Supplemental Digital Content is available in the text.
Keywords: blood pressure, cardiovascular diseases, hypertension, primary prevention, risk assessment
Abstract
Compared with brachial blood pressure (BP), central systolic BP (SBP) can provide a better indication of the hemodynamic strain inflicted on target organs, but it is unclear whether this translates into improved cardiovascular risk stratification. We aimed to assess which of central or brachial BP best predicts cardiovascular risk and to identify the central SBP threshold associated with increased risk of future cardiovascular events. This study included 13 461 participants of CARTaGENE with available central BP and follow-up data from administrative databases but without cardiovascular disease or antihypertensive medication. Central BP was estimated by radial artery tonometry, calibrated for brachial SBP and diastolic BP (type I), and a generalized transfer function (SphygmoCor). The outcome was major adverse cardiovascular events. Cox proportional-hazards models, differences in areas under the curves, net reclassification indices, and integrated discrimination indices were calculated. Youden index was used to identify SBP thresholds. Over a median follow-up of 8.75 years, 1327 major adverse cardiovascular events occurred. The differences in areas under the curves, net reclassification indices, and integrated discrimination indices were of 0.2% ([95% CI, 0.1–0.3] P<0.01), 0.11 ([95% CI, 0.03–0.20] P=0.01), and 0.0004 ([95% CI, −0.0001 to 0.0014] P=0.3), all likely not clinically significant. Central and brachial SBPs of 112 mm Hg (95% CI, 111.2–114.1) and 121 mm Hg (95% CI, 120.2–121.9) were identified as optimal BP thresholds. In conclusion, central BP measured with a type I device is statistically but likely not clinically superior to brachial BP in a general population without prior cardiovascular disease. Based on the risk of major adverse cardiovascular events, the optimal type I central SBP appears to be 112 mm Hg.
Central blood pressure (BP) can be considered to be more representative of the hemodynamic strain inflicted on target organs than brachial BP given the proximity of the aorta with target organs.1 Multiple studies have identified that central BP parameters have a stronger association with cardiovascular disease when compared with brachial BP parameters, mostly in high-risk cohorts.2–6 The role of central BP as a predictor of cardiovascular events independent of brachial BP was previously studied using the Framingham cohort and the International Database of Central Arterial Properties for Risk Stratification database, and both found that central BP was not related to cardiovascular events, although using models that included brachial BP, raising concerns about collinearity.7–9 It was nonetheless suggested that a larger study may have the power to identify a difference between central BP and brachial BP. Thus, it remains unclear whether central BP is a better predictor of cardiovascular disease than brachial BP and if it is, whether the improvement in risk prediction is significant enough to merit its routine assessment for the general population.
In addition, there is clear evidence that central and brachial BP do not always correlate, and some investigators have found that central BP may be useful in guiding hypertensive treatment.10,11 As noninvasive devices measuring central BP become routinely available, the use of central BP to assess cardiovascular risk is limited by the absence of threshold values to guide clinicians. To our knowledge, 2 studies have attempted to define central hypertension and have obtained varying results.3,12
When central BP is measured noninvasively, calibration with the brachial systolic and diastolic pressures (type I calibration) or the mean and diastolic pressures (type II calibration) is necessary. Studies have shown the types of calibration may be associated with significant differences in reported BP values.13,14 Given these differences, special attention should be given to the type of calibration used when comparing central and brachial BP.15
This large population-based cohort exempt of cardiovascular disease and without antihypertensive medication is a unique opportunity to study the potential role of central BP in primary prevention of cardiovascular disease. The first goal of this study was to assess the predictive power of central systolic BP (SBP) compared with brachial SBP in cardiovascular risk prediction. The second goal of this study was to define a central SBP threshold, specific to a type I device and based on the future risk of cardiovascular outcomes.
Methods
Study Population
The CARTaGENE database (https://www.cartagene.qc.ca) is a population-based survey of 19 996 randomly selected individuals between the ages of 40 and 69 years residing in major urban regions of the province of Quebec (Canada). The purpose of this database is to facilitate the study of chronic diseases and their determinants. Details regarding the selection process and data acquisition are available in the Data Supplement.16–19 The data that support the findings of this study are available from the corresponding author upon reasonable request. Health and nutrition questionnaires, medication lists, as well as physical measurements were collected for each participant with the assistance of a nurse to ensure maximal accuracy. Blood and urine samples were collected. Prior cardiovascular disease was determined by participant self-reporting a history of myocardial infarct, angina, stroke, transient ischemic attack, or heart failure. For the purpose of this study, participants taking any antihypertensive medication, with prior cardiovascular disease, missing brachial or central BP readings, or missing follow-up data were excluded. Consent was obtained from all participants. This study adhered to the Declaration of Helsinki and was approved by the local Ethics Review Board.
BP Measurements
All BP measurements were acquired at the enrollment visit. Brachial BP was measured in a seated position, after 10 minutes of rest, in a quiet room with the automated BP monitor Omron 907 L (Omron, Lake Forest, IL). Three measures were acquired at intervals of 2 minutes and averaged. Immediately afterward, central BP was measured by radial applanation tonometry with the SphygmoCor Px device (AtCor Medical, Lisle, IL). Two measurements were taken and averaged. The central SBP and diastolic BP were derived from pulse wave analysis with a generalized transfer function, calibrated to the brachial SBP and diastolic BP (type I calibration).15
Outcomes
Major adverse cardiovascular events (MACE) were defined as a composite of a first occurrence of ischemic or hemorrhagic stroke, fatal or nonfatal myocardial infarction, heart failure requiring hospitalization, and death attributable to cardiovascular disease.20 The incidence of MACE was determined using prospective data obtained from a governmental administrative database—the Régie de l’assurance maladie du Québec—and was available from enrollment (August 2009 to October 2010) to March 31, 2019.21 This database has previously been validated in prospective studies.22 The Régie de l’assurance maladie du Québec administrative database compiles diagnostic codes and procedure codes for both clinic and hospital visits and causes of death using the International Classification of Diseases, Tenth Revision classification—a classification that has also previously been validated.23
Statistical Analyses
Analyses were performed using IBM SPSS Statistics software, version 25 (IBM Corp, Armonk, NY), and R, version 3.6.1. All P values <0.05 were considered significant. Data with normal distribution are presented as mean±SD and compared with Student t tests, whereas non-normally distributed data are expressed as median with interquartile range. Categorical data were compared with Pearson χ2 tests. Multiple imputations to account for missing data was performed with the R package Amelia. Ten copies of the filled-in data set were obtained. Each dataset was analyzed separately, and results were pooled together with the Rubin rule.24 All figures were made with the R package ggplot2.25
Cox Proportional-Hazards Models
Cox proportional-hazards models were constructed (R package RMS, v5.1-4) with the following covariates: age, sex, body mass index, smoking status, diabetes, total cholesterol, HDL cholesterol, estimated glomerular filtration rate, heart rate, statin, and aspirin use. The central SBP model included these variables and central SBP while the brachial SBP model included these variables and brachial SBP. The same analyses were performed for central and brachial pulse pressure and for each component of MACE. Restricted cubic splines to account for nonlinear associations for body mass index and estimated glomerular filtration rate, determined by plotting Martingale residuals for all variables, were performed using 3 knots. The selection of covariates and number of knots was based on the P of the coefficients in the Cox regression models (P≤0.20 considered noteworthy) and the Akaike Information Criteria. Schoenfeld residuals were used to verify the proportional hazards assumption. Calibration of the Cox models was verified.26 Hazard ratios with 95% CIs were calculated per SD for central SBP and brachial SBP.
Discrimination and Reclassification
The presence of collinearity between brachial and central SBP was calculated using the variance inflation factor. A variance inflation factor >10 is considered significant.9 As the inclusion of brachial and central SBP in the same model is not advisable given their presumed high level of collinearity, non-nested models were used to compare central and brachial SBP.27,28 Net reclassification indices (NRI) and integrated discrimination indices for the event group, the nonevent group, and the total cohort were calculated comparing the brachial SBP model to the central SBP model with a 95% CI obtained with bootstrapping (R package NRIcens, v1.6, and R package SurvIDINRI, v1.1-1). Receiver operating characteristic curves for the central SBP and brachial SBP models were also constructed, and the differences in areas under the curves (AUCs) were calculated taking into account the survival data (R package riskRegression). The same analyses were performed for central and brachial pulse pressure and for each component of MACE.
Outcome-Derived SBP Thresholds
Receiver operating characteristic curves for brachial and central SBP were constructed. Youden index aims to identify the BP with the maximal difference between true-positive and false-positive rates with the following formula: sensitivity−[1−specificity] (R package survivalROC).29 Youden index was calculated for each BP between 80 and 160 mm Hg and plotted. The BP corresponding to the Youden index is the optimal threshold.29,30 Bootstrapping was performed to obtain the CI of the Youden index (R package tdROC).31 The Contal and O’Quigly method using log-rank tests was also performed to identify the optimal threshold32 (R package Evaluate Cutpoints33).
Results
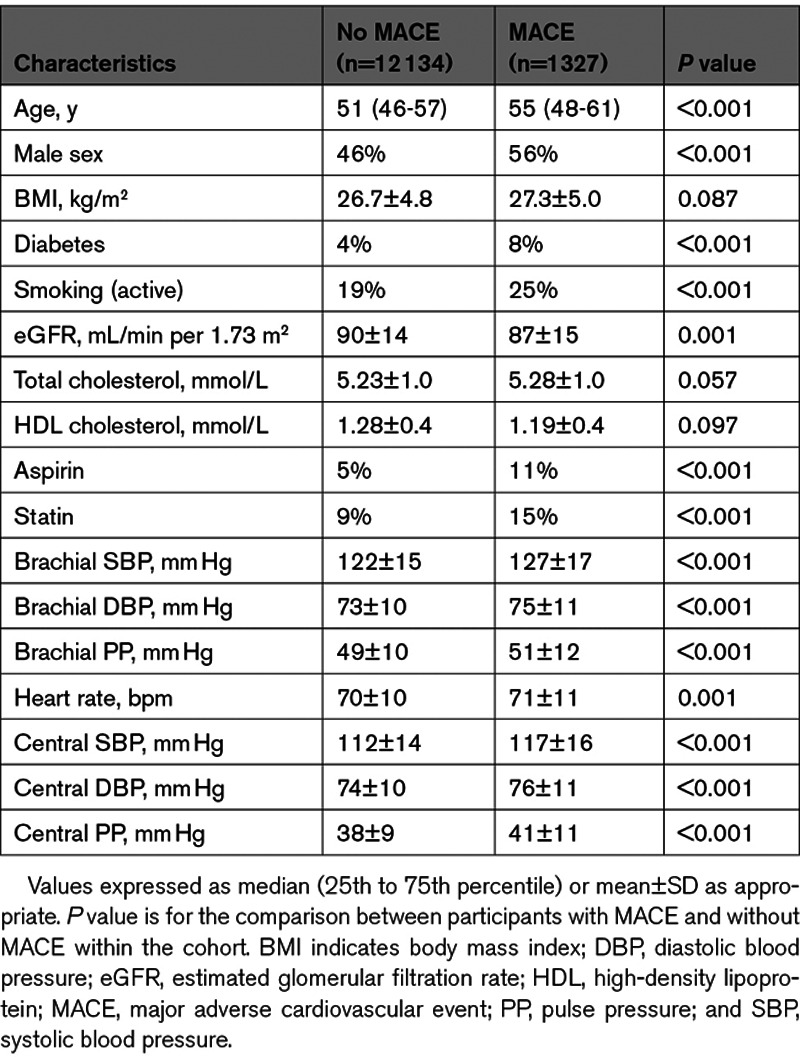
Of the 19 996 CARTaGENE participants, 13 461 were included in the study. Of the participants excluded from the study, 2021 had no pulse wave analysis available, 9 were lost to follow-up, and 4505 had known cardiovascular disease or active antihypertensive medication. Baseline participant characteristics according to the incidence of MACE are available in Table 1. There were 1327 MACEs during a median follow-up of 8.8 years (interquartile range, 8.6–9.0). The MACE events comprised 32 cardiovascular deaths, 705 myocardial infarctions, 357 episodes of heart failure, and 233 strokes. There were significant differences in BP between participants with and without incidence of MACE, for both central SBP (117±16 versus 112±14 mm Hg; P<0.001) and brachial SBP (127±17 versus 122±15 mm Hg; P<0.001). Most baseline parameters were also statistically different between groups (Table 1). Given a variance inflation factor of 12 and an R value of 0.96 for central and brachial SBP, indicating high collinearity, all further analyses were performed by comparing central and brachial SBP in separate models, avoiding the problems associated with the inclusion of both central and brachial SBP in the same model.9
Table 1.
Demographic and Clinical Characteristics of the Cohort
Predictive Value of Central SBP Compared With Brachial SBP
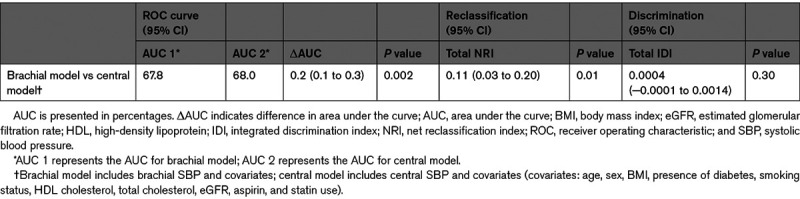
The Cox proportional-hazards models showed that both central and brachial SBP are significantly associated with cardiovascular events (Figure 1). The hazard ratio was of 1.16 (95% CI, 1.10–1.22) for central SBP and 1.15 (95% CI, 1.09–1.22) for brachial SBP for a SD increment (Table 2). Akaike Information Criteria for the central and brachial models were comparable.34 The central SBP model yielded a marginally higher AUC than the brachial SBP model—a difference that was statistically significant; AUCs were 68.0% and 67.8%, respectively, with a ΔAUC of 0.2% (95% CI, 0.1–0.3) and a P of 0.002. The total NRI was of 0.11 (95% CI, 0.03–0.20) with a P of 0.01—a statistically significant difference favoring the central SBP model (Table 3). The integrated discrimination indices of 0.0004 ([95% CI, −0.0001 to 0.0014] P=0.3) did not support any significant difference (Table S1 in the Data Supplement). Comparison of central and brachial pulse pressure yielded similar results (Tables S2 through S4). Subgroup analyses for each MACE component are available in Tables S5 and S6.
Figure 1.
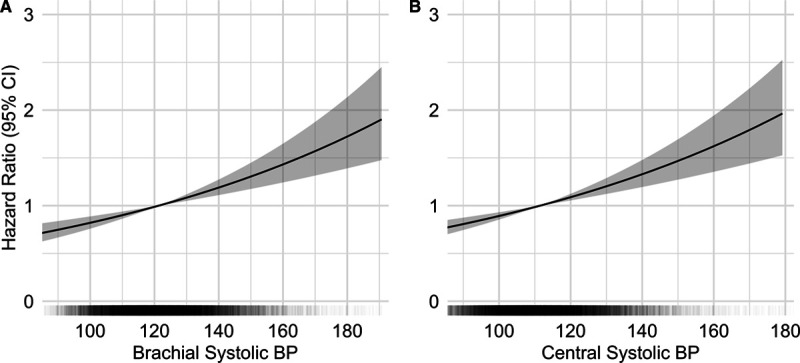
Risk of major adverse cardiovascular events according to brachial and central systolic blood pressure. Multivariate Cox regression analyses of blood pressure (BP) parameters and major adverse cardiovascular events (A) for brachial systolic BP (SBP) and (B) for central SBP. In black: distribution of BP values within the cohort. In gray: 95% CIs. Adjusted for age, sex, body mass index, presence of diabetes, smoking status, HDL (high-density lipoprotein) cholesterol, total cholesterol, estimated glomerular filtration rate, aspirin, and statin use.
Table 2.
Multivariate Cox Regression Analyses for the Association of BP Parameters and Incidence of Major Adverse Cardiovascular Event
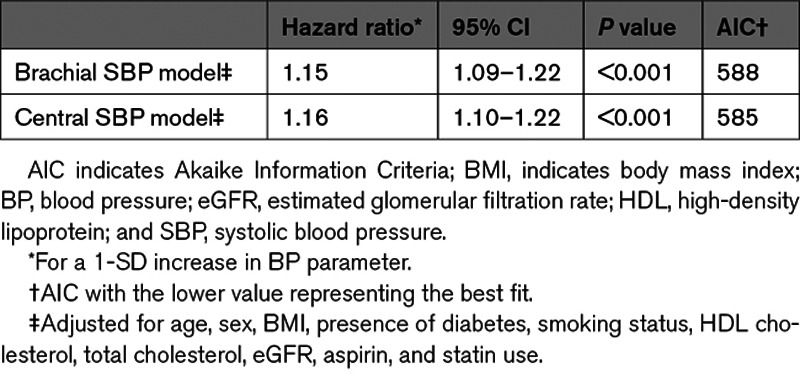
Table 3.
Comparison of the Brachial SBP and Central SBP Models as Predictors of Major Adverse Cardiovascular Event According to the ΔAUC, NRI, and IDI
Outcome-Derived SBP Thresholds
The Youden indices calculated to identify the optimal central and brachial SBP thresholds were the highest at 112 mm Hg (95% CI, 111.2–114.1 mm Hg) and 121 mm Hg ([95% CI, 120.2–121.9 mm Hg] Figure 2). When calculated using the log-rank approach, similar results were obtained (114 and 121 mm Hg for central and brachial SBP; P<0.001).
Figure 2.
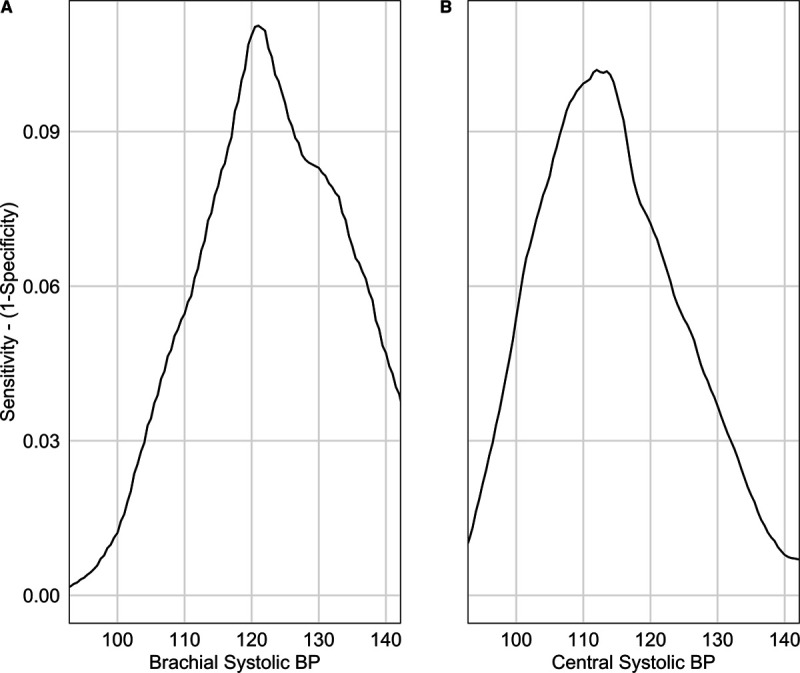
Optimal brachial and central systolic blood pressure thresholds. Youden index according to (A) brachial systolic blood pressure (BP) and (B) central systolic BP. Youden index is the maximal value obtained, a brachial systolic BP threshold of 121 mm Hg (95% CI, 120.2–121.9 mm Hg) and a central systolic BP threshold of 112 mm Hg (95% CI, 111.2–114.1 mm Hg).
Discussion
This large epidemiological study with randomly selected individuals from the general population shows that central SBP and brachial SBP are clinically similar as predictors of cardiovascular risk in a primary prevention cohort, when central SBP is assessed with a type I device such as the SphygmoCor. Both NRI and AUC yield statistically significant differences favoring central SBP but differences that are too small to warrant its routine use in the general population (ΔAUC of 0.2% and NRI of 0.11). Indeed, it has been proposed that NRI values below 0.20 are considered too small to confer a clinically significant difference.35 In this cohort of subjects without prior cardiovascular disease and antihypertensive drugs, the central SBP threshold with maximal effectiveness to detect future cardiovascular events was 112 mm Hg, while the brachial SBP threshold calculated with the same method was of 121 mm Hg.
Since hypertension is the most important modifiable risk factor for the development of cardiovascular disease, proper assessment of BP is crucial.36 However, brachial cuff BP has its inaccuracies and inherent conceptual limitations.37 Central BP has been suggested to be superior to brachial BP for its association with end-organ damage and cardiovascular events in several studies.2–6,12,38–40 In contrast, other studies failed to show the added value of central BP for predicting cardiovascular events above and beyond the brachial BP.7,8,40,41 This study is a unique opportunity to clarify the role of central SBP versus brachial SBP in cardiovascular risk prediction. In contrast with other studies, this study’s cohort is similar to the general population, did not select individuals with a high burden of cardiovascular risk factors,4,6–8,12,41,42 did not include individuals taking antihypertensive medications,2,4,7,8,12,43 and structured the statistical analysis taking into the account the high correlation between central and brachial SBP, avoiding potential errors related to collinearity.7–9
Brachial thresholds defining hypertension have been put into question given recent evidence. A large meta-analysis demonstrated the benefits of lowering BP down to an SBP of 110 mmHg,44 and the Systolic Blood Pressure Intervention trial has shown benefits of targeting an SBP below 120 mm Hg.20 This study thus aimed to establish a central SBP threshold independent of brachial SBP. The central SBP threshold of 112 mm Hg is close to the cutoff between normal and elevated BP according to previous studies.45 Similarly, the brachial threshold of 121 mm Hg is similar to the threshold between the normal (<120 mm Hg) and elevated BP (120–129 mm Hg) categories endorsed by the American Heart Association, among others.46,47
Previous studies have identified 130 and 123 mm Hg as central SBP thresholds, which are much higher than the threshold identified in the present study.3,12 Of note, in the first study, suggesting a central hypertension threshold of 130 mm Hg, the calibration method differed between the derivation cohort (type II calibration on carotid pressure wave) and the validation cohort (type I calibration on radial pressure wave). Therefore, the inherent 10- to 15-mm Hg difference between central SBP estimated with type I versus type II device may explain the difference between the present study and the threshold proposed by Cheng et al.48 Furthermore, this 130-mm Hg threshold was determined by corresponding to the cardiovascular mortality of individuals with a brachial SBP above 140/90 mm Hg, which is now considered far above the brachial BP threshold where the cardiovascular risk significantly increases and where treatment is warranted.46 In the study by Eguchi et al,12 the 123-mm Hg threshold was identified among treated hypertensive individuals, with antihypertensive agents known to have an inconsistent effect on central SBP,43 and using the Omron HEM-9000AI device, which overestimates central SBP compared with the SphygmoCor device.49 This supports the need for a study aiming to identify a central SBP threshold independent of the brachial SBP threshold and in a cohort without prior cardiovascular disease or antihypertensive agents. The present study overcomes these limitations and defines type I central hypertension when assessed with the SphygmoCor device independently of brachial hypertension while avoiding the effects of antihypertensive drugs on BP levels and outcomes. Furthermore, it supports the proposal that a device-specific threshold should be used.
Strengths and Limitations
To our knowledge, this is by far the largest study attempting to identify a difference between central and brachial SBP in the prediction of cardiovascular events. The strengths of this study also include the high number of events while using the most fitting definition of MACE for the objectives of this study.50 The similarity of the cohort in this study with the general population and exclusion of subjects with antihypertensive medication enhances the external validation of its findings.16
Some limitations of this study need to be addressed. As aforementioned, these results do not exclude that central BP may be clearly superior in risk prediction when assessed with other methods of calibration or with other devices. Type II calibration is superior to type I calibration as it gives a more accurate estimation of intraaortic BP, is not as correlated to brachial BP, and is more closely associated with end-organ damage.13,14,48,51 Yet, many major studies on central SBP used devices with type I calibration such as the SphygmoCor,2,10,11,39 and as type I calibration has been used in most of the literature on central BP and is still routinely used, a better understanding of central SBP assessed with type I calibration and its relationship to future cardiovascular events remains relevant. Differences between various devices may also warrant device-specific studies.14,52,53 Devices should follow a standardized approach to estimate the aortic BP and should be able to provide the ability to conserve raw data for future development of novel algorithms to obviate the need for device-specific reference range. Prior cardiovascular disease was self-reported by the participants, which may lead to an information bias, although all individuals taking any antihypertensive drug—a mainstay in the secondary prevention of cardiovascular disease—were excluded. The database included individuals of 40 to 69 years of age, limiting generalizability to this age group.
Perspectives
Central SBP estimated with a type I device statistically improved cardiovascular risk prediction when compared with brachial SBP. However, the increment was marginal and likely not clinically significant. Future studies are required to examine the predictive value of central SBP estimated by other means; notably with the reputed more accurate type II devices or without using a generalized transfer function. When using a type I device such as the SphygmoCor, a central SBP of 112 mm Hg appears to be the optimal SBP cutoff above which the incidence of cardiovascular events begins to significantly increase in this cohort representative of the general population, consequently defining type I central hypertension. Whether targeting a type I central SBP of 112 mm Hg is feasible and beneficial remains to be determined and will need to be weighed against the cost and morbidity associated with the use of antihypertensive drugs.
Acknowledgments
We would like to acknowledge Miguel Chagnon, MSc, PStat, for his guidance with the statistical analyses. R. Goupil holds a research scholarship from Fonds de recherche du Québec–Santé and is a recipient of the Société québécoise d’hypertension artérielle–Bourse Jacques-de-Champlain scholarship. Results from this study were presented, in part, at Kidney Week 2019—the annual meeting of the American Society of Nephrology.
Sources of Funding
None.
Disclosures
None.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AUC
- area under the curve
- BP
- blood pressure
- MACE
- major adverse cardiovascular events
- NRI
- net reclassification index
- SBP
- systolic blood pressure
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.120.16163.
For Sources of Funding and Disclosures, see page 325.
Contributor Information
Florence Lamarche, Email: florence.lamarche2@mail.mcgill.ca.
Mohsen Agharazii, Email: mohsen.agharazii@crchudequebec.ulaval.ca.
François Madore, Email: f.madore@umontreal.ca.
Novelty and Significance
What Is New?
This is the largest study to date examining the incremental value of central systolic blood pressure (SBP) in cardiovascular risk prediction when compared with brachial SBP in the general population without prior cardiovascular disease and antihypertensive drugs.
In this large, healthy population cohort, central SBP estimated with a type I device yields a marginal improvement in cardiovascular risk prediction, when compared with brachial SBP, which is statistically but likely not clinically significant.
A type I central SBP with the maximal potential effectiveness to detect future cardiovascular events was identified independently of the current brachial SBP threshold that defines hypertension. This optimal type I central hypertension threshold was determined to be 112 mm Hg.
What Is Relevant?
Central blood pressure is thought to better reflect hemodynamic strain inflicted on target organs than brachial blood pressure.
Our study puts into perspective the additive value of central SBP in predicting future cardiovascular events, and it defined the critical threshold for central SBP based on future events when using a type I device such as the SphygmoCor.
These findings have a significant impact in the long battle for optimizing blood pressure control—a major modifiable cardiovascular risk factor.
Summary
These findings support that central SBP is highly predictive of cardiovascular disease when estimated with a type I device (SphygmoCor) but does not confer clinically significant improvement compared with brachial SBP. This study will help guide clinicians on the utility of type I central SBP and on the interpretation of SBP values in relation to cardiovascular risk in the general population.
References
- 1.McEniery CM, Cockcroft JR, Roman MJ, Franklin SS, Wilkinson IB. Central blood pressure: current evidence and clinical importance. Eur Heart J. 2014;35:1719–1725. doi: 10.1093/eurheartj/eht565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, Hughes AD, Thurston H, O’Rourke M; CAFE Investigators; Anglo-Scandinavian Cardiac Outcomes Trial Investigators; CAFE Steering Committee and Writing Committee. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113:1213–1225. doi: 10.1161/CIRCULATIONAHA.105.595496 [DOI] [PubMed] [Google Scholar]
- 3.Cheng HM, Chuang SY, Sung SH, Yu WC, Pearson A, Lakatta EG, Pan WH, Chen CH. Derivation and validation of diagnostic thresholds for central blood pressure measurements based on long-term cardiovascular risks. J Am Coll Cardiol. 2013;62:1780–1787. doi: 10.1016/j.jacc.2013.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roman MJ, Devereux RB, Kizer JR, Lee ET, Galloway JM, Ali T, Umans JG, Howard BV. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension. 2007;50:197–203 [DOI] [PubMed] [Google Scholar]
- 5.Dong Y, Jiang L, Wang X, Chen Z, Zhang L, Zhang Z, Zheng C, Kang Y, Wang Z, Cao H, et al. Central rather than brachial pressures are stronger predictors of cardiovascular outcomes: a longitudinal prospective study in a Chinese population. J Clin Hypertens (Greenwich). 2020;22:623–630. doi: 10.1111/jch.13838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pini R, Cavallini MC, Palmieri V, Marchionni N, Di Bari M, Devereux RB, Masotti G, Roman MJ. Central but not brachial blood pressure predicts cardiovascular events in an unselected geriatric population: the ICARe Dicomano Study. J Am Coll Cardiol. 2008;51:2432–2439. doi: 10.1016/j.jacc.2008.03.031 [DOI] [PubMed] [Google Scholar]
- 7.Mitchell GF, Hwang SJ, Larson MG, Hamburg NM, Benjamin EJ, Vasan RS, Levy D, Vita JA. Transfer function-derived central pressure and cardiovascular disease events: the Framingham Heart Study. J Hypertens. 2016;34:1528–1534. doi: 10.1097/HJH.0000000000000968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang QF, Aparicio LS, Thijs L, Wei FF, Melgarejo JD, Cheng YB, Sheng CS, Yang WY, Gilis-Malinowska N, Boggia J, et al. ; IDCARS (International Database of Central Arterial Properties for Risk Stratification) Investigators. Cardiovascular end points and mortality are not closer associated with central than peripheral pulsatile blood pressure components. Hypertension. 2020;76:350–358. doi: 10.1161/HYPERTENSIONAHA.120.14787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dormann CF, Elith J, Bacher S, et al. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography. 2013;36:27–46 [Google Scholar]
- 10.McEniery CM, Yasmin , McDonnell B, Munnery M, Wallace SM, Rowe CV, Cockcroft JR, Wilkinson IB; Anglo-Cardiff Collaborative Trial Investigators. Central pressure: variability and impact of cardiovascular risk factors: the Anglo-Cardiff Collaborative Trial II. Hypertension. 2008;51:1476–1482. doi: 10.1161/HYPERTENSIONAHA.107.105445 [DOI] [PubMed] [Google Scholar]
- 11.Sharman JE, Marwick TH, Gilroy D, Otahal P, Abhayaratna WP, Stowasser M; Value of Central Blood Pressure for Guiding Management of Hypertension Study Investigators. Randomized trial of guiding hypertension management using central aortic blood pressure compared with best-practice care: principal findings of the BP GUIDE study. Hypertension. 2013;62:1138–1145. doi: 10.1161/HYPERTENSIONAHA.113.02001 [DOI] [PubMed] [Google Scholar]
- 12.Eguchi K, Miyashita H, Takenaka T, Tabara Y, Tomiyama H, Dohi Y, Hashimoto J, Ohkubo T, Ohta Y, Hirooka Y, et al. ; ABC-J II Investigator Group. High central blood pressure is associated with incident cardiovascular events in treated hypertensives: the ABC-J II Study. Hypertens Res. 2018;41:947–956. doi: 10.1038/s41440-018-0075-8 [DOI] [PubMed] [Google Scholar]
- 13.Picone DS, Schultz MG, Peng X, Black JA, Dwyer N, Roberts-Thomson P, Qasem A, Sharman JE. Intra-arterial analysis of the best calibration methods to estimate aortic blood pressure. J Hypertens. 2019;37:307–315. doi: 10.1097/HJH.0000000000001902 [DOI] [PubMed] [Google Scholar]
- 14.Papaioannou TG, Karageorgopoulou TD, Sergentanis TN, Protogerou AD, Psaltopoulou T, Sharman JE, Weber T, Blacher J, Daskalopoulou SS, Wassertheurer S, et al. Accuracy of commercial devices and methods for noninvasive estimation of aortic systolic blood pressure a systematic review and meta-analysis of invasive validation studies. J Hypertens. 2016;34:1237–1248. doi: 10.1097/HJH.0000000000000921 [DOI] [PubMed] [Google Scholar]
- 15.Sharman JE, Avolio AP, Baulmann J, Benetos A, Blacher J, Blizzard CL, Boutouyrie P, Chen CH, Chowienczyk P, Cockcroft JR, et al. Validation of non-invasive central blood pressure devices: ARTERY Society task force consensus statement on protocol standardization. Eur Heart J. 2017;38:2805–2812. doi: 10.1093/eurheartj/ehw632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Awadalla P, Boileau C, Payette Y, Idaghdour Y, Goulet JP, Knoppers B, Hamet P, Laberge C; CARTaGENE Project. Cohort profile of the CARTaGENE study: Quebec’s population-based biobank for public health and personalized genomics. Int J Epidemiol. 2013;42:1285–1299. doi: 10.1093/ije/dys160 [DOI] [PubMed] [Google Scholar]
- 17.Lamarche F, Agharazii M, Nadeau-Fredette A-C, Madore F, Goupil R. Central and brachial blood pressures, statins, and low-density lipoprotein cholesterol. Hypertension. 2018;71:415–421. doi: 10.1161/HYPERTENSIONAHA.117.10476 [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Punthakee Z, Goldenberg R, Katz P. Diabetes Canada 2018 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2018;43:S1–S325 [Google Scholar]
- 20.Wright JT, Jr, Williamson JD, Whelton PK, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373:2103–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dupuis ME, Nadeau-Fredette AC, Madore F, Agharazii M, Goupil R. Association of glomerular hyperfiltration and cardiovascular risk in middle-aged healthy individuals. JAMA Netw Open. 2020;3:e202377 doi: 10.1001/jamanetworkopen.2020.2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bérard A, Lacasse A. Validity of perinatal pharmacoepidemiologic studies using data from the RAMQ administrative database. Can J Clin Pharmacol. 2009;16:e360–e369 [PubMed] [Google Scholar]
- 23.McCormick N, Lacaille D, Bhole V, Avina-Zubieta JA. Validity of myocardial infarction diagnoses in administrative databases: a systematic review. PLoS One. 2014;9:e92286 doi: 10.1371/journal.pone.0092286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubin DB. Multiple Imputation for Nonresponse in Survey. 1987. John Wiley & Sons [Google Scholar]
- 25.Wickham H. ggplot2: Elegant Graphics for Data Analysis. 2016. Spinger-Verlag [Google Scholar]
- 26.Harrell FE. Regression Modeling Strategies. 2015. Springer [Google Scholar]
- 27.Thomas LE, O’Brien EC, Piccini JP, D’Agostino RB, Pencina MJ. Application of net reclassification index to non-nested and point-based risk prediction models: a review. Eur Heart J. 2018;40:1880–1887. doi: 10.1093/eurheartj/ehy345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demler OV, Pencina MJ, Cook NR, D’Agostino RB. Asymptotic distribution of ΔAUC, NRIs, and IDI based on theory of U-statistics. Stat Med. 2017;36:3334–3360. doi: 10.1002/sim.7333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J. 2005;47:458–472. doi: 10.1002/bimj.200410135 [DOI] [PubMed] [Google Scholar]
- 30.Tomiyama H, O’Rourke MF, Hashimoto H, Matsumoto C, Odaira M, Yoshida M, Shiina K, Nagata M, Yamashina A. Central blood pressure: a powerful predictor of the development of hypertension. Hypertension Research. 2012;36:19–24. doi: 10.1038/hr.2012.123 [DOI] [PubMed] [Google Scholar]
- 31.Schisterman EF, Perkins N. Confidence intervals for the youden index and corresponding optimal cut-point. Commun Stat- Simul Comput. 2007;36:549–563 [Google Scholar]
- 32.Contal C, O’Quigley J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Comput Stat Data Anal. 1999;30:253–270 [Google Scholar]
- 33.Ogłuszka M, Orzechowska M, Jędroszka D, Witas P, Bednarek AK. Evaluate cutpoints: adaptable continuous data distribution system for determining survival in Kaplan-Meier estimator. Comput Methods Programs Biomed. 2019;177:133–139. doi: 10.1016/j.cmpb.2019.05.023 [DOI] [PubMed] [Google Scholar]
- 34.Richards SA. Testing ecological theory using the information-theoretic approach: examples and cautionary results. Ecology. 2005;86:2805–2814 [Google Scholar]
- 35.Pencina MJ, D’Agostino RB, Pencina KM, Janssens AC, Greenland P. Interpreting incremental value of markers added to risk prediction models. Am J Epidemiol. 2012;176:473–481. doi: 10.1093/aje/kws207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1923–1994. doi: 10.1016/S0140-6736(18)32225-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ; Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142–161. doi: 10.1161/01.HYP.0000150859.47929.8e [DOI] [PubMed] [Google Scholar]
- 38.Wang KL, Cheng HM, Chuang SY, Spurgeon HA, Ting CT, Lakatta EG, Yin FC, Chou P, Chen CH. Central or peripheral systolic or pulse pressure: which best relates to target organs and future mortality? J Hypertens. 2009;27:461–467. doi: 10.1097/hjh.0b013e3283220ea4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kollias A, Lagou S, Zeniodi ME, Boubouchairopoulou N, Stergiou GS. Association of central versus brachial blood pressure with target-organ damage: systematic review and meta-analysis. Hypertension. 2016;67:183–190. doi: 10.1161/HYPERTENSIONAHA.115.06066 [DOI] [PubMed] [Google Scholar]
- 40.Vlachopoulos C, Aznaouridis K, O’Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J. 2010;31:1865–1871. doi: 10.1093/eurheartj/ehq024 [DOI] [PubMed] [Google Scholar]
- 41.Dart AM, Gatzka CD, Kingwell BA, Willson K, Cameron JD, Liang YL, Berry KL, Wing LM, Reid CM, Ryan P, et al. Brachial blood pressure but not carotid arterial waveforms predict cardiovascular events in elderly female hypertensives. Hypertension. 2006;47:785–790. doi: 10.1161/01.HYP.0000209340.33592.50 [DOI] [PubMed] [Google Scholar]
- 42.Safar ME, Blacher J, Pannier B, Guerin AP, Marchais SJ, Guyonvarc’h PM, London GM. Central pulse pressure and mortality in end-stage renal disease. Hypertension. 2002;39:735–738. doi: 10.1161/hy0202.098325 [DOI] [PubMed] [Google Scholar]
- 43.McGaughey TJ, Fletcher EA, Shah SA. Impact of antihypertensive agents on central systolic blood pressure and augmentation index: a meta-analysis. Am J Hypertens. 2016;29:448–457. doi: 10.1093/ajh/hpv134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665 doi: 10.1136/bmj.b1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herbert A, Cruickshank JK, Laurent S, Boutouyrie P; Reference Values for Arterial Measurements Collaboration. Establishing reference values for central blood pressure and its amplification in a general healthy population and according to cardiovascular risk factors. Eur Heart J. 2014;35:3122–3133. doi: 10.1093/eurheartj/ehu293 [DOI] [PubMed] [Google Scholar]
- 46.Whelton Paul K, Carey Robert M, Aronow Wilbert S, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:1269–1324 [DOI] [PubMed] [Google Scholar]
- 47.Nerenberg KA, Zarnke KB, Leung AA, Dasgupta K, Butalia S, McBrien K, Harris KC, Nakhla M, Cloutier L, Gelfer M, et al. ; Hypertension Canada. Hypertension Canada’s 2018 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults and children. Can J Cardiol. 2018;34:506–525. doi: 10.1016/j.cjca.2018.02.022 [DOI] [PubMed] [Google Scholar]
- 48.Wassertheurer S, Hametner B, Mayer CC, Hafez A, Negishi K, Papaioannou TG, Protogerou AD, Sharman JE, Weber T. Aortic systolic pressure derived with different calibration methods: associations to brachial systolic pressure in the general population. Blood Press Monit. 2018;23:134–140. doi: 10.1097/MBP.0000000000000319 [DOI] [PubMed] [Google Scholar]
- 49.Kips JG, Schutte AE, Vermeersch SJ, Huisman HW, Van Rooyen JM, Glyn MC, Fourie CM, Malan L, Schutte R, Van Bortel LM, et al. Comparison of central pressure estimates obtained from SphygmoCor, Omron HEM-9000AI and carotid applanation tonometry. J Hypertens. 2011;29:1115–1120. doi: 10.1097/HJH.0b013e328346a3bc [DOI] [PubMed] [Google Scholar]
- 50.Hupfeld C, Mudaliar S. Navigating the “MACE” in cardiovascular outcomes trials and decoding the relevance of atherosclerotic cardiovascular disease benefits versus Heart Failure benefits. Diabetes Obes Metab. 2019;21:1780–1789. doi: 10.1111/dom.13740 [DOI] [PubMed] [Google Scholar]
- 51.Negishi K, Yang H, Wang Y, Nolan MT, Negishi T, Pathan F, Marwick TH, Sharman JE. Importance of calibration method in central blood pressure for cardiac structural abnormalities. Am J Hypertens. 2016;29:1070–1076. doi: 10.1093/ajh/hpw039 [DOI] [PubMed] [Google Scholar]
- 52.Gotzmann M, Hogeweg M, Seibert FS, Rohn BJ, Bergbauer M, Babel N, Bauer F, Mügge A, Westhoff TH. Accuracy of fully automated oscillometric central aortic blood pressure measurement techniques. J Hypertens. 2020;38:235–242. doi: 10.1097/HJH.0000000000002237 [DOI] [PubMed] [Google Scholar]
- 53.Gotzmann M, Hogeweg M, Bauer F, Seibert FS, Rohn BJ, Mügge A, Babel N, Westhoff TH. The impact of calibration approaches on the accuracy of oscillometric central aortic blood pressure measurement. J Hypertens. 2020;38:2154–2160. doi: 10.1097/HJH.0000000000002563 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


