Abstract
BACKGROUND
Occipital-cervical fusion (OCF) and ventral decompression (VD) may be used in the treatment of pediatric Chiari-1 malformation (CM-1) with syringomyelia (SM) as adjuncts to posterior fossa decompression (PFD) for complex craniovertebral junction pathology.
OBJECTIVE
To examine factors influencing the use of OCF and OCF/VD in a multicenter cohort of pediatric CM-1 and SM subjects treated with PFD.
METHODS
The Park-Reeves Syringomyelia Research Consortium registry was used to examine 637 subjects with cerebellar tonsillar ectopia ≥ 5 mm, syrinx diameter ≥ 3 mm, and at least 1 yr of follow-up after their index PFD. Comparisons were made between subjects who received PFD alone and those with PFD + OCF or PFD + OCF/VD.
RESULTS
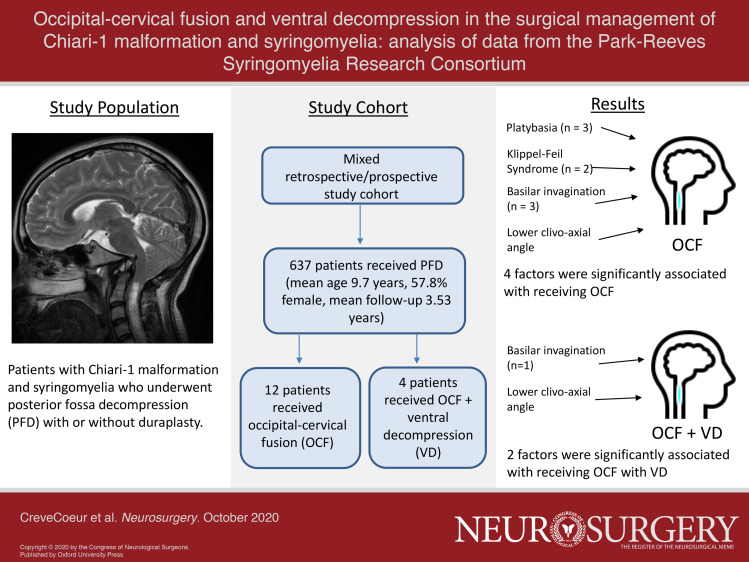
All 637 patients underwent PFD, 505 (79.2%) with and 132 (20.8%) without duraplasty. A total of 12 subjects went on to have OCF at some point in their management (PFD + OCF), whereas 4 had OCF and VD (PFD + OCF/VD). Of those with complete data, a history of platybasia (3/10, P = .011), Klippel-Feil (2/10, P = .015), and basilar invagination (3/12, P < .001) were increased within the OCF group, whereas only basilar invagination (1/4, P < .001) was increased in the OCF/VD group. Clivo-axial angle (CXA) was significantly lower for both OCF (128.8 ± 15.3°, P = .008) and OCF/VD (115.0 ± 11.6°, P = .025) groups when compared to PFD-only group (145.3 ± 12.7°). pB-C2 did not differ among groups.
CONCLUSION
Although PFD alone is adequate for treating the vast majority of CM-1/SM patients, OCF or OCF/VD may be occasionally utilized. Cranial base and spine pathologies and CXA may provide insight into the need for OCF and/or OCF/VD.
Keywords: Chiari malformation, Clivo-axial angle, Craniovertebral junction, Occipital-cervical fusion, pB-C2, Syringomyelia, Ventral brainstem compression
Graphical Abstract
Graphical Abstract.
ABBREVIATIONS
- CXA
clivo-axial angle
- FOR
frontal-occipital ratio
- MRI
magnetic resonance imaging
- OCF
occipital-cervical fusion
- PFD
posterior fossa decompression
- PRSRC
Park-Reeves Syringomyelia Research Consortium
- SM
syringomyelia
- VBSC
ventral brainstem compression
- VD
ventral decompression
Whereas posterior fossa decompression (PFD) is commonly accepted as the primary treatment for pediatric Chiari type I malformation (CM-1) with or without syringomyelia (SM), the indications for and use of occipital-cervical fusion (OCF) and/or ventral decompression (VD) are less clear. Studies have examined variations in craniovertebral anatomy and their relationship to instability with implications for management.1,2,3-10,11-13 Clivo-axial angle (CXA) less than 125°, basilar invagination, medullary kinking, and a diagnosis of Chiari 1.5 malformation14 have been proposed risk factors associated with OCF treatment in a complex subset of CM-1 patients.2,14 Though controversial, the presence of a syrinx and syrinx size may also confer additional risk for requiring OCF.15-21
Patients with CM-1 ± SM may present with symptoms of ventral brainstem compression (VBSC) with associated cranial and cervical neuropathies.3,8,21 Thus, assessment of symptomatic VBSC is important in choosing a treatment approach.10,11 Grabb et al22,23 described an indicator of VBSC, the pB-C2 line, and suggested that this parameter may provide insight into the need for OCF and/or VD; however, pB-C2 may not be sufficient as a stand-alone tool for decision-making.7,23-25
Current studies of OCF and VD in the setting of CM-1 and SM are limited by modest sample sizes and institutional variability. The objective of this current study was to examine factors associated with the use of OCF and VD in a large group of pediatric CM-1/SM patients treated across the United States.
METHODS
Study Design
Subjects were enrolled across 30 Park-Reeves Syringomyelia Research Consortium (PRSRC) sites both retrospectively (n = 369) and prospectively (n = 268) from January 2011 to February 2017. A total of 30 sites contributed patients. This study was approved by the institutional review boards at the host institution and each participating center. All data were de-identified; no patient consent was required. All patients had tonsillar ectopia ≥5 mm, syrinx diameter ≥3 mm, and ≥1-yr postoperative follow-up from their index PFD procedure. Patients who underwent PFD with or without duraplasty were considered in a single cohort (PFD) (duraplasty status specified in Results). The study cohort was divided into three groups: PFD only (referent), PFD + OCF only (OCF group), and PFD + OCF + VD (OCF/VD group). All patients who underwent VD also had OCF.
Variables for Analysis
Demographic data and clinical findings at initial treatment and follow-up encounters were collected and recorded in the PRSRC database. Variables included diagnosis date, symptom onset date, and brain, cranial base, or spine abnormalities identified from clinical history and imaging. Radiological images were reviewed by two blinded, trained readers, including a board certified pediatric neuroradiologist. PRSRC standard magnetic resonance imaging (MRI) measures included: tonsillar position measured in mm below McRae's26 line, maximum antero-posterior syrinx diameter in mm, syrinx length measured in vertebral levels, frontal-occipital ratio27 (FOR), basilar invagination (odontoid process above McRae's26 line), pB-C2,22 and CXA.28 OCF and OCF/VD were recorded as upfront (performed before or ≤30 d after PFD) or delayed (performed after 30 d). Clinical course and additional treatments were documented on initial and subsequent encounters.
Statistical Considerations
Analyses were performed using SPSS version 25 (IBM Corporation, Armonk, New York). Variables were summarized as percentages (categorical variables), mean ± standard deviation (parametric variables), or median with interquartile range (non-parametric distributed numerical variables). Differences between groups were detected using the Mann-Whitney U test and one-way Games-Howell post hoc ANOVA test for continuous variables and Fisher's exact test for frequency data. Analyses were conducted at a predetermined alpha level of 0.05, with Bonferroni correction for clinical symptoms and signs.
RESULTS
Patient Characteristics
A total of 637 patients in the PRSRC database met study inclusion criteria (Table 1, Figure). A total of 12 (1.9%) patients underwent OCF, 9 of whom had upfront OCF, and 3 of whom had delayed OCF at 20.07 ± 17.53 mo following their initial PFD. VD was performed in four patients (0.6%), all of whom also had OCF. Individual patient level data are detailed in Table 2. Four selected case illustrations with figures have been published as Supplemental Digital Content (Text, Supplemental Digital Content 1 and Figures, Supplemental Digital Contents 2-5). No patient in the OCF or OCF/VD groups had preoperative hydrocephalus or craniosynostosis. Regarding clinical presentation, in the OCF cohort, 10/12 patients had some combination of extremity weakness, numbness, and gait ataxia and 8/12 had a combination of dysphagia, choking, vocal cord dysfunction, and apnea. For the OCF/VD cohort, 4/4 had a combination of extremity weakness, numbness, or gait ataxia, and 2/4 had choking and apnea.
TABLE 1.
Characteristics of the Initial Study Cohort (N = 637)
| Variable | Valuea |
|---|---|
| Ageb | 9.67 [5.98-13.33] |
| Gender | |
| Male | 269 (42.2) |
| Female | 368 (57.8) |
| Race | |
| Asian | 10 (1.6) |
| White | 515 (80.7) |
| Black or African American | 62 (9.7) |
| Native Hawaiian or Pacific Islander | 2 (0.3) |
| Other or unknown | 48 (7.5) |
| Primary procedure | |
| PFD | 132 (20.8) |
| PFD with duraplasty (PFDD) | 505 (79.2) |
| Revision PFD or PFDD | 59 |
| PFD → PFD | 1 |
| PFD → PFDD | 18 |
| PFDD → PFDD | 37 |
| PFDD → PFD | 2 |
| OCF onlyc | 12 |
| Upfront | 9 |
| Delayed | 3 |
| VD/OCFc | 4 |
| Upfrontd | 2 |
| Delayede | 2 |
aValues are frequency (percent of entire cohort) unless otherwise specified.
bValues are median (interquartile range).
cTiming defined as upfront if within 30 d of initial PFD procedure, delayed if after 30 d.
dBoth patients underwent VD on the same day as the index PFD and OCF.
eOne patient underwent upfront OCF on the same day as the index PFD, with VD delayed by 6.9 mo. The other patient underwent OCF and VD on the same day 33.6 mo after index PFD.
FIGURE.
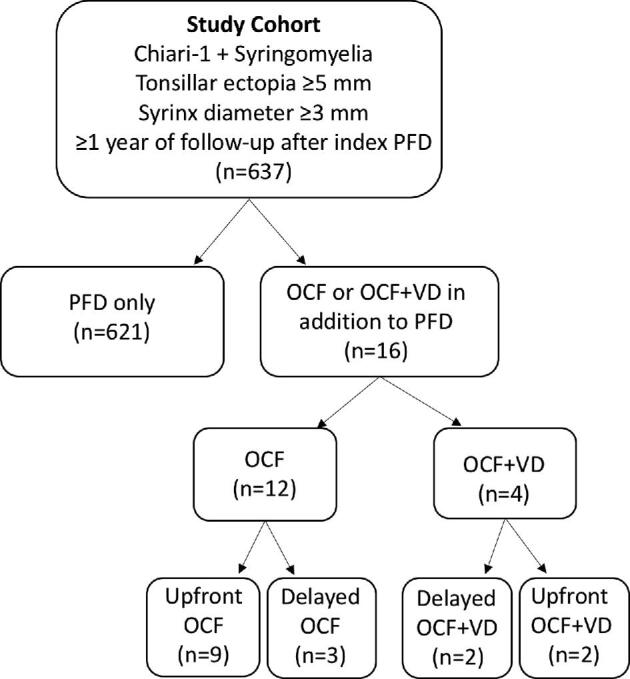
Flowchart depicting the study cohort.
TABLE 2.
Individual Patient Characteristics
| Site | Age diagnosis | Gender | Procedurea | Revision | Revision intervalc | OCF ± VD surgery | OCF timingb | OCF intervalc | VD timingb | VD intervalc |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 | Female | PFDD | – | – | OCF | Upfront | 0 | – | – |
| 2 | 11 | Female | PFDD | – | – | OCF | Upfront | 0 | – | – |
| 2 | 4 | Male | PFDD | – | – | OCF | Upfront | 0 | – | – |
| 2 | 5 | Female | PFD | – | – | VD + OCF | Upfront | 0 | Delayed | 6.9 |
| 2 | 13 | Female | PFD | – | – | VD + OCF | Upfront | 0 | Upfront | 0 |
| 13 | 14 | Male | PFDD | – | – | OCF | Upfront | 0 | – | – |
| 14 | 17 | Male | PFDD | – | – | OCF | Upfront | 0 | – | – |
| 14 | 17 | Male | PFDD | – | – | OCF | Upfront | 0 | – | – |
| 14 | 3 | Male | PFDD | – | – | OCF | Delayed | 45.8 | – | – |
| 14 | 8 | Female | PFDD | PFDD | 11.7 | OCF | Delayed | 11.7 | – | – |
| 17 | 14 | Female | PFDD | – | – | OCF | Upfront | 0 | – | – |
| 24 | 15 | Male | PFDD | PFDD | 13.17 | VD + OCF | Delayed | 33.6 | Delayed | 33.6 |
| 25 | 17 | Female | PFDD | – | – | VD + OCF | Upfront | 22 | Upfront | 22 |
| 27 | 13 | Female | PFD | – | – | OCF | Upfront | 0 | – | – |
| 27 | 7 | Female | PFDD | – | – | OCF | Delayed | 20.2 | – | – |
| 28 | 11 | Female | PFDD | – | – | OCF | Upfront | 0 | – | – |
aValues presented as either PFD with duraplasty (PFDD) or PFD without duraplasty (PFD) at initial procedure.
bTiming defined as “upfront” if within 30 d of initial PFD procedure, “delayed” if after 30 d.
cValues presented in days.
PFDD = PFD with duraplasty.
Clinical Parameters
Table 3 details all clinical characteristics; follow-up data are displayed in Table, Supplemental Digital Content 6. Relative to subjects who did not undergo OCF or VD, the OCF group displayed increased rates of dysphagia (4/12, P = .041) and ataxia (6/12, P = .004). For the OCF/VD group, diplopia (2/4, P = .008) and choking (2/4, P = .013) were increased compared to PFD alone. Neurologic exam findings of weak neck rotation (4/10, P < .001), upper extremity weakness (10/12, P = .042), hyperreflexia (5/12, P = .005), and gait instability (5/8, P = .001) were increased in the OCF cohort over the PFD cohort, whereas for the OCF/VD cohort, only weak gag reflex was significantly increased (2/3, P = .018). With Bonferroni correction, only gait instability and weak neck rotation was found to be significant for OCF (adjusted alpha = .001). As OCF and OCF/VD cases were not performed in all PRSRC centers, further analyses were conducted specifically within centers performing these procedures (Table, Supplemental Digital Content 7).
TABLE 3.
Comparison of Clinical Findings at Initial Treatmenta
| Variable | PFD onlya | OCF | P value | OCF/VD | P value |
|---|---|---|---|---|---|
| Number in group (n) | 621 | 12 | 4 | ||
| Symptoms | |||||
| Headache | 346/621 (55.7) | 8/12 (66.7) | .564 | 2/4 (50.0) | 1.000 |
| Neck pain | 129/621 (20.8) | 4/12 (33.3) | .289 | 0/4 (0.0) | .586 |
| Back pain | 101/621 (16.2) | 1/12 (8.3) | .701 | 0/4 (0.0) | 1.000 |
| Balance/Gait Ataxia | 87/621 (14.0) | 6/12 (50.0) | .004 | 3/4 (75.0) | .010 |
| Dysphagia | 70/621 (11.3) | 4/12 (33.3) | .041 | 0/4 (0.0) | 1.000 |
| UE weakness | 56/621 (9.0) | 1/12 (8.3) | 1.000 | 0/4 (0.0) | 1.000 |
| LE numbness | 55/621 (8.9) | 2/12 (16.6) | .295 | 1/4 (25.0) | .314 |
| LE weakness | 45/621 (7.2) | 1/12 (8.3) | .599 | 0/4 (0.0) | 1.000 |
| UE pain | 42/621 (6.7) | 1/12 (8.3) | .573 | 0/4 (0.0) | 1.000 |
| UE numbness | 42/621 (6.7) | 1/12 (8.3) | .702 | 0/4 (0.0) | .127 |
| Seizure | 36/621 (5.8) | 0/12 (0.0) | 1.000 | 0/4 (0.0) | 1.000 |
| LE pain | 33/621 (5.3) | 1/12 (8.3) | .488 | 0/4 (0.0) | 1.000 |
| Apnea | 32/621 (5.2) | 2/12 (16.6) | .132 | 1/4 (25.0) | .195 |
| Choking | 29/621 (4.7) | 1/12 (8.3) | .445 | 2/4 (50.0) | .013 |
| Diplopia | 22/621 (3.5) | 0/12 (0.0) | 1.000 | 2/4 (50.0) | .008 |
| Pulmonary infections | 12/621 (1.9) | 0/12 (0.0) | 1.000 | 0/4 (0.0) | 1.000 |
| Tremor | 11/621 (1.9) | 0/12 (0.0) | 1.000 | 0/4 (0.0) | 1.000 |
| Hoarseness | 10/621 (1.6) | 0/12 (0.0) | 1.000 | 0/4 (0.0) | 1.000 |
| Trunk pain | 9/621 (1.4) | 0/12 (0.0) | 1.000 | 0/4 (0.0) | 1.000 |
| Face numbness | 9/621 (1.4) | 0/12 (0.0) | 1.000 | 1/4 (25.0) | .063 |
| Vocal cord dysfunction | 8/621 (1.3) | 1/12 (8.3) | .159 | 0/4 (0.0) | 1.000 |
| Face weakness | 6/621 (0.9) | 0/12 (0.0) | 1.000 | 0/4 (0.0) | 1.000 |
| Shortness of breath | 6/621 (0.9) | 0/12 (0.0) | 1.000 | 0/4 (0.0) | – |
| Prior genetic history | 46/621 (7.4) | 3/12 (25.0) | .058 | 0/4 (0.0) | 1.000 |
| Neurologic exam | |||||
| LE weakness | 544/555 (98.0) | 11/12 (91.7) | .228 | 4/4 (100.0) | 1.000 |
| UE weakness | 543/557 (97.5) | 10/12 (83.3) | .042 | 3/4 (75.0) | .103 |
| Pinprick | 30/251 (11.9) | 1/5 (20.0) | .478 | 0/1 (0.0) | 1.000 |
| Gait instability | 61/516 (11.8) | 5/8 (62.5) | .001 | 2/4 (0.0) | .074 |
| Hyperreflexia | 55/493 (11.2) | 5/11 (45.5) | .005 | 2/4 (0.0) | .067 |
| Light touch deficit | 35/422 (8.3) | 1/8 (12.5) | .506 | 0/3 (0.0) | 1.000 |
| Nystagmus | 42/525 (8.0) | 3/12 (25.0) | .071 | 1/4 (25.0) | .288 |
| Clonus | 25/315 (7.9) | 2/6 (33.3) | .083 | 0/1 (0.0) | 1.000 |
| Weak Gag Reflex | 33/436 (7.6) | 1/8 (12.5) | .474 | 2/3 (66.6) | .018 |
| Babinski | 14/233 (6.0) | 1/6 (16.7) | .325 | 0/1 (0.0) | 1.000 |
| Hoffman | 12/219 (5.5) | 0/3 (0.0) | 1.000 | 1/2 (50.0) | .114 |
| Romberg | 8/229 (3.5) | 0/4 (0.0) | 1.000 | 0/1 (0.0) | 1.000 |
| Dysmetria | 9/352 (2.6) | 1/5 (20.0) | .133 | 1/2 (50.0) | .056 |
| Disconjugate gaze | 12/620 (1.9) | 1/12 (8.3) | .222 | 1/4 (25.0) | .081 |
| Weak neck rotation | 9/464 (1.9) | 4/10 (40.0) | <.001 | 0/2 (0.0) | 1.000 |
| Facial sensation | 7/473 (1.5) | 1/11 (0.1) | .169 | 0/3 (0.0) | 1.000 |
| Proprioception | 3/253 (1.2) | 0/5 (0.0) | 1.000 | 0/4 (0.0) | – |
| Facial weakness | 6/549 (1.1) | 0/12 (0.0) | 1.000 | 0/3 (0.0) | 1.000 |
| Hoarseness | 5/461 (1.1) | 0/10 (0.0) | 1.000 | 0/3 (0.0) | 1.000 |
| Extraocular palsy | 5/620 (0.8) | 0/12 (0.0) | 1.000 | 0/4 (0.0) | 1.000 |
| Tongue deviation | 3/548 (0.5) | 1/12 (8.3) | .083 | 0/2 (0.0) | 1.000 |
| eWeak shrug | 1/442 (0.2) | 0/10 (0.0) | 1.000 | 0/2 (0.0) | 1.000 |
Bold value indicate statistically significant P values (p < .05).
Cells show values reported as n of documented cases, with significance tested with Fisher's exact test with PFD only used as referent group. Alpha values set to with Bonferroni correction set to .001. In this table, the PFD Only column included subjects who underwent PFD with or without duraplasty, while the OCF column included subjects treated with PFD/D and OCF, and the VD column included subjects treated with PFD/D, VD, and OCF. UE = upper extremity, LE = lower extremity, DTR = deep tendon reflex.
Initial Radiological Measurements
Radiological and clinical exam findings are summarized in Table 4. Tonsillar position was significantly lower for the OCF group compared with PFD alone (P = .026). There was no difference among groups in syrinx diameter, syrinx length, or pB-C2 distance. For both OCF and OCF/VD groups, the CXA was lower compared to PFD, with mean measurements of 128.8 ± 15.3° (P = .002) and 115.0 ± 11.6° (P = .001) degrees, respectively; however, CXA was not significantly different when comparing OCF and OCF/VD groups (P = .128) Further examination by dichotomizing CXA to ≤125° or >125° showed CXA ≤125° in 60% of the OCF group (P < .001) and 75% of the VD/OCF group (P < .001).2 FOR was not different for any of the groups. History of platybasia (3/10, P = .011), Klippel-Feil (2/10, P = .015), or basilar invagination (3/12, P < .001) all were increased within the OCF group, whereas scoliosis (4/12, P = 1.00) was not different vs the PFD-only group. For the OCF/VD cohort, only basilar invagination (1/4, P < .001) was significantly increased. Of note, only 5/12 OCF and 1/4 OCF/VD patients had cervical spine flexion/extension radiographs available for review. Table, Supplemental Digital Content 8 provides analyses for centers performing all procedure types.
TABLE 4.
Initial Radiologic Findingsa
| Variable | PFD Only | OCF | OCF Mean Difference | P valuee | OCF/VD | OCF/VD Mean Difference | P valuee | OCF vs OCF/VD P valuef |
|---|---|---|---|---|---|---|---|---|
| Number in group (n) | 621 | 12 | 4 | |||||
| Age of surgeryb | 10.09 [7.12-16.74] | 11.51 [7.10-16.74] | 1.42 ± 1.50 | .621 | 12.59 [7.34-15.79] | 2.50 ± 2.54 | .634 | .930 |
| Radiographic findingsc | ||||||||
| Tonsillar Ectopia (mm) | 12.5 ± 4.9 | 17.3 ± 5.4 | −4.8 ± 1.6 | .026 | 17.5 ± 5.8 | −5.00 ± 2.9 | .335 | .999 |
| AP Syrinx diameter (mm) | 7.5 ± 3.1 | 7.1 ± 3.0 | 0.6 ± 0.9 | .811 | 9.5 ± 3.0 | −2.1 ± 1.5 | .442 | .349 |
| Syrinx Levels | 8.9 ± 4.6 | 7.6 ± 5.2 | 1.2 ± 1.7 | .762 | 10.7 ± 6.1 | −1.89 ± 3.5 | .864 | .735 |
| pB-C2 (mm) | 6.9 ± 1.2 | 8.4 ± 2.2 | −1.3 ± 0.7 | .164 | 6.8 ± 0.5 | 0.1 ± 0.3 | .895 | .146 |
| pB-C2 ≥ 9 mmd | 47/510 (9.2) | 3/11 (27.3) | – | .079 | 0/4 (0.0) | – | 1.000 | .516 |
| Clivoaxial angle (°) | 145.5 ± 12.4 | 128.8 ± 15.3 | 16.8 ± 4.4 | .008 | 115.0 ± 11.6 | 30.8 ± 5.8 | .025 | .128 |
| Clivoaxial angle ≤ 125°d | 31/511 (6.0) | 7/11 (63.6) | – | <.001 | 3/4 (75.0) | – | .001 | 1.000 |
| FOR | 0.3 ± 0.04 | 0.3 ± 0.02 | 0.009 ± 0.007 | .501 | 0.3 ± 0.01 | 0.02 ± 0.005 | .226 | .668 |
| Spine Pathologyd | ||||||||
| Platybasia | 12/281 (4.3) | 3/10 (30.0) | – | .011 | 0/3 (0.0) | – | 1.000 | .528 |
| Scoliosis | 230/637 (36.1) | 4/10 (40.0) | – | 1.000 | 1/3 (33.3) | – | .127 | 1.000 |
| Klippel Feil | 4/283 (1.4) | 2/10 (20.0) | – | .015 | 0/3 (0.0) | – | 1.000 | 1.000 |
| Basilar invagination | 11/281 (3.9) | 6/10 (60.0) | – | <.001 | 3/3 (100.0) | – | <.001 | .497 |
Bold value indicate statistically significant P values (p < .05).
aCells show mean ± standard deviation (P-value from one-way ANOVA) for continuous variables and frequency (%, P-value from Fisher's exact test) data, comparing procedure to PFD only.
bValues are reported median [inner quartile range].
cValues are reported as mean ± standard deviation.
dValues reported as n (%) of documented cases.
ePost hoc analysis with Games-Howell tests, values reported as mean difference as compared with PFD only, significance.
fPost hoc analysis with Games-Howell tests, values reported as significance between OCF and OCF/VD mean differences.
Radiologic Follow-up
A total of 359 (57.3%) PFD-only patients, 9 (75%) OCF patients, and 3 (75%) OCF/VD patients had radiologic measurements collected more than 30 d after surgery (Table 5). Mean follow-up was 3.53 ± 1.41 yr. There was a small but significant increase in pB-C2 observed in the PFD alone group on follow-up imaging (preoperative = 6.88 ± 1.24 mm and postoperative = 7.01 ± 1.25 mm, P = .045). There was also a small but significant decrease in CXA in the PFD alone group on follow-up imaging (preoperative = 145.48 ± 12.43° and postoperative = 143.21 ± 11.89° mm, P = .013). Not surprisingly, the OCF and OCF/VD groups still had significantly lower CXA (125.38 ± 20.46°, P = .007, and 121.0 ± 21.95°, P = .033, respectively) compared with the PFD group. When comparing pre- and postoperative groups, only the PFD-only group showed significant improvement for syrinx diameter (P < .001) or syrinx length in levels (P < .001), though these analyses were limited because of the small sample sizes of the OCF and OCF/VD groups. Table, Supplemental Digital Content 6 provides detailed follow-up on individual clinical signs and symptoms.
TABLE 5.
| Variable | PFD only | P value | OCF | P value | OCF/VD | P value |
|---|---|---|---|---|---|---|
| Radiographic findings c | ||||||
| AP Syrinx Diameter (mm) | ||||||
| Preoperative | 7.49 ± 3.09 | 7.10 ± 3.00 | 9.50 ± 3.00 | |||
| Postoperative | 4.06 ± 3.04 | <.001 | 5.33 ± 3.56 | .252 | 4.80 ± 3.03 | .233 |
| Change | −3.76 ± 3.76 | −5.00 ± 3.90 | −3.50 ± 7.78 | |||
| Syrinx length (levels) | ||||||
| Preoperative | 8.94 ± 4.63 | 7.60 ± 5.19 | 10.67 ± 6.11 | |||
| Postoperative | 7.04 ± 4.28 | <.001 | 8.00 ± 5.18 | .956 | 8.50 ± 0.71 | .564 |
| Change | −1.86 ± 4.03 | 2.16 ± 6.37 | −0.50 ± 4.94 | |||
| pb-C2 (mm) | ||||||
| Preoperative | 6.88 ± 1.24 | 8.40 ± 2.17 | 6.75 ± 0.50 | |||
| Postoperative | 7.01 ± 1.25 | .045 | 7.44 ± 1.51 | .057 | 6.83 ± 1.84 | .724 |
| Change | 0.60 ± 8.10 | −1.00 ± 1.87 | 0.33 ± 1.16 | |||
| Clivalaxial angle(°) | ||||||
| Preoperative | 145.48 ± 12.43 | 128.80 ± 15.27 | 115.00 ± 11.55 | |||
| Postoperative | 143.21 ± 11.89 | .013 | 125.38 ± 20.46 | .829 | 121.00 ± 21.95 | .703 |
| Change | −2.15 ± 12.76 | −3.75 ± 11.93 | 3.67 ± 9.29 |
Bold value indicate statistically significant P values (p < .05).
aCells show mean ± standard deviation (P-value from asymptotic two-tailed Mann-Whitney U test) for continuous variables that are statistically tested within each group from preoperative and postoperative.
bPostoperative follow-up refers to the initial radiographic imaging variables taken at least 30 d from primary procedure.
cValues are reported as mean ± standard deviation.
AP = anteroposterior; OCF = occipito-cervical fusion.
Upfront vs Delayed Treatment Timing
Additional analyses regarding timing of either OCF (Table 6) or OCF/VD (Table 7) demonstrated no statistically significant differences in preoperative radiologic, clinical, or historical parameters between upfront and delayed approaches. Out of the 5 patients with delayed OCF (3 received OCF and 2 received OCF + VD), the mean time to OCF was 20.07 ± 17.53 mo. The mean time to delayed OCF/VD was 20.27 ± 18.85 mo for 2 patients, with 1 patient having an upfront OCF and the other delaying VD by 33.6 mo.
TABLE 6.
Comparison Between the Timing of Patients Who Underwent Occipital Fusion Onlya
| Variable | Upfront | Delayed | P value |
|---|---|---|---|
| Number in group | 9 | 3 | |
| Radiographic findings | |||
| Tonsillar Ectopia (mm) | |||
| Preoperativeb | 15.4 ± 7.6 | 19.7 ± 7.4 | .138 |
| Syrinx diameter (mm) | |||
| Preoperative | 6.7 ± 2.1 | 8.0 ± 5.0 | .564 |
| Postoperative | 3.5 ± 3.7 | 8.0 ± 2.8 | .159 |
| Improvement | 4.3 ± 2.5 | 2.5 ± 0.7 | .355 |
| pb-C2 (mm) | |||
| Preoperative | 9.1 ± 2.2 | 6.7 ± 0.6 | .062 |
| Postoperative | 7.7 ± 1.9 | 7.0 ± 1.0 | .789 |
| Improvement | 1.7 ± 1.9 | −0.3 ± 0.6 | .058 |
| Clival angle (°) | |||
| Preoperative | 123.7 ± 13.4 | 140.7 ± 14.5 | .110 |
| Postoperative | 125.2 ± 7.2 | 137.3 ± 22.1 | .456 |
| Improvement | 8.0 ± 11.8 | −3.3 ± 10.0 | .294 |
aPreoperative refers to measurements before index PFD or OCF.
bPostoperative measures for tonsillar ectopia were not recorded due to the post-surgical absence of the opisthion. Cells show mean ± standard deviation (P-value from asymptotic two-tailed Mann-Whitney U test) for continuous variables.
TABLE 7.
Comparison Between the Timing of Patients Who Underwent Occipital Fusion/VDa
| Variable | Upfront | Delayed | P value |
|---|---|---|---|
| Number in group | 2 | 2 | |
| Radiographic findings | |||
| Tonsillar Descent (mm) | |||
| Preoperativeb | 14.5 | 20.5 | .439 |
| AP Syrinx Diameter (mm) | |||
| Preoperative | 10.0 | 9.0 | .683 |
| Postoperative | 3.0 | 8.0 | – |
| Improvement | 9.0 | 2.0 | – |
| pb-C2 (mm) | |||
| Preoperative | 7.0 | 6.5 | .317 |
| Postoperative | 6.0 | 8.0 | .157 |
| Improvement | 1.0 | −1.0 | |
| Clival angle (°) | |||
| Preoperative | 115.0 | 115.0 | 1.000 |
| Postoperative | 116.5 | 125.0 | 1.000 |
| Improvement | 1.5 | 8.0 |
aPreoperative refers to measurements made before index PFD and OCF/VD.
bPostoperative measures for tonsillar ectopia were not recorded because of the post-surgical absence of the opisthion. Cells show mean (P-value from asymptotic two-tailed Mann-Whitney U test) for continuous variables between upfront and delayed.
DISCUSSION
Key Findings
This is the first multicenter study to examine the use and timing of OCF and VD in the management of CM-1/SM patients. Our findings show that OCF ± VD was recommended for a minority of patients with CM-1/SM. Neurological exam deficits of gait instability and weak neck rotation were associated with performing OCF, whereas no clinical findings were associated with the performance of VD (with robust statistical correction). A prior history of basilar invagination was associated with OCF and OCF/VD. These data build upon previous work evaluating tonsillar position, syrinx size, CXA, and pB-C2 in the surgical management of CM-1 and SM.2,16,23,29-31 In this study, tonsil position and CXA were different in those undergoing OCF, whereas pB-C2 was not. When dichotomizing these variables as previously described,2,22 pB-C2 ≥ 9 mm did show a higher (but nonsignificant) incidence in the OCF group, and presence of CXA ≤125° was significantly higher in both OCF and OCF/VD groups.
Interpretation of Findings
CXA has garnered interest as a radiological parameter in formulating risk of craniocervical instability and ventral cord and brain stem compression,2 and our data extend these findings. Henderson et al32 suggest that OCF may be considered in patients with clinical symptoms, Chiari 1 or 0, and a CXA less than 135°. They also indicate that VD may be considered when CXA cannot be re-established to greater than 150°, and there are continued signs of ventral compression.32
Although the OCF/VD group had lower CXA values (115.0 ± 17.5°) than the PFD (145.3 ± 12.7°) and OCF (128.8 ± 15.3°) groups, overall rates of OCF and VD in our purely pediatric study cohort were strikingly low. Improvement in CXA was modest for OCF/VD (121.0 ± 22.0°) and not observed in OCF alone (125.4 ± 20.5°). Variability in CXA improvement following fusion may be related to perioperative reduction or whether operative fusion was undertaken in Situ (eg, for fixed deformities/cervical anomalies, rather than predominantly hereditary connective tissue disorders, as in Henderson et al33). In addition to symptomatic presentation, reducibility of basilar invagination may also dictate whether OCF is adequate to restore craniocervical alignment and improve ventral medullary compression.
Dlouhy, Dahdaleh, and Menezes34 provided an algorithm for treatment of craniovertebral junction pathology, including patients with CM-1. According to these authors, cervicomedullary compression and/or craniovertebral junction instability may be subgrouped, with reducible pathology receiving posterior fusion alone, and irreducible compression or craniovertebral junction instability receiving dorsal decompression (dorsal encroachment) or VD (ventrolateral encroachment). Craniovertebral junction pathologies that remain unstable after decompression should then receive posterior fusion, but this should not occur until decompressions alone have proven insufficient.
In agreement with recent studies,2,10 our data suggest that pB-C2 distance may not be associated with the occurrence of OCF or VD. VBSC may occur in 10% of CM-1 patients, typically presenting as lower cranial nerve neuropathies.35 Interestingly, sleep apnea, a common symptom of medullary compression was not significant in our dataset, though formal sleep studies were neither required nor routinely collected. Goel13 has suggested that atlantoaxial instability may be causative in Chiari malformation, advocating for treatment with atlantoaxial stabilization without foramen magnum decompression. Our study was not designed to evaluate this assertion; rather, our study reflects the current practice of pediatric neurosurgery many centers across the country.
In this study, a history of spinal pathology was more frequent in those undergoing OCF, whereas basilar invagination was associated with OCF/VD. Klippel-Feil syndrome, which was found to be more common in the OCF group (2/12, P = .015), has an estimated occurrence of 3% to 5% of patients with CM-1, and recent linkage analyses implicate growth differentiation factors.36 Klippel-Feil syndrome is also associated with a variety of craniovertebral junction anomalies, such as basilar invagination, platybasia, and C1-C2 instability,37 further supporting our findings. In some cases, complex craniovertebral anomalies may be associated with clinical syndromes, many of which have a known genetic basis.38-40 Our OCF cohort demonstrated a significantly higher rate of genetic findings (25% for OCF, 7.8% for PFD only, and 0.0% for OCF/VD). Plans for further detailed examination of the genetic influences in the PRSRC cohort is underway, as are prospective gene discovery projects for CM-1 and SM.
Limitations
We acknowledge several limitations. The minimum length of follow-up (1 yr) may not be long enough to capture some cases of delayed OCF and VD. The number of patients who underwent OCF or OCF/VD represent a small minority of the entire cohort, limiting the generalizability of the analyses. There is inherent institutional bias or center effect (and institutional experience) in offering these craniovertebral procedures (8 centers performed OCF, whereas 3 performed OCF/VD), and there may be bias towards OCF at certain centers or referral bias to these centers to surgeons who are more likely to perform these procedures. Our database did not capture CM-1 without SM who may have undergone OCF or OCF/VD. Further, there is an inherent bias introduced with upfront procedures, as those with delayed OCF or OCF/VD may have been offered surgery based on the failure of PFD alone to resolve the clinical symptomatology. There was overlap in radiological parameters in upfront procedures; for example, 118 patients in the PFD-only cohort had tonsillar ectopia exceeding the 17.3 and 17.5 mm mean tonsillar ectopia observed in the OCF and OCF/VD groups, respectively. Overlap of results between the PFD-only and OCF/VD cohorts may affect generalizability.
Despite defined PRSRC criteria, clinical history and physical examination data points in this multicenter, mixed retrospective/prospective study are likely to create variability in the data. Indeed, data on 61.5% of the study subjects were collected retrospectively and subject to sampling bias. Imaging was not standardized and certain radiographic (eg, cervical flexion-extension radiographs) and adjunctive tests (eg, sleep studies and swallowing studies) were applied variably across the PRSRC. Rigorous testing of lower cranial nerve function (eg, gag), important in assessing VBSC, may have been inconsistently applied. Clinical and radiological follow-up followed each institution's usual clinical practice and thus varied across centers. Genetic testing for CM-1-related connective tissue disorders was (and remains) limited, which likely limited the number of patients reported to have such disorders in our database. Prospective studies may be able to better define the patient population to be treated with OCF or OCF/VD.
We have investigated a limited number of clinically important and well-characterized craniovertebral morphometrics, which were agreed upon by PRSRC investigator consensus at the consortium's inception. In keeping with other recent findings in the literature,1,2,23 a more comprehensive analysis of additional factors, including brain/spinal cord parameters (eg, obex position/Chiari 1.5), bony parameters (eg, atlanto-occipital assimilation), and posterior fossa volumes is planned in the near future. Similarly, of significant interest are the natural history and outcomes of patients who have complex craniovertebral junction anatomy but who did not undergo these advanced procedures.
CONCLUSION
The vast majority of CM-1/SM patients do not undergo OCF or OCF/VD. Clinical symptoms and signs of lower cranial nerve dysfunction and VBSC were common in patients undergoing OCF and OCF/VD. Patients with significantly greater radiological findings of tonsillar ectopia and lower CXA more often received OCF/VD, and patients with genetic anomalies more frequently received OCF, but most patients with a high degree of tonsillar herniation and a low CXA do not require fusion. Judicious application of OCF and OCF/VD is warranted in the care of pediatric patients with CM-1/SM until more rigorous understanding of outcomes based on uniform indications is established.
Funding
This publication was made possible through the generous support of Sam and Betsy Reeves, the Spears and O’Keefe families, and the many other contributors to the Park-Reeves Syringomyelia Research Consortium. Support was also provided by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under Award Number U54 HD087011 to the Intellectual and Developmental Disabilities Research Center at Washington University.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article. Dr Limbrick has received research funds and/or research equipment for unrelated projects from Medtronic Inc, Karl Storz Inc, and Microbot Medical Inc. Dr Limbrick has received philanthropic equipment contributions for humanitarian relief work from Karl Storz Inc and Aesculap Inc.
Supplementary Material
Contributor Information
Travis S CreveCoeur, Department of Neurological Surgery, Washington University School of Medicine, St. Louis, Missouri.
Alexander T Yahanda, Department of Neurological Surgery, Washington University School of Medicine, St. Louis, Missouri.
Cormac O Maher, Department of Neurosurgery, University of Michigan School of Medicine, Ann Arbor, Michigan.
Gabrielle W Johnson, Department of Neurological Surgery, Washington University School of Medicine, St. Louis, Missouri.
Laurie L Ackerman, Department of Neurological Surgery, Indiana University School of Medicine, Indianapolis, Indiana.
P David Adelson, Division of Pediatric Neurosurgery, Barrow Neurological Institute at Phoenix Children's Hospital, Phoenix, Arizona.
Raheel Ahmed, Department of Neurological Surgery, University of Wisconsin at Madison, Madison, Wisconsin.
Gregory W Albert, Division of Neurosurgery, Arkansas Children's Hospital, Little Rock, Arkansas.
Phillipp R Aldana, Division of Pediatric Neurosurgery, University of Florida College of Medicine, Jacksonville, Florida.
Tord D Alden, Division of Pediatric Neurosurgery, Ann & Robert H. Lurie Children's Hospital of Chicago, Chicago, Illinois.
Richard C E Anderson, Division of Pediatric Neurosurgery, Department of Neurological Surgery, Children's Hospital of New York, Columbia-Presbyterian, New York, New York.
Lissa Baird, Department of Neurological Surgery and Doernbecher Children's Hospital, Oregon Health & Science University, Portland, Oregon.
David F Bauer, Department of Neurosurgery, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire.
Karin S Bierbrauer, Division of Pediatric Neurosurgery, Cincinnati Children's Medical Center, Cincinnati, Ohio.
Douglas L Brockmeyer, Division of Pediatric Neurosurgery, Primary Children's Hospital, Salt Lake City, Utah.
Joshua J Chern, Division of Pediatric Neurosurgery, Children's Healthcare of Atlanta, Atlanta, Georgia.
Daniel E Couture, Department of Neurological Surgery, Wake Forest University School of Medicine, Winston-Salem, North Carolina.
David J Daniels, Department of Neurosurgery, Mayo Clinic, Rochester, Minnesota.
Robert C Dauser, Division of Pediatric Neurosurgery, Texas Children's Hospital, Houston, Texas.
Susan R Durham, Department of Neurosurgery, University of Vermont, Burlington, Vermont.
Richard G Ellenbogen, Division of Pediatric Neurosurgery, Seattle Children's Hospital, Seattle, Washington.
Ramin Eskandari, Department of Neurosurgery, Medical University of South Carolina, Charleston, South Carolina.
Herbert E Fuchs, Department of Neurosurgery, Duke University, Durham, North Carolina.
Timothy M George, Division of Pediatric Neurosurgery, Dell Children's Medical Center, Austin, Texas.
Gerald A Grant, Division of Pediatric Neurosurgery, Lucile Packard Children's Hospital at Stanford, Stanford University School of Medicine, Palo Alto, California.
Patrick C Graupman, Division of Pediatric Neurosurgery, Gillette Children's Hospital, St. Paul, Minnesota.
Stephanie Greene, Divsion of Pediatric Neurosurgery, Children's Hospital of Pittsburgh, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania.
Jeffrey P Greenfield, Department of Neurological Surgery, Weill Cornell Medical College, New York Presbyterian Hospital, New York, New York.
Naina L Gross, Department of Neurosurgery, University of Oklahoma, Oklahoma City, Oklahoma.
Daniel J Guillaume, Department of Neurosurgery, University of Minnesota Medical School, Minneapolis, Minnesota.
Gabe Haller, Department of Neurological Surgery, Washington University School of Medicine, St. Louis, Missouri.
Todd C Hankinson, Department of Neurosurgery, Children's Hospital Colorado, Aurora, Colorado.
Gregory G Heuer, Division of Pediatric Neurosurgery, Children's Hospital of Pennsylvania, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania.
Mark Iantosca, Department of Neurosurgery, Penn State Milton S. Hershey Medical Center, Hershey, Pennsylvania.
Bermans J Iskandar, Department of Neurological Surgery, University of Wisconsin at Madison, Madison, Wisconsin.
Eric M Jackson, Department of Neurosurgery, Johns Hopkins University School of Medicine, Baltimore, Maryland.
Andrew H Jea, Department of Neurological Surgery, Indiana University School of Medicine, Indianapolis, Indiana.
James M Johnston, Division of Pediatric Neurosurgery, University of Alabama at Birmingham, Birmingham, Alabama.
Robert F Keating, Department of Neurosurgery, Children's National Medical Center, Washington, District of Columbia.
Michael P Kelly, Department of Orthopedic Surgery, Washington University School of Medicine, St. Louis, Missouri.
Nickalus Khan, Department of Neurosurgery, Le Bonheur Children's Hospital, Memphis, Tennessee.
Mark D Krieger, Department of Neurosurgery, Children's Hospital of Los Angeles, Los Angeles, California.
Jeffrey R Leonard, Division of Pediatric Neurosurgery, Nationwide Children's Hospital, Columbus, Ohio.
Francesco T Mangano, Division of Pediatric Neurosurgery, Cincinnati Children's Medical Center, Cincinnati, Ohio.
Timothy B Mapstone, Department of Neurosurgery, University of Oklahoma, Oklahoma City, Oklahoma.
J Gordon McComb, Department of Neurosurgery, Children's Hospital of Los Angeles, Los Angeles, California.
Arnold H Menezes, Department of Neurosurgery, University of Iowa Hospitals and Clinics, Iowa City, Iowa.
Michael Muhlbauer, Department of Neurosurgery, Le Bonheur Children's Hospital, Memphis, Tennessee.
W Jerry Oakes, Division of Pediatric Neurosurgery, University of Alabama at Birmingham, Birmingham, Alabama.
Greg Olavarria, Division of Pediatric Neurosurgery, Arnold Palmer Hospital for Children, Orlando, Florida.
Brent R O’Neill, Department of Neurosurgery, Children's Hospital Colorado, Aurora, Colorado.
Tae Sung Park, Department of Neurological Surgery, Washington University School of Medicine, St. Louis, Missouri.
John Ragheb, Department of Neurological Surgery, University of Miami School of Medicine, Miami, Florida.
Nathan R Selden, Department of Neurological Surgery and Doernbecher Children's Hospital, Oregon Health & Science University, Portland, Oregon.
Manish N Shah, Division of Pediatric Neurosurgery, McGovern Medical School, Houston, Texas.
Chevis Shannon, Division of Pediatric Neurosurgery, Monroe Carell Jr Children's Hospital of Vanderbilt University, Nashville, Tennessee.
Joshua S Shimony, Department of Radiology, Washington University School of Medicine, St. Louis, Missouri.
Jodi Smith, Department of Neurological Surgery, Indiana University School of Medicine, Indianapolis, Indiana.
Matthew D Smyth, Department of Neurological Surgery, Washington University School of Medicine, St. Louis, Missouri.
Scellig S D Stone, Division of Pediatric Neurosurgery, Boston Children's Hospital, Boston, Massachusetts.
Jennifer M Strahle, Department of Neurological Surgery, Washington University School of Medicine, St. Louis, Missouri.
Mandeep S Tamber, Department of Neurosurgery, The University of British Columbia, Vancouver, Canada.
James C Torner, Department of Neurosurgery, University of Iowa Hospitals and Clinics, Iowa City, Iowa.
Gerald F Tuite, Department of Neurosurgery, Neuroscience Institute, All Children's Hospital, St. Petersburg, Florida.
Scott D Wait, Carolina Neurosurgery & Spine Associates, Charlotte, North Carolina.
John C Wellons, III, Division of Pediatric Neurosurgery, Monroe Carell Jr Children's Hospital of Vanderbilt University, Nashville, Tennessee.
William E Whitehead, Division of Pediatric Neurosurgery, Texas Children's Hospital, Houston, Texas.
David D Limbrick, Jr, Department of Neurological Surgery, Washington University School of Medicine, St. Louis, Missouri.
Supplemental Digital Content 1. Text. Case descriptions. Selected cases to illustrate scenarios in which patients required upfront/delayed OCF and upfront/delayed OCF/VD.
Supplemental Digital Content 2. Figure. Upfront OCF in a 14-yr-old female with a history of Goldenhar syndrome and Klippel-Feil. Shown is a sagittal T2-weighted MRI demonstrating tonsillar ectopia of 18.6 mm, CXA of 120.2 degrees, pB-C2 of 7.0 mm, basilar invagination, and syrinx diameter of 3.6 mm. She underwent PFD with duraplasty with upfront occiput-C4 fusion and experienced symptomatic relief. No further treatment was necessary.
Supplemental Digital Content 3. Figure. Delayed OCF in a 7-yr-old girl with a history of scoliosis and symptoms of dysphagia and gait ataxia. A, T1-weighted sagittal MRI from her initial MRI demonstrating VBSC with cerebellar tonsillar ectopia of 14 mm, CXA 124°, and pB-C2 of 7.0 mm. B, She initially underwent PFD with duraplasty but later developed sleep disordered breathing, and imaging performed 16 mo after her surgery demonstrated a reduction in CXA to 113°. A total of 20 mo after her initial PFD, she underwent occiput-C2 fusion with symptomatic improvement.
Supplemental Digital Content 4. Figure. Upfront OCF and VD in a 17-yr-old with a history of headaches and gait ataxia. T2-weighted sagittal MRi demonstrating an 8.0 mm holocord syrinx, cerebellar tonsillar ectopia 13.0 mm, pB-C2 7.0 mm, CXA of 101°, and brainstem compression. The patient underwent PFD with duraplasty with occiput-C3 fusion and VD performed 22 d later. She remained stable 36 mo following surgery.
Supplemental Digital Content 5. Figure. Delayed OCF and VD in a 15-yr-old male who presented for evaluation of suboccipital headaches and was found to have nystagmus, disconjugate gaze, gait instability, dysmetria, and hyperreflexia. A, T1-weighted, contrast-enhanced sagittal image from his initial, preoperative MRI demonstrated severe cerebellar ectopia of 26 mm, syrinx diameter of 6.0 mm, CXA of 117°, and a pB-C2 of 7.0 mm. He underwent PFD with duraplasty but returned for follow-up 12 mo postoperatively reporting new headaches and sensory symptoms that were exacerbated by coughing or sneezing. B, He underwent revision PFD with duraplasty without relief. He subsequently developed intractable nausea and vomiting, upper extremity tingling, increased hyperreflexia, and wide-based gait. He underwent VD and occiput-C3 fusion 33 mo after his initial surgery. Afterward, his symptoms stabilized, and his sensory disturbances improved.
Supplemental Digital Content 6. Table. Clinical symptoms with radiologic follow-up.
Supplemental Digital Content 7. Table. Subset analysis of initial clinical symptoms and signs of centers that performed OCF or VD.
Supplemental Digital Content 8. Table. Subset analysis of centers who performed OCF or VD with initial radiologic findings.
COMMENT
Park-Reeves is a high quality, multi-institutional database analyzing a vexing issue in pediatric neurosurgery, the need to standardize treatment for Chiari malformation. In this article the authors present a nice review looking at a large cohort of Chiari patients, and analyzing those patients who required occipital cervical fusion with or without ventral decompression. It should be noted that the rates for requiring fusion are extremely low, at less than 2% of the entire population, and there seemed to be some degree of center bias, with only a few institutions in this cohort having performed most of the procedures. There is also substantial overlap in presentation, so many children with symptoms and radiographic findings that might have been fused at one center were not fused at others, reminding us that surgeons need to analyze each of their patients carefully as opposed to assuming there is a standardized algorithm for performing a fusion. In summary, a very well done study that reminds us the rate of fusion should be extremely low after Chiari decompression.
Mark Proctor
Boston, Massachusetts
REFERENCES
- 1. Martin JE, Bookland M, Moote D, Cebulla C. Standardized method for the measurement of grabb's line and clival-canal angle. J Neurosurg Pediatr. 2017;20(4):352-356. [DOI] [PubMed] [Google Scholar]
- 2. Bollo RJ, Riva-Cambrin J, Brockmeyer MM, Brockmeyer DL. Complex chiari malformations in children: an analysis of preoperative risk factors for occipitocervical fusion: clinical article. J Neurosurg Pediatr. 2012;10(2):134-141. [DOI] [PubMed] [Google Scholar]
- 3. Hankinson TC, Grunstein E, Gardner P, Spinks TJ, Anderson RCE. Transnasal odontoid resection followed by posterior decompression and occipitocervical fusion in children with chiari malformation type I and ventral brainstem compression. J Neurosurg Pediatr. 2010;5(6):549-553. [DOI] [PubMed] [Google Scholar]
- 4. Khalsa SSS, Siu A, DeFreitas TA et al. . Comparison of posterior fossa volumes and clinical outcomes after decompression of chiari malformation type I. J Neurosurg Pediatr. 2017;19(5):511-517. [DOI] [PubMed] [Google Scholar]
- 5. Brockmeyer DL. The complex chiari: issues and management strategies. Neurol Sci. 2011;32(Suppl. 3):S345-S347. [DOI] [PubMed] [Google Scholar]
- 6. Wu T, Zhu Z, Jiang J et al. . Syrinx resolution after posterior fossa decompression in patients with scoliosis secondary to chiari malformation type I. Eur Spine J. 2012;21(6):1143-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fenoy AJ, Menezes AH, Fenoy KA. Craniocervical junction fusions in patients with hindbrain herniation and syringohydromyelia. J Neurosurg Spine. 2008;9(1):1-9. [DOI] [PubMed] [Google Scholar]
- 8. Menezes AH. Current opinions for treatment of symptomatic hindbrain herniation or chiari type I malformation. World Neurosurg. 2011;75(2):226-228. [DOI] [PubMed] [Google Scholar]
- 9. Ladner TR, Dewan MC, Day MA et al. . Posterior odontoid process angulation in pediatric chiari I malformation: an MRI morphometric external validation study. J Neurosurg Pediatr. 2015;16(2):138-145. [DOI] [PubMed] [Google Scholar]
- 10. Ladner TR, Dewan MC, Day MA et al. . Evaluating the relationship of the pB–C2 line to clinical outcomes in a 15-year single-center cohort of pediatric chiari I malformation. J Neurosurg Pediatr. 2014;15(2):178-188. [DOI] [PubMed] [Google Scholar]
- 11. Menezes AH. Craniovertebral junction abnormalities with hindbrain herniation and syringomyelia: regression of syringomyelia after removal of ventral craniovertebral junction compression - Clinical article. J Neurosurg. 2012;116(2):301-309. [DOI] [PubMed] [Google Scholar]
- 12. Alalade AF, Ogando-Rivas E, Forbes J et al. . A dual approach for the management of complex craniovertebral junction abnormalities: endoscopic endonasal odontoidectomy and posterior decompression with fusion. World Neurosurg X. 2019: 2:100010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goel A. Is atlantoaxial instability the cause of chiari malformation? Outcome analysis of 65 patients treated by atlantoaxial fixation. J Neurosurg Spine. 2015;22(2):116-127. [DOI] [PubMed] [Google Scholar]
- 14. Tubbs RS, Iskandar BJ, Bartolucci AA, Oakes WJ. A critical analysis of the chiari 1.5 malformation. J Neurosurg Pediatr. 2004;101(2):179-183. [DOI] [PubMed] [Google Scholar]
- 15. Strahle J, Muraszko KM, Kapurch J, Bapuraj JR, Garton HJL, Maher CO. Chiari malformation type I and syrinx in children undergoing magnetic resonance imaging. J Neurosurg Pediatr. 2011;8(2):205-213. [DOI] [PubMed] [Google Scholar]
- 16. Klekamp J. Neurological deterioration after foramen magnum decompression for chiari malformation type I: old or new pathology? J Neurosurg Pediatr. 2012;10(6):538-547. [DOI] [PubMed] [Google Scholar]
- 17. Arnautovic A, Splavski B, Boop FA, Arnautovic KI. Pediatric and adult chiari malformation type I surgical series 1965–2013: a review of demographics, operative treatment, and outcomes. J Neurosurg Pediatr. 2015;15(2):161-177. [DOI] [PubMed] [Google Scholar]
- 18. Strahle J, Smith BW, Martinez M et al. . The association between chiari malformation type I, spinal syrinx, and scoliosis. J Neurosurg Pediatr. 2015;15(6):607-611. [DOI] [PubMed] [Google Scholar]
- 19. Godzik J, Kelly MP, Radmanesh A et al. . Relationship of syrinx size and tonsillar descent to spinal deformity in chiari malformation type I with associated syringomyelia: clinical article. J Neurosurg Pediatr. 2014;13(4):368-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tubbs RS, Beckman J, Naftel RP et al. . Institutional experience with 500 cases of surgically treated pediatric chiari malformation type I. J Neurosurg Pediatr. 2011;7(3):248-256. [DOI] [PubMed] [Google Scholar]
- 21. Menezes AH, VanGilder JC.. Transoral-transpharyngeal approach to the anterior craniocervical junction. Ten-year experience with 72 patients. J Neurosurg. 1988;69(6):895-903. [DOI] [PubMed] [Google Scholar]
- 22. Grabb PA, Mapstone TB, Oakes WJ. Ventral brain stem compression in pediatric and young adult patients with chiari I malformations. Neurosurgery. 1999;44(3):520-528. [DOI] [PubMed] [Google Scholar]
- 23. Bonney PA, Maurer AJ, Cheema AA et al. . Clinical significance of changes in pB-C2 distance in patients with chiari type I malformations following posterior fossa decompression: a single-institution experience. J Neurosurg Pediatr. 2016;17(3):336-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Greenlee J, Garell PC, Stence N, Menezes AH. Comprehensive approach to chiari malformation in pediatric patients. Neurosurg Focus. 1999;6(6):e4. [DOI] [PubMed] [Google Scholar]
- 25. Menezes AH. Pathogenesis, dynamics, and management of os odontoideum. Neurosurg Focus. 1999;6(6):E4. [DOI] [PubMed] [Google Scholar]
- 26. McRae DL, Barnum AS. Occipitalization of the atlas. Am J Roentgenol Radium Ther Nucl Med. 1953;70(1):23-46. [PubMed] [Google Scholar]
- 27. O’Hayon BB, Drake JM, Ossip MG, Tuli S, Clarke M. Frontal and occipital horn ratio: a linear estimate of ventricular size for multiple imaging modalities in pediatric hydrocephalus. Pediatr Neurosurg. 1998;29(5):245-249. [DOI] [PubMed] [Google Scholar]
- 28. Smoker WR. Craniovertebral junction: normal anatomy, craniometry, and congenital anomalies. Radiographics. 1994;14(2):255-277. [DOI] [PubMed] [Google Scholar]
- 29. Grangeon L, Puy L, Gilard V et al. . Predictive factors of headache resolution after chiari type 1 malformation surgery. World Neurosurg. 2018;110:e, 60-e66. [DOI] [PubMed] [Google Scholar]
- 30. Greenberg JK, Yarbrough CK, Radmanesh A et al. . The chiari severity index: a preoperative grading system for chiari malformation type 1. Neurosurgery. 2015;76(3):279-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thakar S, Sivaraju L, Jacob KS et al. . A points-based algorithm for prognosticating clinical outcome of Chiari malformation Type I with syringomyelia: results from a predictive model analysis of 82 surgically managed adult patients. J Neurosurg Spine. 2018;28(1):23-32. [DOI] [PubMed] [Google Scholar]
- 32. Henderson FC, Henderson FC, Wilson WA, Mark AS, Koby M. Utility of the clivo-axial angle in assessing brainstem deformity: pilot study and literature review. Neurosurg Rev. 2018;41(1):149-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dahdaleh NS, Dlouhy BJ, Menezes AH. Application of neuromuscular blockade and intraoperative 3D imaging in the reduction of basilar invagination: technical note. J Neurosurg Pediatr. 2012;9(2):119-124. [DOI] [PubMed] [Google Scholar]
- 34. Dlouhy BJ, Dahdaleh NS, Menezes AH. Evolution of transoral approaches, endoscopic endonasal approaches, and reduction strategies for treatment of craniovertebral junction pathology: a treatment algorithm update. Neurosurg Focus. 2015;38(4):E8. [DOI] [PubMed] [Google Scholar]
- 35. Pindrik J, Johnston JM.. Clinical presentation of chiari I malformation and syringomyelia in children. Neurosurg Clin N Am. 2015;26(4):509-514. [DOI] [PubMed] [Google Scholar]
- 36. Markunas CA, Soldano K, Dunlap K et al. . Stratified whole genome linkage analysis of chiari type I malformation implicates known Klippel-Feil syndrome genes as putative disease candidates. PLoS One. 2013;8(4):e61521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Smoker WRK, Khanna G. Imaging the craniocervical junction. Child's Nerv Syst. 2008;24(10):1123-1145. [DOI] [PubMed] [Google Scholar]
- 38. Engiz O, Balci S, Unsal M, Ozer S, Oguz KK, Aktas D. 31 cases with oculoauriculovertebral dysplasia (Goldenhar syndrome): clinical, neuroradiologic, audiologic and cytogenetic findings. Genet Couns. 2007;18(3):277-288. [PubMed] [Google Scholar]
- 39. Al Kaissi A, Ben Chehida F, Ganger R, Klaushofer K, Grill F. Distinctive spine abnormalities in patients with goldenhar syndrome: tomographic assessment. Eur Spine J. 2015;24(3):594-599. [DOI] [PubMed] [Google Scholar]
- 40. Menezes AH, Vogel TW.. Specific entities affecting the craniocervical region: syndromes affecting the craniocervical junction. Child's Nerv Syst. 2008;24(10):1155-1163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.