Abstract
Background
Studies in low-income countries have shown that among Bacille Calmette-Guérin (BCG) vaccinated children, those who develop a BCG-scar have significantly better survival than those who do not develop a scar. In a Danish multicenter randomized clinical trial we assessed determinants for developing a BCG-scar and for BCG scar size following neonatal BCG vaccination.
Methods
At three Danish hospitals, newborns were randomized 1:1 to BCG vaccination or no BCG vaccination. The infants were invited for a clinical examination at the ages of 3 and 13 months. At 13 months, the scar site was inspected and scar size measured. We investigated three groups of determinants; external, parental, and individual-level determinants on relative scar prevalence and differences in median scar sizes.
Results
Among 2118 BCG vaccinated infants, 2039 (96 %) were examined at 13 months; 1857 of these (91 %) had developed a BCG-scar. Compared with Copenhagen University Hospital, Hvidovre (85 %), Copenhagen University Hospital, Rigshospitalet had a scar prevalence of 95 % (adjusted Prevalence ratio (aPR) = 1.24 [CI 95 %: 1.18 to 1.30]); it was 93 % at Kolding Hospital (aPR 1.27 [CI 95 %: 1.19 to 1.35]). Increasing vaccine experience was positively associated with developing a scar and with scar size.
Conclusion
Across multiple potential determinants of BCG scaring and size, logistical factors dominated. The results support that injection technique is an important determinant of developing a scar. Given the strong link between having a BCG scar and subsequent health, improved BCG vaccination technique could play a major role for child health.
Keywords: BCG vaccine, BCG scar, Non-specific effects, Heterologous immunity
BCG vaccine; BCG scar; Non-specific effects; Heterologous immunity.
1. Introduction
The Bacille Calmette-Guérin (BCG) vaccine provides protection against tuberculosis (TB) and other mycobacterial infections, and is one of the most used vaccines globally [1]. The vaccine is given routinely in more than 100 countries to newborns as part of the childhood vaccination program. The BCG vaccine contains live attenuated Mycobacterium bovis and following intradermal injection the BCG vaccine elicits a local immune response. This response most often results in an ulcer that heals over weeks and leaves a flat permanent scar at the injection site [2]. The skin lesion has been shown to follow simple dose-response functions; if the dose of the vaccine is halved, the size of the scar will decrease by approximately 1 mm [3]. Also, vaccines with low viability result in smaller scars compared to vaccines with high viability [3].
Whether the development of a BCG scar is associated with TB efficacy is debated [2, 4, 5], but BCG scar size and tuberculin skin test (TST) response seem to correlate [3, 4, 6, 7, 8].
Observational data from a growing number of studies conducted in low-income countries have suggested that the BCG vaccine has strong beneficial non-specific effects (NSEs) beyond the protection against tuberculosis [9, 10, 11, 12, 13]. Recently, these findings were supported in three randomized trials from Guinea-Bissau in West Africa, showing that BCG vaccination at birth to low-birth-weight neonates reduced neonatal mortality by 38 % (95 % CI: 17 %–54 %) [14]. The reduction in neonatal mortality was due to fewer cases of septicemia and pneumonia [14, 15, 16]. Furthermore, among BCG-vaccinated children, those who develop a scar, have 39 % (26–49 %) lower mortality compared with those who do not develop a scar [17].
Understanding the factors, which determine whether BCG vaccination leads to development of a scar, is important for understanding the link between BCG vaccination and specific effects as well as NSEs. We know from observational data from Guinea-Bissau that scar prevalence may differ by vaccinator, whether the vaccine is injected correctly, the fieldworker who conducted the scar reading, sex, season, co-administration of other vaccinations, place of delivery, region, and maternal ethnicity [18], but this has not been tested in a high-income setting.
Within The Danish Calmette Study, a randomized clinical trial, we investigated determinants for developing a BCG scar and for the scar size at 13 months of age among the children allocated to neonatal BCG. We analyzed threes groups of determinants: External determinants such as study site, vaccine batch and season of the year [3, 19]. Parental determinants such as maternal BCG vaccination, parental smoking, and younger children at home and finally, individual factors such as gestational age and anthropometric measures.
2. Methods
2.1. Study design
The Danish Calmette Study was a randomized, prospective, single-blinded, clinical trial conducted at three Danish hospitals; Copenhagen University Hospital, Hvidovre, Copenhagen University Hospital, Rigshospitalet, and Kolding Hospital as described in detail elsewhere [20]. A total of 4262 infants born at the three Danish hospitals were randomized after birth with 1:1 allocation to BCG vaccination or no BCG vaccination. The primary outcomes were hospitalization and development of atopic dermatitis during the first year of life. Secondary outcomes were morbidity including infectious and allergic disease [21, 22, 23, 24, 25]. Children randomized to BCG vaccination received an intradermal BCG vaccine (Danish strain 1331, Statens Serum Institut) in the standard dose of 0.05 ml in the left deltoid region. Two different vaccine batches were used; batch 112032A and batch 111046B. Batch 112032A was used from October 2012 until April 2013. Batch 111046B was used from March 2013 until November 2013. The ratios between Batch 112032A and Batch 111046B were overall equal between the three sites. On Copenhagen University Hospital, Hvidovre the ratio was 1.21. On Copenhagen University Hospital, Rigshospitalet it was 1.36 and on Kolding Hospital it was 1.32. Chi-2 test for difference; p = 0.52.
All study staff involved in randomization were specially trained to administer the vaccine in accordance with the guidelines (Figure 1). In The Danish Calmette Study, 34 trained staff members were administrating the BCG vaccine, but two staff members were responsible for most of the vaccinations.
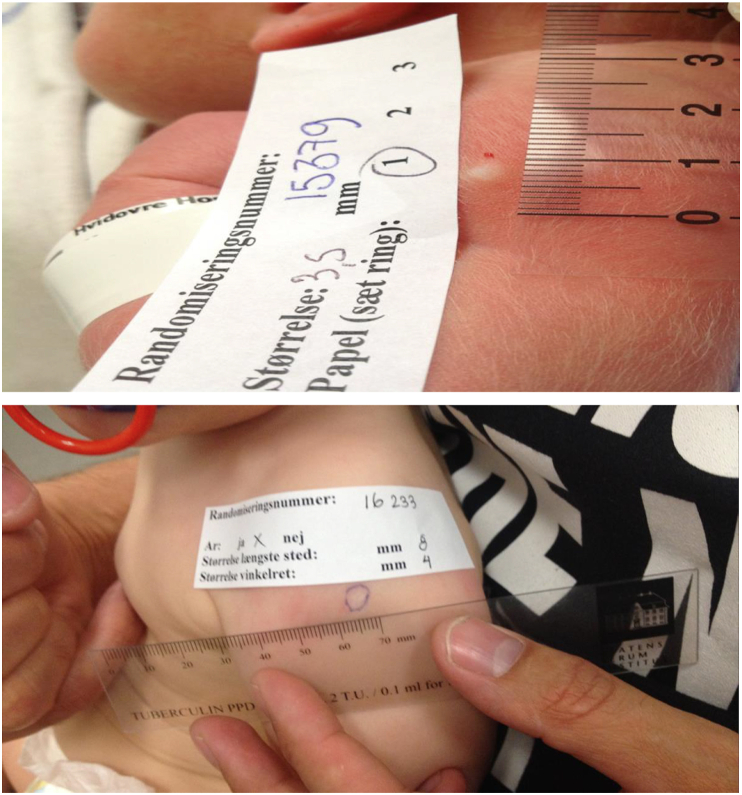
Figure 1.
The skin reaction after BCG vaccination. Upper: Following a correctly applied intradermal BCG vaccination a visible white wheal appears at the injection site. Lower: Measuring of a BCG scar (mm) at the end of the 13-month clinical examination.
2.2. Scar prevalence and size
At 3 and 13 month of age, the parents were invited for a clinical examination of their child. Prior to the clinical examinations the parents were asked to cover the vaccination site in both vaccinated and non-vaccinated children, thereby keeping the examiners blinded to the randomization during the examination. At the end of the 13-month clinical examination, the vaccination site was inspected, and it was registered whether the child had developed a visible BCG scar (yes/no). In case of a scar, the scar size was measured as two perpendicular diameters (mm) with a transparent ruler (Tuberculin PPD RT 21 SSI), and a mean value was calculated. BCG scars were measured with a precision of 0.1 cm (Figure 1).
In a previous paper based on the Calmette trial [26], we reported that 1794 children developed a scar after BCG vaccination and a scar frequency of 84.7 %. It was subsequently discovered that a proportion of the scar assessment data had not been entered, and figured as a “0” in the database, which was erroneously interpreted as “No scar”. The database was updated with the missing scar assessment data for the present study.
2.3. Classification of determinants
The following determinants were included in the analyses: External determinants were study site (Hvidovre hospital, Rigshospitalet, Kolding hospital), vaccine batch (112032A, 111046B), vaccinator (1–34), month of BCG vaccination, vaccinators’ BCG vaccinator experience (0–9; 10–49; 50–99; ≥100 BCG vaccinations given during the duration of the trial). Parental determinants were maternal age at delivery (<27 years, 27–32 years, >32 years), maternal BCG vaccination, parental smoking, atopic disposition and other children < 4 years of age in the home. Individual determinants were sex, prematurity (<37 weeks, ≥37 weeks), birth weight (<2500 g, ≥ 2500 g), caesarean section, child age at BCG vaccination (day 0–1, day 2–7), ethnicity (Danish ethnicity, other ethnicity), exclusively breastfeed in the first 3 months of life, mid-upper arm circumference of left arm (MUAC) (measured at the 13-month clinical examination; quartiles), weight gain from birth to the 13-month clinical examination (quartiles).
2.4. Statistical analysis
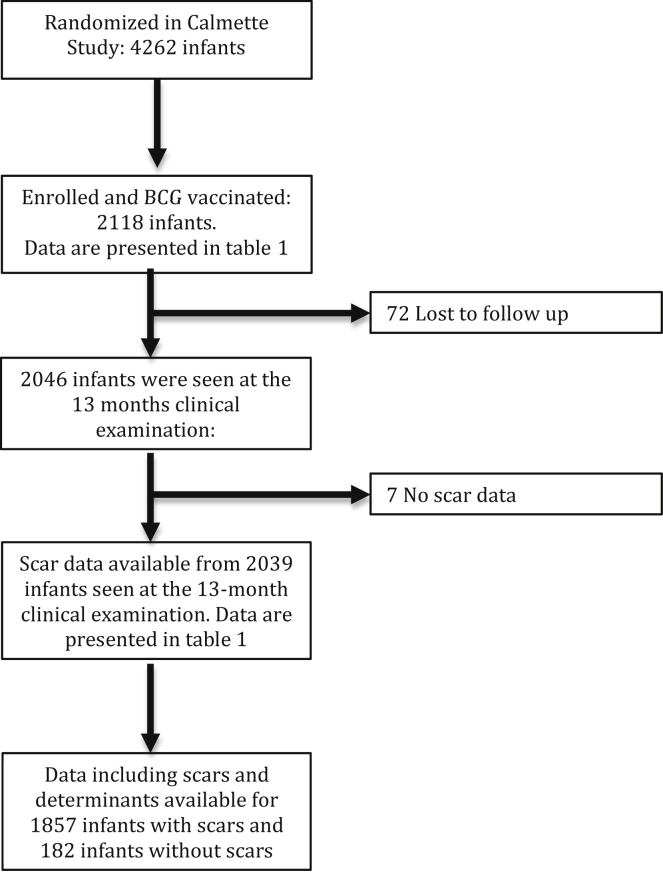
The study population consisted of infants vaccinated as part of The Danish Calmette Study who had been allocated to BCG vaccine at birth and who participated in the 13-month clinical examination (Figure 2). Prevalence ratios (PR) of BCG-scar development were estimated using Poisson regression with robust standard errors providing prevalence rate ratios [27]. Differences in BCG-scar size were estimated by median-based quantile regression (95 % confidence intervals are shown bootstrapped (Table 1) among children who had developed a BCG scar [28]. Estimates are presented crude and mutually adjusted. All adjusted analyses were also adjusted for month of vaccination (Tables 2 and 3). BCG-scar size by vaccinator and study site were visualised in a dot plot.
Figure 2.
Trial profile of infants included into the study.
Table 1.
Baseline measurements and scar size after neonatal BCG vaccination at 13 month among the 1857 BCG-vaccinated children in the Danish Calmette Study who developed a scar. Analysed by bootstrap.
| Determinants | Median (IQR) |
Crude |
Adjusted∗ |
||||||
|---|---|---|---|---|---|---|---|---|---|
| P50 (P25–P75) | Difference in median/cm. | 95 % CI | p-value | Difference in median/cm. | 95 % CI | ∗∗p-value | |||
| External determinants | Study site | Hvidovre hospital | 0.45 (0.35–0.60) | 0 (ref.) | 0 (ref.) | ||||
| Rigshospitalet | 0.50 (0.40–0.60) | 0.05 | (0.01–0.09) | <0.001 | 0.01 | (-0.12 to 0.14) | 0.84 | ||
| Kolding hospital | 0.40 (0.33–0.55) | -0.05 | (-0.09 to -0.01) | 0.05 | (-0.11 to -0.20) | ||||
| Vaccine batch | 1. "112032A″ | 0.45 (0.35–0.55) | 0 (ref.) | 0 (ref.) | |||||
| 2. "111046B″ | 0.50 (0.35–0.60) | 0.05 | (0.03–0.07) | <0.001 | 0.03 | (-0.02 to 0.08) | 0.20 | ||
| Vaccinator experience | 0 to 9 | 0.45 (0.35–0.58) | 0 (ref.) | 0 (ref.) | |||||
| 10 to 49 | 0.44 (0.35–0.55) | 0.00 | (-0.04 to 0.04) | <0.001 | -0.02 | (-0.06 to 0.02) | <0.001 | ||
| 50 to 99 | 0.45 (0.35–0.55) | 0.00 | (-0.05 to 0.05) | 0.01 | (-0.04 to 0.07) | ||||
| ≥ 100 | 0.50 (0.40–0.60) | 0.05 | (0.02–0.08) | 0.06 | (-0.02 to 0.14) | ||||
| Parental determinants | Maternal age at delivery | <27 years | 0.45 (0.35–0.55) | 0 (ref.) | 0 (ref.) | ||||
| 27-32 years | 0.45 (0.35–0.55) | 0.00 | (-0.04 to 0.04) | 1.00 | -0.02 | (-0.04 to 0.02) | 0.38 | ||
| >32 years | 0.45 (0.35–0.60) | 0.00 | (-0.05 to 0.05) | -0.00 | (-0.03 to 0.03) | ||||
| Maternal BCG | Yes | 0.45 (0.35–0.59) | 0 (ref.) | 0 (ref.) | |||||
| No | 0.45 (0.35–0.55) | -0.03 | (-0.07 to 0.02) | 0.28 | -0.01 | (-0.04 to 0.02) | 0.36 | ||
| Parental smokingb | Yes | 0.45 (0.35–0.58) | 0 (ref.) | 0 (ref.) | |||||
| No | 0.45 (0.35–0.55) | 0.00 | (-0.04 to 0.04) | 1.00 | 0.01 | (-0.02 to 0.04) | 0.50 | ||
| Atopic dispositionc | Yes | 0.45 (0.35–0.55) | 0 (ref.) | 0 (ref.) | |||||
| No | 0.45 (0.35–0.58) | 0.00 | (-0.03 to 0.03) | 1.00 | 0.02 | (-0.00 to 0.04) | 0.12 | ||
| Other children <4 years of age in the homed | Yes | 0.45 (0.35–0.55) | 0 (ref.) | 0 (ref.) | |||||
| No | 0.45 (0.35–0.58) | -0.00 | (-0.03 to 0.03) | 1.00 | -0.00 | (-0.03 to 0.02) | 0.78 | ||
| Individual determinants | Sex | Boys | 0.48 (0.35–0.60) | 0 (ref.) | 0 (ref.) | ||||
| Girls | 0.45 (0.35–0.55) | -0.03 | (-0.07 to 0.02) | 0.30 | -0.01 | (-0.03 to 0.01) | 0.26 | ||
| Prematurity | <37 weeks | 0.48 (0.33–0.60) | 0 (ref.) | 0 (ref.) | |||||
| ≥37 weeks | 0.45 (0.35–0.55) | -0.03 | (-0.12 to 0.07) | 0.61 | -0.03 | (-0.11 to 0.05) | 0.44 | ||
| Birth weight | <2500 g | 0.45 (0.31–0.60) | 0 (ref.) | 0 (ref.) | |||||
| ≥2500 g | 0.45 (0.35–0.55) | 0.00 | (-0.09 to 0.09) | 1.00 | 0.02 | (-0.06 to 0.11) | 0.58 | ||
| Caesarean section | Yes | 0.45 (0.35–0.55) | 0 (ref.) | 0 (ref.) | |||||
| No | 0.45 (0.35–0.58) | 0.00 | (-0.04 to 0.04) | 1.00 | 0.01 | (-0.02 to 0.04) | 0.42 | ||
| Child age at BCG vaccination | Day 0-1 | 0.45 (0.35–0.55) | 0 (ref.) | 0 (ref.) | |||||
| Day 2-7 | 0.45 (0.35–0.60) | 0.00 | (-0.03 to 0.03) | 1.00 | -0.00 | (-0.03 to 0.02) | 0.63 | ||
| Ethnicitye | Yes | 0.45 (0.35–0.55) | 0 (ref.) | 0 (ref.) | |||||
| No | 0.45 (0.35–0.58) | 0.00 | (-0.04 to 0.04) | 1.00 | 0.01 | (-0.01 to 0.03) | 0.41 | ||
| Exclusively breastfeeda | Yes | 0.45 (0.35–0.55) | 0 (ref.) | 0 (ref.) | |||||
| No | 0.48 (0.35–0.60) | 0.03 | (-0.01 to 0.06) | 0.18 | -0.00 | (-0.02 to 0.02) | 0.77 | ||
| Mid-upper arm circumferencef | 1 | 0.40 (0.30–0.55) | 0 (ref.) | 0 (ref.) | |||||
| 2 | 0.45 (0.35–0.55) | 0.05 | 0.02 to 0.08) | <0.001 | 0.03 | (-0.00 to 0.06) | 0.27 | ||
| 3 | 0.45 (0.35–0.60) | 0.05 | (0.00–1.00) | 0.02 | (-0.01 to 0.06) | ||||
| 4 | 0.50 (0.38–0.60) | 0.1 | (0.07–0.13) | 0.03 | (-0.01 to 0.07) | ||||
| Δweightg | 1 | 0.43 (0.33–0.55) | 0 (ref.) | 0 (ref.) | |||||
| 2 | 0.45 (0.35–0.55) | 0.03 | (-0.02 to 0.07) | <0.001 | 0.01 | (-0.01 to 0.04) | 0.15 | ||
| 3 | 0.48 (0.35–0.60) | 0.05 | (-0.02 to 0.11) | 0.03 | (0.00–0.06) | ||||
| 4 | 0.50 (0.38–0.60) | 0.08 | (0.03–0.12) | 0.04 | (0.01–0.08) | ||||
Analysis adjusted for study site, vaccine batch, vaccine experience, maternal age at delivery, maternal BCG vaccination, parental smoking, atopic disposition, other children <4 years of age in the home, sex, prematurity, birth weight, caesarean section, child age at BCG vaccination, ethnicity, exclusively breastfeed, mid-upper arm circumference and Δweight.
Wald test.
Exclusively breastfeed: breastfeeding in the first 3 months of life.
Smoking: all types of smoking by parents included as well as inside and outside smoking in the first 12 month of life.
Atopic disposition: at least one first degree relative with atopic disease (physician-diagnosed atopic eczema, asthma or asthmatic bronchitis, allergic rhino conjunctivitis or food allergy).
Any other children <4 years of age in the house at least 5 days a week.
Danish ethnicity or other ethnicity.
Mid upper arm circumference (MUAC) is divided in quartiles: 1) 12.1 cm–14.9 cm, 2) 14.9 cm–15.6 cm, 3) 15.6 cm–16.5 cm and 4) 16.5 cm–20 cm.
Δweight: gain in weight from birth to the 13-month clinical examination; divided in quantiles: 1) 3415 g–6042 g, 2) 6044 g–6688 g, 3) 6689 g–7460 g, 4) 7464 g–10910 g.
Table 2.
The association between month of vaccination and BCG-scar prevalence 13 month after neonatal BCG vaccination among 2039 BCG-vaccinated in the Danish Calmette Study.
| Determinants | (+) BCG, N = 2118 |
Data for scars |
Crude (N = 2039) |
Adjusted∗ (N = 1968) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Number of Infants (%) | Number of infants with scar information (2039), (% with information) | No scar | Scar (%) | Prevalence ratio (95% CI) | p-value | Prevalence ratio (95% CI) | p-value | ||
| Month of vaccination | January | 190 (9) | 188 (99) | 13 | 175 (93) | 1 (ref.) | 1 (ref.) | ||
| February | 166 (8) | 162 (98) | 11 | 151 (93) | 1.00 (0.95–1.06) | 0.96 | 1.01 (0.95–1.08) | 0.68 | |
| March | 179 (8) | 174 (97) | 5 | 169 (97) | 1.04 (1.00–1.09) | 0.07 | 1.02 (0.97–1.07) | 0.43 | |
| April | 158 (7) | 152 (96) | 7 | 145 (95) | 1.02 (0.97–1.08) | 0.92 | 0.94 (0.87–1.00) | 0.07 | |
| May | 158 (7) | 156 (99) | 5 | 151 (97) | 1.04 (0.99–1.09) | 0.11 | 0.91 (0.84–0.98) | 0.01 | |
| June | 165 (8) | 162 (98) | 7 | 155 (96) | 1.03 (0.98–1.08) | 0.29 | 0.91 (0.84–0.99) | 0.02 | |
| July | 177 (8) | 171 (97) | 25 | 146 (85) | 0.92 (0.85–0.99) | 0.02 | 0.81 (0.74–0.90) | 0.00 | |
| August | 178 (8) | 170 (96) | 15 | 155 (91) | 0.98 (0.92–1.04) | 0.51 | 0.87 (0.80–0.94) | 0.00 | |
| September | 158 (7) | 156 (99) | 7 | 149 (96) | 1.03 (0.97–1.08) | 0.33 | 0.89 (0.82–0.96) | 0.01 | |
| October | 196 (9) | 186 (95) | 31 | 155 (83) | 0.90 (0.83–0.97) | 0.00 | 0.82 (0.75–0.90) | 0.00 | |
| November | 227 (11) | 206 (91) | 41 | 165 (80) | 0.86 (0.80–0.93) | 0.00 | 0.87 (0.81–0.95) | 0.00 | |
| December | 166 (8) | 156 (94) | 15 | 141 (90) | 0.97 (0.91–1.04) | 0.37 | 0.98 (0.92–1.04) | 0.44 | |
Analysis adjusted for study site, vaccine batch, vaccine experience, maternal age at delivery, maternal BCG vaccination, parental smoking, atopic disposition, other children <4 years of age in the home, sex, prematurity, birth weight, caesarean section, child age at BCG vaccination, ethnicity, exclusively breastfeed, mid-upper arm circumference and Δweight.
Table 3.
The association between month of vaccination and BCG-scar size 13 month after neonatal BCG vaccination among the 1857 children in the Danish Calmette Study who developed a scar.
| Determinants | Median (IQR) |
Crude (N = 2039) |
Adjusted∗ (N = 1793) |
|||||
|---|---|---|---|---|---|---|---|---|
| P50 (P25–P75) | Difference in median/cm. | 95 % CI | p-value | Difference in median/cm. | 95 % CI | p-value | ||
| Month of vaccination | January | 0.50 (0.40–0.60) | 0 (ref.) | 0 (ref.) | ||||
| February | 0.50 (0.38–0.63) | 0 | (-0.05 to 0.05) | 1.00 | 0.01 | (-0.04 to 0.05) | 0.71 | |
| March | 0.45 (0.35–0.55) | -0.05 | (-0.09 to -0.01) | 0.03 | -0.06 | (-0.10 to -0.01) | 0.01 | |
| April | 0.45 (0.35–0.55) | -0.05 | (-0.10 to 0.00) | 0.04 | -0.06 | (-0.11 to 0.00) | 0.04 | |
| May | 0.45 (0.35–0.58) | -0.05 | (-0.10 to 0.00) | 0.03 | -0.03 | (-0.09 to 0.02) | 0.26 | |
| June | 0.45 (0.35–0.55) | -0.05 | (-0.10 to 0.00) | 0.03 | -0.04 | (-0.10 to 0.01) | 0.13 | |
| July | 0.41 (0.33–0.55) | -0.08 | (-0.12 to -0.03) | 0.00 | -0.06 | (-0.12 to 0.00) | 0.06 | |
| August | 0.45 (0.35–0.55) | -0.05 | (-0.10 to 0.00) | 0.03 | -0.04 | (-0.10 to 0.01) | 0.13 | |
| September | 0.45 (0.35–0.55) | -0.05 | (-0.10 to 0.00) | 0.03 | -0.06 | (-0.11 to 0.00) | 0.05 | |
| October | 0.45 (0.35–0.55) | -0.05 | (-0.10 to 0.00) | 0.03 | -0.05 | (-0.11 to 0.00) | 0.04 | |
| November | 0.40 (0.30–0.55) | -0.1 | (-0.14 to -0.06) | 0.00 | -0.09 | (-0.13 to -0.04) | 0.00 | |
| December | 0.50 (0.40–0.63) | 0 | (-0.05 to 0.05) | 1.00 | -0.01 | (-0.05 to 0.04) | 0.70 | |
Analysis adjusted for study site, vaccine batch, vaccine experience, maternal age at delivery, maternal BCG vaccination, parental smoking, atopic disposition, other children <4 years of age in the home, sex, prematurity, birth weight, caesarean section, child age at BCG vaccination, ethnicity, exclusively breastfeed, mid-upper arm circumference and Δweight.
The statistical analyses were conducted using Stata 13 (StataCorp, Texas, USA) and the visualisation was made using R (Foundation for Statistical Computing, Vienna, Austria).
2.5. Ethics
The Danish Data Protection Board (J.nr. 2009-41-4141), The Danish and the European Medicines Agencies (ref. no. EuDract 2010-021979-85), and The National Committee on Health Research Ethics in Denmark (H-3-2010-087) approved The Danish Calmette Study. The trial was registered at clinicaltrials.org (ref. no. EudraCT2010-021979-85).
3. Results
Between September 2012 and November 2015, 4262 infants were included in The Danish Calmette Study. Of these, 2118 (49.7 %) were BCG vaccinated and 2039 (96 %) were seen at the 13-months examination [20]. Scars were observed in 1857 infants, leading to an overall BCG vaccination scar prevalence of 91 % among children examined at 13 months of age (Figure 2).
3.1. Determinants of BCG scar prevalence
3.1.1. External determinants
Scar prevalence after BCG vaccination varied by study site. The prevalence was 85 % at Copenhagen University Hospital, Hvidovre, compared with 95 % at Copenhagen University Hospital, Rigshospitalet, (crude PR (cPR) 1.11 [95 % CI: 1.08 to 1.16]; adjusted PR (aPR) 1.24 [95 % CI: 1.18 to 1.30]) and 93 % at Kolding hospital (cPR 1.08 [95 % CI: 1.04 to 1.13]; aPR 1.27 [95 % CI: 1.19 to 1.35]). Vaccine batch 2 ″111046B″ resulted in a lower scar prevalence compared to vaccine batch 1 “112032A” (cPR 0.95 [95 % CI 0.92 to 0.98]; aPR 0.90 [95 % CI: 0.85 to 0.96]). Vaccinator experience with more than 100 administrated doses was associated with a higher prevalence (cPR 1.06 [95 % CI 1.01 to 1.10]; aPR 1.16 [95 % CI: 1.09 to 1.22]). At the same time vaccinator experience with 10–49 doses and 50 to 99 doses were associated with a lower prevalence, but only in the adjusted analyses (Table 4). Month of vaccination was also associated with the development of a scar; infants vaccinated from May until November were less likely to develop a scar compared with infants vaccinated in the remaining of the year (Table 2).
Table 4.
Baseline characteristics and BCG-scarification 13 months after neonatal BCG vaccination among the 2118 BCG-vaccinated children in the Danish Calmette Study.
| Determinants | (+) BCG, N = 2118 Numbers of Infants (%) [Missing] |
Data for scars |
Crude |
Adjusted∗ |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of infants with scar information (2039), (% with information) [Missing] | No scar (182) | Scar prevalence (1857) (%) | Prevalence ratio (95 % CI) N = 2039 | p-value∗∗ | Prevalence ratio (95% CI) N = 1968 | p-value∗∗ | ||||
| External determinants | Study site | Hvidovre hospital | 734 (35) | 688 (94) | 101 | 587 (85) | 1 (ref.) | 1 (ref.) | ||
| Rigshospitalet | 761 (36) | 741 (97) | 36 | 705 (95) | 1.11 (1.08–1.16) | <0.001 | 1.24 (1.18–1.30) | <0.001 | ||
| Kolding hospital | 623 (29) | 610 (98) | 45 | 565 (93) | 1.08 (1.04–1.13) | 1.27 (1.19–1.35) | ||||
| Vaccine batch | 1. "112032A″ | 1195 (56) | 1159 (97) | 81 | 1078 (93) | 1 (ref.) | 1 (ref.) | |||
| 2. "111046B″ | 923 (44) | 880 (95) | 101 | 779 (89) | 0.95 (0.92–0.98) | <0.001 | 0.90 (0.85–0.96) | <0.001 | ||
| Vaccinator Experience (number of children vaccinated) | 0 to 9 | 328 (16) | 310 (95) | 31 | 279 (90) | 1 (ref.) | 1 (ref.) | |||
| 10 to 49 | 816 (40) | 790 (97) | 78 | 712 (90) | 1.00 (0.96–1.05) | <0.001 | 0.94 (0.89–0.98) | <0.001 | ||
| 50 to 99 | 262 (13) | 259 (99) | 37 | 218 (85) | 0.95 (0.89–1.01) | 0.93 (0.87–0.99) | ||||
| ≥100 | 643 (31) | 641 (100) | 31 | 589 (95) | 1.06 (1.01–1.10) | 1.16 (1.09–1.22) | ||||
| [69] | [39] | |||||||||
| Parental determinants | Maternal age at delivery | <27 years | 287 (14) | 278 (97) | 24 | 254 (91) | 1 (ref.) | 1 (ref.) | ||
| 27-32 years | 799 (38) | 764 (96) | 67 | 697 (91) | 1.00 (0.96–1.04) | 0.95 | 1.00 (0.96–1.04) | |||
| >32 years | 1032 (49) | 997 (97) | 91 | 906 (91) | 0.99 (0.95–1.04) | 0.99 (0.95–1.03) | 0.93 | |||
| Maternal BCG | Yes | 356 (18) | 357 (96) | 39 | 318 (89) | 1 (ref.) | 1 (ref.) | |||
| No | 1638 (82) | 1661 (96) | 141 | 1520 (92) | 1.03 (0.99–1.07) | 0.18 | 1.03 (0.98–1.07) | 0.17 | ||
| [124] | [19] | |||||||||
| Parental smokingb | Yes | 313 (15) | 301 (96) | 29 | 272 (90) | 1 (ref.) | 1 (ref.) | |||
| No | 1790 (85) | 1738 (97) | 153 | 1585 (91) | 1.01 (0.97–1.05) | 0.61 | 1.00 (0.96–1.03) | 0.85 | ||
| [15] | ||||||||||
| Atopic dispositionc | Yes | 1349 (64) | 1297 (97) | 128 | 1169 (90) | 1 (ref.) | 1 (ref.) | |||
| No | 769 (36) | 742 (97) | 54 | 688 (93) | 1.03 (1.00–1.06) | 0.03 | 1.02 (0.99–1.05) | 0.13 | ||
| Other children <4 years of age in the homed | Yes | 445 (21) | 429 (96) | 39 | 390 (91) | 1 (ref.) | 1 (ref.) | |||
| No | 1656 (79) | 1608 (97) | 142 | 1446 (91) | 1.00 (0.96–1.03) | 0.81 | 0.99 (0.96–1.03) | 0.66 | ||
| [17] | [2] | |||||||||
| Individual determinants | Sex | Boys | 1096 (52) | 1060 (97) | 87 | 973 (92) | 1 (ref.) | 1 (ref.) | ||
| Girls | 1022 (48) | 979 (96) | 95 | 884 (90) | 0.98 (0.96–1.01) | 0.23 | 1.00 (0.97–1.02) | 0.73 | ||
| Prematurity | <37 weeks | 71 (3) | 63 (89) | 2 | 61 (97) | 1 (ref.) | 1 (ref.) | |||
| ≥37 weeks | 2047 (97) | 1976 (97) | 180 | 1796 (91) | 0.94 (0.90–0.98) | 0.01 | 0.96 (0.91–1.01) | 0.11 | ||
| Birth weight | <2500 g | 61 (3) | 53 (87) | 1 | 52 (98) | 1 (ref.) | 1 (ref.) | |||
| ≥2500 g | 2057 (97) | 1986 (97) | 181 | 1805 (91) | 0.92 (0.89–0.96) | <0.001 | 0.98 (0.94–1.03) | 0.52 | ||
| Caesarean section | No | 1694 (80) | 1629 (96) | 141 | 1488 (91) | 1 (ref.) | 1 (ref.) | |||
| Yes | 424 (20) | 410 (97) | 41 | 369 (90) | 0.99 (0.95–1.02) | 0.41 | 0.98 (0.95–1.02) | 0.38 | ||
| Child age at BCG vaccination | Day 2-7 | 554 (26) | 528 (95) | 49 | 479 (91) | 1 (ref.) | 1 (ref.) | |||
| Day 0-1 | 1564 (74) | 1511 (97) | 133 | 1378 (91) | 1.00 (0.96–1.02) | 0.74 | 0.99 (0.95–1.02) | 0.42 | ||
| Danish ethnicitye | Yes | 379 (18) | 357 (94) | 42 | 315 (88) | 1 (ref.) | 1 (ref.) | |||
| No | 1725 (82) | 1671 (97) | 139 | 1532 (92) | 1.04 (1.00–1.09) | 0.15 | 1.02 (0.98–1.06) | 0.60 | ||
| [14] | [11] | |||||||||
| Exclusively breastfeeda | Yes | 1210 (57) | 1168 (96) | 102 | 1066 (91) | 1 (ref.) | 1 (ref.) | |||
| No | 899 (43) | 869 (97) | 80 | 789 (91) | 1.00 (0.97–1.02) | 0.79 | 0.99 (0.96–1.02) | 0.71 | ||
| [9] | [2] | |||||||||
| Mid-upper arm circumferencef | 1 | 519 (25) | 517 (100) | 43 | 474 (92) | 1 (ref.) | 1 (ref.) | |||
| 2 | 521 (26) | 520 (100) | 46 | 474 (91) | 1.00 (0.96–1.03) | 0.88 | 0.98 (0.94–1.02) | 0.51 | ||
| 3 | 524 (26) | 523 (100) | 51 | 472 (90) | 0.98 (0.95–1.02) | 0.98 (0.92–1.01) | ||||
| 4 | 479 (23) | 476 (100) | 42 | 434 (91) | 1.00 (0.96–1.04) | 0.98 (0.93–1.03) | ||||
| [75] | [3] | |||||||||
| ΔWeight,g | 1 | 536 (26) | 533 (100) | 49 | 484 (91) | 1 (ref.) | 1 (ref.) | |||
| 2 | 495 (24) | 495 (100) | 48 | 447 (90) | 0.99 (0.95–1.03) | 0.72 | 0.99 (0.95–1.03) | 0.66 | ||
| 3 | 529 (26) | 526 (100) | 41 | 485 (92) | 1.01 (0.98–1.05) | 1.01 (0.97–1.06) | ||||
| 4 | 486 (24) | 484 (100) | 44 | 440 (91) | 1.00 (0.96–1.04) | 1.01 (0.96–1.07) | ||||
| [72] | [1] | |||||||||
Analysis adjusted for study site, vaccine batch, vaccine experience, maternal age at delivery, maternal BCG vaccination, parental smoking, atopic disposition, other children <4 years of age in the home, sex, prematurity, birth weight, caesarean section, child age at BCG vaccination, ethnicity, exclusively breastfeed, mid-upper arm circumference and Δweight.
Wald test.
Exclusively breastfeed: breastfeeding in the first 3 months of life.
Smoking: all types of smoking by parents included as well as inside and outside smoking in the first 12 month of life.
Atopic disposition: at least one first degree relative with atopic disease (physician-diagnosed atopic eczema, asthma or asthmatic bronchitis, allergic rhino conjunctivitis or food allergy).
Any other children <4 years of age in the house at least 5 days a week.
Danish ethnicity or other ethnicity.
Mid upper arm circumference (MUAC) is divided in quartiles: 1) 12.1 cm–14.9 cm, 2) 14.9 cm–15.6 cm, 3) 15.6 cm–16.5 cm and 4) 16.5 cm–20 cm.
Δweight: gain in weight from birth to the 13-month clinical examination; divided in quantiles: 1) 3415 g–6042 g, 2) 6044 g–6688 g, 3) 6689 g–7460 g, 4) 7464 g–10910 g.
3.1.2. Parental related determinants
Not having an atopic disposition was associated with increased scar prevalence, but only in the crude analysis (cPR 1.03 [95 % CI: 1.00 to 1.06]; aPR 1.02 [95 % CI: 0.99 to 1.05]) (Table 4). None of the other parental related factors were associated with scar prevalence.
3.1.3. Individual related determinants
Among individual determinants on scar prevalence, term born infants and children with birth weight ≥2500 g were less likely to develop a scar in the crude analysis (cPR 0.94 [95 % CI: 0.90 to 0.98]; cPR 0.92 [95 % CI: 0.89 to 0.96]) but the associations were weakened slightly when adjusted for other co-variates (aPR 0.96 [95 % CI: 0.91 to 1.01]; aPR 0.98 [95 % CI: 0.94 to 1.03]) (Table 4). None of the other individual level factors were associated with scar prevalence.
3.2. Determinants of scar size
3.2.1. External determinants
The median BCG scar size was different among infants vaccinated at the three hospitals. At Kolding hospital scar size was on average 0.05 cm smaller in crude analysis (cPR -0.05 [95 % CI: -0.07 to -0.03]), and 0.02 cm smaller in adjusted analysis (aPR -0.02 [95 % CI: -0.05 to 0.02]), compared with Copenhagen University Hospital, Hvidovre (p-value <0.001). BCG batch was associated with difference in scar size in the crude analysis, but the differences did not remain in the adjusted analysis (Table 5). Month of vaccination was associated with difference in scar size; being vaccinated in March, April and from September until November decreased the average scar size between 0.06 cm and 0.09 cm compared with the rest of the year (p-value <0.001) (Table 3).
Table 5.
Baseline characteristics and size of BCG-scar 13 month after neonatal BCG vaccination among the 1857 BCG-vaccinated children in the Danish Calmette Study who developed a scar.
| Determinants | Median (IQR) |
Crude (N = 2039) |
Adjusted∗ (N = 1793) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| P50 (P25–P75) | Difference in median/cm. | 95 % CI | p-value | Difference in median/cm. | 95 % CI | p-value∗∗ | |||
| External determinants | Study site | Hvidovre hospital | 0.45 (0.35–0.60) | 0 (ref.) | 0 (ref.) | ||||
| Rigshospitalet | 0.50 (0.40–0.60) | 0.05 | (0.03–0.07) | <0.001 | 0.04 | (0.01–0.07) | <0.001 | ||
| Kolding hospital | 0.40 (0.33–0.55) | -0.05 | (-0.07 to -0.03) | -0.02 | (-0.05 to 0.02) | ||||
| Vaccine batch | 1. "112032A″ | 0.45 (0.35–0.55) | 0 (ref.) | 0 (ref.) | |||||
| 2. "111046B″ | 0.50 (0.35–0.60) | 0.05 | (0.03–0.07) | <0.001 | 0.03 | (-0.01 to 0.08) | 0.21 | ||
| Vaccinator experience | 0 to 9 | 0.45 (0.35–0.58) | 0 (ref.) | 0 (ref.) | |||||
| 10 to 49 | 0.44 (0.35–0.55) | 0.00 | (-0.03 to 0.03) | <0.001 | -0.01 | (-0.04 to 0.02) | <0.001 | ||
| 50 to 99 | 0.45 (0.35–0.55) | 0.00 | (-0.04 to 0.04) | 0.02 | (-0.02 to 0.05) | ||||
| ≥ 100 | 0.50 (0.40–0.60) | 0.05 | (0.02–0.08) | 0.05 | (0.01–0.09) | ||||
| Parental determinants | Maternal age at delivery | <27 years | 0.45 (0.35–0.55) | 0 (ref.) | 0 (ref.) | ||||
| 27-32 years | 0.45 (0.35–0.55) | 0.00 | (-0.04 to 0.04) | 1.00 | -0.01 | (-0.04 to 0.02) | 0.74 | ||
| >32 years | 0.45 (0.35–0.60) | 0.00 | (-0.04 to 0.04) | -0.00 | (-0.03 to 0.03) | ||||
| Maternal BCG | Yes | 0.45 (0.35–0.58) | 0 (ref.) | 0 (ref.) | |||||
| No | 0.45 (0.35–0.55) | 0.00 | (-0.03 to 0.03) | 0.77 | -0.01 | (-0.03 to 0.02) 0.64 | |||
| Parental smokingb | Yes | 0.45 (0.35–0.58) | 0 (ref.) | 0 (ref.) | |||||
| No | 0.45 (0.35–0.55) | 0.00 | (-0.04 to 0.04) | 1.00 | 0.00 | (-0.02 to 0.03) 0.86 | |||
| Atopic dispositionc | Yes | 0.45 (0.35–0.55) | 0 (ref.) | 0 (ref.) | |||||
| No | 0.48 (0.35–0.58) | 0.03 | (0.00–0.05) | 0.04 | 0.02 | (0.00–0.04) 0.02 | |||
| Other children <4 years of age in the homed | Yes | 0.45 (0.35–0.55) | 0 (ref.) | 0 (ref.) | |||||
| No | 0.45 (0.35–0.58) | 0.00 | (-0.03 to 0.03) | 1.00 | 0.01 | (-0.02 to 0.03) | 0.53 | ||
| Individual determinants | Sex | Boys | 0.48 (0.35–0.60) | 0 (ref.) | 0 (ref.) | ||||
| Girls | 0.45 (0.35–0.55) | -0.03 | (-0.04 to -0.01) | 0.01 | -0.01 | (-0.03 to 0.01) | 0.28 | ||
| Prematurity | <37 weeks | 0.48 (0.33–0.60) | 0 (ref.) | 0 (ref.) | |||||
| ≥37 weeks | 0.45 (0.35–0.55) | -0.03 | (-0.11 to 0.06) | 0.55 | -0.03 | (-0.08 to 0.03) | 0.37 | ||
| Birth weight | <2500 g | 0.45 (0.31–0.60) | 0 (ref.) | 0 (ref.) | |||||
| ≥2500 g | 0.45 (0.35–0.55) | 0.00 | (-0.09 to 0.09) | 1.00 | 0.02 | (-0.05 to 0.08) | 0.61 | ||
| Caesarean section | Yes | 0.45 (0.35–0.55) | 0 (ref.) | 0 (ref.) | |||||
| No | 0.45 (0.35–0.58) | 0.00 | (-0.04 to 0.04) | 1.00 | 0.01 | (-0.02 to 0.03) | 0.61 | ||
| Child age at BCG vaccination | Day 0-1 | 0.45 (0.35–0.55) | 0 (ref.) | 0 (ref.) | |||||
| Day 2-7 | 0.45 (0.35–0.60) | 0.00 | (-0.03 to 0.03) | 1.00 | -0.02 | (-0.04 to 0.01) | 0.14 | ||
| Danish ethnicitye | Yes | 0.45 (0.35–0.55) | 0 (ref.) | 0 (ref.) | |||||
| No | 0.45 (0.35–0.58) | 0.00 | (-0.04 to 0.04) | 0.86 | 0.01 | (-0.01 to 0.04) | 0.53 | ||
| Exclusively breastfeeda | Yes | 0.45 (0.35–0.55) | 0 (ref.) | 0 (ref.) | |||||
| No | 0.45 (0.35–0.60) | 0.00 | (-0.03 to 0.03) | 1.00 | -0.00 | (-0.02 to 0.02) | 0.89 | ||
| Mid-upper arm circumferencef | 1 | 0.40 (0.30–0.55) | 0 (ref.) | 0 (ref.) | |||||
| 2 | 0.45 (0.35–0.55) | 0.05 | 0.03 to 0.07) | <0.001 | 0.03 | (0.00–0.06) | 0.09 | ||
| 3 | 0.45 (0.35–0.60) | 0.05 | (0.03–0.07) | 0.03 | (0.00–0.06) | ||||
| 4 | 0.50 (0.38–0.60) | 0.1 | (0.08–0.12) | 0.04 | (0.00–0.07) | ||||
| Δweightg | 1 | 0.43 (0.33–0.55) | 0 (ref.) | 0 (ref.) | |||||
| 2 | 0.45 (0.35–0.55) | 0.03 | (0.00–0.05) | <0.001 | -0.00 | (-0.03 to 0.03) | 0.20 | ||
| 3 | 0.48 (0.35–0.60) | 0.05 | (0.03–0.07) | 0.02 | (-0.01 to 0.05) | ||||
| 4 | 0.50 (0.38–0.60) | 0.08 | (0.05–0.10) | 0.03 | (-0.00 to 0.07) | ||||
Analysis adjusted for study site, vaccine batch, vaccine experience, maternal age at delivery, maternal BCG vaccination, parental smoking, atopic disposition, other children <4 years of age in the home, sex, prematurity, birth weight, caesarean section, child age at BCG vaccination, ethnicity, exclusively breastfeed, mid-upper arm circumference and Δweight. N = 1793.
Wald test.
Exclusively breastfeed: breastfeeding in the first 3 months of life.
Smoking: all types of smoking by parents included as well as inside and outside smoking in the first 12 month of life.
Atopic disposition: at least one first degree relative with atopic disease (physician-diagnosed atopic eczema, asthma or asthmatic bronchitis, allergic rhino conjunctivitis or food allergy).
Any other children <4 years of age in the house at least 5 days a week.
Danish ethnicity or other ethnicity.
Mid upper arm circumference (MUAC) is divided in quartiles: 1) 12.1 cm–14.9 cm, 2) 14.9 cm–15.6 cm, 3) 15.6 cm–16.5 cm and 4) 16.5 cm–20 cm.
Δweight: gain in weight from birth to the 13-month clinical examination; divided in quantiles: 1) 3415 g–6042 g, 2) 6044 g–6688 g, 3) 6689 g–7460 g, 4) 7464 g–10910 g.
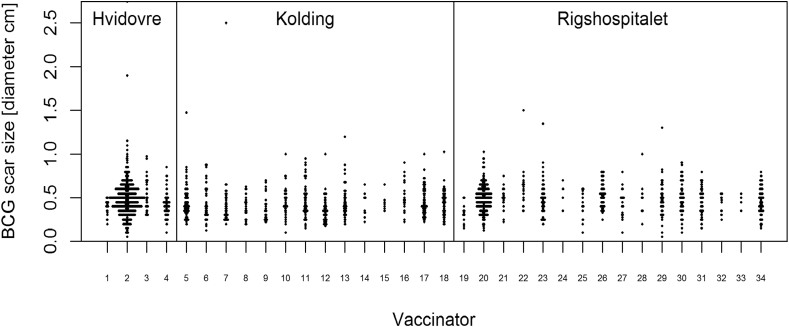
Among vaccinators, the median BCG vaccination scar size varied from 0.28 cm to 0.62 cm (Figure 3). Vaccinators who had vaccinated more than 100 children during the trial had a larger median BCG scar size than those who just vaccinated a few children (Crude median difference 0.05 cm [95 % CI: 0.02 to 0.08], Adjusted median difference 0.05 cm [95 % CI: 0.01 to 0.09] (Table 5).
Figure 3.
Difference in BCG vaccination scars by vaccinator and study site in the 1857 BCG-vaccinated children in the Danish Calmette Study who developed a scar.
3.2.2. Parental related determinants
Not having an atopic disposition tended to be associated with an increase in the median BCG scar with a crude median difference 0.03 cm [95 % CI: 0.00 to 0.05], and 0.02 cm and an adjusted median difference of 0.02 cm [95 % CI: 0.00 to 0.04]. The remaining parental related factors were not associated with median BCG scar size (Table 5).
3.2.3. Individual related determinants
The median BCG scar size difference was smaller for girls compared with boys, the crude median difference 0.03 cm [95 % CI: -0.04 to -0.01], but this estimate was weakened in the adjusted analysis (crude median difference 0.01 cm [95 % CI: -0.03 to 0.01]). MUAC and weight gain from birth to 13 months of age were associated with BCG-scar size in the crude, but not in the adjusted analysis. The remaining factors were not associated with median BCG scar size (Table 5).
4. Discussion
To our knowledge, this is the first study to investigate possible determinants of developing a scar and scar size after BCG vaccination in a high-income country. Development of a scar was significantly associated with study site and vaccinator experience. Furthermore, we found that vaccinator experience was positively associated with scar size. Among the many individual and parental-related factors studied, only very few were associated with differences in scar prevalence and scar size.
4.1. External determinants
It is well known from low-income settings that experience and training of the staff who injected the vaccine as well as BCG vaccination technique influenced the scar formation and the scar size [2, 3, 12, 19]. Certain factors should be considered when vaccinating, such as the specific dose of the vaccine injected, the volume leaking after vaccination, and whether the vaccine is injected intradermally or subcutaneously [13, 29]. Staff conducting vaccination on a regular basis would be expected to achieve higher vaccination skills. In the Calmette study all staff member received the same training at baseline. Predominantly one person were vaccinating at Copenhagen University Hospital, Hvidovre, compared with Copenhagen University Hospital, Rigshospitalet, and Kolding hospital, that had different staff members vaccinating the participants. However, Copenhagen University Hospital, Hvidovre, tended to have a lower scar-prevalence than the other two hospitals. It is possible that the staff conducting vaccinations at Copenhagen University Hospital, Hvidovre, was using a less optimal vaccination technique. It has previously been shown that the size of a wheal after vaccination is associated with the formation of a scar [30]. This supports the fact that vaccination technique is an important predictor for BCG scar size.
Two different vaccine batches were used in the study. Even though both of them are the Danish strain 1331, Statens Serum Institut (SSI), there may still be variation between batches [31]. We found a small but significant difference in scar size between the batches used in the study.
Also month of vaccination seemed to be associated with BCG scar formation and BCG scar size [18]. Though we did control for vaccinator experience, the results may still be biased by the duration of the study as the inclusion started in September 2012 and ended in November 2014.
4.2. Parental related determinants
In our study infants without atopic disposition tended to be more likely to develop a scar after BCG vaccination to have a larger scar compared to infants with atopic disposition. To our knowledge this has not been reported before. The Calmette study had a highly selected study population as 64 % of the children reported atopic disposition; thus, the external validity of this finding may be limited and more studies are warranted.
4.3. Individual related determinants
Earlier studies have investigated a potential sex-specific beneficial effect of BCG vaccination [11, 13, 29, 31, 32]. We did not see any difference in scar prevalence or scar size between boys and girls in the adjusted analyses.
Prematurity and low birth-weight was also analyzed as predictors as both are related to the maturity of the infant including maturity of the immune system. Earlier studies have shown that preterm and low-birth-weight infants have been less likely to develop a scar [19, 33, 34]. We found that preterm and low-birth-weight infants were more likely to develop a scar in the crude analysis, but this was not seen when taking all other covariates into account. In general the nutritional status in western infants is much better compared to infants from low-income countries. Maybe this could be an explanation for the difference in BCG scar frequency seen in this study compared to others. Also, another study that evaluated the BCG vaccine efficacy in preterm infants compared to mature infants did not find a significant difference between the two groups [35]. We also speculated if low-birth-weight infants received a higher dose of vaccine pr. kg, which increased their response and resulted in a higher scar frequency. Although we cannot reject this as an explanatory factor it seems unlikely that this is the only explanation for observed results. We have not been able to find other studies that could provide more insight on this matter.
4.4. Strengths and limitations
It is a strength of the study that only trained staff conducted the BCG vaccinations, and only few were responsible for vaccinating the infants. The consistency in using one BCG strain and only two different batches increases the robustness of our findings.
There was an almost complete follow-up (79 infants included in the sub-study were lost during follow up, 3.7 % of all). It should be noted that precise measurement of BCG scar is difficult [36, 37], hence measuring errors may have occurred. We have attempted to control for confounding in our mutually adjusted estimates. This could lead to an unintended adjustments of effects mediated through some of the covariates, potentially causing our analysis to miss actual effects of determinants (type 2 error). The causal structure of the determinants may be discussed and thus we acknowledge the difficulties in a causal interpretation of some of the mutually adjusted estimates [38].
It is a limitation of the study that we did not use tuberculin skin test, TST, to assess the response to BCG vaccination. As BCG scar, TST response is considered a marker of BCG efficacy [19] and TST has also been shown to correlate with overall mortality [17]. However, TST is more difficult to assess and much fewer children develop a positive TST response than a scar; [39]. We therefore chose to focus on BCG scar in the present trial.
After BCG vaccination, a post injection wheal is supposed to be formed. Earlier work from the Calmette study group have shown that having a post injection wheal is highly correlated to a scar formation [30]. In this study we did not evaluate the post injection wheal.
5. Interpretation
It has been shown that among BCG vaccinated infants in low-income countries, those reacting to the BCG vaccine with a scar have significantly better survival rate [17]. It could therefore be considered that children that develop a scar after BCG vaccination react immunological stronger in general and therefore are more likely to survive; hence a scar is just a marker of a well functioning immune system [12, 31, 32, 33]. However, we know from studies in low-income settings that vaccination technique, type and strain of BCG vaccine, and dose of vaccine injected are important factors for the frequency and size of BCG scarring [13, 29]. Additionally, in the studies finding an association between scar and survival, the scar frequency varied between 52 % and 98 % even within the same country, and scar was still associated with survival, indicating that genetic factors alone cannot explain the association [18]. The present study supports that vaccinator experience rather than parental factors is the most important factor for developing a scar, also in a high-income setting.
6. Conclusion
We found that the probability of developing BCG-scars among Danish BCG vaccinated children in infancy depends on study site of vaccination and vaccinator experience. Like scar prevalence, scar size was influenced by vaccinator experience. Given the strong link between having a BCG scar and subsequent health, improved BCG vaccination technique could play a major role for child health.
Declarations
Author contribution statement
T. Jensen: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
S. Jensen: 2, N. Birk: Conceived and designed the experiments; Performed the experiments.
A. Rieckmann: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
T. Hoffmann, C. Benn, D. Jeppesen: Conceived and designed the experiments.
O. Pryds: Conceived and designed the experiments; Analyzed and interpreted the data.
T. Nissen: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Funding statement
This work was supported by Danmarks Grundforskningsfond.
Data availability statement
The authors do not have permission to share data.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
The clinical trial described in this paper was registered at The European Union Clinical Trials Register under the registration number 2010-021979-85.
Acknowledgements
We gratefully acknowledge the participation of the children and their parents. Special thanks to Birgit Peitersen, Monica Ladekarl, Linda Billetorp and Andreas Andersen for invaluable assistance.
References
- 1.Trunz B.B., Fine P., Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet (London, England) 2006;367:1173–1180. doi: 10.1016/S0140-6736(06)68507-3. [DOI] [PubMed] [Google Scholar]
- 2.Sivarajah N., Sivayogan S., Jegatheesan J., Gnananathan V. BCG vaccination and development of a scar. Ceylon Med. J. 1990;35:75–77. [PubMed] [Google Scholar]
- 3.Vallishayee R.S., Shashidhara a. N., Bunch-Christensen K., Guld J. Tuberculin sensitivity and skin lesions in children after vaccination with 11 different BCG strains. Bull. World Health Organ. 1974;51:489–494. [PMC free article] [PubMed] [Google Scholar]
- 4.Floyd S., Ponnighaus J.M., Bliss L., Warndorff D.K., Kasunga A., Mogha P. BCG scars in northern Malawi: sensitivity and repeatability of scar reading, and factors affecting scar size. Int. J. Tubercul. Lung Dis. 2000;4:1133–1142. [PubMed] [Google Scholar]
- 5.Rani S.H., Vijayalakshmi V., Sunil K., Lakshmi K a, Suman L.G., Murthy K.J. Cell mediated immunity in children with scar-failure following BCG vaccination. Indian Pediatr. 1998;35:123–127. [PubMed] [Google Scholar]
- 6.Sedaghatian M.R., Shana’a I.A. Evaluation of BCG at birth in the United Arab Emirates. Tubercle. 1990;71:177–180. doi: 10.1016/0041-3879(90)90072-g. [DOI] [PubMed] [Google Scholar]
- 7.Chhatwal J., Verma M., Thaper N., Aneja R. Waning of post vaccinial allergy after neonatal BCG vaccination. Indian Pediatr. 1994;31:1529–1533. [PubMed] [Google Scholar]
- 8.Sterne J a, Fine P.E., Pönnighaus J.M., Sibanda F., Munthali M., Glynn J.R. Does bacille Calmette-Guérin scar size have implications for protection against tuberculosis or leprosy? Tuber. Lung Dis. 1996;77:117–123. doi: 10.1016/s0962-8479(96)90025-8. [DOI] [PubMed] [Google Scholar]
- 9.Shann F., Nohynek H., Scott J.A., Hesseling A., Flanagan K.L. Randomized trials to study the nonspecific effects of vaccines in children in low-income countries. Pediatr. Infect. Dis. J. 2010;29:457–461. doi: 10.1097/INF.0b013e3181c91361. [DOI] [PubMed] [Google Scholar]
- 10.Kristensen I., Aaby P., Jensen H. Routine vaccinations and child survival: follow up study in Guinea-Bissau, West Africa. BMJ. 2000;321:1435–1438. doi: 10.1136/bmj.321.7274.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garly M.-L., Martins C.L., Balé C., Baldé M.A., Hedegaard K.L., Gustafson P. BCG scar and positive tuberculin reaction associated with reduced child mortality in West Africa. A non-specific beneficial effect of BCG? Vaccine. 2003;21:2782–2790. doi: 10.1016/s0264-410x(03)00181-6. [DOI] [PubMed] [Google Scholar]
- 12.Roth A., Gustafson P., Nhaga A., Djana Q., Poulsen A., Garly M.-L. BCG vaccination scar associated with better childhood survival in Guinea-Bissau. Int. J. Epidemiol. 2005;34:540–547. doi: 10.1093/ije/dyh392. [DOI] [PubMed] [Google Scholar]
- 13.Roth A., Sodemann M., Jensen H., Poulsen A., Gustafson P., Weise C. Tuberculin reaction, BCG scar, and lower female mortality. Epidemiology. 2006;17:562–568. doi: 10.1097/01.ede.0000231546.14749.ab. [DOI] [PubMed] [Google Scholar]
- 14.Biering-Sørensen S., Aaby P., Lund N., Monteiro I., Jensen K.J., Eriksen H.B. Early BCG-Denmark and neonatal mortality among infants weighing <2500 g: A randomized controlled trial. Clin. Infect. Dis. 2017;65:1183–1190. doi: 10.1093/cid/cix525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aaby P., Roth A., Ravn H., Napirna B.M., Rodrigues A., Lisse I.M. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J. Infect. Dis. 2011;204:245–252. doi: 10.1093/infdis/jir240. [DOI] [PubMed] [Google Scholar]
- 16.Schaltz-Buchholzer F., Biering-Sørensen S., Lund N., Monteiro I., Umbasse P., Fisker A.B. Early BCG vaccination, hospitalizations, and hospital deaths: analysis of a secondary outcome in 3 randomized trials from Guinea-bissau. J. Infect. Dis. 2019;219:624–632. doi: 10.1093/infdis/jiy544. [DOI] [PubMed] [Google Scholar]
- 17.Benn C.S., Roth A., Garly M.L., Fisker A.B., Schaltz-Buchholzer F., Timmermann A. BCG scarring and improved child survival: a combined analysis of studies of BCG scarring. J. Intern. Med. 2020 doi: 10.1111/joim.13084. [DOI] [PubMed] [Google Scholar]
- 18.Storgaard L., Rodrigues A., Martins C., Nielsen B.U., Ravn H., Benn C.S. Development of BCG scar and subsequent morbidity and mortality in rural Guinea-bissau. Clin. Infect. Dis. 2015;61:950–959. doi: 10.1093/cid/civ452. [DOI] [PubMed] [Google Scholar]
- 19.Roth A., Sodemann M., Jensen H., Poulsen A., Gustafson P., Gomes J. Vaccination technique, PPD reaction and BCG scarring in a cohort of children born in Guinea-Bissau 2000-2002. Vaccine. 2005;23:3991–3998. doi: 10.1016/j.vaccine.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 20.Thøstesen L.M., Nissen T.N., Kjærgaard J., Pihl G.T., Birk N.M., Benn C.S. Bacillus Calmette-Guérin immunisation at birth and morbidity among Danish children: a prospective, randomised, clinical trial. Contemp. Clin. Trials. 2015;42:213–218. doi: 10.1016/j.cct.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Kjærgaard J., Birk N.M., Nissen T.N., Thøstesen L.M., Pihl G.T., Benn C.S. Nonspecific effect of BCG vaccination at birth on early childhood infections: a randomized, clinical multicenter trial. Pediatr. Res. 2016;80:681–685. doi: 10.1038/pr.2016.142. [DOI] [PubMed] [Google Scholar]
- 22.Nissen T.N., Birk N.M., Smits G., Jeppesen D.L., Stensballe L.G., Netea M.G. Bacille Calmette-Guérin (BCG) vaccination at birth and antibody responses to childhood vaccines. A randomised clinical trial. Vaccine. 2017;35:2084–2091. doi: 10.1016/j.vaccine.2017.02.048. [DOI] [PubMed] [Google Scholar]
- 23.Nissen T.N., Birk N.M., Blok B.A., Arts R.J.W., Andersen A., Kjærgaard J. Bacillus Calmette-Guérin vaccination at birth and in vitro cytokine responses to non-specific stimulation. A randomized clinical trial. Eur. J. Clin. Microbiol. Infect. Dis. 2018;37:29–41. doi: 10.1007/s10096-017-3097-2. [DOI] [PubMed] [Google Scholar]
- 24.Birk N.M., Nissen T.N., Kjærgaard J., Hartling H.J., Thøstesen L.M., Kofoed P.-E. Effects of Bacillus Calmette-Guérin (BCG) vaccination at birth on T and B lymphocyte subsets: results from a clinical randomized trial. Sci. Rep. 2017;7:12398. doi: 10.1038/s41598-017-11601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thøstesen L.M., Kjaer H.F., Pihl G.T., Nissen T.N., Birk N.M., Kjaergaard J. Neonatal BCG has no effect on allergic sensitization and suspected food allergy until 13 months. Pediatr. Allergy Immunol. 2017;28:588–596. doi: 10.1111/pai.12748. [DOI] [PubMed] [Google Scholar]
- 26.Stensballe L.G., Ravn H., Birk N.M., Kjærgaard J., Nissen T.N., Pihl G.T. BCG vaccination at birth and rate of hospitalization for infection until 15 Months of age in Danish children: a randomized clinical multicenter trial. J. Pediatric. Infect. Dis. Soc. 2019;8:213–220. doi: 10.1093/jpids/piy029. [DOI] [PubMed] [Google Scholar]
- 27.Barros A.J., Hirakata V.N. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med. Res. Methodol. 2003;3:21. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cameron Adrian Colin, PKT . Vol. 2. Stata Press; College Station: 2010. (Microeconometrics using stata). [Google Scholar]
- 29.Stensballe L.G., Nante E., Jensen I.P., Kofoed P.-E., Poulsen A., Jensen H. Acute lower respiratory tract infections and respiratory syncytial virus in infants in Guinea-Bissau: a beneficial effect of BCG vaccination for girls. Vaccine. 2005;23:1251–1257. doi: 10.1016/j.vaccine.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Birk N.M., Nissen T.N., Ladekarl M., Zingmark V., Kjærgaard J., Jensen T.M. The association between Bacillus Calmette-Guérin vaccination (1331 SSI) skin reaction and subsequent scar development in infants. BMC Infect. Dis. 2017;17:540. doi: 10.1186/s12879-017-2641-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roth a. E., Stensballe L.G., Garly M.L., Aaby P. Beneficial non-targeted effects of BCG--ethical implications for the coming introduction of new TB vaccines. Tuberculosis. 2006;86:397–403. doi: 10.1016/j.tube.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Roth A., Garly M.L., Jensen H., Nielsen J., Aaby P. Bacillus Calmette-Guérin vaccination and infant mortality. Expert Rev. Vaccines. 2006;5:277–293. doi: 10.1586/14760584.5.2.277. [DOI] [PubMed] [Google Scholar]
- 33.Roth A., Jensen H., Garly M.-L., Djana Q., Martins C.L., Sodemann M. Low birth weight infants and calmette-guérin Bacillus vaccination at birth. Pediatr. Infect. Dis. J. 2004;23:544–550. doi: 10.1097/01.inf.0000129693.81082.a0. [DOI] [PubMed] [Google Scholar]
- 34.Sedaghatian M.R., Hashem F., Moshaddeque Hossain M. Bacille Calmette Guérin vaccination in pre-term infants. Int. J. Tubercul. Lung Dis. 1998;2:679–682. [PubMed] [Google Scholar]
- 35.Thayyil-Sudhan S., Kumar A., Singh M., Paul V.K., Deorari A.K. Safety and effectiveness of BCG vaccination in preterm babies. Arch. Dis. Child. Fetal Neonatal Ed. 1999;81:F64–F66. doi: 10.1136/fn.81.1.f64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aaby P., Shaheen S.O., Heyes C.B., Goudiaby A., Hall A.J., Shiell A.W. Early BCG vaccination and reduction in atopy in Guinea-Bissau. Clin. Exp. Allergy. 2000;30:644–650. doi: 10.1046/j.1365-2222.2000.00803.x. [DOI] [PubMed] [Google Scholar]
- 37.Fine P.E., Ponnighaus J.M., Maine N. The distribution and implications of BCG scars in northern Malawi. Bull. World Health Organ. 1989;67:35–42. [PMC free article] [PubMed] [Google Scholar]
- 38.Westreich D., Greenland S. The table 2 fallacy: presenting and interpreting confounder and modifier coefficients. Am. J. Epidemiol. 2013;177:292–298. doi: 10.1093/aje/kws412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diness B.R., Fisker A.B., Roth A., Yazdanbakhsh M., Sartono E., Whittle H. Effect of high-dose vitamin A supplementation on the immune response to Bacille Calmette-Guérin vaccine. Am. J. Clin. Nutr. 2007;86:1152–1159. doi: 10.1093/ajcn/86.4.1152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors do not have permission to share data.