Abstract
This review portrays how ambulatory blood pressure (BP) monitoring was established and recommended as the method of choice for the assessment of BP and for the rational use of antihypertensive drugs. To establish much-needed diagnostic ambulatory BP thresholds, initial statistical approaches evolved into longitudinal studies of patients and populations, which demonstrated that cardiovascular complications are more closely associated with 24-hour and nighttime BP than with office BP. Studies cross-classifying individuals based on ambulatory and office BP thresholds identified white-coat hypertension, an elevated office BP in the presence of ambulatory normotension as a low-risk condition, whereas its counterpart, masked hypertension, carries a hazard almost as high as ambulatory combined with office hypertension. What clinically matters most is the level of the 24-hour and the nighttime BP, while other BP indexes derived from 24-hour ambulatory BP recordings, on top of the 24-hour and nighttime BP level, add little to risk stratification or hypertension management. Ambulatory BP monitoring is cost-effective. Ambulatory and home BP monitoring are complimentary approaches. Their interchangeability provides great versatility in the clinical implementation of out-of-office BP measurement. We are still waiting for evidence from randomized clinical trials to prove that out-of-office BP monitoring is superior to office BP in adjusting antihypertensive drug treatment and in the prevention of cardiovascular complications. A starting research line, the development of a standardized validation protocol for wearable BP monitoring devices, might facilitate the clinical applicability of ambulatory BP monitoring.
Keywords: blood pressure, morbidity, mortality, population, risk
In a seminal study published in 1983, Perloff et al1 reported that there was a significant difference in the incidence of fatal and nonfatal cardiovascular events between patients with high and low ambulatory blood pressure (BP), irrespective of the level of baseline office systolic BP (<160 mm Hg versus ≥160 mm Hg) in 1076 patients with mild to moderate hypertension followed up for 5 years.1 Perloff’s study was the first to demonstrate that the association between cardiovascular complications and BP was tighter for ambulatory than office BP measurement, an observation entering the Canadian hypertension guidelines already in 1999.2 Further studies over the next decades3–11 generated irrefutable evidence confirming that the 24-hour ambulatory BP and particularly the nighttime BP5,11 were superior to office BP in predicting total and cardiovascular mortality and overall and cause-specific cardiovascular complications in patients with hypertension3,5–8 and in population cohorts.4,9–11 Moreover, ambulatory BP allows cross-classifying individuals with their office BP, thereby differentiating masked hypertension from office normotension and white-coat hypertension from office hypertension. Another unique feature of ambulatory BP monitoring is that only this approach can reveal BP variation over the whole day and the responsiveness of BP to physical and mental stressors.12 Given all of the evidence, it does not come as a surprise that current guidelines13–16 for the diagnosis and management of hypertension unanimously recommend the use of 24-hour ambulatory BP monitoring as the state-of-the-art technique for BP measurement and as a prerequisite for individualizing hypertension management. The objective of this review is to summarize how over years the building blocks supporting the use of ambulatory BP monitoring fell into place.
Diagnostic Thresholds
BP is continuously distributed. The relation between cardiovascular outcome and BP is log-linear and continuous, irrespective of whether BP is measured at the office,17 or out of the office, either at home or using ambulatory BP monitoring.18 Thus, there is no critical BP level above which cardiovascular risk suddenly starts rising. Thresholds only serve the need of clinicians to use cutoff limits for the diagnosis and management of hypertension. Nevertheless, as for office BP, clinicians need a diagnostic reference frame and operational threshold levels for the ambulatory BP to assess risk and guide treatment decisions. Although the need of diagnostic thresholds was recognized early in bringing ambulatory BP monitoring to clinical practice, it took over 2 decades to mount cohort studies with sufficiently long follow-up to generate outcome-driven limits to categorize individuals along the risk continuum associated with the ambulatory BP. Furthermore, the thresholds, although helpful for diagnosis, are less evidence-based for titration of antihypertensive medications so far.
Statistical Approaches
Thresholds for the clinical application of ambulatory BP monitoring were initially based on the distribution of the ambulatory BP in people with office normotension,19,20 defined as a level of <140 mm Hg systolic and 90 mm Hg diastolic. In a meta-analysis of summary statistics from 23 studies,19 the mean ambulatory systolic/diastolic BP plus twice the SD in 3476 study participants normotensive on office measurement amounted to 139/87, 146/91, and 127/79 mm Hg for the 24-hour, daytime, and nighttime BP, respectively. In a participant-level meta-analysis,20 the thresholds were set at the 95th percentiles of the ambulatory BP distribution among 4577 individuals with office normotension. The ambulatory BP limits so-derived were 133/82, 140/88, and 125/76 mm Hg for the 24-hour, daytime, and nighttime BP, respectively. Thresholds were also generated by regressing the ambulatory on the office BP. Head et al21 applied a least-product fit to regress the ambulatory on the office BP in 8575 Australians. The so-derived thresholds for the 24-hour, daytime, and nighttime BP were 133/84, 136/87, and 121/76 mm Hg, respectively.
Outcome-Driven Thresholds
The aforementioned thresholds relied heavily on the proportion and representativeness of individuals with office normotension in the studies analyzed and were entirely based on a distributional or statistical approach, which ignores what matters most, that is, the association of cardiovascular end points with BP. Verdecchia et al3 and Ohkubo et al4 were the first researchers to propose more robust outcome-driven ambulatory BP thresholds with further reports following until recently.22–25 According to the Ohasama investigators,22 the 24-hour BP associated with the lowest risk of all-cause mortality ranged from 119 to 133 mm Hg systolic and from 65 to 78 mm Hg diastolic. In 2007, the International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcome (IDACO) investigators determined ambulatory BP thresholds resulting in multivariable-adjusted 10-year cardiovascular risks equivalent to those associated with categories of the office BP.23 The upper limits for the 24-hour, daytime, and nighttime BP amounted to 115/75, 120/80, and 100/65 mm Hg for a normal BP, to 125/75, 130/85, and 110/70 mm Hg for a high-normal BP, and to 130/80, 140/85, and 120/70 mm Hg for ambulatory hypertension (Table 1). In the Jackson Heart Study,24 1016 of 5306 Black participants (19.1%) had their office and ambulatory BP measured and the composite of all-cause mortality and cardiovascular disease was analyzed as end point. Diastolic BP was not related to outcome and, therefore, not analyzed. For systolic ambulatory BP, the outcome-driven thresholds corresponding with an office BP of 140 mm Hg were 134, 138, and 129 mm Hg for the 24-hour, daytime, and nighttime, respectively (Table 1).
Table 1.
Proposals for Ambulatory Blood Pressure Thresholds
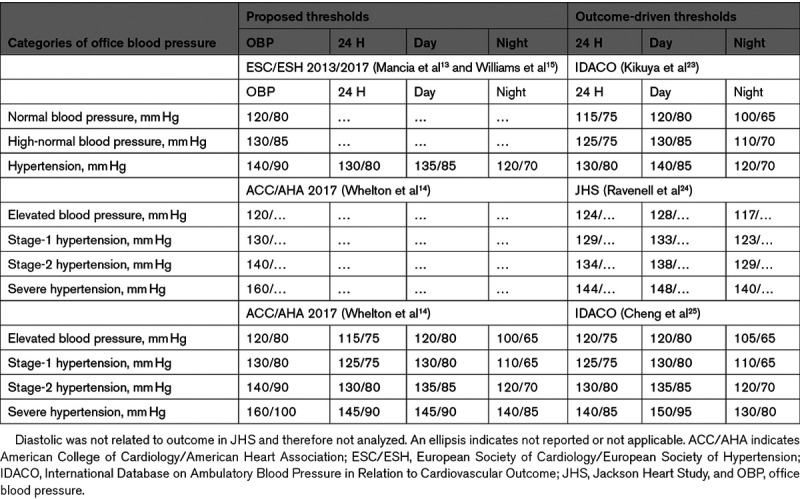
In 2017, the new American College of Cardiology/American Heart Association guideline reclassified office BP and proposed new thresholds for the ambulatory BP, albeit without explicit justification.14,26 The rationale of the proposed thresholds was described in a later separate publication in 2019.27 Thresholds were, therefore, derived from the IDACO database25 that yielded risks equivalent to the new office BP categories.14 Among 11 152 participants representative of 13 populations,25 the thresholds indicating elevated 24-hour, daytime, and nighttime systolic/diastolic BPs were 120/75, 120/80, and 105/65 mm Hg, and for stages 1 and 2, ambulatory hypertension the thresholds were 125/75 and 130/80 mm Hg, 130/80 and 135/85 mm Hg, and 110/65 and 120/70 mm Hg, respectively (Table 1). In general, the thresholds proposed in the European and American guidelines closely approximated to the outcome-driven thresholds. The systolic thresholds derived in the Jackson Heart Study in relation to outcome were substantially higher compared with those proposed by European and American guidelines and those derived from the IDACO database. Ravenell et al24 proposed ethnic differences as a possible explanation.
The Diurnal BP Profile
BP follows a circadian variation, being lower at night than during the day. In 1988, O’Brien et al28 coined the term nondipping referring to the observation that in ≈20% of patients with hypertension the normal decrease in nighttime BP was lost. Nondippers had a significantly higher stroke risk than dippers had (23.8% versus 2.9%).
Dipping Status
The dipping status is not reproducible, depends on environmental (season, temperature, etc) and genetic cues, daytime activity and stress, sleep quality, timing of intake and duration of action of antihypertensive drugs, position of the arm relative to the heart, nocturnal enuresis, differences in the cardiovascular risk profile, and many other factors.29 Researchers contributing to the Spanish Ambulatory Blood Pressure Monitoring Registry,30 recorded the 24-hour ambulatory BP on 2 consecutive days in 611 patients of whom 235 were untreated; from the first to the repeat recording, 24% of patients switched their status from dipper to nondipper, or vice versa. These results were consistent if systolic versus diastolic BP or if treated versus untreated patients were analyzed separately.30 In 512 never-treated patients enrolled in the Edinburgh database,31 who underwent repeat ambulatory monitoring at a median interval of 29 months, dipping status changed in 24% of patients resulting in a κ-coefficient of 0.29. However, when the nocturnal dip was expressed as a continuous variable, the intraclass correlation coefficient of 0.60 indicated moderate reproducibility with no differences depending on the interval between recordings (from 6 to over 36 months).31 Numerous articles addressed the prognostic significance of the nocturnal dipping, in particular the dipping status analyzed as categorical variable. Their results should be taken with skepticism, certainly, when models were not adjusted for the predominant risk factor, that is, the level of the 24-hour ambulatory BP, or when models did not test for collinearity between correlated explanatory BP indexes.11
The Nighttime Predictive Window
With regard to the time of day that is most predictive of adverse health outcomes in relation to the ambulatory BP, studies in patients3,5–8 and populations10,11,22 showed that the nighttime BP by far outperformed the daytime BP, although it should be confirmed in other ethnics.32 In a substudy of the Systolic Hypertension in Europe Trial,5 808 patients were randomized in a double-blind manner to placebo or active BP-lowering treatment. The nighttime systolic BP (midnight to 6 am) was the most accurate predictor of end points. In patients taking placebo, but not in those on active treatment, a 10% increase in the dipping ratio was associated with a multivariable-adjusted hazard ratio for a composite cardiovascular end point of 1.41 (CI, 1.03–1.94).5 These observations illustrate how antihypertensive drug treatment confounds the association of adverse health outcomes with the dipping status. In an analysis of the IDACO database,10 the nighttime BP adjusted for the daytime BP, predicted total, cardiovascular, and noncardiovascular mortality. Conversely, adjusted for nighttime BP, the daytime BP predicted only noncardiovascular mortality, with lower BP levels being associated with increased risk. Antihypertensive drug treatment removed the significant association between cardiovascular events and the daytime BP.10 While a subsequent IDACO publication clarified that both isolated daytime hypertension and isolated nighttime hypertension predicted adverse cardiovascular health outcomes.33
A meta-analysis of both summary statistics and individual-level data, combined studies involving patients with hypertension (N=23 856) separately from those of individuals randomly recruited from populations (N=9641).34 In both patients and populations, in analyses in which the nighttime BP was additionally adjusted for the daytime BP, and vice versa, the nighttime BP was a stronger predictor than the daytime BP was.34 With adjustment for the 24-hour BP, both the dipping ratio and dipping status remained significantly associated with outcome, but as evidenced by the generalized R2 statistic added less than 0.6% to the model fit over and beyond the 24-hour BP.34 Analysis of an updated IDACO database recently demonstrated that higher 24-hour and higher nighttime BP, compared with all other BP indexes, were associated with greater risk of all-cause mortality and a composite cardiovascular outcome, even after adjusting for the manual and automated office BP and after adjusting for the daytime BP and dipping ratio or status.11 Nighttime BP was measured during sleep at the supine position without movement and minimally confounded by antihypertensive drug treatment, usually taken in the morning, which probably explained why the nighttime BP was considered as an individual’s basal BP and a precise prognostic marker. This is in keeping with the concept originally enunciated by Smirk in 1964 that elevation of basal BP obtained following sedation was an accurate marker for adverse health outcomes.35
Cross-Classification With Office BP
A major contribution of ambulatory BP monitoring to the management of hypertension is the cross-classification between the office and ambulatory BP.
White-Coat Hypertension
Building on the work of Mann et al36 at Northwick Park Hospital in London,36 John Floras and Peter Sleight at the John Radcliffe Hospital in Oxford,37 and Dorthee Perloff and Maurice Sokolow at the San Francisco Medical Center,1 in 1984, Kleinert et al38 coined the term white-coat hypertension, referring to patients whose BP was elevated in the medical environment, but not during daytime ambulatory BP monitoring.39–41 Pickering’s articles38,39 raised the hypothesis that patients who showed an exaggerated response to the clinic environment might also exhibit a similar response to more regularly occurring types of stress, a concept that supported the clinical application of BP monitoring. However, a later study by the same group did not confirm the hypothesis that stressor might increase office, but not daytime BP.40
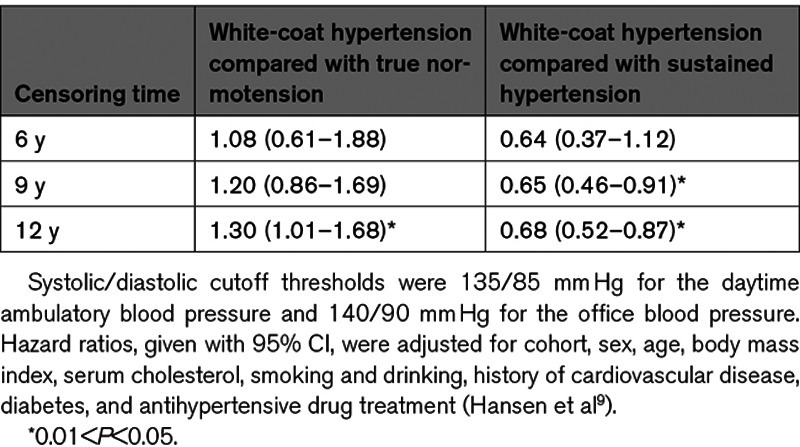
The first longitudinal study on the prognostic values of white-coat hypertension was reported in 1994.3 Based on these early studies1,3,36,37,40,41 and confirmatory reports in patients42 and populations,9,43–46 the currently prevailing point of view is that white-coat hypertension carries little cardiovascular risk.47 A 2007 IDACO analysis addressed the long-term risks associated with white-coat hypertension versus true normotension and sustained hypertension by censoring Cox models for a composite cardiovascular end point for varying follow-up intervals.9 The definition of white-coat hypertension relied on the cross-classification of the office and daytime BP level, irrespective of treatment status.9 The hazard ratios comparing white-coat hypertension with true normotension were 1.08 (P=0.79), 1.20 (P=0.29), and 1.30 (P=0.043), when models were censored at 6, 9, and 12 years (Table 2). The corresponding hazard ratios for white-coat compared with sustained hypertension were 0.64 (P=0.11), 0.65 (P=0.013), and 0.73 (P=0.014), respectively (Table 2).
Table 2.
The Long-Term Risk of a Cardiovascular End Point Associated With White-Coat Hypertension
Mancia and Grassi suggested that white-coat hypertension does carry cardiovascular risk.48 In drawing this conclusion, these investigators ignored the loose criteria usually applied in the literature to diagnose white-coat hypertension, ignoring treatment status, cardiovascular risk factors, target organ damage, a history of cardiovascular disease, and last but not least the wide age range in many studies, for which appropriate adjustment is impossible.49 Baseline ambulatory BP levels in patients with white-coat hypertension were usually higher than normotensives in studies, which suggested that this condition was associated with cardiovascular risk.49 Pierdomenico and Cuccurullo47 reported a meta-analysis of summary statistics to assess the prognostic impact of white-coat hypertension in initially untreated people free of cardiovascular complications. They selected studies, which had adjusted hazard ratios for relevant confounders. Compared with normotension, the pooled hazard ratio of white-coat hypertension for the incidence of cardiovascular events was 0.96 (CI, 0.65–1.42; P=0.85) without any heterogeneity between studies (P>0.65). Follow-up duration did not affect this conclusion.47 This exemplary meta-analysis47 accurately handled confounding by antihypertensive drug treatment and previous cardiovascular disease. A recently published meta-analysis of 27 studies50 also properly addressed the prognosis of white-coat hypertension by antihypertensive treatment status and other confounding factors. In untreated, but not in treated individuals, white-coat hypertension was associated with an increased risk of cardiovascular events and all-cause mortality compared with normotension. However, the risk of white-coat hypertension was attenuated or diminished in studies of individuals younger than 55 years, studies that used 24-hour BP <130/80 to define ambulatory normotension, studies with <5 years of follow-up, and studies that included stroke in the definition of cardiovascular events.50
The prevalence of white-coat hypertension increases exponentially with age. A participant-level meta-analysis combined participants not taking antihypertensive medications enrolled in IDACO (N=7506) and in the study of Genetic and Phenotypic Determinants of Blood Pressure and Other Cardiovascular Risk Factors (GAPP; N=2044).45 The prevalence of white-coat hypertension exponentially increased from 2.2% to 19.5% from age 18 to 30 years to 70 years and over, with little sex differences.45 A case-control study nested within IDACO addressed the age-dependency of white-coat hypertension and its association with risk factors in the prediction of cardiovascular complications (Figure 1). Cardiovascular risk was scored according to the European Society of Hypertension guideline.13 Untreated white-coat hypertensive patients (N=653) were matched with normotensive control by age (within 5 years),44 an approach that is more bias-free than trying to adjust away the huge confounding effect of age. Over a median follow-up of 10.6 years, Kaplan-Meier survival function estimates showed a higher incidence of a composite cardiovascular end point in 159 high-risk white-coat hypertensive patients compared with high-risk normotensive people, but not in 494 low-risk white-coat hypertensive patients compared with low-risk normotensive controls (Figure 1). The corresponding multivariable-adjusted hazard ratios were 2.06 (CI, 1.10–3.84) and 1.06 (CI, 0.66–1.72), respectively. After stratification for age (<60 versus ≥60 years), the increased risk was limited to older white-coat hypertensive patients at high cardiovascular risk.44 Overall, there were 70 incident cardiovascular events in the white-coat hypertensive patients versus 48 in the age-matched normotensive controls, in other words, there was an excess of only 22 new cardiovascular events in 653 white-coat hypertensive patients compared with the cohort- and age-matched normotensive controls. Thus the excess rate was only 3.4%.44 The clinical implication of these findings is that in older high-risk white-coat hypertensive patients priority might be given to addressing the modifiable cardiovascular risk factors. To our knowledge, there is currently no evidence from randomized clinical trials testing whether lowering versus not lowering office BP in white-coat hypertensive might result in benefit. It is unnecessary to intensify antihypertensive treatment in patients with uncontrolled white-coat hypertension,51 as also proposed by the 2017 American guideline.14
Figure 1.
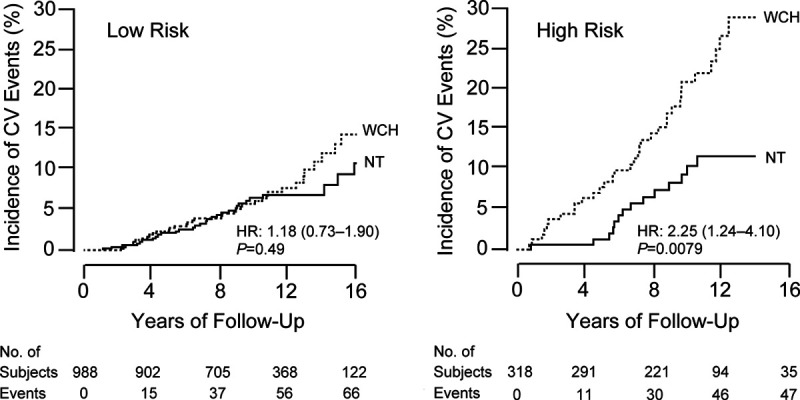
Kaplan-Meier survival estimates for the incidence of a composite cardiovascular end point in 653 subjects with white-coat hypertension (WCH) and their age-matched (within 5 y) normotensive controls (NT). The analysis was stratified by cardiovascular risk according to the 2013 European guidelines: low (left, N=494) and high (right, N=159). The number of incident cardiovascular events in the WCH and NT groups totaled 37 and 32 in the low-risk group and 33 and 16 in the high-risk group. The numbers below the horizontal axis are the number of subjects experiencing a cardiovascular event and the number of subjects still in follow-up at 4-yearly intervals. HR is the unadjusted hazard ratio. Reproduced from Franklin et al44 with permission. Copyright ©2016, Elsevier.
Masked Hypertension
The counterpart of white-coat hypertension is masked hypertension, a disorder characterized by a normal office BP confirmed at repeated clinic visits but a raised daytime, nighttime, or 24-hour ambulatory BP. The probability of having masked hypertension increases with an office BP in the range of 120 to 139 mm Hg systolic or 80 to 89 mm Hg diastolic (odds ratio [OR], 5.1 versus optimal office BP), age 41 years or older versus younger age (OR, 2.5), overweight or obesity (OR, 2.0), alcohol intake (OR, 1.9), diabetes (OR, 1.8), and smoking (OR, 1.5).52 Other risk factors include a family history of hypertension in both parents, patients with multiple risk factors for cardiovascular disease, male sex, and a higher awake heart rate.53 In population studies, the prevalence of masked hypertension is ≈15%.9 In untreated patients, masked hypertension diagnosed by the cross-classification of office with daytime BP is a sustained condition in over 70% of patients.54 Indeed, 45 patients had masked hypertension at baseline, of whom 35 (77.8%) stayed masked hypertensive at 2 weeks of follow-up.54 These observations were consistent if office BP was cross-classified with the 24-hour or nighttime BP.54
Masked hypertension carries a multivariable-adjusted risk almost equivalent to sustained hypertension, that is, hypertension on office and ambulatory BP measurement, irrespective of treatment status.9 An IDACO analysis52 addressed the risk associated with masked hypertension in untreated patients stratified according to categories of office BP, as defined by the JNC7 (the seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure) guidelines. People with normal office and normal daytime ambulatory BP were the reference group (Figure 2). Among participants with office normotension (<120/<80 mm Hg) or office prehypertension (120–139/80–89 mm Hg), respectively, 198 (7.5%) and 900 (29.3%) had masked hypertension. Compared with true normotension, the multivariable-adjusted hazard ratios associated with masked hypertension in participants with office normotension, were 2.11 (P=0.006) for a composite cardiovascular end point and 3.02 (P=0.01) for stroke (Figure 2).52 The corresponding hazard ratios associated with masked hypertension in prehypertensive patients were 2.08 (P<0.0001) and 2.97 (P<0.0001), respectively. Compared with prehypertension without masked hypertension, the hazard ratios associated with masked hypertension in prehypertensive subjects were 1.53 (P=0.0001) for the composite cardiovascular end point and 1.48 (P=0.04) for stroke.52 These findings remained consistent, if masked hypertension was defined based on the 24-hour or the nighttime BP.52 Findings in patients with diabetes were confirmatory, again highlighting the risk carried by masked hypertension.55 In view of the high-risk profile associated with masked hypertension, the 2017 American College of Cardiology/American Heart Association14 and the 2018 European15 hypertension guidelines consistently recommend that masked hypertensive patients should implement lifestyle interventions and be treated with antihypertensive drugs. However, in the absence of supporting evidence from randomized clinical trials, this recommendation rests largely on expert opinion.
Figure 2.
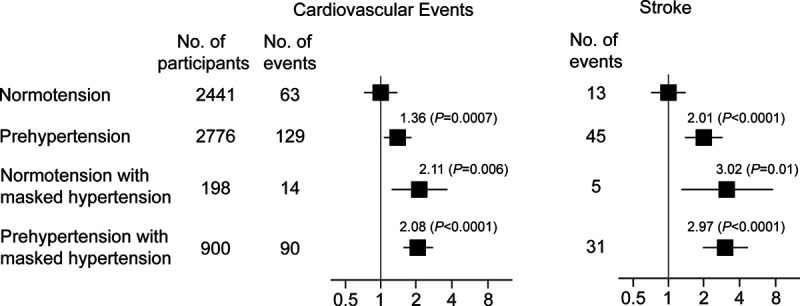
Hazard ratios for cardiovascular events and stroke associated with masked hypertension on daytime blood pressure monitoring in untreated participants with normotension or prehypertension. Participants with sustained normotension are the reference group. Normotension (<120/<80 mm Hg) and prehypertension (120–139/80–89 mm Hg) refer to the classification based on office blood pressure according to the JNC7 guidelines. Thresholds for daytime hypertension were ≥135 mm Hg systolic or ≥85 mm Hg diastolic. The hazard ratios were adjusted for cohort, sex, age, body mass index, smoking and drinking, serum cholesterol, history of cardiovascular complications, and diabetes. Horizontal lines denote the 95% CI. Reproduced from Brguljan-Hitij et al52 with permission. Copyright ©2014, Oxford University Press.
Cost-Effectiveness
Several studies addressed the cost-effectiveness of ambulatory BP monitoring.56–59 Originally, the idea was put forward that ambulatory BP monitoring would reduce health care costs mainly by avoiding antihypertensive drug treatment in white-coat hypertensive patients.57 In 2006, Krakoff computed the cost savings likely to be gained when ambulatory BP monitoring would be implemented in newly detected patients with hypertension.57 In their calculations, the contemporary costs of testing and treatment, the prevalence of white-coat hypertension at baseline, and the incidence of new-onset hypertension after the initial screen were accounted for. The results indicated that using versus not using ambulatory BP monitoring in the 5-year management of hypertension might entail cost savings of 3% per 1000 patients ($45 322 of $1 546 494) to 14% ($210 024) and a treatment-years reduction from 10% to 23% (461–1026 treatment-years).57
In 2011, Lovibond et al58 published a Markov model-based probabilistic cost-effectiveness analysis. These investigators used a hypothetical primary care population aged 40 years or older with a screening office BP >140 mm Hg systolic and 90 mm Hg diastolic and a risk factor prevalence representative for the general population. They compared further BP measurement in the clinic, at home, and with an ambulatory monitor in terms of lifetime costs, quality-of-life-adjusted life-years, and cost-effectiveness. Ambulatory BP monitoring was the most cost-effective strategy for the diagnosis of hypertension in women and men of all ages. It was cost saving in all groups (from −£56 [CI, −105 to −10] in men aged 75 years to −£323 [CI, −389 to −222] in women aged 40 years) and resulted in more quality-of-life-adjusted life-years for women of all ages and for men older than 50 years. These findings were robust when assessed with a wide range of deterministic sensitivity analyses around the base case but was sensitive if home BP monitoring was assumed to have equal test performance to ambulatory BP monitoring.58 However, home BP measurement cannot completely cover what ambulatory monitoring provides in terms of clinical information,60 such as the BP response to the physical and psychological stressors, the night-to-day BP ratio and dipping status, the documentation of an excessive BP drop at night in treated patients, spreading the doses of antihypertensive drugs over the day to have a full 24-hour coverage of the BP-lowering effect, and the unbiased documentation of untowards BP reactions over the whole day.
More recently, Beyhaghi and Viera compared in a primary care setting in the United States the quality-adjusted life-years and lifetime costs associated with clinic, home, and ambulatory BP measurements.59 These investigators applied 2 scenarios, that is, a positive (office hypertension) and a negative (office normotension) initial screen, respectively, reflecting white-coat and masked hypertension. In the screen-positive scenario, ambulatory BP monitoring was the dominant strategy among all age and sex groups. Compared with clinic BP measurement, ambulatory monitoring was associated with cost-savings ranging from $77 (women 80 years of age) to $5013 (women 21 years of age). In the screen-negative scenario, ambulatory BP monitoring was also the dominant strategy in all men and women younger than 80 years and entailed cost savings ranging from $128 (women 70 years of age) to $2794 (women 21 years of age).59
Both health-economic studies referenced above (Staessen et al60 and Pickering61) assumed that ambulatory BP monitoring had 100% sensitivity and specificity and both studies came to alternative conclusions when home BP measurement was assumed to have the same sensitivity and specificity as ambulatory BP monitoring. Moreover, both studies (Staessen et al60 and Pickering61) focused on the diagnostic performance of ambulatory BP monitoring but did not generate any evidence on how the application of ambulatory BP monitoring would affect the costs of hypertension management, an outcome which can only be inferred in the context of a randomized clinical trial comparing the initiation and adjustment of BP-lowering treatment based on office versus ambulatory BP. Thus, from a payers’ perspective, one should be careful in generalizing the health care cost implications of both reports (Staessen et al60 and Pickering61).
Clinical Application
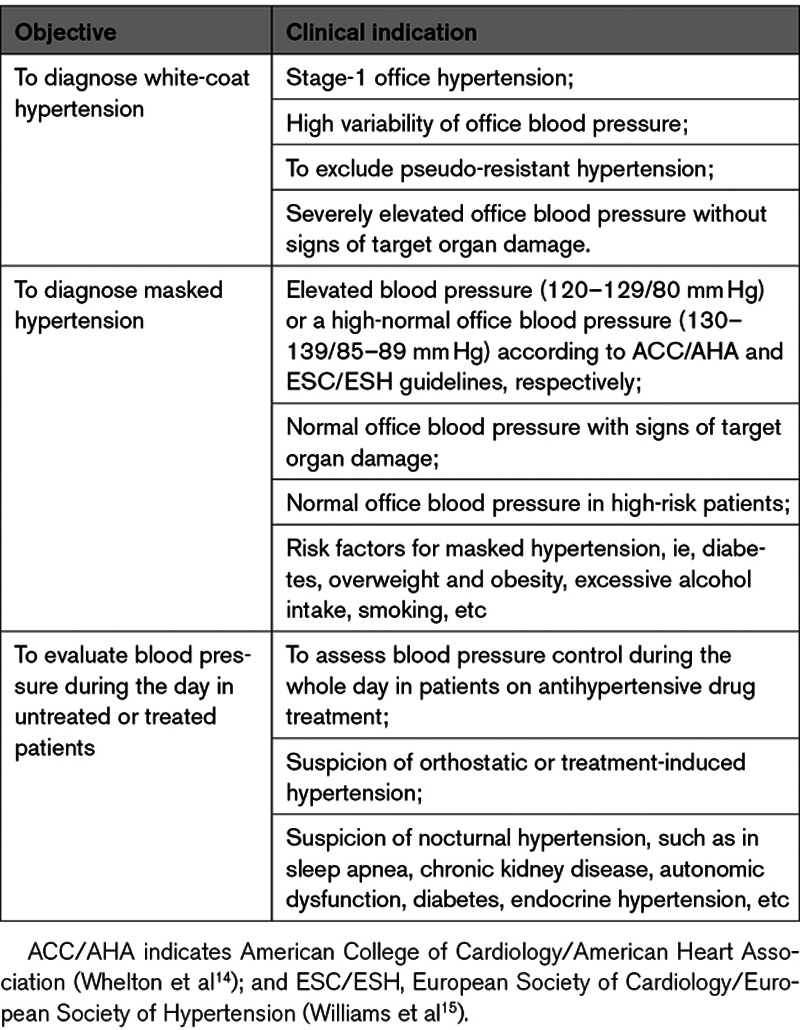
The rational management of hypertension inevitably starts with accurate measurement of BP. American14 and European15 guidelines are unanimous in recommending ambulatory BP monitoring in all patients under consideration for BP-lowering medication. The idea behind these recommendations is to exclude white-coat hypertension and to diagnose masked hypertension, pending on the clinical indications summarized in Table 3. If multiple treatment adjustments are required, as may often be the case, then repeating ambulatory monitoring or other approaches, such as home BP monitoring61 or automated office BP measurement,62 are justified. While uptitrating antihypertensive drug treatment, 24-hour ambulatory BP monitoring allows excluding excessive BP lowering during the night or during activities in the upright position (Table 3). After having optimized treatment, it would seem reasonable to repeat ambulatory monitoring during follow-up to ensure that adequate BP reduction has been achieved.
Table 3.
Clinical Indications for Ambulatory Blood Pressure Monitoring
Ambulatory and home BP monitoring are complimentary techniques, in particular in the follow-up of white-coat hypertensive patients.60 Office BP measurement remains the standard screening method for the diagnosis of hypertension. When office BP is elevated in the absence of target organ damage or when patients with a normal clinic BP show unexplained target organ damage, ambulatory BP monitoring combined with home BP measurement can be applied, as for instance proposed in Figure 3.63 Home BP measurement, especially if combined with telemonitoring, is a powerful instrument in educating and empowering patients.64 In a randomized clinical trial, involving 450 patients recruited at 59 primary care practices in the United Kingdom and followed up for 12 months, self-monitoring and self-titration of antihypertensive dugs lowered systolic BP 8.8 mm Hg more (CI, 4.9–12.7 mm Hg) than usual care based on office BP measurement.64 The 2017 American College of Cardiology/American Heart Association hypertension guideline14 endorses home above ambulatory monitoring in the adjustment of BP-lowering treatment, certainly when BP must be repeatedly measured at short time intervals.
Figure 3.
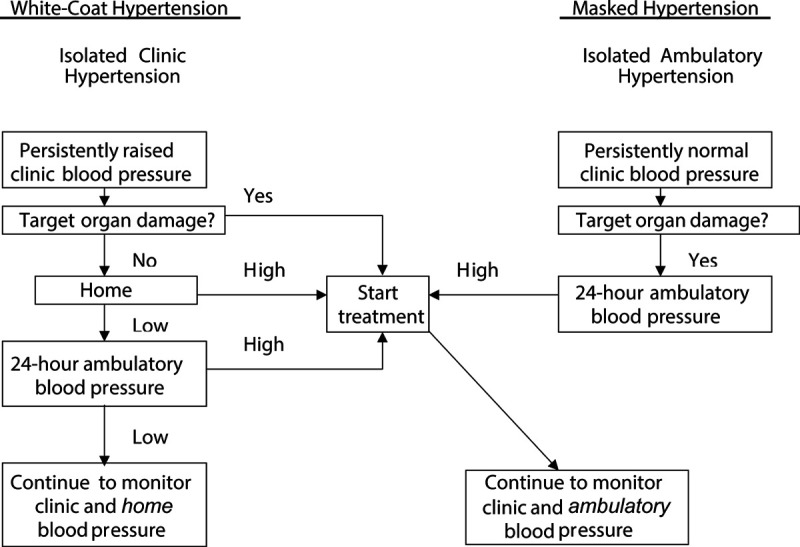
Diagnostic workflow for evaluation of patients by use of office, home, and ambulatory monitoring of blood pressure. Reproduced from Staessen et al63 with permission. Copyright ©2003, Elsevier.
One major limitation is that there is no evidence from randomized clinical trials to prove that out-of-office BP monitoring is superior to office BP in adjusting antihypertensive drug treatment in the prevention of hard cardiovascular complications. The ongoing placebo-controlled ANTIMASK (Antihypertensive Treatment in Masked Hypertension for Target Organ Protection) trial (URL: https://www.clinicaltrials.gov. Unique identifier: NCT02893358) in Chinese patients with masked hypertension will report in 2021, but its sample size is small (n=300) and the primary end point includes only the improvement of target organ damage. The Japanese multicenter Hypertension Objective Treatment Based on Measurement by Electrical Devices of Blood Pressure trial (2001–2010)65 proved the feasibility of using the self-measured home BP as guide for titrating antihypertensive drug treatment aiming at usual and tight BP targets but did not include a control arm, in which treatment adjustments were based on office BP pressure.
Conclusions and Perspectives
Forty years of research consolidated ambulatory BP monitoring as the technique of choice to measure BP. What from a clinical viewpoint matters most is the level of the 24-hour and the nighttime BP. Other BP indexes derived from 24-hour ambulatory BP recordings, such as the night-to-day BP ratio,11 dipping status,11 the morning BP surge,66 24-hour pulse pressure,67 the double-product,68 and BP variability69,70 add little to risk stratification on top of the 24-hour and nighttime BP level. A starting research line is the development of a standardized validation protocol for wearable BP monitoring devices.71 The wearable devices are cuffless and more comfortable for patients but represent a challenge for validation, for which experts in the field must develop standardized protocols producing repeatable results allowing inter-device comparisons.
Acknowledgments
We gratefully acknowledge the clerical assistance of Vera De Leebeeck and Renilde Wolfs, Research Unit Hypertension and Cardiovascular Epidemiology, KU Leuven Department of Cardiovascular Sciences, University of Leuven, Leuven, Belgium.
Sources of Funding
NPA Alliance for the Promotion of Preventive Medicine (htpps://www.appremed.org) received a nonbinding grant from OMRON Healthcare Co Ltd, Kyoto, Japan. Q.-F. Huang and Y. Li are supported by the National Natural Science Foundation of China (grants 81770455 and 81970353), the Ministry of Science and Technology (grants 2018YFC1704902 and 2016YFC0900902), Beijing, China, and by the Shanghai Commissions of Science and Technology (grants 19DZ2340200 and 19ZR1443300), and Health (grants 2017BR025 and 201940297), Shanghai, China.
Disclosures
None.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- BP
- blood pressure
- IDACO
- International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcome
- OR
- odds ratio
This paper was sent Robert M. Carey, Consulting Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 262.
Contributor Information
Qi-Fang Huang, Email: qifanghuang@hotmail.com.
Wen-Yi Yang, Email: etaloc@163.com.
Kei Asayama, Email: kei@asayama.org.
Zhen-Yu Zhang, Email: zhenyu.zhang@kuleuven.be.
Lutgarde Thijs, Email: lutgarde.thijs@kuleuven.be.
Yan Li, Email: liyanshcn@yahoo.com.
Eoin O’Brien, Email: profeobrien@icloud.com.
References
- 1.Perloff D, Sokolow M, Cowan R. The prognostic value of ambulatory blood pressures. JAMA. 1983;249:2792–2798 [PubMed] [Google Scholar]
- 2.Myers MG, Haynes RB, Rabkin SW. Canadian hypertension society guidelines for ambulatory blood pressure monitoring. Am J Hypertens. 1999;1211pt 11149–1157. doi: 10.1016/s0895-7061(99)00199-5 [DOI] [PubMed] [Google Scholar]
- 3.Verdecchia P, Porcellati C, Schillaci G, Borgioni C, Ciucci A, Battistelli M, Guerrieri M, Gatteschi C, Zampi I, Santucci A, et al. Ambulatory blood pressure. An independent predictor of prognosis in essential hypertension. Hypertension. 1994;24:793–801. doi: 10.1161/01.hyp.24.6.793 [DOI] [PubMed] [Google Scholar]
- 4.Ohkubo T, Imai Y, Tsuji I, Nagai K, Watanabe N, Minami N, Kato J, Kikuchi N, Nishiyama A, Aihara A, et al. Relation between nocturnal decline in blood pressure and mortality. The Ohasama Study. Am J Hypertens. 1997;10:1201–1207. doi: 10.1016/s0895-7061(97)00274-4 [DOI] [PubMed] [Google Scholar]
- 5.Staessen JA, Thijs L, Fagard R, O’Brien ET, Clement D, de Leeuw PW, Mancia G, Nachev C, Palatini P, Parati G, et al. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. Systolic Hypertension in Europe Trial Investigators. JAMA. 1999;282:539–546. doi: 10.1001/jama.282.6.539 [DOI] [PubMed] [Google Scholar]
- 6.Clement DL, De Buyzere ML, De Bacquer DA, de Leeuw PW, Duprez DA, Fagard RH, Gheeraert PJ, Missault LH, Braun JJ, Six RO, et al. ; Office versus Ambulatory Pressure Study Investigators. Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. N Engl J Med. 2003;348:2407–2415. doi: 10.1056/NEJMoa022273 [DOI] [PubMed] [Google Scholar]
- 7.Dolan E, Stanton A, Thijs L, Hinedi K, Atkins N, McClory S, Den Hond E, McCormack P, Staessen JA, O’Brien E. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension. 2005;46:156–161. doi: 10.1161/01.HYP.0000170138.56903.7a [DOI] [PubMed] [Google Scholar]
- 8.Ben-Dov IZ, Kark JD, Ben-Ishay D, Mekler J, Ben-Arie L, Bursztyn M. Predictors of all-cause mortality in clinical ambulatory monitoring: unique aspects of blood pressure during sleep. Hypertension. 2007;49:1235–1241. doi: 10.1161/HYPERTENSIONAHA.107.087262 [DOI] [PubMed] [Google Scholar]
- 9.Hansen TW, Kikuya M, Thijs L, Björklund-Bodegård K, Kuznetsova T, Ohkubo T, Richart T, Torp-Pedersen C, Lind L, Jeppesen J, et al. ; IDACO Investigators. Prognostic superiority of daytime ambulatory over conventional blood pressure in four populations: a meta-analysis of 7,030 individuals. J Hypertens. 2007;25:1554–1564. doi: 10.1097/HJH.0b013e3281c49da5 [DOI] [PubMed] [Google Scholar]
- 10.Boggia J, Li Y, Thijs L, Hansen TW, Kikuya M, Björklund-Bodegård K, Richart T, Ohkubo T, Kuznetsova T, Torp-Pedersen C, et al. ; International Database on Ambulatory blood pressure monitoring in relation to Cardiovascular Outcomes (IDACO) investigators. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet. 2007;370:1219–1229. doi: 10.1016/S0140-6736(07)61538-4 [DOI] [PubMed] [Google Scholar]
- 11.Yang WY, Melgarejo JD, Thijs L, Zhang ZY, Boggia J, Wei FF, Hansen TW, Asayama K, Ohkubo T, Jeppesen J, et al. ; International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes (IDACO) Investigators. Association of office and ambulatory blood pressure with mortality and cardiovascular outcomes. JAMA. 2019;322:409–420. doi: 10.1001/jama.2019.9811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boggia J, Luzardo L, Lujambio I, Sottolano M, Robaina S, Thijs L, Olascoaga A, Noboa O, Struijker-Boudier HA, Safar ME, et al. The diurnal profile of central hemodynamics in a general uruguayan population. Am J Hypertens. 2016;29:737–746. doi: 10.1093/ajh/hpv169 [DOI] [PubMed] [Google Scholar]
- 13.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–2219. doi: 10.1093/eurheartj/eht151 [DOI] [PubMed] [Google Scholar]
- 14.Whelton PK, Carey RM, Aronow WS, Casey DE, Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127–e248. doi: 10.1016/j.jacc.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 15.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. ; ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339 [DOI] [PubMed] [Google Scholar]
- 16.National Institute for Health and Clinical Excellence (NICE). Hypertension in adults: diagosis and management. NICE guideline [NG136]. Updated August 28, 2019. Accessed October 19, 2020. https://www.nice.org.uk/guidance/ng136.
- 17.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8 [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Thijs L, Zhang ZY, Asayama K, Hansen TW, Boggia J, Björklund-Bodegård K, Yang WY, Niiranen TJ, Ntineri A, et al. ; International Database on Ambulatory and Home Blood Pressure in Relation to Cardiovascular Outcome Investigators. Opposing age-related trends in absolute and relative risk of adverse health outcomes associated with out-of-office blood pressure. Hypertension. 2019;74:1333–1342. doi: 10.1161/HYPERTENSIONAHA.119.12958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Staessen JA, Fagard RH, Lijnen PJ, Thijs L, Van Hoof R, Amery AK. Mean and range of the ambulatory pressure in normotensive subjects from a meta-analysis of 23 studies. Am J Cardiol. 1991;67:723–727. doi: 10.1016/0002-9149(91)90529-t [DOI] [PubMed] [Google Scholar]
- 20.Staessen JA, O’Brien ET, Amery AK, Atkins N, Baumgart P, De Cort P, Degaute JP, Dolenc P, De Gaudemaris R, Enström I, et al. Ambulatory blood pressure in normotensive and hypertensive subjects: results from an international database. J Hypertens. 1994;12suppl 7S1–S12 [PubMed] [Google Scholar]
- 21.Head GA, Mihailidou AS, Duggan KA, Beilin LJ, Berry N, Brown MA, Bune AJ, Cowley D, Chalmers JP, Howe PR, et al. ; Ambulatory Blood Pressure Working Group of the High Blood Pressure Research Council of Australia. Definition of ambulatory blood pressure targets for diagnosis and treatment of hypertension in relation to clinic blood pressure: prospective cohort study. BMJ. 2010;340:c1104 doi: 10.1136/bmj.c1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kikuya M, Ohkubo T, Asayama K, Metoki H, Obara T, Saito S, Hashimoto J, Totsune K, Hoshi H, Satoh H, et al. Ambulatory blood pressure and 10-year risk of cardiovascular and noncardiovascular mortality: the Ohasama study. Hypertension. 2005;45:240–245. doi: 10.1161/01.HYP.0000152079.04553.2c [DOI] [PubMed] [Google Scholar]
- 23.Kikuya M, Hansen TW, Thijs L, Björklund-Bodegård K, Kuznetsova T, Ohkubo T, Richart T, Torp-Pedersen C, Lind L, Ibsen H, et al. ; International Database on Ambulatory blood pressure monitoring in relation to Cardiovascular Outcomes Investigators. Diagnostic thresholds for ambulatory blood pressure monitoring based on 10-year cardiovascular risk. Circulation. 2007;115:2145–2152. doi: 10.1161/CIRCULATIONAHA.106.662254 [DOI] [PubMed] [Google Scholar]
- 24.Ravenell J, Shimbo D, Booth JN, 3rd, Sarpong DF, Agyemang C, Beatty Moody DL, Abdalla M, Spruill TM, Shallcross AJ, Bress AP, et al. Thresholds for ambulatory blood pressure among African Americans in the Jackson Heart Study. Circulation. 2017;135:2470–2480. doi: 10.1161/CIRCULATIONAHA.116.027051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng YB, Thijs L, Zhang ZY, Kikuya M, Yang WY, Melgarejo JD, Boggia J, Wei FF, Hansen TW, Yu CG, et al. Outcome-driven thresholds for ambulatory blood pressure based on the New American College of Cardiology/American Heart Association Classification of Hypertension. Hypertension. 2019;74:776–783. doi: 10.1161/HYPERTENSIONAHA.119.13512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muntner P, Shimbo D, Carey RM, Charleston JB, Gaillard T, Misra S, Myers MG, Ogedegbe G, Schwartz JE, Townsend RR, et al. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension. 2019;73:e35–e66. doi: 10.1161/HYP.0000000000000087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muntner P, Carey RM, Jamerson K, Wright JT, Jr, Whelton PK. Rationale for ambulatory and home blood pressure monitoring thresholds in the 2017 American College of Cardiology/American Heart Association Guideline. Hypertension. 2019;73:33–38. doi: 10.1161/HYPERTENSIONAHA.118.11946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Brien E, Sheridan J, O’Malley K. Dippers and non-dippers. Lancet. 1988;2:397 doi: 10.1016/s0140-6736(88)92867-x [DOI] [PubMed] [Google Scholar]
- 29.Sheng CS, Cheng YB, Wei FF, Yang WY, Guo QH, Li FK, Huang QF, Thijs L, Staessen JA, Wang JG, et al. Diurnal blood pressure rhythmicity in relation to environmental and genetic cues in untreated referred patients. Hypertension. 2017;69:128–135. doi: 10.1161/HYPERTENSIONAHA.116.07958 [DOI] [PubMed] [Google Scholar]
- 30.Hernández-del Rey R, Martin-Baranera M, Sobrino J, Gorostidi M, Vinyoles E, Sierra C, Segura J, Coca A, Ruilope LM; Spanish Society of Hypertension Ambulatory Blood Pressure Monitoring Registry Investigators. Reproducibility of the circadian blood pressure pattern in 24-h versus 48-h recordings: the Spanish Ambulatory Blood Pressure Monitoring Registry. J Hypertens. 2007;25:2406–2412. doi: 10.1097/HJH.0b013e3282effed1 [DOI] [PubMed] [Google Scholar]
- 31.McGowan NJ, Gough K, Padfield PL. Nocturnal dipping is reproducible in the long term. Blood Press Monit. 2009;14:185–189. doi: 10.1097/MBP.0b013e32832ff4e1 [DOI] [PubMed] [Google Scholar]
- 32.Kidambi S, Wang T, Chelius T, Nunuk I, Agarwal P, Laud P, Mattson D, Cowley AW, Jr, Liang M, Kotchen T. Twenty-four-hour versus clinic blood pressure levels as predictors of long-term cardiovascular and renal disease outcomes among African Americans. Sci Rep. 2020;10:11685 doi: 10.1038/s41598-020-68466-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fan HQ, Li Y, Thijs L, Hansen TW, Boggia J, Kikuya M, Björklund-Bodegård K, Richart T, Ohkubo T, Jeppesen J, et al. ; International Database on Ambulatory Blood Pressure In Relation to Cardiovascular Outcomes Investigators. Prognostic value of isolated nocturnal hypertension on ambulatory measurement in 8711 individuals from 10 populations. J Hypertens. 2010;28:2036–2045. doi: 10.1097/HJH.0b013e32833b49fe [DOI] [PubMed] [Google Scholar]
- 34.Hansen TW, Li Y, Boggia J, Thijs L, Richart T, Staessen JA. Predictive role of the nighttime blood pressure. Hypertension. 2011;57:3–10. doi: 10.1161/HYPERTENSIONAHA.109.133900 [DOI] [PubMed] [Google Scholar]
- 35.Smirk FH. Observations on mortality of 270 treated and 199 untreated retinal grade I and II hypertensive patients followed in all instances for 5 years. N Z Med J. 1964;63:413–443 [Google Scholar]
- 36.Mann S, Millar Craig MW, Raftery EB. Superiority of 24-hour measurement of blood pressure over clinic values in determining prognosis in hypertension. Clin Exp Hypertens A. 1985;7:279–281. doi: 10.3109/10641968509073547 [DOI] [PubMed] [Google Scholar]
- 37.Coats AJ, Conway J, Sleight P, Meyer TE, Somers VK, Floras JS, Vann Jones J. Interdependence of blood pressure and heart period regulation in mild hypertension. Am J Hypertens. 1991;43pt 1234–238. doi: 10.1093/ajh/4.3.234 [DOI] [PubMed] [Google Scholar]
- 38.Kleinert HD, Harshfield GA, Pickering TG, Devereux RB, Sullivan PA, Marion RM, Mallory WK, Laragh JH. What is the value of home blood pressure measurement in patients with mild hypertension? Hypertension. 1984;6:574–578. doi: 10.1161/01.hyp.6.4.574 [DOI] [PubMed] [Google Scholar]
- 39.Pickering TG, Harshfield GA, Kleinert HD, Blank S, Laragh JH. Blood pressure during normal daily activities, sleep, and exercise. Comparison of values in normal and hypertensive subjects. JAMA. 1982;247:992–996 [PubMed] [Google Scholar]
- 40.Pickering TG, James GD, Boddie C, Harshfield GA, Blank S, Laragh JH. How common is white coat hypertension? JAMA. 1988;259:225–228 [PubMed] [Google Scholar]
- 41.O’Brien E. First Thomas Pickering memorial lecture: ambulatory blood pressure measurement is essential for the management of hypertension. J Clin Hypertens. 2012;14:836–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fagard RH, Van Den Broeke C, De Cort P. Prognostic significance of blood pressure measured in the office, at home and during ambulatory monitoring in older patients in general practice. J Hum Hypertens. 2005;19:801–807. doi: 10.1038/sj.jhh.1001903 [DOI] [PubMed] [Google Scholar]
- 43.Franklin SS, Thijs L, Hansen TW, Li Y, Boggia J, Kikuya M, Björklund-Bodegård K, Ohkubo T, Jeppesen J, Torp-Pedersen C, et al. ; International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes Investigators. Significance of white-coat hypertension in older persons with isolated systolic hypertension: a meta-analysis using the International Database on Ambulatory Blood Pressure Monitoring in Relation to Cardiovascular Outcomes population. Hypertension. 2012;59:564–571. doi: 10.1161/HYPERTENSIONAHA.111.180653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Franklin SS, Thijs L, Asayama K, Li Y, Hansen TW, Boggia J, Jacobs L, Zhang Z, Kikuya M, Björklund-Bodegård K, et al. ; IDACO Investigators. The cardiovascular risk of white-coat hypertension. J Am Coll Cardiol. 2016;68:2033–2043. doi: 10.1016/j.jacc.2016.08.035 [DOI] [PubMed] [Google Scholar]
- 45.Conen D, Aeschbacher S, Thijs L, Li Y, Boggia J, Asayama K, Hansen TW, Kikuya M, Björklund-Bodegård K, Ohkubo T, et al. Age-specific differences between conventional and ambulatory daytime blood pressure values. Hypertension. 2014;64:1073–1079. doi: 10.1161/HYPERTENSIONAHA.114.03957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Asayama K, Thijs L, Li Y, Gu YM, Hara A, Liu YP, Zhang Z, Wei FF, Lujambio I, Mena LJ, et al. ; International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes (IDACO) Investigators. Setting thresholds to varying blood pressure monitoring intervals differentially affects risk estimates associated with white-coat and masked hypertension in the population. Hypertension. 2014;64:935–942. doi: 10.1161/HYPERTENSIONAHA.114.03614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pierdomenico SD, Cuccurullo F. Prognostic value of white-coat and masked hypertension diagnosed by ambulatory monitoring in initially untreated subjects: an updated meta analysis. Am J Hypertens. 2011;24:52–58. doi: 10.1038/ajh.2010.203 [DOI] [PubMed] [Google Scholar]
- 48.Mancia G, Grassi G. The heterogeneous nature of white-coat hypertension. J Am Coll Cardiol. 2016;68:2044–2046. doi: 10.1016/j.jacc.2016.08.043 [DOI] [PubMed] [Google Scholar]
- 49.Mancia G, Facchetti R, Bombelli M, Grassi G, Sega R. Long-term risk of mortality associated with selective and combined elevation in office, home, and ambulatory blood pressure. Hypertension. 2006;47:846–853. doi: 10.1161/01.HYP.0000215363.69793.bb [DOI] [PubMed] [Google Scholar]
- 50.Cohen JB, Lotito MJ, Trivedi UK, Denker MG, Cohen DL, Townsend RR. Cardiovascular events and mortality in white coat hypertension: a systematic review and meta-analysis. Ann Intern Med. 2019;170:853–862. doi: 10.7326/M19-0223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Asayama K, Li Y, Franklin SS, Thijs L, O’Brien E, Staessen JA. Cardiovascular risk associated with white-coat hypertension: con side of the argument. Hypertension. 2017;70:676–682. doi: 10.1161/HYPERTENSIONAHA.117.08902 [DOI] [PubMed] [Google Scholar]
- 52.Brguljan-Hitij J, Thijs L, Li Y, Hansen TW, Boggia J, Liu YP, Asayama K, Wei FF, Bjorklund-Bodegard K, Gu YM, et al. ; International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcome Investigators. Risk stratification by ambulatory blood pressure monitoring across JNC classes of conventional blood pressure. Am J Hypertens. 2014;27:956–965. doi: 10.1093/ajh/hpu002 [DOI] [PubMed] [Google Scholar]
- 53.Fagard RH, Stolarz K, Kuznetsova T, Seidlerova J, Tikhonoff V, Grodzicki T, Nikitin Y, Filipovsky J, Peleska J, Casiglia E, et al. Sympathetic activity, assessed by power spectral analysis of heart rate variability, in white-coat, masked and sustained hypertension versus true normotension. J Hypertens. 2007;25:2280–2285. doi: 10.1097/HJH.0b013e3282efc1fe [DOI] [PubMed] [Google Scholar]
- 54.Wei FF, Li Y, Zhang L, Shan XL, Cheng YB, Wang JG, Yang CH, Staessen JA. Persistence of masked hypertension in chinese patients. Am J Hypertens. 2016;29:326–331. doi: 10.1093/ajh/hpv106 [DOI] [PubMed] [Google Scholar]
- 55.Franklin SS, Thijs L, Li Y, Hansen TW, Boggia J, Liu Y, Asayama K, Björklund-Bodegård K, Ohkubo T, Jeppesen J, et al. ; International Database on Ambulatory blood pressure in Relation to Cardiovascular Outcomes Investigators. Masked hypertension in diabetes mellitus: treatment implications for clinical practice. Hypertension. 2013;61:964–971. doi: 10.1161/HYPERTENSIONAHA.111.00289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krakoff LR, Eison H, Phillips RH, Leiman SJ, Lev S. Effect of ambulatory blood pressure monitoring on the diagnosis and cost of treatment for mild hypertension. Am Heart J. 1988;116:1152–1154. doi: 10.1016/0002-8703(88)90180-9 [DOI] [PubMed] [Google Scholar]
- 57.Krakoff LR. Cost-effectiveness of ambulatory blood pressure: a reanalysis. Hypertension. 2006;47:29–34. doi: 10.1161/01.HYP.0000197195.84725.66 [DOI] [PubMed] [Google Scholar]
- 58.Lovibond K, Jowett S, Barton P, Caulfield M, Heneghan C, Hobbs FD, Hodgkinson J, Mant J, Martin U, Williams B, et al. Cost-effectiveness of options for the diagnosis of high blood pressure in primary care: a modelling study. Lancet. 2011;378:1219–1230. doi: 10.1016/S0140-6736(11)61184-7 [DOI] [PubMed] [Google Scholar]
- 59.Beyhaghi H, Viera AJ. Comparative cost-effectiveness of clinic, home, or ambulatory blood pressure measurement for hypertension diagnosis in US Adults. Hypertension. 2019;73:121–131. doi: 10.1161/HYPERTENSIONAHA.118.11715 [DOI] [PubMed] [Google Scholar]
- 60.Staessen JA, Li Y, Hara A, Asayama K, Dolan E, O’Brien E. Blood Pressure Measurement anno 2016. Am J Hypertens. 2017;30:453–463. doi: 10.1093/ajh/hpw148 [DOI] [PubMed] [Google Scholar]
- 61.Pickering T. Recommendations for the use of home (self) and ambulatory blood pressure monitoring. American society of hypertension ad hoc panel. Am J Hypertens. 1996;9:1–11. doi: 10.1016/0895-7061(95)00341-x [DOI] [PubMed] [Google Scholar]
- 62.Roerecke M, Kaczorowski J, Myers MG. Comparing automated office blood pressure readings with other methods of blood pressure measurement for identifying patients with possible hypertension: a systematic review and meta-analysis. JAMA Intern Med. 2019;179:351–362. doi: 10.1001/jamainternmed.2018.6551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Staessen JA, Wang J, Bianchi G, Birkenhäger WH. Essential hypertension. Lancet. 2003;361:1629–1641. doi: 10.1016/S0140-6736(03)13302-8 [DOI] [PubMed] [Google Scholar]
- 64.McManus RJ, Mant J, Haque MS, Bray EP, Bryan S, Greenfield SM, Jones MI, Jowett S, Little P, Penaloza C, et al. Effect of self-monitoring and medication self-titration on systolic blood pressure in hypertensive patients at high risk of cardiovascular disease: the TASMIN-SR randomized clinical trial. JAMA. 2014;312:799–808. doi: 10.1001/jama.2014.10057 [DOI] [PubMed] [Google Scholar]
- 65.Asayama K, Ohkubo T, Metoki H, Obara T, Inoue R, Kikuya M, Thijs L, Staessen JA, Imai Y; Hypertension Objective Treatment Based on Measurement by Electrical Devices of Blood Pressure (HOMED-BP). Cardiovascular outcomes in the first trial of antihypertensive therapy guided by self-measured home blood pressure. Hypertens Res. 2012;35:1102–1110. doi: 10.1038/hr.2012.12522895063 [Google Scholar]
- 66.Li Y, Thijs L, Hansen TW, Kikuya M, Boggia J, Richart T, Metoki H, Ohkubo T, Torp-Pedersen C, Kuznetsova T, et al. ; International Database on Ambulatory Blood Pressure Monitoring in Relation to Cardiovascular Outcomes Investigators. Prognostic value of the morning blood pressure surge in 5645 subjects from 8 populations. Hypertension. 2010;55:1040–1048. doi: 10.1161/HYPERTENSIONAHA.109.137273 [DOI] [PubMed] [Google Scholar]
- 67.Gu YM, Thijs L, Li Y, Asayama K, Boggia J, Hansen TW, Liu YP, Ohkubo T, Björklund-Bodegård K, Jeppesen J, et al. ; International Database on Ambulatory blood pressure in relation to Cardiovascular Outcomes (IDACO) Investigators. Outcome-driven thresholds for ambulatory pulse pressure in 9938 participants recruited from 11 populations. Hypertension. 2014;63:229–237. doi: 10.1161/HYPERTENSIONAHA.113.02179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schutte R, Thijs L, Asayama K, Boggia J, Li Y, Hansen TW, Liu YP, Kikuya M, Björklund-Bodegård K, Ohkubo T, et al. ; on behalf of the International Database on Ambulatory blood pressure in relation to Cardiovascular Outcomes (IDACO) investigators. Double product reflects the predictive power of systolic pressure in the general population: evidence from 9937 participants. Hypertension. 2013;26:665–672. doi: 10.1093/ajh/hps119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schutte R, Thijs L, Liu YP, Asayama K, Jin Y, Odili A, Gu YM, Kuznetsova T, Jacobs L, Staessen JA. Within-subject blood pressure level–not variability–predicts fatal and nonfatal outcomes in a general population. Hypertension. 2012;60:1138–1147. doi: 10.1161/HYPERTENSIONAHA.112.202143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Asayama K, Wei FF, Hara A, Hansen TW, Li Y, Staessen JA. Prognosis in relation to blood pressure variability: con side of the argument. Hypertension. 2015;65:1170–1179. doi: 10.1161/HYPERTENSIONAHA.115.04808 [DOI] [PubMed] [Google Scholar]
- 71.Hung K, Zhang YT, Tai B. Wearable medical devices for tele-home healthcare. Conf Proc IEEE Eng Med Biol Soc. 2004;2004:5384–5387. doi: 10.1109/IEMBS.2004.1404503 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


