Abstract
Context
A strictly controlled diet (often involving enteral tube feeding (ETF)) is part of the treatment of many inherited metabolic diseases (IMDs).
Objective
To describe the use of ETF in a large cohort of patients with IMDs.
Design
A retrospective analysis of ETF in patients with urea cycle disorders (UCDs), organic aciduria (OA), maple syrup disease (MSUD), glycogen storage diseases (GSDs) or fatty acid oxidation disorders (FAODs) diagnosed before the age of 12 months.
Setting
The reference center for IMDs at Necker Hospital (Paris, France).
Results
190 patients born between January 1991 and August 2017 were being treated for OA (n = 60), UCDs (n = 55), MSUD (n = 32), GSDs (n = 26) or FAODs (n = 17). Ninety-eight of these patients (52%) received ETF (OA subgroup: n = 40 (67%); UCDs: n = 12 (22%); MSUD: n = 9 (28%); GSDs: n = 23 (88%); FAODs: n = 14 (82%)). Indications for ETF were feeding difficulties in 64 (65%) patients, cessation of fasting in 39 (40%), and recurrent metabolic decompensation in 14 (14%). Complications of ETF were recorded in 48% of cases, more frequently with nasogastric tube (NGT) than with gastrostomy. Among patients in whom ETF was withdrawn, the mean duration of ETF was 5.9 (SD: 4.8) years (range: 0.6–19.8 years). The duration of ETF was found to vary from one disease subgroup to another (p = 0.051). While the longest median duration was found in the GSD subgroup (6.8 years), the shortest one was found in the UCD subgroup (0.9 years).
Conclusion
ETF is an integral part of the dietary management of IMDs. The long duration of ETF and the specific risks of NGT highlights the potential value of gastrostomy.
In this study at a French tertiary hospital, we documented the indications, modalities, duration and complications of enteral tube feeding in a cohort of patients with inherited metabolic diseases.
1. Introduction
Inherited metabolic diseases (IMDs) are individually rare but collectively frequent disorders, the estimated incidence of which ranges from 1/784 to 1/2500 live births [1,2]. The management of amino- and organic-acid-related disorders (organic aciduria (OA)) [3], maple syrup urine disease (MSUD) [4,5], and urea cycle disorders (UCDs) [[6], [7], [8]], glycogen storage diseases (GSDs) [[9], [10], [11]] and fatty acid oxidation disorders (FAODs) [12,13] is based on a nutrient-controlled diet and caloric support. These diseases require continuous enteral tube feeding (ETF) during the catabolic phase, in order to provide a high-energy diet (i.e. enough energy for metabolic demands [14]) and prevent proteolysis- and lipolysis-related decompensations. To avoid nocturnal lipolysis and fasting, infants with OA, FAOD or GSD can also be dependent on long-term overnight ETF at home. Thus, ETF is a key treatment component for children suffering from these diseases. Regardless of the type of disease, life-saving home ETF programs are being increasingly implemented worldwide to contribute to improving quality of life [15,16]. Many studies have focused on the composition of diet in different IMDs, whereas others have looked at the safety and complications of ETF at home [[17], [18], [19], [20], [21], [22]]. However, only few studies have been devoted to report the proportion of patients with IMDs receiving ETF, its indications (and notably the prevalence of feeding difficulties as an indication for ETF), and the duration of ETF in these contexts [12,23,24].
The objective of the present study is to describe the use of ETF in a large cohort of patients with IMDs diagnosed during the first year of life, who also require dietary treatment. We focused on its indications, modalities (i.e. a nasogastric tube (NGT) vs. gastrostomy), duration, and complications in a population treated in one French National reference center for IMDs.
2. Patients and methods
2.1. Patients
The main inclusion criterion was the dietary treatment of an IMD diagnosed in the first year of life (i.e. neonatal forms up to the age of one month, and delayed forms at an age ranging from one to 12 months) in the Metabolic Disease Reference Center at Necker Children's Hospital (Paris, France). These IMDs included UCDs (N-acetylglutamate synthetase (NAGS), carbamylphosphate synthetase (CPS), ornithine transcarbamylase (OTC), argininosuccinate synthetase (ASS), argininosuccinate lyase (ASL), arginase and carbonic anhydrase deficiencies (the latest is not properly UCD, but has a main impact on urea cycle)), OA (methylmalonic aciduria (MA), propionic aciduria (PA), and isovaleric aciduria (IVA)), MSUD, GSD types 0, I and III, and FAOD (carnitine palmitoyltransferase II (CPT2), very long-chain acyl-CoA dehydrogenase (VLCAD), long-chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD), medium-chain acyl-CoA dehydrogenase (MCAD) and short-chain acyl-CoA dehydrogenase (SCAD) deficiencies). The diagnosis was based on biochemical assay data and then confirmed by genetic testing. The included children were born between January 1991 and August 2017.
The main exclusion criteria were late-onset IMDs (i.e. diagnosed after the first year of life), death before or during the study period, loss to follow-up, severe neurological impairments, and birth after August 2017. Late-onset IMDs and birth after August 2017 exclusion criteria were chosen in order to have the most homogeneous population regarding feeding particularities and to have a certain time of follow-up. Similarly, severe neurological impairments were chosen as a criterion to avoid counting for the cases in which the prevention of autonomous oral feeding was independent of IMD. Each patient was treated with appropriate drugs and dietary treatments according to the underlying disease and individual tolerability.
3. Methods
Data were retrospectively collected from the hospital medical records up until August 2017. This comprises type of IMD, demographic characteristics (age, sex, and living area), nutritional management (such as the use of maintenance ETF, i.e. outside an emergency context), and the characteristics of the ETF (ages at initiation and discontinuation, indications, duration, modalities (an NGT or gastrostomy), tolerability, and complications).
From 2010 onwards, two groups could be considered: i) a group of children for whom ETF was systematically given, at least during the night, in order to avoid fasting. This group includes PA and MA accordingly to recommendations [14], and GSDs before possible utilization of cornstarch [25]. Moreover, since we have no systematic neonatal screening in France, FAODs discovered before the age of one year old by a clinical distress were considered as severe, usually requiring a nocturnal nutrition. Therefore, the systematic implementation of ETF in the absence of another cause was considered as fasting intolerance; ii) a second group of children who required ETF only for specific indications, including UCDs, MSUD and IVA. The indications for ETF in this group ii were feeding difficulties or metabolic decompensations. Feeding difficulties were defined by growth stagnation and/or less than 50% of recommended calorie intake taken orally and/or systematic very long and laborious meals. Similarly, metabolic decompensation was defined by clinical and/or biochemical criteria of iterative metabolic decompensation specific to each pathology.
ETF was systematically introduced in hospitalization and initiated with NGT. Parents were progressively trained by caregivers and service providers at the hospital and then at home. After few months, the indication of gastrostomy was considered for those cases in which ETF has to be prolonged. Thus, gastrostomy was always preceded by NGT.
Importantly, caloric intakes have not significantly changed with the time for 30 years (data not shown).
We also collected summary data on the children's psychomotor development (normal or delayed) and the type of school education (i.e. normal classes, special classes in conventional schools, or classes in a specialist institution for children with severe developmental delay). The study was approved by the local institutional ethical committee at Necker Children's Hospital (2017-PDL-15). Clinical data were registered with the Clinical Research Department at the Paris Public Hospital Group (Assistance Publique, Hôpitaux de Paris), after the provision of written, informed consent.
3.1. Statistical analysis
Quantitative variables were described as the mean (standard deviation (SD)) and range or as the median [25th and 75th percentiles (P25-P75)]. Qualitative variables were described as the number (percentage). To evaluate changes in practice, we classified patients into three groups, according to their date of birth: before 2002, between 2002 and 2009, and after 2009. Similarly, we defined four age groups: under 6 years of age, from 6 to 12 years old, from 13 to 17 years old, and 18 years old and above. Fisher's exact and chi-squared tests were used to examine relationships between categorical variables. Distributions of a continuous variable across categories of another variable were compared using the Kruskal-Wallis test.
Statistical analyses were performed with Stata software (version 14.0, StataCorp, College Station, TX, USA) and R software (version 3.4.1, R Foundation for Statistical Computing, Vienna, Austria).
4. Results
4.1. Study population and diagnoses
Table 1 shows the main characteristics of the 190 patients included in the study population. The patients were residents of the Île-de-France region (n = 87, 46%), elsewhere in France (n = 98, 52%), or abroad (n = 5, 2%). The sex ratio was 1.1 (91 girls and 99 boys). In August 2017, the patients were 11.9 years old on average (SD: 6.9 years, range: 0.3–26.3 years, median [P25-P75]: 10.9 [6.5–16.4] years). Fifty-eight patients were born before 2002, 74 were born between 2002 and 2009, and 58 were born after 2009. Accordingly, 41 patients were under the age of 6 years, 65 were aged from 6 to 12 years, 44 were aged from 13 to 17 years, and 40 were aged 18 years and above.
Table 1.
Characteristics of the study population: 190 patients with inherited metabolic diseases.
| Characteristics | n (%) or mean (standard deviation) |
|---|---|
| Sex | |
| Male | 99 (52.1) |
| Female | 91 (47.9) |
| Year of birth | |
| Before 2002 | 58 (30.5) |
| Between 2002 and 2009 | 74 (39.0) |
| After 2009 | 58 (30.5) |
| Age in August 2017 (in years) | 11.9 (6.9) |
| < 6 years of age | 41 (21.6) |
| 6–12 years of age | 65 (34.2) |
| 13–17 years of age | 44 (23.2) |
| > 18 years of age | 40 (21.0) |
| Area of residence | |
| Île-de-France region | 87 (45.8) |
| Elsewhere in France | 98 (51.6) |
| Outside France | 5 (2.6) |
| Inherited metabolic disease | |
| Organic aciduria | 60 (31.6) |
| Methylmalonic aciduria | 29/60 (48.4) |
| Propionic aciduria | 17/60 (28.3) |
| Isovaleric aciduria | 14/60 (23.3) |
| Urea cycle disorder | 55 (28.9) |
| N-acetylglutamate synthase deficiency | 3/55 (5.4) |
| Carbamylphosphate synthetase deficiency | 5/55 (9.1) |
| Ornithine transcarbamylase deficiency | 14/55 (25.4) |
| Argininosuccinate synthetase deficiency | 9/55 (16.4) |
| Argininosuccinate lyase deficiency | 19/55 (34.6) |
| Arginase deficiency | 3/55 (5.5) |
| Carbonic anhydrase deficiency | 2/55 (3.6) |
| Maple syrup urine | 32 (16.8) |
| Glycogen storage disease | 26 (13.7) |
| Type 0 | 3/26 (11.5) |
| Type Ia | 11/26 (42.3) |
| Type Ib | 8/26 (30.8) |
| Type III | 4/26 (15.4) |
| Fatty acid oxidation deficiency | 17 (9.0) |
| CPT2 | 2/17 (11.8) |
| VLCAD | 4/17 (23.5) |
| LCHAD | 8/17 (47.0) |
| MCAD | 2/17 (11.8) |
| SCAD | 1/17 (5.9) |
| Age at diagnosis | |
| Antenatal period | 9 (4.7) |
| One month or less | 123 (64.8) |
| Two months or more | 58 (30.5) |
The cohort included 60 patients with OA (MA: 29; PA: 17; IVA: 14), 55 with a UCD (NAGS: 3; CPS: 5; OTC: 14; ASS: 19; ASL: 9; arginase: 3; carbonic anhydrase: 2), 32 with an MSUD, 26 with a GSD (GSD I: 19; GSD III: 4; GSD 0: 3) and 17 with an FAOD (CPT2: 2; VLCAD: 4; LCHAD: 8; MCAD: 2; SCAD: 1).
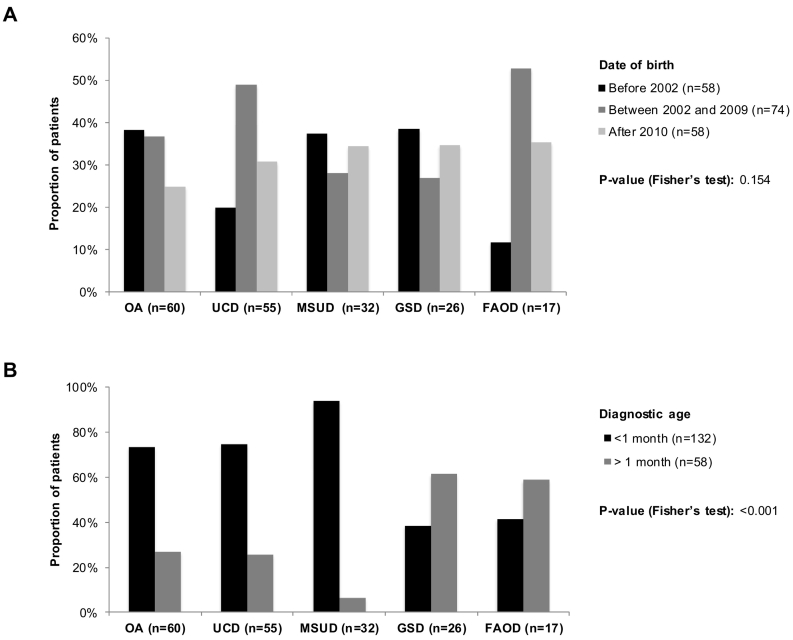
Fig. 1A shows the proportion of patients by disease and by year of birth. Although the proportion of patients with an FAOD was lower before 2000, the relationship between the disease type and the year of birth was not significant (p = 0.154).
Fig. 1.
Distribution of inherited metabolic diseases, by year of birth (A) and by age at diagnosis (B) in the whole study population (N = 190).
Abbreviations: FAOD, fatty acid oxidation deficiency; GSD, glycogen storage disease; MSUD, maple syrup urine disease; OA, organic aciduria; UCD, urea cycle disorder.
P-value is from the Fisher's exact test that examines the relationship between the disease type and the year of birth (A) and the age at diagnosis (B).
Fig. 1B presents the proportion of patients by disease and by age at diagnosis. A total of 132 patients were diagnosed within one month of birth, and 58 patients were diagnosed within one to 12 months of birth. The disease type was significantly related to the age at diagnosis (neonatal vs. non‑neonatal; p < 0.001). Neonatal presentation was more frequent for amino- and organic-acid-related disorders (OA: 73%, UCDs: 75%, MSUD: 94%) whereas late presentation was more frequent for energy disorders (FAODs: 59%, GSDs: 62%).
Psychomotor development was normal in 138 patients (73%) and delayed in 52 patients (27%). Looking at patients older than 3 years with available data on schooling (n = 162), the proportion of patients receiving special schooling (special classes or education in a specialist institution) was higher in the OA subgroup (n = 23/52, 44%) than in the other IMD subgroups (FAODs: n = 2/16, 13%; UCDs: n = 8/47, 17%; GSDs: n = 2/21, 10%; MSUD: n = 4/26, 15%; p = 0.002).
4.2. Characteristics of the ETF regimens
4.2.1. The requirement for ETF as a function of the type of IMD
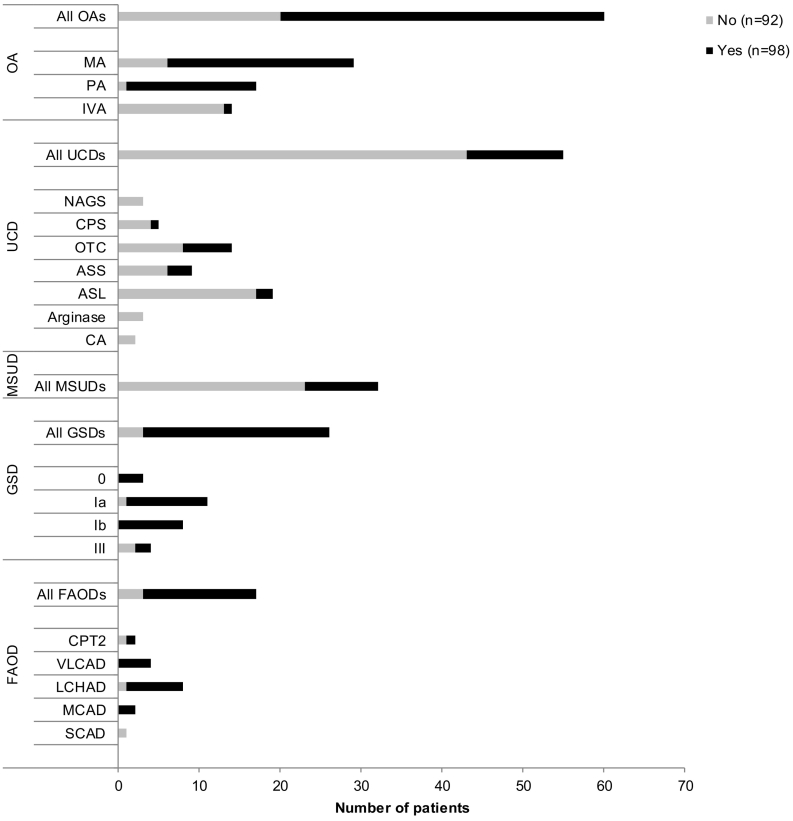
Fig. 2 shows the requirement for ETF by type of IMD, and Table 2 details the characteristics of ETF. A total of 98 patients (52%) required maintenance ETF, whereas 92 patients (48%) did not receive ETF support (other than in emergency situations). Maintenance ETF was required for 40 of the 60 patients in the OA subgroup (MA: 23/29; PA: 16/17; IVA: 1/14), 23 of the 26 in the GSD subgroup (GSD Ia: 10/11; GSD Ib: 8/8; GSD 0: 3/3; GSD III: 2/4), 14 of the 17 in the FAOD subgroup (CPT2: 1/2; VLCAD: 4/4; LCHAD: 7/8; MCAD: 2/2), 12 of the 55 in the UCD subgroup (CPS: 1/5; OTC: 6/14; ASS: 3/9; ASL: 2/19), and 9 in the 32 MSUD subgroup. We observed a significant relationship between the disease type and the requirement for ETF. Although a large majority of the patients receiving ETF came from the OA group (41%), many of the patients not receiving ETF had UCDs (47%; p < 0.001) - particularly ASL deficiency.
Fig. 2.
The requirement for enteral tube feeding, by type of inherited metabolic disease in the whole study population (N = 190).
Abbreviations: ASL, argininosuccinate lyase deficiency; ASS, argininosuccinate synthetase deficiency; CA, Carbonic anhydrase deficiency; CPS, carbamylphosphate synthetase deficiency; FAOD, fatty acid oxidation deficiency; GSD, glycogen storage disease; IVA, isovaleric acidemia; MA, methylmalonic acidemia; MSUD, maple syrup urine disease; NAGS, N-acetylglutamate synthase deficiency; OA, organic aciduria; OTC, ornithine transcarbamylase deficiency; PA, propionic acidemia; UCDs, urea cycle disorder.
Table 2.
Characteristics of enteral tube feeding regimens in the study population (N = 190).
| Inherited metabolic disease | Enteral tube feeding |
Gastrostomy |
|||
|---|---|---|---|---|---|
| n (%) | Median [P25-P75] age (in years) | n (%) | Median [P25-P75] age (in years) | ||
| Organic aciduria | Disease | ||||
| All OAs (n = 60) | 40 (66.7) | 0.2 [0.1–1.0] | 14 (23.3) | 2.3 [1.4–4.5] | |
| MA (n = 29) | 23 (79.3) | 0.1 [0.1–0.5] | 7 (24.1) | 2.6 [1.5–4.5] | |
| PA (n = 17) | 16 (94.1) | 0.3 [0.1–1.6] | 6 (35.3) | 2.3 [1.3–4.6] | |
| IVA (n = 14) | 1 (7.1) | 0.3 [0.3–0.3] | 1 (7.1) | 1.9 [1.9–1.9] | |
| Age at diagnosis | |||||
| <1 month (n = 44) | 28 (63.6) | 0.1 [0.1–0.3] | 10 (23) | 2.1 [1.5–4.5] | |
| >1 month (n = 16) | 12 (75.0) | 1.0 [0.3–3.7] | 4 (25) | 2.6 [1.3–6.6] | |
| Urea cycle disorder | Disease | ||||
| All UCDs (n = 55) | 12 (21.8) | 1.1 [0.7–2.0] | 6 (10.9) | 2.4 [2.3–3.1] | |
| NAGS (n = 3) | 0 (0) | – | – | – | |
| CPS (n = 5) | 1 (20.0) | 1.0 [1.0–1.0] | 1 (20.0) | 2.4 [2.4–2.4] | |
| OTC (n = 14) | 6 (42.9) | 1.6 [0.6–2.1] | 2 (14.3) | 2.5 [1.9–3.1] | |
| ASS (n = 9) | 3 (33.3) | 0.7 [0.7–1.2] | 1 (11.1) | 2.3 [2.3–2.3] | |
| ASL (n = 19) | 2 (11) | 1.7 [0.7–2.6] | 2 (10.5) | 3.1 [2.5–3.8] | |
| Arginase (n = 3) | 0 (0) | – | – | – | |
| CA (n = 2) | 0 (0) | – | – | – | |
| Age at diagnosis | |||||
| <1 month (n = 41) | 8 (19.5) | 0.9 [0.7–1.6] | 5 (12) | 2.5 [2.4–3.1] | |
| >1 month (n = 14) | 4 (28.6) | 1.7 [0.9–5.2] | 1 (7) | 2.3 [2.3–2.3] | |
| Maple syrup urine disease | Disease | ||||
| All MSUDs (n = 32) | 9 (28.1) | 1.1 [0.4–1.7] | 2 (6.3) | 2.7 [0.8–4.6] | |
| Age at diagnosis | |||||
| <1 month (n = 30) | 8 (26.7) | 0.8 [0.3–1.5] | 1 (3.3) | 0.8 [0.8–0.8] | |
| >1 month (n = 2) | 1 (50.0) | 3.5 [3.5–3.5] | 1 (50.0) | 4.6 [4.6–4.6] | |
| Glycogen storage disease | Disease | ||||
| All GSDs (n = 26) | 23 (88.5) | 0.3 [0.1–0.9] | 8 (30.8) | 1.4 [0.4–2.3] | |
| 0 (n = 3) | 3 (100) | 0.9 [0.6–0.9] | 0 (0) | – | |
| Ia (n = 11) | 10 (90.9) | 0.2 [0.1–0.4] | 7 (63.6) | 1.5 [0.5–3.2] | |
| Ib (n = 8) | 8 (100) | 0.2 [0.1–1.2] | 1 (12.5) | 0.3 [0.3–0.3] | |
| III (n = 4) | 2 (50.0) | 0.6 [0.2–1.1] | 0 (0) | – | |
| Age at diagnosis | |||||
| <1 month (n = 10) | 10 (100) | 0.1 [0.1–0.1] | 4 (40) | 0.4 [0.3–1.8] | |
| >1 month (n = 16) | 13 (81.3) | 0.9 [0.4–1.0] | 4 (25) | 1.5 [1.4–3.4] | |
| Fatty acid oxidation deficiency | Disease | ||||
| All FAODs (n = 17) | 14 (82.4) | 0.4 [0.1–0.9] | 5 (29.4) | 1.5 [1.4–1.5] | |
| CPT2 (n = 2) | 1 (50.0) | 0.1 [0.1–0.1] | 1 (50.0) | 1.3 [1.3–1.3] | |
| VLCAD (n = 4) | 4 (100) | 0.3 [0.1–0.9] | 2 (50.0) | 1.4 [1.4–1.5] | |
| LCHAD (n = 8) | 7 (87.5) | 0.4 [0.1–0.8] | 2 (25.0) | 2.3 [1.5–3.0] | |
| MCAD (n = 2) | 2 (100) | 0.9 [0.9–1.0] | 0 (0) | – | |
| SCAD (n = 1) | 0 (0) | – | – | – | |
| Age at diagnosis | |||||
| <1 month (n = 7) | 6 (85.7) | 0.1 [0.1–0.1] | 3 (43) | 1.4 [1.3–1.5] | |
| >1 month (n = 10) | 8 (80.0) | 0.7 [0.4–0.9] | 2 (20) | 2.3 [1.5–3.0] | |
| ALL PATIENTS (N = 190) | 98 (51.6) | 0.4 [0.1–1.1] | 35 (18.4) | 1.9 [1.4–3.1] | |
Abbreviations: ASL, argininosuccinate lyase deficiency; ASS, argininosuccinate synthetase deficiency; CA, carbonic anhydrase deficiency; CPS, carbamylphosphate synthetase deficiency; FAOD, fatty acid oxidation deficiency; GSD, glycogen storage disease; IVA, isovaleric acidemia; MA, methylmalonic acidemia; MSUD, maple syrup urine disease; NAGS, N-acetylglutamate synthase deficiency; OA, organic aciduria; OTC, ornithine transcarbamylase deficiency; P25-P75, 25th and 75th percentiles; PA, propionic acidemia; UCD, urea cycle disorder.
Gastrostomy feeding was always preceded by NGT feeding, thus gastrostomy patients are also included in ETF patients. For example, among 26 GSD patients, 23 had ETF and 8 of them had gastrostomy.
The mean age at ETF initiation was 1.1 (SD: 2.6) years, and ranged from birth to 20.5 years. The median [P25-P75] age was 0.4 [0.1–1.1] years. The age at ETF initiation differed according to the disease type (p = 0.012). The median [P25-P75] age at ETF initiation in the OA, GSD, FAOD, UCD, an MSUD subgroups was 0.2 [0.1–1.0], 0.3 [0.1–0.9], 0.4 [0.1–0.9], 1.1 [0.7–2.0], and 1.1 [0.4–1.7] years, respectively. Regardless of the age at diagnosis, the initiation of ETF was always done with NGT. Then gastrostomy could be proposed, and accepted or not by the parents and/or the child.
4.2.2. Indications for ETF
The three most frequent indications were (total or partial) feeding difficulties and failure to thrive (in 64 patients (65%)), fasting intolerance (including systematic ETF in patients with recently diagnosed OA, GSD or severe FAOD) in 39 patients (40%)), and metabolic decompensation (in 14 patients (14%)). Some patients had more than one indication for treatment (e.g. feeding difficulties leading to metabolic decompensation).
In the OA subgroup, the indication for ETF was feeding difficulties in 36 cases, recurrent metabolic decompensation in 11, and/or fasting intolerance in 8. In the GSD subgroup, the indication for ETF was fasting intolerance in 22 cases, feeding difficulties in three cases and recurrent decompensation with lactic acidosis in one case. In the FAOD subgroup, because all the patients were treated with ETF in order to avoid nocturnal fasting, indications for ETF were fasting intolerance in all, and feeding difficulties in 5. All nine patients with MSUD and 11 of the 12 patients with UCD were treated with ETF for feeding difficulties, while one patient with UCD suffered from recurrent metabolic decompensation. In one of the patients with MSUD, feeding difficulties led to recurrent decompensation (plasma leucine levels >5 mg/dL).
4.2.3. Durations and modalities of ETF
A total of 63 patients received ETF only via an NGT (64%), and 35 patients received ETF via a gastrostomy tube after an NGT (36%). The mean age at ETF initiation was 1.4 (SD: 3.1) years in the NGT-only subgroup and 0.6 (SD: 0.8) years in the gastrostomy subgroup. In the latter, gastrostomy was given to patients at a mean age of 2.4 (SD: 1.6) years.
As showed in Table 3, ETF was discontinued in 38 patients (OA: 12; FAODs: 8; GSDs: 8; UCDs: 5; MSUD: 5). At the end of the follow-up period, they were 15.8 (SD: 5.7) years old (range: 5.1–25.4, median [P25-P75]: 14.9 [11.1–21.8] years). The median [P25-P75] age in the disease subgroups MSUD, GSD, OA, FAOD and UCD was 22.3 [18.2–23.0], 15.3 [13.6–22.7], 14.9 [9.5–19.6], 13.6 [11.1–18.6], and 11.1 [10.2–16.9] years, respectively. The mean duration of ETF in these patients was 5.9 (SD: 4.8) years. The duration of ETF differed from one disease subgroup to another but the differences were only borderline (p = 0.051). The median [P25-P75] duration of ETF in the GSD subgroup, the OA, FAOD, MSUD and UCD patients, was 6.8 [4.9–13.6], 5.3 [3.0–9.1], 5.2 [1.8–7.6], 3.6 [2.7–4.2], and 0.9 [0.9–1.4] years, respectively.
Table 3.
Characteristics of enteral tube feeding according to treatment discontinuation (N = 98).
| Patients who discontinued enteral tube feeding (n = 38) |
Patients who did not discontinue enteral tube feeding (n = 60) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inherited metabolic disease | n | Age at last follow-up (y) | Age at initiation (y) | Age at discontinuation (y) | Duration (y) |
Indication |
n | Age at last follow-up (y) | Age at initiation (y) | Indication |
||||
| Feeding difficulties | Imbalance | Fasting intolerance | ||||||||||||
| Feeding difficulties | Imbalance | Fasting intolerance | ||||||||||||
| Organic aciduria | 12 | 14.9 [9.5–19.6] | 1.0 [1.0–3.7] | 8.2 [5.4–12.6] | 5.3 [3.0–9.1] | 11 | 3 | 3 | 28 | 12.1 [4.6–16.7] | 0.1 [0.1–0.5] | 25 | 8 | 5 |
| MA | 6 | 16.7 [9.0–20.6] | 1.4 [1.0–7.0] | 10.7 [7.1–13.3] | 6.1 [4.8–7.5] | 5 | 1 | 1 | 17 | 10.9 [3.6–12.6] | 0.1 [0.1–0.2] | 15 | 4 | 5 |
| PA | 6 | 13.6 [10.1–17.7] | 0.6 [0.1–2.4] | 5.9 [5.3–9.3] | 4.2 [3.0–9.1] | 6 | 2 | 2 | 10 | 17.2 [8.7–21.0] | 0.3 [0.1–1.1] | 9 | 4 | 0 |
| IVA | 0 | – | – | – | – | – | – | – | 1 | 14.4 [14.4–14.4] | 0.3 [0.3–0.3] | 1 | 0 | 0 |
| Urea cycle disorder | 5 | 11.1 [10.2–16.9] | 0.7 [0.7–0.7] | 2.1 [1.6–2.7] | 0.9 [0.9–1.4] | 4 | 1 | 0 | 7 | 8.1 [2.7–9.9] | 1.3 [1.0–2.6] | 7 | 0 | 0 |
| CPS | 0 | – | – | – | – | – | – | – | 1 | 3.1 [3.1–3.1] | 1.0 [−-] | 1 | 0 | 0 |
| OTC | 2 | 14.1 [5.1–23.2] | 1.2 [0.3–2.1] | 2.0 [1.3–2.7] | 0.8 [0.6–0.9] | 1 | 1 | 0 | 4 | 9.2 [5.5–11.4] | 1.6 [0.9–5.1] | 4 | 0 | 0 |
| ASS | 2 | 10.6 [10.2–11.1] | 0.7 [0.7–0.8] | 1.9 [1.6–2.1] | 1.2 [0.9–1.4] | 2 | 0 | 0 | 1 | 2.7 [2.7–2.7] | 1.2 [1.2–1.2] | 1 | 0 | 0 |
| ASL | 1 | 16.9 [16.9–16.9] | 0.7 [0.7–0.8] | 15.0 [15.0–15.0] | 14.3 [14.3–14.3] | 1 | 0 | 0 | 1 | 8.1 [8.1–8.1] | 2.7 [2.7–2.7] | 1 | 0 | 0 |
| Maple syrup urine disease | 5 | 22.3 [18.2–23.0] | 1.3 [1.1–1.7] | 4.0 [4.0–6.0] | 3.6 [2.7–4.2] | 5 | 0 | 0 | 4 | 8.8 [4.1–10.8] | 0.3 [0.1–2.0] | 4 | 1 | 0 |
| Glycogen storage disease | 8 | 15.3 [13.6–22.7] | 0.5 [0.1–0.9] | 7.6 [5.2–14.1] | 6.8 [4.9–13.6] | 1 | 0 | 8 | 15 | 6.5 [2.2–16.2] | 0.2 [0.1–1.0] | 2 | 1 | 14 |
| 0 | 3 | 15.9 [14.7–25.4] | 0.9 [0.6–0.9] | 7.2 [4.3–8.0] | 6.6 [3.4–7.1] | 0 | 0 | 3 | 0 | – | – | – | – | – |
| Ia | 2 | 18.2 [13.2–23.1] | 0.2 [0–0.4] | 12.5 [5.3–19.6] | 12.2 [4.9–19.6] | 0 | 0 | 2 | 8 | 7.2 [4.0–13.5] | 0.2 [0.1–0.6] | 1 | 0 | 8 |
| Ib | 1 | 22.4 [22.4–22.4] | 0.1 [0.1–0.1] | 19.8 [19.8–19.8] | 19.8 [19.8–19.8] | 1 | 0 | 1 | 7 | 6.5 [1.1–16.2] | 0.4 [0.1–1.4] | 1 | 1 | 6 |
| III | 2 | 12.0 [10.0–14.0] | 0.6 [0.2–1.1] | 6.8 [5.0–8.6] | 6.2 [4.8–7.5] | 0 | 0 | 2 | 0 | – | – | – | – | – |
| Fatty acid deficiency | 8 | 13.6 [11.1–18.6] | 0.8 [0.4–1.0] | 6.1 [2.9–8.1] | 5.2 [1.8–7.6] | 2 | 0 | 6 | 6 | 5.7 [5.1–7.6] | 0.1 [0.1–0.3] | 3 | 0 | 3 |
| CPT2 | 0 | – | – | – | – | – | – | – | 1 | 7.6 [−-] | 0.1 [−-] | 1 | 0 | 0 |
| VLCAD | 2 | 15.4 [8.0–22.9] | 0.9 [0.6–1.2] | 4.0 [3.3–4.6] | 3.0 [2.1–4.0] | 1 | 0 | 1 | 2 | 4.4 [3.3–5.5] | 0.1 [0.1–0.1] | 1 | 0 | 1 |
| LCHAD | 4 | 13.6 [12.1–18.1] | 0.4 [0.2–0.8] | 8.1 [7.6–9.8] | 7.6 [6.8–9.5] | 1 | 0 | 3 | 3 | 5.9 [5.1–7.6] | 0.3 [0.1–0.8] | 1 | 0 | 2 |
| MCAD | 2 | 13.1 [10.7–15.5] | 0.9 [0.9–1.0] | 2.3 [2.0–2.5] | 1.3 [1.1–1.5] | 0 | 0 | 2 | 0 | – | – | – | – | – |
| ALL PATIENTS | 38 | 14.9 [11.1–21.8] | 0.9 [0.4–1.2] | 6.1 [4.0–9.3] | 4.8 [2.7–7.5] | 23 | 4 | 17 | 60 | 8.2 [3.5–14.4] | 0.2 [0.1–1.0] | 41 | 10 | 22 |
Ages and durations are quoted as the median [25th and 75th percentiles], and indications are quoted as the number. Some patients had more than one indication.
Abbreviations: ASS, argininosuccinate synthetase deficiency; ASL, argininosuccinate lyase deficiency; MA, methylmalonic acidemia; PA, propionic acidemia; OTC, ornithine transcarbamylase deficiency; LCHAD: Long-chain 3-hydroxyacyl-CoA dehydrogenase, VLCAD: Very long-chain acyl-CoA dehydrogenase, CPT2: Carnitine palmitoyltransferase II, MCAD: Medium-chain acyl-CoA dehydrogenase, SCAD: Short-chain acyl-CoA dehydrogenase deficiencies).
Patients who continued to receive ETF (n = 60) were 9.4 (SD: 6.5) years old at the end of the follow-up period. According to the disease type, the median [P25-P75] age at the end of the follow-up period was 12.1 [4.6–16.7], 8.8 [4.1–10.8], 8.1 [2.7–9.9], 6.5 [2.2–16.2], and 5.7 [5.1–7.6] years in the OA, MSUD, UCD, GSD, and FAOD subgroup, respectively. Weaning off ETF failed in 13 patients (7 patients with OA, 1 with UCD, and 5 with GSDs). The reasons were refusal by the patient in 2 cases (a child with GSD Ib and a child with GSD Ia), metabolic decompensations in 7 children with OA, fasting intolerance in 1 child with GSD Ib, feeding difficulties with failure to thrive for one patient with UCD and one patient with GSD Ia, and gastrointestinal symptoms in one GSD Ib patient.
Importantly, no difference in ETF practices (none, NGT only or gastrostomy tube after NGT) was observed over time (p = 0.447, Table 4), and disease-by-disease (all P-values >0.05, Table 5). With regard to changes in practice among the patients receiving NGT alone or NGT then gastrostomy, a gastrostomy tube was implemented in 7 of the 29 ETF patients (24%) born before 2002 (accounting for 12% of all patients born before 2002). Gastrostomy was implemented in 15 of the 41 ETF patients (37%) born between 2002 and 2009 (accounting for 20% of all patients born during this period), and 13 of the 28 ETF patients (46%) born after 2009 (accounting for 22% of all patients born after 2009). Gastrostomy was significantly performed earlier in life after 2009 than before 2009 (at a median age of 1.4 years versus 2.8 years, p = 0.002).
Table 4.
Enteral tube feeding (ETF) practices (none, nasogastric tube [NGT] only or gastrostomy tube after NGT) over time (N = 190).
| n (%) | No ETF (N = 92) | NGT only (N = 63) | Gastrostomy (N = 35) | P-value |
|---|---|---|---|---|
| Before 2002 | 29 (50.0) | 22 (37.9) | 7 (12.1) | 0.447 |
| 2002–2009 | 33 (44.5) | 26 (35.2) | 15 (20.3) | |
| After 2009 | 30 (51.7) | 15 (25.9) | 13 (22.4) |
P-value of Chi-2 test.
Table 5.
Enteral tube feeding (ETF) practices (none, nasogastric tube [NGT] only or gastrostomy tube after NGT) before and after 2009, by the type of inherited disease (N = 190).
| n (%) | No ETF (N = 92) | NGT only (N = 63) | Gastrostomy (N = 35) | P-value |
|---|---|---|---|---|
| Organic aciduria | ||||
| Before 2009 | 15 (33.3) | 20 (44.5) | 10 (22.2) | 0.930 |
| After 2009 | 5 (33.3) | 6 (40.0) | 4 (26.7) | |
| Urea cycle disorder | ||||
| Before 2009 | 30 (79.0) | 5 (13.2) | 3 (7.8) | 0.458 |
| After 2009 | 13 (76.5) | 1 (5.8) | 3 (17.7) | |
| Maple syrup urine disease | ||||
| Before 2009 | 14 (66.7) | 5 (23.8) | 2 (9.5) | 0.840 |
| After 2009 | 9 (81.8) | 2 (18.2) | 0 | |
| Glycogen storage disease | ||||
| Before 2009 | 2 (11.8) | 11 (64.7) | 4 (23.5) | 0.704 |
| After 2009 | 1 (11.2) | 4 (44.4) | 4 (44.4) | |
| Fatty acid oxidation deficiency | ||||
| Before 2009 | 1 (9.1) | 7 (63.6) | 3 (27.3) | 0.440 |
| After 2009 | 2 (33.4) | 2 (33.3) | 2 (33.3) | |
| All patients | ||||
| Before 2009 | 62 (47.0) | 48 (36.4) | 22 (16.6) | 0.325 |
| After 2009 | 30 (51.7) | 15 (25.9) | 13 (22.4) |
P-value of Chi-2 test (for all patients) or Fisher's exact test (for each disease).
4.2.4. Oral feeding
Oral feeding difficulties (e.g. pre-existing eating disorders) became less severe in 39 of the 98 patients receiving ETF, neither more severe or less severe in 59 patients (OA: 30; FAODs: 5; UCDs: 8; GSDs: 8; MSUD: 8), and more severe in 7 patients (following gastrostomy in all cases; FAOD: 3, GSD: 2, OA: 1; MSUD: 1).
4.2.5. Complications of ETF
Overall, ETF was well supported and considered as an alleviation when the indications were feeding difficulties, metabolic decompensations and fasting intolerance.
However, forty-seven patients of the ETF group (48%) experienced one or more complications. These concerned the NGT in 42 patients (43%) and the gastrostomy tube in 13 patients (37%).
The NGT-related complications included (i) medical problems such as vomiting (n = 9), recurrent ear, nose and throat infections (n = 5), cough (n = 3), vasovagal syncope (n = 1), and skin lesions related to NGT fixation (n = 1); (ii) difficulties inserting the tube (n = 28), frequent removal of the NGT (n = 8), rejection of NGT leading to an indication for gastrostomy (n = 1); and (iii) complications related to pump or tubing failures (n = 4, including two cases of severe hypoglycemia with seizure and neurological sequelae in 2 patients with GSDs).
The gastrostomy-related complications were worsening of feeding difficulties (according to the parents; n = 7), skin lesions (n = 2), local discomfort and esthetic issues (n = 2), leakage (n = 1), vomiting (n = 1), and frequent removal of the gastrostomy button (n = 1).
4.2.6. Psychomotor development and ETF
Out of the 52 patients with delayed psychomotor development, 35 patients (67%) received ETF and 17 (33%) did not. Conversely, in the 138 patients (73%) with normal psychomotor development, 63 patients (46%) received ETF and 75 patients (54%) did not. This could suggest a possible negative impact of ETF on psychomotor development (p = 0.008), however delayed psychomotor development is probably, above all, related to the disease and its complications.
5. Discussion
Diet is crucial to the management of many IMDs. In amino- and organic-acid-related disorders, the main objective of ETF, when proposed, is to prevent the accumulation of toxic products upstream of the enzyme deficiency by limiting protein intake and promoting anabolism through sufficient energy inputs [6,7,14,23,24]. In OA, ETF also limits nocturnal lipolysis. In energy disorders, ETF limits fasting and thus prevents hypoglycemia and/or decompensation - notably in GSD I/III and FAOD [[9], [10], [11], [12], [13],26]. The present study is the first devoted to describe the use of ETF and its modalities in a large cohort of patients with a variety of diet-dependent IMDs, followed in a reference center. Importantly, the aim of this work was not to define the indications for ETF, which are given by collective guidelines or recommendations. To the best of our knowledge, our follow-up is the longest yet for this type of study. At the end of the follow-up (August 2017), our patients were 11.9 (SD: 6.9) years old on average, whereas the patients in Evan's studies in the UK were 4.1 years old in 2007 [18], 7.5 years old in 2011 [19], and 5.3 years old in 2012 [20].
Half of our IMD patients diagnosed in the first year of life received ETF. This corresponded to 89% of the patients in the GSD subgroup, 82% in the FAOD subgroup, 67% in the OA subgroup, 28% in the MSUD subgroup, and 22% in the UCD subgroup. Relative to the literature data, the prevalence of ETF in our cohort was higher for most of the studied IMDs. In a UK cohort published in 2012, 25% of the patients with UCD received ETF [24]. Similarly, Pinto et al. found that 8 of their 133 patients with IVA required ETF (6%) [23]; this proportion was 7% (1 out of 14) in our study.
In our cohort, the indications for ETF were feeding difficulties in 65% of the patients (90% for OA, 92% for UCDs, 100% for MSUD, 13% for GSDs and 36% for FAODs), cessation of fasting in 40% (64% for FAODs and 96% for GSDs), and recurrent metabolic decompensation in 14%.
In agreement with previous reports [6,24,27,28], we observed severe feeding difficulties in children with protein restrictions. Evans et al. compared feeding behaviors in 20 children with an IMD and 15 healthy children. Their results show that the IMD had a higher incidence of eating disorders such as low appetite, limited food variety, and negative eating behavior with a tendency to vomit frequently [28]. These may well have been preexisting conditions [27] that worsened after the diagnosis by the restrictive diet, with low intake and marked food selection. Difficulties in oral feeding have also been reported in the literature for patients with FAOD (particularly those with LCHAD deficiency) [29] and those with GSDs [30]. In both cases, these difficulties appear to be inherently linked to the pathology. In a UK study of 90 hospital admissions for decompensation of a UCD with confirmed precipitating factors, Gardeitchik et al. attributed the decompensation to an insufficient calorie intake in 9 cases, and to an intercurrent infection in 76 (resulting in catabolism per se but also in a greatly diminished food intake) [27].
Technical progress led to the development of percutaneous endoscopic gastrostomy techniques, along with a shift in care provision from acute settings to community settings. This resulted in a worldwide increase in the prevalence of home enteral and parenteral nutrition programs for children as well as for adults. Home ETF has been available for more than 40 years to treat adults and for around 30 years for children, and is associated with improved quality of life 9/30/2020 6:47:00 PM. This change in practice was widely adopted, regardless of diseases (including IMDs). The indication for ETF in IMDs has not changed since the early 2000s. In our study, we did not observe any difference in the proportion of patients with ETF when comparing those born before 2002 and those born afterwards. However, the age at ETF initiation decreased over time (1.9 year before 2002 and 0.7 years afterwards). This reflects the current and systematic use of ETF in some diseases – at least for nighttime feeding [28]., especially in the context of MA, PA [18] and FAODs [20]. Regardless of the indication or underlying disease, gastrostomy was performed increasingly earlier in life. The current guidelines recommend a gastrostomy tube as a safe and simple device for long-term ETF (i.e. for more than 3 months) [22,33,34]. Indeed, the proportion of patients having undergone gastrostomy was 24% before 2002 and 41% afterwards. This is consistent with the literature data [33]. However, we observed that gastrostomy could be refused by patients when it was proposed to them in their teenage years. Thus, four teenagers in our cohort refused to switch to gastrostomy because they inserted the tube themselves in the evening and therefore did not suffer from any visible disease stigma during daytime. In order to relieve local discomfort and esthetic issues, one teenager even decided to remove his gastrostomy port. Therefore, in our cohort, the teenagers had been using an NGT for many years and were able to insert and use it by themselves. Although a comparison of the quality of life in patients with NGT vs. gastrostomy tube might be of value, we considered that such a study would be outdated and against to current recommendations (i.e. favouring gastrostomy when ETF has to be prolonged for more than 3 months). According to the literature [35], parents and caregivers have a positive perception of ETF via a gastrostomy port.
It is important to assess and prevent the complications associated with NGTs and gastrostomy tubes, including psychological consequences related to esthetic issues. About half of our patients with ETF experienced complications related to NGT and/or gastrostomy. These complications, which are more acute in the NGT subgroup than in the gastrostomy subgroup, have had a huge impact on the children's and family's quality of life. In 2007, Evans et al. highlighted the fact that both NGTs and gastrostomy tubes were associated with complications in everyday life for the families of children receiving ETF [18]. In their publication, they show that out of the 34 families interviewed, all reported poor sleep, 50% reported pump dysfunctions, and 45% reported tube blockages [18]. Moreover, Colomb et al. reported that the main complications of ETF were vomiting (as in our study) and diarrhea [34]. The incidence of digestive complications may have been underestimated in our study because this type of complications may have been present even before the initiation (i.e. as an oral feeding disorder) or may have been related to decompensation (especially in young children with concomitant viral infections) [36]. The NGT-related complications were mainly related to introduction of the tube, which can be difficult and/or painful. As mentioned above, these difficulties decreased with age. Many children started to insert the NGT themselves as they grew up. In agreement with the literature [15,18,33,34,37], technical complications (such as tube obstruction, disconnection or pump dysfunction) with potentially serious consequences were observed in 14% of our patients receiving ETF, including frequent removal and technical errors in 10% of the patients. Metabolic complications caused by the abrupt discontinuation of ETF, such as hypoglycemia, were observed in four patients with GSD. This led to neurological sequelae in two of the four patients. Although respiratory complications, mainly related to a poorly positioned NGT, have been reported in the literature, they were not observed in our cohort. On the other hand, we detected recurrent ENT infections (Ear Nose and Throat infections) in 12% of patients with an NGT. Again, these complications may have been underestimated because infections can be attributed to usual childhood infections [34]. Gastrostomy seems to be an efficient, safe technique - even in young children. Complications of gastrostomy were reported in 44% of cases, mainly buried bumper syndrome, but also encountered technical problems [38]. In our cohort, technical problems affecting the gastrostomy device were observed in 4 cases (accounting for 31% of the gastrostomy-related complications). The other observed complications were related to feeding (with the worsening of feeding difficulties) and surgery (including buried bumper syndrome and reflux).
Overall, the complications associated with ETF point to the importance of providing patients with the necessary information and training on inserting the NGT (which remains a potentially dangerous procedure) and handling gastrostomy ports and tubes. Many researchers have mentioned the need of providing families of children receiving ETF with training programs. In 2012, for example, Evans et al. questioned 32 parents of children with IMDs about their knowledge of ETF [20]. They found that the quality of implementation (concerning the preparation of solutes, hygiene, technical checks, and treatment administration) decreased over time, especially after the first three years of ETF. It is therefore imperative to insist on the importance of providing parents and patients with a continuous education program to ensure optimal safety during the use of EFT [[18], [19], [20], [21],39].
Interestingly, several of the parents in our study reported a decrease in oral feeding and total feeding difficulties after gastrostomy. This can be due to the fact that after gastrostomy the child no longer had the need of eating. However, the parents also reported an improvement in their quality of life following gastrostomy – mainly among those who had difficulties with tube insertion.
Despite the information obtained over the years of our study, to interpret the relationship between psychomotor development and ETF was not straightforward. We found that the proportion of children with normal schooling was higher among the non-ETF group (76%) than among the ETF group (54%). Moreover, the proportion of children in specialist institutions was higher among patients receiving ETF. However, this approach has an important confounding factor that might relate developmental delay to the underlying disease, rather than to the disease's nutritional impact.
6. Conclusion
Approximately half of the patients in our cohort were treated with long-term ETF - emphasizing the major role of ETF in the dietary management of IMDs. The indications for ETF (i.e. fasting intolerance, feeding difficulties or metabolic decompensation) were found to vary depending on the disease. The duration of ETF was found to be relatively long, regardless of the indication. Our analysis highlights the difficulty of weaning ETF. Gastrostomy is now routinely proposed by physicians as an alternative to NGT when this is indicated, and is rarely declined by new patients.
Acknowledgments
Acknowledgements
The authors thank Rosalba Montealegre for her careful proofreading and the precious help given to this work.
Details of the contributions of individual authors
CM Bérat: Conceptualization, investigations, patient management, writing manuscript;
Roda: Conceptualization, statistical analysis, writing manuscript;
Patient management: Brassier, Bouchereau, Wicker, Servais, Dubois, Assoun, Belloche, Barbier, Leboeuf, Touati, Talbotec, Campeotto.
Biological investigations: Petit, Gaignard, Lebigot, Pontoizeau, Ottolenghi.
PJ Bérat: investigations, statistical analysis.
Arnoux: patient management, writing manuscript.
De Lonlay: Conceptualization, investigations, patient management, writing manuscript, Supervision, Funding Acquisition, Guarantor.
References
- 1.Sanderson S., Green A., Preece M.A., Burton H. The incidence of inherited metabolic disorders in the West Midlands, UK. Arch. Dis. Child. 2006;91(11):896–899. doi: 10.1136/adc.2005.091637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dionisi-Vici C., Rizzo C., Burlina A.B. Inborn errors of metabolism in the Italian pediatric population: a national retrospective survey. J. Pediatr. 2002;140(3):321–329. doi: 10.1067/mpd.2002.122394. [DOI] [PubMed] [Google Scholar]
- 3.European Registry and Network for Introduction Type Metabolic Diseases (E-IMD). Isovaleric Acidemia: Quick Reference Guide. 2014. http://www.e-imd.org/rc/e-imd/htm/Article/2014/e-imd-20140716-085102-695/src/htm_fullText/en/IVA%20guideline_Quick%20reference%20guide_Ensenauer_201408.pdf Published online.
- 4.Couce M.L., Ramos F., Bueno M.A. Evolution of maple syrup urine disease in patients diagnosed by newborn screening versus late diagnosis. Eur. J. Paediatr. Neurol. 2015;19(6):652–659. doi: 10.1016/j.ejpn.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Servais A., Arnoux J.B., Lamy C. Treatment of acute decompensation of maple syrup urine disease in adult patients with a new parenteral amino-acid mixture. J. Inherit. Metab. Dis. 2013;36(6):939–944. doi: 10.1007/s10545-012-9570-2. [DOI] [PubMed] [Google Scholar]
- 6.Boneh A. Dietary protein in urea cycle defects: how much? Which? How? Mol. Genet. Metab. 2014;113(1–2):109–112. doi: 10.1016/j.ymgme.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Adam S., Almeida M.F., Assoun M. Dietary management of urea cycle disorders: European practice. Mol. Genet. Metab. 2013;110(4):439–445. doi: 10.1016/j.ymgme.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Singh R.H. Nutritional management of patients with urea cycle disorders. J. Inherit. Metab. Dis. 2007;30(6):880–887. doi: 10.1007/s10545-007-0718-4. [DOI] [PubMed] [Google Scholar]
- 9.Heller S., Worona L., Consuelo A. Nutritional therapy for glycogen storage diseases. J. Pediatr. Gastroenterol. Nutr. 2008;47:S15. doi: 10.1097/MPG.0b013e3181818ea5. [DOI] [PubMed] [Google Scholar]
- 10.Derks T.G.J., Martens D.H., Sentner C.P. Dietary treatment of glycogen storage disease type Ia: uncooked cornstarch and/or continuous nocturnal gastric drip-feeding? Mol. Genet. Metab. 2013;109(1):1–2. doi: 10.1016/j.ymgme.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Sentner C.P., Hoogeveen I.J., Weinstein D.A. Glycogen storage disease type III: diagnosis, genotype, management, clinical course and outcome. J. Inherit. Metab. Dis. 2016;39(5):697–704. doi: 10.1007/s10545-016-9932-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karall D., Brunner-Krainz M., Kogelnig K. Clinical outcome, biochemical and therapeutic follow-up in 14 Austrian patients with long-chain 3-Hydroxy acyl CoA dehydrogenase deficiency (LCHADD) Orphanet J Rare Dis. 2015;10(1):21. doi: 10.1186/s13023-015-0236-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilcken B. Fatty acid oxidation disorders: outcome and long-term prognosis. J. Inherit. Metab. Dis. 2010;33(5):501–506. doi: 10.1007/s10545-009-9001-1. [DOI] [PubMed] [Google Scholar]
- 14.Baumgartner M.R., Hörster F., Dionisi-Vici C. Proposed guidelines for the diagnosis and management of methylmalonic and propionic acidemia. Orphanet J Rare Dis. 2014;9(1) doi: 10.1186/s13023-014-0130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colomb V., Goulet O., Ricour C. Home enteral and parenteral nutrition in children. Baillieres Clin Gastroenterol. 1998;12(4):877–894. doi: 10.1016/s0950-3528(98)90012-4. [DOI] [PubMed] [Google Scholar]
- 16.Daveluy W., Guimber D., Mention K. Home enteral nutrition in children: an 11-year experience with 416 patients. Clin. Nutr. 2005;24(1):48–54. doi: 10.1016/j.clnu.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Holden C.E., Puntis J.W., Charlton C.P., Booth I.W. Nasogastric feeding at home: acceptability and safety. Arch. Dis. Child. 1991;66(1):148–151. doi: 10.1136/adc.66.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans S., MacDonald A., Daly A., Hopkins V., Holden C. Home enteral tube feeding in patients with inherited metabolic disorders: safety issues. J Hum Nutr Diet Off J Br Diet Assoc. 2007;20(5):440–445. doi: 10.1111/j.1365-277X.2007.00816.x. [DOI] [PubMed] [Google Scholar]
- 19.Evans S., Preston F., Daly A., Neville C., MacDonald A. Accuracy of home enteral feed preparation for children with inherited metabolic disorders. J Hum Nutr Diet Off J Br Diet Assoc. 2011;24(1):68–73. doi: 10.1111/j.1365-277X.2010.01132.x. [DOI] [PubMed] [Google Scholar]
- 20.Evans S., Preston F., Daly A., Ashmore C., Holden C., MacDonald A. Home enteral tube feeding in children with inherited metabolic disorders: a review of long-term carer knowledge and technique. J Hum Nutr Diet Off J Br Diet Assoc. 2012;25(6):520–525. doi: 10.1111/j.1365-277X.2012.01274.x. [DOI] [PubMed] [Google Scholar]
- 21.Evans S., Shelton F., Holden C., Daly A., Hopkins V., MacDonald A. Monitoring of home safety issues in children on enteral feeds with inherited metabolic disorders. Arch. Dis. Child. 2010;95(9):668–672. doi: 10.1136/adc.2008.148338. [DOI] [PubMed] [Google Scholar]
- 22.FröHlich T., Richter M., Carbon R., Barth B., KöHler H. Review article: percutanous endoscopic gastrostomy in infants and children. Aliment Pharmacol Ther. Published online January. 2010 doi: 10.1111/j.1365-2036.2010.04246.x. [DOI] [PubMed] [Google Scholar]
- 23.Pinto A., Daly A., Evans S. Dietary practices in isovaleric acidemia: a European survey. Mol Genet Metab Rep. 2017;12:16–22. doi: 10.1016/j.ymgmr.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adam S., Champion H., Daly A. Dietary management of urea cycle disorders: UK practice. J Hum Nutr Diet Off J Br Diet Assoc. 2012;25(4):398–404. doi: 10.1111/j.1365-277X.2012.01259.x. [DOI] [PubMed] [Google Scholar]
- 25.Rake J., Visser G., Labrune P., Leonard J., Ullrich K., Smit P. Glycogen storage disease type I: diagnosis, management, clinical course and outcome. Results of the European study on glycogen storage disease type I (ESGSD I) Eur. J. Pediatr. 2002;161(0):S20–S34. doi: 10.1007/s00431-002-0999-4. [DOI] [PubMed] [Google Scholar]
- 26.Hochuli M., Christ E., Meienberg F., Lehmann R., Krützfeldt J., Baumgartner M.R. Alternative nighttime nutrition regimens in glycogen storage disease type I: a controlled crossover study. J. Inherit. Metab. Dis. 2015;38(6):1093–1098. doi: 10.1007/s10545-015-9864-2. [DOI] [PubMed] [Google Scholar]
- 27.Gardeitchik T., Humphrey M., Nation J., Boneh A. Early clinical manifestations and eating patterns in patients with urea cycle disorders. J. Pediatr. 2012;161(2):328–332. doi: 10.1016/j.jpeds.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Evans S., Alroqaiba N., Daly A., Neville C., Davies P., Macdonald A. Feeding difficulties in children with inherited metabolic disorders: a pilot study. J Hum Nutr Diet Off J Br Diet Assoc. 2012;25(3):209–216. doi: 10.1111/j.1365-277X.2012.01229.x. [DOI] [PubMed] [Google Scholar]
- 29.Lund A.M., Leonard J.V. Feeding difficulties in long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency. Arch. Dis. Child. 2001;85(6):487–488. doi: 10.1136/adc.85.6.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinez C.C., Tonon T., Nalin T., Refosco L.F., de Souza C.F.M., Schwartz I.V.D. Feeding difficulties and Orofacial Myofunctional disorder in patients with hepatic glycogen storage diseases. JIMD Rep. 2018;45:21–27. doi: 10.1007/8904_2018_131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daveluy W., Guimber D., Mention K. Home enteral nutrition in children: an 11-year experience with 416 patients. Clin Nutr Edinb Scotl. 2005;24(1):48–54. doi: 10.1016/j.clnu.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Colomb V. Nutrition entérale chez l'enfant. /data/revues/00079960/00370005/344/. Published online February 16, 2008. Accessed February 6, 2019. https://www.em-consulte.com/en/article/78965.
- 35.Brotherton A., Abbott J., Hurley M., Aggett P.J. Home enteral tube feeding in children following percutaneous endoscopic gastrostomy: perceptions of parents, paediatric dietitians and paediatric nurses. J Hum Nutr Diet Off J Br Diet Assoc. 2007;20(5):431–439. doi: 10.1111/j.1365-277X.2007.00811.x. [DOI] [PubMed] [Google Scholar]
- 36.Holden C.E., Puntis J.W., Charlton C.P., Booth I.W. Nasogastric feeding at home: acceptability and safety. Arch. Dis. Child. 1991;66(1):148–151. doi: 10.1136/adc.66.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Evans S., Holden C., MacDonald A. Home enteral feeding audit 1 year post-initiation. J. Hum. Nutr. Diet. 2006;19(1):27–29. doi: 10.1111/j.1365-277X.2006.00662.x. [DOI] [PubMed] [Google Scholar]
- 38.Fröhlich T., Richter M., Carbon R., Barth B., Köhler H. Review article: percutaneous endoscopic gastrostomy in infants and children. Aliment. Pharmacol. Ther. 2010;31(8):788–801. doi: 10.1111/j.1365-2036.2010.04246.x. [DOI] [PubMed] [Google Scholar]
- 39.Siddiq S., Wilson B.J., Graham I.D. Experiences of caregivers of children with inherited metabolic diseases: a qualitative study. Orphanet J Rare Dis. 2016;11(1):168. doi: 10.1186/s13023-016-0548-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further Reading
- 31.Ojo O. The challenges of home enteral tube feeding: a global perspective. Nutrients. 2015;7(4):2524–2538. doi: 10.3390/nu7042524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hebuterne X., Bozzetti F., Moreno Villares J.M. Home enteral nutrition in adults: a European multicentre survey. Clin Nutr Edinb Scotl. 2003;22(3):261–266. doi: 10.1016/s0261-5614(03)00005-0. [DOI] [PubMed] [Google Scholar]