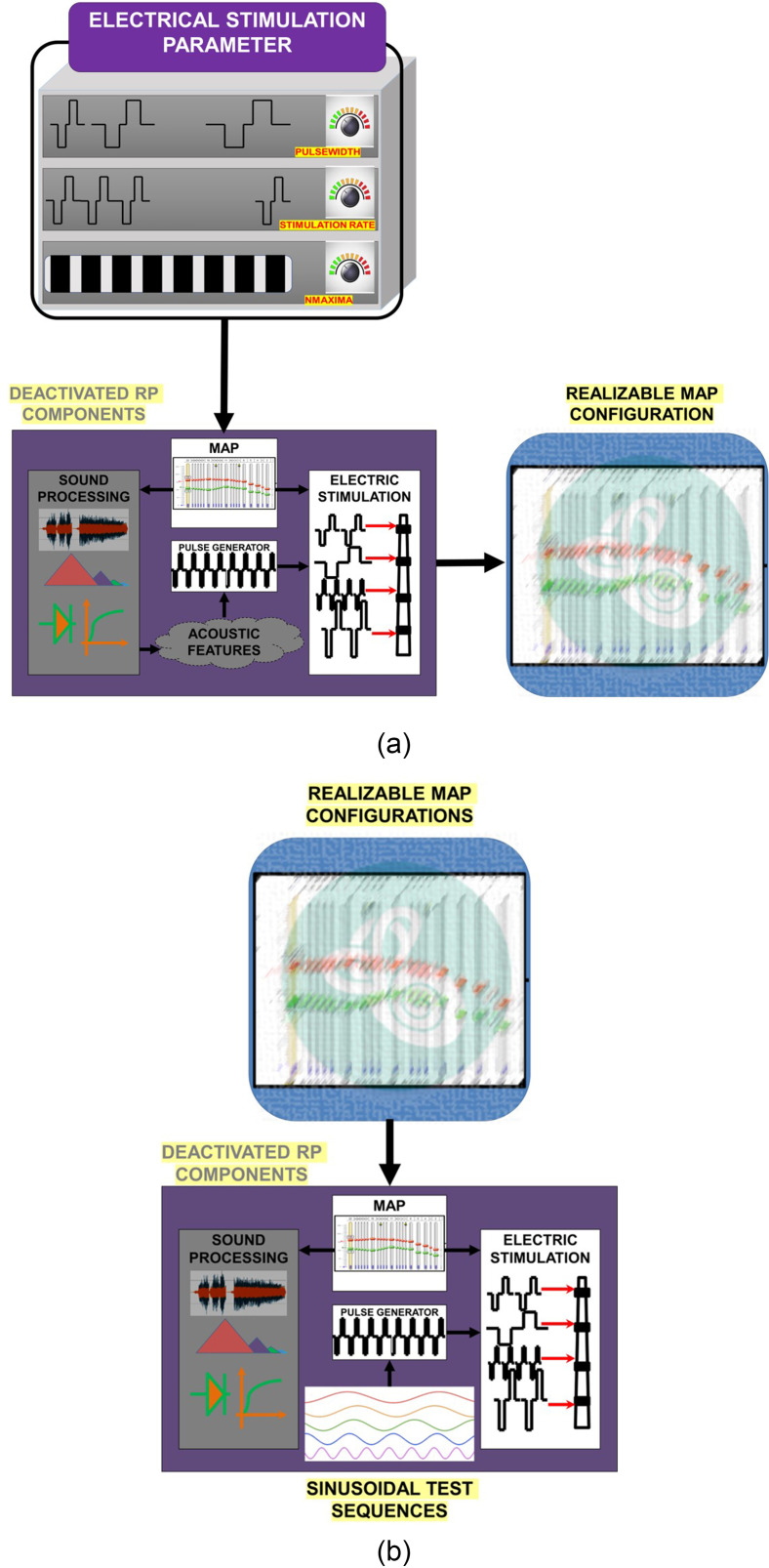
B. Electrical parameter analysis and stimulation evaluation phase
The CI electrical pulse generation includes characteristics, such as rate of pulse generation, pulse width (PW), IPG, pulse amplitude, and others, that influence electrical stimulation. These electrical stimulation characteristics are controlled using customizable electrical stimulation parameters. Since researchers want full control of these parameters, it is important to primarily determine if the stimulation parameters entered by the researcher are physically realizable and, additionally verify if the resulting pulse sequence maintains safe operation for the CI recipient.
Electrical stimulation parameters enable individualized hearing restoration and perceptual listening experience. Customization of one parameter may therefore influence and constrain the choice of others. As such, many MAP configurations may not be physically realizable. Therefore, electrical stimulation evaluation is performed in two stages. In stage one, the electrical MAP stimulation parameters are assessed for realizability, as shown in Fig. 3(a). Every electrical stimulation parameter, within its operational limits, can be varied and combined with other electrical stimulation parameters to generate a distinct MAP configuration corresponding to a pulse stimulation sequence. Every MAP configuration can then be assessed for realizability; since the researcher has the flexibility to enter any MAP configuration, the testing protocol should determine if the RP actually stimulates unsafe, unverified physically unrealizable stimulation configurations. The realizability test also reveals the nature of the interdependence between individual electrical stimulation parameters. To conduct a realizability test, it may be necessary to subsample the stimulation configuration space by taking discrete and meaningful parameter steps.
FIG. 3.
(Color online) (a) Proposed electrical pulse realizability analysis for all the possible combinations of electrical MAP stimulation parameters. (b) Proposed stimulation evaluation of realizable MAP configurations.
In the evaluation phase, realizable electrical pulses can be modulated by test clinical levels instead of using acoustical features. Clinical levels can be derived by sampling a standard test signal like sinusoids, as shown in Fig. 3(b).The safety and performance of RP can be evaluated by assessing the electrical stimuli.
III. WORKING PRINCIPLE, CHARACTERISTICS, AND ASSESSMENT USING CCi-MOBILE RP
Having established the general properties and acoustic design space for a general burn-in test protocol, we now turn to an example evaluation of a new CI/HA research platform. The CCi-MOBILE RP is a versatile and computationally powerful speech and SP emulator developed by the Cochlear Implant Processing Laboratory—Center for Robust Speech Systems (CI-CRSS) at UT-Dallas (Hansen et al., 2019). A software suite has been developed using matlab, with connective support using a PC/tablet or android to enable signal processing (sound coding) development and support clinical and engineering-based research. The CCi-MOBILE research platform is designed to support acoustic and/or electrical stimulation and hence allows researchers to carry out investigations for both scientific studies and advance assistive hearing devices.
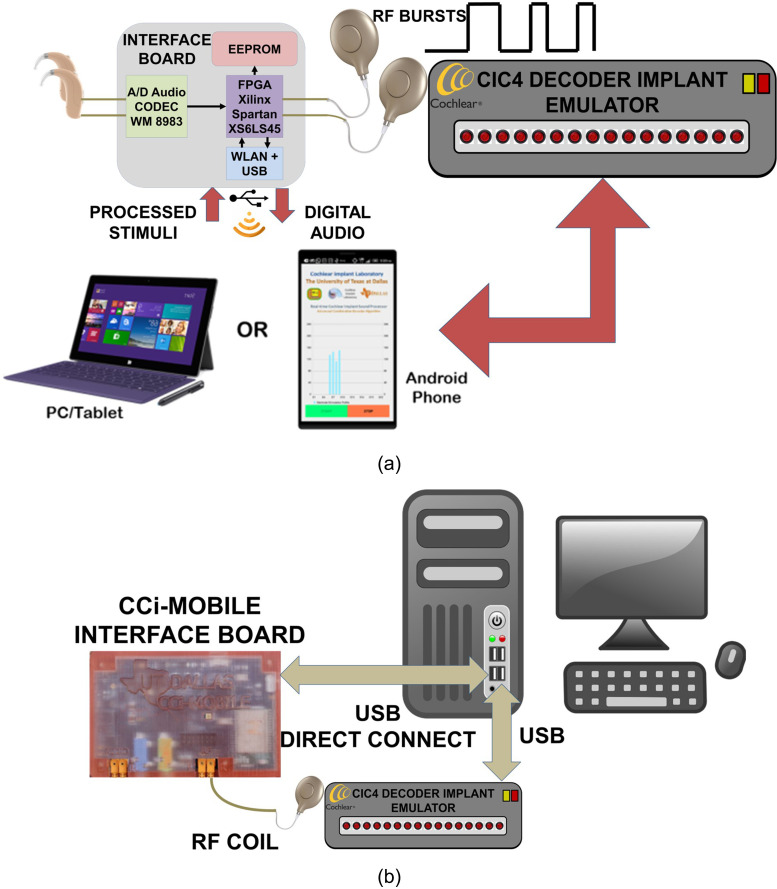
A. Functional setup
The CCi-MOBILE RP is controlled using a PC loaded with CCi-MOBILE software suite (matlab toolkit), as shown in Fig. 4(a). The CCi-MOBILE RP can be used either in direct-connect via universal serial bus (USB) or in wireless configuration via WiFi. In real-time (online) mode, behind the ear (BTE) microphones are used to capture the input acoustic signal, and in offline mode, the audio, stored on the PC, is used as input for CI/HA processing. Radio frequency (RF) transmission coils are used to deliver the processed electrical signal to CI subjects through an interface board as described in Shekar et al. (2018). A decoder implant emulator (DIET; manufactured by Cochlear Corp.) is used to interpret RF transmission and measure the electrical current evoked across all electrodes from the audio input. The RF coil is placed over a marked surface, on the DIET box surface, to establish magnetic contact and enable reception of the electrical stimuli. The DIET can be connected to the PC via USB to decode and log the stimulation data. python and c++ libraries enable data logging of electrical stimuli.
FIG. 4.
(Color online) (a) CCi-MOBILE RP: Functional setup and connectivity with PC/smartphone device and DIET box. (b) CCi-MOBILE RP: Working principle and sound processing for PC/smartphone device and DIET-based setup.
B. Processing flow
CCi-MOBILE processing is illustrated in Fig. 4(b). For offline processing, the matlab-based CCi-MOBILE software suite processes audio, which is already stored on the PC. In real-time mode, the CCi-MOBILE software suite processes input audio from the BTE microphone. Audio is processed frame by frame. Electrical stimuli are generated by mapping the acoustical information to appropriate electrical current values measured in clinical units. The electrical stimuli are streamed to the field programmable gate arrays (FPGAs) on the RP using a USB in direct-connect mode. The FPGA receives electroacoustical stimulation (EAS) and encodes output stimuli in clinical units to the appropriate current levels or bit values for output RF transmission. The FPGA streams time-synchronous acoustic data to the CI/HA transducers using each RF coil. A DIET box is used to interpret the electrical stimuli from the RF transmission. It is possible to correlate the electric current induced at the implant and generated stimuli using the DIET. Here, the PC accesses the DIET unit to record and store the electrical stimuli for analysis and evaluation. This output DIET configuration is only needed for RP testing and evaluation. Typical lab and field use with CCi-MOBILE would have the CI subject's receiver coil connected to the CCi-MOBILE output RF coil.
C. Sound processing and electrical stimulation components
The incoming acoustic signal, from the BTE microphones, is converted to digital form based on a sampling frequency (Fs). The sampling frequency is set at 16 kHz and a pre-emphasis followed by frame-by-frame decomposition are applied on the sampled signal every 8 ms as shown in Fig. 5(a). Every audio frame is processed using an M-channel filter bank to extract acoustical features that reflect the spectral envelope and energy. For Cochlear Corp. devices, M is 22 channels. Logarithmic compression is applied on these acoustic features using the threshold-saturation levels, which are determined from MAP (Loizou, 1998, 1999, 2006). Based on the electrical stimulation strategy, the acoustical information may be used to modulate electrical pulses. These electrical pulses are associated with parameters such as clinical level/units (A) and the channel number (electrode number) denoted by E (electrode). For analysis and evaluation of the CCi-MOBILE RP, we employ biphasic symmetric (50% duty cycle) electrical pulse stimulation using a CIS strategy. In CIS, biphasic carriers are time interleaved between electrodes to avoid simultaneous stimulation. Hence, every channel/electrode is allocated a specific amount of time for electrical stimulation. Electrical stimulation parameters of the biphasic carrier pulses are as follows. M represents the electrical pulse type (biphasic); StimRatech indicates the pulse rate per channel per second. It describes the rate at which these biphasic carriers are presented in every channel. PW represents the pulse width of biphasic pulse. IPG represents the interphase gap between anodic and cathodic pulses. PD represents the pulse duration, which describes the total amount of stimulation time available for every channel per audio frame. Typically, stimulation strategies such as ACE impose a restriction on stimulating only Nmaxima (N < M= 22) select number of electrodes per audio frame.
FIG. 5.
(Color online) (a) CCi-MOBILE RP: Signal path for acoustic to electric mapping and electrodogram visualization. (b) CCi-MOBILE RP: Biphasic electrical pulse characteristics and electrical stimuli.
D. Electrical characteristics of stimulation
The CCi-MOBILE RP complies with the stimulation standards established by Cochlear Corp. (Claussen et al., 2019). The relationship between the electrical stimulation parameters can be established for biphasic electrical pulses, as shown in Fig. 5(b). The MAP can be configured to activate Nmaxima electrodes and generate the needed biphasic electrical pulses in every channel at a certain rate prescribed by StimRatech. Next, the available PD can be determined using this relationship,
| (1) |
However, for a biphasic pulse, PD also defines the full time taken to stimulate: cathodic phase, IPG, and anodic phase. Cochlear Corp. and the FDA have established minimum requirements for stimulating biphasic pulse safely. Therefore, a minimum threshold is needed for valid and safe electrical stimulation, and hence PD is constrained to a minimum PDTh by the parameters of the biphasic carrier pulses: PW for anodic and cathodic phases of the pulse and the IPG as shown here,
| (2) |
A stable electrical stimulation is realizable only if the PD determined by the specified StimRatech and Nmaxima is larger than the minimum PDTh established from Eq. (2). Therefore, the criterion for realizable pulses is as follows:
| (3) |
A standard MAP stimulation configuration, quite popular in clinical research (Ali et al., 2017) is a stimulation rate of StimRatech = 1000 pps, PW of 25 μs, a limit on the number of electrodes given by Nmaxima = 8 channels/audio frame, and a standard IPG of 8 μs. In our evaluations, we suggest this as a reasonable default setting.
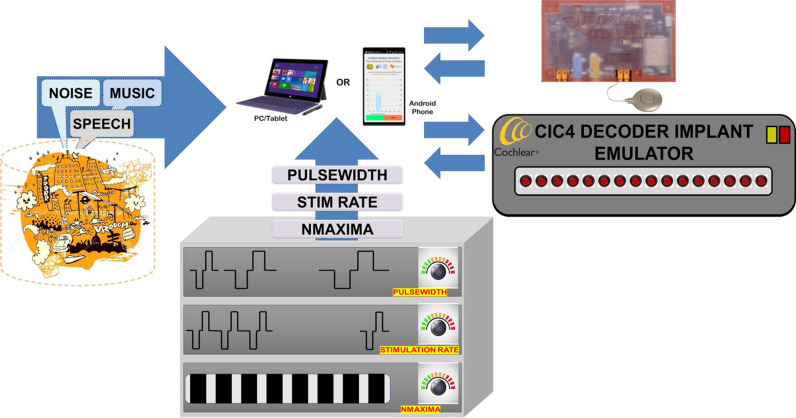
E. Observation, analysis, and evaluation with CCi-MOBILE
The experimental setup for analysis and evaluation of CCi-MOBILE RP is shown in Fig. 6. External resources include acoustic sound/speech databases and customizable MAP parameter settings that are used during burn-in analysis and performance evaluation of CCi-MOBILE RP. The CIC4 Decoder Implant Emulator (DIET) (manufactured by Cochlear Corp.) is used to interpret the electrical stimuli received through RF transmission by RP. DIET is controlled by the PC via a USB interface and is programmed to record and log all electric stimuli data. The recorded electrical stimulation data are used for post burn-in analysis and performance evaluation of CCi-MOBILE.
FIG. 6.
(Color online) CCi-MOBILE RP: Analysis and evaluation in diverse acoustical conditions and electrical MAP stimulation configurations.
Stimulated intracochlear current (SIC) (Shekar et al., 2018) describes the average amount of electric current stimulated across all active electrodes in a stimulation cycle. SIC describes the electrical activity as a result of the audio processed using the DSP units. For CCi-MOBILE, 0 clinical units corresponds to a cochlear electrode current of 17.5 μA, whereas 255 clinical units corresponds to a cochlear electrode current of 1750 μA. The amount of charge associated with the pulse is described in Eq. (4). SIC is therefore estimated across all active electrodes as described in Eq. (5),
| (4) |
| (5) |
The electrical stimulation performance of the CCi-MOBILE research platform can be evaluated by measuring the discrepancies between the researcher specified stimulation parameters and the observed electrical components of the recorded RF stimuli output . This includes (i) inter-phase gap discrepancy (ΔIPG), the difference between the IPG observed in the recording and that specified by the researcher; (ii) the total PD discrepancy (ΔT), the difference between the PD observed from the output recording and the PD corresponding to the researcher specified stimulation rate; (iii) the PW discrepancy (ΔPW), the difference between the PW observed in the output recording and the PW corresponding to the researcher specified stimulation rate; and (iv) the PW imbalance (PWΔAC), the difference between the anodic and cathodic PWs observed in the output recording.
IV. EXPERIMENT I: ACOUSTIC-BASED SOUND PROCESSING EVALUATION PHASE
The analysis and evaluation are carried out for CCi-MOBILE RP in two stages: (i) at an individual component level for SP and (ii) a comprehensive evaluation at system level by applying acoustical conditions. In the individual component level analysis, all SP components that are part of the primary signal processing block are assessed. Similar assessment can be carried out for custom research algorithms. The overall system level analysis and performance evaluation is carried out using a test battery compiled from many different acoustic corpora. For overall system level evaluation, the CCi-MOBILE RP is configured with a standard MAP stimulation configuration.
A. SP analysis
1. Pre-emphasis
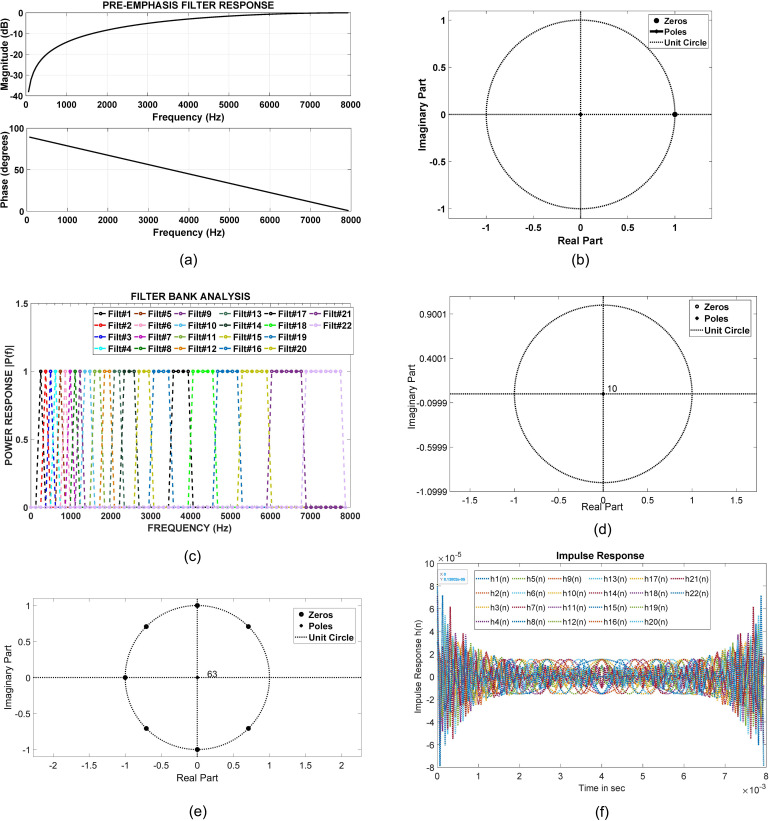
The CCi-MOBILE software suite implements a pre-emphasis filter as a finite impulse response (FIR) filter. The frequency characteristics are shown in Fig. 7(a). Since this is a FIR filter, the poles are located at zero, as shown in the pole zero plot. Hence, the implemented pre-emphasis filter is BIBO stable for all bounded input conditions, as shown in Fig. 7(b).
FIG. 7.
(Color online) (a) CCi-MOBILE RP: Analysis of frequency response characteristics of pre-emphasis. (b) CCi-MOBILE RP: Analysis of pole zero plot for stability assessment of pre-emphasis. (c) CCi-MOBILE RP: Analysis of frequency response characteristics of M-channel filter bank. (d) CCi-MOBILE RP: Analysis of pole zero plot for stability assessment of M-channel filter bank, Filter #9. (e) CCi-MOBILE RP: Analysis of pole zero plot for stability assessment of M-channel filter bank, Filter #22; (f) CCi-MOBILE RP: Analysis of impulse response of M-channel filter bank.
2. Windowing
The CCi-MOBILE software suite provides several standard windowing options, such as Hanning, Hamming, and Blackman. Standard window implementations are also BIBO stable for all bounded input conditions.
3. Frequency decomposition using an M-channel filter bank
CCi-MOBILE software suite implements an M-channel filter bank, where M = 22 and every filter is implemented as a FIR filter. The frequency characteristics of all 22 channels are shown in Fig. 7(c). Since this filter bank is implemented using FIR filters, all poles are located at zero, as seen in the pole zero plot for filter components 9 and 22 shown in Figs. 7(d) and 7(e). Hence, the M-channel filter bank implementation is also BIBO stable for all bounded input conditions. The impulse responses of all the filters in the filter bank are as shown in Fig. 7(f).
4. Logarithmic compression
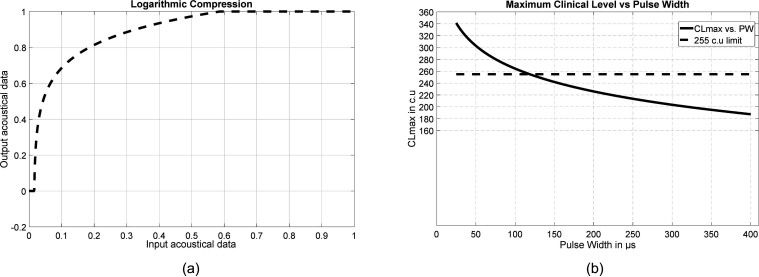
To achieve logarithmic compression, the loudness growth function model is used, and the exponential factor is determined using the compression parameter set in the MAP. The logarithmic compression is achieved by scaling and compressing all acoustical information between base level and saturation level, as defined in the MAP. Figure 8(a) shows the implementation of logarithmic compression. In Fig. 8(a), for all normalized input acoustical data in [0,1], the logarithmic compression, with a compression factor of 20, is applied for acoustical data in [base level = 0.0156, saturation level = 0.556]. The compressed output is therefore maintained between 0 and 1.
FIG. 8.
(a) CCi-MOBILE RP: Plot of output signal level achieved by applying logarithmic compression against input signal level. (b) CCi-MOBILE RP: Plot of maximum clinical level allowed in order to ensure safety compliance against specified PW.
5. Clinical level safety compliance
CCi-MOBILE RP is designed to electrically stimulate CI subjects with clinical units prescribed in their individual MAP. The maximum current, and hence charge, that can be safely stimulated is dependent on the PW and clinical level. Hence, CI manufacturers (Cochlear Corp.) prescribe control parameters for safe electrical stimulation and compliance with Shannon's limit (Shannon, 1992). Hence, the maximum clinical level that is permissible and compliant with Shannon's safety limit is determined as follows:
| (6) |
where represents the inherent control parameters for a specific implant type. Therefore, Eq. (6) is implemented to verify if the maximum clinical level set in the MAP complies with the Shannon limit. CCi-MOBILE software suite is designed to provide interactive notification in order to alert CI subjects with maximum and threshold clinical levels and therefore verifies if electrical stimuli generated in each channel are constrained between maximum and threshold clinical levels, as shown in Fig. 8(b).
B. Comprehensive evaluation using acoustical data
CCi-MOBILE RP is evaluated under diverse audio, sound, and speech acoustic conditions, which can be categorized into three groups: speech, music, and noise.
1. Materials and methods
a. Test battery.
To assess the CCi-MOBILE research platform under diverse acoustical conditions, a test battery is prepared by selecting audio from numerous databases listed as follows: (i) Arizona BioIndustry (AzBio), (ii) Institute of Electrical and Electronics Engineers (IEEE) sentences, (iii) Consonant Nucleus Consonant (CNC), (iv) NOIZEUS, (v) Language Recognition Evaluation (LRE), (vi) Texas Instruments and MIT Lincoln Lab corpus (TIMIT), (vii) Defense Advanced Research Project Agency (DARPA) Robust Automatic Transcription of Speech (RATS), (viii) Musical Analysis Retrieval and Synthesis for Audio Signals (MARSYAS)–George TZANetakis (GTZAN), (ix) Environmental Sound Classification (ESC), (x) Freesound Project, (xi) UrbanSounds, (xii) Gunshots corpus. The audio test battery is prepared by considering diversity in various acoustical characteristics, such as language, environmental, background ambience, emotion, statistics, temporal properties, production, sources, and numerous other factors. The diverse test battery consists of nearly 380 h of audio, comprising 260 h of speech, 46 h of music, and 76 h of noise/environmental sounds.
b. Test setup.
CCi-MOBILE RP is initialized with a standard MAP stimulation configuration as described in Sec. II A. The CCi-MOBILE software suite is programmed to periodically submit/play audio from the available sound test battery for generation of electrical stimuli. The output RF coil is placed on the DIET unit to capture output pulse stimulation data. The electrical stimuli are transmitted to the FPGA on the research platform, over a USB interface. The output RF signal contains electrical stimulus information that is transmitted through the RF coil, which is placed on the DIET. The PC is programmed to monitor the DIET output and record stimuli observations for all audio in the test battery. For all acoustic conditions in the test battery, the recorded observations are assessed to verify if recorded electric stimuli are biphasic as required for safe operation. The overall reliability and performance of the CCi-MOBILE are then determined by analyzing the discrepancies between the specified stimulation parameters and the observed electrical components of the output: ΔIPG, ΔT, ΔPW, and PWΔAC. The SIC therefore describes the electrical activity of CCi-MOBILE under diverse acoustical conditions.
2. Results
For the entire 380 h of acoustic data, CCi-MOBILE RP exhibited no stimuli over the safety limits for all components of the electrical stimuli, and it was limited to the specified threshold and comfort levels. Electrical pulse type detected using DIET was found to maintain the desired standard biphasic structure. The observed current level was always limited to the operational range, and only Nmaxima electrodes were ever active as channels per frame, as specified in the standard MAP configuration.
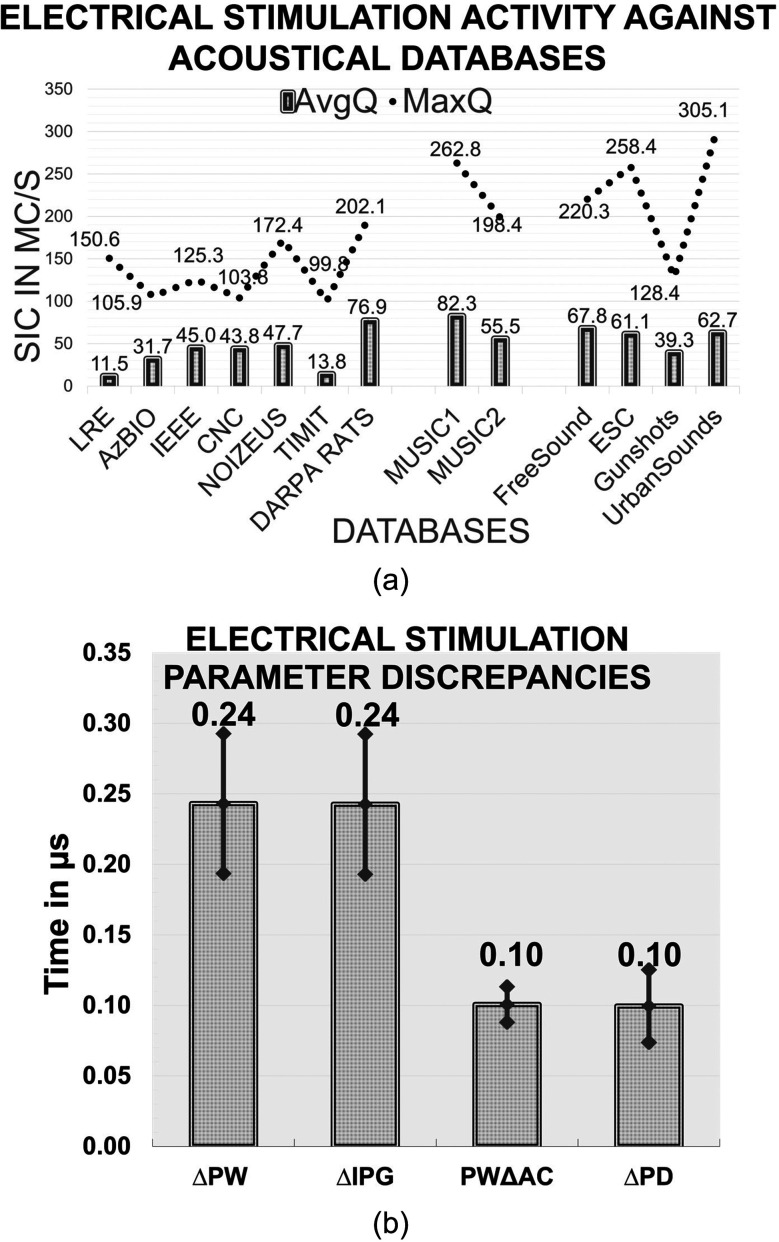
The absolute mean and maximum SICs (in mC/s), are then tabulated against all available databases in the test battery, as shown in Fig. 9(a). The electrical activity was found to be significantly higher for noise when compared to music and speech. Music recorded a higher SIC vs speech. The performance and reliability of CCi-MOBILE in delivering electrical stimulation is measured by comparing the observed electrical stimuli with the standard MAP parameters. Across all acoustical data, error discrepancies were maintained at a relatively consistent mean with very minimal standard deviation, as shown in Fig. 9(b). PWΔAC and ΔPD each accounted for a mean 0.1 μs (0.4% of PW = 25 μs, and 0.08% of PD = 125 μs, respectively), whereas ΔPW and ΔIPG have a relatively higher mean of 0.24 μs (0.96% of PW = 25 μs, and 3% of IPG = 8 μs). Apart from ΔIPG, the remaining measures had a relative lower impact. It is noted that IPG was constrained to be low to accommodate longer PWs, and therefore ΔIPG is lower, and the impact on the MAP stimulation configurations with higher stimulation rates and larger PWs is minimal. A higher SIC indicates an increase in perceived loudness. The discrepancies observed in the RF pulse characteristics, ΔPW, ΔIPG, PWΔAC, and ΔT, induce an exponential increment in perceived loudness. Residual non-zero PWΔAC also contributes toward irreversible corrosion of the electrodes and potential deposit of metal oxides at the electrode–tissue interface. A non-zero PWΔAC worsens the quality of CI stimulation and reduces the life of the CI system itself.
FIG. 9.
(a) CCi-MOBILE RP: Evaluation of average electrical current stimulation activity observed across all electrodes by assessing SIC (mC/s) against all the acoustical databases. (b) CCi-MOBILE RP: Evaluation of error discrepancy in ΔIPG, ΔT, ΔPW, and PWΔAC stimulation factors, observed across all diverse acoustical conditions.
V. EXPERIMENT II: ELECTRICAL PARAMETER ANALYSIS AND STIMULATION EVALUATION PHASE
CCi-MOBILE RP is compliant with CI technology developed at Cochlear Corp. Hence, the customizable electrical stimulation parameters of the MAP are typically fixed to values/operational ranges stipulated by Cochlear Corp. Biphasic electrical pulse stimulation can be designed using electrical stimulation parameters such as stimulation rate, PW, IPG, Nmaxima, and clinical level. The IPG is usually fixed at 8 μs. The clinical level is only dependent upon the acoustical information, and the clinical level is constrained to be presented between comfort and threshold levels. For the realizability assessment, only electrical stimulation parameters (stimulation rate, PW, and Nmaxima) are considered. The physical realizability of electrical stimulation is determined by evaluating the PD using Eqs. (1)–(3). Once an optimal set of physically realizable electrical stimulation parameters are determined, they are loaded onto the CCi-MOBILE RP. This unique and optimal set of MAP configurations are then used to assess the overall reliability and performance of CCi-MOBILE.
A. Electrical parameter analysis and realizable space assessment
1. Materials and methods
a. Test battery.
CCi-MOBILE is assessed for physical realizability of researcher defined electrical stimulation using a standard IPG of 8 μs and by varying the electrical stimulation of each parameter in the MAP, such as StimRatech, PW, and Nmaxima. CCi-MOBILE has lower and upper operating limits on the stimulation parameters compliant with stimulation design constraints established by Cochlear Corp., and they are specified as follows: StimRatech, 125–14 400 pps; PW, 25–400 μs; and Nmaxima, 1–22 channels. For testing, all parameters are discretized, with respective incremental step sizes as follows: StimRatech, 125 pps; PW, 5 μs; and Nmaxima, 1 channel. This results in a total of 242 880 possible MAP configurations.
b. Procedure.
CCi-MOBILE software suite is programmed to only assess the electrical stimulation, and hence the SP units are deactivated during this evaluation. The StimRatech, PW, and Nmaxima are varied from a lower bound to highest possible value by applying individual parameter incremental step sizes. Every MAP configuration is assessed to verify if the pulse duration constraints established in Eq. (3) are satisfied. MAP configurations that are physically realizable are referred to as valid MAP configurations. If the PD constraints are not satisfied, then CCi-MOBILE software suite applies a minimum plausible adjustment to StimRatech and PW and then reevaluates this adjusted configuration for realizability. Due to the constraints on Nmaxima, the MAP configurations may still remain unrealizable, and these are referred to as invalid MAP configurations and set aside.
2. Results
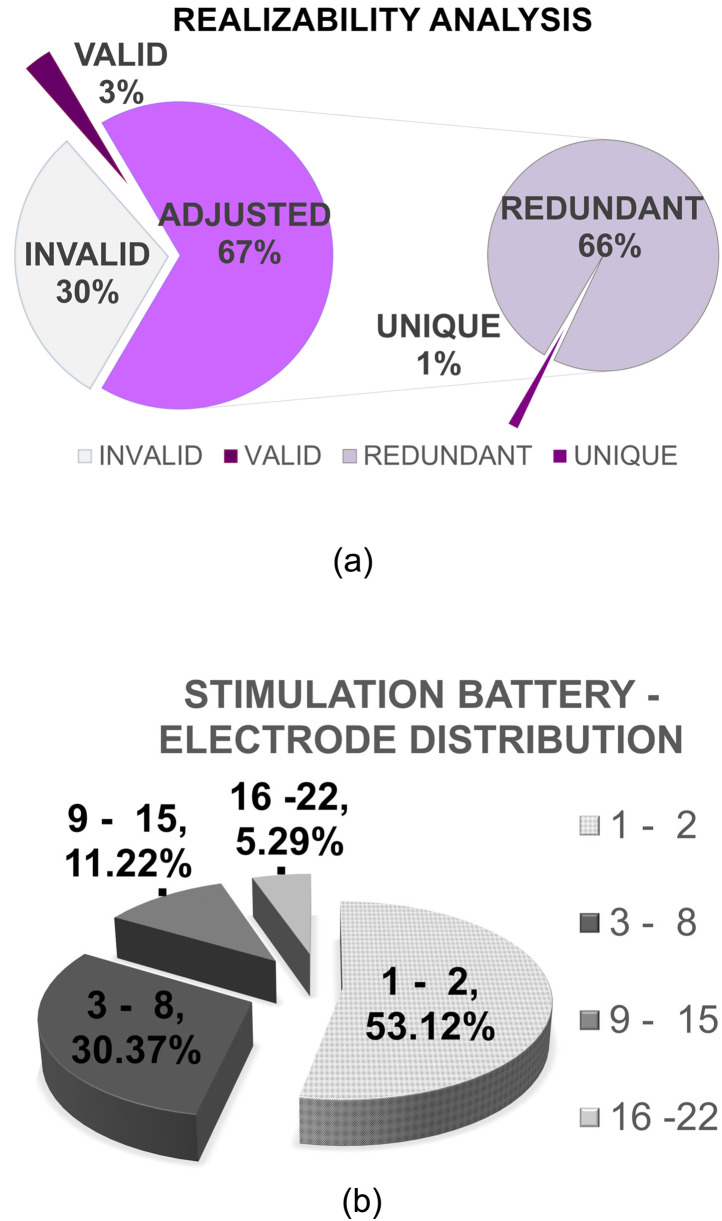
As shown in Fig. 10(a), approximately 70% of the possible MAP configurations are physically realizable and are valid. Only 3% of the MAP configurations are directly realizable, whereas 67% of MAP configurations are validated only after applying adjustment on StimRatech and PW. CCi-MOBILE software suite applies modification on the StimRatech and PW such that they undergo minimum change, and the adjusted MAP still remains closer to the desired realizable MAP configuration. For every Nmaxima, the adjustment algorithm determines cutoff values for StimRatech and PW. So any MAP configuration that does not fall within the valid StimRatech and/or PW values undergoes adjustment, and the cutoff values get used. Therefore, most of the adjusted MAP are configurations containing cutoff StimRatech and/or PW, and hence they are not unique. Among all the adjusted MAP configurations, only 1% of them are found to be unique, and the remaining are found to be duplicates and hence redundant. Here, 30% of MAP configurations are unrealizable and therefore set aside as invalid. Only 9655, which account for 4% of the 242 880 MAP configurations (3% valid + 1% adjusted), are found to be distinctly realizable. This analysis significantly reduces the necessary researcher defined parameter setting based on the burn-in setting.
FIG. 10.
(Color online) (a) CCi-MOBILE RP: Analysis of MAP configurations that result in valid, realizable, unique, and invalid sets of electrical pulses. (b) CCi-MOBILE RP: Analysis of stimulation test battery of CCi-MOBILE RP across Nmaxima for all the realizable MAP configurations.
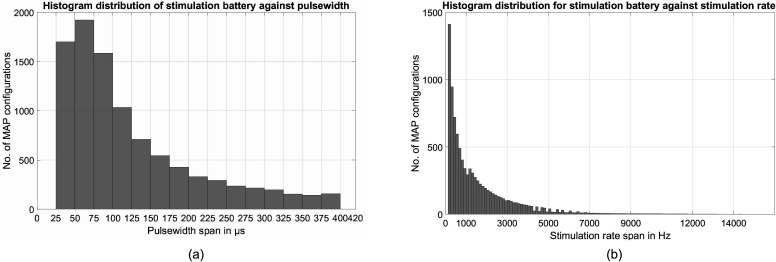
Figure 10(b) depicts the distribution of MAP configurations in the test battery with respect to the electrical stimulation parameter Nmaxima, or the number of active electrodes per frame. Nearly 54% of possible MAP configurations in the test battery have only one or two electrodes active per frame. Approximately 84% of MAP configurations in the test battery have at most eight electrodes active per frame. Therefore, with an increase in the number of electrodes per audio frame, the number of valid MAP configurations is greatly reduced. As a result, only a small population of MAP configurations in test battery, approximately 16%, have nine or more electrodes active in a frame. A similar analysis of distribution of MAP configurations in the test battery is considered with respect to the stimulation parameters PW and StimRatech, as shown in Figs. 11(a) and 11(b). A trend similar to Nmaxima can be observed, where fewer valid MAP configurations contain larger values of PW and StimRatech. In an overall exponentially decreasing trend, odd peaks can be attributed to the characteristic nature of adjustment in the CCi-MOBILE software suite. Typically, the PW is lowered to satisfy and accommodate a higher desired stimulation rate.
FIG. 11.
(a) CCi-MOBILE RP: Analysis of stimulation test battery of CCi-MOBILE RP across PW for all the realizable MAP configurations. (b) CCi-MOBILE RP: Analysis of stimulation test battery of CCi-MOBILE RP across stimulation rate for all the realizable MAP configurations.
B. Electrical stimulation evaluation phase
1. Materials and methods
a. Test battery.
The optimal set of distinctly realizable MAP configurations is used to configure CCi-MOBILE for electrical stimulation evaluation. All SP components that involve audio processing and acoustic-to-electric mapping modules are deactivated. The clinical levels are synthetically generated by sampling a sinusoidal tone at a sampling rate of 100 Hz. These sinusoidal samples are equally applied across all electrodes.
b. Procedure.
To assess safety and operational performance, the CCi-MOBILE is setup as described in Secs. III A and III B. For every MAP configuration from the distinctly realizable set, synthesized sinusoidal samples are applied as clinical levels across all electrodes, and output electrical stimuli are generated. The CIC4 DIET is used to record and store all the electric stimuli observations as described in Sec. III D.
2. Results
Observations of electrical stimuli revealed that stimulation was always found to be biphasic, as stipulated, for all MAP configurations in the test battery. Current levels recorded in the electrical stimulus observations matched with discrete synthesized sinusoidal clinical levels specified, and hence the integrity of clinical levels was never compromised. Only the specified Nmaxima electrodes were ever found to be active for every audio frame (e.g., never greater).
VI. SYNOPTIC ANALYSIS AND EVALUATION FOR RPS
Here, analysis and evaluation of elementary components of RPs are carried out in two phases: (i) acoustic-based sound processing evaluation phase and (ii) electrical stimulation evaluation phase. These phases involve comprehensive analysis and evaluation, requiring longer test intervals on the order of hundreds/thousands of hours, due to larger test batteries. However, a researcher may have to configure the RP with a new customized MAP (i.e., filter bank cutoff frequencies, clinical levels, stimulation parameters and strategies) for every CI subject on a trial-by-trial basis. Hence, there is a need to reduce the overall duration for testing and analysis of RP.
A synoptic test protocol is proposed to quickly sample and assess the entire test space, using a reduced test battery set. The proposed condensed SP evaluation phase is carried out using the minimum acoustical test battery (Advanced Bionics LLC et al., 2011), and the proposed condensed electrical stimulation evaluation phase is carried out using a min-max stimuli test battery.
A. Minimum test battery
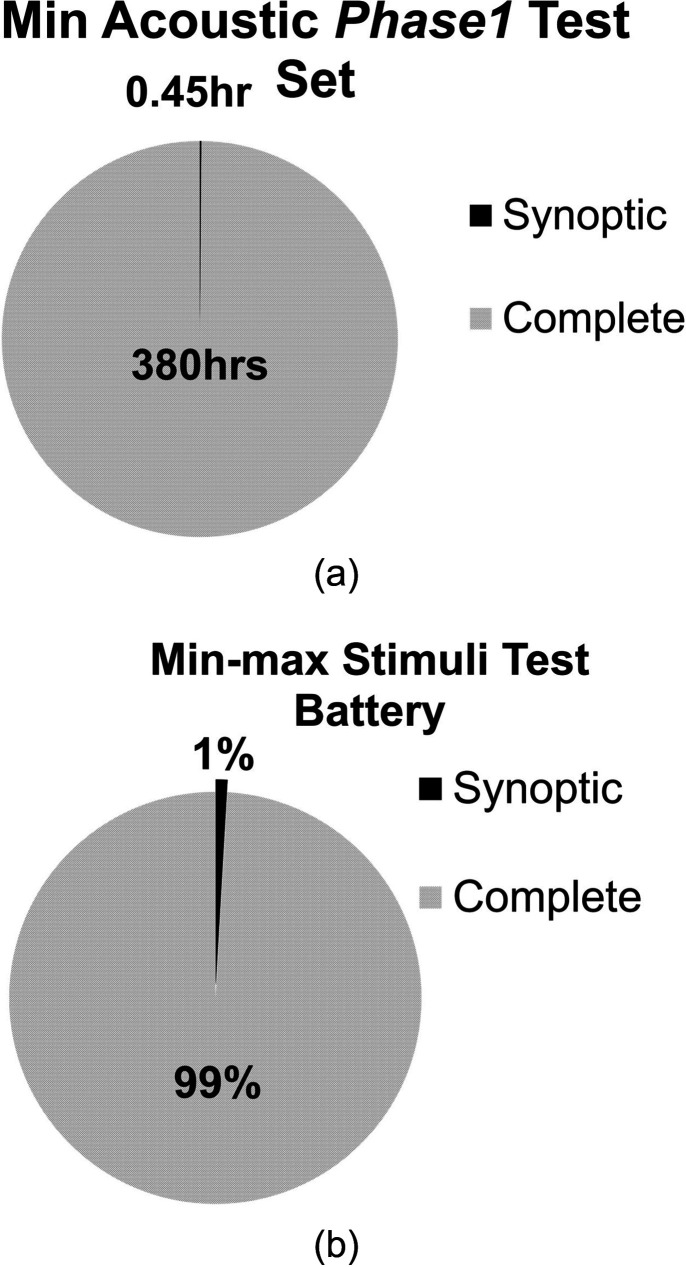
The FDA has developed the minimum test battery for CI subjective studies. The minimum speech and noise test battery can be formed using the following: one 20-sentence list of AzBio sentences presented in quiet; one 20-sentence list of AzBio sentences presented in noise; one 50-word list of CNC words; one 20-sentence list-pair (10 sentences per list) of the Bamford-Kowal-Bench--Speech-in-Noise (BKB-SIN). Based on the requirements of the experiment, a researcher can expand the test battery to include databases that are part of the experimental trial. As an example, a minimum speech and music test battery can be prepared using three music samples per genre, totaling 30 music samples across all ten genres from George Tzanetakis (GTZan) genre. Here, 30 tracks, out of 120 tracks, and one music sample each from the Music Speech GTZan database are employed. The proposed minimum test battery consumes only 0.45 h vs the complete set of 380 h, as shown in Fig. 12(a).
FIG. 12.
(a) CCi-MOBILE RP: Assessment of testing time for full acoustical test battery vs minimum test battery. (b) CCi-MOBILE RP: Assessment of testing time using min-max stimulation test battery vs all the realizable MAP configurations.
B. Min-max stimuli test battery
The comprehensive electrical stimulation test battery can be greatly reduced by determining the minimum and the maximum cutoff values of PW and stimulation rates for every electrode-limit, Nmaxima electrodes per frame. For CIS implementation with CCi-MOBILE RP, four test configurations corresponding to the maximum and minimum possible stimulation rate and pulse width, respectively, are selected. Here, 88 stimulation configurations corresponding to 22 possible Nmaxima are used to generate the min-max stimuli test battery. This condensed stimulation test battery requires only 15 min for evaluating all 88 stimulation configurations. Therefore, the amount of time consumed in conducting the proposed min-max stimuli test battery consumes only 1% of the total possible required time, as shown in Fig. 12(b). Further, based on the experimental requirements, the researcher can expand the test battery to include stimulation configurations that are of interest.
VII. CONCLUSION
To our knowledge, this study is one of the first efforts to develop a test protocol framework to assess and analyze research platforms for safety in a systematic manner. This study has proposed a two-phase burn-in analysis and evaluation paradigm for assessing safety and reliability for RPs based on (i) an acoustic phase 1 step and (ii) a parameter phase 2 step. Having established the general burn-in analysis and evaluation protocol, the proposed solution was successfully demonstrated with an evaluation of CCi-MOBILE RP. In acoustic phase 1, for CCi-MOBILE RP, all components were found to be BIBO stable, and implementation of logarithmic compression did not generate any errors. The Shannon limit was established by applying a limit on the maximum clinical level in accordance with specified PW and electrode parameters. In the acoustic-based stimulation evaluation, 11 databases approximately amounting to 380 h+, were applied on CCi-MOBILE RP, and stimulation was evaluated. CCi-MOBILE RP was found to operate in safe conditions and resulted in no errors in output acoustic/stimulation characteristics. The acoustic phase 1-based burn-in evaluation established the safety of experimental trials with CI subjects by ensuring that RPs never exceed a safe/expected level of operation for naturalistic speech, audio, music, and sound exposure in naturalistic settings. In parameter phase 2, using the CCi-MOBILE RP with a CIS strategy, the realizable MAP space for pulse stimulation was first determined by performing parameter realizability assessment. This was achieved by exhaustively considering all possible researcher selected stimulation parameter setting combinations. For all MAP configurations in the realizable space, it was found that most realizable MAP configurations had very minimal discrepancies. Although a few configurations exhibited rare large deviations from specifications, repeated testing found no additional evidence of repeat observations of such discrepancies. Using CCi-MOBILE RP as an example, this study has also established guidelines for developing a reduced set of test conditions to more efficiently evaluate RPs (useful if many examples of the same RP must be tested). With this reduced-set test protocol, only minor modifications, such as custom algorithms and/or stimulation, MAP individualization, and others, are introduced. This study has therefore shown an effective evaluation protocol for CI/HA RPs, such as CCi-MOBILE, to be safe with well benchmarked performance characteristics. The proposed analysis and evaluation protocol can be easily migrated to support any generic AHDs (i.e., both CIs and HAs) to assess safety, reliability, and performance of electrical stimulation. Extensions to assess alternate modes of operations, including real-time/offline, bilateral/bimodal, and others, are also possible. Application and adaptation of the proposed protocol to other RPs is expected to support other testing conditions/scenarios and help toward establishing a more universal overall RP testing standard.
ACKNOWLEDGMENTS
This work was supported by Grant No. R01 DC016839-02 (PI: Hansen) from the National Institute on Deafness and Other Communication Disorders, National Institutes of Health, and partially by the University of Texas at Dallas from Distinguished University Chair in Telecommunications Engineering held by J.H.L.H.
References
- 1.Advanced Bionics LLC (2020). https://advancedbionics.com/us/en/home.html (Last viewed June 21, 2020).
- 2.Advanced Bionics LLC, Cochlear Americas, and MED-EL Corp. (2011). Minimum Speech Test Battery (MSTB) for Adult Cochlear Implant Users ( Advanced Bionics LLC, Cochlear Americas, MED-EL Corp.). [Google Scholar]
- 3. Agnew, W. F. , Yuen, T. G. H. , McCreery, D. B. , and Bullara, L. A. (1986). “ Histopathologic evaluation of prolonged intracortical electrical stimulation,” Exp. Neurol. 92(1), 162–185. 10.1016/0014-4886(86)90132-9 [DOI] [PubMed] [Google Scholar]
- 4. Ali, H. , Ammula, S. , Saba, J. , and Hansen, J. H. L. (2017). “ CCi mobile platform for cochlear implant and hearing aid research,” in Proceedings of the 1st Conference on Challenges in Hearing Aid Assistive Technology, August 19, Stockholm, Sweden, pp. 21–23. [Google Scholar]
- 5. Ali, H. , Lobo, A. P. , and Loizou, P. C. (2013). “ Design and evaluation of a personal digital assistant-based research platform for cochlear implants,” IEEE Trans. Biomed. Eng. 60(11), 3060–3073. 10.1109/TBME.2013.2262712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Backus, B. , Adiloğlu, K. , and Herzke, T. (2015). “ A binaural CI research platform for Oticon Medical SP/XP implants enabling ITD/ILD and variable rate processing,” Trends Hear. 19, 2331216515618655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buchman, C. A. , Gifford, R. H. , Haynes, D. S. , Lenarz, T. , O'Donoghue, G. , Adunka, O. , Biever, A. , Briggs, R. J. , Carlson, M. L. , Dai, P. , and Driscoll, C. L. (2020). “ Unilateral cochlear implants for severe, profound, or moderate sloping to profound bilateral sensorineural hearing loss: A systematic review and consensus statements,” JAMA Otolaryngol. Head Neck Surg. 146(10), 942–953. 10.1001/jamaoto.2020.0998 [DOI] [PubMed] [Google Scholar]
- 8. Chasin, M. (2012). “ Music and hearing aids—An introduction,” Trends Amplif. 16(3), 136–139. 10.1177/1084713812468512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chatterjee, M. , Fu, Q. J. , and Shannon, R. V. (2000). “ Effects of phase duration and electrode separation on loudness growth in cochlear implant listeners,” J. Acoust. Soc. Am. 107(3), 1637–1644. 10.1121/1.428448 [DOI] [PubMed] [Google Scholar]
- 10. Chatterjee, M. , and Zwislocki, J. J. (1998). “ Cochlear mechanisms of frequency and intensity coding. II. Dynamic range and the code for loudness,” Hear. Res. 124(1–2), 170–181. 10.1016/S0378-5955(98)00135-X [DOI] [PubMed] [Google Scholar]
- 11. Claussen, A. D. , Quevedo, R. V. , Mostaert, B. , Kirk, J. R. , Dueck, W. F. , and Hansen, M. R. (2019). “ A mouse model of cochlear implantation with chronic electric stimulation,” PLoS One 14(4), e0215407. 10.1371/journal.pone.0215407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cochlear Corp. (2020). https://www.cochlear.com/us/en/home (Last viewed June 21, 2020).
- 13.Cochlear Corp. (2006). NIC v2 Software Interface Specification E11318RD ( Cochlear Corp, Sydney, Australia: ). [Google Scholar]
- 14. Cooper, W. B. , Tobey, E. , and Loizou, P. C. (2008). “ Music perception by cochlear implant and normal hearing listeners as measured by the Montreal Battery for Evaluation of Amusia,” Ear Hear. 29(4), 618–626. 10.1097/AUD.0b013e318174e787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CRC and HearWorks (2003a). “ SPEAR3 3rd generation speech processor for electrical and acoustic research,” in Product Brief ( University of Melbourne, Victoria, Australia: ). [Google Scholar]
- 16.CRC and HearWorks (2003b). “ SPEAR3 speech processing system,” in Product Brief ( University of; Melbourne, Victoria, Australia: ). [Google Scholar]
- 17. Crew, J. D. , and Galvin, J. J. III (2012). “ Channel interaction limits melodic pitch perception in simulated cochlear implants,” J. Acoust. Soc. Am. 132(5), EL429–EL435. 10.1121/1.4758770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Damen, G. W. , Beynon, A. J. , Krabbe, P. F. , Mulder, J. J. , and Mylanus, E. A. (2007). “ Cochlear implantation and quality of life in postlingually deaf adults: Long-term follow-up,” Otolaryngol. Head Neck Surg. 136(4), 597–604. 10.1016/j.otohns.2006.11.044 [DOI] [PubMed] [Google Scholar]
- 19. Deller, J. R. , Proakis, J. G. , and Hansen, J. H. (2000). Discrete-Time Processing of Speech Signals, Institute of Electrical and Electronics Engineers ( Macmillan, New York: ). [Google Scholar]
- 20. Dudley, W. H. (1940). “ vocoder--Electrical re-creation of speech,” J. Soc. Motion Pict. Eng. 34(3), 272–278. 10.5594/J10096 [DOI] [Google Scholar]
- 21. Eddington, D. K. , Dobelle, W. H. , Brackmann, D. E. , Mladevosky, M. G. , and Parkin, J. L. (1978). “ Auditory prosthesis research with multiple channel intracochlear stimulation in man,” Ann. Otol. Rhinol. Laryngol. 87, 1–39. 10.1177/00034894780870S602 [DOI] [PubMed] [Google Scholar]
- 22. Fant, G. (1970). Acoustic Theory of Speech Production ( Mouton & Co, The Hague: ). [Google Scholar]
- 23. Flanagan, J. L. , and Golden, R. M. (1966). “ Phase vocoder,” Bell Syst. Tech. J. 45(9), 1493–1509. 10.1002/j.1538-7305.1966.tb01706.x [DOI] [Google Scholar]
- 24. Flynn, M. C. , Dowell, R. C. , and Clark, G. M. (1998). “ Aided speech recognition abilities of adults with a severe or severe-to-profound hearing loss,” J. Speech Lang. Hear. Res. 41(2), 285–299. 10.1044/jslhr.4102.285 [DOI] [PubMed] [Google Scholar]
- 25. Francart, T. , Van Wieringen, A. , and Wouters, J. (2008). “ APEX 3: A multi-purpose test platform for auditory psychophysical experiments,” J. Neurosci. Methods 172(2), 283–293. 10.1016/j.jneumeth.2008.04.020 [DOI] [PubMed] [Google Scholar]
- 26. Fu, Q. J. , and Shannon, R. V. (2000). “ Effect of stimulation rate on phoneme recognition by Nucleus-22 cochlear implant listeners,” J. Acoust. Soc. Am. 107(1), 589–597. 10.1097/01.aud.0000261689.35445.20 [DOI] [PubMed] [Google Scholar]
- 27. Gfeller, K. , Woodworth, G. , Robin, D. A. , Witt, S. , and Knutson, J. F. (1997). “ Perception of rhythmic and sequential pitch patterns by normally hearing adults and adult cochlear implant users,” Ear Hear. 18(3), 252–260. 10.1097/00003446-199706000-00008 [DOI] [PubMed] [Google Scholar]
- 28. Green, R. A. , Matteucci, P. B. , Dodds, C. W. D. , Palmer, J. , Dueck, W. F. , Hassarati, R. T. , and Suaning, G. J. (2014). “ Laser patterning of platinum electrodes for safe neurostimulation,” J. Neural Eng. 11(5), 056017. 10.1088/1741-2560/11/5/056017 [DOI] [PubMed] [Google Scholar]
- 29. Hansen, J. H. L. , Ali, H. , Saba, J. N. , Charan, M. R. , Mamun, N. , Ghosh, R. , Brueggeman, A. , and CRSS-CI Lab: Center for Robust Speech Systems – Cochlear Implant Processing Lab (2019). “ CCI-MOBILE: Design and evaluation of a cochlear implant and hearing aid research platform for speech scientists and engineers,” in 2019 IEEE EMBS International Conference on Biomedical & Health Informatics (BHI), May 19–22, Chicago ( Institute of Electrical and Electronics Engineers, New York: ), pp. 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hinderink, J. B. , Krabbe, P. F. , and Van Den Broek, P. (2000). “ Development and application of a health-related quality-of-life instrument for adults with cochlear implants: The Nijmegen cochlear implant questionnaire,” Otolaryngology--Head Neck Surg. 123(6), 756–765. [DOI] [PubMed] [Google Scholar]
- 31. Huang, C. Q. , and Shepherd, R. K. (1999). “ Reduction in excitability of the auditory nerve following electrical stimulation at high stimulus rates. IV. Effects of stimulus intensity,” Hearing Res. 132(1–2), 60–68. 10.1016/S0378-5955(99)00034-9 [DOI] [PubMed] [Google Scholar]
- 32. Kąkol, K. , and Kostek, B. (2016). “ A study on signal processing methods applied to hearing aids,” in 2016 Signal Processing: Algorithms, Architectures, Arrangements, and Applications (SPA), September 21–23, Poznan, Poland ( Institute of Electrical and Electronics Engineers, New York: ), pp. 219–224. [Google Scholar]
- 33. Kiefer, J. , Hohl, S. , Stürzebecher, E. , Pfennigdorff, T. , and Gstöettner, W. (2001). “ Comparison of speech recognition with different speech coding strategies (SPEAK, CIS, and ACE) and their relationship to telemetric measures of compound action potentials in the nucleus CI 24M cochlear implant system,” Audiology 40(1), 32–42. 10.3109/00206090109073098 [DOI] [PubMed] [Google Scholar]
- 34. Knutson, J. F. , Gantz, B. J. , Hinrichs, J. V. , Schartz, H. A. , Tyler, R. S. , and Woodworth, G. (1991). “ Psychological predictors of audiological outcomes of multichannel cochlear implants: Preliminary findings,” Ann. Otol. Rhinol. Laryngol. 100, 817–822. 10.1177/000348949110001006 [DOI] [PubMed] [Google Scholar]
- 35. Koch, D. B. , Osberger, M. J. , Segel, P. , and Kessler, D. (2004). “ HiResolution and conventional sound processing in the HiResolution bionic ear: Using appropriate outcome measures to assess speech recognition ability,” Audiol. Neurotol. 9(4), 214–223. 10.1159/000078391 [DOI] [PubMed] [Google Scholar]
- 36. Leung, R. T. , Shivdasani, M. N. , Nayagam, D. A. , and Shepherd, R. K. (2014). “ In vivo and in vitro comparison of the charge injection capacity of platinum macroelectrodes,” IEEE Trans. Biomed. Eng. 62(3), 849–857. 10.1109/TBME.2014.2366514 [DOI] [PubMed] [Google Scholar]
- 37. Litovsky, R. Y. , Goupell, M. J. , Kan, A. , and Landsberger, D. M. (2017). “ Use of research interfaces for psychophysical studies with cochlear-implant users,” Trends Hear. 21, 1–15. 10.1177/2331216517736464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Loizou, P. C. (1998). “ Mimicking the human ear,” IEEE Signal Process. Mag. 15(5), 101–130. 10.1109/79.708543 [DOI] [Google Scholar]
- 39. Loizou, P. C. (1999). “ Signal-processing techniques for cochlear implants,” IEEE Eng. Med. Biol. Mag. 18(3), 34–46. 10.1109/51.765187 [DOI] [PubMed] [Google Scholar]
- 40. Loizou, P. C. (2006). “ Speech processing in vocoder-centric cochlear implants,” Adv. Otorhinolaryngol. 64, 109–143. 10.1159/000094648 [DOI] [PubMed] [Google Scholar]
- 41. Looi, V. , McDermott, H. , McKay, C. , and Hickson, L. (2008a). “ Music perception of cochlear implant users compared with that of hearing aid users,” Ear Hear. 29(3), 421–434. 10.1097/AUD.0b013e31816a0d0b [DOI] [PubMed] [Google Scholar]
- 42. Looi, V. , McDermott, H. , McKay, C. , and Hickson, L. (2008b). “ The effect of cochlear implantation on music perception by adults with usable pre-operative acoustic hearing,” Int. J. Audiol. 47(5), 257–268. 10.1080/14992020801955237 [DOI] [PubMed] [Google Scholar]
- 43. McCreery, D. B. , Agnew, W. F. , Yuen, T. G. , and Bullara, L. (1990). “ Charge density and charge per phase as cofactors in neural injury induced by electrical stimulation,” IEEE Trans. Biomed. Eng. 37(10), 996–1001. 10.1109/10.102812 [DOI] [PubMed] [Google Scholar]
- 44. McCreery, D. B. , Agnew, W. F. , Yuen, T. G. H. , and Bullara, L. A. (1992). “ Damage in peripheral nerve from continuous electrical stimulation: Comparison of two stimulus waveforms,” Med. Biol. Eng. Comput. 30(1), 109–114. 10.1007/BF02446202 [DOI] [PubMed] [Google Scholar]
- 45. McCreery, D. B. , Agnew, W. F. , Yuen, T. G. H. , and Bullara, L. A. (1995). “ Relationship between stimulus amplitude, stimulus frequency and neural damage during electrical stimulation of sciatic nerve of cat,” Med. Biol. Eng. Comput. 33, 426–429. 10.1007/BF02510526 [DOI] [PubMed] [Google Scholar]
- 46. McCreery, D. B. , Bullara, L. A. , and Agnew, W. F. (1986). “ Neuronal activity evoked by chronically implanted intracortical microelectrodes,” Exp. Neurol. 92(1), 147–161. 10.1016/0014-4886(86)90131-7 [DOI] [PubMed] [Google Scholar]
- 47. McDermott, H. J. , Mckay, C. M. , and Vandali, A. E. (1992). “ A new portable sound processor for the University of Melbourne/Nucleus Limited multielectrode cochlear implant,” J. Acoust. Soc. Am. 91(6), 3367–3371. 10.1121/1.402826 [DOI] [PubMed] [Google Scholar]
- 48. McDermott, H. J. , Vandali, A. E. , Van Hoesel, R. J. , McKay, C. M. , Harrison, J. M. , and Cohen, L. T. (1993). “ A portable programmable digital sound processor for cochlear implant research,” IEEE Trans. Rehabil. Eng. 1(2), 94–100. 10.1109/86.242423 [DOI] [Google Scholar]
- 49. McKay, C. M. , and Henshall, K. R. (2003). “ The perceptual effects of interphase gap duration in cochlear implant stimulation,” Hear. Res. 181(1–2), 94–99. 10.1016/S0378-5955(03)00177-1 [DOI] [PubMed] [Google Scholar]
- 50. McKay, C. M. , Remine, M. D. , and McDermott, H. J. (2001). “ Loudness summation for pulsatile electrical stimulation of the cochlea: Effects of rate, electrode separation, level, and mode of stimulation,” J. Acoust. Soc. Am. 110(3), 1514–1524. 10.1121/1.1394222 [DOI] [PubMed] [Google Scholar]
- 51.MED-EL (2020). https://www.medel.com/ (Last viewed June 21, 2020).
- 52. Mo, B. , Lindbæk, M. , and Harris, S. (2020). “ Cochlear implants and quality of life: A prospective study,,” Ear Hear. 26(2), 186–194. [DOI] [PubMed] [Google Scholar]
- 53. Normann, R. A. , Maynard, E. M. , Rousche, P. J. , and Warren, D. J. (1999). “ A neural interface for a cortical vision prosthesis,” Vis. Res. 39(15), 2577–2587. 10.1016/S0042-6989(99)00040-1 [DOI] [PubMed] [Google Scholar]
- 54. Patrick, J. F. , Busby, P. A. , and Gibson, P. J. (2006). “ The development of the Nucleus® Freedom™ cochlear implant system,” Trends Amplif. 10(4), 175–200. 10.1177/1084713806296386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Prado-Guitierrez, P. , Fewster, L. M. , Heasman, J. M. , McKay, C. M. , and Shepherd, R. K. (2006). “ Effect of interphase gap and pulse duration on electrically evoked potentials is correlated with auditory nerve survival,” Hear. Res. 215(1–2), 47–55. 10.1016/j.heares.2006.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ramekers, D. , Versnel, H. , Strahl, S. B. , Smeets, E. M. , Klis, S. F. , and Grolman, W. (2014). “ Auditory-nerve responses to varied inter-phase gap and phase duration of the electric pulse stimulus as predictors for neuronal degeneration,” J. Assoc. Res. Otolaryngol. 15(2), 187–202. 10.1007/s10162-013-0440-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Reed, C. M. , and Delhorne, L. A. (2005). “ Reception of environmental sounds through cochlear implants,” Ear Hear. 26(1), 48–61. 10.1097/00003446-200502000-00005 [DOI] [PubMed] [Google Scholar]
- 58. Shannon, R. V. (1983). “ Multichannel electrical stimulation of the auditory nerve in man. II. Channel interaction,” Hear. Res. 12(1), 1–16. 10.1016/0378-5955(83)90115-6 [DOI] [PubMed] [Google Scholar]
- 59. Shannon, R. V. (1992). “ A model of safe levels for electrical stimulation,” IEEE Trans. Biomed. Eng. 39(4), 424–426. 10.1109/10.126616 [DOI] [PubMed] [Google Scholar]
- 60. Shannon, R. V. , Adams, D. D. , Ferrel, R. L. , Palumbo, R. L. , and Grandgenett, M. (1990). “ A computer interface for psychophysical and speech research with the Nucleus cochlear implant,” J. Acoust. Soc. Am. 87(2), 905–907. 10.1121/1.398902 [DOI] [PubMed] [Google Scholar]
- 61. Shannon, R. V. , Fu, Q. J. , Chatterjee, M. , Galvin, J. J., III , Friesen, L. , Cruz, R. , Wygonski, J. , and Robert, M. E. (2002). “ Speech processors for auditory prostheses,” NIH Quarterly Progress Report, QPR No. 12—Final Report (House Ear Institute, Los Angeles).
- 62. Shannon, R. V. , Zeng, F. G. , Fu, Q. J. , Chatterjee, M. , Wygonski, J. , Galvin, J., III , Robert, M. , and Wang, X. (1999). “ Speech processors for auditory prostheses,” NIH Quarterly Progress Report, QPR No. 1 (House Ear Institute, Los Angeles).
- 63. Shekar, R. C. M. C. , Ali, H. , and Hansen, J. H. L. (2018). “ Testing paradigms for assistive hearing devices in diverse acoustic environments,” in ISCA Interspeech-2018, September 2–6, Hyderabad, India, pp. 1686–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shepherd, R. K. , Clark, G. M. , and Black, R. C. (1983). “ Chronic electrical stimulation of the auditory nerve in cats: Physiological and histopathological results,” Acta Oto-Laryngol. 95(Sup399), 19–31. [DOI] [PubMed] [Google Scholar]
- 65. Shokouhi, N. , and Hansen, J. H. L. (2017). “ Teager–Kaiser energy operators for overlapped speech detection,” IEEE/ACM Trans. Audio Speech Language Process. 25(5), 1035–1047. 10.1109/TASLP.2017.2678684 [DOI] [Google Scholar]
- 66. Shokouhi, N. , Ziaei, A. , Sangwan, A. , and Hansen, J. H. L. (2015). “ Robust overlapped speech detection and its application in word-count estimation for Prof-Life-Log data,” in 2015 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), April 19–24, South Brisbane, Queensland, Australia ( Institute of Electrical and Electronics Engineers, New York: ) pp. 4724–4728. [Google Scholar]
- 67. Skinner, M. W. , Ketten, D. R. , Vannier, M. W. , Gates, G. A. , Yoffie, R. L. , and Kalender, W. A. (1994). “ Determination of the position of nucleus cochlear implant electrodes in the inner ear,” Am. J. Otol. 15(5), 644–651. [PubMed] [Google Scholar]
- 68. Spitzer, J. B. , Kessler, M. A. , and Bromberg, B. (1992). “ Longitudinal findings in quality of life and perception of handicap following cochlear implantation,” Semin. Hear. 13(3), 260–268. 10.1055/s-0028-1085162 [DOI] [Google Scholar]
- 69. Stohl, J. S. , Throckmorton, C. S. , and Collins, L. M. (2008). “ Developing a flexible SPEAR3-based psychophysical research platform for testing cochlear implant users,” Technical Report (Duke University, Durham, NC, USA).
- 70. Tye-Murray, N. , Tyler, R. S. , Woodworth, G. G. , and Gantz, B. J. (1992). “ Performance over time with a Nucleus or Ineraid cochlear implant,” Ear Hear. 13(3), 200–209. 10.1097/00003446-199206000-00010 [DOI] [PubMed] [Google Scholar]
- 71. Tyler, R. S. , and Kelsay, D. (1990). “ Advantages and disadvantages reported by some of the better cochlear-implant patients,” Am. J. Otol. 11(4), 282–289. [PubMed] [Google Scholar]
- 72. Tyler, R. S. , and Lowder, M. W. (1992). “ Audiological management and performance of adult cochlear-implant patients,” Ear Nose Throat J. 71(3), 117–128. 10.1177/014556139207100302 [DOI] [PubMed] [Google Scholar]
- 73.University of Innsbruck (2001). RIB: Research Interface Box System V1.0 Manual ( University of Innsbruck, Innsbruck, Austria: ). [Google Scholar]
- 74. Van Immerseel, L. , Peeters, S. , Dykmans, P. , Vanpoucke, F. , and Bracke, P. (2005). “ SPAIDE: A real-time research platform for the Clarion CII/90K cochlear implant,” EURASIP J. Adv. Signal Process. 2005(18), 764821. 10.1155/ASP.2005.3060 [DOI] [Google Scholar]
- 75. Vandali, A. E. , Sucher, C. , Tsang, D. J. , McKay, C. M. , Chew, J. W. , and McDermott, H. J. (2005). “ Pitch ranking ability of cochlear implant recipients: A comparison of sound-processing strategies,” J. Acoust. Soc. Am. 117(5), 3126–3138. 10.1121/1.1874632 [DOI] [PubMed] [Google Scholar]
- 76. Walsh, S. M. , and Leake-Jones, P. A. (1982). “ Chronic electrical stimulation of auditory nerve in cat: Physiological and histological results,” Hear. Res. 7(3), 281–304. 10.1016/0378-5955(82)90041-7 [DOI] [PubMed] [Google Scholar]
- 77. Wilson, B. S. , and Dorman, M. F. (2008). “ Cochlear implants: A remarkable past and a brilliant future,” Hear. Res. 242(1–2), 3–21. 10.1016/j.heares.2008.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wilson, B. S. , Finley, C. C. , Farmer, J. C., Jr. , Lawson, D. T. , Weber, B. A. , Wolford, R. D. , Kenan, P. D. , White, M. W. , Merzenich, M. M. , and Schindler, R. A. (1988). “ Comparative studies of speech processing strategies for cochlear implants,” Laryngoscope 98, 1069–1077. 10.1288/00005537-198810000-00009 [DOI] [PubMed] [Google Scholar]
- 79. Wilson, B. S. , Finley, C. C. , Lawson, D. T. , Wolford, R. D. , Eddington, D. K. , and Rabinowitz, W. M. (1991). “ Better speech recognition with cochlear implants,” Nature 352, 236–238. 10.1038/352236a0 [DOI] [PubMed] [Google Scholar]
- 80.World Health Organization (2015). “1.1 billion people at risk of hearing loss,” http://www.who.int/mediacentre/news/releases/2015/ear-care/en/ (Last viewed July 29, 2019).
- 81. Zeng, F. G. (2008). “ Role of temporal fine structure in speech perception,” J. Acoust. Soc. Am. 123, 3710. 10.1121/1.2935141 [DOI] [Google Scholar]
- 82. Zeng, F. G. , and Shannon, R. V. (1994). “ Loudness-coding mechanisms inferred from electric stimulation of the human auditory system,” Science 264(5158), 564–566. 10.1126/science.8160013 [DOI] [PubMed] [Google Scholar]
- 83. Zhao, F. , Stephens, S. D. G. , Sim, S. W. , and Meredith, R. (1997). “ The use of qualitative questionnaires in patients having and being considered for cochlear implants,” Clin. Otolaryngol. Allied Sci. 22(3), 254–259. 10.1046/j.1365-2273.1997.00036.x [DOI] [PubMed] [Google Scholar]