Supplemental digital content is available in the text.
Key Words: ENDURANCE EXERCISE, AEROBIC EXERCISE, EXERCISE RECOVERY, SUPPLEMENT
ABSTRACT
Introduction/Purpose
Evidence suggests that carbohydrate and protein (CHO-PRO) ingestion after exercise enhances muscle glycogen repletion to a greater extent than carbohydrate (CHO) alone. However, there is no consensus at this point, and results across studies are mixed, which may be attributable to differences in energy content and carbohydrate intake relative to body mass consumed after exercise. The purpose of this study was determine the overall effects of CHO-PRO and the independent effects of energy and relative carbohydrate content of CHO-PRO supplementation on postexercise muscle glycogen synthesis compared with CHO alone.
Methods
Meta-analysis was conducted on crossover studies assessing the influence of CHO-PRO compared with CHO alone on postexercise muscle glycogen synthesis. Studies were identified in a systematic review from PubMed and Cochrane Library databases. Data are presented as effect size (95% confidence interval [CI]) using Hedges’ g. Subgroup analyses were conducted to evaluate effects of isocaloric and nonisocaloric energy content and dichotomized by median relative carbohydrate (high, ≥0.8 g·kg−1⋅h−1; low, <0.8 g·kg−1⋅h−1) content on glycogen synthesis.
Results
Twenty studies were included in the analysis. CHO-PRO had no overall effect on glycogen synthesis (0.13, 95% CI = −0.04 to 0.29) compared with CHO. Subgroup analysis found that CHO-PRO had a positive effect (0.26, 95% CI = 0.04–0.49) on glycogen synthesis when the combined intervention provided more energy than CHO. Glycogen synthesis was not significant (−0.05, 95% CI = −0.23 to 0.13) in CHO-PRO compared with CON when matched for energy content. There was no statistical difference of CHO-PRO on glycogen synthesis in high (0.07, 95% CI = −0.11 to 0.22) or low (0.21, 95% CI = −0.08 to 0.50) carbohydrate content compared with CHO.
Conclusion
Glycogen synthesis rates are enhanced when CHO-PRO are coingested after exercise compared with CHO only when the added energy of protein is consumed in addition to, not in place of, carbohydrate.
It is well known that modulating postexercise nutrition is an effective approach to facilitate replenishment of muscle glycogen stores in athletes or military service members who train, compete, and perform sustained physically demanding tasks multiple times per day (1). To maximally stimulate glycogen synthesis, current sport nutrition recommendations are to consume 1.2 g of carbohydrate per kilogram body mass per hour for 4–6 h postexercise (1). Consumption of 0.3 g·kg−1 body mass of a high-quality protein is also recommended to aid in postexercise recovery by stimulating muscle protein synthesis and repair (1). Some evidence suggests that coingestion of carbohydrate and protein (CHO-PRO) after exercise may stimulate greater glycogen synthesis during recovery compared with carbohydrate (CHO) alone (2,3). This greater glycogen synthesis has been attributed to the insulinotropic effects of amino acids, such as leucine, on pancreatic release of insulin (4,5), resulting in higher circulating insulin concentrations thereby increasing muscle glucose uptake when CHO-PRO is consumed compared with CHO alone (6,7). Furthermore, consuming CHO-PRO after exercise may enhance glycogen synthesis to a greater extent than CHO alone by upregulating markers of glycogen synthase activity (8).
Despite evidence of greater glucose uptake and molecular regulation of glycogen synthesis, the observed effect of CHO-PRO ingestion on postexercise glycogen synthesis as compared with CHO alone is inconsistent. Both positive (9–11) and null (12–14) effects of CHO-PRO ingestion compared with CHO have been reported. The discordant results across studies may arise because some used isocaloric CHO-PRO and CHO interventions, whereas others used nonisocaloric interventions. van Loon et al. (15) reported that when interventions were matched for carbohydrate and the addition of protein resulted in greater energy content (i.e., nonisocaloric), CHO-PRO increased glycogen synthesis compared with CHO. In the same study, when interventions were matched for energy content (i.e., isocaloric), glycogen synthesis was similar between CHO-PRO and CHO (15). However, in a subsequent study by Howarth et al. (13), postexercise glycogen synthesis was similar between CHO-PRO and CHO regardless if interventions were isocaloric or nonisocaloric. These result may suggests that other factors beyond energy content might need to be considered when assessing the influence of CHO-PRO on glycogen synthesis. One such factor is the relative (g·kg−1 body mass·h−1) amount of carbohydrate consumed in a CHO-PRO supplement (2,3,16). Specifically, the additive effect of CHO-PRO on glycogen synthesis as compared with CHO alone may only be evident when carbohydrate content is <0.8 g·kg−1⋅h−1 (2,3). When CHO intakes are >0.8 g·kg−1⋅h−1, glycogen synthesis is already maximally stimulated, thereby negating any potential effect of added protein (2,3).
The objective of this systematic review and meta-analysis was to aggregate results from multiple studies to characterize the effects of CHO-PRO on glycogen synthesis during recovery from exercise compared with CHO alone. In addition, subgroup analyses were performed to examine if energy content (isocaloric and nonisocaloric) or carbohydrate content modulates the effects of CHO-PRO and CHO on glycogen synthesis. We hypothesized that there would be no overall significant effect in postexercise glycogen synthesis in CHO-PRO compared with CHO. We further hypothesized that subgroup analysis would identify increased postexercise glycogen synthesis with higher energy content (i.e., nonisocaloric) in CHO-PRO compared with PRO.
METHODS
Literature search strategy
Publication abstracts identified in PubMed (http://www.ncbi.nlm.nih.gov/pubmed) and the Cochrane Library (https://www.cochranelibrary.com) were organized using the Abstrackr citation program (http://abstrackr.cebm.brown.edu) and reviewed for relevance by researchers (JTA, AHM, and LMM). The initial search took place on July 12, 2019, and was not restricted by publication date (see Table, Supplemental Digital Content 1, Search terms used to identify relevant articles, http://links.lww.com/MSS/C94). A second search was conducted on March 24, 2020, to assess if any new relevant manuscripts had been published since the first search was performed. No new articles were identified. The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) search strategy and further reference narrowing is described in Figure 1 (17). The population, intervention, control, and outcome for this meta-analysis were healthy, trained or untrained men or women, CHO-PRO, CHO only, and glycogen synthesis, respectively. Reference lists from these publications were hand searched for relevant manuscripts missed in the database search. One relevant manuscript (9) was identified. There were no language restrictions although English search terms were used. Publications were reviewed and data were extracted by two researchers (JTA and AHM). The final decision on manuscript inclusion was conducted by LMM.
FIGURE 1.
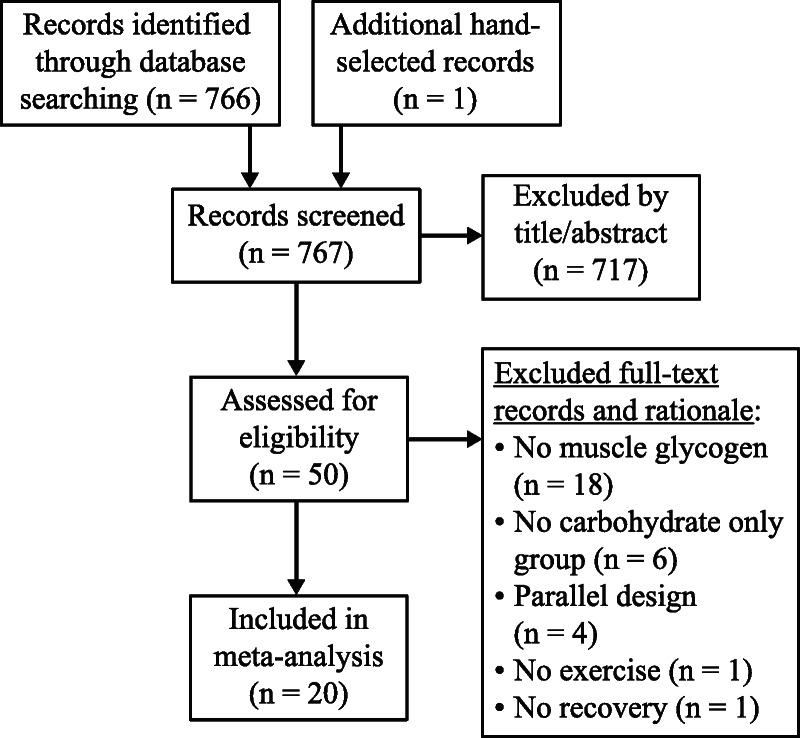
PRISMA meta-regression search strategy diagram.
Inclusion criteria
Randomized and nonrandomized crossover controlled trials assessing the effect of CHO-PRO and CHO on postexercise muscle glycogen synthesis in healthy, trained or untrained men or women were included.
Exclusion criteria
Studies were excluded if there was no exercise or recovery component, if there was no CHO-only group, or if both groups consumed protein after exercise. Studies comparing CHO-PRO to CHO for outcomes other than muscle glycogen synthesis were excluded. To control heterogeneity across studies, parallel study designs were excluded (18).
Bias and limitations
Bias was assessed by LMM in accordance with PRISMA guidelines recommended by Sterne et al. (19). Ratings including low, some, or high concern of randomization, intervention, outcomes, and reporting bias were assigned to each study (see Table, Supplementary Digital Content 2, Risk of bias for publications included in the meta-analysis, http://links.lww.com/MSS/C95).
Data extraction
Data were extracted from 20 articles that met the inclusion and exclusion criteria (9–15,20–32). Three studies (9,13,15) had multiple groups, comparing isocaloric and nonisocaloric interventions. Each intervention was treated as an independent group. The final data extraction was conducted on 23 groups. Sex, age, weight, V˙O2max, and training status were extracted to provide volunteer descriptive characteristics. Muscle glycogen measurements were extracted from each study. Energy, carbohydrate, and protein intake from study interventions were extracted. Data that were not presented numerically were obtained using an image analysis software (Image J, version 1.52a; National Institutes of Health, Bethesda, MD) by digitally measuring the height of data points and error bars and calculating relative to measured y-axis units in histograms.
Statistical analysis
Meta-Essentials by van Rhee et al. (33) with Microsoft Excel 2010 (Microsoft Corp., Redmond, WA) was used to conduct the meta-analysis. Effect sizes (ES) for the difference in delta (recovery minus immediately postexercise) glycogen synthesis were determined as standard mean difference between CHO-PRO and CHO divided by the pooled standard deviation. Sample sizes, glycogen mean and SD, and a correlation coefficient (r) for within-participant measurements were imputed. Because of a lack of individual data presented in publications, a correlation coefficient of r = 0.80 generated from muscle glycogen data by our laboratory (34) was used across all studies. To confirm that outcomes were not the result of our selection of r = 0.80, sensitivity analysis was conducted imputing an r value at a low of 0.50 and high of 0.90. There was no difference in our results based on the select r value. As such, all ES data were calculated using r = 0.80. To account for heterogeneity, random effects were applied and ES were generated as Hedges’ g (35). To determine heterogeneity, both Q and I2 statistics were used to assess between-study variations in ES (35). Publication bias was determined using the Egger regression (36). Subgroup analysis was conducted to assess the influence of energy content (isocaloric and nonisocaloric) on postexercise glycogen synthesis. Additional subgroup analysis was performed using median intake across studies to dichotomize carbohydrate content (high, ≥0.8 g·kg−1⋅h−1; low, <0.8 g·kg−1⋅h−1). Glycogen data are presented as ES (95% confidence intervals [CI]). To confirm results of subgroup analysis, regression analysis was performed with ES as the dependent variable and isocaloric or nonisocaloric energy content, low or high carbohydrate content, relative carbohydrate intake (g·kg−1⋅h−1), and relative protein intake (g·kg−1⋅h−1) as independent variables. In addition, chi-square analysis was used, setting categories as significant increase in glycogen synthesis (yes or no) and energy content (isocaloric or nonisocaloric) or carbohydrate intake (high or low) as categorical data using IBM SPSS Statistics for Windows Version 26.0 (IBM Corp., Armonk, NY). All other data are presented as mean values. Statistical significance was set at P < 0.05.
RESULTS
Study characteristics
A total of 767 studies were identified and screened for inclusion (Fig. 1). Of these studies, 20 met the inclusion criteria, and within these 20 studies, 176 individuals participated (158 men and 18 women) (see Table, Supplemental Digital Content 3, Volunteer characteristics, http://links.lww.com/MSS/C96). For subgroup analysis, 23 groups were identified, with 10 groups ingesting isocaloric interventions (Table 1) and 13 ingesting nonisocaloric interventions (Table 2). Energy, carbohydrate, and protein intake for subgroup analyses are presented in Table 3.
TABLE 1.
Study design for isocaloric interventions.
| Study | Sample Size (n) | Exercise Type | Exercise Time (min) | Recovery Time (h) | Treatment | Energy (kcal) | CHO (g) | Pro (g) | CHO (g·kg−1⋅h−1) | Pro (g·kg−1⋅h−1) | Glycogen Synthesis | Glycogen Measurement Method |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alghannam et al. (20) | 6 (5M, 1F) | Treadmill running; Time to exhaustion 70% V˙O2max | 82 | 4 | CHO | 1277 | 319.2 | 1.20 | 37.4a | Plate assay, dry weight | ||
| CHO-PRO | 1277 | 212.8 | 106.4 | 0.80 | 0.40 | 41.7a | ||||||
| Carrithers et al. (23) | 7 (7M) | Cycle ergometry 70% V˙O2max; sprint to exhaustion | 82.5 | 4 | CHO | 1206 | 301.6 | 1.00 | 31a | Plate assay, dry weight | ||
| CHO-PRO | 1206 | 259.3 | 42.2 | 0.86 | 0.14 | 28a | ||||||
| Cogan et al. (12) | 11 (11M) | Cycle ergometry 70% V˙O2max | 120 | 4 | CHO | 720 | 180 | 0.60 | 0.58b | Plate assay, wet weight | ||
| CHO-PRO | 720 | 156 | 24 | 0.52 | 0.08 | 0.52b | ||||||
| Detko et al. (24) | 7 (7M) | Intermittent cycle ergometry; 70% and 120% V˙O2max | 120 | 4 | CHO | 1516 | 379 | 1.20 | 5.8c | Magnetic resonance spectroscopy | ||
| CHO-PRO | 1516 | 253 | 126 | 0.80 | 0.40 | 3.7c | ||||||
| Ferguson-Stegall et al. (25) | 10 (5M, 5F) | Cycle ergometry 70% V˙O2max; intervals 45% and 90% V˙O2max | 100 | 4 | CHO | 949 | 181.8 | 0.67 | 30.6d | Plate assay, wet weight | ||
| CHO-PRO | 949 | 137.8 | 44 | 0.51 | 0.16 | 23.9d | ||||||
| Howarth et al. (13) | 6 (6M) | Intermittent cycle ergometer; 50%–80% V˙O2max | 120 | 4 | CHO | 1728 | 432 | 1.20 | 25a | Plate assay, dry weight | ||
| CHO-PRO | 1728 | 324 | 108 | 0.90 | 0.30 | 25a | ||||||
| Ivy et al. (9) | 7 (7M) | Cycle ergometry 65%–75% V˙O2max; sprint to exhaustion | 150 | 4 | CHO | 640 | 160 | 0.54 | 8.4c | Magnetic resonance spectroscopy | ||
| CHO-PRO | 864 | 160 | 56 | 0.54 | 0.19 | 12.0c | ||||||
| Lunn et al. (28) | 6 (6M) | Treadmill running 65% V˙O2max | 45 | 3 | CHO | 296 | 74 | 0.32 | −0.1e | Plate assay, wet weight | ||
| CHO-PRO | 296 | 58 | 16 | 0.25 | 0.07 | 0e | ||||||
| Roy et al. (29) | 10 (10M) | Whole-body resistance exercise: 9 exercises/3 sets 80% 1RM | NR | 4.5 | CHO | 694 | 173.6 | 0.44 | 19.3a | Plate assay, dry weight | ||
| CHO-PRO | 694 | 114.5 | 40 | 0.29 | 0.10 | 23.0a | ||||||
| van Loon et al. (15) | 8 (8M) | Intermittent cycle ergometer; 50%–90% V˙O2max | 100 | 5 | CHO | 1680 | 420 | 1.20 | 44.8a | Plate assay, dry weight | ||
| CHO-PRO | 1680 | 280 | 140 | 0.80 | 0.40 | 35.4a |
Values are presented as mean.
ammol·kg−1 muscle dry weight·h−1.
bμg·mg−1 muscle wet weight·h−1.
cmmol·L−1·h−1.
dμmol·g−1 muscle wet weight·h−1.
eg per 100 g wet muscle weight·h−1.
M, males; F, females.
TABLE 2.
Study design for nonisocaloric interventions.
| Study | Sample Size (n) | Exercise Type | Exercise Time (min) | Recovery Time (h) | Treatment | Energy (kcal) | CHO (g) | Pro (g) | CHO (g·kg−1⋅h−1) | Pro (g·kg−1⋅h−1) | Glycogen Synthesis | Glycogen Measurement Method |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beelen et al. (21) | 14 (14M) | Intermittent cycle ergometer; 50%–90% V˙O2max | 106 | 4 | CHO | 2062 | 515.5 | 1.80 | 31a | Plate assay, dry weight | ||
| CHO-PRO | 2578 | 515.5 | 128.9 | 1.80 | 0.45 | 34a | ||||||
| Betts et al. (22) | 6 (6M) | Treadmill running; 70% V˙O2max | 90 | 4 | CHO | 945 | 236.2 | 0.80 | 12.3a | Plate assay, dry weight | ||
| CHO-PRO | 1299 | 236.2 | 88.6 | 0.80 | 0.30 | 12.1a | ||||||
| Howarth et al. (13) | 6 (6M) | Intermittent cycle ergometer; 50%–80% V˙O2max | 120 | 4 | CHO | 1296 | 324 | 0.90 | 23a | Plate assay, dry weight | ||
| CHO-PRO | 1728 | 324 | 108 | 0.90 | 0.30 | 25a | ||||||
| Ivy et al. (9) | 7 (7M) | Cycle ergometry 70% V˙O2max; sprint to exhaustion | 150 | 4 | CHO | 864 | 216 | 0.73 | 7.3b | Magnetic resonance spectroscopy | ||
| CHO-PRO | 864 | 160 | 56 | 0.54 | 0.19 | 12.0b | ||||||
| Jentjens et al. (26) | 8 (8M) | Intermittent cycle ergometer 50%–90% V˙O2max | 135 | 3 | CHO | 1002 | 250.6 | 1.20 | 40a | Plate assay, dry weight | ||
| CHO-PRO | 1336 | 250.6 | 83.5 | 1.20 | 0.40 | 25a | ||||||
| Kammer et al. (27) | 12 (8M, 4F) | Cycle ergometry 60%–65% V˙O2max | 120 | 2 | CHO | 314 | 78.5 | 1.10 | 6.2c | Plate assay, wet weight | ||
| CHO-PRO | 410 | 77 | 19.5 | 1.08 | 0.27 | 7.3c | ||||||
| Tarnopolsky et al. (30) | 16 (8M, 8F) | Cycle ergometry 65% V˙O2max | 90 | 4 | CHO | 536 | 134 | 0.50 | 37.2a | Plate assay, dry weight | ||
| CHO-PRO | 480 | 100.5 | 13.4 | 0.38 | 0.05 | 24.6a | ||||||
| van Loon et al. (8) | 8 (8M) | Intermittent cycle ergometer; 50%–90% V˙O2max | 100 | 5 | CHO | 1120 | 280 | 0.80 | 16.4a | Plate assay, dry weight | ||
| CHO-PRO | 1680 | 280 | 140 | 0.80 | 0.40 | 35.4a | ||||||
| Van Hall et al. (14) | 5 (5M) | Intermittent cycle ergometer 50%–90% V˙O2max | NR | 4 | CHO | 492 | 123 | 0.42 | 39.8a | Plate assay, dry weight | ||
| CHO-PRO | 640 | 123 | 37 | 0.42 | 0.13 | 36.5a | ||||||
| Van Hall et al. (31) | 8 (8M) | Intermittent cycle ergometer 50%–90% V˙O2max | NR | 3 | CHO | 692 | 173 | 0.80 | 28.0a | Plate assay, dry weight | ||
| CHO-PRO | 952 | 173 | 65 | 0.80 | 0.30 | 34.0a | ||||||
| Williams et al. (10) | 8 (8M) | Intermittent cycle ergometer 65%–85% V˙O2max | 120 | 4 | CHO | 168 | 42 | 0.16 | 17.3a | Plate assay, dry weight | ||
| CHO-PRO | 536 | 106 | 28 | 0.39 | 0.10 | 39.8a | ||||||
| Yaspelkis et al. (32) | 12 (12M) | Intermittent cycle ergometer 50%–80% V˙O2max | 120 | 3 | CHO | 864 | 216 | 1.00 | 6.0c | Plate assay, wet weight | ||
| CHO-PRO | 933 | 216 | 17.3 | 1.00 | 0.08 | 8.2c | ||||||
| Zawadzki et al. (11) | 9 (9M) | Intermittent cycle ergometer 50%–85% V˙O2max | 120 | 4 | CHO | 896 | 224 | 0.77 | 26.7d | Plate assay, wet weight | ||
| CHO-PRO | 1222 | 224 | 81.4 | 0.77 | 0.28 | 35.5d |
Values are presented as mean.
ammol·kg−1 muscle dry weight·h−1.
bmmol·L−1·h−1.
cμmol·g−1 muscle wet weight·h−1.
dμmol·g−1 muscle protein concentration.
M, males; F, females.
TABLE 3.
Energy, protein, and carbohydrate intake for subgroup analysis.
| Subgroup | Treatment | Energy (kcals) | Carbohydrate (g) | Protein (g) | Carbohydrate (g·kg−1⋅h−1) | Protein (g·kg−1⋅h−1) |
|---|---|---|---|---|---|---|
| Isocaloric | CHO | 1093 ± 469 | 268 ± 120 | – | 0.86 ± 0.34 | – |
| CHO-PRO | 1093 ± 469 | 195 ± 84 | 70 ± 45 | 0.63 ± 0.24 | 0.22 ± 0.14 | |
| Nonisocaloric | CHO | 848 ± 485 | 212 ± 121 | – | 0.84 ± 0.41 | – |
| CHO-PRO | 1128 ± 613 | 214 ± 117 | 67 ± 43 | 0.85 ± 0.39 | 0.26 ± 0.14 | |
| High carbohydrate | CHO | 1208 ± 483 | 302 ± 117 | – | 1.10 ± 0.27 | – |
| CHO-PRO | 1409 ± 519 | 262 ± 100 | 90 ± 43 | 0.98 ± 0.28 | 0.33 ± 0.12 | |
| Low carbohydrate | CHO | 625 ± 256 | 151 ± 59 | – | 0.52 ± 0.19 | – |
| CHO-PRO | 727 ± 263 | 134 ± 45 | 40 ± 21 | 0.46 ± 0.15 | 0.24 ± 0.14 |
Values are presented as mean ± SD. High carbohydrate, ≥0.8 g·kg−1⋅h−1; low carbohydrate, <0.8 g·kg−1⋅h−1.
Glycogen synthesis
Overall, CHO-PRO had no significant effect (0.13, 95% CI = −0.04 to 0.29) on glycogen synthesis during recovery from exercise compared with CHO. There was substantial (Q = 76, I2 = 71%) heterogeneity across all studies. In subgroup analysis for energy content, CHO-PRO had a positive effect (0.26, 95% CI = 0.04–0.49) on glycogen synthesis when the energy content in CHO-PRO was higher (nonisocaloric) than CHO (Fig. 2). When the energy content (isocaloric) was matched, there was no significant effect in CHO-PRO (−0.05, 95% CI = −0.23 to 0.13) compared with CHO (Fig. 2). There was substantial heterogeneity in nonisocaloric studies (Q = 47, I2 = 74%) and moderate heterogeneity in isocaloric studies (Q = 18, I2 = 50%). In subgroup analysis for carbohydrate content, there was no significant effect of CHO-PRO in high (0.07, 95% CI = −0.11 to 0.25) or low (0.21, 95% CI = −0.08 to 0.50) carbohydrate content compared with CHO (Fig. 3). Substantial heterogeneity was detected in high (Q = 31, I2 = 61%) and low (Q = 44, I2 = 80%) carbohydrate content studies.
FIGURE 2.
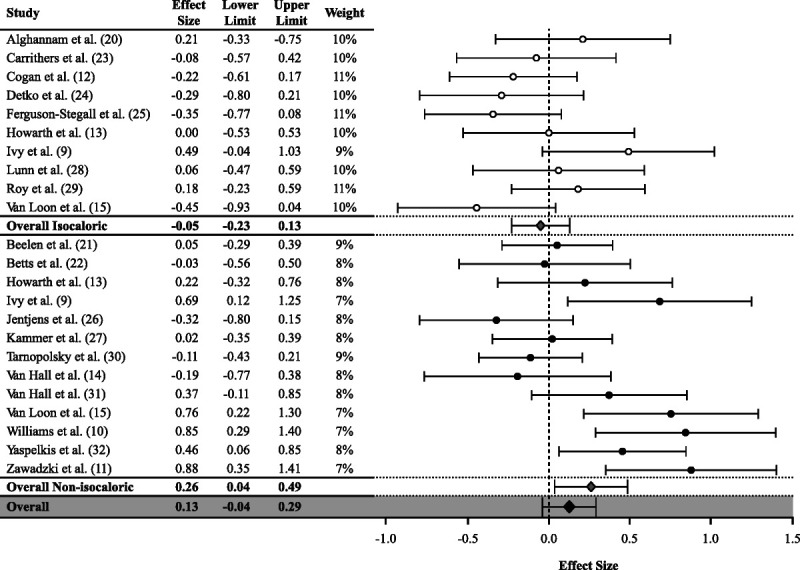
Values are presented as ES (95% CI) stratified by energy content.
FIGURE 3.
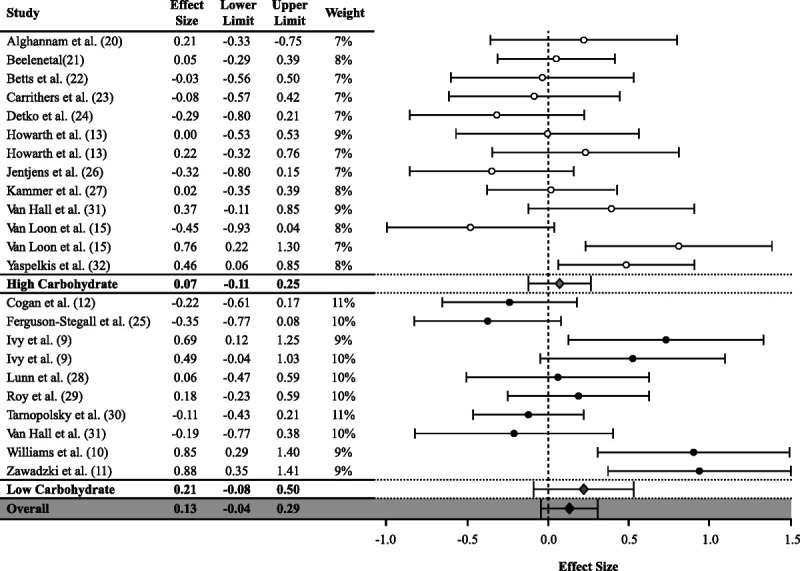
Values are presented as ES (95% CI) stratified by median relative carbohydrate content (high carbohydrate, ≥0.8 g·kg−1⋅h−1; low carbohydrate, <0.8 g·kg−1⋅h−1).
Regression analysis identified energy content (isocaloric or isocaloric) as a significant (P = 0.03) independent variable explaining 17% (r2 = 0.174) of the variance in glycogen synthesis ES. Carbohydrate content (low or high), relative carbohydrate intake, and relative protein intake were not significant in the regression analysis model. Similarly, chi-square analysis was significant (P = 0.03) when categorizing significant increase in glycogen synthesis (yes or no) and energy content (isocaloric or nonisocaloric). Chi-square analysis was not significant when categorizing significant increase in glycogen synthesis (yes or no) and carbohydrate content (high or low).
Publication bias
Publication bias was identified (P = 0.03) in the 23 groups included in this meta-analysis (see Figure, Supplemental Digital Content 4, Funnel plots of publication bias, http://links.lww.com/MSS/C97). No publication bias was identified (P = 0.35) in assessing isocaloric studies alone. Publication bias was identified (P = 0.03) when assessing nonisocaloric studies alone. Publication bias was driven by positive effects reported in multiple studies (9–11,15). No publication bias was identified in the overall data set (P = 0.81) or nonisocaloric subgroup (P = 0.85) when these studies were removed from the data set. However, there did not appear to be any inherit experimental or methodological flaws to warrant exclusion of these studies from the current investigation. Rather publication bias appeared to be the result of studies reporting only positive or null effects, with no studies reporting negative effects. As such, these investigations were included in the final analysis.
DISCUSSION
The primary outcome of this meta-analysis was that coingestion of CHO-PRO had no overall significant effect on postexercise glycogen synthesis compared with CHO alone. When studies were dichotomized into high or low relative carbohydrate intake, CHO-PRO had no significant effect on glycogen synthesis compared with CHO. However, postexercise glycogen synthesis was enhanced when the combined CHO-PRO treatment provided more total energy than CHO alone. These data indicate that increasing the energy content of postexercise nutrition by adding protein to carbohydrate, and not replacing carbohydrate for protein, is likely a primary stimulus for enhanced glycogen synthesis during recovery from exercise when CHO-PRO are consumed together.
Our meta-analysis demonstrated a significant positive effect of combined CHO-PRO ingestion when the treatment provided more energy than CHO alone. Our findings are in agreement with van Loon et al. (15), who assessed postexercise glycogen synthesis in individuals consuming 0.8 g CHO·kg−1⋅h−1 (low CHO), 1.2 g CHO·kg−1⋅h−1 (high CHO), or 0.8 g CHO·kg−1⋅h−1 + 0.4 g PRO·kg−1⋅h−1 (CHO-PRO). In the study of van Loon et al. (15), postexercise glycogen synthesis was greater after ingesting CHO-PRO compared with low CHO (nonisocaloric comparison), but not different than high CHO (isocaloric comparison). The authors suggested that the greater glycogen synthesis rates observed after ingesting CHO-PRO compared with the lower energy low CHO treatment was due to an 88% higher postprandial insulin response (15). The greater insulin response with CHO-PRO would, in theory, enhance GLUT4 translocation, glucose uptake, and glycogen synthase activity (15,37). Although van Loon et al. did not explore these mechanisms, data from small animal studies support this hypothesis and show that amino acids alone can increase AKT and AS160 phosphorylation status, which are upstream regulators of GLUT4 translocation (38,39). However, no studies have clearly delineated the mechanistic effects of the additional energy content in combined CHO-PRO ingestion from the potential insulinotropic effects provided by protein. The study of van Loon et al. and our aggregate results do show that when CHO-PRO and CHO are matched for total energy, there is no direct benefit of displacing carbohydrate for protein. By contrast, adding protein to matched amounts of carbohydrate will enhance glycogen synthesis.
Dichotomizing studies by high (≥0.8 g·kg−1⋅h−1) or low (<0.8 g·kg−1⋅h−1) relative carbohydrate intake in the current meta-analysis had no significant effect on glycogen synthesis in CHO-PRO compared with CHO. This result appears to contradict previous assertions that insulinotropic effects of dietary protein may only be observed when postexercise carbohydrate intake is <0.8 g·kg−1⋅h−1 (2,3). However, our analysis may not definitively rule out an improvement in glycogen synthesis when coingestion of protein occurs with lower relative carbohydrate intake. Because of a limited number of studies, we could not conduct subgroup analysis on high and low relative carbohydrate intake between isocaloric and nonisocaloric studies. Similarly, there is no individual study that has used a multigroup approach to assess the effect of CHO-PRO compared with CHO using high and low carbohydrate with isocaloric and nonisocaloric interventions. Furthermore, although not statistically significant, the ES was higher in low (0.21) compared with high (0.07) relative carbohydrate subgroups, suggesting that there may be some benefit of CHO-PRO on glycogen synthesis when carbohydrate intake is <0.8 g·kg−1⋅h−1. Further investigation is needed to gain an understanding how manipulating both carbohydrate and energy intake (high energy [high CHO + PRO, low CHO + PRO]; low energy [high CHO + PRO, low CHO + PRO]) affects muscle glycogen synthesis.
Moderate to substantial levels of heterogeneity were observed across studies in the overall and subgroup analysis. Heterogeneity between studies is common and unavoidable with most meta-analysis (40). Differences in exercise mode (running vs cycling vs whole-body resistance exercise), assessment of muscle glycogen (dry muscle mass vs wet muscle mass vs 13C-magnetic resonance spectroscopy), carbohydrate and protein sources, postexercise recovery duration, and timing of supplement intake may have contributed to the observed heterogeneity. Subgroup analysis identified that the use of isocaloric versus nonisocaloric interventions resulted in different ES, suggesting that the differences in study interventions explain some degree of the overall variance between studies. In addition, caution should be taken when high levels of heterogeneity are detected if the direction of individual study ES (e.g., positive or negative) vary across studies and/or when individual CI do not overlap. Given that all studies included in the current meta-analysis reported a null or positive effect of CHO-PRO compared with CHO and that the majority of CI overlapped between studies, it is unlikely that the level of variance interfered with the present results and interpretations.
Although consuming isocaloric CHO-PRO and CHO promoted the same glycogen synthesis rates in recovery from exercise, a secondary advantage of coingesting carbohydrate and protein, which is often overlooked in the context of aerobic exercise, is the primary effects of protein on muscle protein synthesis (41). There is no debate that increases in extracellular and intracellular amino acids when consuming CHO-PRO postexercise increases muscle protein synthesis to a greater extent than CHO (13,28). The fact that lower carbohydrate intake with isocaloric CHO-PRO does not impair postexercise glycogen synthesis, and that ingesting PRO will increase muscle protein synthesis, indicates that CHO-PRO can produce improved overall muscle recovery (i.e., glycogen synthesis, repair, remodeling, and protein accretion) from exercise compared with CHO alone. In the current study, similar glycogen synthesis rates were achieved despite a ~30% lower carbohydrate intake in CHO-PRO compared with CHO. In the context of current recommendations to consume carbohydrate at 1.2 g·kg−1⋅h−1 to replenish glycogen stores postexercise, the present analysis indicates that carbohydrate can be consumed at 0.9 g·kg−1⋅h−1 and protein at 0.3 g·kg−1⋅h−1. Matching energy intake at 1.2 g·kg−1⋅h−1 with coingestion of CHO-PRO ensures adequate glycogen synthesis and that postexercise protein requirements (0.25–0.3 g protein per kilogram per meal postexercise) (1) are met to optimize muscle protein recovery.
The results of this meta-analysis should be interpreted in the context of the population and environment in which data were collected. The studies included in the current meta-analysis were performed under well-controlled laboratory settings. Failure to consider the environmental conditions under which skeletal muscle recovers and the necessity for postexercise fueling may limit the extension of our analysis. For example, recovery in environmental extremes such as heat and cold results in reduced postexercise glycogen synthesis (42,43). In addition, unacclimatized exposure to environmental conditions, such as heat and high altitude, increase glycogenolysis and decrease the use of exogenous carbohydrate for fuel during aerobic exercise (44,45). These changes in postexercise glycogen synthesis, glycogenolysis, and exogenous glucose oxidation during and in recovery from exercise may affect postexercise recovery nutritional needs. However, the effect of CHO-PRO on glycogen synthesis postexercise under environmental extremes, such as heat, cold, and high altitude, has not been examined. In addition, individual studies were primarily conducted using male participants. Some research has indicated that substrate oxidation differs by sex, with women oxidizing more fat and less carbohydrate compared with men during aerobic exercise (46–49). However, there appear to be minimal differences between sex postexercise, with men and women exhibiting similar rates of glycogen synthesis postexercise (30,50). This may suggest similar response in men and women to CHO-PRO on glycogen synthesis postexercise. It should also be noted that these studies were primarily conducted with participants in a fasted state. Glycogen oxidation during exercise is lower in fed compared with fasted states and affects the rate of postexercise glycogen synthesis (51). It is unclear if consuming a meal before exercise would affect the influence of CHO-PRO on postexercise glycogen synthesis. Future investigation is needed to assess the application of recovery nutrition prescriptions for how environment, sex, and feeding state (fasted or fed) may alter response to CHO-PRO on postexercise glycogen synthesis.
Although outside the scope of the current meta-analysis, increased glycogen synthesis with nonisocaloric CHO-PRO is uncertain, as limited studies assess both muscle glycogen and physical performance. In the current meta-analysis, Williams et al. (10) was the only study using a nonisocaloric intervention to assess both glycogen synthesis and physical performance, reporting a 55% increase in time-to-exhaustion performance immediately after the 4-h recovery period in CHO-PRO compared with CHO. However, recent systematic reviews have stated that there is no overall benefit to physical performance when consuming CHO-PRO compared with CHO (52,53). It should be noted that these systematic reviews included performance after consumption of isocaloric and nonisocaloric CHO-PRO compared with CHO. As isocaloric CHO-PRO supplementation did not result in a significant increase in glycogen synthesis postexercise compared with CHO in the current meta-analysis, an ergogenic effect would not be anticipated. Similar to glycogen synthesis, the consumption of protein at the expense of carbohydrate was not reported to result in a negative effect on physical performance in past systematic reviews (52,53). Again this suggests that some carbohydrate can be replaced with dietary protein postexercise to obtain the benefit of protein on muscle mass.
CONCLUSION
In conclusion, results from this meta-analysis indicate that postexercise glycogen synthesis is enhanced by a higher energy intake when coingesting CHO-PRO compared with the same amount of CHO alone. Equally important is our observation that ingesting CHO-PRO did not impair muscle glycogen synthesis when compared with an isocaloric amount of CHO only. As such, we contend that matching the energy content provided in current sport nutrition recommendations for optimal glycogen recovery of 1.2 g·kg−1⋅h−1 by lowering carbohydrate (0.9 g·kg−1⋅h−1) and adding back the equivalent amount of protein (0.3 g·kg−1⋅h−1) may yield the most complete postexercise recovery by not only maximizing glycogen synthesis but also by stimulating muscle protein synthesis.
Supplementary Material
Acknowledgments
The authors acknowledge Dr. Andrew Young for his critical review of this manuscript as well as the subjects and authors of the papers included in this meta-analysis. The authors also acknowledge Mr. Phil Niro for generating the figures for the manuscript.
This work was supported by the U.S. Army Medical Research and Development Command.
The investigators adhered to the policies for protection of human subjects as prescribed in Army Regulation 70-25, and the research was conducted in adherence with the provisions of 32 CFR part 219. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Army or the Department of Defense. Any citations of commercial organizations and trade names in this report do not constitute an official Department of the Army endorsement of approval of the products or services of these organizations.
The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. Results of the present study do not constitute endorsement by the American College of Sports Medicine.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.acsm-msse.org).
Contributor Information
JILLIAN T. ALLEN, Email: jillian.t.allen.ctr@mail.mil.
ADRIENNE HATCH-MCCHESNEY, Email: adrienne.m.mcchesney.civ@mail.mil.
STEFAN M. PASIAKOS, Email: stefan.m.pasiakos.civ@mail.mil.
REFERENCES
- 1.Thomas DT, Erdman KA, Burke LM. American College of Sports Medicine Joint Position Statement: nutrition and athletic performance. Med Sci Sports Exerc. 2016;48(3):543–68. [DOI] [PubMed] [Google Scholar]
- 2.Alghannam AF, Gonzalez JT, Betts JA. Restoration of muscle glycogen and functional capacity: role of post-exercise carbohydrate and protein co-ingestion. Nutrients. 2018;10(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betts JA, Williams C. Short-term recovery from prolonged exercise: exploring the potential for protein ingestion to accentuate the benefits of carbohydrate supplements. Sports Med. 2010;40(11):941–59. [DOI] [PubMed] [Google Scholar]
- 4.Floyd JC, Jr, Fajans SS, Conn JW, Knopf RF, Rull J. Stimulation of insulin secretion by amino acids. J Clin Invest. 1966;45(9):1487–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Floyd JC, Jr, Fajans SS, Conn JW, Thiffault C, Knopf RF, Guntsche E. Secretion of insulin induced by amino acids and glucose in diabetes mellitus. J Clin Endocrinol Metab. 1968;28(2):266–76. [DOI] [PubMed] [Google Scholar]
- 6.Doi M, Yamaoka I, Nakayama M, Mochizuki S, Sugahara K, Yoshizawa F. Isoleucine, a blood glucose-lowering amino acid, increases glucose uptake in rat skeletal muscle in the absence of increases in AMP-activated protein kinase activity. J Nutr. 2005;135(9):2103–8. [DOI] [PubMed] [Google Scholar]
- 7.Doi M, Yamaoka I, Nakayama M, Sugahara K, Yoshizawa F. Hypoglycemic effect of isoleucine involves increased muscle glucose uptake and whole body glucose oxidation and decreased hepatic gluconeogenesis. Am J Physiol Endocrinol Metab. 2007;292(6):E1683–93. [DOI] [PubMed] [Google Scholar]
- 8.Ivy JL, Ding Z, Hwang H, Cialdella-Kam LC, Morrison PJ. Post exercise carbohydrate-protein supplementation: phosphorylation of muscle proteins involved in glycogen synthesis and protein translation. Amino Acids. 2008;35(1):89–97. [DOI] [PubMed] [Google Scholar]
- 9.Ivy JL, Goforth HW, Jr, Damon BM, McCauley TR, Parsons EC, Price TB. Early postexercise muscle glycogen recovery is enhanced with a carbohydrate-protein supplement. J Appl Physiol (1985). 2002;93(4):1337–44. [DOI] [PubMed] [Google Scholar]
- 10.Williams M, Raven PB, Fogt DL, Ivy JL. Effects of recovery beverages on glycogen restoration and endurance exercise performance. J Strength Cond Res. 2003;17(1):12–9. [DOI] [PubMed] [Google Scholar]
- 11.Zawadzki KM, Yaspelkis BB, 3rd, Ivy JL. Carbohydrate–protein complex increases the rate of muscle glycogen storage after exercise. J Appl Physiol (1985). 1992;72(5):1854–9. [DOI] [PubMed] [Google Scholar]
- 12.Cogan KE Evans M Iuliano E, et al. Co-ingestion of protein or a protein hydrolysate with carbohydrate enhances anabolic signaling, but not glycogen resynthesis, following recovery from prolonged aerobic exercise in trained cyclists. Eur J Appl Physiol. 2018;118(2):349–59. [DOI] [PubMed] [Google Scholar]
- 13.Howarth KR, Moreau NA, Phillips SM, Gibala MJ. Coingestion of protein with carbohydrate during recovery from endurance exercise stimulates skeletal muscle protein synthesis in humans. J Appl Physiol (1985). 2009;106(4):1394–402. [DOI] [PubMed] [Google Scholar]
- 14.van Hall G, Shirreffs SM, Calbet JA. Muscle glycogen resynthesis during recovery from cycle exercise: no effect of additional protein ingestion. J Appl Physiol (1985). 2000;88(5):1631–6. [DOI] [PubMed] [Google Scholar]
- 15.van Loon LJ, Saris WH, Kruijshoop M, Wagenmakers AJ. Maximizing postexercise muscle glycogen synthesis: carbohydrate supplementation and the application of amino acid or protein hydrolysate mixtures. Am J Clin Nutr. 2000;72(1):106–11. [DOI] [PubMed] [Google Scholar]
- 16.Burke LM, van Loon LJC, Hawley JA. Postexercise muscle glycogen resynthesis in humans. J Appl Physiol (1985). 2017;122(5):1055–67. [DOI] [PubMed] [Google Scholar]
- 17.Moher D Liberati A Tetzlaff J Altman DG, PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011. [Google Scholar]
- 19.Sterne JAC Savović J Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 20.Alghannam AF, Jedrzejewski D, Bilzon J, Thompson D, Tsintzas K, Betts JA. Influence of post-exercise carbohydrate-protein ingestion on muscle glycogen metabolism in recovery and subsequent running exercise. Int J Sport Nutr Exerc Metab. 2016;26(6):572–80. [DOI] [PubMed] [Google Scholar]
- 21.Beelen M, Kranenburg Jv, Senden JM, Kuipers H, Loon LJ. Impact of caffeine and protein on postexercise muscle glycogen synthesis. Med Sci Sports Exerc. 2012;44(4):692–700. [DOI] [PubMed] [Google Scholar]
- 22.Betts JA, Williams C, Boobis L, Tsintzas K. Increased carbohydrate oxidation after ingesting carbohydrate with added protein. Med Sci Sports Exerc. 2008;40(5):903–12. [DOI] [PubMed] [Google Scholar]
- 23.Carrithers JA, Williamson DL, Gallagher PM, Godard MP, Schulze KE, Trappe SW. Effects of postexercise carbohydrate-protein feedings on muscle glycogen restoration. J Appl Physiol (1985). 2000;88(6):1976–82. [DOI] [PubMed] [Google Scholar]
- 24.Detko E O’Hara JP Thelwall PE, et al. Liver and muscle glycogen repletion using 13C magnetic resonance spectroscopy following ingestion of maltodextrin, galactose, protein and amino acids. Br J Nutr. 2013;110(5):848–55. [DOI] [PubMed] [Google Scholar]
- 25.Ferguson-Stegall L McCleave EL Ding Z, et al. Postexercise carbohydrate-protein supplementation improves subsequent exercise performance and intracellular signaling for protein synthesis. J Strength Cond Res. 2011;25(5):1210–24. [DOI] [PubMed] [Google Scholar]
- 26.Jentjens RL, van Loon LJ, Mann CH, Wagenmakers AJ, Jeukendrup AE. Addition of protein and amino acids to carbohydrates does not enhance postexercise muscle glycogen synthesis. J Appl Physiol (1985). 2001;91(2):839–46. [DOI] [PubMed] [Google Scholar]
- 27.Kammer L, Ding Z, Wang B, Hara D, Liao YH, Ivy JL. Cereal and nonfat milk support muscle recovery following exercise. J Int Soc Sports Nutr. 2009;6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lunn WR Pasiakos SM Colletto MR, et al. Chocolate milk and endurance exercise recovery: protein balance, glycogen, and performance. Med Sci Sports Exerc. 2012;44(4):682–91. [DOI] [PubMed] [Google Scholar]
- 29.Roy BD, Tarnopolsky MA. Influence of differing macronutrient intakes on muscle glycogen resynthesis after resistance exercise. J Appl Physiol (1985). 1998;84(3):890–6. [DOI] [PubMed] [Google Scholar]
- 30.Tarnopolsky MA, Bosman M, Macdonald JR, Vandeputte D, Martin J, Roy BD. Postexercise protein-carbohydrate and carbohydrate supplements increase muscle glycogen in men and women. J Appl Physiol (1985). 1997;83(6):1877–83. [DOI] [PubMed] [Google Scholar]
- 31.van Hall G, Saris WH, van de Schoor PA, Wagenmakers AJ. The effect of free glutamine and peptide ingestion on the rate of muscle glycogen resynthesis in man. Int J Sports Med. 2000;21(1):25–30. [DOI] [PubMed] [Google Scholar]
- 32.Yaspelkis BB, 3rd, Ivy JL. The effect of a carbohydrate–arginine supplement on postexercise carbohydrate metabolism. Int J Sport Nutr. 1999;9(3):241–50. [DOI] [PubMed] [Google Scholar]
- 33.van Rhee HJ. User Manual for Meta-Essentials: Workbooks for Meta-analysis. Rotterdam (The Netherlands): Erasmus Research Institute of Management; 2015. Available from: Erasmus Research Institute of Management. [Google Scholar]
- 34.Margolis LM Wilson MA Whitney CC, et al. Exercising with low muscle glycogen content increases fat oxidation and decreases endogenous, but not exogenous carbohydrate oxidation. Metabolism. 2019;97:1–8. [DOI] [PubMed] [Google Scholar]
- 35.Borenstein M, Hedges LV, Higgins JPT. Introduction to Meta-Analysis. Chichester (UK): John Wiley & Sons; 2009. [Google Scholar]
- 36.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ivy JL, Kuo CH. Regulation of GLUT4 protein and glycogen synthase during muscle glycogen synthesis after exercise. Acta Physiol Scand. 1998;162(3):295–304. [DOI] [PubMed] [Google Scholar]
- 38.Bernard JR Liao YH Ding Z, et al. An amino acid mixture improves glucose tolerance and lowers insulin resistance in the obese Zucker rat. Amino Acids. 2013;45(1):191–203. [DOI] [PubMed] [Google Scholar]
- 39.Kleinert M, Liao YH, Nelson JL, Bernard JR, Wang W, Ivy JL. An amino acid mixture enhances insulin-stimulated glucose uptake in isolated rat epitrochlearis muscle. J Appl Physiol (1985). 2011;111(1):163–9. [DOI] [PubMed] [Google Scholar]
- 40.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Margolis LM, Pasiakos SM. Optimizing intramuscular adaptations to aerobic exercise: effects of carbohydrate restriction and protein supplementation on mitochondrial biogenesis. Adv Nutr. 2013;4(6):657–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tucker TJ, Slivka DR, Cuddy JS, Hailes WS, Ruby BC. Effect of local cold application on glycogen recovery. J Sports Med Phys Fitness. 2012;52(2):158–64. [PubMed] [Google Scholar]
- 43.Naperalsky M, Ruby B, Slivka D. Environmental temperature and glycogen resynthesis. Int J Sports Med. 2010;31(8):561–6. [DOI] [PubMed] [Google Scholar]
- 44.Jentjens RL, Wagenmakers AJ, Jeukendrup AE. Heat stress increases muscle glycogen use but reduces the oxidation of ingested carbohydrates during exercise. J Appl Physiol (1985). 2002;92(4):1562–72. [DOI] [PubMed] [Google Scholar]
- 45.Margolis LM Wilson MA Whitney CC, et al. Acute hypoxia reduces exogenous glucose oxidation, glucose turnover, and metabolic clearance rate during steady-state aerobic exercise. Metabolism. 2019;103:154030. [DOI] [PubMed] [Google Scholar]
- 46.Carter SL, Rennie C, Tarnopolsky MA. Substrate utilization during endurance exercise in men and women after endurance training. Am J Physiol Endocrinol Metab. 2001;280(6):E898–907. [DOI] [PubMed] [Google Scholar]
- 47.Devries MC, Hamadeh MJ, Phillips SM, Tarnopolsky MA. Menstrual cycle phase and sex influence muscle glycogen utilization and glucose turnover during moderate-intensity endurance exercise. Am J Physiol Regul Integr Comp Physiol. 2006;291(4):R1120–8. [DOI] [PubMed] [Google Scholar]
- 48.Tarnopolsky MA, Rennie CD, Robertshaw HA, Fedak-Tarnopolsky SN, Devries MC, Hamadeh MJ. Influence of endurance exercise training and sex on intramyocellular lipid and mitochondrial ultrastructure, substrate use, and mitochondrial enzyme activity. Am J Physiol Regul Integr Comp Physiol. 2007;292(3):R1271–8. [DOI] [PubMed] [Google Scholar]
- 49.Tarnopolsky MA, Atkinson SA, Phillips SM, MacDougall JD. Carbohydrate loading and metabolism during exercise in men and women. J Appl Physiol (1985). 1995;78(4):1360–8. [DOI] [PubMed] [Google Scholar]
- 50.Flynn S, Rosales A, Hailes W, Ruby B. Males and females exhibit similar muscle glycogen recovery with varied recovery food sources. Eur J Appl Physiol. 2020;120(5):1131–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Bock K Richter EA Russell AP, et al. Exercise in the fasted state facilitates fibre type-specific intramyocellular lipid breakdown and stimulates glycogen resynthesis in humans. J Physiol. 2005;564(Pt 2):649–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCartney D, Desbrow B, Irwin C. Post-exercise ingestion of carbohydrate, protein and water: a systematic review and meta-analysis for effects on subsequent athletic performance. Sports Med. 2018;48(2):379–408. [DOI] [PubMed] [Google Scholar]
- 53.McLellan TM, Pasiakos SM, Lieberman HR. Effects of protein in combination with carbohydrate supplements on acute or repeat endurance exercise performance: a systematic review. Sports Med. 2014;44(4):535–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


