ABSTRACT
In invertebrates, UNC-45 regulates myosin stability and functions. Vertebrates have two distinct isoforms of the protein: UNC-45B, expressed in muscle cells only, and UNC-45A, expressed in all cells and implicated in regulating both non-muscle myosin II (NMII)- and microtubule (MT)-associated functions. Here, we show that, in vitro and in human and rat cells, UNC-45A binds to the MT lattice, leading to MT bending, breakage and depolymerization. Furthermore, we show that UNC-45A destabilizes MTs independent of its C-terminal NMII-binding domain and even in the presence of the NMII inhibitor blebbistatin. These findings identified UNC-45A as a novel type of MT-severing protein with a dual non-mutually exclusive role in regulating NMII activity and MT stability. Because many human diseases, from cancer to neurodegenerative diseases, are caused by or associated with deregulation of MT stability, our findings have profound implications in the biology of MTs, as well as the biology of human diseases and possible therapeutic implications for their treatment.
This article has an associated First Person interview with the joint first authors of the paper.
KEY WORDS: UNC-45A, Microtubules, Microtubule lattice, Microtubule-destabilizing proteins, Tau
Highlighted Article: Analyses in vitro and in human and rat cells reveal that UNC-45A is a microtubule-destabilizing protein that acts on microtubules independently of its effects on non-muscle myosin II.
INTRODUCTION
UNC-45, a highly conserved member of the UCS (UNC-45/CRO1/She4p) family, was discovered in the early 1970s, when mutations in its encoding gene caused Caenorhabditis elegans to have uncoordinated movements, hence the term ‘UNC’ (Epstein and Thomson, 1974). It was later discovered that this phenotype was due to the role of UNC-45 as a myosin chaperone (Barral et al., 1998, 2002; Bernick et al., 2010; Etard et al., 2007; Etheridge et al., 2002; Geach and Zimmerman, 2010; Hutagalung et al., 2002; Lee et al., 2011). In the early 2000s, it was shown that, unlike lower organisms, vertebrates have two isoforms of UNC-45: UNC-45A and UNC-45B. The latter’s encoding gene is located on chromosome 17 and is expressed in muscle cells only, where – similarly to UNC-45 – it is required for striated muscle myosin folding and assembly (Bujalowski et al., 2014). The gene encoding UNC-45A, on the other hand, is located on chromosome 15, is expressed in all cells and has multiple functions.
We and others have shown that, in mammalian cells, UNC-45A binds to and colocalizes with non-muscle myosin II (NMII) (Bazzaro et al., 2007; Iizuka et al., 2015, 2017a), and the C-terminal NMII-binding domain of UNC-45A is required for myosin II folding, myosin II binding with actin (Guo et al., 2011; Ni et al., 2011) and stress fiber assembly (Lehtimäki et al., 2017). This includes studies from our laboratory showing that UNC-45A plays a role in regulating NMII-assisted functions, including cytokinesis (Bazzaro et al., 2007), exocytosis in immune cells (Iizuka et al., 2015), axonal growth of neurons (Iizuka et al., 2017b) and tunneling nanotube formation (Lou et al., 2018).
In addition to its myosin-dependent functions, UNC-45A was recently found to colocalize with gamma-tubulin at the microtubule (MT)-organizing center (MTOC), biochemically co-fractionates with gamma-tubulin (Jilani et al., 2015), and fractionates with well-known MT-associated and destabilizing proteins, including katanin and MCAK (also known as FIF2C) (Itzhak et al., 2016). Additionally, we have recently shown that UNC-45A is a novel MT-associated-protein (MAP) with MT-destabilizing properties, and that UNC-45A is overexpressed in paclitaxel-resistant human cancer cells, where it counteracts the MT-stabilizing effects of paclitaxel (Habicht et al., 2019; Mooneyham et al., 2019). Although UNC-45A has been most commonly associated with actomyosin-related function, recent studies contribute to the growing body of evidence that UNC-45A has myosin-independent functions and regulates MT stability.
A number of MAPs are involved in regulating MT stability via either promoting polymerization or depolymerization from MT ends (Goodson and Jonasson, 2018). A special class of MT-destabilizing proteins, known as MT-severing proteins, exerts destabilization from the MT lattice. MT-severing proteins bind along the length of MTs, causing morphological changes to the MT lattice, which is followed by MT breakage into shorter fragments that eventually depolymerize (Hartman et al., 1998; Hartman and Vale, 1999).
In this study, we show for the first time that UNC-45A depolymerizes paclitaxel-stabilized MTs by binding to their lattice and weakening it, which causes kinks and breakages along the MT length. This is followed by MT depolymerization in a dose-dependent manner. We also show for the first time that, both in vitro and in cells, the C-terminal NMII-binding domain of UNC-45A is not required for MT binding or for their destabilization. Lastly, we show that, in cells, UNC-45A depolymerizes MTs independent of its effect on NMII because UNC-45A-mediated MT depolymerization occurs even in the presence of the NMII inhibitor blebbistatin. Taken together, our study results support the role of UNC-45A as a novel member of the MT-destabilizing protein family via weakening, bending and breaking the MT lattice, and its dual and not mutually exclusive role in regulating actomyosin and MT activities.
RESULTS
In vitro, UNC-45A-mediated MT destabilization is preceded by kinks and breakages in the MT body
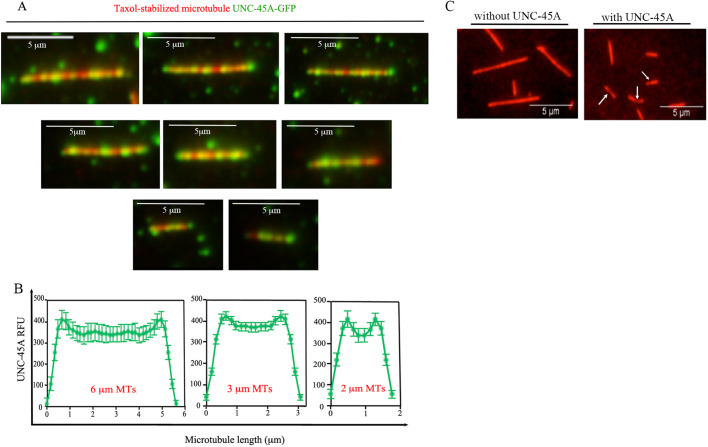
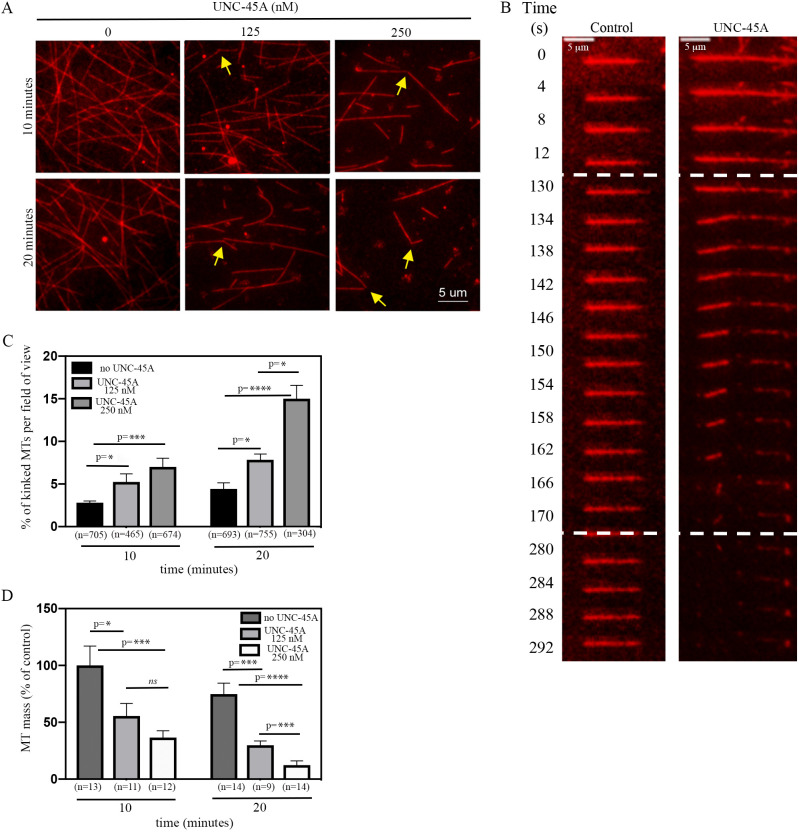
We have recently shown that UNC-45A is a novel MAP that binds and destabilizes MTs in vitro and in vivo in cancer cells and in fibroblasts (Habicht et al., 2019; Mooneyham et al., 2019). To gain insights into the mechanism through which UNC-45A regulates MT stability, we used total internal reflection fluorescence (TIRF) microscopy to examine the localization of UNC-45A on paclitaxel-stabilized rhodamine-labeled MTs of varying lengths. By sorting the MTs by length and then quantifying the average localization of UNC-45A-GFP on the MTs, we found that UNC-45A-GFP binds robustly along the length of the in vitro MTs, with a slight increase in UNC45A-GFP binding at MT ends (Fig. 1A,B). This suggests that, in vitro, UNC-45A might not act like a plus-end MT-destabilizing protein. While performing these experiments, we noticed that not only did MTs shorten in the presence of UNC-45A in a dose- and time-dependent fashion (Mooneyham et al., 2019), but they also appeared to be kinked and have gaps that were generally unapparent in MTs in the absence of UNC-45A (Fig. 1C, arrows). Because this suggests that UNC-45A may destabilize the MT lattice, we next examined the events that preceded MT depolymerization in the presence of UNC-45A. For this, paclitaxel-stabilized, rhodamine-labeled bovine-brain MTs were incubated in the absence and presence of 125 nM or 250 nM UNC-45A-GFP, and the presence of kinks and overall MT mass were recorded at 10 min and 20 min of incubation. We found that the UNC-45A causes a dose- and time-dependent increase in the numbers of MTs with kinks (Fig. 2A) and a decrease in MT mass, compared to the control condition (absence of UNC-45A). Time-lapse TIRF microscopy of paclitaxel-stabilized, rhodamine-labeled bovine-brain MTs in the presence of 250 nM GFP-UNC-45A also showed that, in the presence (but not absence) of UNC-45A, MT breakages and depolymerization are preceded by MT kinks (Fig. 2B). Quantification of the number of kinked MTs and MT mass in the absence and presence of increasing concentrations of UNC-45A over time is given in Fig. 2C and D, respectively. Taken together, these results suggest that UNC-45A binds, kinks and destabilizes paclitaxel-stabilized MTs in a manner that is consistent with destabilizing MTs along their length.
Fig. 1.
UNC-45A binds to microtubules (MTs) independent of their length. (A) Sample images of paclitaxel-stabilized MTs (red) and UNC-45A-GFP (green) for different MT lengths. (B) Quantification of UNC-45A-GFP localization on MTs, for three different average MT lengths (errors bars, s.e.m.; n=115, n=138 and n=134, respectively). (C) Sample image of MTs with kinks (indicated by arrows) in the presence of 250 nM UNC-45A. RFU, relative fluorescence units.
Fig. 2.
UNC-45A binds, kinks and destabilizes MTs in vitro in a concentration-dependent manner. (A) Sample images of paclitaxel-stabilized MTs in the absence and presence of 125 nM or 250 nM UNC-45A-GFP for 10 min and 20 min. Arrows indicate MTs with kinks. (B) Sample images of time-lapse TIRF microscopy of paclitaxel-stabilized MTs in the presence or absence (control) of 250 nM UNC-45A-GFP. Dashed lines indicate the timeframe in which MTs kink and break. (C) MTs with kinks evaluated in the absence and presence of increasing concentrations of UNC-45A at 10 min and 20 min of incubation. Results are expressed as percentage of kinked MTs per field of view (n=MTs evaluated per condition). (D) MT mass in the absence and presence of increasing concentrations of UNC-45A at 10 min and 20 min of incubation, and determined by measuring the average MT fluorescent intensity from three individual areas per field of view (n=field of view measured per condition). Results are expressed as a percentage of control. *P<0.05, ***P<0.001, ****P<0.0001; ns, not significant.
UNC-45A breaks paclitaxel-stabilized MTs in vitro independent of its C-terminal NMII-binding domain
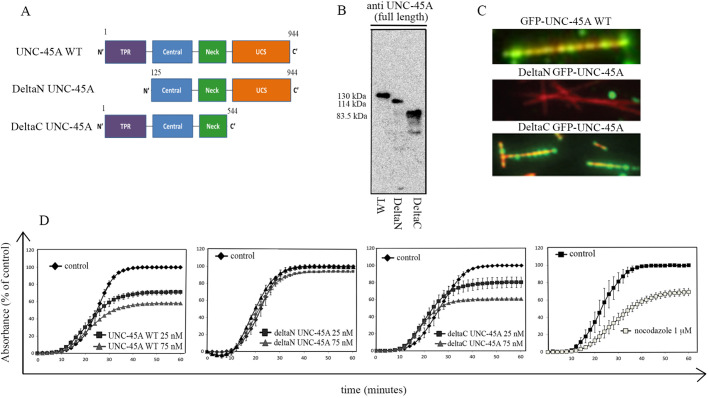
Structurally, UNC-45A can be divided into four domains: an N-terminal domain, which contains three tetratricopeptide repeat (TPR) sequences and has been shown to bind to Hsp90 (also known as HSP90AA1); a central domain of largely unknown function; a neck domain, which has been recently proposed to be required for UNC-45 (worm isoform of UNC-45A) oligomerization; and a C-terminal, UCS domain that is critical for interaction with NMII (Barral et al., 1998; Ni et al., 2011; Shi and Blobel, 2010) (Fig. 3A). We have shown that UNC-45A destabilizes paclitaxel-stabilized MTs in the absence of any additional cellular components, including NMII, suggesting that, in vitro, UNC-45A destabilizes MTs independent of the presence of NMII (Mooneyham et al., 2019). Here, we set out to determine which domain of UNC-45A is required for the binding and destabilization of paclitaxel-stabilized MTs in vitro. We started by evaluating the MT-binding abilities of recombinant UNC-45A wild type (WT), deltaN-UNC-45A or deltaC-UNC-45A (Fig. 3B) via TIRF microscopy. As we have previously shown (Mooneyham et al., 2019), UNC-45A is capable of directly binding MTs in a purified in vitro system without any additional cellular factors. The binding pattern is punctate and visible along the entire length of the MTs (Fig. 3C, top). Similarly, and despite being approximately half the size of the UNC-45A WT, the deltaC-UNC-45A retained its ability to bind MTs efficiently and with a similar punctate pattern (Fig. 3C, bottom). Conversely, deletion of the N-terminus domain completely abrogated the MT binding ability of UNC-45A (Fig. 3C, middle). This suggests that the NMII-interacting domain is not required for the MT-associated function of UNC-45A and that the UNC-45A MT-interacting domain is located in the first 124 amino acids (aa) at the amino terminus. To correlate these findings with MT-destabilizing activity, we determined the ability of recombinant full-length (WT) UNC-45A, deltaN-UNC-45A or deltaC-UNC-45A to limit net polymerization in a standard turbidimetric assay (Shelanski, 1973; Shelanski et al., 1973). We found that both WT and deltaC-UNC-45A caused a dose-dependent inhibition of net MT assembly in vitro (Fig. 3D, first and third panels), whereas deltaN-UNC-45A did not (Fig. 3D, second panel). Importantly, UNC-45A acts on MTs at concentrations similar to the ones reported for other MT-depolymerizing proteins when used in this assay (Di Paolo et al., 1997; Ng et al., 2006). Nocodazole was used as a positive control (Fig. 3D, last panel). Furthermore, both full-length (WT) UNC-45A and deltaC-UNC-45A do not seem to interfere with either nucleation or the elongation phase, but rather with the MT steady-state levels, suggesting that UNC-45A acts to destabilize polymerized MTs. Importantly, because in this assay MTs are not stabilized by paclitaxel, this also suggests that UNC-45A does not exclusively depolymerize MTs by disrupting paclitaxel stabilization. Taken together, these results support the idea that UNC-45A has multiple distinct roles mediated by distinct protein domains.
Fig. 3.
UNC-45A breaks MTs in vitro independent of its C-terminal NMII-binding domain. (A) Schematic of full-length (WT) UNC-45A (1–944 aa), deltaN-UNC-45A (125–944 aa) or deltaC UNC-45A (1–544 aa). B. Recombinant full-length (WT) UNC-45A-GFP (130 kDa), deltaN UNC-45A-GFP (114 kDa) and deltaC UNC-45A-GFP (83.5 kDa) were separated on a 4–15% SDS gel, transferred onto PVDF membrane and blotted against a rabbit polyclonal antibody raised against full-length human UNC-45A. (C) Representative images of WT (top), C-terminally deleted (middle) or N-terminally deleted (bottom) UNC-45A (GFP-tagged) and rhodamine-labeled paclitaxel-stabilized MTs obtained via TIRF microscopy. (D) Tubulin (5 mg/ml) was incubated in the absence (vehicle) or presence of full-length (WT) UNC-45A (first panel), deltaN-UNC-45A (second panel) or deltaC-UNC-45A (third panel) or nocodazole (last panel) at the indicated concentrations at 4°C. MT polymerization was induced at 37°C and optical density was recorded over a period of 1 h. Results are expressed as absorbance as a percentage of control. All experiments were performed in triplicate.
The C-terminal NMII-binding domain of UNC-45A is not required for MT binding in cells
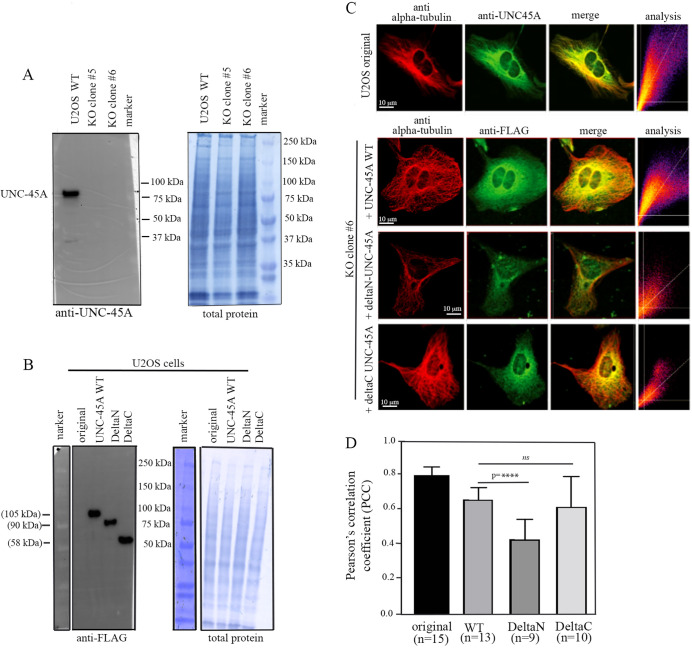
Having shown that in a cell-free system the N-terminal domain of UNC-45A is required for MT binding, we next asked whether the same is true in a cellular system. In order to test whether the N-terminal domain on UNC-45A is required for localization with MTs in cells, we took advantage of the previously characterized UNC-45A knockout (KO) U2OS cells (Lehtimäki et al., 2017). This is for two main reasons: (1) given the relatively high expression levels of UNC-45A in cells, ectopic expression of dominant negatives in UNC-45A WT cells is likely to result in high background; and (2) ectopic expression of MAPs in general is known to cause ‘off-target’ subcellular localization (Lehtimäki et al., 2017). First, we confirmed UNC-45A KO in U2OS cells via western blot analysis. As shown in Fig. 4A, in both the clones used (clone #5 and clone #6) (Lehtimäki et al., 2017) UNC-45A protein is not detected. We then ectopically expressed UNC-45A WT and its deltaN and deltaC mutants as FLAG-tag proteins in UNC-45A KO U2OS cell clone #6, and found that both UNC-45A WT and its mutants were expressed at similar levels in cells (Fig. 4B). Next, we determined the subcellular localization of endogenously expressed UNC-45A (U2OS original) and ectopically expressed UNC-45A WT and its mutants with respect to MTs via immunofluorescence (IF) microscopy as we have done previously (Habicht et al., 2019; Mooneyham et al., 2019). As shown in Fig. 4C (top row) we found that, similarly to what we have previously described in other cell types, UNC-45A colocalizes with MTs in U2OS cells. Of note, we found that, in this particular cell type, the MT network is particularly dense in the perinuclear region. We also found that, similarly to endogenous UNC-45A, ectopically expressed FLAG-tagged UNC-45A and deltaC-UNC-45A colocalize with MTs in U2OS cells (Fig. 4C, second and bottom rows, respectively). However, deletion of the N-terminal resulted in a significant decrease in the levels of colocalization with MTs (Fig. 4C, third row). Quantification of colocalization between UNC-45A and its mutants and MTs is shown in Fig. 4D. Thus, even if to a lesser extent than in the cell-free system (Fig. 3C, middle), the N-terminus of UNC-45A seems to be important for UNC-45A localization to MTs in cells, strengthening our results that the NMII-binding domain of UNC-45A is not required for MT binding.
Fig. 4.
UNC-45A and its C-deleted but not N-deleted mutant colocalize with MTs in cells. (A) Left: western blot analysis of the levels of UNC-45A in WT and UNC-45A knockout (KO) U2OS cells using anti-UNC-45A antibody. Two different clones were used. Right: total protein (Amido Black staining) was used as loading control. (B) Left: western blot analysis of the levels of UNC-45A in original and UNC-45A KO clone #6 ectopically expressing either UNC-45A WT or deltaN-UNC-45A or deltaC-UNC-45A as FLAG-tagged proteins using an anti-FLAG antibody. Right: total protein (Amido Black staining) was used as a loading control. (C) Top row: representative images of tubulin (red) and UNC-45A (green) in original U2OS cells. Second row: representative images of tubulin (red) and UNC-45A WT (FLAG-tagged; green) ectopically expressed in UNC-45A KO U2Os (clone #6). Third row: representative images of tubulin (red) and deltaN-UNC-45A (FLAG-tagged; green) ectopically expressed in UNC-45A KO U2Os (clone #6). Bottom row: representative images of tubulin (red) and deltaC-UNC-45A (FLAG-tagged; green) ectopically expressed in UNC-45A KO U2Os (clone #6). In all rows, merge is in yellow. Colocalization is expressed as Pearson's correlation coefficient (PCC). (D) Quantification of colocalization between UNC-45A and its mutants and MTs as determined via PCC. n=number of cells evaluated per condition (three different areas per cell were evaluated). ****P<0.0001; ns, not significant.
UNC-45A overexpression in RFL-6 cells leads to loss of MT mass and increase in MT breakages
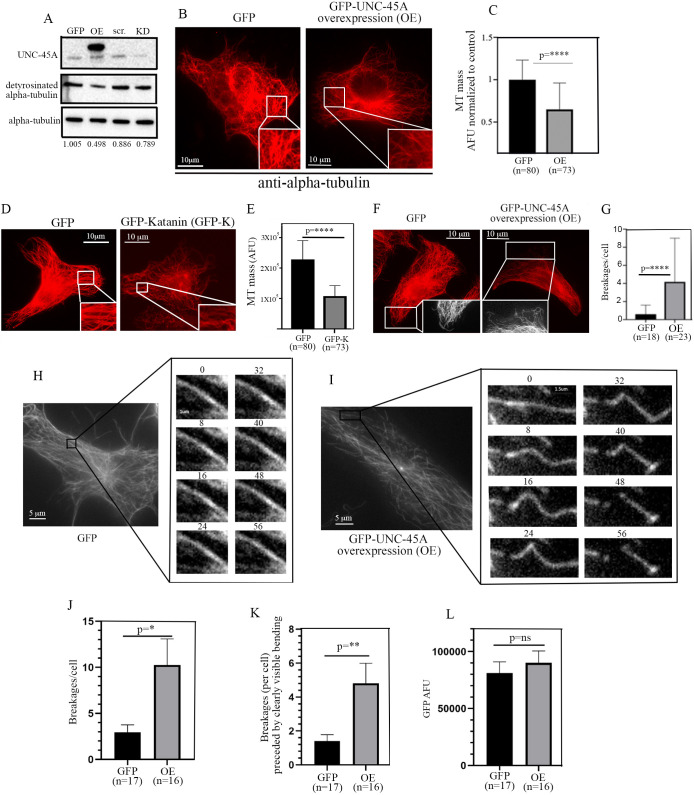
We first determined whether, similarly to what we have shown for other cell types (Habicht et al., 2019; Mooneyham et al., 2019), UNC-45A destabilizes MT in rat lung fibroblast RLF-6 cells. We used these cells because they have a very distinct MT network that facilitates analysis of MTs and their mass (Baas and Sudo, 2010; Sudo and Baas, 2010). As shown in Fig. 5A, overexpression of UNC-45A led to a ∼50% decrease in the levels of detyrosinated alpha-tubulin, a marker of long-lived MTs, in lysates of RFL-6 cells. Complementary experiments showed no differences in the levels of detyrosinated tubulin in UNC-45A knockdown (KD) cells versus control (Fig. 5A). This could be due to the fact that RFL-6 has a relatively stable morphology compared to other cell types, and there is a direct correlation between stable morphology and MT stability (Baas et al., 2016; Qiang et al., 2006). Next, we determined the effects of UNC-45A overexpression in RFL-6 via IF by quantifying the MT mass in control versus overexpressing cells. Specifically, following GFP or UNC-45A-GFP overexpression, cells were fixed, extracted and stained with anti-alpha-tubulin antibody 24 h post-infection. Extraction was performed as previously described to minimize the background caused by free tubulin within cells (Sudo and Baas, 2011). As shown in Fig. 5B, we found that ectopic expression of UNC-45A results in a significant decrease in total MT mass compared to that in control cells. Quantification of MT mass per condition is shown in Fig. 5C. Interestingly, we found no difference in MT mass in ‘low’ versus ‘high’ UNC-45A-expressing RFL-6 cells (Fig. S1A,B). The difference in GFP expression levels in ‘low’ and ‘high’ GFP-UNC45A-expressing RFL-6 cells was consistently >60%. Taken together, these results suggest that even a modest increase in UNC-45A is sufficient to cause a saturating effect. The loss of MT mass following UNC-45A overexpression was similar to that observed after overexpressing the known MT-severing protein katanin (Qiang et al., 2006) in the same cell type (Fig. 5D,E). Importantly, we found that, also similarly to what has been previously reported for katanin (Qiang et al., 2006), UNC-45A overexpression resulted in MT breakages at the edges of RLF-6 cells compared to the control condition (Fig. 5F,G; Fig. S1C). Lastly, we confirmed that UNC-45A overexpression leads to MT breakages in live cells. Time-lapse microscopy of Tubulin Tracker Deep Red-labeled MTs shows that GFP-UNC-45A overexpression in RFL-6 cells results in increased MT bending and breakages compared to GFP-transfected control cells (Fig. 5H,I; Movies 1 and 2). Quantification of the number of MT breakages per cell (three fields of view per cell were counted) in each condition is shown in Fig. 5J. We found that not all MT breakages were preceded by visible MT bending, but that the number of MT breakages that were preceded by bending was significantly higher in GFP-UNC-45A-overexpressing cells compared to control cells. Quantification of MT bending and breakages per condition was performed in cells expressing a similar amount of GFP (shown in Fig. 5L). Importantly, the experiments in live cells were conducted in the presence of 500 nM taxol (personal communication from the Tubulin Tracker Deep Red manufacturer regarding the taxol concentration corresponding to the dilution of Tubulin Tracker Deep Red used), confirming our earlier results that, in cells, UNC-45A counteracts the MT-stabilizing effect of paclitaxel (Mooneyham et al., 2018).
Fig. 5.
UNC-45A overexpression leads to loss of MT mass and increase in MT breakages in RFL-6 cells. (A) Western blot analysis of the levels of UNC-45A and detyrosinated alpha-tubulin (here used as a marker of MT stability) in GFP-UNC-45A-overexpressing (OE) or UNC-45A knockdown (KD) RFL-6 cells compared to their relative controls GFP and scramble (scr.). Total alpha-tubulin was used as a loading control. Numbers represent the ratio between detyrosinated alpha-tubulin and total alpha-tubulin per condition. (B) Representative images of GFP- and GFP-UNC-45A OE RFL-6 cells fixed and stained with an anti-alpha-tubulin antibody to visualize MT mass. All images were taken using the same exposure time in cells expressing similar amounts of GFP. (C) Quantification of MT mass per condition expressed as arbitrary fluorescence units (AFU). n=number of cells analyzed per condition (three different areas per cell were evaluated). (D) Representative images of GFP- and GFP-katanin (GFP-K)-expressing RFL-6 cells fixed and stained with an anti-alpha-tubulin antibody to visualize MT mass. All images were taken using the same exposure time in cells expressing similar amounts of GFP. (E) Quantification of MT mass per condition expressed as AFU. n=number of cells analyzed per condition (three different areas per cell were evaluated). (F) Representative images of GFP- and GFP-UNC-45A OE RFL-6 cells fixed and stained with an anti-alpha-tubulin antibody to visualize MTs and their breakages. All images were taken using the same exposure time in cells expressing similar amounts of GFP. Insets show black and white details. (G) Quantification of MT breakages per cell per condition. n=number of cells analyzed per condition. (H) Live-cell sample images of MTs (shown in white) in GFP-transfected RFL-6 cells. Eight sequential time-lapse frames are shown taken 8 s apart. (I) Live-cell sample images of MTs (shown in white) in GFP-UNC-45A OE RFL-6 cells. Eight sequential time-lapse frames are shown taken 8 s apart. (J) Quantification of MT breakages per cell per condition. (K) Quantification of MT breakages per cell that were preceded by clearly visible bending per condition. n=number of cells analyzed. (L) Quantification of GFP (green signal) intensity in GFP and GFP-UNC-45A cells expressed as AFU. *P<0.05, **P<0.01, ****P<0.0001; ns, not significant.
In RFL-6 cells, UNC-45A breaks the MT lattice in the absence of its C-terminal NMII-binding domain, including in the presence of the NMII inhibitor blebbistatin
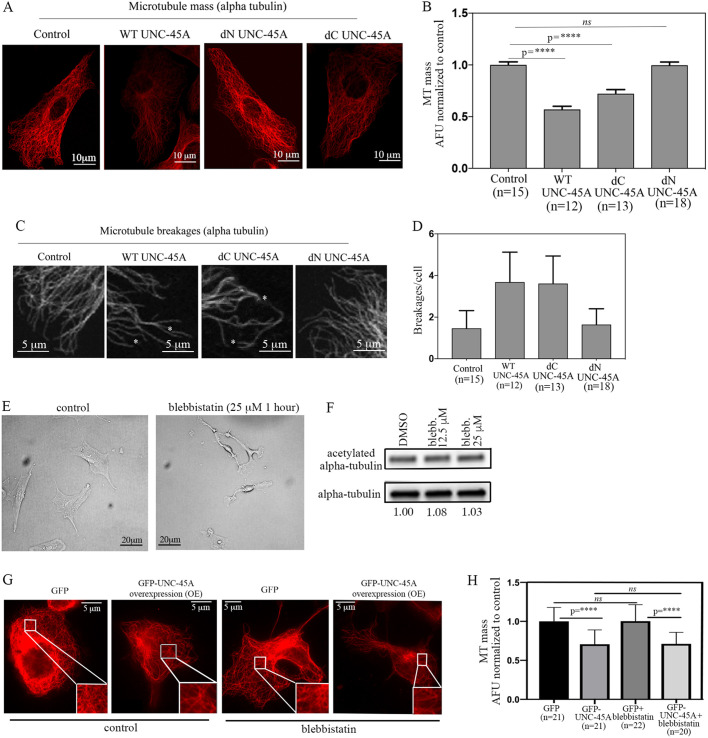
Having established that, in RFL-6 cells, UNC-45A destabilizes MTs via weakening and bending of the MT lattice, we next asked whether the C-terminal NMII-binding domain of UNC-45A is required for this effect. While performing these experiments, we noticed that although UNC-45A WT is overexpressed very efficiently in RFL-6 cells, both mutants were expressed less efficiently (Fig. S2A). For this reason, rather than assessing MT stability biochemically at the population level (via western blotting, for example), we evaluated MT stability in cells that expressed comparable levels (as determined by anti-FLAG IF staining; Fig. S2B) of either UNC-45A or its mutants, as determined by IF. Specifically, following overexpression of UNC-45A and its C-terminally or N-terminally deleted mutants, cells were fixed, extracted and stained with anti-alpha-tubulin antibody 24 h post-infection. As shown in Fig. 6A, we found that ectopic expression of both UNC-45A and its C-terminally deleted mutant resulted in a significant decrease in total MT mass compared to that in control and in N-terminally deleted cells. Quantification of MT mass per condition is shown in Fig. 6B. We also looked at the possible effects of overexpression of UNC-45A and its mutant on MT breakages. Although the numbers did not reach statistical significance (possibly due to the less efficient expression of the FLAG-tagged proteins used for these experiments and compared to the GFP-tagged proteins used in Fig. 5), we found a clear trend for breakages being more frequent in WT UNC-45A- and deltaC-UNC-45A-expressing cells compared to control and deltaN-UNC-45A-expressing cells (Fig. 6C,D).
Fig. 6.
UNC-45A breaks MT in the absence of its C-terminal NMII-binding domain and in the presence of the NMII inhibitor blebbistatin. (A) Representative images of RFL-6 cells transduced with either empty vector (FLAG), WT UNC-45A, C-terminally deleted UNC-45A (dC UNC-45A), or N-terminally deleted UNC-45A (dN UNC-45A) FLAG-tagged proteins and stained for alpha-tubulin (red). All images were taken using the same exposure time. (B) Quantification of MT mass per condition expressed as AFU. n=number of cells analyzed per condition (three different areas per cell were evaluated). (C) Representative images of RFL-6 cells transduced with either empty vector (FLAG), WT UNC-45A (WT), C-terminally deleted UNC-45A (dC UNC-45A) or N-terminally deleted UNC-45A (dN UNC-45A) FLAG-tagged proteins stained for alpha-tubulin (red) to visualize MTs and their breakages. All images were taken using the same exposure time. Black and white images are shown for better visualization. Asterisks indicate MT breakages. (D) Quantification of MT breakages per cell per condition. n=number of cells analyzed per condition. Differences between values for conditions were non-significant. (E) Representative image of RFL-6 cells either mock treated or treated with the indicated concentration of blebbistatin over 1 h. (F) Levels of acetylated tubulin versus total tubulin (numbers indicate the ratio) in control (DMSO) versus blebbistatin-treated RFL-6 cells. (G) Representative images of MT mass in control (GFP) and GFP-UNC-45A OE RFL-6 cells (24 h post-infection) with or without blebbistatin treatment (25 µM for 1 h) after staining with an anti-alpha-tubulin antibody to visualize MTs. All images were taken using the same exposure time. (H) Quantification of MT mass per condition. n=number of cells evaluated per condition (three different areas per cell were evaluated). ****P<0.0001; ns, not significant.
To directly confirm that, in cells, UNC-45A destabilizes MTs independent of its effect on NMII, we investigated whether the effects of UNC-45A on MT destabilization (Figs 5 and 6) occurs even in the presence of the NMII inhibitor blebbistatin (Allingham et al., 2005). For these experiments, we first treated RFL-6 cells with either control dimethyl sulfoxide (DMSO) or 12.5 μm and 25 µM of the NMII inhibitor blebbistatin over a period of 1 h. The amount and duration of this treatment has been shown to potently and specifically inhibit NMII in a number of cell types (Kovács et al., 2004). As expected and previously shown (Kovács et al., 2004), blebbistatin treatment resulted in profound changes in cell morphology, consistent with its effect on decreasing actomyosin contractility (Fig. 6E). We then biochemically determined whether blebbistatin treatment led to changes in the levels of acetylated alpha-tubulin. We found no differences in the levels of acetylated alpha-tubulin in treated versus untreated cells (Fig. 6F). This indicates that, unlike what happens following UNC-45A KD (Fig. 5A; Mooneyham et al., 2019), inhibition of NMII does not lead to an increase in the levels of the more stable MT population. Next, we reasoned that if UNC-45A destabilizes MTs independent of its effect on myosin then it should still have an effect when NMII is inhibited. To this end, GFP- and GFP-UNC-45A-expressing RFL-6 cells were treated with or without 25 µM blebbistatin over a period of 1 h. Cells were next fixed, extracted and stained with anti-alpha-tubulin antibody 24 h post-infection. As shown in Fig. 6G, we found that ectopic expression of UNC-45A (as visualized by the green channel; Fig. S2C), resulted in a significant decrease in total MT mass compared to that in the control, even in the presence of blebbistatin treatment. Quantification of MT mass per condition is shown in Fig. 6H. Taken together, these results indicate that UNC-45A destabilizes MTs independent of its effects on NMII activity.
DISCUSSION
MTs are highly dynamic components of the cytoskeleton and crucial for a variety of cellular functions. A number of MAPs are involved in regulating MT stability via promoting either MT polymerization or depolymerization (Goodson and Jonasson, 2018). There are three main classes of MT-destabilizing proteins: sequestering, plus-end depolymerizing and severing. MT-sequestering proteins do not bind to MTs but cause their destabilization by binding to the pool of free tubulin in the cells, which becomes then unavailable for MT polymerization (Belmont and Mitchison, 1996). MT plus-end-depolymerizing proteins can either bind to the plus end of the MT directly or bind along the length of MT and then travel to their plus end, where they cause destabilization by preventing incorporation of tubulin or by causing morphological changes that cause MT depolymerization for the plus end (Howard and Hyman, 2007). MT-severing proteins, on the other hand, use ATP hydrolysis to ‘wedge’ or ‘pull’ tubulin dimers off the body of MTs (Roll-Mecak and McNally, 2010). This leads to a local destabilization of the MTs, followed by breakages of the MTs along their lattice and creation of new plus ends from which further rapid depolymerization occurs. Katanin, the first MT-severing protein discovered, owes its name to its ability to ‘cut’ MTs along their length in the same fashion as a sword (‘katana’, in Japanese) would do (Quarmby, 2000).
We have previously shown that UNC-45A is an MAP with MT-destabilizing properties in vitro and in vivo in a variety of mammalian cells, and that it is able to counteract the MT-stabilizing effects of taxol in cancer cells (Habicht et al., 2019; Mooneyham et al., 2018). Here, we show that, in vitro, UNC-45A binds to the MT lattice and causes bends and breakages along the length of paclitaxel-stabilized MTs. This is followed by MT depolymerization in a dose-dependent manner. Furthermore, we show that UNC-45A overexpression causes a dramatic loss of MT mass and increase in MT breakages in RFL-6 cells. Importantly, RFL-6 cells lack the well-known MT-stabilizing protein tau (also known as MAPT), which has been recently proposed to prevent the activity of MT-severing proteins (Baas and Qiang, 2019; Tan et al., 2019). Taken together, our data suggest that UNC-45A acts via interacting with the MT lattice and causing its weakening and destabilization, which leads to MT breakages and destabilization. Although additional studies are needed to understand the exact mechanism through which UNC-45A breaks MTs, it is likely that the mechanism is different from that of MT-severing proteins. In fact, UNC-45A has no known ATP-ase domain and is capable of destabilizing paclitaxel-stabilized MTs in vitro independent of ATP (Bailey et al., 2016). Furthermore, and perhaps as a consequence of its ATP independence, UNC-45A requires a higher concentration and longer time, compared to other ATP-dependent MT-destabilizing proteins, to destabilize paclitaxel-stabilized MTs in vitro (Jiang et al., 2017). This is also consistent with the fact that, in cells, UNC-45A is ∼20-fold more abundant (0.4 μM) than other MT-destabilizing proteins, suggesting that UNC-45A might have a dominant role in the regulation of cellular MTs (Itzhak et al., 2016). Interestingly, MT-depolymerizing proteins are known to exist as oligomers (including dimers, hexamers and dodecameric stacked rings), and their oligomerization is required for MT binding and/or MT depolymerization (Hertzer et al., 2006; Lupas and Martin, 2002; Neuwald et al., 1999; Sharp and Ross, 2012; Vale, 1991). Studies conducted using UNC-45 revealed that the protein exists as oligomers (dimers, tetramers, pentamers in C. elegans and dodecamers in Drosophila) in vitro and in vivo (Gazda et al., 2013). The sequence similarity between human and worm UNC-45A is over 50%, and the homology between human and fly UNC-45A is over 60%. This suggests that, similarly to known MT-severing proteins, UNC-45A may exist and act on MTs in an oligomeric state.
Interactions between actomyosin and MT systems are well known, and many cytoskeletal-associated proteins are known to independently regulate both. The calcium-binding family member S100A4, for instance, is overexpressed in a number of human cancers and promotes cancer metastasis (Bresnick et al., 2015). This effect seems to be due to S100A4 binding to NMIIA (also known as MYH9) and promoting its depolymerization, and its myosin II-independent effect consistent with MT depolymerization (Dulyaninova et al., 2018). Furthermore, one of the most well-known cell cycle regulators, the cyclin-dependent kinase inhibitor 1B (p27), is both an MT- and an NMII-associated and colocalizing protein and has been shown to independently regulate both NMII activity and MT stability in normal and cancer cells (Baldassarre et al., 2005; Besson et al., 2004; Bezanilla et al., 2000; Fabris et al., 2015; Godin et al., 2012; Murthy and Wadsworth, 2005; Serres et al., 2012). Similarly to, and perhaps because of its worm homolog UNC-45, UNC-45A has been mostly studied in the context of its role in regulating NMII activity. This includes studies from our and other groups, showing that UNC-45A binds to and colocalizes with NMII and regulates its activity, including the formation of stress fibers (Bazzaro et al., 2007; Guo et al., 2011; Iizuka et al., 2015, 2017a; Lehtimäki et al., 2017). Over the past 5 years, however, it became evident that UNC-45A also regulates MT stability. This not only includes our above-mentioned work (Habicht et al., 2019; Mooneyham et al., 2018), but also work from others showing that UNC-45A colocalizes and co-fractionates with gamma-tubulin, the main component of the MTOC (Jilani et al., 2015), and is abundant in subcellular fractions containing other MAPs with MT-destabilizing properties, including katanin and MCAK (Itzhak et al., 2016).
We have previously shown that UNC-45A binds MTs in vitro in the absence of NMII (Mooneyham et al., 2019). Here, we show that, in the same in vitro system, UNC-45A binds to MTs even in the absence of its C-terminal NMII-binding domain. Further, we show that in a turbidimetric assay, UNC-45A lacking its C-terminal NMII-binding assay is still able to interfere with net MT polymerization in a time- and dose-dependent fashion, similarly to UNC-45A WT. Because in this particular assay tubulin polymerization occurs spontaneously and it is not promoted or maintained by taxol (Shelanski et al., 1973), this result suggests that UNC-45A does not destabilize MTs by simply competing or interfering with taxol binding to MTs. Also, interestingly, in this turbidimetric assay, UNC-45A and its mutant lacking the C-terminal-deleted myosin II do not interfere with the elongation phase of the MTs as nocodazole does, but, rather, they decrease the steady-state levels of polymerized MTs. This is in accordance with our previous results (Mooneyham et al., 2019) indicating that UNC-45A does not destabilize MTs by sequestering free tubulin but rather destabilizes already formed MTs. We also show that, both in vitro and in U2OS cells, UNC-45A lacking the C-terminal NMII-binding domain is still able to bind and destabilize interphase MTs similarly to UNC-45 WT. Importantly, this is the same cell type in which UNC-45A was found to localize at the MTOC, to co-fractionate with gamma-tubulin, and to track with MTs close to the MTOC in metaphase cells (Jilani et al., 2015).
The effects of the actomyosin system on MT stability are generally modest and in reverse direction. Specifically, although inhibition of myosin II leads to modest increase in MT stability (Kadir et al., 2011), we have previously shown that loss of UNC-45A leads to increased myosin II activity (Iizuka et al., 2017a). Here, we show that treatment with the NMII inhibitor blebbistatin induces a profound change in the morphology of RLF-6 cells, with cells becoming more elongated due to a reduction in actomyosin contractility (Allingham et al., 2005; Kovács et al., 2004). This, however, did not result in a change in the expression levels of acetylated alpha-tubulin, suggesting that, at least in this cell type, loss of NMII activity is not accompanied by increased MT stability. Lastly, we show that, in RFL-6 cells, UNC-45A destabilizes MTs even in the presence of blebbistatin, confirming that UNC-45A acts on MTs independently of its effects on NMII, and has a dual non-mutually exclusive role in regulating actomyosin and MT stability.
MATERIALS AND METHODS
Preparation of paclitaxel-stabilized, rhodamine-labeled MTs
The paclitaxel-stabilized rhodamine-labeled MTs were prepared as we have previously described (Mooneyham et al., 2019). Briefly, unlabeled tubulin (Cytoskeleton, T240) and rhodamine-labeled tubulin (Cytoskeleton, TL590M) were mixed in a 5:1 ratio in Brb80 buffer with 1 mM dithiothreitol (DTT) and 1 mM GTP then incubated on ice for 5 min. Next, the mixture was incubated at 37°C, and 1 μM, 10 μM and 100 μM paclitaxel (Cytoskeleton, TXD01) were added sequentially to 1/10 of the reaction mixture volume for 5 min each to create labeled, taxol-stabilized MTs at a final concentration of 40 μM. After incubation, the MT mixture was diluted with warm Brb80 solution containing 100 μM paclitaxel and 1 mM DTT to be flowed into the TIRF chamber. All MTs were prepared on the same day of the experiment.
Construction and preparation of flow chambers for TIRF imaging
A 22×50 mm glass coverslip was used as the base of the chamber and an 18×18 mm coverslip was used as the top. Prior to chamber assembly, coverslips were thoroughly cleaned and silanized. Three narrow strips of parafilm were stacked in three equidistant columns on the base coverslip to create two separate experimental chambers per slide. Once assembled, vacuum assistance was used to flow an anti-rhodamine antibody solution in Brb80 into the chambers. After 10 min of room temperature incubation, the antibody solution was flushed out with two chamber volumes of Brb80 and a blocking solution of 1% PF127 in Brb80 was introduced. After 10 min of incubation, the chambers were flushed with two channel volumes of Brb80, and the rhodamine-labeled MT mixture was added and allowed to attach to the antibody-coated coverslip for 15 min.
UNC-45A-GFP MT binding and impact on MT kinking and MT mass
For UNC-45A-GFP MT binding, a final reaction mixture, containing 1× imaging buffer and 0.6 µM final concentration of UNC-45A-GFP, was introduced into the imaging chamber, and the interaction between UNC-45A-GFP and paclitaxel-stabilized MT was visualized via 488 nm and 561 nm lasers generated from a Nikon TI-TIRF-PAU illuminator, which provided TIRF illumination. The images were collected from a Nikon CFI Apo TIRF 100× oil objective using an Andor iXon EMCCD camera. For the impact of UNC-45A-GFP on MT kinking and MT mass, a final reaction mixture, containing imaging buffer and 125 nM or 250 nM final concentration of UNC-45A-GFP, was introduced into the TIRF imaging chamber, and the interaction between UNC-45A-GFP and paclitaxel-stabilized MTs was visualized via time-lapse imaging with 488 nm and 561 nm lasers generated from the Zeiss TIRF microscope at 100× magnification. Alternatively, images were taken after 10 min and 20 min incubation with UNC-45A-GFP in each condition. A kinked MT was defined as an angle observed within an individual MT. MT mass was measured as average MT fluorescent intensity from three individual areas per field of view. Laser power and exposure time were minimized and TIRF angle was maximized, to avoid photobleaching and photodamage.
Recombinant protein
GFP-tagged UNC-45A full-length (UNC-45A WT; 1–944 aa) and its C-terminally (deltaC-UNC-45A; 1–554 aa) or N-terminally (deltaN-UNC-45A; 125–944 aa) deleted mutants were cloned into pGEX-2TK to generate the GST-GFP-UNC-45A protein. The protein was expressed in Rosetta (DE3) pLysS, and, following GST removal, it was affinity purified and dialyzed, as we have previously described (Mooneyham et al., 2019).
In vitro tubulin polymerization assay
The tubulin polymerization assay was performed as instructed in the Cytoskeleton Tubulin Polymerization Assay Kit (BK006P) with the following modifications: no glycerol was used as a polymerization enhancer due to its known interference with tubulin ligand binding; instead, a 5 mg/ml starting concentration of purified tubulin was used to enhance spontaneous tubulin polymerization. The assay was performed with 25 nM or 75 nM purified UNC-45A protein (WT, C-terminally deleted or N-terminally deleted) and 1 μM nocodazole as a depolymerization control. Each polymerization curve was analyzed as a percentage of the control polymerization curve. All experiments were performed in triplicate using at least two different batches of recombinant protein (specifically, we used three different deltaN-UNC-45A batches and two different batches of WT UNC-45A and deltaC-UNC-45A).
Cell culture
Original and UNC-45A KO human osteosarcoma U2OS cells were a generous gift from Drs Jaakko Lehtimäki and Pekka Lappalainen (University of Helsinki, Helsinki, Finland) and were cultured as previously described (Lehtimäki et al., 2017). Rat RFL-6 fibroblasts were purchased from the American Type Culture Collection (ATCC) and cultured in Ham's F-12K medium (Thermo Fisher Scientific) supplemented with 20% fetal bovine serum as previously described (Qiang et al., 2006). All cells were recently authenticated and routinely tested negative for mycoplasma.
Antibodies and chemicals
Rabbit anti-UNC-45A (Protein Tech, 1956-1-AP) raised against the C-terminus of the human UNC-45A (Protein Tech, 1956-1-AP) was used for immunofluorescence (IF) analysis as previously described (Habicht et al., 2019; Mooneyham et al., 2019). Mouse polyclonal anti-UNC-45A raised against full-length UNC-45A (Abnova, H00055898-B01P; 1:1000) was used for western blot (WB) analysis to detect recombinant UNC-45A and its mutants (Fig. 3B). Other primary antibodies used were as follows: mouse anti-α-tubulin (Sigma-Aldrich, T6074; 1:1000, WB; 1:2000, IF), rabbit polyclonal anti-α-tubulin (Abcam, ab18251; 1:1000, WB), mouse monoclonal anti-detyrosinated-α-tubulin (EMD Millipore, AB3201; 1:1000, WB), rabbit monoclonal anti-GFP (Thermo Fisher Scientific, G10362; 1:1000, WB), mouse monoclonal anti-FLAG (Sigma-Aldrich, F1804; 1:1000, WB; 1:200, IF). Secondary antibodies used were: peroxidase-linked anti-mouse IgG and peroxidase-linked anti-rabbit IgG (both Cytiva, formerly known as GE Healthcare Bio-Sciences, NA931 and NA934; 1:5000), Alexa Fluor 594-conjugated donkey anti-mouse IgG (1:250) and FITC-conjugated goat anti-rabbit IgG (1:200) (both Jackson ImmunoResearch Laboratories, 715-585-150 and 111-095-003, respectively). Paclitaxel was purchased from Teva Pharmaceuticals. Blebbistatin was purchased from Sigma-Aldrich. Tubulin Tracker Deep Red was purchased from Thermo Fisher Scientific (T34077).
Modulation of UNC-45A and katanin expression levels in cells
For UNC-45A silencing and overexpression, scramble and UNC-45A shRNAs, lentiviral supernatant, or empty vector control and UNC-45A-GFP lentiviral supernatants were prepared and used to infect RFL-6 cells as previously described (Iizuka et al., 2015, 2017a; Mooneyham et al., 2019). For ectopic expression, RFL-6 or U2OS cells were infected with lentiviral particle carrying FLAG only (control) or FLAG-tagged UNC-45A WT, deltaN-UNC-45A and deltaC-UNC-45A, and expression levels were evaluated 24 h or 48 h post-infection. For ectopic expression of UNC-45A in RFL-6 cells, cells were infected with either GFP or GFP-UNC-45A lentiviral supernatants as previously described (Mooneyham et al., 2019). For ectopic expression of katanin, RFL-6 cells were transduced with GFP or GFP-katanin plasmids using Fugene HD.
Western blot analysis
Total cellular protein (10–50 µg) from each sample was separated by SDS-PAGE, transferred to PVDF membranes and subjected to western blot analysis using the specified antibodies. Amido Black staining was performed to confirm equal protein loading.
Blebbistatin treatment
RFL-6 cells were treated with either 12.5 μM or 25 µM blebbistatin or DMSO for 1 h before lysates were made using RIPA buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS) as previously described (Joo and Yamada, 2014).
IF microscopy, image acquisition and analysis
For colocalization analysis of UNC-45A and its mutants and MTs, U2OS cells were fixed in cold methanol for 5 min at −20°C. After blocking with 5% bovine serum albumin (BSA) in PBST, cells were stained with anti-UNC-45A and anti-α-tubulin primary antibodies followed by fluorescein isothiocyanate (FITC)- or Alexa Fluor 594-conjugated secondary antibodies and analyzed via confocal fluorescence microscopy. Images were taken with an Olympus BX2 upright microscope. A UPlanApo N 60×/1.42 NA objective was used. FITC was excited with a 488 nm laser, and emission was collected between 505 nm and 525 nm. For Alexa Fluor, a 594 nm laser was used for excitation, and emission was collected between 560 nm and 660 nm. Images were taken with sequential excitation. Colocalization analysis was performed using the Fiji 2 software Coloc 2 plugin and Pearson's correlation coefficient (PCC) and calculated as previously described (Dunn et al., 2011). For studies on MT morphology (breakages) and mass following UNC-45A overexpression, GFP-control for GFP-UNC-45A-expressing RFL-6 cells were extracted in an MT-stabilizing buffer with 0.5% Triton-X for 30 s to remove free tubulin and then fixed with 0.5% glutaraldehyde for 10 min. After fixation, cells were quenched for 7 min with 0.1% NaBH4 and then rehydrated in PBS before blocking in AbDil (2% TBST with 2% BSA and 0.1% sodium azide) for 30 min as previously described (Ritter et al., 2017). After blocking, cultures were stained with anti-α-tubulin and anti-GFP primary antibody followed by Alexa Fluor 594- and FITC-conjugated secondary antibodies. Cells were then rinsed in TBST and mounted in a medium that reduces photobleaching. Images were obtained on an Axiovert 200 microscope (Zeiss) equipped with a high-resolution CCD camera. All images were obtained using identical camera, microscope and imaging criteria, such as gain, brightness, contrast and exposure time. Digital gray values of image pixels representing arbitrary fluorescence units (AFU) were obtained using Fiji software. For MT mass measurement in RFL-6 cells, quantification was performed on three comparable, equally sized areas per cell. For the analysis of MT breakages, MT free ends were counted per cell as previously described (Ahmad et al., 1999).
Live imaging of MTs in RFL-6 cells
To determine the effects of UNC-45A overexpression on MT bending and breakage in live cells, GFP-control for GFP-UNC-45A-overexpressing RFL-6 cells were treated with Tubulin Tracker Deep Red according to the manufacturer’s recommendation. Tubulin Tracker Deep Red was diluted 1:2000. This dilution corresponds to 500 nM taxol (personal communication from the Thermo Fisher Scientific technical support team). Cells (GFP and GFP-UNC-45A) were treated with Tubulin Tracker Deep Red sequentially so that imaging was performed under the same conditions. Time-lapse fluorescent images were collected with a Zeiss Axio observer Z1 inverted microscope using a 100×/1.46 NA objective lens. Digital images were collected at 8 s intervals over a period of 8 min using a Cy5 filter cube. Laser power and exposure time were minimized to avoid photobleaching and photodamage, and all images (for GFP and GFP-UNC-45A) were taken under the same conditions. A bending MT was defined as a MT in which bending was observable throughout the time of imaging.
Statistical analysis
Results are reported as mean±s.d. of three or more independent experiments. Unless otherwise indicated, statistical significance of difference was assessed with an unpaired two-tailed Student's t-test using Prism (V.4 GraphPad) and Excel (Microsoft). The level of significance was set at P<0.05.
Supplementary Material
Acknowledgements
We thank Drs Jaakko Lehtimäki and Pekka Lappalainen (Institute of Biotechnology, University of Helsinki, Helsinki, Finland) for the generous gift of the UNC-45A KO U2OS clones. We thank Dr Valentino Clemente (University of Minnesota) and Guillermo Marques (University of Minnesota Imaging Center) for assistance with image analysis.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: A.H., M.K.G., M.B.; Methodology: J.H., A.M., A.H., M.S., X.Z., E.E., Q.Y., C.C., M.K.G., M.B.; Software: J.H., A.M., A.H., M.B.; Validation: J.H., A.M., A.H., M.B.; Formal analysis: J.H., A.M., A.H., M.S., M.B.; Investigation: J.H., M.K.G., M.B.; Resources: M.B.; Data curation: A.H., M.B.; Writing - original draft: M.B.; Writing - review & editing: J.H., A.M., M.S., M.K.G., M.B.; Visualization: M.K.G.; Supervision: M.K.G., M.B.; Project administration: M.B.; Funding acquisition: M.B.
Funding
This work was supported by the US Department of Defense Ovarian Cancer Research Program (OC160377), the Minnesota Ovarian Cancer Alliance, the Randy Shaver Cancer Research and Community Fund and the National Institute of General Medical Sciences (R01-GM130800 to M.B.; R35-GM126974 to M.K.G.). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at https://jcs.biologists.org/lookup/doi/10.1242/jcs.248815.supplemental
Peer review history
The peer review history is available online at https://jcs.biologists.org/lookup/doi/10.1242/jcs.248815.reviewer-comments.pdf
References
- Ahmad F. J., Yu W., McNally F. J. and Baas P. W. (1999). An essential role for katanin in severing microtubules in the neuron. J. Cell Biol. 145, 305-315. 10.1083/jcb.145.2.305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allingham J. S., Smith R. and Rayment I. (2005). The structural basis of blebbistatin inhibition and specificity for myosin II. Nat. Struct. Mol. Biol. 12, 378-379. 10.1038/nsmb908 [DOI] [PubMed] [Google Scholar]
- Baas P. W. and Qiang L. (2019). Tau: it's not what you think. Trends Cell Biol. 29, 452-461. 10.1016/j.tcb.2019.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas P. W. and Sudo H. (2010). More microtubule severing proteins: more microtubules. Cell Cycle 9, 2273 10.4161/cc.9.12.12026 [DOI] [PubMed] [Google Scholar]
- Baas P. W., Rao A. N., Matamoros A. J. and Leo L. (2016). Stability properties of neuronal microtubules. Cytoskeleton (Hoboken) 73, 442-460. 10.1002/cm.21286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey M. E., Jiang N., Dima R. I. and Ross J. L. (2016). Invited review: Microtubule severing enzymes couple atpase activity with tubulin GTPase spring loading. Biopolymers 105, 547-556. 10.1002/bip.22842 [DOI] [PubMed] [Google Scholar]
- Baldassarre G., Belletti B., Nicoloso M. S., Schiappacassi M., Vecchione A., Spessotto P., Morrione A., Canzonieri V. and Colombatti A. (2005). p27(Kip1)-stathmin interaction influences sarcoma cell migration and invasion. Cancer Cell 7, 51-63. 10.1016/j.ccr.2004.11.025 [DOI] [PubMed] [Google Scholar]
- Barral J. M., Bauer C. C., Ortiz I. and Epstein H. F. (1998). Unc-45 mutations in Caenorhabditis elegans implicate a CRO1/She4p-like domain in myosin assembly. J. Cell Biol. 143, 1215-1225. 10.1083/jcb.143.5.1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral J. M., Hutagalung A. H., Brinker A., Hartl F. U. and Epstein H. F. (2002). Role of the myosin assembly protein UNC-45 as a molecular chaperone for myosin. Science 295, 669-671. 10.1126/science.1066648 [DOI] [PubMed] [Google Scholar]
- Bazzaro M., Santillan A., Lin Z., Tang T., Lee M. K., Bristow R. E., Shih I.-M. and Roden R. B. S. (2007). Myosin II co-chaperone general cell UNC-45 overexpression is associated with ovarian cancer, rapid proliferation, and motility. Am. J. Pathol. 171, 1640-1649. 10.2353/ajpath.2007.070325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmont L. D. and Mitchison T. J. (1996). Identification of a protein that interacts with tubulin dimers and increases the catastrophe rate of microtubules. Cell 84, 623-631. 10.1016/S0092-8674(00)81037-5 [DOI] [PubMed] [Google Scholar]
- Bernick E. P., Zhang P.-J. and Du S. (2010). Knockdown and overexpression of Unc-45b result in defective myofibril organization in skeletal muscles of zebrafish embryos. BMC Cell Biol. 11, 70 10.1186/1471-2121-11-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson A., Gurian-West M., Schmidt A., Hall A. and Roberts J. M. (2004). p27Kip1 modulates cell migration through the regulation of RhoA activation. Genes Dev. 18, 862-876. 10.1101/gad.1185504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla M., Wilson J. M. and Pollard T. D. (2000). Fission yeast myosin-II isoforms assemble into contractile rings at distinct times during mitosis. Curr. Biol. 10, 397-400. 10.1016/S0960-9822(00)00420-6 [DOI] [PubMed] [Google Scholar]
- Bresnick A. R., Weber D. J. and Zimmer D. B. (2015). S100 proteins in cancer. Nat. Rev. Cancer 15, 96-109. 10.1038/nrc3893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujalowski P. J., Nicholls P. and Oberhauser A. F. (2014). UNC-45B chaperone: the role of its domains in the interaction with the myosin motor domain. Biophys. J. 107, 654-661. 10.1016/j.bpj.2014.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo G., Antonsson B., Kassel D., Riederer B. M. and Grenningloh G. (1997). Phosphorylation regulates the microtubule-destabilizing activity of stathmin and its interaction with tubulin. FEBS Lett. 416, 149-152. 10.1016/S0014-5793(97)01188-5 [DOI] [PubMed] [Google Scholar]
- Dulyaninova N. G., Ruiz P. D., Gamble M. J., Backer J. M. and Bresnick A. R. (2018). S100A4 regulates macrophage invasion by distinct myosin-dependent and myosin-independent mechanisms. Mol. Biol. Cell 29, 632-642. 10.1091/mbc.E17-07-0460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn K. W., Kamocka M. M. and McDonald J. H. (2011). A practical guide to evaluating colocalization in biological microscopy. Am. J. Physiol. Cell Physiol. 300, C723-C742. 10.1152/ajpcell.00462.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein H. F. and Thomson J. N. (1974). Temperature-sensitive mutation affecting myofilament assembly in Caenorhabditis elegans. Nature 250, 579-580. 10.1038/250579a0 [DOI] [PubMed] [Google Scholar]
- Etard C., Behra M., Fischer N., Hutcheson D., Geisler R. and Strahle U. (2007). The UCS factor Steif/Unc-45b interacts with the heat shock protein Hsp90a during myofibrillogenesis. Dev. Biol. 308, 133-143. 10.1016/j.ydbio.2007.05.014 [DOI] [PubMed] [Google Scholar]
- Etheridge L., Diiorio P. and Sagerstrom C. G. (2002). A zebrafish unc-45-related gene expressed during muscle development. Dev. Dyn. 224, 457-460. 10.1002/dvdy.10123 [DOI] [PubMed] [Google Scholar]
- Fabris L., Berton S., Pellizzari I., Segatto I., D'Andrea S., Armenia J., Bomben R., Schiappacassi M., Gattei V., Philips M. R. et al. (2015). p27kip1 controls H-Ras/MAPK activation and cell cycle entry via modulation of MT stability. Proc. Natl. Acad. Sci. USA 112, 13916-13921. 10.1073/pnas.1508514112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazda L., Pokrzywa W., Hellerschmied D., Löwe T., Forné I., Mueller-Planitz F., Hoppe T. and Clausen T. (2013). The myosin chaperone UNC-45 is organized in tandem modules to support myofilament formation in C. elegans. Cell 152, 183-195. 10.1016/j.cell.2012.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geach T. J. and Zimmerman L. B. (2010). Paralysis and delayed Z-disc formation in the Xenopus tropicalis unc45b mutant dicky ticker. BMC Dev. Biol. 10, 75 10.1186/1471-213X-10-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin J. D., Thomas N., Laguesse S., Malinouskaya L., Close P., Malaise O., Purnelle A., Raineteau O., Campbell K., Fero M. et al. (2012). p27(Kip1) is a microtubule-associated protein that promotes microtubule polymerization during neuron migration. Dev. Cell 23, 729-744. 10.1016/j.devcel.2012.08.006 [DOI] [PubMed] [Google Scholar]
- Goodson H. V. and Jonasson E. M. (2018). Microtubules and microtubule-associated proteins. Cold Spring Harb. Perspect Biol. 10, a022608 10.1101/cshperspect.a022608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Chen D., Fan Z. and Epstein H. F. (2011). Differential turnover of myosin chaperone UNC-45A isoforms increases in metastatic human breast cancer. J. Mol. Biol. 412, 365-378. 10.1016/j.jmb.2011.07.012 [DOI] [PubMed] [Google Scholar]
- Habicht J., Mooneyham A., Shetty M., Zhang X., Shridhar V., Winterhoff B., Zhang Y., Cepela J., Starr T., Lou E. et al. (2019). UNC-45A is preferentially expressed in epithelial cells and binds to and co-localizes with interphase MTs. Cancer Biol. Ther. 20, 1304-1313. 10.1080/15384047.2019.1632637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman J. J. and Vale R. D. (1999). Microtubule disassembly by ATP-dependent oligomerization of the AAA enzyme katanin. Science 286, 782-785. 10.1126/science.286.5440.782 [DOI] [PubMed] [Google Scholar]
- Hartman J. J., Mahr J., McNally K., Okawa K., Iwamatsu A., Thomas S., Cheesman S., Heuser J., Vale R. D. and McNally F. J. (1998). Katanin, a microtubule-severing protein, is a novel AAA ATPase that targets to the centrosome using a WD40-containing subunit. Cell 93, 277-287. 10.1016/S0092-8674(00)81578-0 [DOI] [PubMed] [Google Scholar]
- Hertzer K. M., Ems-McClung S. C., Kline-Smith S. L., Lipkin T. G., Gilbert S. P. and Walczak C. E. (2006). Full-length dimeric MCAK is a more efficient microtubule depolymerase than minimal domain monomeric MCAK. Mol. Biol. Cell 17, 700-710. 10.1091/mbc.e05-08-0821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. and Hyman A. A. (2007). Microtubule polymerases and depolymerases. Curr. Opin. Cell Biol. 19, 31-35. 10.1016/j.ceb.2006.12.009 [DOI] [PubMed] [Google Scholar]
- Hutagalung A. H., Landsverk M. L., Price M. G. and Epstein H. F. (2002). The UCS family of myosin chaperones. J. Cell Sci. 115, 3983-3990. 10.1242/jcs.00107 [DOI] [PubMed] [Google Scholar]
- Iizuka Y., Cichocki F., Sieben A., Sforza F., Karim R., Coughlin K., Isaksson Vogel R., Gavioli R., McCullar V., Lenvik T. et al. (2015). UNC-45A is a nonmuscle myosin IIA chaperone required for NK cell cytotoxicity via control of lytic granule secretion. J. Immunol. 195, 4760-4770. 10.4049/jimmunol.1500979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka Y., Mooneyham A., Sieben A., Chen K., Maile M., Hellweg R., Schutz F., Teckle K., Starr T., Thayanithy V. et al. (2017a). UNC-45A is required for neurite extension via controlling NMII activation. Mol. Biol. Cell 28, 1337-1346. 10.1091/mbc.e16-06-0381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka Y., Mooneyham A., Sieben A., Chen K., Maile M., Hellweg R., Schutz F., Teckle K., Starr T., Thayanithy V. et al. (2017b). UNC-45A is required for neurite extension via controlling NMII activation. Mol. Biol. Cell 28, 1337-1346. 10.1091/mbc.e16-06-0381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhak D. N., Tyanova S., Cox J. and Borner G. H. (2016). Global, quantitative and dynamic mapping of protein subcellular localization. Elife 5, e16950 10.7554/eLife.16950.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang K., Rezabkova L., Hua S., Liu Q., Capitani G., Altelaar A. F. M., Heck A. J. R., Kammerer R. A., Steinmetz M. O. and Akhmanova A. (2017). Microtubule minus-end regulation at spindle poles by an ASPM-katanin complex. Nat. Cell Biol. 19, 480-492. 10.1038/ncb3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilani Y., Lu S., Lei H., Karnitz L. M. and Chadli A. (2015). UNC45A localizes to centrosomes and regulates cancer cell proliferation through ChK1 activation. Cancer Lett. 357, 114-120. 10.1016/j.canlet.2014.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo E. E. and Yamada K. M. (2014). MYPT1 regulates contractility and microtubule acetylation to modulate integrin adhesions and matrix assembly. Nat. Commun. 5, 3510 10.1038/ncomms4510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadir S., Astin J. W., Tahtamouni L., Martin P. and Nobes C. D. (2011). Microtubule remodelling is required for the front-rear polarity switch during contact inhibition of locomotion. J. Cell Sci. 124, 2642-2653. 10.1242/jcs.087965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács M., Tóth J., Hetényi C., Málnási-Csizmadia A. and Sellers J. R. (2004). Mechanism of blebbistatin inhibition of myosin II. J. Biol. Chem. 279, 35557-35563. 10.1074/jbc.M405319200 [DOI] [PubMed] [Google Scholar]
- Lee C. F., Melkani G. C., Yu Q., Suggs J. A., Kronert W. A., Suzuki Y., Hipolito L., Price M. G., Epstein H. F. and Bernstein S. I. (2011). Drosophila UNC-45 accumulates in embryonic blastoderm and in muscles, and is essential for muscle myosin stability. J. Cell Sci. 124, 699-705. 10.1242/jcs.078964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtimäki J. I., Fenix A. M., Kotila T. M., Balistreri G., Paavolainen L., Varjosalo M., Burnette D. T. and Lappalainen P. (2017). UNC-45a promotes myosin folding and stress fiber assembly. J. Cell Biol. 216, 4053-4072. 10.1083/jcb.201703107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou E., Zhai E., Sarkari A., Desir S., Wong P., Iizuka Y., Yang J., Subramanian S., McCarthy J., Bazzaro M.. et al. (2018). Cellular and molecular networking within the ecosystem of cancer cell communication via tunneling nanotubes. Front. Cell Dev. Biol. 6, 95 10.3389/fcell.2018.00095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupas A. N. and Martin J. (2002). AAA proteins. Curr. Opin. Struct. Biol. 12, 746-753. 10.1016/S0959-440X(02)00388-3 [DOI] [PubMed] [Google Scholar]
- Mooneyham A., Iizuka Y., Yang Q., Coombes C., McClellan M., Shridhar V., Emmings E., Shetty M., Chen L., Ai T. et al. (2018). UNC-45A is a novel microtubule-associated protein and regulator of paclitaxel sensitivity in ovarian cancer cells. Mol. Cancer Res. 17, 370-383. 10.1158/1541-7786.MCR-18-0670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooneyham A., Iizuka Y., Yang Q., Coombes C., McClellan M., Shridhar V., Emmings E., Shetty M., Chen L., Ai T. et al. (2019). UNC-45A is a novel microtubule-associated protein and regulator of paclitaxel sensitivity in ovarian cancer cells. Mol. Cancer Res. 17, 370-383. 10.1158/1541-7786.MCR-18-0670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy K. and Wadsworth P. (2005). Myosin-II-dependent localization and dynamics of F-actin during cytokinesis. Curr. Biol. 15, 724-731. 10.1016/j.cub.2005.02.055 [DOI] [PubMed] [Google Scholar]
- Neuwald A. F., Aravind L., Spouge J. L. and Koonin E. V. (1999). AAA+: A class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 9, 27-43. [PubMed] [Google Scholar]
- Ng D. C., Lin B. H., Lim C. P., Huang G., Zhang T., Poli V. and Cao X. (2006). Stat3 regulates microtubules by antagonizing the depolymerization activity of stathmin. J. Cell Biol. 172, 245-257. 10.1083/jcb.200503021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni W., Hutagalung A. H., Li S. and Epstein H. F. (2011). The myosin-binding UCS domain but not the Hsp90-binding TPR domain of the UNC-45 chaperone is essential for function in Caenorhabditis elegans. J. Cell Sci. 124, 3164-3173. 10.1242/jcs.087320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang L., Yu W., Andreadis A., Luo M. and Baas P. W. (2006). Tau protects microtubules in the axon from severing by katanin. J. Neurosci. 26, 3120-3129. 10.1523/JNEUROSCI.5392-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarmby L. (2000). Cellular Samurai: katanin and the severing of microtubules. J. Cell Sci. 113, 2821-2827. [DOI] [PubMed] [Google Scholar]
- Ritter B., Ferguson S. M., De Camilli P. and McPherson P. S. (2017). A lentiviral system for efficient knockdown of proteins in neuronal cultures [version 1; referees: 2 approved]. MNI Open Res. 1, 10.12688/mniopenres.12766.1 10.12688/mniopenres.12766.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll-Mecak A. and McNally F. J. (2010). Microtubule-severing enzymes. Curr. Opin. Cell Biol. 22, 96-103. 10.1016/j.ceb.2009.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serres M. P., Kossatz U., Chi Y., Roberts J. M., Malek N. P. and Besson A. (2012). p27(Kip1) controls cytokinesis via the regulation of citron kinase activation. J. Clin. Invest. 122, 844-858. 10.1172/JCI60376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp D. J. and Ross J. L. (2012). Microtubule-severing enzymes at the cutting edge. J. Cell Sci. 125, 2561-2569. 10.1242/jcs.101139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelanski M. L. (1973). Chemistry of the filaments and tubules of brain. J. Histochem. Cytochem. 21, 529-539. 10.1177/21.6.529 [DOI] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F. and Cantor C. R. (1973). Microtubule assembly in the absence of added nucleotides. Proc. Natl. Acad. Sci. USA 70, 765-768. 10.1073/pnas.70.3.765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H. and Blobel G. (2010). UNC-45/CRO1/She4p (UCS) protein forms elongated dimer and joins two myosin heads near their actin binding region. Proc. Natl. Acad. Sci. USA 107, 21382-21387. 10.1073/pnas.1013038107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo H. and Baas P. W. (2010). Acetylation of microtubules influences their sensitivity to severing by katanin in neurons and fibroblasts. J. Neurosci. 30, 7215-7226. 10.1523/JNEUROSCI.0048-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo H. and Baas P. W. (2011). Strategies for diminishing katanin-based loss of microtubules in tauopathic neurodegenerative diseases. Hum. Mol. Genet. 20, 763-778. 10.1093/hmg/ddq521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan R., Lam A. J., Tan T., Han J., Nowakowski D. W., Vershinin M., Simo S., Ori-McKenney K. M. and McKenney R. J. (2019). Microtubules gate tau condensation to spatially regulate microtubule functions. Nat. Cell Biol. 21, 1078-1085. 10.1038/s41556-019-0375-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R. D. (1991). Severing of stable microtubules by a mitotically activated protein in Xenopus egg extracts. Cell 64, 827-839. 10.1016/0092-8674(91)90511-V [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.