Supplemental Digital Content is available in the text.
Keywords: atrial fibrillation, blood pressure, brain, magnetic resonance imaging, white matter
Abstract
The association of blood pressure (BP) and hypertension with the presence of different types of brain lesions in patients with atrial fibrillation is unclear. BP values were obtained in a multicenter cohort of patients with atrial fibrillation. Systolic and diastolic BP was categorized in predefined groups. All patients underwent brain magnetic resonance imaging and neurocognitive testing. Brain lesions were classified as large noncortical or cortical infarcts, small noncortical infarcts, microbleeds, or white matter lesions. White matter lesions were graded according to the Fazekas scale. Overall, 1738 patients with atrial fibrillation were enrolled in this cross-sectional analysis (mean age, 73 years, 73% males). Mean BP was 135/79 mm Hg, and 67% of participants were taking BP-lowering treatment. White matter lesions Fazekas ≥2 were found in 54%, large noncortical or cortical infarcts in 22%, small noncortical infarcts in 21%, and microbleeds in 22% of patients, respectively. Compared with patients with systolic BP <120 mm Hg, the adjusted odds ratios (95% CI) for Fazekas≥2 was 1.25 (0.94–1.66), 1.41 (1.03–1.93), and 2.54 (1.65–3.95) among patients with systolic BP of 120 to 140, 140 to 160, and ≥160 mm Hg (P for linear trend<0.001). Per 5 mm Hg increase in systolic and diastolic BP, the adjusted β-coefficient (95% CI) for log-transformed white matter lesions was 0.04 (0.02–0.05), P<0.001 and 0.04 (0.01–0.06), P=0.004. Systolic BP was associated with small noncortical infarcts (odds ratios [95% CI] per 5 mm Hg 1.05 [1.01–1.08], P=0.006), microbleeds were associated with hypertension, but large noncortical or cortical infarcts were not associated with BP or hypertension. After multivariable adjustment, BP and hypertension were not associated with neurocognitive function. Among patients with atrial fibrillation, BP is strongly associated with the presence and extent of white matter lesions, but there is no association with large noncortical or cortical infarcts.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT02105844.
Hypertension is one of the most important cardiovascular risk factors and strongly associated with major cardiovascular events, such as atrial fibrillation (AF), congestive heart failure, myocardial infarction, and stroke.1–4 AF and hypertension often coexist and the prevalence of both conditions is expected to increase in the future.5,6 AF is a risk factor of cognitive impairment and dementia, independent of clinical stroke.7,8 AF may contribute to vascular dementia either by causing embolic strokes or via shared risk factors for small vessel disease, such as hypertension or diabetes. However, the relationship of hypertension with various types of brain lesions observed in patients with AF is poorly understood.
Brain parenchymal damage, especially white matter lesions (WML), is frequently detected on brain magnetic resonance imaging (bMRI), mainly in elderly individuals. The prevalence and volume of different clinical and subclinical brain lesions has been reported to be significant both in patients with and without AF.9–11 In the general population, increased blood pressure (BP) was associated with the occurrence and the progression of WML.12–14 In elderly individuals, hypertension was not only associated with WML, but also with MRI-detected subclinical brain infarcts.10 In a small study of patients with lacunar strokes, a positive association was found between ambulatory BP and cerebral microbleeds.15 Although smaller studies exist in different patient groups without AF,10,12–15 the association between BP and clinical and subclinical brain lesions has never been investigated thoroughly in patients with AF. Whether hypertensive patients with AF show a different pattern of brain lesions compared to normotensive patients with AF is unknown. The generalizability of the current evidence in general populations to patients with AF is not clear and patients with AF might have a different susceptibility for brain lesions due to several reasons. First, patients with AF often suffer from multiple comorbidities and have a high risk of brain lesions due to AF and other cardiovascular comorbidities. Second, AF per se might be a risk modifier regarding BP-related brain lesions for example due to high beat-to-beat BP variability or cerebral hypoperfusion. In addition, the majority of patients with AF are on oral anticoagulation for stroke prevention. However, its effect on the association between BP and brain lesions is unknown. Finally, the shape of the association between BP and brain lesions in patients with AF is unclear. Therefore, the aim of this analysis was to investigate brain lesion types according to BP and BP control in an unselected cohort of patients with AF.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Data of the ongoing, prospective, observational, multicenter Swiss-AF (Swiss Atrial Fibrillation Cohort Study) were used for this cross-sectional analysis. The study design of the Swiss-AF study was published previously.16 In brief, patients ≥65 years with documented AF were enrolled in 14 study centers in Switzerland between 2014 and 2017. A group of 250 patients with AF <65 years were enrolled, as indicated in the study protocol. Exclusion criteria were short episodes of AF (eg, after cardiac surgery, or severe sepsis), any acute illness within the past 4 weeks, or inability to sign the informed consent. The study protocol was approved by the local Ethics committee. Written informed consent was obtained from all patients and the study was conducted in accordance with the Helsinki declaration.
Of 2415 patients enrolled in this study, 667 patients had to be excluded due to missing bMRI (mainly due to the presence of a cardiac device or claustrophobia), 5 patients due to a missing MRI sequence (fluid-attenuated inversion recovery), and 5 patients due to missing BP measurement, resulting in the 1738 patients used for this analysis.
BP and Hypertension
Systolic and diastolic BP was measured 3× in a supine position after 5 minutes of rest using a validated device. For this analysis, the mean of all available BP measurements was used. If only 2 or 1 BP measurements were available, we used the mean of 2 or even a single measurement (n=2 with 2 and n=4 with only one BP measurement). Hypertension was defined according to current guidelines,17,18 and all definitions are presented in Table S1 in the Data Supplement.
Other Study Variables
Information on cardiovascular risk factors, lifestyle factors, medical history, and medication was assessed using standardized questionnaires. Weight and height were taken and body mass index was calculated as the ratio of the weight in kg and the height in m2. Smoking status was categorized into current, former, or never smoking. Educational status was classified according to the highest degree achieved. To assess neurocognitive function, the Montreal Cognitive Assessment (MoCA) score, the Trail Making Test (part A and B), the Semantic Fluency Test, and the Digit Symbol Substitution Test were performed in all patients. In brief, the validated MoCA score is scaled from 0 (worse) to 30 (best) and is representing global cognitive functioning. Patients with <12 years of education receive an additional point if they have <30 points. Details regarding the methodology of the other cognitive tests are presented in Table S2.19–24
Brain Magnetic Resonance Imaging
A bMRI without application of contrast agents was obtained according to a standardized protocol in all eligible study patients. The standardized protocol included a sagittal 3-dimensional T-weighted magnetization-prepared rapid gradient echo (spatial resolution 1.0×1.0×3.0 mm3), an axial 2-dimensional fluid-attenuated inversion recovery (spatial resolution 1.0×1.0×3.0 mm3), and an axial 2-dimensional diffusion-weighted imaging (spatial resolution 1.0×1.0×3.0 mm3) sequence with whole-brain coverage, no gaps and without interpolation. All bMRI data were sent to and analyzed in a specialized imaging core laboratory (Medical Imaging Analysis Center AG, Basel, Switzerland). The scans were analyzed by blinded expert raters. Brain lesions were marked and segmented in a standardized fashion using an in-house procedure approved for international clinical studies. Board-certified neuroradiologists confirmed all ratings.
The Fazekas scale was used to grade hyperintense white matter lesions. A score of ≥2 in either the periventricular or the deep white matter was defined as at least moderate disease.25 Ischemic brain lesions were classified as large noncortical or cortical infarcts (LNCCI) or small noncortical infarcts (SNCI). Large noncortical infarcts are defined as noncortical infarcts >20 mm. Cortical infarcts are defined as hyperintense lesions on fluid-attenuated inversion recovery involving the cortex independent of the size of the lesion. Both lesion types in combination are defined as LNCCI. SNCIs are defined as noncortical infarcts<20 mm.26 The cause of LNCCI and SNCI cannot be proven based on the bMRI. However, in general, LNCCIs might represent embolic lesions, whereas SNCI might correspond to microvascular brain damage. Microbleeds were identified and counted as nodular, hypointense lesion on either T2*-weighted or susceptibility-weighted imaging.
Statistical Analysis
Baseline characteristics were stratified by groups of systolic BP (<120, 120–140, 140–160, and ≥160 mm Hg) and hypertension categories (normotension, controlled hypertension, and uncontrolled hypertension). Continuous data are presented as mean±SD or median (interquartile range) and categorical data as numbers (percentages). Continuous and categorical data were compared across categories using ANOVA, Kruskal-Wallis tests or χ2 tests, as appropriate.
To assess the associations of BP and hypertension with the prevalence of vascular brain lesions, multivariable-adjusted logistic regression analyses were performed using the presence of Fazekas score ≥2, LNCCI, SNCI, or microbleeds as the outcome variable. In a second step, the association of BP and hypertension with the volume of brain lesions was calculated using multivariable-adjusted linear regression analysis. Due to the skewed distribution, the volumes of the respective brain lesions were log-transformed. Results are presented as odds ratios (OR) or β-coefficients with the corresponding 95% CI, and 2-sided P-values were calculated. The volume of microbleeds was not quantified due to an expected blooming effect of the volume when using the current methods. All regression analyses were adjusted for age and sex. A second model was additionally adjusted for educational status, body mass index, smoking status, diabetes, history of stroke, history of heart failure, and history of coronary heart disease, AF type, oral anticoagulation, and antithrombotic treatment. When using BP as the predicting variable, we additionally adjusted the models for antihypertensive treatment (yes/no).
The association of BP and hypertension with the MoCA score and the other neurocognitive tests was calculated using multivariable-adjusted linear regression models. Subgroup analysis for the presence or absence of the respective brain lesion was done for the association of BP or hypertension with the MoCA score. The model was adjusted as described above. We tested interacting effects of lesion presence and BP or hypertension by including the respective interaction terms in the models.
P values were not corrected for multiple comparisons due to the exploratory nature of this analysis. All analyses were performed with R version 3.5.2 (2018-12-20; R Core Team, 2019).
Results
Baseline Characteristics
Baseline characteristics stratified by systolic BP categories and hypertension are presented in Table 1 and Table S3, respectively. The mean age of the population was 73±8 years, and 73% were male. Mean SBP and DBP were 135±19 and 79±12 mm Hg, respectively, and 1158 (67%) patients were on antihypertensive treatment. A history of stroke was present in 230 (13%) patients. Across increasing systolic BP categories, patients were older, more often female, and had a higher CHA2DS2-VASc score. The prevalence of recurrent falls across systolic BP categories was not different, even when looking at patients with low systolic BP (Table 1 and Table S4). Normotension, controlled hypertension and uncontrolled hypertension according to the criteria of the European Society of Cardiology were present in 387 (22%), 642 (37%), and 709 patients (41%), respectively (Table S3).
Table 1.
Baseline Characteristics Stratified by Blood Pressure Categories

Prevalence and Volume of Brain Lesions
On the bMRI, 99% of the patients presented WMLs, 54% had a Fazekas score ≥2, 22% had LNCCIs, 21% SNCIs and 22% microbleeds. In patients without a history of stroke or transient ischemic attack, 50% had a Fazekas score ≥2, 15% had LNCCIs, 18% SNCIs and 20% microbleeds. As an example, the bMRI of 2 different patients with AF is presented in Figure 1, showing one patient with few brain lesions (Fazekas <2) and one patient with multiple brain lesions (Fazekas ≥2, SNCI, and LNCCI). There was a linear increase of the prevalence of Fazekas ≥2, SNCIs, and microbleeds as well as volume of WML across systolic BP categories (P value for all <0.02; Table 2 and Figure 2). However, the prevalence and volume of brain lesions did not differ across categories of diastolic BP (Table 2). The prevalence and volume of WMLs, LNCCIs, SNCIs, and microbleeds stratified by hypertension are presented in Table S5.
Figure 1.

Brain magnetic resonance imaging (fluid-attenuated inversion recovery) of 2 different patients with atrial fibrillation. A, Normotensive man, 72 y old with a small amount of white matter lesions (Fazekas score <2, arrows). B, Uncontrolled hypertensive man, 74 y old with white matter lesions inferior (Fazekas score ≥2, arrows), lacunar lesions (circle), and cortical infarcts (right bottom).
Table 2.
Prevalence and Volume of Brain Lesions Stratified by Blood Pressure Categories

Figure 2.
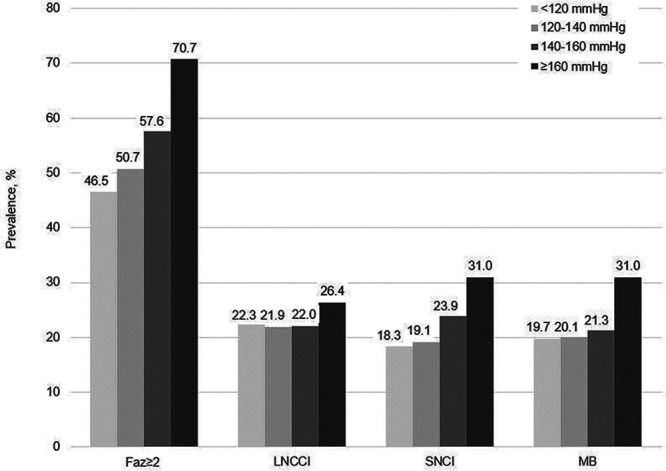
Prevalence of brain lesions stratified by systolic blood pressure categories. Faz indicates Fazekas; LNCCI, large noncortical or cortical infarcts; MB, microbleeds; and SNCI, small noncortical infarcts.
Association of BP and Hypertension With White Matter Lesions
The multivariable-adjusted ORs for Fazekas≥2 among patients with systolic BP of 120 to 140, 140 to 160 and ≥160 mm Hg were 1.25 (0.94–1.66), 1.41 (1.03–1.93), and 2.54 (1.65–3.95), compared with patients with a systolic BP <120 mm Hg (P<0.001). In addition, we found an association between continuous BP and Fazekas ≥2. Per 5 mm Hg increase in systolic and diastolic BP, the odds of Fazekas ≥2 increased by 7% (OR [95% CI], 1.07 [1.03–1.10], P<0.001 and 1.07 [1.02–1.12], P=0.005, respectively; Table 3). There was a linear increase in WML volume across systolic and diastolic BP categories (P value for trend <0.001 for systolic and P value for trend 0.004 for diastolic BP; Table 3 and Figure 3). Results of the association between hypertension and WML are presented in Table S6.
Table 3.
Association Between Blood Pressure and WML

Figure 3.

Scatterplot of the age and sex-adjusted association of blood pressure with volume of white matter lesions. The solid line represents the model-based predicted values, and the dotted lines represent the 95% pointwise CI.
Association of BP and Hypertension With Large Noncortical or Cortical Infarcts, Small Noncortical Infarcts, and Microbleeds
Associations of BP and hypertension with the presence and volume of LNCCIs are presented in Tables S7 and S8. No association was found between systolic and diastolic BP and the presence and volume of LNCCIs. In addition, the presence and volume of LNCCI was not associated with controlled and uncontrolled hypertension, compared with normotensive patients. Systolic BP was associated with the presence of SNCI (OR [95% CI] per 5 mm Hg 1.05 [1.01–1.08], P=0.006), as shown in Table S9. Compared with patients with a systolic BP <120 mm Hg, the multivariable-adjusted OR (95% CI) for patients with a systolic BP of 120 to 140, 140 to 160, and ≥160 mm Hg was 1.07 (0.76–1.51), 1.31 (0.91–1.90), and 1.89 (1.20–2.97), respectively (P for linear trend=0.003). However, we found no consistent relationship between BP and hypertension with volume of SNCI (Tables S9 and S10). Finally, the presence of microbleeds was associated with hypertension (Table S11) but not with either systolic or diastolic BP (Table S12).
BP and Neurocognitive Function
Across increasing categories of BP, the mean MoCA score was different for diastolic (P=0.006) but not for systolic BP (P=0.10). Results of the Digit Symbol Substitution Test, Trail Making Test A, and Trail Making Test B were different across systolic and diastolic BP categories (Table S13). Using multivariable-adjusted regression models, we found no association between BP and neurocognitive function when using the MoCA score or the other neurocognitive tests as the outcome variables (Table S14). There was an interaction of the association between hypertension and the MoCA score for the presence/absence of Fazekas≥2 (P for interaction=0.007), with an inverse association in patients with a Fazekas≥2, but no association in patients with a Fazekas<2. None of the other interaction analyses were significant (Table S15).
Discussion
In this cross-sectional analysis of patients with AF, we identified several important results. First, most patients with AF (78%) had either controlled (37%) or uncontrolled hypertension (41%) and only few patients were normotensive without treatment. Second, the prevalence of different brain lesions was unexpectedly high, and a relevant part of these brain lesions were clinically silent. Third, BP and hypertension were linearly associated with moderate to severe white matter disease and its volume. Fourth, whereas SBP was positively associated with SNCIs, BP and hypertension were not associated with LNCCIs. Fifth, uncontrolled hypertension was associated with the presence of microbleeds in this population of mostly anticoagulated AF patients. Our results suggest that BP and hypertension have an impact on microvascular disease in patients with AF, but not on lesions of presumed embolic origin such as LNCCIs. Finally, SBP and DBP were not associated with neurocognitive function, when using different neurocognitive tests.
From a prognostic standpoint, WMLs are important because patients with WMLs face an increased risk of stroke,27 cognitive impairment, and vascular dementia.11,28,29 For example, different types of brain lesions have been found to be associated with cognitive decline in a general population29 and large infarcts and WML have been shown to be associated with lower cognitive function in our cohort of patients with AF.11 Moderate to severe WMLs (defined as Fazekas ≥2), present in over half of our study population, are known to be common in elderly populations30 and their extent by volume in our study is high. Our findings are in line with studies in patients without AF showing an association of BP or hypertension with WMLs.13 One study in patients of similar age compared to our cohort (65–75 years) but with poorly controlled hypertension, also showed a significantly higher risk of severe WMLs compared to normotensive patients.12 Even in young adults in the Framingham Heart Study (mean age, 39 years), an association between SBP and white matter integrity was found, emphasizing the importance of BP control early in life.31 WMLs are thought to be microangiopathic in origin and located mainly in the periventricular and deep white brain region. WMLs in patients with AF in our cohort most likely represent BP-induced end-organ damage and reflect the hypertensive burden as a composite of severity of hypertension, quality of BP control, and time since diagnosis. However, whether the presence or absence of AF modifies the extent of WML cannot be answered due to the lack of a control group. Nevertheless, an additional impact of AF on the association between hypertension and brain lesions is conceivable for example due to the large beat-to-beat cycle length variations in patients with AF, which result in BP peaks followed by very low BP values. A hypothesis could be that the extreme BP variations increase the risk of different types of brain lesions, mainly those of microvascular origin. If so, this could constitute further evidence supporting rhythm control in patients with AF.
LNCCI and SNCI were present in >20% in this population of patients with AF. LNCCIs are thought to represent an event of cardioembolic or arterioembolic origin. These mechanisms are considered the main underlying mechanisms of cerebral infarcts in patients with AF. In our study, the volume affected by LNCCIs is markedly higher compared to the volume of SNCIs, but much less than that of WMLs. Neither BP nor hypertension were associated with LNCCIs. This could be explained by potential competing mechanisms involved in the pathogenesis of LNCCIs as well as the protective effect of the oral anticoagulation, which is commonly prescribed in patients with AF based on risk stratification schemes such as the CHA2DS2-VASc score encompassing cardiovascular comorbidities (eg, hypertension).32 In contrast, SNCIs are, at least to some extent, caused by cerebral small vessel disease and this assumption is supported by the association between SBP and the presence of SNCI found in this analysis.
Patients with microbleeds face an increased risk of intracerebral hemorrhage. In this cohort, ≈20% of patients had at least one microbleeds, which is in line with previous studies in elderly subjects.9,33 Hypertension has been shown to be strongly associated with the presence of microbleeds in healthy adults, in hypertensive adults, and also in patients with cerebrovascular diseases (including ischemic stroke and intracerebral hemorrhage).15,33,34 This association was partly confirmed in our population of mostly anticoagulated AF patients. Different pathophysiological mechanisms may explain the occurrence of microbleeds in patients with hypertension, including ruptured arteriosclerotic microvessels,35 lipohyalinosis, and amyloid deposits in cerebral amyloid angiopathy, which may increase the vessel permeability and result in leakage of blood into the brain parenchyma.36
The question whether more aggressive BP control in patients with AF and bMRI-detected lesions (WML, LNCCIs, SNCIs, or microbleeds) is associated with better outcomes or whether these lesions can be prevented cannot be answered with our study. The SPRINT-MIND study (Systolic Blood Pressure Intervention Trial-Memory and Cognition in Decreased Hypertension) showed slower WML progression but a greater decrease in total brain volume in patients with intensive antihypertensive treatment compared to patients with a standard antihypertensive treatment.37 Whether the decrease in WML with more intensive BP lowering is beneficial in light of the more pronounced brain atrophy currently remains unclear.38 Optimal BP treatment is always a trade-off between achieving the beneficial effect of antihypertensive treatment and avoiding potential side-effects, including hypotension, syncope, or electrolyte abnormalities.39
Whether BP is independently associated with cognitive decline or dementia is controversially discussed, as stated in the scientific statement from the American Heart Association.40 Based on data of an observational study from the general population, higher systolic BP and lower diastolic BP seem to be associated with a faster cognitive decline in global cognition over 8 years.41 Another study found no significant associations of having ideal BP values and different neurocognitive functions.42 In the randomized SPRINT-MIND study, the authors found a lower risk of mild cognitive impairment in patients of the intensive treatment group (systolic BP goal of <120 mm Hg) compared with patients in the standard treatment group (systolic BP goal of <140 mm Hg).38 However, there was no association of BP treatment with probable dementia, which might be explained with the early termination of the SPRINT study. In this cross-sectional study, BP was not associated with neurocognitive function after multivariable adjustment, suggesting that this association is affected by multiple other factors, including age, sex, or educational status. However, we found potential indication for Fazekas≥2 to be an effect modifier of the association between hypertension and the MoCA score, with an inverse association of hypertension and neurocognitive function in patients with a Fazekas ≥2 but no association in patients with a Fazekas <2.
Strengths and Limitations
A main strength of this analysis is the large sample of well-characterized patients with AF. All patients had a standardized bMRI, which was centrally analyzed according to a standardized protocol. However, some limitations should be considered when interpreting the results. First, most patients in our study were of European origin, and the generalizability of our results to other populations is not clear. Second, as Swiss-AF is an observational study, residual confounding might be possible, although we adjusted our models for a comprehensive set of potential confounders. Potential unmeasured confounders, including the time since diagnosis of hypertension, AF burden, arterial stiffness, brain perfusion, and genetic determinants might have an impact on the results. Third, no information on brain perfusion is available based on the performed brain MRI scans. It is assumed that cerebral hypoperfusion could be a plausible mechanism for cognitive impairment. Therefore, advanced brain MRI might be of added value. Finally, office BP was measured 3× at one time point, making it possible for white coat hypertension to be present or masked hypertension to be missed.
Perspective
BP and hypertension in patients with AF are strongly associated with WML and to a lesser extent with SNCIs, but not with LNCCIs. Our data suggest that the presence and extent of WMLs are altered by the burden of hypertension. Further studies are needed to assess the effect of more aggressive BP control on brain lesions in patients with AF.
Sources of Funding
The Swiss-AF study (Swiss Atrial Fibrillation Cohort Study) is supported by grants of the Swiss National Science Foundation (Grant numbers 33CS30_148474, 33CS30_177520, 32473B_176178), the Swiss Heart Foundation, the Foundation for Cardiovascular Research Basel and the University of Basel. D. Conen holds a McMaster University Department of Medicine Mid-Career Research Award.
Disclosures
C. Sticherling has received speaker honoraria from Biosense Webster, Boston Scientific and Medtronic and research grants from Biosense Webster, Daiichi Sankyo, and Medtronic; He is a proctor for Medtronic (Cryoballoon); C.S. Zuern reports a research grant from Medtronic and speaker fees from Vifor Pharma and Novartis; D. Conen has received consultant/speaker fees from Servier Canada outside of the submitted work; J.H. Beer reports grants from the Swiss National Foundation of Science, The Swiss Heart Foundation, grants from Bayer, lecture fees from Sanofi Aventis, and Amgen, to the institution outside the submitted work; J. Wuerfel is an employee of MIAC AG, has received funding from EU (Horizon2020), Else-Kröner-Fresenius Foundation, Novartis Foundation, and consultancy, steering committee, advisory board, and speaker honoraria from Actelion, Bayer, Biogen, Idorsia, Roche, Sanofi-Genzyme, and Teva; L.H. Bonati received grants from the Swiss National Science Foundation (PBBSB-116873, 33CM30-124119, 32003B-156658; Berne, Switzerland), The Swiss Heart Foundation (Berne, Switzerland), and the University of Basel (Basel, Switzerland). L.H. Bonati has received an unrestricted research grant from AstraZeneca, and consultancy or advisory board fees or speaker’s honoraria from Amgen, Bayer, Bristol-Myers Squibb (BMS), and Claret Medical, and travel grants from AstraZeneca and Bayer; L. Roten receives speaker honoraria from Abbott/SJM and consulting honoraria from Medtronic; M. Kühne reports personal fees from Bayer, personal fees from Böhringer Ingelheim, personal fees from Pfizer BMS, personal fees from Daiichi Sankyo, personal fees from Medtronic, personal fees from Biotronik, personal fees from Boston Scientific, personal fees from Johnson&Johnson, grants from Bayer, grants from Pfizer BMS, grants from Boston Scientific; N. Rodondi received a grant from the Swiss Heart Foundation; T. Burkard reports personal fees from Servier, Amgen, Takeda, Menarini, MSD, Sanofi, and Vifor, outside the submitted work. The other authors report no conflicts.
Supplementary Material
Appendix
List of all Swiss-AF investigators according to participating center:
University Hospital Basel and Basel University: Chloé Auberson, Selinda Ceylan, Simone Doerpfeld, Marc Girod, Elisa Hennings, Philipp Krisai, Andreas U. Monsch, Christian Müller, Anne Springer, Gian Voellmin. University Hospital Bern: Drahomir Aujesky, Urs Fischer, Juerg Fuhrer, Simon Jung, Heinrich Mattle, Luise Adam, Carole Elodie Aubert, Martin Feller, Axel Loewe, Claudio Schneider, Tanja Flückiger, Cindy Groen, Lukas Ehrsam, Sven Hellrigl, Alexandra Nuoffer, Damiana Rakovic, Nathalie Schwab, Rylana Wenger. Stadtspital Triemli Zurich: Andreas Müller, Christopher Beynon, Roger Dillier, Michèle Deubelbeiss, Franz Eberli, Christine Franzini, Isabel Juchli, Claudia Liedtke, Jacqueline Nadler, Thayze Obst, Jasmin Roth, Fiona Schlomowitsch, Xiaoye Schneider, Katrin Studerus, Noreen Tynan, Dominik Weishaupt. Kantonspital Baden: Simone Fontana, Silke Kuest, Karin Scheuch, Denise Hischier, Nicole Bonetti, Alexandra Grau, Jonas Villinger, Eva Laube, Philipp Baumgartner, Mark Filipovic, Marcel Frick, Giulia Montrasio, Stefanie Leuenberger, Franziska Rutz. Cardiocentro Lugano: Tiziano Moccetti, Angelo Auricchio, Adriana Anesini, Cristina Camporini, Giulio Conte, Maria Luce Caputo, Francois Regoli. Kantonsspital St. Gallen: Peter Ammann, Roman Brenner, David Altmann, Michaela Gemperle. Hôpital Cantonal Fribourg: Daniel Hayoz, Mathieu Firmann, Sandrine Foucras, Martine Rime. Luzerner Kantonsspital: Richard Kobza, Benjamin Berte, Virgina Justi, Frauke Kellner-Weldon, Brigitta Mehmann, Sonja Meier, Myriam Roth, Andrea Ruckli-Kaeppeli, Ian Russi, Kai Schmidt, Mabelle Young, Melanie Zbinden. Ente Ospedaliero Cantonale Lugano: Jane Frangi-Kultalahti, Anica Pin, Luisa Vicari University Hospital Geneva: Dipen Shah, Georg Ehret, Hervé Gallet, Elise Guillermet, Francois Lazeyras, Karl-Olof Lovblad, Patrick Perret, Philippe Tavel, Cheryl Teres. University Hospital Lausanne: Jürg Schläpfer, Nathalie Lauriers, Marie Méan, Sandrine Salzmann. Bürgerspital Solothurn: Frank-Peter Stephan, Andrea Grêt, Jan Novak, Sandra Vitelli. Ente Ospedaliero Cantonale Bellinzona: Marcello Di Valentino, Jane Frangi-Kultalahti, Augusto Gallino. University of Zurich/University Hospital Zurich: Fabienne Witassek, Matthias Schwenkglenks. Medical Image Analysis Center AG Basel: Anna Altermatt, Michael Amann, Petra Huber, Esther Ruberte, Vanessa Zuber. Clinical Trial Unit Basel: Pascal Benkert, Gilles Dutilh, Milica Markovic, Pia Neuschwander, Patrick Simon. Schiller AG Baar: Ramun Schmid.
Nonstandard Abbreviation and Acronyms
- AF
- atrial fibrillation
- bMRI
- brain magnetic resonance imaging
- BP
- blood pressure
- LNCCI
- large noncortical or cortical infarcts
- MoCA
- Montreal Cognitive Assessment
- SNCI
- small noncortical infarcts
- Swiss-AF
- Swiss Atrial Fibrillation Cohort Study
- WML
- white matter lesions
A list of all Swiss-AF Investigators members is given in the Appendix.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.120.16025.
For Sources of Funding and Disclosures, see page 670.
Contributor Information
Stefanie Aeschbacher, Email: stefanie.aeschbacher@usb.ch.
Steffen Blum, Email: steffen.blum@usb.ch.
Pascal B. Meyre, Email: pascal.meyre@usb.ch.
Michael Coslovsky, Email: michael.kuehne@usb.ch.
Annina S. Vischer, Email: annina.vischer@usb.ch.
Tim Sinnecker, Email: tim.sinnecker@usb.ch.
Nicolas Rodondi, Email: nicolas.rodondi@insel.ch.
Jürg H. Beer, Email: hansjuerg.beer@ksb.ch.
Giorgio Moschovitis, Email: giorgio.moschovitis@eoc.ch.
Elisavet Moutzouri, Email: elisavet.moutzouri@extern.insel.ch.
Christof Hunkeler, Email: christof.hunkeler@stud.unibas.ch.
Thilo Burkard, Email: thilo.burkard@usb.ch.
Ceylan Eken, Email: ceylan.eken@usb.ch.
Laurent Roten, Email: laurent.roten@insel.ch.
Christine S. Zuern, Email: christine.zuern@gmx.de.
Christian Sticherling, Email: christian.sticherling@usb.ch.
Jens Wuerfel, Email: jw@miac.ch.
Leo H. Bonati, Email: leo.bonati@usb.ch.
David Conen, Email: conend@mcmaster.ca.
Stefan Osswald, Email: sosswald@uhbs.ch.
Novelty and Significance
What Is New?
Blood pressure (BP) and hypertension are associated with white matter lesions but not with large noncortical or cortical infarcts in patients with atrial fibrillation (AF).
BP and hypertension are not associated with neurocognitive function after multivariable adjustment.
What Is Relevant?
Optimal BP control is important to potentially prevent brain lesions in patients with atrial fibrillation.
Summary
Among patients with atrial fibrillation, BP is strongly associated with the presence and extent of white matter lesions, but there is no association with large noncortical or cortical infarcts. Whether more aggressive BP control may prevent different types of brain lesions in patients with atrial fibrillation has to be investigated.
References
- 1.Conen D, Ridker PM, Buring JE, Glynn RJ. Risk of cardiovascular events among women with high normal blood pressure or blood pressure progression: prospective cohort study. BMJ. 2007;335:432 doi: 10.1136/bmj.39269.672188.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conen D, Tedrow UB, Koplan BA, Glynn RJ, Buring JE, Albert CM. Influence of systolic and diastolic blood pressure on the risk of incident atrial fibrillation in women. Circulation. 2009;119:2146–2152. doi: 10.1161/CIRCULATIONAHA.108.830042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8 [DOI] [PubMed] [Google Scholar]
- 4.Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S, White IR, Caulfield MJ, Deanfield JE, Smeeth L, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1·25 million people. Lancet. 2014;383:1899–1911. doi: 10.1016/S0140-6736(14)60685-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1 [DOI] [PubMed] [Google Scholar]
- 6.Krijthe BP, Kunst A, Benjamin EJ, Lip GY, Franco OH, Hofman A, Witteman JC, Stricker BH, Heeringa J. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;34:2746–2751. doi: 10.1093/eurheartj/eht280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalantarian S, Stern TA, Mansour M, Ruskin JN. Cognitive impairment associated with atrial fibrillation: a meta-analysis. Ann Intern Med. 2013;1585 pt 1338–346. doi: 10.7326/0003-4819-158-5-201303050-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Bruijn RF, Heeringa J, Wolters FJ, Franco OH, Stricker BH, Hofman A, Koudstaal PJ, Ikram MA. Association between atrial fibrillation and dementia in the general population. JAMA Neurol. 2015;72:1288–1294. doi: 10.1001/jamaneurol.2015.2161 [DOI] [PubMed] [Google Scholar]
- 9.Poels MM, Vernooij MW, Ikram MA, Hofman A, Krestin GP, van der Lugt A, Breteler MM. Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam scan study. Stroke. 2010;4110 supplS103–S106. doi: 10.1161/STROKEAHA.110.595181 [DOI] [PubMed] [Google Scholar]
- 10.Prabhakaran S, Wright CB, Yoshita M, Delapaz R, Brown T, DeCarli C, Sacco RL. Prevalence and determinants of Subclinical brain infarction: the Northern Manhattan Study. Neurology. 2008;70:425–430. doi: 10.1212/01.wnl.0000277521.66947.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conen D, Rodondi N, Müller A, Beer JH, Ammann P, Moschovitis G, Auricchio A, Hayoz D, Kobza R, Shah D, et al. ; Swiss-AF Study Investigators. Relationships of overt and silent brain lesions with cognitive function in patients with atrial fibrillation. J Am Coll Cardiol. 2019;73:989–999. doi: 10.1016/j.jacc.2018.12.039 [DOI] [PubMed] [Google Scholar]
- 12.van Dijk EJ, Breteler MM, Schmidt R, Berger K, Nilsson LG, Oudkerk M, Pajak A, Sans S, de Ridder M, Dufouil C, et al. ; CASCADE Consortium. The association between blood pressure, hypertension, and cerebral white matter lesions: cardiovascular determinants of dementia study. Hypertension. 2004;44:625–630. doi: 10.1161/01.HYP.0000145857.98904.20 [DOI] [PubMed] [Google Scholar]
- 13.Dufouil C, de Kersaint-Gilly A, Besançon V, Levy C, Auffray E, Brunnereau L, Alpérovitch A, Tzourio C. Longitudinal study of blood pressure and white matter hyperintensities: the EVA MRI Cohort. Neurology. 2001;56:921–926. doi: 10.1212/wnl.56.7.921 [DOI] [PubMed] [Google Scholar]
- 14.Verhaaren BF, Vernooij MW, de Boer R, Hofman A, Niessen WJ, van der Lugt A, Ikram MA. High blood pressure and cerebral white matter lesion progression in the general population. Hypertension. 2013;61:1354–1359. doi: 10.1161/HYPERTENSIONAHA.111.00430 [DOI] [PubMed] [Google Scholar]
- 15.Klarenbeek P, van Oostenbrugge RJ, Rouhl RP, Knottnerus IL, Staals J. Higher ambulatory blood pressure relates to new cerebral microbleeds: 2-year follow-up study in lacunar stroke patients. Stroke. 2013;44:978–983. doi: 10.1161/STROKEAHA.111.676619 [DOI] [PubMed] [Google Scholar]
- 16.Conen D, Rodondi N, Mueller A, Beer J, Auricchio A, Ammann P, Hayoz D, Kobza R, Moschovitis G, Shah D, et al. Design of the Swiss Atrial Fibrillation Cohort Study (Swiss-AF): structural brain damage and cognitive decline among patients with atrial fibrillation. Swiss Med Wkly. 2017;147:w14467 doi: 10.4414/smw.2017.14467 [DOI] [PubMed] [Google Scholar]
- 17.Williams B, Mancia G, Spiering W, Rosei EA, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. [2018 ESC/ESH Guidelines for the management of arterial hypertension]. Kardiol Pol. 2019;77:71–159. doi: 10.5603/KP.2019.0018 [DOI] [PubMed] [Google Scholar]
- 18.Whelton PK, Carey RM, Aronow WS, Casey DE, Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127–e248. doi: 10.1016/j.jacc.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 19.Bowie CR, Harvey PD. Administration and interpretation of the trail making test. Nat Protoc. 2006;1:2277–2281. doi: 10.1038/nprot.2006.390 [DOI] [PubMed] [Google Scholar]
- 20.Arbuthnott K, Frank J. Trail making test, part B as a measure of executive control: validation using a set-switching paradigm. J Clin Exp Neuropsychol. 2000;22:518–528. doi: 10.1076/1380-3395(200008)22:4;1-0;FT518 [DOI] [PubMed] [Google Scholar]
- 21.Lopes M, Brucki SMD, Giampaoli V, Mansur LL. Semantic verbal fluency test in dementia: preliminary retrospective analysis. Dement Neuropsychol. 2009;3:315–320. doi: 10.1590/S1980-57642009DN30400009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schweizer TA, Alexander MP, Susan Gillingham BA, Cusimano M, Stuss DT. Lateralized cerebellar contributions to word generation: a phonemic and semantic fluency study. Behav Neurol. 2010;23:31–37. doi: 10.3233/BEN-2010-0269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baldo JV, Schwartz S, Wilkins D, Dronkers NF. Role of frontal versus temporal cortex in verbal fluency as revealed by voxel-based lesion symptom mapping. J Int Neuropsychol Soc. 2006;12:896–900. doi: 10.1017/S1355617706061078 [DOI] [PubMed] [Google Scholar]
- 24.Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403 [DOI] [PubMed] [Google Scholar]
- 25.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351 [DOI] [PubMed] [Google Scholar]
- 26.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O’Brien JT, Barkhof F, Benavente OR, et al. ; STandards for ReportIng Vascular changes on nEuroimaging (STRIVE v1). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM; Rotterdam Scan Study. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke. 2003;34:1126–1129. doi: 10.1161/01.STR.0000068408.82115.D2 [DOI] [PubMed] [Google Scholar]
- 28.de Groot JC, de Leeuw FE, Oudkerk M, van Gijn J, Hofman A, Jolles J, Breteler MM. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol. 2000;47:145–151. doi: 10.1002/1531-8249(200002)47:2<145::aid-ana3>3.3.co;2-g [DOI] [PubMed] [Google Scholar]
- 29.Windham BG, Griswold ME, Wilkening SR, Su D, Tingle J, Coker LH, Knopman D, Gottesman RF, Shibata D, Mosley TH. Midlife smaller and larger infarctions, white matter hyperintensities, and 20-year cognitive decline: A Cohort Study. Ann Intern Med. 2019;171:389–396. doi: 10.7326/M18-0295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Leeuw FE, de Groot JC, Achten E, Oudkerk M, Ramos LM, Heijboer R, Hofman A, Jolles J, van Gijn J, Breteler MM. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry. 2001;70:9–14. doi: 10.1136/jnnp.70.1.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maillard P, Seshadri S, Beiser A, Himali JJ, Au R, Fletcher E, Carmichael O, Wolf PA, DeCarli C. Effects of systolic blood pressure on white-matter integrity in young adults in the Framingham Heart Study: a cross-sectional study. Lancet Neurol. 2012;11:1039–1047. doi: 10.1016/S1474-4422(12)70241-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, et al. ; ESC Scientific Document Group. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210 [DOI] [PubMed] [Google Scholar]
- 33.Cordonnier C, Al-Shahi Salman R, Wardlaw J. Spontaneous brain microbleeds: systematic review, subgroup analyses and standards for study design and reporting. Brain. 2007;130pt 81988–2003. doi: 10.1093/brain/awl387 [DOI] [PubMed] [Google Scholar]
- 34.Henskens LH, van Oostenbrugge RJ, Kroon AA, de Leeuw PW, Lodder J. Brain microbleeds are associated with ambulatory blood pressure levels in a hypertensive population. Hypertension. 2008;51:62–68. doi: 10.1161/HYPERTENSIONAHA.107.100610 [DOI] [PubMed] [Google Scholar]
- 35.Tanaka A, Ueno Y, Nakayama Y, Takano K, Takebayashi S. Small chronic hemorrhages and ischemic lesions in association with spontaneous intracerebral hematomas. Stroke. 1999;30:1637–1642. doi: 10.1161/01.str.30.8.1637 [DOI] [PubMed] [Google Scholar]
- 36.Fazekas F, Kleinert R, Roob G, Kleinert G, Kapeller P, Schmidt R, Hartung HP. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. AJNR Am J Neuroradiol. 1999;20:637–642 [PMC free article] [PubMed] [Google Scholar]
- 37.Nasrallah IM, Pajewski NM, Auchus AP, Chelune G, Cheung AK, Cleveland ML, Coker LH, Crowe MG, Cushman WC, Cutler JA, et al. Association of intensive vs standard blood pressure control with cerebral white matter lesions. JAMA. 2019;322:524–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williamson JD, Pajewski NM, Auchus AP, Bryan RN, Chelune G, Cheung AK, Cleveland ML, Coker LH, Crowe MG, Cushman WC, et al. Effect of intensive vs standard blood pressure control on probable dementia: a Randomized Clinical Trial. JAMA. 2019;321:553–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wright JT, Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iadecola C, Yaffe K, Biller J, Bratzke LC, Faraci FM, Gorelick PB, Gulati M, Kamel H, Knopman DS, Launer LJ, et al. ; American Heart Association Council on Hypertension; Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Stroke Council. Impact of hypertension on cognitive function: a scientific statement from the american heart association. Hypertension. 2016;68:e67–e94. doi: 10.1161/HYP.0000000000000053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levine DA, Galecki AT, Langa KM, Unverzagt FW, Kabeto MU, Giordani B, Cushman M, McClure LA, Safford MM, Wadley VG. Blood pressure and cognitive decline over 8 years in middle-aged and older black and white americans. Hypertension. 2019;73:310–318. doi: 10.1161/HYPERTENSIONAHA.118.12062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gardener H, Wright CB, Dong C, Cheung K, DeRosa J, Nannery M, Stern Y, Elkind MS, Sacco RL. Ideal cardiovascular health and cognitive aging in the northern manhattan study. J Am Heart Assoc. 2016;5:e002731 doi: 10.1161/JAHA.115.002731 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


