Abstract
BACKGROUND
Deep brain stimulation (DBS) is a standard of care treatment for multiple neurologic disorders. Although 3-tesla (3T) magnetic resonance imaging (MRI) has become the gold-standard modality for structural and functional imaging, most centers refrain from 3T imaging in patients with DBS devices in place because of safety concerns. 3T MRI could be used not only for structural imaging, but also for functional MRI to study the effects of DBS on neurocircuitry and optimize programming.
OBJECTIVE
To use an anthropomorphic phantom design to perform temperature and voltage safety testing on an activated DBS device during 3T imaging.
METHODS
An anthropomorphic 3D-printed human phantom was constructed and used to perform temperature and voltage testing on a DBS device during 3T MRI. Based on the phantom assessment, a cohort study was conducted in which 6 human patients underwent MRI with their DBS device in an activated (ON) state.
RESULTS
During the phantom study, temperature rises were under 2°C during all sequences, with the DBS in both the deactivated and activated states. Radiofrequency pulses from the MRI appeared to modulate the electrical discharge from the DBS, resulting in slight fluctuations of voltage amplitude. Six human subjects underwent MRI with their DBS in an activated state without any serious adverse events. One patient experienced stimulation-related side effects during T1-MPRAGE scanning with the DBS in an ON state because of radiofrequency-induced modulation of voltage amplitude.
CONCLUSION
Following careful phantom-based safety testing, 3T structural and functional MRI can be safely performed in subjects with activated deep brain stimulators.
Keywords: Deep brain stimulation, Magnetic resonance imaging, Phantom, Psychiatric surgery, Radiofrequency heating
ABBREVIATIONS
- 3T
3-tesla
- ASL
arterial spin labeling
- BOLD
blood oxygenation level dependent
- DBS
deep brain stimulation
- DTI
diffusion tensor imaging
- EPI
echo planar imaging
- fMRI
functional MRI
- FoV
field of view
- IPG
implantable pulse generator
- MFB
medial forebrain bundle
- MPRAGE
magnetization prepared rapid gradient echo
- MRI
magnetic resonance imaging
- NAc
nucleus accumbens
- rms
root mean square
- SAR
specific absorption rate
- SCC
subcallosal cingulate
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
- SWI
susceptibility-weighted imaging
- T1
longitudinal relaxation time
- T2
transverse relaxation time
- TE
echo time
- TI
inversion time
- TR
repetition time
Deep brain stimulation (DBS) is now a standard of care treatment for advanced Parkinson disease, essential tremor, dystonia, and obsessive compulsive disorder and is under active investigation for the treatment of numerous other neurological and neuropsychiatric illnesses.1 Although the mechanisms underlying DBS are not yet fully understood, it is becoming increasingly apparent that DBS results in widespread changes in the structure and function of the brain.2 Millimeter changes in DBS placement and subtle changes in the choice of contact or stimulation settings have drastic effects on long-term outcomes, further highlighting the crucial role of structural and functional connectivity.3
Magnetic resonance imaging (MRI) is the medical imaging modality of choice for nearly all neurological illnesses. Noninvasive and normally imposing minimal attributable risk, without the use of ionizing radiation, MRI permits high-resolution (millimeter-scale) anatomic imaging as well as functional interrogation of neural circuitry. Specifically, functional MRI (fMRI) allows for tracking changes in the blood oxygenation level-dependent (BOLD) signal, a surrogate for neural activity, and diffusion tensor imaging (DTI) allows for imaging the diffusion of water molecules to delineate white matter tracts within the brain. Anatomical imaging, fMRI, and DTI can be acquired within a single MRI exam, in under 45 min depending on the acquisition parameters. Three Tesla (3T) magnetic field strength is the gold standard for such imaging of the central nervous system, offering improved signal-to-noise ratio when compared with 1.5 Tesla (1.5T) (ScanMed).4
Although MRI is normally a safe procedure, legitimate concerns are raised when imaging is contemplated of patients with implanted electrically conductive medical devices. To date, patients with DBS devices in place have largely been restricted to 1.5T MRI. Medtronic, Abbott, and Boston Scientific all have DBS systems available now which are approved for 1.5T imaging with specific absorption rate (SAR) restrictions which limit the types of MRI sequences that can be performed.5,6 These same devices are not approved for 3T imaging under any circumstance. The primary safety concerns involve MRI-induced heating of tissue surrounding the electrode tip and also MRI-induced interference with the electrical stimulation and programming of the device.7-10 Numerical modeling suggests that this risk is greatest to the electrode located contralateral to the implantable pulse generator (IPG), because of the longer course of the associated lead along the skull.11,12 Over the past decade, manufacturers have modified the implantable hardware to mitigate these concerns and thus improve MRI safety. Specifically, there is now reduced ferromagnetic material used to make DBS devices, thus minimizing the potential effects of magnetic forces,13 and device programming is more robust to prevent interference from MRI.14 Despite these advancements, 3T MRI is still not vendor approved nor widely performed at DBS centers of excellence.
In an effort to move the field forward, there have been several studies to probe the safety of MRI at various field strengths in the presence of DBS hardware. The most common approach involves phantom studies, in which a DBS device is implanted in a substance intended to mimic brain tissue, and imaging is performed while monitoring temperature and/or electrical changes.9,15-19 The results of these studies have been highly variable, likely because of variations in phantom construction, DBS hardware, MRI systems, and acquisition protocols.
In this article, we present novel phantom safety testing of DBS hardware in a Siemens 3T MRI system. Importantly, an anthropomorphic realistic phantom design was used, closely mimicking the conditions under which DBS MRI will occur in humans. Temperature change and voltage deviations were measured during imaging with the device activated. After demonstrating safety, we then imaged 6 patients with DBS devices in place, with the DBS in both the inactivated (OFF) and activated (ON) state. This work supports the notion that 3T MRI can be performed in patients with DBS devices in place, provided prior safety testing is performed. In addition, several novel considerations are highlighted when undertaking this type of workflow.
METHODS
A realistic 3-D printed, anthropomorphic phantom model was used for testing (Figure 1). A detailed description of the phantom construction is reported elsewhere.20 Briefly, right and left burr holes were drilled in a human cadaveric skull, approximately 1 cm anterior to the coronal suture and 4 cm lateral to the midline bilaterally. A model of a human head and torso made of clear plastic was created using 3-dimensional printing. The skull and the head/torso model were filled with gelatin, with similar electromagnetic properties as gray matter.21 Fiberoptic thermometers were sutured tip to tip to Medtronic (Dublin, Ireland) 3389 electrodes, and then were inserted through the bilateral holes to a depth of 7 cm. Given that the skull was filled with set gelatin, a stylet was necessary to insert/advance the electrodes, which was then removed to allow connection with 37 086 extension wires and an Activa PC IPG (Medtronic). The electrode leads were each looped once around the burr hole site to mimic our clinical practice, in accordance with evidence suggesting that this reduces MRI-induced heating.11 Additional fiberoptic thermometers were placed at the site of the left burr hole, along the left-sided extension in the right mastoid region, on the posterior surface of the IPG, and over the left mastoid (negative control). Using the standard Medtronic clinician-programmer device, the DBS setup was interrogated to ensure low impedances at all contacts.
Figure 1.
Phantom design. A, Electrodes (blue arrows) were inserted to approximately 7-cm depth through holes drilled approximately 1 cm anterior to the coronal suture and 3 to 4 cm lateral from midline. Fiberoptic leads were used for real-time temperature monitoring at the electrode tips (red arrowheads), and a sensor was also placed at the site of the left hole adjacent to the electrode (black arrowhead). A fiberoptic temperature sensor was also placed against the dorsal surface of the implanted pulse generator (not shown). B, Fiberoptic temperature sensors were sutured to the tips of Medtronic 3389 electrodes prior to electrode insertion. C, Phantom setup assembly. The black arrow indicates the implantable pulse generator, and the green arrowhead indicates the exit point of the fiberoptic cables.
The phantom model underwent 3T MRI on a research-dedicated system (Magnetom Prisma, Siemens Healthineers, Erlangen, Germany) using a 20-channel head/neck receiver coil and built-in body transmitter coil, with fiberoptic thermometers in place and the DBS turned OFF. Temperatures were recorded continuously throughout scanning, and maximal temperatures were recorded for each sequence. This was repeated with the DBS then turned ON at 4 V bilaterally, at 130 Hertz and 90 μs pulse width, as this is a common stimulation setting at our institution. The following imaging sequences were performed: 3-plane localizer, T1-MPRAGE, T2*EPI, DTI, SWI, ASL, and T2 TSE (see Table 1 for scanning parameters and definitions of acronyms).
TABLE 1.
MRI Sequences
| TR (ms) | TE (ms) | TI (ms) | Flip angle (0) | FoV (mm x mm) | Slice thickness (mm) | Slice number | Duration (min) | |
|---|---|---|---|---|---|---|---|---|
| 3-plane localizer | 20 | 5 | N/A | 40 | 240 × 240 | 5 | 15 | 0:40 |
| T1-MPRAGE | 1800 | 2.21 | 900 | 10 | 256 × 256 | 1 | 176 | 4:15 |
| T2*EPI | 1750 | 30 | N/A | 40 | 200 × 200 | 2.5 | 60 | 6:09 |
| DTI | 3400 | 51 | N/A | 90 | 256 × 256 | 2 | 72 | 4:22 |
| SWI | 27 | 20 | N/A | 15 | 224 × 203 | 1.5 | 96 | 6:32 |
| ASL | 4000 | 15.54 | 1990 | 155 | 224 × 224 | 4 | 36 | 6:30 |
| T2 TSE | 6000 | 100 | N/A | 165 | 220 × 220 | 5 | 24 | 8:50 |
ASL = arterial spin labeling (pulsed); DTI = diffusion tensor imaging; EPI = echo planar imaging; FoV = field of view; MPRAGE = magnetization prepared rapid gradient echo; SWI = susceptibility-weighted imaging; T1 = longitudinal relaxation time; T2 = transverse relaxation time; TE = echo time; TI = inversion time; TR = repetition time; TSE = turbo spin echo.
In the next phase of the phantom testing, voltages were measured between the ventral-most electrode contact and the IPG case surface (ground). To do this, a fine enameled copper wire with the insulation removed at its tip was inserted into each of the DBS battery ports. A third wire was placed against the posterior surface of the IPG case. These 3 wires were connected to an oscilloscope, capable of continuous voltage measurement during MRI. T1-MPRAGE and EPI sequences were run with the DBS turned ON and OFF.
Based on the phantom safety data generated from the above set of experiments, 6 patients with bilateral DBS devices (Medtronic 3389 or 3387 electrodes, 37 086 extensions, and Activa PC IPGs) underwent MRI. All patients signed an informed consent form to participate in this 3T MRI study, which was approved by the Research Ethics Board at Sunnybrook Health Sciences Centre. All scanning was conducted in January and February 2020. Imaging was performed with the DBS device both ON and OFF, using only the T1-MPRAGE, DTI, and EPI sequences. Patients had electrodes in the medial forebrain bundle (MFB, n = 2), subcallosal cingulate (SCC, n = 1), and nucleus accumbens (NAc, n = 3). During the ON stage, the patients’ usual DBS settings were used. Patients were examined neurologically following the scanning. In addition, the impedance of their device was checked before and after the session. The STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines for cohort studies were followed in the writing of this report.
RESULTS
Phantom Temperature Testing
The therapy impedances of the right and left electrodes in the phantom were 690 Ω and 736 Ω, respectively. The peak temperature rise observed during each imaging sequence is listed in Table 2. Temperature rises were calculated as the change measured in relation to the baseline temperature at the start of each imaging sequence. At all sites, T2 and ASL sequences were associated with the greatest temperature rise. The maximal temperature rises during the DBS-OFF experiments occurred at the right electrode tip during T2 TSE imaging (1.1°C). The maximal temperature rises during the DBS-ON experiments occurred at the left electrode tip during T2 TSE imaging (0.9°C).
TABLE 2.
Temperature Monitoring During Phantom Scanning
| DBS system OFF | ||||||
|---|---|---|---|---|---|---|
| Left electrode | Right electrode | Left burr hole | Extension cables | IPG | Left postauricular | |
| Localizer | 0.0 | 0.1 | 0.0 | 0.1 | 0.0 | 0.1 |
| T1-MPRAGE | 0.0 | 0.2 | 0.0 | 0.0 | 0.2 | 0.2 |
| EPI | 0.0 | 0.2 | 0.0 | 0.1 | 0.3 | 0.2 |
| DTI | 0.1 | 0.4 | 0.1 | 0.1 | 0.3 | 0.3 |
| SWI | 0.1 | 0.3 | 0.1 | 0.1 | 0.4 | 0.3 |
| ASL | 0.1 | 0.4 | 0.2 | 0.26 | 0.4 | 0.53 |
| T2 | 0.3 | 1.1 | 0.5 | 0.36 | 0.5 | 0.37 |
| DBS system turned ON to 4 V, 130 Hz, 90 μs (C+ 0-/4-) | ||||||
| Left electrode | Right electrode | Left burr hole | Extension cables | IPG | Left postauricular | |
| Localizer | 0.2 | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 |
| T1-MPRAGE | 0.3 | 0.2 | 0.0 | 0.0 | 0.0 | 0.1 |
| EPI | 0.4 | 0.2 | 0.0 | 0.0 | 0.0 | 0.1 |
| DTI | 0.5 | 0.4 | 0.1 | 0.0 | 0.2 | 0.1 |
| SWI | 0.5 | 0.3 | 0.1 | 0.1 | 0.2 | 0.2 |
| ASL | 0.6 | 0.6 | 0.2 | 0.2 | 0.2 | 0.3 |
| T2 | 0.9 | 0.4 | 0.5 | 0.3 | 0.3 | 0.7 |
Temperature increases are relative to the start of each sequence.
See Table 1 for the definition of MRI sequence acronyms. IPG = implantable pulse generator.
C+: case positive; electrodes listed left/right, with in ventral-dorsal order (0-3, 4-7).
Phantom Voltage Testing
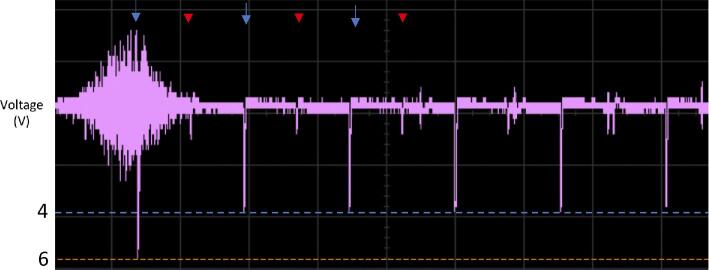
The voltage time series from T1-MPRAGE and EPI are displayed in Figure 2. DBS pulses could be clearly visualized at the expected frequency and amplitude. Interestingly, when recording from either electrode, the discharge emitted from the contralateral electrode could be appreciated, which occurred 180 degrees out of phase. Interference from the MRI system's radiofrequency pulses is also appreciable, particularly during high flip-angle pulses, such as the inversion pulse at the beginning of the MPRAGE sequence (Figure 2). These radiofrequency pulses can sometimes overlap with the DBS discharge to result in constructive interference and a greater-than-expected amplitude (Figure 3).
Figure 2.

Oscilloscope tracings from the right DBS electrode relative to the IPG (ground) during MRI of the phantom. The blue arrows mark the stimulation pulses on the right electrode, and the red arrows indicate stimulation pulses from the left (contralateral) electrode, detected 180 degrees out of phase. The open-ended bracket indicates a period of interference because of the MRI system's radiofrequency pulse. Two traces are shown: one for the T1-MPRAGE sequence and the other for EPI. See Table 1 for definition of MRI sequence acronyms.
Figure 3.
Oscilloscope trace captured during T1-MPRAGE imaging of the phantom. The blue arrows mark the stimulation pulses on the right electrode, and the red arrowheads indicate stimulation pulses detected from the left (contralateral) electrode. The pulse amplitude can be seen to increase from approximately 4 to 6 V when overlapping with a high flip-angle radiofrequency pulse.
3T Imaging in Humans
Six patients with fully internalized DBS devices were imaged (Table 3, Figure 4). There were no serious adverse events, clinical or radiographic. Electrode impedances remained stable between, before, and after the imaging sequences. The SAR and B1+ root mean square (rms) estimates from the MRI system are displayed in Table 4. One patient with MFB DBS experienced a sensation of rhythmic vertigo during T1-MPRAGE imaging, when his DBS was ON. The sensation stopped immediately when imaging ceased and also improved upon closing his eyes. Changing his DBS settings to 1 V bilaterally completely alleviated this issue.
TABLE 3.
Patients Undergoing 3T Scanning
| Age/sex | Target | Stimulation settings | Impedance (ohms) | |
|---|---|---|---|---|
| 1 | 67/M | Nucleus accumbens | C+ 0-/4- 3.75 V | R: 757 L: 928 |
| 2 | 48/F | Nucleus accumbens | C+ 1-/5- 3.0 V | R: 672 L: 624 |
| 3 | 56/M | Nucleus accumbens | C+ 1-/5- 4.0 V | R: 808 L: 857 |
| 4 | 50/M | Subcallosal cingulate | C+ 1-/5- 3.0 V | R: 935 L: 936 |
| 5 | 56/M | Medial forebrain bundle | C+ 2-/6- 1.0 Va | R: 1413 L: 1633 |
| 6 | 63/M | Medial forebrain bundle | C+ 1-/5- 1.5 V | R: 1412 L: 1502 |
aSubject was adjusted from 2.0 to 1.0 V to prevent vertigo during imaging.
C+: case positive; electrodes listed left/right, with in ventral-dorsal order (0-3, 4-7); R: right electrode, L: left electrode.
Figure 4.

X-ray image illustrating the electrode/extension configuration used at our institution for the purpose of decreasing MRI-related heating. Each electrode is looped once about its respective burr hole. Further excess wire is coiled deep to the IPG.
TABLE 4.
Specific Absorption Rate and B1 + rms Values During Phantom and Human Imaging (n = 6)
| Phantom | Human | ||
|---|---|---|---|
| B1 + rms (μT) | SAR (W/kg) | SAR (W/kg) | |
| EPI | 0.6 | 0.1 | 0.1 (0.10-0.12) |
| T1-MPRAGE | 1.1 | 0.1 | 0.2 (0.17-0.24) |
| DTI | 1.7 | 0.9 | 0.6 (0.46-0.70) |
For human data, mean is followed by the range in parentheses. B1 + rms was constant across subjects.
DISCUSSION
A realistic phantom model was used to perform temperature and voltage safety measurements during 3T MRI. Temperature rises were within a safe range (<1°C) for T1-MPRAGE, DTI, and EPI sequences. T1-MPRAGE imaging resulted in periodic deviations in voltage, but these were relatively minor (<3 V). Based on the phantom results, 6 patients with fully internalized DBS hardware underwent 3T MRI with the aforementioned T1-MPRAGE, DTI, and EPI sequences. There were no serious adverse events.
The Medtronic 3387 and 3389 devices, in common use today, remain MRI conditional for 1.5T imaging and are not supported for 3T. The new Medtronic Percept available in Europe is 3T conditional and hopefully will become commercially available in North America over the coming years, although this will not benefit the thousands of patients with existing Medtronic 3387 and 3389 electrodes. In an effort to begin imaging patients with indwelling DBS hardware, it is important to emphasize that a priori safety testing is necessary prior to 3T imaging. Although we have demonstrated the safety of these T1-MPRAGE, EPI, and DTI sequences on a 3T Siemens Prisma scanner (Siemens Healthineers), the same may not hold true for other sequence parameters, MRI system models, or with other types of DBS hardware.
Different phantom designs have been implemented in the testing of DBS devices.15 An advantage of the phantom setup used in the present study is the use of a human skull and realistic contouring of the head and torso, made possible through 3-dimensional printing. The phantom appeared to accurately reflect the SAR experienced by human subjects (Table 4). It should be noted though that the addition of temperature monitoring during phantom testing is necessary as SAR estimates are known to vary between vendors.22
Attention must also be paid to the configuration of DBS electrodes and extension wires. In the process of implanting a DBS system, there is invariably excess DBS cable, and depending on surgeon/institutional practice, these wires are looped and placed in different regions—surrounding the burr holes, in the right retro-auricular region, and/or deep to the IPG in the chest. At our institution, the neurosurgeons (N.L. and C.H.) adopt the practice of looping each electrode/extension around the burr hole once before tunneling (Figure 4). It is important to note that because both the phantom and clinical data presented here utilized this burr hole loop design, these results should not be extrapolated to other electrode configurations. Evidence from computer modeling suggests that this arrangement reduces the SAR at electrode leads along that segment, which is exposed to the greatest tangential electric field during MRI.12 This effect is amplified in 3T MRI systems compared with 1.5T systems and is especially important for the contralateral electrode, which travels a greater distance across the scalp.11 The importance of consistent electrode/extension configuration is supported by a recent phantom study demonstrating substantial variation in MRI-induced heating depending on the configuration.16 This same work also suggested unilateral DBS devices may be much more likely to undergo heating, especially during T2 and ASL scanning.16
The only negative event which occurred during human imaging was a patient experiencing rhythmic vertigo during the T1-MPRAGE imaging with the DBS activated (ON). Our hypothesis is that this occurred as a result of rhythmic increases in discharge voltage stimulating the nearby oculomotor nucleus/nerve because of constructive interference between DBS discharges and the T1-MPRAGE radiofrequency pulses. This hypothesis is supported by the fact that the symptoms lessened when the patient closed his eyes, and abolished with lowering his stimulation level (from 2 to 1 V). It is now our practice to turn the DBS device off prior to T1-MPRAGE imaging to avoid this. In theory, similar constructive interference could occur during EPI, but given the low maximal artifact seen during phantom EPI tests (<0.5V) and the low radiofrequency pulse amplitudes used in EPI for fMRI, such small fluctuations would be unexpected to result in any clinically appreciable change.
The phantom study revealed an unexpected finding. On common programming settings (monopolar stimulation, pulse width = 90 μs, and frequency = 130 Hz), the Medtronic Activa PC device uses out-of-phase stimulation between the 2 electrodes (rather than the electrodes firing simultaneously). This was a surprise to our group, and upon corresponding with other colleagues in the field, we found this may not be a widely known fact. In theory, bilateral DBS could result in perfectly de-phasing the activity between 2 structures (ie, left and right subthalamic nuclei). With our ability to now perform EPI sequences in these patients, we intend to further study this concept using resting-state fMRI with seed-based analyses.
Limitations
Although the phantom design used in this study was intended to closely replicate real-world tissue interfaces, it is important to acknowledge that additional biological complexities, such as the role of blood vessels and biological heat transfer, were not accounted for. This study is also limited by the inability to perform temperature or voltage testing in the human patients; MR thermometry, for instance, would be significantly hampered by distortion artifact at the DBS-brain interface, interfering with the reliable detection of proton resonance shifts.23 Through a thorough neurological examination and confirming unchanged DBS impedances, we can be confident that no serious adverse events occurred, but it is not possible to rule out excessive heating. Furthermore, given the small sample size, the positive safety results should be interpreted with caution. All of the DBS targets tested in vivo are common psychiatric surgery targets (NAc, MFB, and SCC), but these results also need to be tested in common motor targets, such as the subthalamic nucleus and ventral intermediate nucleus of the thalamus. Although these findings should provide encouragement for other centers to pursue phantom testing, these results are DBS and scanner specific.
CONCLUSION
Following phantom testing and with carefully selected sequences, 3T MRI can be performed safely on patients with common Medtronic DBS devices using a Siemens Magnetom Prisma MRI system (Siemens Healthineers), with the sequence parameters listed herein. Imaging can also be performed with the stimulator turned ON, allowing for functional imaging sequences to interrogate differences in activation in ON and OFF states. Radiofrequency pulses are capable of increasing the amplitude of electrical discharge, and therefore, care should be taken when imaging patients with activated electrodes. Manufacturers are now working to produce 3T-compatible DBS devices, but in the meantime, through site- and configuration-specific phantom testing, 3T anatomical and functional MRI is possible.
Funding
This study was partially funded by an operating grant from the Canadian Institutes of Health Research (CIHR). Neither they nor any other organization had any role in the analysis, writing, or decision to publish this work.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Contributor Information
Benjamin Davidson, Division of Neurosurgery, Sunnybrook Health Sciences Centre, University of Toronto, Toronto, Canada; Harquail Centre for Neuromodulation, Hurvitz Brain Sciences Program, Sunnybrook Research Institute, Toronto, Canada; Sunnybrook Research Institute, Toronto, Canada.
Fred Tam, Physical Sciences Platform, Sunnybrook Research Institute, Toronto, Canada.
Benson Yang, Physical Sciences Platform, Sunnybrook Research Institute, Toronto, Canada.
Ying Meng, Division of Neurosurgery, Sunnybrook Health Sciences Centre, University of Toronto, Toronto, Canada; Harquail Centre for Neuromodulation, Hurvitz Brain Sciences Program, Sunnybrook Research Institute, Toronto, Canada; Sunnybrook Research Institute, Toronto, Canada.
Clement Hamani, Division of Neurosurgery, Sunnybrook Health Sciences Centre, University of Toronto, Toronto, Canada; Harquail Centre for Neuromodulation, Hurvitz Brain Sciences Program, Sunnybrook Research Institute, Toronto, Canada; Sunnybrook Research Institute, Toronto, Canada.
Simon J Graham, Sunnybrook Research Institute, Toronto, Canada; Physical Sciences Platform, Sunnybrook Research Institute, Toronto, Canada; Department of Medical Biophysics, University of Toronto, Toronto, Canada.
Nir Lipsman, Division of Neurosurgery, Sunnybrook Health Sciences Centre, University of Toronto, Toronto, Canada; Harquail Centre for Neuromodulation, Hurvitz Brain Sciences Program, Sunnybrook Research Institute, Toronto, Canada; Sunnybrook Research Institute, Toronto, Canada.
REFERENCES
- 1. Harmsen IE, Elias GJB, Beyn ME et al. Clinical trials for deep brain stimulation: current state of affairs. Brain Stimul. 2020;13(2):378-385. [DOI] [PubMed] [Google Scholar]
- 2. Horn A. The impact of modern-day neuroimaging on the field of deep brain stimulation. Curr Opin Neurol. 2019;32(4):511-520. [DOI] [PubMed] [Google Scholar]
- 3. Horn A, Wenzel G, Irmen F et al. Deep brain stimulation induced normalization of the human functional connectome in parkinson's disease. Brain. 2019;142(10):3129-3143. [DOI] [PubMed] [Google Scholar]
- 4. Boutet A, Gramer R, Steele CJ et al. Neuroimaging technological advancements for targeting in functional neurosurgery. Curr Neurol Neurosci Rep. 2019;19(7):42. [DOI] [PubMed] [Google Scholar]
- 5. Boston Scientific. ImageReady MRI Guidelines for Boston Scientific Deep Brain Stimulation Systems. Marlborough, MA: Boston Scientific; 2017. [Google Scholar]
- 6. Medtronic MRI Guidelines for Medtronic Deep Brain Stimulation Systems. Minneapolis, MN: 2015. [Google Scholar]
- 7. Gleason CA, Kaula NF, Hricak H, Schmidt RA, Tanagho EA. The effect of magnetic resonance imagers on implanted neurostimulators. Pacing Clin Electrophysiol. 1992;15(1):81-94. [DOI] [PubMed] [Google Scholar]
- 8. Rezai AR, Baker KB, Tkach JA et al. Is magnetic resonance imaging safe for patients with neurostimulation systems used for deep brain stimulation? Neurosurgery. 2005;57(5):1056-1062; discussion 1056-1062. [DOI] [PubMed] [Google Scholar]
- 9. Rezai AR, Finelli D, Nyenhuis JA et al. Neurostimulation systems for deep brain stimulation: in vitro evaluation of magnetic resonance imaging-related heating at 1.5 tesla. J Magn Reson Imaging. 2002;15(3):241-250. [DOI] [PubMed] [Google Scholar]
- 10. Spiegel J, Fuss G, Backens M et al. Transient dystonia following magnetic resonance imaging in a patient with deep brain stimulation electrodes for the treatment of parkinson disease. Case report. J Neurosurg. 2003;99(4):772-774. [DOI] [PubMed] [Google Scholar]
- 11. Golestanirad L, Angelone LM, Iacono MI, Katnani H, Wald LL, Bonmassar G. Local SAR near deep brain stimulation (DBS) electrodes at 64 and 127 MHz: a simulation study of the effect of extracranial loops. Magn Reson Med. 2017;78(4):1558-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Golestanirad L, Kirsch J, Bonmassar G et al. RF-induced heating in tissue near bilateral DBS implants during MRI at 1.5T and 3T: the role of surgical lead management. Neuroimage. 2019;184:566-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shellock FG, Fischer L, Fieno DS. Cardiac pacemakers and implantable cardioverter defibrillators: in vitro magnetic resonance imaging evaluation at 1.5-tesla. J Cardiovasc Magn Reson. 2007;9(1):21-31. [DOI] [PubMed] [Google Scholar]
- 14. Sommer T, Naehle CP, Yang A et al. Strategy for safe performance of extrathoracic magnetic resonance imaging at 1.5 tesla in the presence of cardiac pacemakers in non-pacemaker-dependent patients: a prospective study with 115 examinations. Circulation. 2006;114(12):1285-1292. [DOI] [PubMed] [Google Scholar]
- 15. Sammartino F, Krishna V, Sankar T et al. 3-Tesla MRI in patients with fully implanted deep brain stimulation devices: a preliminary study in 10 patients. J Neurosurg. 2017;127(4):892-898. [DOI] [PubMed] [Google Scholar]
- 16. Boutet A, Hancu I, Saha U et al. 3-Tesla MRI of deep brain stimulation patients: safety assessment of coils and pulse sequences. J Neurosurg. 2019;132(2):586-594. [DOI] [PubMed] [Google Scholar]
- 17. Bhidayasiri R, Bronstein JM, Sinha S et al. Bilateral neurostimulation systems used for deep brain stimulation: in vitro study of MRI-related heating at 1.5 T and implications for clinical imaging of the brain. Magn Reson Imaging. 2005;23(4):549-555. [DOI] [PubMed] [Google Scholar]
- 18. Carmichael DW, Pinto S, Limousin-Dowsey P et al. Functional MRI with active, fully implanted, deep brain stimulation systems: safety and experimental confounds. Neuroimage. 2007;37(2):508-517. [DOI] [PubMed] [Google Scholar]
- 19. Boutet A, Elias GJB, Gramer R et al. Safety assessment of spine MRI in deep brain stimulation patients. published online: February 14, 2020. J Neurosurg Spine. (doi:10.3171/2019.12.SPINE191241). [DOI] [PubMed] [Google Scholar]
- 20. Yang B, Tam F, Davidson B et al. Technical note: an anthropomorphic phantom with implanted neurostimulator for investigation of MRI safety. Med Phys. 2020;47(8):3745-3751. [DOI] [PubMed] [Google Scholar]
- 21. Lazebnik M, Madsen EL, Frank GR, Hagness SC. Tissue-mimicking phantom materials for narrowband and ultrawideband microwave applications. Phys Med Biol. 2005;50(18):4245-4258. [DOI] [PubMed] [Google Scholar]
- 22. Baker KB, Tkach JA, Nyenhuis JA et al. Evaluation of specific absorption rate as a dosimeter of MRI-related implant heating. J Magn Reson Imaging. 2004;20(2):315-320. [DOI] [PubMed] [Google Scholar]
- 23. Shrivastava D, Abosch A, Hughes J et al. Heating induced near deep brain stimulation lead electrodes during magnetic resonance imaging with a 3 t transceive volume head coil. Phys Med Biol. 2012;57(17):5651-5665. [DOI] [PMC free article] [PubMed] [Google Scholar]