Abstract
In the adult brain, self-renewing radial-glia like (RGL) progenitor cells have been shown to reside in the subventricular zone and the subgranular zone of the hippocampus. A large body of evidence shows that experiences such as learning, enriched environment and stress can alter proliferation and differentiation of RGL progenitor cells. The progenitor cells present in the subgranular zone of the hippocampus divide to give rise to newborn neurons that migrate to the dentate gyrus where they differentiate into adult granule neurons. These newborn neurons have been found to have a unique role in certain types of hippocampus-dependent learning and memory, including goal-directed behaviors that require pattern separation. Experimental traumatic brain injury (TBI) in rodents has been shown to alter hippocampal neurogenesis, including triggering the acute loss of newborn neurons, as well as progenitor cell hyperproliferation. In this review, we discuss the role of hippocampal neurogenesis in learning and memory. Furthermore, we review evidence for the molecular mechanisms that contribute to newborn neuron loss, as well as increased progenitor cell proliferation after TBI. Finally, we discuss strategies aimed at enhancing neurogenesis after TBI and their possible therapeutic benefits.
Keywords: context discrimination, hippocampus, pattern separation, subgranular zone, traumatic brain injury
Introduction:
For much of the history of modern neuroscience, there was a long-held dogma that neurogenesis was restricted to prenatal development. The first experimental evidence suggesting new neurons were generated in the adult brain was obtained in the early 1960s by Altman and Das, who tracked the incorporation of [3H]-thymidine into the DNA of dividing cells in young rats (Altman and Das, 1965). Their findings showed [3H]-thymidine incorporation in discrete brain areas, and from these experiments it was concluded that neurogenesis occurred in the adult rat brain. Further evidence of hippocampal neurogenesis tracking incorporation of the non-radioactive thymidine analog bromodeoxyuridine (BrdU) was later found in adult macaque monkeys (Gould etal., 1999a; Gould et al., 1999b; Kornack and Rakic, 1999). Several additional studies demonstrated evidence of adult hippocampal neurogenesis in rats up to 26 months old, although the rate of neurogenesis appeared to be age-dependent (Cameron and McKay, 1999; Snyder and Drew, 2020). Taken together, these and other related studies have provided strong experimental evidence for the existence of ongoing neurogenesis into adulthood in multiple species, and opened up new areas of investigation into the mechanisms that control neurogenesis and the role(s) these adult-born neurons play in health and disease.
Adult hippocampal radial-glia like (RGL) progenitor cells in the subgranular zone go through several developmental stages before they become functionally incorporated into neural circuits (Kempermann et al., 2015; Toda et al., 2019). In their initial developmental stage (referred to as type 1), these cells have been found in close proximity to blood vessels (a so-called neurogenic niche; see Figure 1), where they reside in a quiescent state until activated (Filippov et al., 2003; Moreno-Jimenez etal., 2019; Vicidomini et al., 2020). Upon activation, type 1 cells proliferate rapidly, giving rise to intermediate progenitors, or type 2a cells. Like type 1 cells, type 2a cells retain self-renewal capacity. These cells then undergo asymmetric cell division and give rise to fate-specified daughter cells (type 2b cells). Type 2b cells can further give rise to neuroblasts (type 3 cells) that subsequently differentiate into mature granule neurons (Goncalves et al., 2016).
Figure 1. Differentiation of progenitor cells into mature granule neurons.
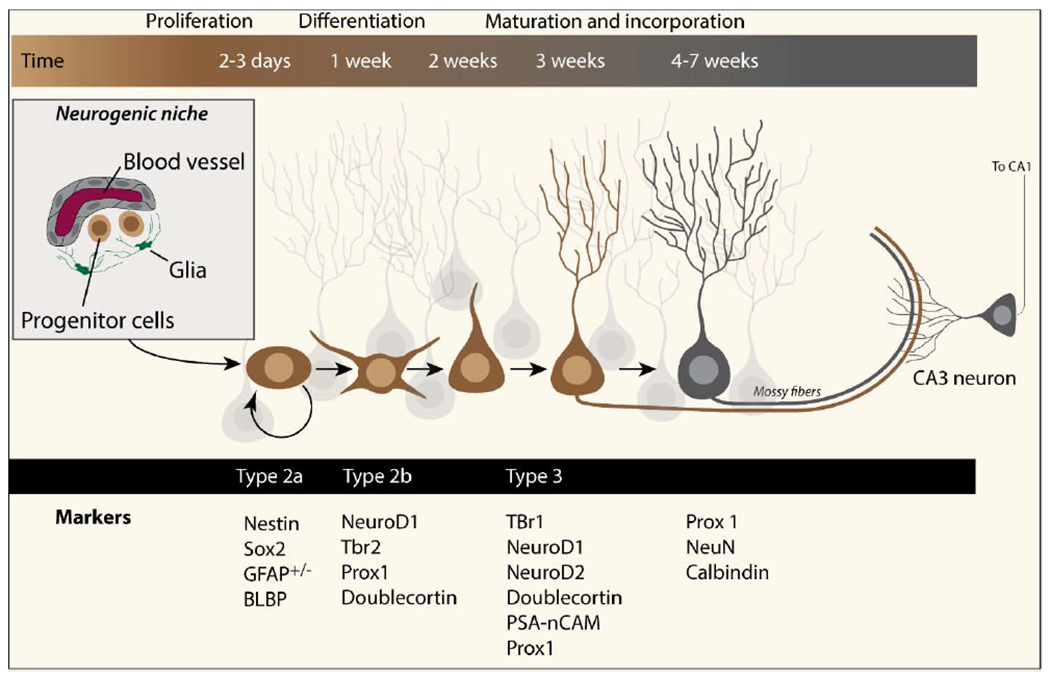
The timeline of maturation is indicated above each stage. Each stage of progenitor cell differentiation can be studied using antibodies to the indicated markers. The axons of mature neurons join the mossy fibers where they make synapses with CA3 neurons.
While it has been reported that newborn neurons contribute to approximately 30% of the neural network in the adult rodent hippocampus (Ninkovic et al., 2007), the rate of adult neurogenesis in the hippocampus of humans remains controversial. By measuring the post-mortem concentration of [14C] in genomic DNA of post-mortem brain tissue from humans exposed to the fallout from nuclear bomb tests, it was calculated that approximately 700 new neurons are generated per day in the adult human hippocampus (Spalding et al., 2013). Similarly, it was observed in autopsy specimens (14-79 years of age) that although the rate of neurogenesis remained relatively constant across the aging process of healthy adults, it declined in persons afflicted with neurodegenerative diseases such as Alzheimer’s (Boldrini et al., 2018; Moreno-Jimenez et al., 2019). In contrast, a recent report by Sorrells etal. used immunohistochemistry for proliferating cell markers, as well as electron microscopy, to show that neurogenesis in the human hippocampus declined rapidly over the first few years of life before becoming undetectable in the adult human brain (Sorrells et al., 2018). Technical limitations, including tissue processing and differences in the sensitivity and specificity of the various techniques employed to detect neurogenesis in the adult human brain, likely contribute to the discrepancies between the degree of post-natal neurogenesis reported in published studies (Gage, 2019). Consequently, questions surrounding the overall rate of adult neurogenesis, the mechanism(s) regulating post-natal neurogenesis, and the role(s) newborn neurons may play in cognition are areas of active investigation.
Migration and maturation of newborn neurons
Adult neurogenesis has been found to primarily occur in only a few regions of the brain, including the subventricular zone (within the lateral walls of the lateral ventricles) and the subgranular zone of the hippocampus. RGL progenitor cells resident to the subventricular zone undergo asymmetric neurogenic cell division to produce daughter cells that will differentiate into restricted neuroblasts, which then migrate via the rostral migratory stream to the olfactory bulb (Ming and Song, 2005). Progenitor cells located in the subgranular zone produce daughter cells that migrate the relatively short distance to the dentate gyrus subfield. After reaching their final destination, restricted neuroblasts differentiate into immature neurons (van Praag et al., 2000), and over time develop the morphological and electrophysiological characteristics of mature neurons (Johnson et al., 2007). While there are reports of newborn neurons being generated in other brain structures such as the cortex, amygdala, and striatum (Bedard et al., 2006; Jhaveri et al., 2018), for the purpose of this review, we will focus primarily on the role of hippocampal neurogenesis and its roles in TBI pathophysiology and outcome.
Hippocampal neurogenesis and cognition
As described above, newborn neurons in the adult hippocampus are generated from RGL cells that reside in the subgranular region of the dentate gyrus. As they differentiate and mature into granule neurons, these newborn neurons begin to extend dendrites and axons to form functional connections with existing neurons. In rodents, it has been estimated that the entire process from birth to a fully incorporated mature neuron of takes approximately 4-7 weeks (Han et al., 2017; Johnson et al., 2007; Lee et al., 2007; Perrier et al., 2004). The various stages of differentiation and maturation are associated with characteristic phenotypic changes, and can be monitored by evaluating the expression levels of key regulatory proteins (Figure 1). Hippocampal neurogenesis has been suggested to play a role in learning and memory, including long-term spatial memory retention, context fear memory, cognitive flexibility, pattern separation, and systems consolidation (Besnard and Sahay, 2016; Jessberger et al., 2009; Kheirbek et al., 2012a; Kheirbek et al., 2012b; McHugh et al., 2007; Terranova et al., 2019). The proposed mechanism(s) by which these newborn neurons may participate in learning and memory processes has been the subject of intense debate.
Newborn neurons up to 7 weeks after generation have been shown to predominately activate inhibitory neurons that regulate the activity of mature granule neurons within the dentate gyrus (Drew et al., 2016). Recent studies examining the electrophysiological properties of newborn neurons also found that they exhibit lower thresholds for triggering action potentials, and can make functional connections with the CA3 subfield prior to full maturation (Brunner et al., 2014; Marin-Burgin et al., 2012; Schmidt-Hieber et al., 2004; Yau et al., 2015). However, once they have fully matured, these adult born neurons become indistinguishable from other resident granule neurons in the dentate gyrus. It has been hypothesized that the changing properties of newborn neurons as they mature may play a role in how they participate in, or regulate, specific behaviors (Gage, 2000). These newly generated neurons may preferentially play a role in new learning and memory, suggesting that disrupting ongoing neurogenesis may underlie some of the cognitive problems associated with neurological disorders and brain injury.
As the dentate gyrus is more heavily populated and densely packed compared to the input and output structures it communicates with, it has been suggested that a distributed pattern of granule cell activation occurs during learning that allows for non-overlapping responses to different learning events (Amaral et al., 2007). This function is thought to be critical for pattern separation, which is the ability to make distinct representations from similar input information. Immature newborn neurons are thought to play a critical role in pattern separation through their ability to modulate the activity of inhibitory interneurons and decrease the number of mature granule neurons responding to a specific stimuli (Aimone et al., 2011). Thus, evidence suggests that even prior to their full maturation and incorporation, these newly generated neurons participate in hippocampus-dependent behaviors.
The behavioral manifestation of pattern separation is an improvement in the ability to distinguish between highly similar environments. That is, pattern separation allows for a context-appropriate response based on recall of associative memory, even when two sensory stimuli are highly similar. Several behavioral tasks have been designed to examine pattern separation that are sensitive to perturbations of neurogenesis (Figure 2). The context fear discrimination task (Figure 2A) examines an animal’s ability to differentiate between two similar, but distinct, environments. In this task, animals with intact adult neurogenesis perform significantly better than animals with impaired neurogenesis, and are able to differentiate between similar contexts as demonstrated by displaying more freezing behavior when placed in a chamber in which a mild foot shock was delivered com pared to a similar context in which no foot shock was delivered (Besnardand Sahay, 2016; Kheirbek et al., 2012a; Kheirbek et al., 2012b; McHugh et al., 2007).
Figure 2. Examples of cognitive tasks used to assess the role of adult hippocampal neurogenesis.
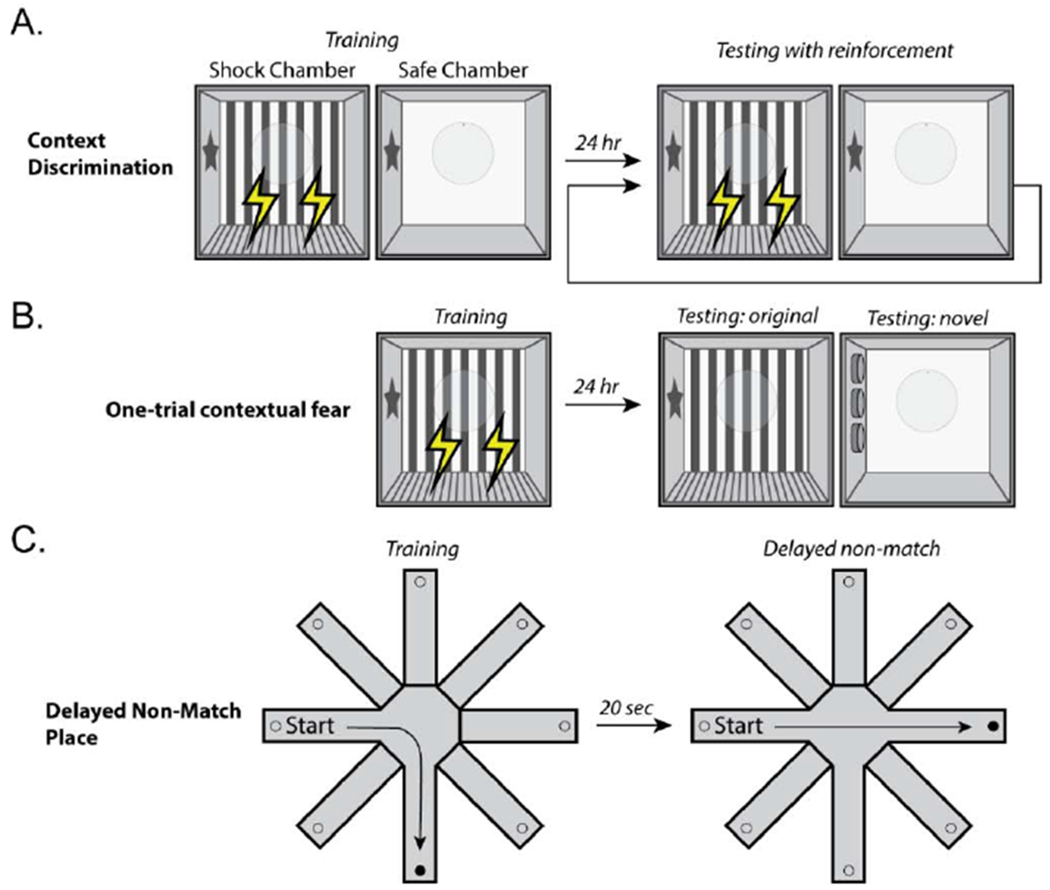
A) Context fear discrimination task (Frankland et al., 1998; Huckleberry et al., 2016). In this task, a test animal is repeatedly exposed to two chambers that share some features, but are different in others. In the “shock” chamber, the animal receives a mild foot shock in the absence of any salient cues (e.g. tone or light), while no shock is delivered in the “safe” chamber. The ability to differentiate between the “shock” and “safe” chambers is assessed by monitoring the freezing response over the course of training. B) One-trial context fear (Drew et al., 2010). In this task, animals are trained to fear a training chamber in the absence of a salient cue. After a 1-2 minute exploratory period, a single foot shock is delivered. Fear memory is tested by placing the animal back into the training chamber after a delay and monitoring freezing behavior. The specificity of the fear is assessed by measuring freezing behavior in a novel environment. C) Delayed non-match to place (Clelland et al., 2009). This a variant of the radial arm maze task in which animals are trained over a period of days to find a food pellet placed in a randomly chosen arm. All other arms are blocked during training. During the non-matching phase of the task, animals can choose a newly opened arm that is baited, or the original arm, which is not baited.
Similarly, Saxe et al., showed that performance in a one-trial contextual fear task is also dependent on hippocampal neurogenesis (Saxe et al., 2007). Interestingly, ablating neurogenesis impaired performance in this task when tested 4-6 weeks, but not 1-2 weeks, after the treatment (Drew et al., 2010; Seo et al., 2015). Using low-dose x-ray-irradiation, Clelland et al., demonstrated that neurogenesis is required for fine discrimination in a delayed non-match to place task (Clelland et al., 2009). Animals with impaired neurogenesis make more errors when the choice arm is proximal to the original arm (separated by 2 or less arms), but has no demonstrable effect when the arm separation is large (i.e. choice and original arm are separated by 3 or more arms).
It has also been proposed that adult neurogenesis may play a role in forgetting. The integration of new neurons may modify the pattern of dentate gyrus connections, thereby altering hippocampal circuits to make recently stored memories harder to access (Frankland et al., 2013). Some supporting evidence for this hypothesis has been observed in mice and guinea pigs. Increasing neurogenesis via exercise (running on a treadmill) soon after memory formation induced forgetting (Akers et al., 2014; Epp et al., 2016), although conflicting results have been observed (Kodali et al., 2016). It has been hypothesized that this role for neurogenesis may be related to the finite storage capacity of the hippocampus, requiring that older memories become destabilized so that new memories with their own distinct circuit can be incorporated (Toda et al., 2019). Consistent with this hypothesis, Akers et al., demonstrated that while increasing neurogenesis disrupted a newly learned memory, it enhanced subsequent learning and memory (Akers et al., 2014). However, once a memory had become hippocampus-independent, enhancing neurogenesis had no effect (Gao et al., 2018).
Hippocampal neurogenesis and disease
Altered neurogenesis has been observed in a wide variety of physiological and pathological conditions, including depression (Sahay and Hen, 2007; Tanti and Belzung, 2013), contextual memory (Aimone et al., 2011; Sahay et al., 2011), pattern separation (Anacker and Hen, 2017; Clelland et al., 2009), forgetting (Akers et al., 2014), Alzheimer’s disease (Jin et al., 2004), Down’s syndrome (Wisniewski et al., 1984) and stress (Anacker et al., 2018). For example, increased brain glucocorticoid levels and steroid signaling arising from stress or aging has been shown to decrease both the proliferation of progenitor cells and the differentiation of newborn neurons into mature neurons (Anacker et al., 2013). Consistent with this, removing the adrenal gland, the primary source for glucocorticoids in rats, enhances neurogenesis (Cameron and McKay, 1999). In depression, it has been postulated that antidepressants, in addition to directly increasing monoamine levels in synapses, may also improve depression indirectly through increasing hippocampal neurogenesis (the neurogenic hypothesis) (Eisch and Petrik, 2012). Supporting evidence includes data showing that antidepressant efficacy can be blocked by radiation-induced ablation of hippocampal progenitor cell proliferation and triggering the apoptosis of newborn neurons (Drewet al., 2010; Seo et al., 2015). Recently, using stereological cell counts, Moreno-Jimenez et al., demonstrated a progressive decline in the number of doublecortin-positive newborn neurons in the dentate gyrus of persons with Alzheimer’s disease as compared to age-matched healthy controls. Interestingly, this reduction appeared prior to the appearance of neurofibrillary tangles and Aß deposition in the hippocampus (Moreno-Jimenez et al., 2019). Taken together, these studies suggest that a fuller understanding of the mechanism(s) by which neurogenesis is altered by pathological disease or insults may inform treatments to help reduce resulting hippocampal dysfunction.
Hippocampal neurogenesis is enhanced following experimental TBI
A study by Dash et al., first reported that moderate-severe controlled cortical impact (CCI) enhances the incorporation of BrdU into newborn neurons in rats (Dash et al., 2001). This enhanced neurogenesis could be observed as early as 24 hr post-injury and continued for 7 days. This study also showed that some of these newborn neurons differentiated into mature neurons that expressed NeuN. Several other studies have corroborated these findings following CCI, and have extended these result to show that while most of the newly generated neurons do not survive, some extend their axons through the mossy fibers and make synaptic connections with CA3 neurons (Blaya et al., 2019; Dash et al., 2001; Yan et al., 2018; Zhang et al., 2020). Interestingly, this neurogenic effect appears to be related to the magnitude of injury employed, as mild CCI did not have a demonstrable effect on hippocampal neurogenesis (Wang et al., 2016).
Using the fluid percussion injury (FPI) model of experimental brain injury, Dietrich and colleagues demonstrated enhanced BrdU incorporation in several brain regions including the injured cortex, white matter tracts, subventricular zone and the dentate gyrus of the hippocampus (Urrea et al., 2007). While most of these proliferating cells matured to express astrocytic and microglial markers, enhanced neurogenesis was observed in the hippocampus. Similarly, Sun et al., demonstrated that hippocampal neurogenesis is increased after FPI in both juvenile and adult rats, with the degree of neurogenesis and number of new neurons generated being significantly higher in juvenile animals (Sun et al., 2005). This same group subsequently showed that of the newly generated neurons that survived for 10 weeks, approximately 30% could be labeled by the retrograde tracer Fluorogold when injected into the CA3 subfield, suggesting they integrated into the hippocampal circuit (Sun et al., 2007).
Although the mechanism(s) underlying the increased proliferation of progenitor cells and enhanced neurogenesis after TBI remain to be elucidated, a variety of morphogens, growth factors, transcription factors and epigenetic mechanisms have been implicated in the regulation of neurogenesis (Liu and Song, 2016). For example, bone morphogenic protein (BMP) signaling has been implicated in the regulation of neural stem cells proliferation both in vitro and in vivo (Mira et al., 2010). Over expression of Noggin, an endogenous BMP inhibitor, promotes neural stem cell proliferation (Gobeske et al., 2009). Using a mouse model of CCI, Logan et al., examined the expression of BMPs and Noggin in the subventricular and subgranular zones (Logan et al., 2013). Contrary to expectations, these authors reported that TBI increased the expression of many BMP family members without any significant change in Noggin levels.
A large body of evidence shows that neuronal activity can alter hippocampal neurogenesis (Kempermann et al., 1998; Voss et al., 2019; Yun et al., 2018). For example, the expression of the DNA demethylase GADD45b is increased in mature granule neurons in response to neuronal activity (Ma et al., 2009). Increased GADD45b has been shown to demethylate the regulatory regions of the FGF and BDNF genes, thereby increasing their expression and release where they can act on neuronal progenitor cells to promote neurogenesis (Ma et al., 2009). Consistent with this, when FGF-2 knockout mice were subjected to controlled cortical impact injury, the number of newly generated neurons (labeled with BrdU) was significantly reduced compared to injured wild-type controls (Yoshimura et al., 2003). In contrast, overexpression of FGF-2 increased the numbers of dividing cells and BrdU-positive neurons in injured wild-type mice (Yoshimura et al., 2003). Similarly, when mice lacking the BDNF gene were subjected to moderate TBI, the survival of adult-born immature neurons was significantly compromised (Gao and Chen, 2009). Interestingly, direct infusion of BDNF into the adult rat hippocampus not only resulted in a significant increase in BrdU labeled neurons in the granule layer, but also increased the ectopic localization of newborn neurons in the hilar region (Scharfman et al., 2005), a phenomenon that has been previously observed after TBI (Robinson et al., 2016; Shapiro, 2017).
Mossy cells, which reside in the hilar region of the hippocampus, are glutamatergic and are known to form synapses with hilar inhibitory interneurons (Scharfman and Myers, 2012). Chemogenetic activation of mossy cells causes GABA release (via stimulation of inhibitory neurons) onto neural stem cells, promoting quiescence. In contrast, mossy cell inhibition facilitates proliferation by reducing GABA release onto stem cells (Yeh et al., 2018). As we and others have shown that TBI can cause the death of hilar GABAergic neurons (Lowenstein et al., 1992; Santhakumar et al., 2000; Zhao et al., 2018), the loss of these inhibitory neurons is likely to remove the inhibitory constraint on local neural stem cells and facilitate their proliferation after TBI.
In the developing brain, the regulation of neurogenesis, axon pathfinding, dendritic development, and synaptic assembly are critically dependent on signaling by the Wnt family of proteins (Salinas, 2012). Recently, it has been appreciated that modulation of Wnt signaling cascades could also be important in stimulating neurogenesis, repair of the blood-brain barrier, and cognitive recovery after brain injury (Lambert et al., 2016; Piccin and Morshead, 2011). Consistent with this, it has been shown that intravenous administration of recombinant Wnt3a to brain injured mice enhances neurogenesis (Zhao et al., 2016). Taken together, these results suggest that TBI-triggered alterations in neurogenesis may be the result of a complex interplay of growth factor and morphogen signaling, neurotransmitter imbalances, and endogenous cell loss.
Functional consequences of altered neurogenesis after TBI
Based on the observation that TBI alters hippocampal neurogenesis, several studies have investigated the functional consequences of enhancing/impairing neurogenesis in the injured brain. Lu et al., was one of the first studies to show that increasing neurogenesis was associated with improved neurological outcome after TBI (Lu et al., 2003). In this study, the nitric oxide donor DETA/NONOate was administered daily to rats after CCI injury. Compared to injured rats that received vehicle, animals treated with DETA/NONOate showed increased proliferation, survival, migration and differentiation of neural progenitor cells. These neurogenic effects were associated with improved neurological functional outcome. Similarly, Bullock and colleagues reported that post-injury infusion of S100B, a neurotrophic protein secreted by astrocytes, enhanced neurogenesis and improved the performance of injured rats in the Morris water maze task (Kleindienst et al., 2005). Likewise, administration of basicfibroblast growth factor, erythropoietin, epidermal growth factor, insulin-like growth factor 1, thymosin β(4), antidepressants, statins, and other proneurogenic compounds, have all been demonstrated to enhance neurogenesis and improve cognitive outcome (Blaya et al., 2014; Carlson and Saatman, 2018; Han et al., 2011; Kleindienst et al., 2005; Lu et al., 2005; Sun et al., 2010; Sun et al., 2009; Xiong et al., 2011; Yoshimura et al., 2003). While these studies support the premise that treatments can be used to improve outcomes after TBI by targeting neurogenesis, the effects of these treatments on other cell types cannot be excluded.
To address this knowledge gap, Kernie and colleagues demonstrated that disruption of neurogenesis (using ganciclovir) after TBI results in exacerbated hippocampus-dependent learning and memory dysfunction (Blaiss et al., 2011). Similarly, Hamm and colleagues used the antimitotic agent arabinofuranosyl cytidine (Ara-C) to directly assess the role of injury-induced hippocampal neurogenesis and cognitive recovery after fluid percussion injury (Sun et al., 2015). These authors found that Ara-C reduced the injury-triggered proliferative response of hippocampal progenitor cells and abolished the innate recovery of hippocampus-dependent learning and memory when tested weeks after the discontinuation of treatment.
Although these and other studies indicate that increased neurogenesis after TBI is beneficial, detrimental effects have been reported, including reduced proliferative capacity (Atkins et al., 2010; Neuberger et al., 2017; Wang et al., 2011), and aberrant migration and sprouting (Ibrahim et al., 2016; Robinson et al., 2016; Villasana et al., 2015). For example, using doublecortin promotor-driven reporter mice, Villasana et al., reported that neurons generated after TBI have increased dendritic complexity including enhanced dendritic branching proximal to the soma and widely splayed dendritic branches (Villasana et al., 2015). Similarly, newborn neurons that failed to properly migrate, or migrated beyond the dentate gyrus, have also been observed (Robinson et al., 2016; Shapiro, 2017). As newborn neurons mature into excitatory neurons, aberrant migration and formation of inappropriate synaptic connections may be a part of the mechanisms leading to seizure susceptibility in animals following TBI. Consistent with this, post-CCI treatment of injured mice with the mTOR antagonist rapamycin was found to attenuate mossy fiber sprouting and reduce the incidence of post-traumatic seizures (Butler et al., 2015, 2017). Likewise, administration of the VEGFR2 antagonist SU1498 to suppress injury-induced neurogenesis after lateral fluid percussion injury was found to reduce seizure susceptibility (Neuberger et al., 2017).
Controlled cortical impact injury causes the acute loss of newborn neurons
In addition to enhancing hippocampal neurogenesis, CCI has been shown to cause the acute death of doublecortin-positive newborn neurons (peaking within two to three days after injury) as well as reducing the dendritic arborization of newborn neurons that survive (Figure 3) (Gibb et al., 2015; Rola et al., 2006). The death of doublecortin-positive newborn neurons appears to be apoptotic in nature, as has been demonstrated using TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) staining (Li et al., 2016; Xiong et al., 2008). Consistent with this, it has been reported that downregulation of Survivin, a member of the inhibitor of apoptosis (IAP) protein family, exacerbates the loss of newborn neurons (Zhang et al., 2015).
Figure 3. Consequences of CCI and FPI on adult hippocampal neurogenesis.
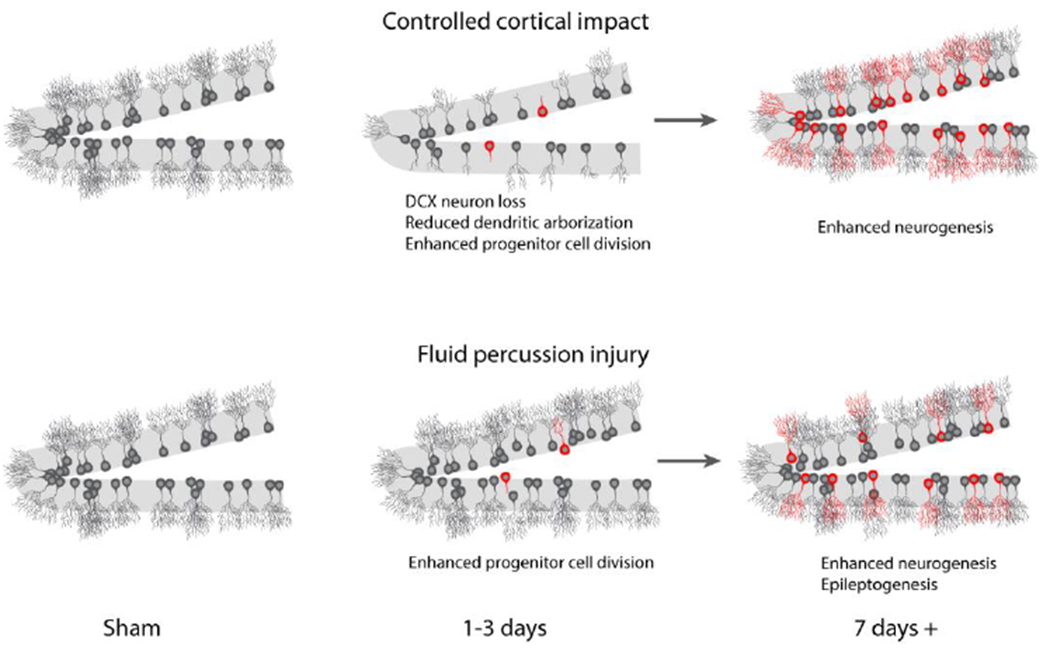
A large body of literature indicates that controlled cortical im pact injury reduces the survival of newborn neurons within 1-3 days of injury. This reduction in DCX-positive neurons (shown in grey) has been observed in rats, mice, and New Zealand rabbits both ipsilateral and contralateral to the injury. Furthermore, CCI also reduces dendritic arborization of the surviving newborn neurons at these acute time points. Concurrent with this loss of newborn neurons, examination of BrdU incorporation (indicated in red) has revealed that enhanced proliferation of neural progenitor cells occurs as early as 24 hrs post-injury. A large percentage of these cells fail to make synaptic connections with CA3 neurons, and undergo apoptosis. Both the surviving newborn neurons (generated prior to injury) and the newly generated neurons (indicated in red color) can mature into granule neurons and incorporate into the hippocampal circuit by 3-6 weeks post-generation. Fluid percussion injury does not appear to cause acute loss of doublecortin-positive cells, but does result in enhanced progenitor cell proliferation. Although generally thought to be a reparative mechanism, enhanced neurogenesis has been reported to potentially reduce the threshold for seizure activity.
As newborn neurons are involved in specific forms of learning and memory, their loss is anticipated to contribute to learning and memory dysfunction after TBI. Thus, determining the mechanisms underlying the apoptosis of newborn neurons, and the identification of strategies to reduce their loss, is an area of continued research. Using conditional BDNF knockouts, Gao and Chen showed that loss of BDNF in the dentate gyrus exacerbated the death of newborn neurons, indicating that the survival of these neurons may be enhanced by augmenting BDNF signaling after TBI (Gao and Chen, 2009). Vascular endothelial growth factor (VEGF), a key pro-angiogenic factor, has been shown to increase neurogenesis in response to enriched environment, exercise, and antidepressant treatment (Cao et al., 2004; Fabel et al., 2003; Schmidt and Duman, 2007). Using a rat FPI model, Lee and Agoston reported that intraventricular administration VEGF increased the number of BrdU and Prox1-positive neurons. This increase appeared to have resulted from a reduction in the death of BrdU+ newly generated neurons rather than increased proliferation of neural progenitor cells (Lee and Agoston, 2010).
Recently, we have demonstrated that endoplasmic reticulum (ER) stress plays a role in the apoptotic death of newborn neurons after TBI (Hood et al., 2018). Specifically, our study showed that prolonged ER stress activated the pro-apoptotic transcription factor C/EBP homologous protein (CHOP), and that inactivation of CHOP enhanced the survival of doublecortin positive newborn neurons. Post-injury administration of guanabenz, an agent which reduces ER stress by increasing transcription of a number of cytoprotective genes including growth arrest and DNA damage 34 (GADD34) protein, was found to protect against doublecortin-positive neurons loss (without effecting proliferation), to preserve dendritic arborization, and also improved one-trial fear conditioning tested 4 weeks after the injury (Hood et al., 2018).
Finally, it has been proposed that some of the protective effects observed following stem cell administration are related to their ability to promote cell survival via release of paracrine factors. For example, systemic administration of BMSCs (or isolated mesenchymal stem cells, MSCs) does not result in any significant infiltration of these cells into the injured brain (Fischer et al., 2009; Harting et al., 2009). However, these cells influence a number of TBI pathologies including reducing blood-brain barrier permeability, attenuating inflammation, and increasing the survival of newborn neurons (Lu et al., 2001; Mahmood et al., 2003; Mahmood et al., 2002; Shin et al., 2016). Interestingly, one of the molecules secreted by these cells was identified as the matrix protease inhibitor Timp3 (Menge et al., 2012). Post-TBI intravenous administration of recombinant Timp3 reproduced many of the actions of stem cell administration, including reducing the loss of hippocampal neural stem cells and preserving newborn neuron dendritic outgrowth (Gibb et al., 2015).
Perspective (Summary).
There is accumulating evidence that neurogenesis continues beyond development, and persists in the adult brain. In experimental animal studies, it has been shown that adult neurogenesis contributes to various hippocampal functions, including long-term spatial memory retention, context fear memory, cognitive flexibility, pattern separation, systems consolidation, and forgetting. It is still a subject of debate whether adult neurogenesis in the human hippocampus contributes to these cognitive functions. TBI influences hippocampal neurogenesis in three major ways. (1) It increases the rate of neurogenesis. A portion of these newborn neurons survive and integrate into the hippocampal circuit, making synaptic connections with CA3 pyramidal neurons, as well as perforant fibers from the entorhinal cortex. The mechanism(s) underlying the proliferation of neural stem cells have not been well elucidated, although multiple lines of evidence point to contributions by multiple factors. Although multiple neurotropic and growth factors have been shown to enhance neurogenesis, it remains to be determined if the expression of these factors are altered in the injured brain. Based on emerging data, it is likely that a combination of these factors act in concert to maximally increase neurogenesis. (2) Depending on injury type and severity, TBI can also trigger the acute loss of pre-existing newborn neurons. Apoptosis appears to the mechanism underlying the loss of doublecortin-positive newborn neurons. This apoptosis is likely to have been triggered, at least in part, by ER stress. Recent studies have reported that treatments that decrease the acute apoptotic loss of newborn neurons after injury can improve cognitive function in experimental TBI models. (3) TBI alters the dendritic arborization of surviving newborn neurons, with both reduced dendritic complexity and aberrant sprouting observed. Although it has been reported that enhanced post-injury neurogenesis may increase seizure susceptibility, possibly due to aberrant sprouting and migration, it is generally thought that ongoing neurogenesis plays a role in recovery of the injured brain and restoration of cognitive function.
Acknowledgements.
The work described herein was supported by grants from the National Institutes of Health (NS090935, NS086301, NS088298). Salary support for E.U. was provided by a T32 grant (2T32GM008792-16) from NIGMS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aimone JB, Deng W, Gage FH, 2011. Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron 70, 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akers KG, Martinez-Canabal A, Restivo L, Yiu AP, De Cristofaro A, Hsiang HL, Wheeler AL, Guskjolen A, Niibori Y, Shoji H, Ohira K, Richards BA, Miyakawa T, Josselyn SA, Frankland PW, 2014. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science 344, 598–602. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD, 1965. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 124, 319–335. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Scharfman HE, Lavenex P, 2007. The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies). Prog Brain Res 163, 3–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker C, Cattaneo A, Luoni A, Musaelyan K, Zunszain PA, Milanesi E, Rybka J, Berry A, Cirulli F, Thuret S, Price J, Riva MA, Gennarelli M, Pariante CM, 2013. Glucocorticoid-related molecular signaling pathways regulating hippocampal neurogenesis. Neuropsychopharmacology 38, 872–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker C, Hen R, 2017. Adult hippocampal neurogenesis and cognitive flexibility - linking memory and mood. Nat Rev Neurosci 18, 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker C, Luna VM, Stevens GS, Millette A, Shores R, Jimenez JC, Chen B, Hen R, 2018. Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus. Nature 559, 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins CM, Truettner JS, Lotocki G, Sanchez-Molano J, Kang Y, Alonso OF, Sick TJ, Dietrich WD, Bramlett HM, 2010. Post-traumatic seizure susceptibility is attenuated by hypothermia therapy. Eur J Neurosci 32, 1912–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard A, Gravel C, Parent A, 2006. Chemical characterization of newly generated neurons in the striatum of adult primates. Exp Brain Res 170, 501–512. [DOI] [PubMed] [Google Scholar]
- Besnard A, Sahay A, 2016. Adult Hippocampal Neurogenesis, Fear Generalization, and Stress. Neuropsychopharmacology 41,24–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaiss CA, Yu TS, Zhang G, Chen J, Dimchev G, Parada LF, Powell CM, Kernie SG, 2011. Temporally specified genetic ablation of neurogenesis impairs cognitive recovery after traumatic brain injury. J Neurosci 31,4906–4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaya MO, Bramlett HM, Naidoo J, Pieper AA, Dietrich WD, 2014. Neuroprotective efficacy of a proneurogenic compound after traumatic brain injury. J Neurotrauma 31,476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaya MO, Wasserman JM, Pieper AA, Sick TJ, Bramlett HM, Dietrich WD, 2019. Neurotherapeutic capacity of P7C3 agents for the treatment of Traumatic Brain Injury. Neuropharmacology 145, 268–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, Rosoklija GB, Stankov A, Arango V, Dwork AJ, Hen R, Mann JJ, 2018. Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem Cell 22, 589–599.e585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner J, Neubrandt M, Van-Weert S, Andrasi T, Kleine Borgmann FB, Jessberger S, Szabadics J, 2014. Adult-born granule cells mature through two functionally distinct states. Elife 3, e03104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler CR, Boychuk JA, Smith BN, 2015. Effects of Rapamycin Treatment on Neurogenesis and Synaptic Reorganization in the Dentate Gyrus after Controlled Cortical Impact Injury in Mice. Front Syst Neurosci 9, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler CR, Boychuk JA, Smith BN, 2017. Brain Injury-Induced Synaptic Reorganization in Hilar Inhibitory Neurons Is Differentially Suppressed by Rapamycin. eNeuro 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, McKay RD, 1999. Restoring production of hippocampal neurons in old age. Nat Neurosci 2, 894–897. [DOI] [PubMed] [Google Scholar]
- Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ, 2004. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet 36, 827–835. [DOI] [PubMed] [Google Scholar]
- Carlson SW, Saatman KE, 2018. Central Infusion of Insulin-Like Growth Factor-1 Increases Hippocampal Neurogenesis and Improves Neurobehavioral Function after Traumatic Brain Injury. J Neurotrauma 35, 1467–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD Jr., Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ, 2009. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325, 210–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PK, Mach SA, Moore AN, 2001. Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. J Neurosci Res 63, 313–319. [DOI] [PubMed] [Google Scholar]
- Drew LJ, Kheirbek MA, Luna VM, Denny CA, Cloidt MA, Wu MV, Jain S, Scharfman HE, Hen R, 2016. Activation of local inhibitory circuits in the dentate gyrus by adult-born neurons. Hippocampus 26, 763–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MR, Denny CA, Hen R, 2010. Arrest of adult hippocampal neurogenesis in mice impairs single-but not multiple-trial contextual fear conditioning. Behav Neurosci 124, 446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ, Petrik D, 2012. Depression and hippocampal neurogenesis: a road to remission? Science 338, 72–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epp JR, Silva Mera R, Kohler S, Josselyn SA, Frankland PW, 2016. Neurogenesis-mediated forgetting minimizes proactive interference. Nat Commun 7, 10838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabel K, Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD, 2003. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci 18, 2803–2812. [DOI] [PubMed] [Google Scholar]
- Filippov V, Kronenberg G, Pivneva T, Reuter K, Steiner B, Wang LP, Yamaguchi M, Kettenmann H, Kempermann G, 2003. Subpopulation of nestin-expressing progenitor cells in the adult murine hippocampus shows electrophysiological and morphological characteristics of astrocytes. Mol Cell Neurosci 23, 373–382. [DOI] [PubMed] [Google Scholar]
- Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, Laine GA, Cox CS Jr., 2009. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev 18, 683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, Cestari V, Filipkowski RK, McDonald RJ, Silva AJ, 1998. The dorsal hippocampus is essential for context discrimination but not for contextual conditioning. Behav Neurosci 112, 863–874. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Kohler S, Josselyn SA, 2013. Hippocampal neurogenesis and forgetting. Trends Neurosci 36, 497–503. [DOI] [PubMed] [Google Scholar]
- Gage FH, 2000. Mammalian neural stem cells. Science 287, 1433–1438. [DOI] [PubMed] [Google Scholar]
- Gage FH, 2019. Adult neurogenesis in mammals. Science 364, 827–828. [DOI] [PubMed] [Google Scholar]
- Gao A, Xia F, Guskjolen AJ, Ramsaran AI, Santoro A, Josselyn SA, Frankland PW, 2018. Elevation of Hippocampal Neurogenesis Induces a Temporally Graded Pattern of Forgetting of Contextual Fear Memories. J Neurosci 38, 3190–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Chen J, 2009. Conditional knockout of brain-derived neurotrophic factor in the hippocampus increases death of adult-born immature neurons following traumatic brain injury. J Neurotrauma 26, 1325–1335. [DOI] [PubMed] [Google Scholar]
- Gibb SL, Zhao Y, Potter D, Hylin MJ, Bruhn R, Baimukanova G, Zhao J, Xue H, Abdel-Mohsen M, Pillai SK, Moore AN, Johnson EM, Cox CS Jr., Dash PK, Pati S, 2015. TIMP3 Attenuates the Loss of Neural Stem Cells, Mature Neurons and Neurocognitive Dysfunction in Traumatic Brain Injury. Stem Cells 33, 3530–3544. [DOI] [PubMed] [Google Scholar]
- Gobeske KT, Das S, Bonaguidi MA, Weiss C, Radulovic J, Disterhoft JF, Kessler JA, 2009. BMP signaling mediates effects of exercise on hippocampal neurogenesis and cognition in mice. PLoS One 4, e7506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves JT, Schafer ST, Gage FH, 2016. Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior. Cell 167, 897–914. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ, 1999a. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci 2, 260–265. [DOI] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Graziano MS, Gross CG, 1999b. Neurogenesis in the neocortex of adult primates. Science 286, 548–552. [DOI] [PubMed] [Google Scholar]
- Han X, Tong J, Zhang J, Farahvar A, Wang E, Yang J, Samadani U, Smith DH, Huang JH, 2011. Imipramine treatment improves cognitive outcome associated with enhanced hippocampal neurogenesis after traumatic brain injury in mice. J Neurotrauma 28, 995–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Yu L, Ren J, Wang M, Liu Z, Hu X, Hu D, Chen Y, Chen L, Zhang Y, Liu Y, Zhang X, He H, Gao Z, 2017. Efficient and Fast Differentiation of Human Neural Stem Cells from Human Embryonic Stem Cells for Cell Therapy. Stem Cells Int 2017, 9405204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harting MT, Jimenez F, Xue H, Fischer UM, Baumgartner J, Dash PK, Cox CS, 2009. Intravenous mesenchymal stem cell therapy for traumatic brain injury. J Neurosurg 110, 1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood KN, Zhao J, Redell JB, Hylin MJ, Harris B, Perez A, Moore AN, Dash PK, 2018. Endoplasmic Reticulum Stress Contributes to the Loss of Newborn Hippocampal Neurons after Traumatic Brain Injury. J Neurosci 38, 2372–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckleberry KA, Ferguson LB, Drew MR, 2016. Behavioral mechanisms of context fear generalization in mice. Learn Mem 23, 703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim S, Hu W, Wang X, Gao X, He C, Chen J, 2016. Traumatic Brain Injury Causes Aberrant Migration of Adult-Born Neurons in the Hippocampus. Sci Rep 6, 21793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S, Clark RE, Broadbent NJ, Clemenson GD Jr., Consiglio A, Lie DC, Squire LR, Gage FH, 2009. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem 16, 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhaveri DJ, Tedoldi A, Hunt S, Sullivan R, Watts NR, Power JM, Bartlett PF, Sah P, 2018. Evidence for newly generated interneurons in the basolateral amygdala of adult mice. Mol Psychiatry 23, 521–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Peel AL, Mao XO, Xie L, Cottrell BA, Henshall DC, Greenberg DA, 2004. Increased hippocampal neurogenesis in Alzheimer’s disease. Proc Natl Acad Sci U S A 101, 343–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MA, Weick JP, Pearce RA, Zhang SC, 2007. Functional neural development from human embryonic stem cells: accelerated synaptic activity via astrocyte coculture. J Neurosci 27, 3069–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH, 1998. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci 18, 3206–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Song H, Gage FH, 2015. Neurogenesis in the Adult Hippocampus. Cold Spring Harb Perspect Biol 7, a018812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirbek MA, Klemenhagen KC, Sahay A, Hen R, 2012a. Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders. Nat Neurosci 15, 1613–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirbek MA, Tannenholz L, Hen R, 2012b. NR2B-dependent plasticity of adult-born granule cells is necessary for context discrimination. J Neurosci 32, 8696–8702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleindienst A, McGinn MJ, Harvey HB, Colello RJ, Hamm RJ, Bullock MR, 2005. Enhanced hippocampal neurogenesis by intraventricular S100B infusion is associated with improved cognitive recovery after traumatic brain injury. J Neurotrauma 22, 645–655. [DOI] [PubMed] [Google Scholar]
- Kodali M, Megahed T, Mishra V, Shuai B, Hattiangady B, Shetty AK, 2016. Voluntary Running Exercise-Mediated Enhanced Neurogenesis Does Not Obliterate Retrograde Spatial Memory. J Neurosci 36, 8112–8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornack DR, Rakic P, 1999. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc Natl Acad Sci U S A 96, 5768–5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert C, Cisternas P, Inestrosa NC, 2016. Role of Wnt Signaling in Central Nervous System Injury. Mol Neurobiol 53, 2297–2311. [DOI] [PubMed] [Google Scholar]
- Lee C, Agoston DV, 2010. Vascular endothelial growth factor is involved in mediating increased de novo hippocampal neurogenesis in response to traumatic brain injury. J Neurotrauma 27, 541–553. [DOI] [PubMed] [Google Scholar]
- Lee H, Shamy GA, Elkabetz Y, Schofield CM, Harrsion NL, Panagiotakos G, Socci ND, Tabar V, Studer L, 2007. Directed differentiation and transplantation of human embryonic stem cell-derived motoneurons. Stem Cells 25, 1931–1939. [DOI] [PubMed] [Google Scholar]
- Li YQ, Cheng Z, Wong S, 2016. Differential Apoptosis Radiosensitivity of Neural Progenitors in Adult Mouse Hippocampus. Int J Mol Sci 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Song N, 2016. Molecular Mechanism of Adult Neurogenesis and its Association with Human Brain Diseases. J Cent Nerv Syst Dis 8, 5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan TT, Villapol S, Symes AJ, 2013. TGF-beta superfamily gene expression and induction of the Runx1 transcription factor in adult neurogenic regions after brain injury. PLoS One 8, e59250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein DH, Thomas MJ, Smith DH, McIntosh TK, 1992. Selective vulnerability of dentate hilar neurons following traumatic brain injury: a potential mechanistic link between head trauma and disorders of the hippocampus. J Neurosci 12, 4846–4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Li Y, Wang L, Chen J, Mahmood A, Chopp M, 2001. Intraarterial administration of marrow stromal cells in a rat model of traumatic brain injury. J Neurotrauma 18, 813–819. [DOI] [PubMed] [Google Scholar]
- Lu D, Mahmood A, Qu C, Goussev A, Schallert T, Chopp M, 2005. Erythropoietin enhances neurogenesis and restores spatial memory in rats after traumatic brain injury. J Neurotrauma 22, 1011–1017. [DOI] [PubMed] [Google Scholar]
- Lu D, Mahmood A, Zhang R, Copp M, 2003. Upregulation of neurogenesis and reduction in functional deficits following administration of DEtA/NONOate, a nitric oxide donor, after traumatic brain injury in rats. J Neurosurg 99, 351–361. [DOI] [PubMed] [Google Scholar]
- Ma DK, Jang MH, Guo JU, Kitabatake Y, Chang ML, Pow-Anpongkul N, Flavell RA, Lu B, Ming GL, Song H, 2009. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science 323, 1074–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood A, Lu D, Lu M, Chopp M, 2003. Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery 53, 697–702; discussion 702-693. [DOI] [PubMed] [Google Scholar]
- Mahmood A, Lu D, Wang L, Chopp M, 2002. Intracerebral transplantation of marrow stromal cells cultured with neurotrophic factors promotes functional recovery in adult rats subjected to traumatic brain injury. J Neurotrauma 19, 1609–1617. [DOI] [PubMed] [Google Scholar]
- Marin-Burgin A, Mongiat LA, Pardi MB, Schinder AF, 2012. Unique processing during a period of high excitation/inhibition balance in adult-born neurons. Science 335, 1238–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S, 2007. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science 317, 94–99. [DOI] [PubMed] [Google Scholar]
- Menge T, Zhao Y, Zhao J, Wataha K, Gerber M, Zhang J, Letourneau P, Redell J, Shen L, Wang J, Peng Z, Xue H, Kozar R, Cox CS Jr., Khakoo AY, Holcomb JB, Dash PK, Pati S, 2012. Mesenchymal stem cells regulate blood-brain barrier integrity through TIMP3 release after traumatic brain injury. Sci Transl Med 4, 161ra150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming GL, Song H, 2005. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci 28, 223–250. [DOI] [PubMed] [Google Scholar]
- Mira H, Andreu Z, Suh H, Lie DC, Jessberger S, Consiglio A, San Emeterio J, Hortiguela R, Marques-Torrejon MA, Nakashima K, Colak D, Gotz M, Farinas I, Gage FH, 2010. Signaling through BMPR-IA regulates quiescence and long-term activity of neural stem cells in the adult hippocampus. Cell Stem Cell 7, 78–89. [DOI] [PubMed] [Google Scholar]
- Moreno-Jimenez EP, Flor-Garcia M, Terreros-Roncal J, Rabano A, Cafini F, Pallas-Bazarra N, Avila J, Llorens-Martin M, 2019. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat Med 25, 554–560. [DOI] [PubMed] [Google Scholar]
- Neuberger EJ, Swietek B, Corrubia L, Prasanna A, Santhakumar V, 2017. Enhanced Dentate Neurogenesis after Brain Injury Undermines Long-Term Neurogenic Potential and Promotes Seizure Susceptibility. Stem Cell Reports 9, 972–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninkovic J, Mori T, Gotz M, 2007. Distinct modes of neuron addition in adult mouse neurogenesis. J Neurosci 27, 10906–10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier AL, Tabar V, Barberi T, Rubio ME, Bruses J, Topf N, Harrison NL, Studer L, 2004. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci U S A 101, 12543–12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccin D, Morshead CM, 2011. Wnt signaling regulates symmetry of division of neural stem cells in the adult brain and in response to injury. Stem Cells 29, 528–538. [DOI] [PubMed] [Google Scholar]
- Robinson C, Apgar C, Shapiro LA, 2016. Astrocyte Hypertrophy Contributes to Aberrant Neurogenesis after Traumatic Brain Injury. Neural Plast 2016, 1347987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rola R, Mizumatsu S, Otsuka S, Morhardt DR, Noble-Haeusslein LJ, Fishman K, Potts MB, Fike JR, 2006. Alterations in hippocampal neurogenesis following traumatic brain injury in mice. Exp Neurol 202, 189–199. [DOI] [PubMed] [Google Scholar]
- Sahay A, Hen R, 2007. Adult hippocampal neurogenesis in depression. Nat Neurosci 10, 1110–1115. [DOI] [PubMed] [Google Scholar]
- Sahay A, Wilson DA, Hen R, 2011. Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron 70, 582–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas PC, 2012. Wnt signaling in the vertebrate central nervous system: from axon guidance to synaptic function. Cold Spring Harb Perspect Biol 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhakumar V, Bender R, Frotscher M, Ross ST, Hollrigel GS, Toth Z, Soltesz I, 2000. Granule cell hyperexcitability in the early post-traumatic rat dentate gyrus: the ‘irritable mossy cell’ hypothesis. J Physiol 524 Pt 1, 117–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe MD, Malleret G, Vronskaya S, Mendez I, Garcia AD, Sofroniew MV, Kandel ER, Hen R, 2007. Paradoxical influence of hippocampal neurogenesis on working memory. Proc Natl Acad Sci U S A 104, 4642–4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S, 2005. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol 192, 348–356. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Myers CE, 2012. Hilar mossy cells of the dentate gyrus: a historical perspective. Front Neural Circuits 6, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J, 2004. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature 429, 184–187. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Duman RS, 2007. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol 18, 391–418. [DOI] [PubMed] [Google Scholar]
- Seo DO, Carillo MA, Chih-Hsiung Lim S, Tanaka KF, Drew MR, 2015. Adult Hippocampal Neurogenesis Modulates Fear Learning through Associative and Nonassociative Mechanisms. J Neurosci 35, 11330–11345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro LA, 2017. Altered Hippocampal Neurogenesis during the First 7 Days after a Fluid Percussion Traumatic Brain Injury. Cell Transplant 26, 1314–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin MS, Park HK, Kim TW, Ji ES, Lee JM, Choi HS, Kim MY, Kim YP, 2016. Neuroprotective Effects of Bone Marrow Stromal Cell Transplantation in Combination With Treadmill Exercise Following Traumatic Brain Injury. Int Neurourol J 20, S49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Drew MR, 2020. Functional neurogenesis over the years. Behav Brain Res 382, 112470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrells SF, Paredes MF, Cebrian-Silla A, Sandoval K, Qi D, Kelley KW, James D, Mayer S, Chang J, Auguste KI, Chang EF, Gutierrez AJ, Kriegstein AR, Mathern GW, Oldham MC, Huang EJ, Garcia-Verdugo JM, Yang Z, Alvarez-Buylla A, 2018. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 555, 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Bostrom E, Westerlund I, Vial C, Buchholz BA, Possnert G, Mash DC, Druid H, Frisen J, 2013. Dynamics of hippocampal neurogenesis in adult humans. Cell 153, 1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Bullock MR, Altememi N, Zhou Z, Hagood S, Rolfe A, McGinn MJ, Hamm R, Colello RJ, 2010. The effect of epidermal growth factor in the injured brain after trauma in rats. J Neurotrauma 27, 923–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Bullock MR, McGinn MJ, Zhou Z, Altememi N, Hagood S, Hamm R, Colello RJ, 2009. Basic fibroblast growth factor-enhanced neurogenesis contributes to cognitive recovery in rats following traumatic brain injury. Exp Neurol 216, 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Colello RJ, Daugherty WP, Kwon TH, McGinn MJ, Harvey HB, Bullock MR, 2005. Cell proliferation and neuronal differentiation in the dentate gyrus in juvenile and adult rats following traumatic brain injury. J Neurotrauma 22, 95–105. [DOI] [PubMed] [Google Scholar]
- Sun D, Daniels TE, Rolfe A, Waters M, Hamm R, 2015. Inhibition of injury-induced cell proliferation in the dentate gyrus of the hippocampus impairs spontaneous cognitive recovery after traumatic brain injury. J Neurotrauma 32, 495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, McGinn MJ, Zhou Z, Harvey HB, Bullock MR, Colello RJ, 2007. Anatomical integration of newly generated dentate granule neurons following traumatic brain injury in adult rats and its association to cognitive recovery. Exp Neurol 204, 264–272. [DOI] [PubMed] [Google Scholar]
- Tanti A, Belzung C, 2013. Neurogenesis along the septo-temporal axis of the hippocampus: are depression and the action of antidepressants region-specific? Neuroscience 252, 234–252. [DOI] [PubMed] [Google Scholar]
- Terranova JI, Ogawa SK, Kitamura T, 2019. Adult hippocampal neurogenesis for systems consolidation of memory. Behav Brain Res 372, 112035. [DOI] [PubMed] [Google Scholar]
- Toda T, Parylak SL, Linker SB, Gage FH, 2019. The role of adult hippocampal neurogenesis in brain health and disease. Mol Psychiatry 24, 67–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urrea C, Castellanos DA, Sagen J, Tsoulfas P, Bramlett HM, Dietrich WD, 2007. Widespread cellular proliferation and focal neurogenesis after traumatic brain injury in the rat. Restor Neurol Neurosci 25, 65–76. [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH, 2000. Neural consequences of environmental enrichment. Nat Rev Neurosci 1, 191–198. [DOI] [PubMed] [Google Scholar]
- Vicidomini C, Guo N, Sahay A, 2020. Communication, Cross Talk, and Signal Integration in the Adult Hippocampal Neurogenic Niche. Neuron 105, 220–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villasana LE, Kim KN, Westbrook GL, Schnell E, 2015. Functional Integration of Adult-Born Hippocampal Neurons after Traumatic Brain Injury(1,2,3). eNeuro 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Soto C, Yoo S, Sodoma M, Vivar C, van Praag H, 2019. Exercise and Hippocampal Memory Systems. Trends Cogn Sci 23, 318–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Gao X, Michalski S, Zhao S, Chen J, 2016. Traumatic Brain Injury Severity Affects Neurogenesis in Adult Mouse Hippocampus. J Neurotrauma 33, 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Neumann M, Hansen K, Hong SM, Kim S, Noble-Haeusslein LJ, Liu J, 2011. Fluoxetine increases hippocampal neurogenesis and induces epigenetic factors but does not improve functional recovery after traumatic brain injury. J Neurotrauma 28, 259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski KE, Laure-Kamionowska M, Wisniewski HM, 1984. Evidence of arrest of neurogenesis and synaptogenesis in brains of patients with Down’s syndrome. N Engl J Med 311, 1187–1188. [DOI] [PubMed] [Google Scholar]
- Xiong K, Luo DW, Patrylo PR, Luo XG, Struble RG, Clough RW, Yan XX, 2008. Doublecortin-expressing cells are present in layer II across the adult guinea pig cerebral cortex: partial colocalization with mature interneuron markers. Exp Neurol 211, 271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Meng Y, Zhang Y, Zhang ZG, Morris DC, Chopp M, 2011. Treatment of traumatic brain injury with thymosin beta(4) in rats. J Neurosurg 114, 102–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R, Zhang L, Li M, Liu X, Yang X, Chen L, 2018. Hes1 negatively regulates neurogenesis in the adult mouse dentate gyrus following traumatic brain injury. Exp Ther Med 16, 2267–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau SY, Li A, So KF, 2015. Involvement of Adult Hippocampal Neurogenesis in Learning and Forgetting. Neural Plast 2015, 717958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh CY, Asrican B, Moss J, Quintanilla LJ, He T, Mao X, Casse F, Gebara E, Bao H, Lu W, Toni N, Song J, 2018. Mossy Cells Control Adult Neural Stem Cell Quiescence and Maintenance through a Dynamic Balance between Direct and Indirect Pathways. Neuron 99, 493–510.e494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura S, Teramoto T, Whalen MJ, Irizarry MC, Takagi Y, Qiu J, Harada J, Waeber C, Breakefield XO, Moskowitz MA, 2003. FGF-2 regulates neurogenesis and degeneration in the dentate gyrus after traumatic brain injury in mice. J Clin Invest 112, 1202–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun S, Reynolds RP, Petrof I, White A, Rivera PD, Segev A, Gibson AD, Suarez M, DeSalle MJ, Ito N, Mukherjee S, Richardson DR, Kang CE, Ahrens-Nicklas RC, Soler I, Chetkovich DM, Kourrich S, Coulter DA, Eisch AJ, 2018. Stimulation of entorhinal cortex-dentate gyrus circuitry is antidepressive. Nat Med 24, 658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Ishrat S, O’Bryan M, Klein B, Saraswati M, Robertson CL, Kannan S, 2020. Pediatric traumatic brain injury causes long-term deficits in adult hippocampal neurogenesis and cognition. J Neurotrauma. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Wang H, Jin Z, Cai X, Gao N, Cui X, Liu P, Zhang J, Yang S, Yang X, 2015. Downregulation of survivin regulates adult hippocampal neurogenesis and apoptosis, and inhibits spatial learning and memory following traumatic brain injury. Neuroscience 300, 219–228. [DOI] [PubMed] [Google Scholar]
- Zhao J, Hylin MJ, Kobori N, Hood KN, Moore AN, Dash PK, 2018. Post-Injury Administration of Galantamine Reduces Traumatic Brain Injury Pathology and Improves Outcome. J Neurotrauma 35, 362–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Gibb SL, Zhao J, Moore AN, Hylin MJ, Menge T, Xue H, Baimukanova G, Potter D, Johnson EM, Holcomb JB, Cox CS Jr., Dash PK, Pati S, 2016. Wnt3a, a Protein Secreted by Mesenchymal Stem Cells Is Neuroprotective and Promotes Neurocognitive Recovery Following Traumatic Brain Injury. Stem Cells 34, 1263–1272. [DOI] [PubMed] [Google Scholar]


