ABSTRACT
Purpose
We recently reported that oral ketone ester (KE) intake before and during the initial 30 min of a 3 h 15 min simulated cycling race (RACE) transiently decreased blood pH and bicarbonate without affecting maximal performance in the final quarter of the event. We hypothesized that acid–base disturbances due to KE overrules the ergogenic potential of exogenous ketosis in endurance exercise.
Methods
Nine well-trained male cyclists participated in a similar RACE consisting of 3 h submaximal intermittent cycling (IMT180′) followed by a 15-min time trial (TT15′) preceding an all-out sprint at 175% of lactate threshold (SPRINT). In a randomized crossover design, participants received (i) 65 g KE, (ii) 300 mg·kg−1 body weight NaHCO3 (BIC), (iii) KE + BIC, or (iv) a control drink (CON), together with consistent 60 g·h−1 carbohydrate intake.
Results
KE ingestion transiently elevated blood D-ß-hydroxybutyrate to ~2–3 mM during the initial 2 h of RACE (P < 0.001 vs CON). In KE, blood pH concomitantly dropped from 7.43 to 7.36 whereas bicarbonate decreased from 25.5 to 20.5 mM (both P < 0.001 vs CON). Additional BIC resulted in 0.5 to 0.8 mM higher blood D-ß-hydroxybutyrate during the first half of IMT180′ (P < 0.05 vs KE) and increased blood bicarbonate to 31.1 ± 1.8 mM and blood pH to 7.51 ± 0.03 by the end of IMT180′ (P < 0.001 vs KE). Mean power output during TT15′ was similar between KE, BIC, and CON at ~255 W but was 5% higher in KE + BIC (P = 0.02 vs CON). Time to exhaustion in the sprint was similar between all conditions at ~60 s (P = 0.88). Gastrointestinal symptoms were similar between groups.
Discussion
The coingestion of oral bicarbonate and KE enhances high-intensity performance at the end of an endurance exercise event without causing gastrointestinal distress.
Key Words: KETONE, BICARBONATE, EXERCISE PERFORMANCE, KETOACIDOSIS
The popularity of oral ketone supplementation in athletic populations has exponentially grown over recent years. From a scientific perspective, this popularity was originally triggered by a seminal publication by Cox and his coworkers (1), showing that exogenous ketosis generated by high-dose ingestion of the ketone ester (R)-3-hydroxybutyl (R)-3-hydroxybutyrate (KE) improved performance in a 30-min time trial for maximum distance, after a 1-h constant-load cycling bout at 75% of Wmax. More recently, we also demonstrated that consistent ingestion of KE after training sessions during a 3-wk training overload period not only markedly suppressed the development of overreaching symptoms but also significantly enhanced exercise tolerance as well as improved endurance exercise performance (2).
Nonetheless, the effects of acute exogenous ketosis in endurance exercise performance remain a matter of considerable debate. In contrast to the original findings by Cox et al. (1), follow-up studies failed to demonstrate an ergogenic effect of exogenous ketosis on shuttle run time to exhaustion (3), repeated sprint performance (3,4), 5- and 10-km running performance (5,6), 4–60 min cycling time-trial performance (7–10), and during an incremental cycle test (11). We recently also found that KE intake during the initial phase of a 3-h 15-min simulated cycling race did not enhance the 15-min time-trial performance, nor sprint performance in the final part of the event (12). These conflicting results are at least partly explained by different sources of ketone supplements used. For instance, poor gastrointestinal tolerance of ketone salts, i.e., sodium-potassium or magnesium-calcium ß-hydroxybutyrate (4,9,10), ketone precursors (6,7), or ketone diester (8), limits the actual elevation of circulating ketone body concentrations above 2 mmol·L−1, which has been speculated by some to be required to potentially affect exercise performance (13). Conversely, KE is in general well tolerated (14,15) and allows to reach blood ketone concentrations of ~2–5 mmol·L−1 during exercise (1,11). However, in contrast to the aforementioned study by Cox et al. (1), other studies failed to demonstrate an ergogenic effect of acute KE administration either in a maximal incremental cycling test (11) or in a 10-km all-out run preceded by a 1-h submaximal constant-load running bout (5). Different physiological mechanisms are probably implicated in the modulation of endurance exercise performance during exogenous ketosis. First, the aforementioned study by Cox et al. (1) showed that high-dose KE intake during 120-min cycling at 70% of V˙O2max resulted in glycogen sparing, which may exert an ergogenic action whenever glycogen availability per se limits performance. However, this occurred in the presence of 40% lower oral carbohydrate intake in KE than in the control condition, which per se may have induced lower fractional contribution of carbohydrates in the KE condition (16–18). Moreover, exercise testing was performed in the fasted state which shifts energy metabolism from carbohydrate to fat metabolism (19,20). This may have provoked the observed ergogenic effect of KE, as under conditions of high carbohydrate availability, reduced glycolytic flux will rather impair high-intensity performance given its reliance on carbohydrate utilization for energy provision (5,16). Hence, we recently observed that the glycogen sparing effect as well as the ergogenic effect of KE was entirely negated by providing identical high carbohydrate intake before and during (60 g·h−1) endurance exercise (12).
Exogenous ketosis may also affect performance by shifting acid–base balance. Early studies have shown that the acid load caused by the ingestion of 20–40 g KE decreases blood pH by about 0.05–0.10 U at rest (21) as well as during submaximal exercise (11). For comparison, in the conditions of our aforementioned study involving a 3-h 15-min simulated cycling race, blood pH transiently decreased by ~0.05 U after KE ingestion. Importantly, the intermittent ingestion of KE also decreased blood alkaline reserve in the approach of the final stage of the event. Moreover, the participants reported higher degree of fatigue perception during the early phase of the event when blood pH was slightly reduced because of KE. Although the precise role of [H+] in the development of muscular fatigue is still a matter of debate (22), a recent meta-analysis clearly indicated that extracellular acidosis negatively affects exercise performance (23), even at moderately decreased pH values (24) as observed following KE ingestion.
Taking literature data (11,21) and our own recent observations (12) together, it is reasonable to assume that the potential of exogenous ketosis to enhance endurance exercise performance via beneficial metabolic regulation may be overruled by ketosis-induced acid–base dysregulation. Hence, we postulated that the prevention of KE-induced acidosis by concomitant oral bicarbonate (NaHCO3) ingestion may unlock the ergogenic potential of KE. It is well established that oral NaHCO3 ingestion before exercise can counteract exercise-induced pH drop and thereby stimulate performance in short all-out exercise (23,25,26). In addition, we have recently also demonstrated that consistent low-dose NaHCO3 ingestion during prolonged submaximal exercise can elevate alkaline reserve without causing gastrointestinal distress (Dalle et al., unpublished observations). Against this background, the aim of the present study was to investigate whether NaHCO3 intake and exogenous ketosis produced by oral KE administration may be synergistic in improving endurance exercise performance.
METHODS
Ethical approval and participants
Nine well-trained male cyclists (mean ± SD; age = 29 ± 5 yr, height = 1.81 ± 0.07 m, body mass = 71 ± 7 kg; V˙O2max = 61.0 ± 2.9 mL·kg−1·min−1, range = 55–64 mL·kg−1·min−1, cycling activity = 11.2 ± 4.6 h·wk−1, range = 7–20 h·wk−1) volunteered in this study, which was approved by the KU Leuven Biomedical Ethics Committee (B322201939080), and conforms to the Declaration of Helsinki. Participants gave their written informed consents after they were informed of the content and potential risks involved with the experimental procedures. Exclusion criteria for participation were smoking and intake of any medication or ergogenic supplement during the last 3 months before the start of the study. Participants were instructed to maintain their habitual physical activity level and diet during the full study period.
General study design
The research design (Fig. 1) was adopted from our recent study in which we investigated the effect of KE in endurance exercise performance (12). The study was of a double-blind, placebo-controlled, crossover design and involved four experimental sessions, each separated by a 1-wk washout period. All experimental sessions were conducted at the same time of the day and involved a simulated cycling race (RACE). The race started with 3 h submaximal intermittent cycling (IMT180′) followed by a 15-min time trial (TT15′) and an all-out exercise bout at 175% of the anaerobic threshold (SPRINT). On each of the experimental days, the participants were allocated to one of the following four supplemental conditions in a stratified, randomized order (stratum: average power output during TT15′ in the second familiarization session): (i) KE, (ii) NaHCO3 (BIC), (iii) KE + BIC, or (iv) placebo (CON). Briefly, participants were ranked based on their average power output during TT15′ in the second familiarization session, and the 24 possible sequences of the four experimental conditions were assigned to the nine subjects by lottery and by a person who was otherwise not involved in the experiments. Whenever needed, the lottery for a given subject was repeated to eventually obtain a well-balanced distribution of the stratum as well as the four experimental conditions over the four experimental sessions. In the KE conditions (KE, KE + BIC), participants received a 65-g (922 ± 85 mg·kg−1, range = 790–1087 mg·kg−1) KE dose in the form of a drink [96% (R)-3-hydroxybutyl (R)-3-hydroxybutyrate; TdeltaS Ltd., Thame, Oxfordshire, UK). Drinks were provided in three aliquots (25–20–20 g) to be ingested (i) 60 and (ii) 20 min before IMT180′ and (iii) at minute 30 of IMT180′. In line with our earlier study (12), we aimed to induce physiological ketosis (2–5 mM βHB) during the initial 1.5–2 h of IMT180′. The other conditions (CON and BIC) received a taste and viscosity-matched placebo, which was prepared by dissolving collagen (12.5% w/v; 6d Sports Nutrition, Oudenaarde, Belgium) and 1 mM bitter sucrose octaacetate (Sigma-Aldrich, Bornem, Belgium) in water. Both drinks were provided in nontransparent 50-mL tubes to avoid the visual identification of the treatments. In the BIC conditions (BIC and KE + BIC), participants received a total of 300 mg·kg−1 body weight (BW) NaHCO3 using a similar protocol as previously implemented (Dalle et al., unpublished observations). They ingested 90 mg·kg−1 with breakfast, followed by two boluses of 30 mg·kg−1 at 75 and 30 min before RACE in the form of 700-mg gelatin capsules. Throughout IMT180′, participants received NaHCO3 at a rate of 50 mg·kg−1·h−1 dissolved in 500 mL of a 6% maltodextrin solution. Conversely, in the other conditions (CON and KE), participants ingested an appearance-matched placebo (NaCl), containing an equimolar amount of sodium. Furthermore, during IMT180′, participants received one energy bar delivering 30 g carbohydrates per hour (6d Sports Nutrition) to obtain a carbohydrate intake of 60 g·h−1 in each experimental arm. All supplements used were badge controlled by an independent laboratory to contain no contaminating agents. All exercise tests were performed in an air-conditioned laboratory (18°C, 60% relative humidity) and on the participant’s own bicycle, which was mounted on a calibrated cycle ergometer (Avantronic Cyclus II, Leipzig, Germany).
FIGURE 1.
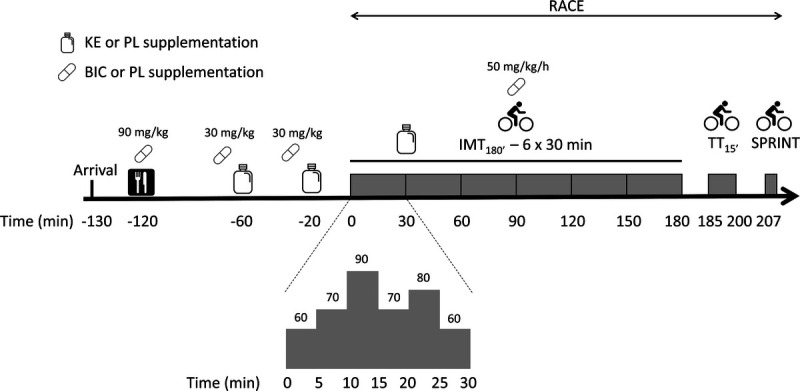
Schematic overview of the experimental sessions. The study involved four experimental sessions in a double-blind, randomized crossover design. At each occasion, participants (n = 9) participated in a 6 × 30-min simulated intermittent cycling race (IMT180′) while ingesting 60 g carbohydrates per hour, followed by a 15-min simulated time trial (TT15′) and a ~1‐min all-out exercise bout at 175% of LT (SPRINT). Before and during exercise, the participants received placebo (CON), KE, bicarbonate (BIC), or KE plus bicarbonate supplements (KE + BIC). Intensities during IMT180′ were set relative to each individual’s LT. PL placebo.
Preliminary testing
Two weeks before the first experimental session, the participants participated in two familiarization trials with 4 d in between. During the first visit, participants performed a maximal incremental cycling test to determine their lactate threshold (LT) and maximal oxygen uptake rate (V˙O2max). Initial workload was set at 100 W and was increased by 40 W per 8 min until volitional exhaustion. Respiratory gas exchange was measured during the final phase of the test (Cortex Metalyzer II, Leipzig, Germany), and the highest oxygen uptake measured over a 30-s period was defined as the V˙O2max. At minutes 4 and 8 of each intensity block, capillary blood samples were obtained from a hyperemic earlobe for the determination of blood lactate (Lactate Pro2, Arkray, Japan). LT was determined as the lowest workload corresponding to a ≥ 1 mM blood lactate increment from minutes 4 to 8 within the same stage, indicating onset of lactate non–steady state. After 15 min of active recovery by cycling at 100 W, the participants performed the final 2 h of the simulated cycling race (see below) to simulate an identical exercise duration (~3 h 15 min) compared with the experimental sessions. On the second visit, the participants completed the full simulated cycling race as to be performed during the later experimental trials.
Experimental sessions
On the evening before each experimental session, the participants received a standardized carbohydrate-rich dinner (~5600 kJ; 69% carbohydrate [3.28 ± 0.30 g·kg−1], 16% fat [0.34 ± 0.03 g·kg−1], and 15% protein [0.71 ± 0.07 g·kg−1]). After an overnight fast, the participants reported to the laboratory 2 h before the start of RACE (between 6:00 am and 10:00 am) and received a standardized breakfast (~2600 kJ; 72% carbohydrate [1.59 ± 0.15 g·kg−1], 15% fat [0.15 ± 0.01 g·kg−1], and 13% protein [0.29 ± 0.03 g·kg−1]) followed by 500 mL of a 6% carbohydrate drink (6d Sports Nutrition) 90 min later. RACE (Fig. 1) started with IMT180′ during which the intensity was varied between 60% and 90% (5-min stages) of the previously determined LT. Upon the completion of IMT180′ and an ensuing 5-min rest period, participants started TT15′ in which they aimed for the highest possible mean power output. During the initial 3 min (t0–t3), workload was fixed at the mean power output affected during TT15′ at the occasion of the last familiarization session. From t3 to t12, participants could voluntarily increase or decrease the workload at 3-min intervals according to their perception of fatigue. From t12 to t15, 1-min adjustments were allowed to establish full exhaustion by the end of TT15′. After completing TT15′, participants rested for 5 min followed by 2 min cycling at 50 W. Thereafter, participants performed a constant-power (175% LT) all-out exercise bout (SPRINT) to exhaustion, which was defined as drop of cadence to <70 rpm. Test–retest reliability in well-trained cyclists for TT15′ (+2.0% ± 2.8%, mean ± SD) and SPRINT (+1.2% ± 5.4%) after IMT180′ has previously been determined by measuring the percent change in performance between two subsequent sessions without previous familiarization (12). During the first experimental session, water was provided ad libitum to the participants, and intake was recorded to allow replication during the ensuing sessions. Throughout each exercise session, a countdown timer was shown, but power output and heart rate were blinded to the participants. During SPRINT, participants received no information about the elapsed time.
Respiratory gas measurements
Minute ventilation (V˙E), oxygen consumption (V˙O2), and carbon dioxide production (V˙CO2) were measured using breath-by-breath indirect calorimetry (Cortex Metalyzer II) at the beginning (t40–t55) and end (t160–t175) of IMT180′. The reported values for both 15-min periods represent the overall average of the last minute of each 5-min block. Gas analyzers were calibrated before every measurement using a 5% CO2–17% O2 gas mixture and a 3-L calibration syringe (Cortex Metalyzer).
Assessment of perceived exertion, gastrointestinal distress and appetite perception
Subjective scores of the RPE were assessed halfway IMT180′ and immediately after IMT180′, TT15′, and SPRINT using a 6–20 Borg Scale (27). Gastrointestinal discomfort was rated 6 min after the completion of SPRINT by means of a validated 0–8 Likert scale questionnaire (28). The questionnaire evaluated distress at the systemic (dizziness, headache, muscle cramp, urge to urinate), upper (reflux, bloating, nausea, vomiting), and lower abdominal (cramps, flatulence, abdominal pain, diarrhea) level. Subsequently, “appetite sensations” were determined using a validated 10-point visual analog scale (29) for the following four questions: “How hungry do you feel?” “How full do you feel?” “How satisfied do you feel?” “How much do you think you could eat now?”
Capillary blood sampling and analyses
Capillary blood samples were obtained from a hyperemic earlobe for the evaluation of blood D-ß-hydroxybutyrate (ßHB) and glucose concentrations (Glucomen Lx plus-meter with Lx ß-ketone and Lx glucose strips; Menarini Diagnostics, Firenze, Italy) immediately before and 30 min after ingestion of the first KE bolus, as well as at 30-min intervals during IMT180′ and at the end of TT15′. Blood lactate concentrations were determined (Lactate Pro 2, Arkray, Japan) in capillary blood obtained at regular intervals during IMT180′ and TT15′, and 5 min after the completion of SPRINT. Reliability of the blood lactate, ßHB, and glucose meters was assessed at the start of each experimental day by verifying the consistency between the two Glucomen Lx PLUS meters and four Lactate Pro 2 devices used during the experiments. Measurements for a given participant were performed with the same device during each experimental condition. In addition, a 70-μL capillary blood sample from a preheated earlobe was collected into a safeCLINITUBE (Radiometer Medical APS, Copenhagen, Denmark) (i) before breakfast, (ii) immediately before the ingestion of the first KE bolus, (iii) at the start of IMT180′, (iv) in the middle of IMT180′, and (v) at the end of IMT180′, (vi) and 3 min after SPRINT. Thereafter, samples were mixed during 10 s followed by the determination of acid–base balance, blood gasses, and electrolytes using an automated acid–base laboratory (ABL90 Flex analyzer, Radiometer Medical Aps). The acid–base laboratory was calibrated before the start of the study, and quality controls for each parameter were performed before the start and every 2 h of each experimental day using the in-build quality management system of the ABL90 Flex analyzer.
Urine sampling and analyses
Urine was collected in flasks from breakfast up to 1 h postexercise. Total urinary output volume was noted, and urinary ketone excretion was evaluated using ketone reagent strips (Ketostix, Ascensia Diabetes Care). Blood and urinary ßHB concentrations as well as blood acid–base balance were assessed by an investigator who was otherwise not involved in the experimental testing, to ensure double-blindness during the experiments.
Study outcomes and statistical analyses
The primary outcome was mean power output during TT15′, and the secondary outcomes were measurements of blood acid–base balance and time to exhaustion in the SPRINT. Differences between conditions and over time were evaluated using a three-way (time–KE–BIC) repeated-measures ANOVA, whereas differences between conditions at one time point were assessed using a two-way (KE–BIC) repeated-measures ANOVA (GraphPad Prism version 8.3.0, La Jolla, CA). When the sphericity assumption was violated (Mauchly’s test), a Geisser–Greenhouse correction was used. If a significant interaction effect was detected, post hoc analyses were performed using Bonferroni correction. A P value <0.05 was defined as statistically significant. All data are presented as mean ± SD; 95% confidence intervals (CI) were included in the text for the primary outcome variables. Effect sizes were presented as Cohen’s d for pairwise comparisons and as partial eta-squared (η2) for main and interaction effects. Cohen’s d effect sizes were interpreted using thresholds of <0.25, >0.25, ≥0.5, and ≥1.0 for trivial, small, medium, and large, respectively (30). An a priori power analysis in G*Power (version 3.1) indicated that to detect a significant interaction effect (P < 0.05) for mean power output during TT15′ (primary outcome) in a two-way repeated-measures ANOVA (η2 of ~0.33 [2] and statistical power of 0.80) required eight participants.
RESULTS
Blood ßHB concentration and urinary ketone excretion
There was a significant time–KE–BIC interaction effect for blood ßHB concentrations (P < 0.01) (Fig. 2A). Initial blood ßHB concentrations were below 0.4 mM in each condition (P = 0.99) and remained stable in CON and BIC. Conversely, the ingestion of KE elevated blood ßHB to ~2–3 mM during the initial 2 h of IMT180′ (P < 0.001 vs baseline), but by the end of IMT180′, ßHB had reverted to 0.5 ± 0.5 mM (P = 0.02 vs CON and BIC). The coingestion of BIC even further increased blood ßHB levels resulting in 0.5–0.8 mM higher values for KE + BIC compared with KE from 30 min before the start of RACE until halfway IMT180′ (95% CI of difference = +0.4 to +0.8 mM, d = 1.30, all P < 0.05 KE vs KE + BIC). Only a minor fraction (0.2 ± 0.2 g) of the ketone bodies ingested in the form of the KE (65 g) was excreted via urine, whereas ketone bodies were undetectable (<0.05 g·L−1) in urine during CON and BIC (main effect of KE: P < 0.001, η2 = 0.94). Urine volume was similar between all conditions as indicated by the absence of a main or interaction effect (CON = 663 ± 440, KE = 448 ± 353, BIC = 493 ± 381, KE + BIC = 538 ± 472 mL).
FIGURE 2.
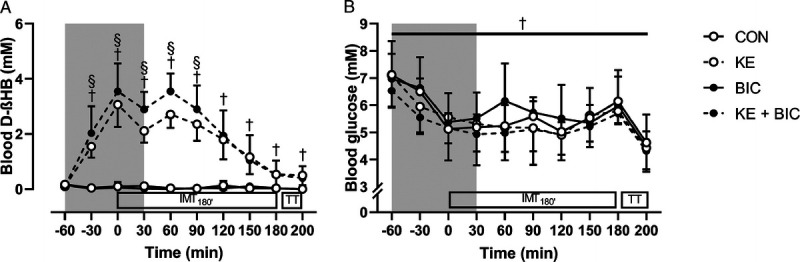
Effect of KE intake, alone or combined with bicarbonate supplementation, on blood D-βHB and glucose during the simulated cycling race. Data are presented as mean ± SD (n = 9) and represent blood D-βHB (A) and glucose (B) concentrations. Participants participated in a simulated cycling race consisting of 3 h intermittent submaximal cycling (IMT180′) followed by a 15-min simulated time trial (TT). Before and during the initial phase of IMT180′ (gray zone), the participants received control (, CON), KE (, KE), bicarbonate (, BIC), or KE plus bicarbonate supplements (, KE + BIC). †P < 0.05 for effect of KE (KE and KE + BIC vs CON and BIC). §P < 0.05 for KE + BIC vs KE.
Blood glucose and lactate concentrations
Baseline blood glucose and lactate concentrations were similar between conditions (Figs. 2B and 3A). There was a main effect of time (P < 0.001, η2 = 0.76) and KE (P = 0.047, η2 = 0.17) for glucose, indicating that KE as well as KE + BIC slightly (−0.3 ± 0.6 mM) reduced blood glucose concentrations (d = 0.76). Yet post hoc analyses did not reveal a significant difference between conditions at any time point. KE did not affect blood lactate concentrations during RACE (main effect of KE, P = 0.21; time–KE, P = 0.97). However, there was a time–BIC interaction effect (P < 0.01), indicating that blood lactate concentrations during the high-intensity blocks of IMT180′ and by the end of TT15′ and SPRINT were ~1–2 mM higher in BIC and KE + BIC than in CON and KE (all P < 0.05).
FIGURE 3.
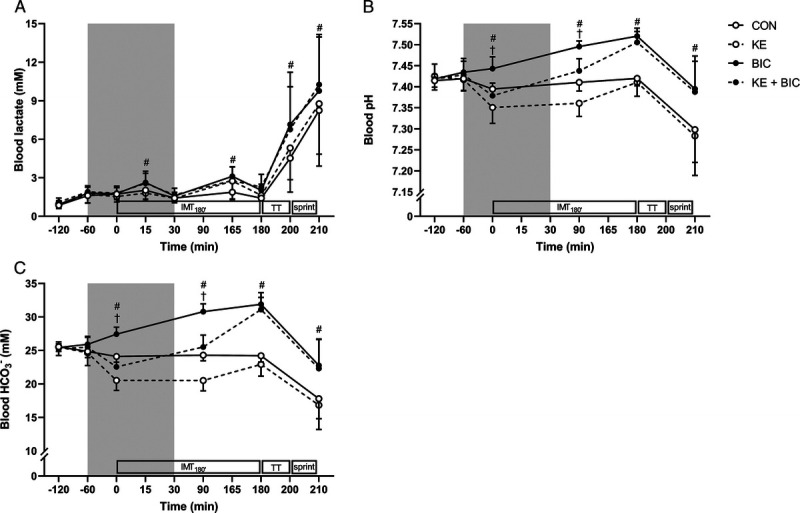
Effect of KE and/or bicarbonate supplementation on blood lactate, pH, and bicarbonate concentration. Data are presented as mean ± SD for blood lactate (A), pH (B), and bicarbonate (HCO3−) concentration (C). In a crossover design, participants (n = 9) received control (, CON), KE (, KE), bicarbonate (, BIC), or KE and bicarbonate (, KE + BIC) before and during exercise (gray zone). #P < 0.05 for effect of BIC (BIC and KE + BIC vs CON and KE). †P < 0.05 for effect of KE (KE and KE + BIC vs CON and BIC). IMT180′, 3 h submaximal intermittent cycling; TT, 15-min time trial; sprint, ~1‐min all-out exercise bout at 175% LT.
Acid–base balance and arterial PCO2
A time–KE and a time–BIC interaction effect was detected for both blood pH and [HCO3−] (all P < 0.001; Fig. 3B and C). Baseline blood pH (~7.42, range = 7.37–7.45) and [HCO3−] (25.4 mM, range = 23.7–28.3 mM) were similar between conditions (all P > 0.99). KE caused blood pH (7.36 ± 0.03) and [HCO3−] (20.5 ± 1.6 mM) to drop by halfway IMT180′ (both P < 0.001 KE vs no KE). However, the intake of BIC fully negated this mild KE-induced acidosis (P < 0.001 BIC vs no BIC). Moreover, compared with CON and KE, BIC alone or in conjunction with KE resulted in higher blood pH (BIC: 7.52 ± 0.02, KE + BIC: 7.51 ± 0.03) and [HCO3−] (BIC = 31.9 ± 1.7, KE + BIC = 31.1 ± 1.8 mM) by the end of IMT180′ (both P < 0.001). TT15′ and SPRINT caused similar pH drop between groups, yet due to the higher initial value, at the end of the exercise protocol, blood pH still was ~0.1 U higher in BIC and KE + BIC than in CON and KE (d = 1.27, P < 0.001). Conversely, from start TT15′ to end of SPRINT, blood [HCO3−] dropped more in BIC (−9.1 ± 3.7 mM) and KE + BIC (−8.8 ± 3.0 mM) than in CON (−6.4 ± 2.9 mM) and KE (−6.1 ± 2.4 mM; main effect of BIC: P < 0.01, d = 0.92, 95% CI of difference = −4.0 to −1.5 mM). A KE–BIC interaction effect (P = 0.03) was detected for PCO2. Post hoc comparisons indicated that the transient pH drop caused by KE ingestion during IMT180′ was associated with ~10 mm Hg lower PCO2 compared with CON (d = 0.90, P < 0.01), BIC (d = 1.08, P < 0.001), and KE + BIC (d = 0.74, P < 0.01).
Plasma electrolytes
For plasma calcium concentrations, a time–BIC effect was detected (both P < 0.001), together with a tendency for a time–KE effect (P = 0.06) (Fig. 4). Post hoc analyses indicated that plasma calcium levels were increased halfway IMT180′ after KE (KE vs no KE: d = 0.52, P = 0.01). The concomitant ingestion of BIC significantly decreased plasma calcium concentrations throughout RACE, resulting in 0.08 mM lower values at the end of the SPRINT in KE + BIC compared with KE (d = 2.55, P < 0.001). For plasma sodium and chloride, a tendency for a main effect of KE was observed (η2 = 0.15, P = 0.08, and η2 = 0.41, P = 0.05, respectively), indicating that plasma sodium (+1.1 mM) and chloride (+1.2 mM) concentrations tended to be higher in KE compared with CON. BIC did not affect sodium concentrations as evidenced by the absence of a main effect for BIC (P = 0.68) or a time–BIC effect (P = 0.31). Conversely, a time–BIC interaction effect (P < 0.001) was observed for plasma chloride levels, indicating that BIC resulted in ~9 mM lower plasma chloride concentrations by the end of the RACE irrespective of KE (d = 4.43, P < 0.001). Only a time effect (P < 0.001) was observed for plasma potassium concentrations, indicating that no differences between any of the experimental conditions were present.
FIGURE 4.
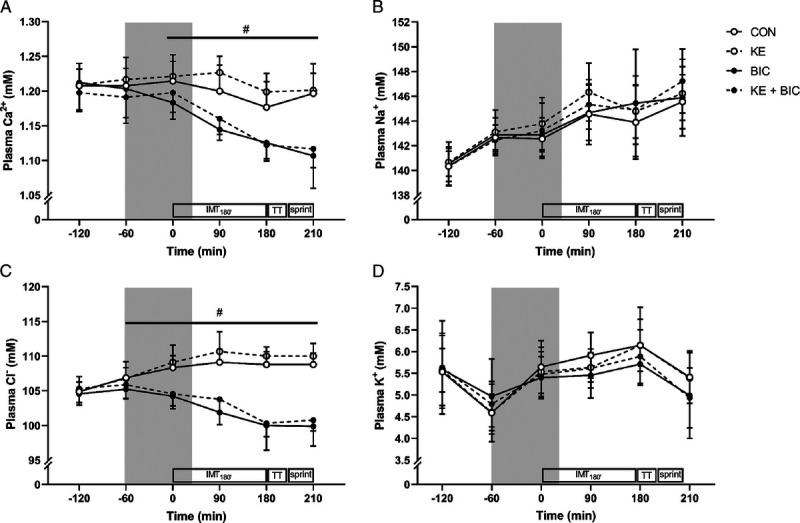
Effect of KE and/or bicarbonate supplementation on plasma electrolytes. Data are presented as mean ± SD and represent plasma calcium (Ca2+) (A), sodium (Na+) (B), chloride (Cl−) (C), and potassium (K+) (D) concentration. In a randomized crossover design, participants (n = 9) received control (, CON), KE (, KE), bicarbonate (, BIC), or KE and bicarbonate (, KE + BIC) before and during exercise (gray zone). #P < 0.05 effect of BIC (BIC and KE + BIC vs CON and KE). IMT180′, 3 h submaximal intermittent cycling; TT, 15-min time trial; sprint, ~1-min all-out exercise bout at 175% LT.
Exercise performance and RPE
A KE–BIC interaction effect was detected for mean power output during TT15′ (P = 0.04, η2 = 0.44; Fig. 5A). Post hoc analyses indicated that mean power output during TT15′ was similar (~254 W) for KE and BIC compared with CON (both P > 0.99). Conversely, mean power output in KE + BIC was 5% (+12 W) higher than in CON (95% CI = +2 to +22 W, d = 0.93, P = 0.02) and KE (95% CI = +0 to +23 W, d = 0.77, P = 0.049) and tended to be 5% higher compared with BIC (95% CI = −3 to +31 W, d = 1.01, P = 0.11; Fig. 5B). Time to exhaustion in SPRINT after TT15′ was similar between conditions as no main (KE: P = 0.96, η2 < 0.01; BIC: P = 0.44, η2 = 0.15) or interaction (KE–BIC: P = 0.88, η2 < 0.01) effect was detected (CON: 55 ± 21; KE: 55 ± 19; BIC: 58 ± 19; KE + BIC: 58 ± 21 s; Fig. 5C and D). No order effect was observed for both the performance outcomes (P = 0.30 and P = 0.66 for mean power output during TT15′ and time to exhaustion in SPRINT, respectively). RPE was similar between experimental conditions at all times as only a time effect was detected (P < 0.001; Table 1).
FIGURE 5.
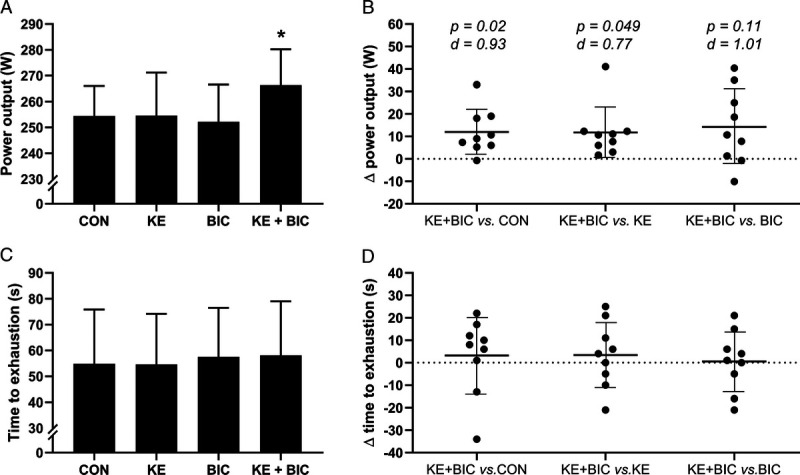
Effect of KE and/or bicarbonate supplementation on exercise performance. Data are presented as mean ± SD for average power output during the 15-min simulated time trial (TT15′) (A) and individual differences between KE + BIC vs CON, KE, and BIC together with mean ± 95% CI (B). Mean ± SD for time to exhaustion in the SPRINT (C), and individual differences between KE + BIC vs CON, KE, and BIC together with mean ± 95% CI (D). Participants (n = 9) received in a crossover design control (, CON), KE (, KE), bicarbonate (, BIC), or KE and bicarbonate (, KE + BIC) before and during exercise. *P < 0.05 for indicated condition vs CON.
TABLE 1.
Effect of KE and/or bicarbonate supplementation on RPE and heart rate.
| CON | KE | BIC | KE + BIC | |
|---|---|---|---|---|
| RPE (6–20) | ||||
| 90′ | 12.0 ± 1.7 | 12.4 ± 1.4 | 12.3 ± 1.7 | 12.3 ± 2.4 |
| 180′ | 13.3 ± 1.9 | 13.9 ± 2.4 | 13.6 ± 1.6 | 13.8 ± 3.0 |
| TT15′ | 16.0 ± 1.4 | 17.2 ± 1.2 | 16.6 ± 2.1 | 17.2 ± 1.4 |
| Sprint | 16.9 ± 1.3 | 17.4 ± 2.2 | 17.2 ± 1.4 | 17.6 ± 0.7 |
| HR (bpm) | ||||
| 40–55′ | 148 ± 13 | 153 ± 14 | 153 ± 13 | 153 ± 11 |
| 160–175′ | 152 ± 13 | 154 ± 12 | 158 ± 13 | 155 ± 11 |
| TT15′ | 160 ± 12 | 160 ± 10 | 162 ± 12 | 161 ± 9 |
| Sprint | 171 ± 12 | 176 ± 11 | 180 ± 9* | 177 ± 9* |
Values are presented as mean ± SD for RPE halfway (90′) and at the end of IMT180′ (180′), and after TT15′ and Sprint. Heart rate (HR) is represented as average values from minutes 40 to 55 (TW1) and from minutes 160 to 175 (TW2) of IMT180′, and during TT15′ and Sprint. In a crossover design, subjects (n = 9) received control (CON), ketone (KE), bicarbonate (BIC), or KE and bicarbonate (KE + BIC) before and during exercise.
*P < 0.05 for indicated condition vs CON.
Respiratory measurements and heart rate
Pulmonary gas exchange and heart rate were measured from minutes 40 to 55 and from minutes 165 to 175 during IMT180′. The first time window (TW1) represents the episode of peak blood βHB concentration upon KE intake, whereas blood βHB had nearly returned to baseline by the start of the second time window immediately before TT15′ (TW2). A time–BIC interaction effect was detected for heart rate (P = 0.047; Table 1). Post hoc analyses indicated that heart rate during IMT180′ and TT15′ was similar between all conditions. However, peak heart rate elicited by SPRINT on average was 6 ± 10 bpm (95% CI = 0 to +11) higher in BIC and KE + BIC (~179 bpm) than in CON and KE (~173 bpm, d = 0.54, P < 0.001). For V˙O2 (P = 0.04), V˙CO2 (P = 0.049), and V˙E (P < 0.01), a time–KE–BIC interaction effect was detected (Table 2). Compared with CON, V˙E during TW1 was ~15% (95% CI = +8% to +21%) higher in KE (d = 0.94, P < 0.001) and KE + BIC (d = 0.85, P < 0.001). By contrast, during TW2, V˙E was higher in KE (95% CI = 0% to +13%, d = 0.43, P = 0.049 vs CON) but not in KE + BIC (95% CI = −6% to +7%, P > 0.99 vs CON). The higher V˙E during TW1 in KE and KE + BIC also resulted in a ~10% higher V˙O2 (d = 0.66, P < 0.01 for KE vs CON and d = 0.61, P < 0.01 for KE + BIC vs CON) and V˙CO2 (d = 0.72, P < 0.01 for KE vs CON and d = 0.70, P < 0.01 for KE + BIC vs CON). In BIC, compared with CON, V˙O2 (d = 0.62, P < 0.001) and V˙CO2 (d = 0.70, P < 0.01) also increased during TW1, but this increase occurred in the absence of alterations in V˙E (P = 0.59). Only a time effect was observed for the RER (P < 0.001; Table 2), indicating similar values between conditions at all times.
TABLE 2.
Effect of KE and/or bicarbonate supplementation on respiration during exercise.
| CON | KE | BIC | KE + BIC | |
|---|---|---|---|---|
| V˙E (L·min−1) | ||||
| 40–55′ | 75.8 ± 13.1 | 87.6 ± 12.0* | 78.4 ± 15.7 | 87.5 ± 14.4* |
| 160–175′ | 84.3 ± 15.7 | 89.9 ± 10.5* | 80.0 ± 13.9 | 83.8 ± 14.4 |
| V˙O2 (L·min−1) | ||||
| 40–55′ | 2.89 ± 0.42 | 3.15 ± 0.36* | 3.18 ± 0.51* | 3.15 ± 0.44* |
| 160–175′ | 3.05 ± 0.35 | 3.01 ± 0.36 | 3.07 ± 0.34 | 3.05 ± 0.42 |
| V˙CO2 (L·min−1) | ||||
| 40–55′ | 2.71 ± 0.47 | 2.97 ± 0.34* | 3.02 ± 0.51* | 2.99 ± 0.45* |
| 160–175′ | 2.79 ± 0.39 | 2.76 ± 0.36 | 2.83 ± 0.32 | 2.84 ± 0.43 |
| RER | ||||
| 40–55′ | 0.94 ± 0.03 | 0.94 ± 0.03 | 0.95 ± 0.02 | 0.95 ± 0.02 |
| 160–175′ | 0.91 ± 0.03 | 0.92 ± 0.03 | 0.92 ± 0.02 | 0.93 ± 0.02 |
Values are presented as mean ± SD for V˙E, V˙O2, V˙CO2, and RER from minutes 40 to 55 (TW1) and from minutes 160 to 175 (TW2) of the 3-h intermittent cycling bout (IMT180′). In a crossover design, subjects (n = 9) received control (CON), KE, bicarbonate (BIC), or KE and bicarbonate (KE + BIC) before and during exercise.
*P < 0.05 for indicated condition vs CON.
Gastrointestinal comfort and appetite perception
The incidence and severity of gastrointestinal symptoms was low to moderate (mean score of 17 on a 96-point scale, range = 2–43), and there was no difference between conditions as indicated by the absence of a main or interaction effect. There was a significant KE–BIC interaction effect for “perception of hunger” and “desire to eat” (both P = 0.02). Post hoc analyses indicated that KE, but not KE + BIC or BIC, suppressed the “perception of hunger” (KE = 3.8 ± 1.1 vs CON = 5.6 ± 0.6, d = 1.16, P = 0.04; KE + BIC = 5.9 ± 1.5 and BIC = 4.9 ± 1.8, both P > 0.99 vs CON) as well as the “desire to eat” (KE = 4.0 ± 0.9 vs CON = 5.8 ± 1.7, d = 1.31, P = 0.02; KE + BIC = 5.8 ± 2.0 and BIC = 5.2 ± 1.4, both P > 0.99 vs CON) after exercise. By contrast, “perceived fullness” and “nutritional satisfaction” were similar between conditions as no main or interaction effects were observed.
Identification of experimental conditions
After the final experimental session, the participants were asked to identify the order of their experimental conditions. None of the participants was able to correctly identify the four experimental conditions, indicating successful blinding. Two participants correctly identified the CON or BIC condition, whereas three and four subjects correctly identified KE and KE + BIC, respectively.
DISCUSSION
The acute ergogenic effect of exogenous ketosis in endurance exercise is currently being debated. In fact, apart from a single study yielding positive results in a 30-min time trial (1), most studies have failed to demonstrate acute ketogenic supplement intake before or during exercise to enhance performance (3–11). From a physiological perspective, one could even logically speculate that high-dose oral KE intake may negatively affect high-intensity endurance exercise performance by glycolytic inhibition on the one hand (31) and by causing metabolic acidosis on the other hand. Indeed, it is well documented that oral KE intake in sufficient dosage to establish circulating βHB concentrations at ~3–5 mM during exercise results in a significant pH drop (11,21). Therefore, in this study, we investigated whether counteracting KE-induced acidosis by NaHCO3 coingestion may improve endurance exercise performance. We recently found that KE administration alone and in conjunction with adequate high rates of carbohydrate intake before and during exercise did not enhance the capacity to sustain high power outputs in the final phase of a 3-h 15-min simulated cycling race (12). Here we repeated this experiment but administered KE either alone or in conjunction with NaHCO3. In line with our earlier findings, KE alone did not beneficially affect performance. However, concomitant NaHCO3 administration, which was effective to fully counteract the anticipated ketoacidosis due to KE, resulted in 5% higher power outputs during the final 15 min of the race simulation. This effect was specifically due to a KE–NaHCO3 interaction because neither KE alone nor NaHCO3 alone improved performance. Thus, our findings for the first time show that counteracting pH drop due to the intake of exogenous ketones can unmask the ergogenic effect of acute exogenous ketosis in endurance exercise.
Previous studies showed that blood pH and blood HCO3− content decrease for up to 1 h postingestion of KE both at rest (21) and during submaximal exercise (11). In line with these observations, we observed that the ingestion of 65 g KE over a 90-min period before and during the initial half an hour of a 3-h 15-min simulated cycling race resulted in a transient mild ketoacidosis (pH ~7.35) with concomitant drop of alkaline reserve (see Fig. 3B and C). However, following the final KE bolus at minute 30, values gradually restored to normal within the next 150 min. KE induced hyperventilation to compensate for the metabolic acidosis, which has also been observed in a previous study (11). Two earlier studies found no clear effect of KE on V˙O2 and V˙CO2 during exercise in the fasted state (1,11). However, in the conditions of the current study, KE induced hyperventilation, which reduced PCO2 by ~10 mm Hg while slightly elevating both V˙O2 and V˙CO2 at unchanged RER. Conversely, Cox et al. (1) previously reported KE to suppress RER during exercise in the fasted state and with 40% higher rate of carbohydrate intake in KE compared with the control condition. It is not possible to exactly calculate substrate oxidation rates as coincident ketone oxidation confounds the interpretation of RER (32). Nonetheless, the unaltered RER values are in line with our previous observation that during submaximal exercise under exogenous ketosis, and against the background of ample exogenous carbohydrate supply before and during exercise, ketone body oxidation does not substitute for muscle glycogen oxidation (12). Hence, the contrasting results for RER and potentially also V˙O2 and V˙CO2 most likely are explained by different nutritional conditions before and during exercise between the studies.
Aiming to elucidate the physiological mechanisms underlying the ergogenic effect of KE + BIC in TT15′, we evaluated both the respiratory and the metabolic effects of the interventions by the end of IMT180′. Interestingly, compared with KE alone, KE + BIC counteracted the increase in V˙E at the end of IMT180′. However, V˙O2, V˙CO2, or RER were not different between KE and KE + BIC. This indicates that alterations in either pulmonary ventilation or substrate hierarchy did not account for the increased power output seen in TT15′. However, the addition of NaHCO3 ingestion to KE produced moderate alkalosis (pH ~7.51, +0.1 vs KE) in conjunction with elevated alkaline reserve (blood [HCO3−] ~31.1 mM, +8.2 mM vs KE) by the start of TT15′. Expectedly, the elevated preexercise alkaline reserve resulted in ~50% more HCO3− utilization during TT15′ and SPRINT in KE + BIC than in KE. Unfortunately, we did not assess blood acid–base balance at the end of TT15′, i.e., before the start of SPRINT. However, blood lactate was measured at repeated times before and during TT15′ and SPRINT. Clearly, TT15′ increased circulating blood lactate concentration at a much faster rate during KE + BIC and BIC than during KE alone, resulting in 50% higher blood lactate by the end of TT15′ in the former conditions. These data are in line with previous findings, indicating that elevated extracellular alkaline reserve due to bicarbonate ingestion facilitates the cotransport of H+ and La− out of skeletal muscle cells during exercise (33). However, previous research is inconclusive on whether this may result in an attenuation of exercise-induced intramuscular acidosis (33,34). Still, it cannot be excluded that the elevated La− values also resulted from higher glyco(geno)lytic rate (34).
There is also some evidence to indicate that the modulation of intra- and extracellular ion distributions, most prominently potassium, may be implicated in the ergolytic effect of exercise-induced acidosis (35). To date, one study has reported that KE intake decreases extracellular potassium concentrations at rest (21), which might depress muscular contractility during submaximal exercise (35). By contrast, we found KE and KE + BIC to affect plasma [K+] concentration neither at rest nor during exercise. Furthermore, we found calcium and chloride concentrations at the start of TT15′ to be significantly lower in KE + BIC than in KE. However, this effect was independent of KE because NaHCO3 alone caused a similar drop in extracellular calcium and chloride without affecting performance.
Interestingly, we observed that the coingestion of NaHCO3 and KE resulted in ~0.5–0.8 mM higher blood ßHB levels compared with KE alone. This effect was specifically due to an interaction of NaHCO3 and KE, as NaHCO3 alone did not increase blood [ßHB]. This is in line with a previous study showing that the infusion of 16.8 g NaHCO3 in patients with diabetic ketoacidosis delays the fall in blood ketones after insulin infusion (36). Moreover, in a subsequent experiment, the authors observed that perfusing a rat liver preparation with NaHCO3 doubled the hepatic production of ketone bodies (36). This suggests that the higher blood [ßHB] concentrations in KE + BIC compared with KE observed in our study may be the result of increased hepatic ketone production. However, it cannot be excluded that the coingestion of NaHCO3 also altered the oxidation of ketone bodies by extrahepatic tissues.
Based on the current and previous findings, it is impossible to pinpoint the precise physiological mechanisms underlying the ergogenic effect of KE + BIC in TT15′. Nonetheless, we can exclude a potential thermodynamic advantage resulting from stimulation of ketone body oxidation (37) because the ergogenic effect of KE + BIC occurred at a time when blood ketone levels had returned to <1 mM. For sure the ergogenic effect resulted from a direct interaction between KE and NaHCO3 ingestion because either agent alone failed to affect performance. Such interaction is also supported by the fact that for a given dose of KE, the coingestion of NaHCO3 yielded higher circulating βHB levels. Nonetheless, by the start of TT15′, all blood parameters measured, including blood βHB concentration, were identical between KE alone and KE + BIC. This seems to indicate that the ergogenic effect was produced by metabolic events happening during KE + NaHCO3 intake early in RACE, yet yielding residual effects during the final TT. In this regard, it has been demonstrated that metabolic alkalosis, produced by NaHCO3 loading, affects the regulation of flux-generating enzymes in carbohydrate degradation, including glycogen phosphorylase, phosphofructokinase, and pyruvate dehydrogenase (38). These effects conceivably may also alter the βHB-induced inhibition of glycolysis (31). Furthermore, our current (see Fig. 4) and previous findings (12) clearly demonstrate that KE affects exercise-induced ion fluxes, which among others results in elevated extracellular Na+ and Ca++ concentrations. Changes in sarcolemmal electrolyte gradients formed eventually may affect electrophysiological events related to neuromuscular transmission and excitation–contraction coupling. Interestingly, there is also strong evidence to indicate that the ergogenic effect of NaHCO3 in short high-intensity exercise is at least partly due to alterations in transmembrane strong ion gradients in muscle fibers (34). However, further studies are needed to elucidate the concerted actions of KE and BIC on the regulation of energy substrate metabolism and ion fluxes in muscle cells during contractions. In addition, potential ketosis-induced regulation at neuromuscular level (3,39,40), either central or peripheral, should also be considered.
In apparent contrast with the present findings, we recently demonstrated that oral NaHCO3 supplementation before and during a 3-h simulated cycling race improved power output by ~3% in a 90-s all-out exercise bout at the end of the event (Dalle et al., unpublished observations). However, the final SPRINT in the current study was preceded by TT15′, which already raised blood lactate from ~2 to ~7 mM and thus conceivably also significantly reduced blood pH and blood alkaline reserve before the start of SPRINT. Hence, the potential benefit of elevated pre-SPRINT pH and alkaline reserve probably was largely absorbed during TT15′ (41), leaving hardly any benefit in terms of buffering capacity available for SPRINT.
We and others recently also reported that acute exogenous ketosis by KE ingestion suppresses hunger and the desire to eat both at rest (42) and immediately postexercise (12). Hence, KE might suppress food intake postexercise, which in turn might impair muscle repair and training adaptation (43). The data of the current study confirm these previous observations. Although the precise mechanism by which KE decreases appetite remains to be confirmed, it is the prevailing opinion that the appetite-suppressing effect of ketosis is directly caused by ßHB. ßHB has been shown to decrease appetite both via central actions in the brain (44) as well as by decreasing ghrelin concentrations in the blood (42). However, the reduced appetite after KE may also result from ketoacidosis (45). In support of the latter hypothesis, here we show that the coingestion of bicarbonate completely negated the effect of KE to suppress appetite.
Gastrointestinal distress is a potential source of concern whenever considering KE or bicarbonate supplementation before and during exercise. However, in line with recent studies from our group (2) and others (14), KE resulted in similar gastrointestinal discomfort as the ingestion of carbohydrate drinks alone. Moreover, the addition of NaHCO3 intake did not increase symptoms. Thus, in the conditions of the current study, the coingestion of KE and NaHCO3, in conjunction with high rate carbohydrate intake during exercise, was well tolerated.
The effect of KE on most parameters measured (e.g., exercise performance, blood metabolites and acid–base balance, plasma electrolytes) was fully in line with our previous study in which an identical exercise protocol was used (12). However, we could not statistically replicate the KE-induced decrease in urinary output observed before (12). Nonetheless, urinary output on average was 33% lower in KE than in CON (mean ± SD of difference = −216 ± 292 mL, range = −4 to −910 mL). In addition, upon pooling of the results from both experiments, urinary output emerged to be significantly lower in KE compared with CON (P < 0.01, 95% CI of difference = −359 to −79 mL). Further studies will need to elucidate the physiological mechanism underlying this antidiuretic effect of KE during exercise.
In conclusion, in this study, we for the first time demonstrate that the coingestion of KE and NaHCO3 induces a transient exogenous ketosis in the absence of ketoacidosis. The coingestion of KE and NaHCO3 appears to be a well-tolerated nutritional intervention to stimulate high-intensity exercise performance in the final stage of a prolonged endurance exercise event.
Acknowledgments
The authors wish to thank all participants for their dedicated cooperation in this demanding trial.
This study was funded by Research Fund Flanders (Fonds voor Wetenschappelijk Onderzoek–Vlaanderen; research grant no. G080117N). The authors declare that they have no competing interests. The study results are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation and do not constitute endorsement by the American College of Sports Medicine.
Author contributions are as follows: C. P. and P. H., conception and design of the study; C. P.,M. R., and S. B., data collection; C. P. and P. H., analysis and interpretation of the data and manuscript drafting. All authors critically evaluated the manuscript and approved for submission. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Contributor Information
CHIEL POFFÉ, Email: chiel.poffe@kuleuven.be.
MONIQUE RAMAEKERS, Email: monique.ramaekers@kuleuven.be.
STIJN BOGAERTS, Email: stijn.bogaerts@kuleuven.be.
REFERENCES
- 1.Cox PJ Kirk T Ashmore T, et al. Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Cell Metab. 2016;24(2):256–68. [DOI] [PubMed] [Google Scholar]
- 2.Poffé C, Ramaekers M, Van Thienen R, Hespel P. Ketone ester supplementation blunts overreaching symptoms during endurance training overload. J Physiol. 2019;597(12):3009–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans M, Egan B. Intermittent running and cognitive performance after ketone ester ingestion. Med Sci Sports Exerc. 2018;50(11):2330–8. [DOI] [PubMed] [Google Scholar]
- 4.Waldman HS Basham SA Price FG, et al. Exogenous ketone salts do not improve cognitive responses after a high-intensity exercise protocol in healthy college-aged males. Appl Physiol Nutr Metab. 2018;43(7):711–7. [DOI] [PubMed] [Google Scholar]
- 5.Evans M, McSwiney FT, Brady AJ, Egan B. No benefit of ingestion of a ketone monoester supplement on 10-km running performance. Med Sci Sports Exerc. 2019;51(12):2506–15. [DOI] [PubMed] [Google Scholar]
- 6.Scott BE Laursen PB James LJ, et al. The effect of 1,3-butanediol and carbohydrate supplementation on running performance. J Sci Med Sport. 2019;22(6):702–6. [DOI] [PubMed] [Google Scholar]
- 7.Shaw DM, Merien F, Braakhuis A, Plews D, Laursen P, Dulson DK. The effect of 1,3-butanediol on cycling time-trial performance. Int J Sport Nutr Exerc Metab. 2019;29(5):466–73. [DOI] [PubMed] [Google Scholar]
- 8.Leckey JJ, Ross ML, Quod M, Hawley JA, Burke LM. Ketone diester ingestion impairs time-trial performance in professional cyclists. Front Physiol. 2017;8:806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Malley T, Myette-Cote E, Durrer C, Little JP. Nutritional ketone salts increase fat oxidation but impair high-intensity exercise performance in healthy adult males. Appl Physiol Nutr Metab. 2017;42(10):1031–5. [DOI] [PubMed] [Google Scholar]
- 10.Rodger S, Plews D, Laursen P, Driller M. Oral β-hydroxybutyrate salt fails to improve 4-minute cycling performance following submaximal exercise. J Sci Cycl. 2017;6(1):26–31. [Google Scholar]
- 11.Dearlove DJ, Faull OK, Rolls E, Clarke K, Cox PJ. Nutritional ketoacidosis during incremental exercise in healthy athletes. Front Physiol. 2019;10:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poffé C, Ramaekers M, Bogaerts S, Hespel P. Exogenous ketosis impacts neither performance nor muscle glycogen breakdown in prolonged endurance exercise. J Appl Physiol. 2020;128(6):1643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Margolis LM, O’fallon KS. Utility of ketone supplementation to enhance physical performance: a systematic review. Adv Nutr. 2020;11(2):412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stubbs BJ, Cox PJ, Kirk T, Evans RD, Clarke K. Gastrointestinal effects of exogenous ketone drinks are infrequent, mild, and vary according to ketone compound and dose. Int J Sport Nutr Exerc Metab. 2019;29(6):596–603. [DOI] [PubMed] [Google Scholar]
- 15.Cox PJ, Clarke K. Acute nutritional ketosis: implications for exercise performance and metabolism. Extrem Physiol Med. 2014;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawley JA, Leckey JJ. Carbohydrate dependence during prolonged, intense endurance exercise. Sports Med. 2015;45(1 Suppl):S5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeukendrup AE. Carbohydrate intake during exercise and performance. Nutrition. 2004;20(7–8):669–77. [DOI] [PubMed] [Google Scholar]
- 18.Tsintzas K, Williams C. Human muscle glycogen metabolism during exercise. Effect of carbohydrate supplementation. Sports Med. 1998;25(1):7–23. [DOI] [PubMed] [Google Scholar]
- 19.De Bock K Richter EA Russell AP, et al. Exercise in the fasted state facilitates fibre type-specific intramyocellular lipid breakdown and stimulates glycogen resynthesis in humans. J Physiol. 2005;564:649–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Proeyen K, Szlufcik K, Nielens H, Ramaekers M, Hespel P. Beneficial metabolic adaptations due to endurance exercise training in the fasted state. J Appl Physiol. 2011;110:236–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stubbs BJ Cox PJ Evans RD, et al. On the metabolism of exogenous ketones in humans. Front Physiol. 2017;8:848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Westerblad H. Acidosis is not a significant cause of skeletal muscle fatigue. Med Sci Sports Exerc. 2016;48(11):2339–42. [DOI] [PubMed] [Google Scholar]
- 23.Carr AJ, Hopkins WG, Gore CJ. Effects of acute alkalosis and acidosis on performance: a meta-analysis. Sports Med. 2011;41(10):801–14. [DOI] [PubMed] [Google Scholar]
- 24.Robergs R, Hutchinson K, Hendee S, Madden S, Siegler J. Influence of pre-exercise acidosis and alkalosis on the kinetics of acid–base recovery following intense exercise. Int J Sport Nutr Exerc Metab. 2005;15(1):59–74. [DOI] [PubMed] [Google Scholar]
- 25.McNaughton L, Curtin R, Goodman G, Perry D, Turner B, Showell C. Anaerobic work and power output during cycle ergometer exercise: effects of bicarbonate loading. J Sports Sci. 1991;9(2):151–60. [DOI] [PubMed] [Google Scholar]
- 26.Dalle S, De Smet S, Geuns W, Rompaye BV, Hespel P, Koppo K. Effect of stacked sodium bicarbonate loading on repeated all-out exercise. Int J Sports Med. 2019;40(11):711–6. [DOI] [PubMed] [Google Scholar]
- 27.Borg G. Psychophysical scaling with applications in physical work and the perception of exertion. Scand J Work Environ Health. 1990;16(1 Suppl):55–8. [DOI] [PubMed] [Google Scholar]
- 28.Pfeiffer B, Cotterill A, Grathwohl D, Stellingwerff T, Jeukendrup AE. The effect of carbohydrate gels on gastrointestinal tolerance during a 16-km run. Int J Sport Nutr Exerc Metab. 2009;19(5):485–503. [DOI] [PubMed] [Google Scholar]
- 29.Woods AL Rice AJ Garvican-Lewis LA, et al. The effects of intensified training on resting metabolic rate (RMR), body composition and performance in trained cyclists. PLoS One. 2018;13(2):e0191644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rhea MR. Determining the magnitude of treatment effects in strength training research through the use of the effect size. J Strength Cond Res. 2004;18(4):918–20. [DOI] [PubMed] [Google Scholar]
- 31.Newsholme EA, Randle PJ, Manchester KL. Inhibition of the phosphofructokinase reaction in perfused rat heart by respiration of ketone bodies, fatty acids and pyruvate. Nature. 1962;193:270–1. [DOI] [PubMed] [Google Scholar]
- 32.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol. 1983;55(2):628–34. [DOI] [PubMed] [Google Scholar]
- 33.Spriet LL, Lindinger MI, Heigenhauser GJ, Jones NL. Effects of alkalosis on skeletal muscle metabolism and performance during exercise. Am J Physiol. 1986;251(5 Pt 2):R833–9. [DOI] [PubMed] [Google Scholar]
- 34.Siegler JC, Marshall PW, Bishop D, Shaw G, Green S. Mechanistic insights into the efficacy of sodium bicarbonate supplementation to improve athletic performance. Sports Med Open. 2016;2(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cairns SP, Lindinger MI. Do multiple ionic interactions contribute to skeletal muscle fatigue? J Physiol. 2008;586(17):4039–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okuda Y, Adrogue HJ, Field JB, Nohara H, Yamashita K. Counterproductive effects of sodium bicarbonate in diabetic ketoacidosis. J Clin Endocrinol Metab. 1996;81(1):314–20. [DOI] [PubMed] [Google Scholar]
- 37.Sato K Kashiwaya Y Keon CA, et al. Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J. 1995;9(8):651–8. [DOI] [PubMed] [Google Scholar]
- 38.Hollidge-Horvat MG, Parolin ML, Wong D, Jones NL, Heigenhauser GJ. Effect of induced metabolic alkalosis on human skeletal muscle metabolism during exercise. Am J Physiol Endocrinol Metab. 2000;278(2):E316–29. [DOI] [PubMed] [Google Scholar]
- 39.Murray AJ Knight NS Cole MA, et al. Novel ketone diet enhances physical and cognitive performance. FASEB J. 2016;30(12):4021–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim DY Davis LM Sullivan PG, et al. Ketone bodies are protective against oxidative stress in neocortical neurons. J Neurochem. 2007;101(5):1316–26. [DOI] [PubMed] [Google Scholar]
- 41.Gough LA, Deb SK, Sparks SA, McNaughton LR. Sodium bicarbonate improves 4 km time trial cycling performance when individualised to time to peak blood bicarbonate in trained male cyclists. J Sports Sci. 2018;36(15):1705–12. [DOI] [PubMed] [Google Scholar]
- 42.Stubbs BJ, Cox PJ, Evans RD, Cyranka M, Clarke K, de Wet H. A ketone ester drink lowers human ghrelin and appetite. Obesity. 2018;26(2):269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.American Dietetic Association; Dietitians of Canada; American College of Sports Medicine; Rodriguez NR, Di Marco NM, Langley S. American College of Sports Medicine position stand. Nutrition and athletic performance. Med Sci Sports Exerc. 2009;41(3):709–31. [DOI] [PubMed] [Google Scholar]
- 44.Laeger T, Pöhland R, Metges CC, Kuhla B. The ketone body β-hydroxybutyric acid influences agouti-related peptide expression via AMP-activated protein kinase in hypothalamic GT1-7 cells. J Endocrinol. 2012;213(2):193–203. [DOI] [PubMed] [Google Scholar]
- 45.Zheng ZH, Anderstam B, Yu X, Qureshi AR, Heimbürger O, Lindholm B. Bicarbonate-based peritoneal dialysis solution has less effect on ingestive behavior than lactate-based peritoneal dialysis solution. Perit Dial Int. 2009;29(6):656–63. [PubMed] [Google Scholar]


