To the Editor,
Since the SARS-CoV-2 emergence in December 2019, one of the major concerns has been the duration of immune protection after a first episode. This question is of paramount importance for healthcare workers (HCWs), who are a highly exposed population and among the first targets of vaccination programmes. To date, the persistence of SARS-CoV-2 antibodies in HCWs 6 months after disease onset (ADO) has not been studied with both a virus neutralisation test and commercial assays.
HCWs who experienced COVID-19 during the early phase of the pandemic were included in a prospective study conducted at the University Hospital of Lyon, France [1]. Serum samples collected 6 months ADO were tested using three commercial assays: the Wantai Ab assay, which detects total antibodies against the receptor binding domain (RBD) of the S protein, the bioMérieux Vidas assay, which detects IgG to the RBD, and the Abbott Architect assay, which detects IgG to the N protein. The neutralizing antibody (NAb) titre was determined by a virus neutralization assay (VNA) using live virus as previously described [2].
A total of 296 HCWs were included; the median (interquartile range, IQR) age was 41 (32–51) years and 17.2% (51/296) were male. The median duration between symptom onset and inclusion was 186 (180–196) days. Of note, 8/296 HCWs (2.7%) were asymptomatic and the onset of disease was established on the basis of the median date of the RT-PCR positive result of the ward cluster. All participants were tested positive for SARS-CoV-2 serology at least 2 weeks ADO. The SARS-CoV-2 infection was also documented by RT-PCR test in 170 patients.
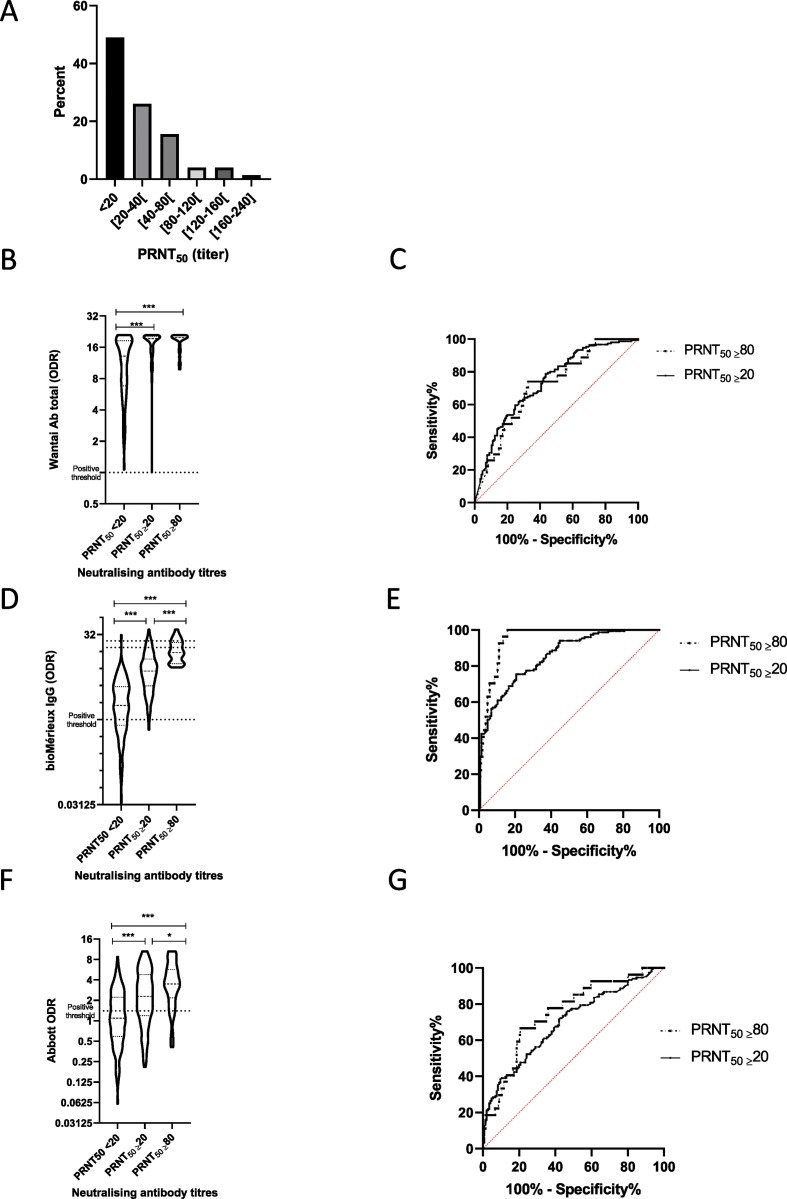
The positivity rate at 6 months ADO was 100% with the Wantai assay, 84.8% with the Vidas assay and 55.4% with the Architect assay. Only 51% of HCWs were positive for the presence of NAbs. Positive NAb titres ranged from 20 to 240. Only 27/296 (9.1%) had a NAb ≥80 (Fig. 1 A, please see supplementary information for raw data). No difference in positivity rates with any assay was observed between patients with a SARS-CoV-2 infection documented by RT-PCR and the rest of the cohort.
Fig. 1.
(A) Distribution of neutralisation antibody titres in convalescent subjects (n = 296) 6 months after SARS-CoV-2 infection. (B, D, F) Violin plots describing ODR according to neutralising antibody titres. Dotted lines described positive threshold recommended by each manufacturer. Comparisons was performed using the Kruskal Wallis test followed by Dunn's test. ∗∗∗p < 0.001, ∗p < 0.05. (C, E, G) ROC curves were built to estimate the performance of Wantai (C), bioMérieux (E) and Abbott (G) assays for detecting the presence of neutralising antibodies (PRNT50 ≥20, continuous line) and high neutralizing antibody titre (PRNT50 ≥80, dotted line). ODR, optical density ratio; PRNT, plaque reduction neutralization titres.
Of the 296 HCWs, six (2.0%) developed a clinical form requiring hospitalization; all were positive with the three serological assays and for the presence of NAb with a median titre of 40 (range 30–160). By contrast, in asymptomatic HCWs, 8/8, 5/8 and 4/8 were positive with Wantai, Vidas and Architect assays, respectively, and only 3/8 exhibited NAbs with low titres (range 30–60).
The area under the ROC curve (AUC) was estimated for assessing the performance of serological assays for two NAb titres (PRNT50 ≥ 20 or PRNT50 ≥ 80; (Fig. 1C, E, G). The highest AUCs were found with the Vidas assay: 0.85 (95% CI 0.81–0.89) and 0.95 (0.92–0.97), respectively. The Wantai and Abbott assays had AUCs of, respectively, 0.73 (0.68–0.79) and 0.70 (0.64–0.76) for PRNT50 ≥ 20, and 0.71 (0.62–0.81), 0.75 (0.66–0.85) for PRNT50 ≥ 80. These results suggest that an optimized ratio with some commercial serological assay could be found to maximize the positive predictive value enabling to select individuals with a NAb titres ≥80. For instance, with the Vidas assay, the median (IQR) ratio for samples with PRNT50 ≥ 80 was 15.4 (9.7–22.7) vs. 5.9 (3.3–9.2) for samples with a titre between 20 and 80 and 1.8 (0.8–3.8) for samples without NAb (Fig. 1F). Among the 27 samples with NAb titre ≥80, all had a Vidas ratio above 8 compared with 31.5% and 3.5% of the samples with a titre between 20 and 80 or without NAb, respectively.
The findings of the present study indicate that, 6 months after infection, NAbs were no longer detected in about half of HCWs who presented mainly mild COVID-19. Overall, the detection of SARS-CoV-2 Abs with commercial tests was higher despite important heterogeneity between the assays evaluated herein. In a previous study [3], about 40% of asymptomatic subjects became negative for IgG to the N protein within 3–6 months, which is consistent with that presented herein for the Architect assay. This suggests that assays detecting only antibodies against the N protein must not be used in long-term seroprevalence surveys. By contrast, the Wantai assay could be very useful for epidemiological purposes as 100% of the HCWs were still positive at 6 months ADO. Although VNA should remain the reference standard to assess the protective antibody response, the data presented herein suggest that some commercial assays could be useful for first-line screening of long-term presence of NAbs as previously reported within 4 months ADO [2,4].
Despite these observations of the decrease in NAbs in patients with mild COVID-19, it is important to note that they do not preclude the protective role of an anamnestic antibody response in previously exposed subjects, nor that of the long-term cellular immunity [5].
Transparency declaration
Antonin Bal has received grant from bioMérieux and has served as consultant for bioMérieux for work and research not related to this manuscript. Sophie Trouillet-Assant has received research grant from bioMérieux concerning previous works not related to this manuscript. The other authors have no relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
Ethics
Written informed consent was obtained from all participants; ethics approval was obtained from the national review board for biomedical research in April 2020 (Comité de Protection des Personnes Sud Méditerranée I, Marseille, France; ID RCB 2020-A00932-37), and the study was registered on ClinicalTrials.gov (NCT04341142).
COVID-SER study group
Adnot Jérôme, Alfaiate Dulce, Bal Antonin, Bergeret Alain, Boibieux André, Bonnet Florent, Bourgeois Gaëlle, Brunel-Dalmas Florence, Caire Eurydice, Charbotel Barbara, Chiarello Pierre, Cotte Laurent, d'Aubarede Constance, Durupt François, Escuret Vanessa, Fascia Pascal, Fassier Jean-Baptiste, Fontaine Juliette, Gaillot-Durand Lucie, Gaymard Alexandre, Gillet Myriam, Godinot Matthieu, Gueyffier François, Guibert Nicolas, Josset Laurence, Lahousse Matthieu, Lina Bruno, Lozano Hélène, Makhloufi Djamila, Massardier-Pilonchéry Amélie, Milon Marie-Paule, Moll Frédéric, Morfin Florence, Narbey David, Nazare Julie-Anne, Oria Fatima, Paul Adèle, Perry Marielle, Pitiot Virginie, Prudent Mélanie, Rabilloud Muriel, Samperiz Audrey, Schlienger Isabelle, Simon Chantal, Trabaud Mary-Anne, Trouillet-Assant Sophie, Valette Martine.
Funding source
This research is being supported by Hospices Civils de Lyon and by Fondation des Hospices Civils de Lyon.
Acknowledgements
We thank all the personnel of the occupational health and medicine department of Hospices Civils de Lyon who contributed to the samples collection. Human biological samples and associated data were obtained from NeuroBioTec (CRB HCL, Lyon France, Biobank BB-0033-00046). We thank Karima Brahami and all members of the clinical research and innovation department for their reactivity (DRCI, Hospices Civils de Lyon). We thank Philip Robinson (DRCI, Hospices Civils de Lyon) for his help in manuscript preparation.
Editor: L. Kaiser
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.01.003.
Contributor Information
COVID SER Study Group:
Adnot Jérôme, Alfaiate Dulce, Bal Antonin, Bergeret Alain, Boibieux André, Bonnet Florent, Bourgeois Gaëlle, Brunel-Dalmas Florence, Caire Eurydice, Charbotel Barbara, Chiarello Pierre, Cotte Laurent, Constance d'Aubarede, Durupt François, Escuret Vanessa, Fascia Pascal, Fassier Jean-Baptiste, Fontaine Juliette, Gaillot-Durand Lucie, Gaymard Alexandre, Gillet Myriam, Godinot Matthieu, Gueyffier François, Guibert Nicolas, Josset Laurence, Lahousse Matthieu, Lina Bruno, Lozano Hélène, Makhloufi Djamila, Massardier-Pilonchéry Amélie, Milon Marie-Paule, Moll Frédéric, Morfin Florence, Narbey David, Nazare Julie-Anne, Oria Fatima, Paul Adèle, Perry Marielle, Pitiot Virginie, Prudent Mélanie, Rabilloud Muriel, Samperiz Audrey, Schlienger Isabelle, Simon Chantal, Trabaud Mary-Anne, Trouillet-Assant Sophie, and Valette Martine
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Trouillet-Assant S., Albert Vega C., Bal A., Nazare J.A., Fascia P., Paul A. Assessment of serological techniques for screening patients for COVID-19 (COVID-SER): a prospective, multicentric study. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-041268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bal A., Pozzetto B., Trabaud M.-A., Escuret V., Rabilloud M., Langlois-Jacques C. Evaluation of high-throughput SARS-CoV-2 serological assays in a longitudinal cohort of mild COVID-19 patients: sensitivity, specificity and association with virus neutralization test. Clin Chem. 2021:hvaa336. doi: 10.1093/clinchem/hvaa336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ripperger T.J., Uhrlaub J.L., Watanabe M., Wong R., Castaneda Y., Pizzato H.A. Orthogonal SARS-CoV-2 serological assays enable surveillance of low-prevalence communities and reveal durable humoral immunity. Immunity. 2020;53:925–933. doi: 10.1016/j.immuni.2020.10.004. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muecksch F., Wise H., Batchelor B., Squires M., Semple E., Richardson C. Longitudinal analysis of serology and neutralizing antibody levels in COVID19 convalescents. J Infect Dis. 2020:jiaa659. doi: 10.1093/infdis/jiaa659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodda L.B., Netland J., Shehata L., Pruner K.B., Morawski P.A., Thouvenel C.D. Functional SARS-CoV-2-specific immune memory persists after mild COVID-19. Cell. 2020 doi: 10.1016/j.cell.2020.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.