Highlights
-
•
Controls and binge drinkers (BDs) do not differ in their behavioral performance.
-
•
BDs show increased neural activity during attention, working memory and inhibition.
-
•
Augmented P3 amplitude in BDs was the most solid electrophysiological finding.
-
•
Evidence does not support specific gender vulnerabilities to the effects of BD.
-
•
Memory, emotional processing and decision-making processes need further exploration.
Keywords: Binge drinking, Electroencephalography, EEG, Adolescents, Young adults, Event-related potentials, Systematic review
Abstract
Research on neurophysiological impairments associated with binge drinking (BD), an excessive but episodic alcohol use pattern, has significantly increased over the last decade. This work is the first to systematically review –following PRISMA guidelines- the empirical evidence regarding the effects of BD on neural activity –assessed by electroencephalography- of adolescents and young adults. A systematic review was conducted in 34 studies (N = 1723). Results indicated that binge drinkers (BDs) showed similar behavioral performance as non/low drinkers. The most solid electrophysiological finding was an augmented P3 amplitude during attention, working memory and inhibition tasks. This increased neural activity suggests the recruitment of additional resources to perform the task at adequate/successful levels, which supports the neurocompensation hypothesis. Similar to alcoholics, BDs also displayed increased reactivity to alcohol-related cues, augmented resting-state electrophysiological signal and reduced activity during error detection –which gives support to the continuum hypothesis. Evidence does not seem to support greater vulnerability to BD in females. Replication and longitudinal studies are required to account for mixed results and to elucidate the extent/direction of the neural impairments associated with BD.
1. Introduction
Alcohol use has an important social component among young people, since it is an essential part of academic traditions and parties (Dormal et al., 2019, Patrick et al., 2016). The excessive alcohol consumption that frequently occurs in these contexts is often associated with major social and health consequences, such as poor academic performance, motor vehicle accidents, sexual assault, liver and heart damage, and ultimately, death (Eurobarometer, 2010, National Institute of Alcohol Abuse and Alcoholism, 2020). As such, alcohol misuse has been associated with more than 30% of deaths among American and European males aged between 15 and 29 years old (World Health Organization, 2011).
The detrimental effects of alcohol use on the brain have been broadly documented (Bernardin et al., 2014, Rangaswamy and Porjesz, 2014, Voon et al., 2020). Despite the focus has mainly been on alcohol dependence, the last decade has seen a significant increase in the studies concerning binge drinking (BD) (López-Caneda et al., 2019a). This pattern is commonly defined as the consumption of four (or more) drinks for women and five (or more) drinks for men in two hours, which results in a blood alcohol concentration of 0.08 g/dl or above (National Institute of Alcohol Abuse and Alcoholism, 2004). According to recent surveys from European and American national health agencies, BD is highly prevalent among adolescents and young adults, with around 35–40% of college students reporting at least one BD episode in the last month (Kraus et al., 2016, Substance Abuse and Mental Health Services Administration (SAMHSA), 2018).
These data become even more worrying when considering the special vulnerability of adolescence and youth to the neurotoxic effects of alcohol, particularly due to the undergoing structural and functional brain changes at this stage (Bava and Tapert, 2010, Jones et al., 2018). This developmental window is characterized by the maturation and refinement of several cognitive functions, especially higher order executive processes including cognitive flexibility, working memory and inhibitory control, which are mainly linked to the maturation of frontal areas (Boelema et al., 2014, Crone and Ridderinkhof, 2011, Luna et al., 2015).
Perhaps partially related to this increased vulnerability, BD during adolescence and youth has been associated with impaired cognitive performance, alterations in brain structure, and neurofunctional abnormalities (Carbia et al., 2018, Cservenka and Brumback, 2017, Jones et al., 2018, Lannoy et al., 2019, Lees et al., 2019, Petit et al., 2014a). In this sense, neuropsychological studies have reported that BD is mainly related to deficits in verbal memory and executive functions, particularly poor inhibitory control (Carbia et al., 2018). In addition, evidence from neuroimaging studies showed disruptions –reductions and/or increases- in the prefrontal cortex and subcortical structures (Kvamme et al., 2015, Sousa et al., 2020, Squeglia et al., 2015, Doallo et al., 2014, Howell et al., 2013, Morris et al., 2018). At the functional level, neuroimaging data revealed impaired –frequently increased- neural activity during attentional, working memory and response inhibition tasks (Lees et al., 2019, Lannoy et al., 2019).
Another concerning aspect regarding this population is the possibility that BD and alcohol-dependence constitute two stages of the same phenomenon, a postulate known as the continuum hypothesis (Enoch, 2006, Parsons, 1998). This hypothesis has been supported by evidence showing that BDs exhibit impairments similar to those observed in alcoholics (Crego et al., 2010, López-Caneda et al., 2017b, Maurage et al., 2009, Petit et al., 2014a, Sanhueza et al., 2011), and that the engagement in this pattern during adolescence may constitute a first step towards the development of alcohol abuse during adulthood (Bonomo et al., 2004, McCambridge et al., 2011, McCarty et al., 2004). However, the validity of this proposal is still to be tested as, to the best of our knowledge, no neurophysiological study has directly explored the evolution –and its derivative effects- from BD to alcohol-dependence (Lannoy et al., 2014).
In the research on the potential cerebral effects of BD, the electroencephalography (EEG) –i.e., the study of the brain electrical activity by electrodes placed at the scalp- has gained considerable importance over the last decade. Contrarily to other imaging techniques, which have a coarse temporal resolution, EEG allows to explore the brain activity in the order of milliseconds, thus providing an optimal way for studying the neural dynamics that underlie the numerous cognitive stages occurring between a stimulus and a response (Campanella, 2013, Luck, 2014). The signal obtained can be analyzed in the time domain –e.g. event-related brain potentials (ERPs)-, the frequency domain -e.g. spectral power- or both –i.e. the time–frequency domain. Importantly, these measures have proven to be highly sensitive to the acute and chronic effects of alcohol (Brion et al., 2016, Kamarajan, 2019, Rangaswamy et al., 2007). Thus, given the subclinical nature of the BD pattern, EEG has emerged as a valuable approach for disentangling potential anomalies not observable at the behavioral level as well as for determining possible neural markers of risk for alcohol abuse.
Nevertheless, despite the importance of EEG for detecting underlying neural impairments associated with BD, the growing literature examining the electrophysiological impairments linked to this pattern and the previous efforts in providing an overview of the BD’s EEG profile (Lees et al., 2019, Lannoy et al., 2019), no study to date has systematically reviewed the existing research on this topic. Thus, the main objective of the present study is to provide a qualitative synthesis of the available empirical evidence on the effects of BD in the brain electrical activity of adolescents and young adults. Additionally, we will discuss the general strengths and limitations of these studies and recommend areas of interest for future research.
2. Methods
2.1. Search strategy and article selection
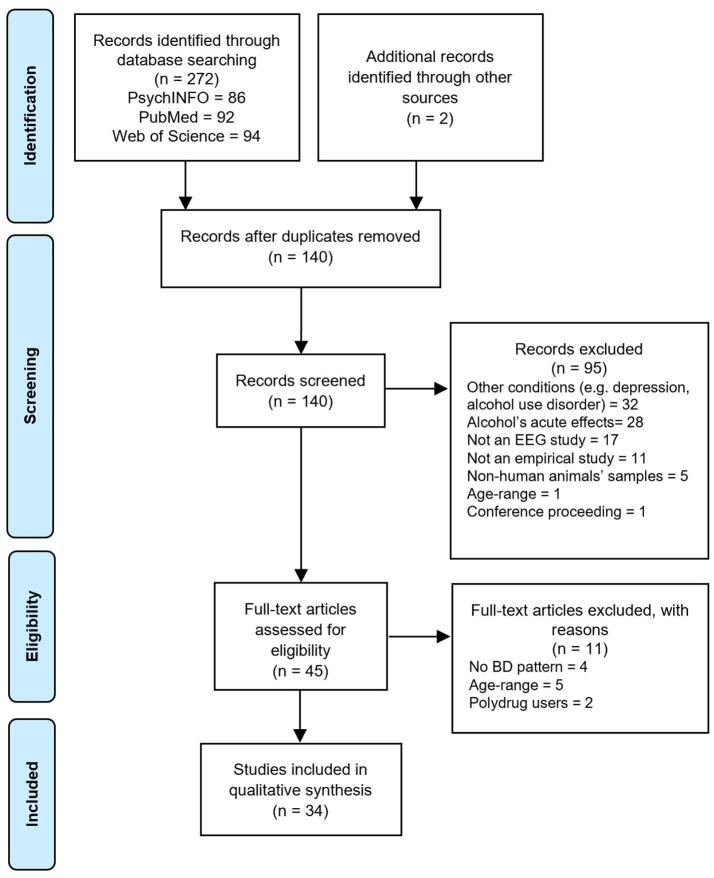
The present systematic review follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher et al., 2015), and the protocol was registered at PROSPERO International Prospective Register of Systematic Reviews of the University of York (registration number CRD42019118301). The literature review was conducted using PsycINFO, Web of Science, and PubMed databases. Articles were retrieved using the key terms: (binge/heavy/college drinking, binge/heavy/college/social drinkers, heavy episodic drinking, adolescen*, youth*, teen*, young, young adults and college/university students) and (ERP/ERPs, event-related, evoked potentials/evoked potential, electroencephalograph*, EEG and time–frequency). As additional inclusion criteria were defined: human observational studies, published in English between 2000 and 2020 (i.e. 1 January 2000–1 July 2020). Article’s search, screening, eligibility and inclusion, was independently conducted by two of the authors using the Covidence systematic review software (Veritas Health Innovation, 2016). Authors resolved disagreements through discussion and consensus, and any remaining divergence (e.g. when one of the authors did not consider an inclusion/exclusion criteria) was resolved by a third author. Fig. 1 represents the PRISMA flow diagram displaying the number of studies included at each phase of the selection process, and the reasoning for inclusion/exclusion.
Fig. 1.
PRISMA flow diagram of the study selection process.
The eligibility criteria are summarized in Table 1. Participants’ age ranged from 12 to 30, namely from early adolescence to the end of young adulthood (Blakemore, 2012, Fuhrmann et al., 2015, Schulenberg et al., 2019). While variations in the definition of BD might exist (e.g. regarding frequency), we followed the standard definition of the NIAAA, which does not include a specific criterion regarding frequency (National Institute of Alcohol Abuse and Alcoholism, 2004). We excluded studies using other functional techniques such as functional magnetic resonance imaging (fMRI) or Magnetoencephalography (MEG). Additionally, we did not include studies whose main aim was to examine the relationship between psychiatric conditions (e.g. depression, anxiety) and the BD pattern or studies exploring other forms of alcohol consumption (e.g. acute ethanol effects).
Table 1.
Inclusion/Exclusion Criteria.
| 1. Articles in English |
| 2. Peer-reviewed journals indexed in Journal Citation Reports (JCR) |
| 3. Published since January 2000 |
| 4. Human empirical studies |
| 5. Participants aged between 12 and 30 years old |
| 6. Participants must have a BD pattern as defined by NIAAA (2004), i.e. ≥4 (females) or 5 (males) drinks in two hours. |
| 7. Healthy young people without an alcohol use disorder diagnosis or any substance use disorder |
| 8. Participants must not be polydrug users, apart from tobacco and non-regular cannabis use |
| 9. Healthy young people without history of psychiatric disorders (e.g. schizophrenia, depression) |
| 10. Studies aimed at determining the neurofunctional impairments associated with BD assessed by EEG |
Note. BD: Binge Drinking; EEG: Electroencephalography.
The electrophysiological studies included in the present review are summarized in Table 2. The Cochrane-recommended National Heart, Lung, and Blood Institute (NHLBI) Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies was used to assess the methodological quality of the studies (Heart and Lung, and Blood Institute (NHLBI), 2014) (for detailed information on assessment procedures see Table 3). Questions 5 and 14 of the NHLBI were adapted in order to better capture the strengths and weaknesses of the EEG studies (see supplementary Table S1 for further details). There was a high total agreement (31/34 = 91.2%) between raters in the assessment of the studies. The inter-rater reliability, measured using Kappa coefficient of Cohen, was strong (K = 0.85) (McHugh, 2012).
Table 2.
Summary of electrophysiological studies with adolescents and young adults BDs.
| Study & Design | Population (N) | Age (Mean ± SD) | BD criteria | Exclusion Criteria | Task | Main findings | Conclusions |
|---|---|---|---|---|---|---|---|
|
Affan et al., 2018 Cross-sectional USA |
N/LDs: 31 (16♂, 15♀)BDs: 30 (15♂, 15♀) |
N/LDs: 23.3 ± 3.4 BDs: 23.4 ± 3.5 |
N/LDs: ≤ 1 BD episode in the previous 6 monthsBDs: ≥ 5 BD episodes [≥ 6 (♂)/5(♀) SADs/2h] in the previous 6 months |
Illicit drug or tobacco use at least one month prior to the study; History of brain injury, or other neuropsychiatric or medical problems; and Medications use at the time of the study | Resting state with eyes-open and eyes-closed | BDs: Alpha peak frequency was slower by 0.7 Hz; ↑ frontal theta and beta powerNegative correlation between alpha peak frequency and drinking variables (e.g. n° of drinking days/week, n° of BD episodes in the previous 6 months) Positive correlation between theta power and drinking history during both resting conditions |
BD among young adults is associated with augmented spontaneous electrophysiological signal |
|
Bauer and Ceballos, 2014 Cross-sectional USA |
Infrequent BDs: 55 ♀ (30 never and 25 less than monthly). Frequent BDs: 42 ♀ (28 monthly and 14 weekly). |
Infrequent BDs: 19.5 ± 1.3 Frequent BDs: 19.4 ± 1.1 |
Infrequent BDs: infrequent episodes with ≥ 6 drinks/occasion at least once a month Frequent BDs: frequent episodes with ≥ 6 drinks/occasion at least once a week |
Past year pregnancy; Psychosis; or Medical major disorders | Motor time estimation task | Frequent BD: ↑ Slow Potentials amplitude (more negative) in the right parietal cortex than infrequent BDs No group differences in Motor Potentials Negative correlation between Slow Potentials amplitude and AUDIT score |
Augmented neural activity in BDs may reflect a compensatory over- activation of the circuit to perform the task at successful level |
|
Blanco-Ramos et al., 2019 Cross-sectional Europe |
N/LDs: 80 (28♂, 43♀)BDs: 71 (42♂, 38♀) |
18–19 | N/LDs: < 6 BD episodes over the last 6 months and cannabis consumption < 12 units over the last 3 months BDs: ≥ 6 BD episodes over the last 6 months and cannabis consumption < 12 units over the last 3 months |
Chronic neurocognitive pathologies; History of neurological disorder or brain injury with LoC > 20 min; SCL-90-R > 90th percentile on GSI or ≥ 90 in 2 symptom dimensions; Family and/or personal history of psychopathology/alcoholism; Regular use of psychoactive drugs; Illegal drugs use (except cannabis) in the last 6 months; Non-corrected sensory/motor deficits | Go/NoGo task with alcoholic and non-alcoholic stimuli | BDs: ↑ N2-NoGo for non-alcoholic than for alcoholic stimuli; Similar P3-NoGo amplitude for non-alcoholic and for alcoholic stimuli (only in males) Negative correlation between: - N2-NoGo for non-alcoholic and the n° of BD episodes and total n° of alcoholic drinks in the last 180 days -P1 for Go-Alcohol and NoGo-NoAlcohol and age of onset drinking - n° of BD episodes and reaction time of false alarms Positive correlation between N2-NoGo for NoAlcohol and reaction time for false alarms |
BDs seem to need increased activation to monitor conflict with the aim of compensate the affective-automatic system overactivation caused by alcohol-related bias |
| Study & Design | Population (N) | Age (Mean ± SD) | BD criteria | Exclusion Criteria | Task | Main findings | Conclusions |
|---|---|---|---|---|---|---|---|
|
Courtney and Polich, 2010 Cross-sectional USA |
N/LDs: 32 (16♂, 16♀)LBDs: 32 (16♂, 16♀)HBDs: 32 (16♂, 16♀) |
N/LDs: ♂ 21.8 ± 0.8 ♀ 21.4 ± 1.3 LBDs: ♂ 20.5 ± 1.0 ♀ 20.4 ± 1.1 HBDs: ♂ 20.8 ± 2.0 ♀ 19.9 ± 1.1 |
N/LDs: 1–4 (♀)/5(♂) alcoholic drinks/<2h in the past 6 months LBDs: 5–4(♀)/7–6 (♂) alcoholic drinks/<2h at least once in the last 6 months HBDs: ≥ 10 alcoholic drinks/< 2h at least once in the last 6 months |
Not use of alcohol, tobacco and psychiatric medication; Serious health problems (e.g. asthma, heart condition, etc.); Family and/or personal history of alcoholism; Neurologic/Psychiatric disorders; and Recent drug use | Resting state with eyes open | HBDs: ↑ spectral power in the delta (0–4 Hz) and fast beta (20–35 Hz) bands in comparison with N/LDs and LBDs | BDs exhibit augmented brain activity at rest similarly to alcohol-dependent individuals |
|
Crego et al., 2009 Cross-sectional Europe |
N/LDs: 53 (27♂, 26♀)BDs: 42 (21♂, 21♀) |
N/LDs: 18.7 ± 0.5 BDs: 18.9 ± 0.5 |
N/LDs: < 6 SADs/occasion and ≤ 2 SADs/hour BDs: ≥ 6 SADs/occasion at least once a month, ≥ 3 SADs/hour at least once a month |
AUDIT > 20; Non-corrected sensory deficits; LoC > 20 min; History of traumatic brain injury or neurological disorder; Family and/or personal history of psychopathology; Drug use (except tobacco and cannabis); Alcohol use disorder; SCL-90-R > 90 on GSI or ≥ 2 symptom dimensions | Visual identical-pairs continuous performance task |
BDs: ↑ N2 amplitude in central and parietal regions for the matching stimuli than N/LDs Cs: ↑ P3 amplitude in frontal, central and parietal regions for the matching than for the nonmatching stimuli (but not in BDs) No behavioral differences between groups |
BDs require higher levels of attentional effort to perform the task at adequate levels. Also, they seem to have a deficiency in the electrophysiological differentiation between relevant and irrelevant information |
|
Crego et al., 2010 Cross-sectional Europe |
N/LDs: 53 (27♂, 26♀)BDs: 42 (21♂, 21♀) |
N/LDs: 18.7 ± 0.5 BDs: 18.9 ± 0.5 |
(Same as above) | (Same as above) | (Same as above) | BDs: ↓ LPC amplitude in frontal and central regions for matching condition than N/LDs, which was associated with hypoactivation of the right anterior prefrontal cortex No behavioral differences between groups |
BDs display decreased electrophysiological activity during recognition and evaluation of the working memory processes |
|
Crego et al., 2012 Cross-sectional Europe |
N/LDs: 53 (28♂, 25♀)BDs: 32 (17♂, 15♀) |
N/LDs: 18.5 ± 0.5 BDs: 18.8 ± 0.6 |
(Same as above) | Same asCrego et al. (2009) and Left-handedness | Simple visual oddball task | BDs: ↑P3b amplitude in all regions (frontal, central and parietal) than N/LDs No significant differences in N2 No behavioral differences between groups |
BDs seem to recruit broader brain areas linked to attentional processes to properly execute the task, which support the neurocompensation hypothesis |
| Study & Design | Population (N) | Age (Mean ± SD) | BD criteria | Exclusion Criteria | Task | Main findings | Conclusions |
|---|---|---|---|---|---|---|---|
|
Folgueira-Ares et al., 2017 Cross-sectional Europe |
N/LDs: 25 (13♂, 12♀)BDs: 25 (14♂, 11♀) |
N/LDs: 20.5 ± 0.6 BDs: 20.8 ± 0.7 |
(Same as above) | Non-corrected sensory deficits; LoC> 20 min; History of traumatic brain injury or neurological disorder; Family and/or personal history of psychopathology; Use of illegal drugs (except cannabis); and AUDIT > 20 | Visual face–name association memory task | BDs: Similar neural activity for successful and unsuccessful encoding (no Dm effect); ↑ VPP amplitude at C3 and Cz electrodes than N/LDs Cs: Dm effect in posterior regions in the 350–650 ms latency range No significant differences in N170 No behavioral differences between groups |
BDs display abnormal pattern of brain activity during the encoding phase, suggesting a different neural signature of successful memory encoding |
|
Holcomb et al., 2019 CS USA |
LDs: 32 (16♂, 16♀)BDs: 29 (14♂, 15♀) |
LDs: 23.41 ± 3.4 BDs: 23.41 ± 3.5 |
LDs: < 1 BD episode in the past six months BDs: ≥ 3 BD episodes in the past 6 months with at least one episode in the last month |
Same asAffan et al. (2018) | Visual Go/NoGo task | BDs: ↓ theta (4-7Hz) and early beta (15-25Hz) power during NoGo trials than LDs Negative correlation between NoGo theta power and n° BD episodes, daily alcohol intake, and the average n° of weekly drinking days No behavioral differences between groups |
The results are consistent with deficits in the inhibitory control circuitry and are suggestive of allostatic neuroadaptive changes associated with BD |
|
Huang et al., 2018 CS USA |
LDs: 32 (16♂, 16♀)BDs: 32 (16♂, 16♀)- LBDs: 17 (9♂)- HBDs: 15 (7♂) |
LDs: 23.4 ± 3.4 BDs: 23.2 ± 3.3 |
LDs: 1 BD episode in the last 6 months. BDs: ≥ 5 BD episodes in the last 6 months. - LBDs: ≤ 10 BD episodes over the previous 6 months. - HBDs: ≥ 12 BD episodes over the previous 6 months |
Drug or tobacco use for at least 1 month prior to the study; History of seizures, brain injury, neurological or neuropsychiatric disorders; Vision or hearing problems; learning difficulties; and Medications use at the time of the study | Emotional rating task | LDs: theta poweremotional > theta powerneutral (no differences in BDs) BDs: ↑ theta during erotic pictures compared to the other emotions HBDs: ↓ emotional modulation of theta and ↓ theta power to negative and positive photos in contrast with LDs Negative correlation between emotion-induced theta and n° of BD occasions within the past 6 months |
BDs show diminished sensitivity of event-related theta to emotional salience, namely for negative and positive emotions |
|
Kiat and Cheadle, 2018 CS USA |
26 (6♂, 20♀) − 13: no BD episodes (past 30 days) − 13: 1–2 BD episodes (9 subjects) or 3–5 BD episodes (4 subjects) |
20.0 ± 1.7 | BD: > 5 alcoholic drinks in a row within a few hours | Not reported | Crocodile dentist (aversive risk-taking task) | ↑ Late Positive Potential amplitude was associated with higher risk levels of BD | BD frequency seems to be linked to increased levels of anticipatory risk-taking reactivity |
| Study & Design | Population (N) | Age (Mean ± SD) | BD criteria | Exclusion Criteria | Task | Main findings | Conclusions |
|---|---|---|---|---|---|---|---|
|
Kim and Kim, 2019 CS Asia |
NBDs: 25♀ BDs: 25♀ |
NBDs: 21.7±2.4 BDs: 21.4±1.9 |
NBDs: AUDIT-K≤ 8, < 4 glasses in the last 2 weeks, and drank < 1 glass/hour. BDs: AUDIT-K= 12–26; ≥ 4 glasses more than once in the previous 2 weeks; and drank >2 glasses/hour |
Left-handedness, Ambidexterity and History of psychiatric disorders | Flanker task (modified) | BDs: ↓ ERN amplitude than NBDs No differences in Pe amplitude or latency Positive correlation between ERN amplitude and total AUDIT-K and Alcohol Use Questionnaire scores Behavioral: BDs: ↑ error rates and ↓ reaction times in congruent and incongruent conditions than NBDs |
BD seem to be associated with an impaired capacity to automatically monitor errors, reflected by decreased neural activity |
|
Lannoy et al., 2017 Cross-sectional Europe |
N/LDs: 20 (7♂, 13♀)BDs: 20 (8♂, 12♀) |
N/LDs: 21.2 ± 2.6 BDs: 20.3 ± 1.6 |
BD score formula: [(4*consumption speed) + drunkenness frequency + (0.2*drunkenness)] N/LDs: BD score ≤ 16 BDs: BD score > 16 |
Family and/or personal history of alcoholism; Positive psychological or neurological disorder; Current medication; Major medical problems; Past/current drug consumption (excepting alcohol and tobacco) | Visual speeded Go/NoGo task Balloon Analogue Risk task |
BDs: ↑ ERN amplitude for false alarms than slow hits at Fz (not in N/LDs); ↑ Pe latency for slow hits at Cz during the Go/NoGo task No differences in FRN and P3 No behavioral differences between groups |
BDs seem to have an impaired performance monitoring, showing an abnormal automatic processing of response errors and a decreased processing of their motivational significance |
|
Lannoy et al., 2018 Cross-sectional Europe |
N/LDs: 19 (11♂, 8♀)MDs: 17 (9♂, 8♀)BDs: 17 (10♂, 7♀) |
N/LDs: 20.4 ± 2.8 MDs: 21.0 ± 2.7 BDs: 20.2 ± 1.6 |
N/LDs: BD score = 0, no consumption; MDs: BD score = 1–12; ≤3 doses/occasion; consumption speed 0.33–2; ≤3 drinking occasions/week. BDs: BD score ≥ 16; ≥ 6 doses/occasion; consumption speed ≥2; 2–4 drinking occasions/week |
Same asLannoy et al. (2017) and Non-corrected visual and auditory problems | Emotional crossmodal task | N/LDs: N1 latencyhappiness > N1 latencyanger (no differences in BDs) BDs: Congruent trialsSP3b amplitude for happy than anger faces (no differences in N/LDs and MDs) ; ↑P3b latency than MDs and N/LDs Incongruent trials ↑P3b amplitude than MDs; ↑ second positive component latency when anger voices were presented than MDs; ↑ third positive component amplitude when anger faces were presented than MDs |
BDs present higher electrophysiological activity in the absence of behavioral deficits, which could be associated with a potential compensation process |
|
Lannoy et al., 2020 Cross-sectional Europe |
N/LDs: 25 (13♂, 12♀)BDS: 25 (10♂, 15♀) |
N/LDs: 21.7 ± 1.8 BDs: 20.9 ± 1.7 |
N/LDs: BD score < 12; ≤ 4 drinking occasions/week; < 3 doses/occasion BDs: BD score ≥ 16; 2–4 drinking occasions/week; ≥ 6 doses/occasion |
Severe alcohol use disorders; Family history of alcohol‐use disorders; Psychological and neurological disorders; Past and current drug consumption (except alcohol and tobacco). | Go/NoGo task with alcoholic and non-alcoholic stimuli | BDs: ↓ NoGo-N2 amplitude for alcohol‐related at F3 than F4 electrode. Behavioral: BDs: ↓ inhibition performance for explicit than implicit processing when compared to N/LDs |
BD may be associated with impaired attentional/inhibitory processes in the presence of alcohol cues |
| Study & Design | Population (N) | Age (Mean ± SD) | BD criteria | Exclusion Criteria | Task | Main findings | Conclusions |
|---|---|---|---|---|---|---|---|
|
López-Caneda et al., 2012 Longitudinal Europe |
N/LDs: 25 (11♂, 14♀)BDs: 23 (13♂, 10♀) |
1st evaluation N/LDs: 18.6 ± 0.5 BDs: 18.8 ± 0.5 2nd evaluation (2-year follow-up) N/LDs: 20.3 ± 0.5 BDs: 20.7 ± 0.6 |
N/LDs: < 6 SADs/occasion and ≤ 2 SADs/hour. BD: ≥ 6 SADs/occasion at least once a week or ≥ 6 SADs/occasion, ≥ 3 SADs/hour at least once a month |
Family history of alcoholism; Family and/or personal history of psychopathology; drugs use (except cannabis); LoC> 20 min; history of traumatic brain injury or neurological disorder; non-corrected sensory deficits; and AUDIT> 20 | Visual Go/NoGo Task | BDs: ↑Go-P3 amplitude in central and parietal regions in the 1st and 2nd evaluations ↑No/Go-P3 amplitude in all regions (frontal, central and parietal) in the 2nd evaluation, associated with hyperactivation of the right inferior frontal cortex during successful inhibition No significant differences in N2 No behavioral differences between groups |
BDs show increased neural activity in inhibitory control regions during response inhibition, which could reflect a compensatory mechanism to perform the task efficiently |
|
López-Caneda et al., 2013 Longitudinal Europe |
N/LDs: 31 (15♂, 16♀)BDs: 26 (15♂, 11♀) |
1st evaluation N/LDs: 18.5±0.5 BDs: 18.8±0.5 2ndevaluation (2-year follow-up) N/LDs: 20.4±0.6 BDs: 20.8±0.6 |
(Same as above) | (Same as above) | Simple visual oddball task | BDs: ↑P3 amplitude at both evaluation times than N/LDs, with more pronounced differences in the follow-up evaluation Positive correlation between P3b amplitude and quantity and intensity of alcohol consumption No behavioral differences between groups |
The increased neural activity linked to attentional/working memory processes, suggesting the recruitment of additional resources to perform the task at adequate levels |
|
López-Caneda et al., 2014b Longitudinal Europe |
N/LDs: 25 (11♂, 14♀)BDs: 22 (11♂, 11♀)Ex-BDs: 10 (3♂, 7♀) |
1st evaluation 18–19 2ndevaluation (2-year follow-up) 20–21 |
(Same as above) Ex-BDs: BD criteria in the 1st but not in the 2nd evaluation |
(Same as above) | Visual Go/NoGo Task | BDs: ↑NoGo-P3 amplitudes in the 2nd evaluation than N/LDs; ↑Go-P3 amplitudes than N/LDs; Ex-BDs: intermediate position between BDs and N/LDs Frontal NoGo-P3 amplitude in the 2nd evaluation: - correlated negatively with the age of onset of regular drinking - correlated positively with speed of alcohol consumption and weekly quantity of alcohol consumed |
BD lead to impairments in the neural functioning involved in inhibitory control, and the cessation of BD could act as a brake on the neurophysiological impairments related to response inhibition |
|
López-Caneda et al., 2017a Cross-sectional Europe |
N/LDs: 40 (21♂, 19♀)BDs: 40 (20♂, 20♀) |
N/LDs: 18.1 ± 0.3 BDs: 18.1 ± 0.3 |
Cs: never BAC ≥ 0.08 g/dL BDs: BAC ≥ 0.08 g/dL at least once during the last month |
(Same as above) Use of medical drugs with psychoactive effects |
Resting-state with eyes-open and eyes-closed conditions | BDs: ↑ beta power over the right temporal lobe (parahippocampal and fusiform gyri) during eyes-open resting state ↑ theta power over the bilateral occipital cortex (cuneus and lingual gyrus) during eyes-closed resting condition |
BDs seem to present cortical hyperexcitability and potential difficulties in the information processing capacity |
| Study & Design | Population (N) | Age (Mean ± SD) | BD criteria | Exclusion Criteria | Task | Main findings | Conclusions |
|---|---|---|---|---|---|---|---|
|
López-Caneda et al., 2017b Cross-sectional Europe |
N/LDs: 36 (17♂, 19♀) - Abstainers: 20 (12♂, 8♀)BDs: 36 (20♂, 16♀) |
N/LDs: 18.1 ± 0.3 BDs: 18.1 ± 0.3 |
N/LDs: never reached a BAC of 0.08 g/dL. BDs: BAC ≥ 0.08 g/dL at least once during the last month |
Same asLópez-Caneda et al. (2012) and use of psychoactive medical drugs during the week before the assessment | Visual equiprobable Go/NoGo Task | BDs: ↓ beta and theta during Go and NoGo conditions than N/LDs. No behavioral differences between groups |
BDs appear to show decreased neural oscillations linked to motor inhibition and execution similar to those observed in alcohol-dependent subjects |
|
Maurage et al., 2009 Longitudinal Europe |
N/LDs: 18 (7♂, 11♀)BDs: 18 (7♂, 11♀) |
N/LDs: 18.2 ± 0.3 BDs: 18.2 ± 0.4 |
N/LDs: expected alcohol use 9 months after the 1st evaluation < 3 SADs/week. BDs: expected alcohol consumption 9 months after the 1st evaluation > 20 SADs/week |
Family history of alcoholism; High past alcohol consumption or BD habits; Past or current drug use; Major medical problems; CNS disease; Auditory impairment; Moderate/high depression/anxiety; and Personal history of psychopathology | Auditory task based on emotionalvalence detection (negative or positive) |
BDs: ↑ P1, N2 and P3b latency No differences in amplitude Positive correlation between mean alcohol intake and latency of each component No behavioral differences between groups |
Short-term BD can produce marked cerebral dysfunction undetectable by behavioral measures alone. Specifically, BD seems to be associated with a slowed cerebral activity |
|
Maurage et al., 2012 Cross-sectional Europe |
N/LDs: 20 (11♂, 9♀)DDs: 20 (11♂, 9♀)LBDs: 20 (11♂, 9♀)HBDs: 20 (11♂, 9♀) |
N/LDs: 21.6 ± 2.4 DDs:22.1 ± 2.2 LBDs: 21.0 ± 2.2 HBDs: 21.2 ± 2.0 |
N/LDs: non-drinkersDDs: 3–5 SADs/occasion, < 2 SADs/h, 5–7 times/week and 15–29 SADs/week LBDs: 5–12 SADs/occasion, > 3 SADs/h, 2–3 times/week and 15–29 SADs/week HBDs: > 10 SADs/occasion, > 3 SADs/h, 3–4 times/week and > 30 SADs/week |
(Same as above) | Visual oddball task with face-detection | LBDs and HBDs: ↑P3b latency and ↓N1, P1, N2b amplitude than the other 3 groups; ↓P3b amplitude than N/LDs and DDs HBDs: ↑ N1, P1, N2b, P3a latency and ↓N170, P2 amplitude than the other 3 groups; ↑ P3b latency than DDs LBDs: ↓N170 amplitude than N/LDs; ↓P2 amplitude than N/LDs and DDs DDs: No significant differences with N/LDs |
BDs present early and global electrophysiological impairments (characterized by reduced and slower activity), affecting low-level (perception and attention) as well as high-level (decision) cognitive stages |
|
Na et al., 2019 Cross-sectional Asia |
N/LDs: 23♀ BDs: 27♀ |
N/LDs: 22.0 ± 2.0 BDs: 21.4 ± 2.0 |
(Same asKim and Kim, 2019) | AUDIT-K score > 26; No psychiatric disorder; Score ≥ 6 on the Children of Alcoholics Screening Test (family history of alcohol use disorder); Left-handed and ambidextrous | Iowa Gambling Task (modified) | BDs: ↓ ΔFRN than N/LDs; No differences in P3 amplitude Behavioral: BDs: ↓ total net score than the N/LDs |
Female BDs seem to have difficulties in early evaluation of positive or negative feedback which seem to be associated with decision-making deficits |
| Study & Design | Population (N) | Age (Mean ± SD) | BD criteria | Exclusion Criteria | Task | Main findings | Conclusions |
|---|---|---|---|---|---|---|---|
|
Park and Kim, 2018 Cross-sectional Asia |
N/LDs: 25 (8♂, 17♀)BDs: 25 (8♂, 17♀) |
N/LDs: 22.2± 2.4 BDs: 22.1±2.2 |
N/LDs: same as above BDs: AUDIT-K = 12–26; > 4(♀)/5(♂) glasses at least one time during the previous 2 weeks; > 2(♀)/3(♂) glasses/hour |
CAST-K score > 6; Drug/alcohol abuse; Left-handed and ambidextrous; and History of neurological/psychiatric disorders | Spatial 2-back task with congruent, incongruent, and lure conditions | BDs: ↑P3 amplitude than N/LDs. NBDs: ↑P3 amplitude for congruent stimuli compared to the incongruent and lure stimuli (no differences in BDs) No group significant differences in N2. |
BDs show increased cognitive effort to perform the task effectively. Additionally, they were less efficient in differentiating between relevant and irrelevant information |
|
Petit et al., 2012 Cross-sectional Europe |
N/LDs: 18 (8♂, 10♀)BDs: 18 (12♂, 6♀) |
N/LDs: 21.9±3.1 BDs: 21.3±1.7 |
N/LDs: <6 SADs/occasion and < 3 SADs/hour. BDs: ≥6 SADs/occasion, ≥ 3 SADs/h and ≥ 1–4 times/week |
Major medical problems; History of CNS disease; Visual impairment, Past or current drug use; Family history of alcoholism; Very low alcohol consumption; BD habits before starting university studies | Visual oddball task with neutral stimuli and alcoholic and non-alcoholic pictures as target deviant stimuli. | BDs: ↑P1 amplitude for alcoholic pictures than for the non-alcoholic pictures Positive correlation between P1 amplitudes for alcoholic pictures and duration of BD habits, and n° of doses consumed/week No group significant differences in N2b and P3 No behavioral differences between groups |
BDs exhibit signs of prioritizing processing of alcohol-related information |
|
Petit et al., 2013 Cross-sectional Europe |
N/LDs: 27 (10♂, 17♀)BDs: 29 (15♂, 14♀) |
N/LDs: ♂ 22.1 ± 2.5 ♀ 20.5 ± 1.2 BDs: ♂ 22.5 ± 3.7 ♀ 21.9 ± 2.3 |
(Same as above) | Major medical problems; CNS conditions; Visual impairment; Past or current drug consumption (except alcohol, cannabis and tobacco); and Alcohol abstinence | (Same as above) | BDs: ↑P3 amplitude for alcoholic pictures than for the non-alcoholic pictures (only in males) No behavioral differences between groups |
BDs seem to present an enhanced motivational response to alcoholic stimuli |
|
Petit et al., 2014b Longitudinal Europe |
Cs: 15 (4♂, 11♀)BDs: 15 (11♂, 4♀) |
1st evaluation N/LDs: 22.0±2.13 BDs: 22.0±1.72 2nd evaluation (1-year follow-up) N/LDs: 23.0±2.2 BDs: 23.0±1.6 |
(Same as above) | (Same as above) | (Same as above) | BDs: ↓ P1 amplitude for both types of stimuli in the 2nd than in the 1st evaluation ↓ P3 amplitude for non-alcoholic cues in the 2nd than in the 1st evaluation No behavioral differences between groups |
The continuation of BD over one year is associated with the development of brain functional abnormalities as well as ↑ reactivity to alcoholic stimuli and/or ↓ reactivity to non-alcoholic stimuli |
| Study & Design | Population (N) | Age (Mean ± SD) | BD criteria | Exclusion Criteria | Task | Main findings | Conclusions |
|---|---|---|---|---|---|---|---|
|
Ryerson et al., 2017 Cross-sectional USA |
62 − 41: 1–8 BD episodes − 21: no BD episodes in the last month |
18.9 ± 1.1 | BDs: ≥ 5 SADs/occasion (males); ≥ 4 SADs/occasion (females) | Not reported | Alcoholic and neutral pictures following a global or local attentional scope manipulation | BD correlated positively with N1 amplitude to alcoholic pictures, but not to neutral pictures | Individuals with greater BD experience demonstrate increased neural response to alcoholic pictures, but not neutral pictures |
|
Schroder et al, 2019 Cross-sectional Europe |
N/LDs: 24 (11♂, 13♀)BDs: 25 (13♂, 12♀) |
N/LDs: 26.8 ± 9.3 BDs: 24.0 ± 2.4 |
N/LDs: drank 1–30 days/month, but never > 5 SADs/occasion and ≤ 2 SADs/h BDs: ≥ 6 SADs occasion and ≥ 2 SADs/h |
Major medical issues, CNS conditions (e.g. epilepsy and a prior history of brain injury); Visual impairments; and Past/current drug consumption | N-back task with numbers | BDs: ↑ P3 and P600 amplitude than N/LDs N/LDs: ↑ P2 and N4 amplitude than BDs No behavioral differences between groups |
BDs require higher processing intensity throughout the information-processing stream to perform the task at the same level as controls. |
|
Smith and Mattick, 2013 Cross-sectional Australia |
N/LDs: 17 ♀ BDs: 13 ♀ |
N/LDs: 20.1 ± 1.2 BDs: 20.0 ± 1.2 |
N/LDs: no regular (less than once a month) consumption of ≥ 4 SADs/occasion BDs: ≥ 4 SADs/occasion at least once a month |
Epileptic seizure, serious head injury or LoC; Uncorrected hearing/vision problems; and Regular (≥ 2/month) use of other drugs | Stop signal task | BDs: ↓P3 amplitude for failed inhibitions than N/LDs; ↑P3 amplitude in FCz for successful than failed inhibition trials; ↓ERN amplitude in Fz than N/LDs; Positive correlation between P3 amplitude at FCz with AUDIT scores Behavioral: BDs: ↑Stop signal reaction time than N/LDs |
Young female BDs have large deficits in inhibitory control and performance monitoring, and they may have to work harder in order to successfully inhibit a response |
|
Smith et al., 2015 Cross-sectional Australia |
N/LDs: 35 (18♂, 17♀)BDs: 31 (16♂, 15♀) |
N/LDs: ♂ 22.1 ± 2.4 ♀ 21.4 ± 2 BDs: ♂ 23.0 ± 2.2 ♀ 21.0 ± 2.3 |
N/LDs: ≥ 5 SADs/occasion less than once a month BDs: ≥ 5 SADs/occasion at least once a month |
Psychotropic medication; Epileptic seizure, head injury or LoC; Uncorrected vision problems; and Regular (>1/month) use of drugs (except for alcohol or tobacco) | Eriksen flanker task (Visual conflict monitoring task) |
BDs: ↑conflict adaptation for N2 amplitude than N/LDs (females); ↓ N2 amplitude and no differences in the conflict adaptation effect than N/LDs (males); No differences in P3 (indexing inhibitory control) Behavioral: BDs: ↑errors than N/LDs |
Results are suggestive of a compensatory response in female BDs, as they seem to need to increase their ongoing performance monitoring to properly execute the task |
| Study & Design | Population (N) | Age (Mean ± SD) | BD criteria | Exclusion Criteria | Task | Main findings | Conclusions |
|---|---|---|---|---|---|---|---|
|
Smith et al., 2016 Cross-sectional Australia |
N/LDs: 37 (20♂, 17♀)BDs: 34 (21♂, 13♀) |
N/LDs: ♂ 20.1 ± 1.1 ♀ 20.1 ± 1.2 BDs: ♂ 19.8 ± 1.2 ♀ 20.0 ± 1.2 |
N/LDs: no regular (less than once a month) consumption of ≥ 4 SADs/occasion BDs: ≥ 4 SADs/occasion at least once a month preceding 12 months |
(Same asSmith and Mattick, 2013) | Visual stop-signal task | BDs: ↑P3 amplitude for successful than failed inhibition trials (marginally significant); ↑P3 latency for failed than successful inhibitions (only in females); ↓ERN amplitude than N/LDs (marginally significant) Behavioral: BDs: ↑ Stop signal reaction time than N/LDs (only in females) |
Electrophysiological deficits during response inhibition and performance monitoring seem to be common to both sexes; however females also show to be more vulnerable at behavioral level |
|
Smith et al., 2017a Cross-sectional Australia |
N/LDs: 35 (18♂, 17♀)BDs: 25 (12♂, 13♀) |
N/LDs: 21.8 ± 2.2 BDs: 22.2 ± 2.5 |
(Same as above) |
(Same asSmith et al., 2015) |
Error awareness (Stroop Go/NoGo) task | No ERPs differences between groups Behavioral: BDS: ↑inhibitory errors |
BDs commit more inhibitory errors, suggesting deficits in inhibitory control, but they not display failures in error awareness |
|
Smith et al., 2017b Cross-sectional Australia |
Study 1: N/LDs: 13♂ BDs: 12♂ CU: 8♂ Study 2: N/LDs: 45 (25♂, 20♀)BDs: 39 (23♂, 16♀)Cannabis users: 20 (11♂, 9♀) |
Study 1: 17.2±0.7 Study 2: N/LDs: ♂ 20.0 ± 1.1 ♀ 19.9 ± 1.2 BDs: ♂ 19.7 ± 1.2 ♀ 20.0 ± 1.2 Cannabis users: ♂ 20.6 ± 1.2 ♀ 20.1 ± 1.2 |
N/LDs: non-regular use of cannabis and non-regular heavy drinking BDs: ≥ 4 SADs per occasion at least once a month in the past year Cannabis < 2 times/month in the past year Cannabis users: Cannabis ≥ 2 times/month in the preceding 12 months |
Regular use of drugs (except cannabis/tobacco); Uncorrected hearing/vision problems; Use of psychoactive medications; Seizure, serious head injury or LoC. |
Rey Auditory Verbal Learning Test | BDs: ↑P540 than N/LDs. Cannabis users: ↓N340 than BDs Behavioral: BDs: poorer delayed recall relative to N/LDs (Study 2) |
The results indicated alterations in recognition memory processing which, even in the absence of overt behavioral impairment, underline the potential for neural dysfunction with early exposure to alcohol |
Note. AUDIT = Alcohol Use Disorders Identification Test; AUDIT-K = AUDIT - Korean; BAC = Blood Alcohol Concentration; BD = Binge Drinking; BDs = Binge Drinkers; CNS = Central Nervous System; DDs = Daily Drinkers; ERN = Error-Related Negativity; FRN = Feedback-related Negativity; GSI = Global Severity Index; HBDs = High Binge Drinkers; LBDs = Light Binge Drinkers; N/LDs = Non/Light Drinkers; LoC = Loss of Consciousness; LPC = Late Positive Component; MDs = Moderate Drinkers; N = sample size; NBDs = Non-Binge Drinkers; NDs = Non-Drinkers; Pe = Error-positivity; SCL-90-R = Symptom Checklist-90-Revised; SD = Standard Deviation; SADs = Standard Alcoholic Drinks; ↑= larger/higher; ↓= lower/reduced; ΔFRN = difference in amplitudes of feedback-related negativity between gain and loss feedback; VPP = Vertex Positive Potential.
Table 3.
Quality assessment scores according to the NHLBI Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies.
| Study | Q1 | Q2 | Q3 | Q4 | Q5 na/justification b | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Q14 | Quality Rating |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Affan et al., 2018 | Yes | No | NR | Yes | Yes/No | No | No | No | Yes | No | Yes | NR | NA | No | Poor |
| Bauer and Ceballos, 2014 | Yes | Yes | NR | Yes | Yes/Yes | No | No | No | Yes | No | Yes | NR | NA | No | Fair |
| Blanco-Ramos et al., 2019 | Yes | Yes | NR | Yes | Yes/No | No | No | No | Yes | No | Yes | NR | NA | Yes | Good |
| Courtney and Polich, 2010 | Yes | Yes | NR | Yes | Yes/No | No | No | Yes | Yes | No | Yes | NR | NA | No | Fair |
| Crego et al., 2009 | Yes | Yes | NR | Yes | Yes/No | No | No | No | Yes | No | Yes | NR | NA | Yes | Good |
| Crego et al., 2010 | Yes | Yes | NR | Yes | Yes/No | No | No | No | Yes | No | Yes | NR | NA | Yes | Good |
| Crego et al., 2012 | Yes | Yes | NR | Yes | Yes/No | No | No | No | Yes | No | Yes | NR | NA | Yes | Good |
| Folgueira-Ares et al., 2017 | Yes | Yes | NR | Yes | Yes/No | No | No | No | Yes | No | Yes | NR | NA | Yes | Good |
| Holcomb et al., 2019 | Yes | Yes | NR | Yes | Yes/No | No | No | No | Yes | No | Yes | NR | NA | No | Fair |
| Huang et al., 2018 | Yes | No | NR | Yes | Yes/No | No | No | No | Yes | No | Yes | NR | NA | No | Poor |
| Kiat and Cheadle, 2018 | Yes | No | NR | No | No (BD = 13)/Yes | No | No | No | Yes | No | Yes | NR | NA | No | Poor |
| Kim and Kim, 2019 | Yes | Yes | NR | Yes | Yes/No | No | No | No | Yes | No | Yes | NR | NA | No | Fair |
| Lannoy et al., 2017 | Yes | Yes | NR | Yes | Yes/No | No | No | No | Yes | No | Yes | NR | NA | No | Fair |
| Lannoy et al., 2018 | Yes | Yes | NR | Yes | No (BD = 17)/No | No | No | Yes | Yes | No | Yes | NR | NA | Yes | Good |
| Lannoy et al., 2020 | Yes | Yes | NR | Yes | Yes/No | No | No | No | Yes | No | Yes | NR | NA | Yes | Good |
| López-Caneda et al., 2012 | Yes | Yes | NR | Yes | Yes/No | No | Yes | No | Yes | Yes | Yes | NR | Yes | Yes | Good |
| Study | Q1 | Q2 | Q3 | Q4 | Q5 na/justification b | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Q14 | Quality Rating |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| López-Caneda et al., 2013 | Yes | Yes | NR | Yes | Yes/No | No | Yes | No | Yes | Yes | Yes | NR | Yes | Yes | Good |
| López-Caneda, et al., 2014b | Yes | Yes | NR | Yes | Yes/No | No | Yes | Yes | Yes | Yes | Yes | NR | Yes | Yes | Good |
| López-Caneda et al., 2017a | Yes | Yes | NR | Yes | Yes/No | No | No | No | Yes | No | Yes | NR | NA | No | Fair |
| López-Caneda et al., 2017b | Yes | Yes | NR | Yes | Yes/No | No | No | No | Yes | No | Yes | NR | NA | Yes | Good |
| Maurage et al., 2009 | Yes | Yes | NR | Yes | No (BD=18)/No | Yes | Yes | No | Yes | Yes | Yes | NR | Yes | Yes | Good |
| Maurage et al., 2012 | Yes | Yes | NR | Yes | Yes/No | No | No | Yes | Yes | No | Yes | NR | NA | Yes | Good |
| Na et al., 2019 | Yes | Yes | NR | Yes | Yes/No | No | No | No | Yes | No | Yes | NR | NA | No | Fair |
| Park and Kim, 2018 | Yes | Yes | NR | Yes | Yes/No | No | No | No | Yes | No | Yes | NR | NA | No | Fair |
| Petit et al., 2012 | Yes | Yes | NR | Yes | No (BD=18)/No | No | No | No | Yes | No | Yes | NR | NA | Yes | Fair |
| Petit et al., 2013 | Yes | Yes | NR | Yes | Yes/No | No | No | No | Yes | No | Yes | NR | NA | Yes | Good |
| Petit et al., 2014b | Yes | Yes | NR | Yes | No/No | No | Yes | No | Yes | Yes | Yes | NR | Yes | Yes | Good |
| Ryerson et al., 2017 | Yes | Yes | NR | No | Yes/No | No | No | No | Yes | No | Yes | NR | NA | No | Poor |
| Schroder et al., 2019 | Yes | Yes | NR | Yes | Yes/No | No | No | No | Yes | No | Yes | NR | NA | No | Fair |
| Smith and Mattick, 2013 | Yes | Yes | NR | Yes | No/No | No | No | No | Yes | No | Yes | NR | NA | No | Poor |
| Smith et al., 2015 | Yes | Yes | NR | Yes | Yes/No | No | No | No | Yes | No | Yes | NR | NA | No | Fair |
| Smith et al., 2016 | Yes | Yes | NR | Yes | Yes/No | No | No | No | Yes | No | Yes | NR | NA | No | Fair |
| Smith et al., 2017a | Yes | Yes | NR | Yes | Yes/No | No | No | No | Yes | No | Yes | NR | NA | No | Fair |
| Smith et al., 2017b | Yes | Yes | NR | Yes | Yes/No | No | No | No | Yes | No | Yes | NR | NA | No | Fair |
Note. na = refers to having a reasonable sample size (≥20). Justificationb = refers to statistical justification of sample size, estimates of effect size, etc. NA = not applicable; NR = not reported; NHLBI = National Heart, Lung, and Blood Institute
3. Results
3.1. Main findings
The database search resulted in the identification of 272 articles and Additional records identified through other sources. From these, 134 duplicated papers were excluded using the Zotero software (Ahmed and Al Dhubaib, 2011). The titles and abstracts of the remaining 140 articles were scrutinized and ninety-five studies were excluded (see Fig. 1). In case of doubt, the manuscripts were submitted to full-text reading. Following the final screening of 45 full texts, a total of 34 articles fulfilled the inclusion criteria, being included a total of 1723 individuals (57.1% female).
3.2. Study characteristics
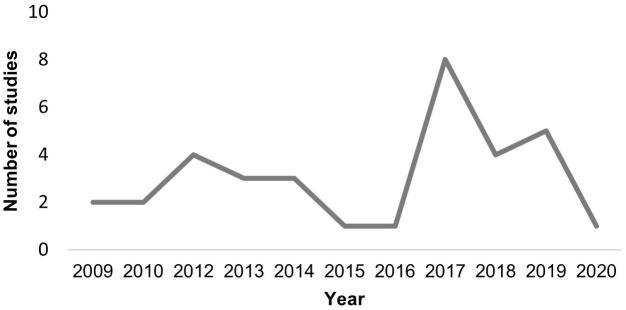
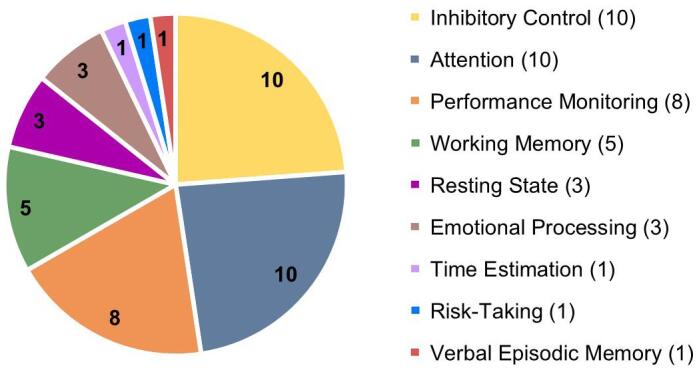
The majority of the studies included in this review (94.1%) were published after 2009, being 58.8% of them published between 2015 and 2020 (see Fig. 2). More than half of the studies (55.9%) were conducted in Europe, 20.6% in the United States, 14.7% in Australia, and the remaining 8.8% in Asia. Five studies (14.7%) were longitudinal, involving two assessments, but only one of them began before BD onset. Studies’ samples were mostly composed of college students. The cognitive processes most frequently assessed were inhibitory control (29.4%), attention (29.4%) and performance monitoring (23.5%). Fig. 3 depicts the proportion of studies that evaluated different cognitive functions. Regarding attention, the oddball paradigm was the most widely used task (6 of 10 studies). Similarly, in the studies assessing inhibitory control the paradigm most frequently employed was the Go/NoGo task (7/10). More variability was observed in the performance monitoring studies, in which four paradigms were used, including the Go/NoGo (3/8), Stop-Signal (2/8), Iowa Gambling (1/8), and Flanker tasks (2/8).
Fig. 2.
Number of EEG studies exploring the neurofunctional impairments associated with BD conducted per year.
Fig. 3.
Number of articles included in the systematic review and the type of cognitive process analyzed.
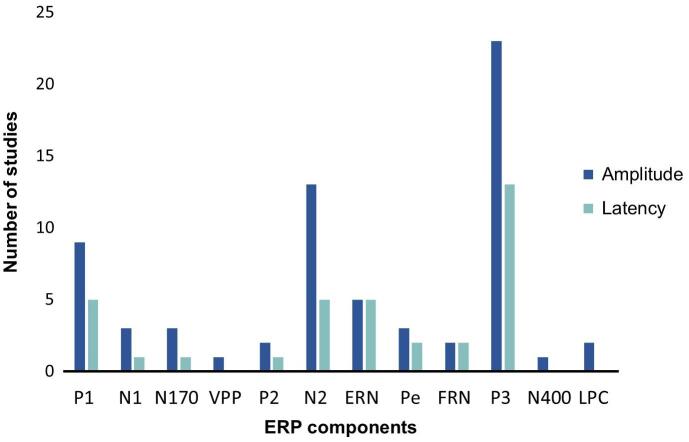
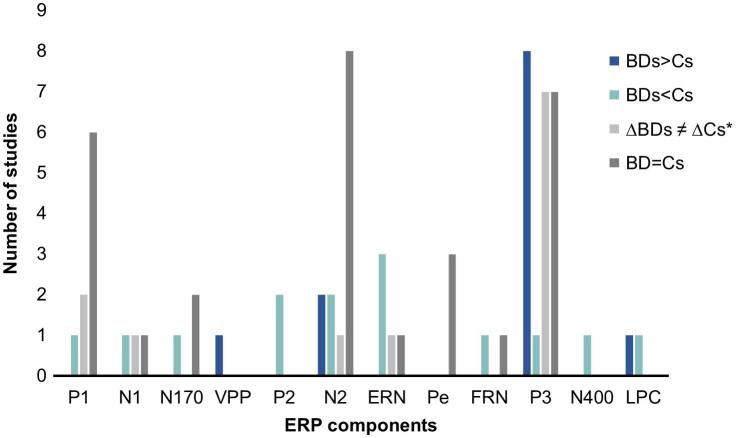
From the studies reviewed, 29 explored ERPs, three studies analyzed EROs and three assessed the EEG rhythms during resting-state (see supplementary Figure S1). The ERPs analyzed throughout the various studies are described in Table 4, including their time windows, cortical/subcortical sources and functional correlates. As reflected in Fig. 4, P3 was the most studied component, being explored by 23 out of the 29 ERP studies. In addition, alterations in P3 amplitude were the most consistently reported ERP disruption in BDs (see Fig. 5). For similar specifications regarding each cognitive function see supplementary Figure S2.
Table 4.
Details of the main ERP components assessed by the studies included in the systematic review.
| ERP | Time window | Polarity | Distribution | Source | Functional Meaning |
|---|---|---|---|---|---|
| 1P1 | Auditory ≈ 50 ms post-stimulus onset Visual ≈ 100 ms post-stimulus onset |
Positive | Auditory: Fronto-Central Visual: Occipital |
Auditory: superior temporal gyrus and medial frontal cortex Visual: extrastriate occipital cortex and posterior parietal regions |
P1 represents a basic perceptual processing of the stimulus and provides a quantitative measure of the functional integrity of the sensory pathways. Additionally, it is usually interpreted as a neurophysiological indicator of preferential attention to sensory inputs and as an index of alertness status and attention. |
| 2N1 | Auditory: 75–150 ms post-stimulus onset Visual: 100–175 ms post-stimulus onset |
Negative | Auditory: Fronto-Central Visual: Temporo-Occipital |
Auditory: Primary auditory cortex and frontal regions. Visual: Inferior occipital cortex and the occipito-temporal junction |
N1 is assumed to reflect selective attention to basic stimulus characteristics, an initial selection for later pattern recognition, which is modulated by the arousal and emotional salience of the stimulus. |
| 3N170 | 125–225 ms post-stimulus onset | Negative | Occipito-Temporal | Posterior fusiform and inferior-temporal gyri | N170 is a component mainly associated with visual processing of human faces. It reflects the identification and structural encoding of faces and eyes, being considerably reduced for non-facial stimuli. |
| 4VPP | 140–180 ms post-stimulus onset | Positive | Central | Inferior temporal cortex | VPP is evoked during the processing of single images, exhibiting its largest amplitude response for faces. The functional similarity and the temporal coincidence with N170 have led to consider that both are flip sides of the same neural generators. |
| 5P2 | 150–250 ms post-stimulus onset | Positive | Auditory: Central Visual: Frontal |
Auditory: Primary and associative auditory cortex. Visual: inferior occipital cortex |
P2 represents higher order perceptual processing modulated by attention and linked to memory. This component is part of a cognitive matching system that compares sensory inputs with stored memory, being involved in stimulus classification and attention modulation of nontarget stimuli. |
| N2 | 200–350 ms post-stimulus onset | Negative | N2 is considered to be a family of responses that differ in their distribution, source and interpretation, based on the features of the eliciting task, possibly reflecting task demands. However, all of them are part of the attentional processing and appear to indicate a detection of a deviation between a particular stimulus and the participant’s expectation. | ||
| 6N2b | 200–350 ms post-stimulus onset | Negative | Fronto-Central | Anterior cingulate, frontal and superior temporal cortex | N2b is mainly elicited during the Stop Signal, Eriksen Flanker and Go/NoGo (NoGo-N2) tasks. It is associated with several processes such detection of response conflict (conflict monitoring), response inhibition or error detection. It is larger for non-targets (which do not require response) and it is usually observed along with the P3a component. |
| 7N2c | 200–350 ms post-stimulus onset | Negative | Auditory: Central Visual: Parieto-Occipital |
Auditory: supratemporal auditory cortex Visual: occipito-temporal regions |
N2c is most frequently elicited during the Continuous Performance and Oddball tasks. It partly reflects the conscious allocation of attentional resources to stimuli indicated as salient, as well as the voluntary switch of attention operated. It is larger for targets and observed along with P3b component. |
| ERP | Time window | Polarity | Distribution | Source | Interpretation |
|---|---|---|---|---|---|
| 8ERN | 80–150 ms after an erroneous response | Negative | Fronto-Central | Anterior cingulate cortex | ERN represents the early automatic detection of an error. This component is considered to reflect a process involved in evaluating the need for, or in implementing, control. The Flanker, Go/NoGo, Stop Signal and Stroop tasks (i.e. paradigms requiring speeded responses) are the most commonly used paradigms for assessing error processing. |
| 9Pe | 200–400 ms after response onset | Positive | Caudal and rostral portions of anterior cingulate cortex | Pe is a slow wave that reflects conscious error recognition. Similar to the ERN, the Flanker, Go/NoGo, Stop Signal and Stroop tasks are the most commonly used paradigms for eliciting this component. | |
| 10FRN | 250–300 ms after feedback presentation | Negative | Fronto-Central | Anterior mid cingulate cortex | FRN follows the performance feedback, being linked to its valence and magnitude. Indexes an early evaluation, through a bottom-up mechanism of the feedback provided by the environment. It may also be an indicator of reward prediction and expectancy violation. |
| 11P3 | 250–600 ms post-stimulus onset | Positive | P3 (or P300) is considered to index a wide variety of neurocognitive processes, including context processing, attention, working memory, response selection, stimulus salience, response inhibition and reward or emotional processing, depending on the type of cognitive processes required by the task. This component is usually divided into two subcomponents: P3a and P3b. | ||
| P3a | 250–350 ms after the stimulus onset | Positive | Fronto-Central | Prefrontal cortex | P3a is mainly elicited by novelty oddball paradigms. This waveform has been associated with the involuntary attention orienting in response to changes in the environment. It reflects the bottom-up saliency that is determined by the novelty of the stimulus. |
| P3b | 300–600 ms after the stimulus onset | Positive | Parietal | Temporo-parietal junction and deeper sources in the thalamus and hippocampus | P3b is frequently elicited by oddball and stimulus selection paradigms. This component has been associated with the voluntary attention and the updating of the stimulus representation in the working memory–usually indicating the top-down classification of the stimulus as relevant or target. In Go/NoGo paradigms, P3b elicited by NoGo stimulus may reflects response inhibition (NoGo-P3) and involve prefrontal regions. Sometimes this component is simply called P3. |
| 12N400 | 300–600 ms post stimulus | Negative | Centro-Parietal | Anterior pre-frontal, superior temporo-parietal cortex and hippocampus and cingulate regions | N400 is a component commonly related to semantic incongruence in language paradigms, it is also typically observed in recognition–recall memory paradigms and is often referred to as an old–new effect. This component is associated with stimulus familiarity and memory trace strength. |
| 13LPC | 500–800 ms post stimulus | Positive | Centro-Parietal | Prefrontal cortex | LPC is related to higher order cognitive processes, such as recognition of the stimuli and decision accuracy/confidence. This component reflects the selection of a response category and the evaluation of the success of a category-related decision or memory match. Sometimes this component is also called P600. |
Fig. 4.
Number of studies exploring the amplitude and latency of each ERP component. Note. VPP: Vertex Positive Potential; ERN: Error-Related Negativity; FRN: Feedback-Related Negativity; LPC: Late Positive Component; Pe: Error Positivity.
Fig. 5.
Number of studies, for each ERP component, that found increased (BDs > Cs), decreased (BDs < Cs), different (ΔBDs ≠ ΔCs), and similar (BDs = Cs) amplitude in BDs when compared to Cs (control group or non/low drinkers). Note. *Significant differences between conditions only in one group.
3.3. Quality assessment
Most of the studies included were of high (44.1%) or intermediate quality (41.2%), while five studies were rated as having poor quality (14.7%). The main limitation of the studies was the disregard of potential confounding factors that could influence the results, such as not having relevant and clearly specified exclusion criteria, and lack of statistical control over confounders. The use of other drugs (including cannabis) and psychoactive substances as well as family history of alcoholism were the most common unspecified confounding factors. Specifically, a total of 13/34 studies (38.2%) did not mention or specifically clarify the consumption of illicit drugs in the sample.
3.4. Resting-state
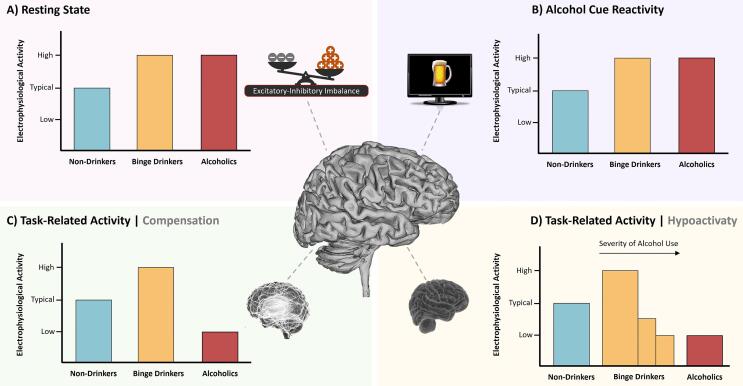
Three studies explored resting-state brain activity –i.e. the electrophysiological recording of oscillatory brain activity while the person is relaxed- and all of them showed spontaneous EEG signal alterations associated with the BD pattern (Affan et al., 2018, Courtney and Polich, 2010, López-Caneda et al., 2017a). Courtney and Polich (2010) compared the brain activity of low-, high- and non-BDs during passive viewing. High-BDs (n = 32; Mage_males = 20.8; Mage_females = 19.9) presented greater spectral power over the frontal, central and parietal regions (namely, the midline electrodes Fz, Cz and Pz) in the delta (0–4 Hz), and fast-beta (20–35 Hz) frequency bands, suggesting that high-BDs display an EEG spectral pattern similar to that observed in alcoholic patients.
The other two studies explored the neural activity of BDs during eyes-open and eyes-closed conditions. The results of López-Caneda et al. (2017a) showed that BDs (n = 40; Mage = 18.1) –comparatively to non/light drinkers- exhibited higher theta and beta power over the bilateral occipital cortex and the right temporal lobe respectively, which was suggestive of a potential neural disinhibition resulting from an excitatory-inhibitory imbalance. Similarly, Affan et al. (2018) found a slower Alpha Peak Frequency as well as increased frontal theta and beta power in the BD (n = 30; Mage = 23.4) relative to the light drinking group. According to the authors, the power increase in theta and beta bands might indicate disrupted excitatory-inhibitory homeostasis in BDs, resembling again the neural activity displayed by alcohol-dependent individuals (e.g. Rangaswamy et al., 2002, Rangaswamy et al., 2003).
Collectively, resting-state EEG studies seem to indicate that BDs, similarly to alcoholic individuals, present abnormal spontaneous EEG signal, mainly characterized by increased power in slow (delta/theta) and fast (beta) frequency bands. These results suggest that BD is associated with a brain overactivity eventually caused by an excitatory-inhibitory imbalance resulting from alterations in the neurotransmitter systems, including the GABAergic and glutamatergic systems (Ward et al., 2009).
3.5. Attention
Ten EEG studies included in this review examined attentional processes by mean of neutral (four studies) and alcohol-related (six studies) stimuli. Six of these studies used different versions of the visual oddball paradigm, which requires the detection of an unexpected stimulus among frequent/repetitive ones, involving bottom-up and top-down aspects of attention (Bledowski et al., 2004). While the former relates to the capacity of a salient stimulus to hold our attention regardless of our intentional goals, the top-down processes refer to our ability to screen the external information considering our current goals, and thus classifying the stimulus as a target (Katsuki and Constantinidis, 2013).
Crego et al., 2012, López-Caneda et al., 2013 used a visual oddball task with neutral stimuli (stars and circles). Crego et al. (2012) explored the amplitude and latency of N2(7) and P3b(11) components (see table 4) and showed that BDs (n = 32; Mage = 18.8) exhibited larger P3b amplitude than controls, specifically for the target condition, with no differences in the N2 component. In a follow-up study that used part of the sample of Crego et al.’s study, López-Caneda et al. (2013) also found larger P3b amplitude in BDs (n = 26; Mage = 18.8) when compared to the control group, a difference that was more pronounced after two years maintaining the BD pattern. According to the authors, these findings seem to indicate that, when compared to healthy subjects, BDs display abnormal neural activity associated with attentional processes (tentatively, an increased attentional resource allocation). Moreover, this anomalous activity tends to increase with the maintenance of BD over the years (López-Caneda et al., 2013).
Contrarily, Maurage et al. (2012) –using a visual oddball task with neutral faces-reported that BDs presented delayed latency and reduced amplitude in the ERP components associated with both early (perception and attention) and later (decision) stages of the cognitive processing. Specifically, when compared to controls, both low-BDs (n = 20) and high-BDs (n = 20; Mage = 21.2) exhibited lower (less negative) N1(2) and lower (less positive) P1(1) amplitude (with high-BDs showing in turn delayed latency in these components), suggesting an impaired early processing of visual stimuli in BDs. Both groups also had reduced N2b(6) amplitude relative to controls, and high-BDs exhibited larger N2b and P3a(11) latencies than the other three groups. These components are associated with adjustment of attention towards stimuli changes (Folstein and Van Petten, 2008), indicating that BD may lead to impairments in attentional control processes. Furthermore, delayed latency and reduced amplitude of the P3b component were also observed in both BD groups, reflecting impairments in the top-down (attentional/working memory) mechanisms involved in stimulus categorization. Lastly, low- and high-BDs displayed decreased N170(3)/P2(5) amplitude (an index of the perceptual processing of human faces), indicating diminished processing of high social value stimuli in BDs. However, the results of Folgueira-Ares et al. (2017) –employing a memory paradigm instead of an attentional task- were not congruent with these findings as they reported absence of differences in N170 amplitude and increased amplitude in the vertex positive potential (VPP)(4), an ERP component also related to the processing of human faces.
Six studies –three from the same research group- tried to examine the attentional bias to alcohol stimuli. Petit et al., 2012, Petit et al., 2014a, and Petit et al. (2014b) used a visual oddball task with neutral pictures as standard stimuli and alcohol and non-alcohol-related pictures as target deviant stimuli. The first study (Petit et al., 2012) showed that in the BD group (n = 18; Mage = 21.3), alcoholic stimuli elicited larger P1 amplitude than non-alcoholic stimuli. This enhanced electrophysiological reactivity at early perceptual level was considered an index of unconscious shift in attention (i.e. an attentional bias) toward alcohol pictures. Similarly, the study of Petit et al. (2013) showed that BDs (n = 29; Mage_females = 21.9; Mage_males = 22.5) tended to elicit higher P3b amplitude to alcohol-related cues, which points again toward an enhanced motivational response to alcohol-related stimuli in these individuals. Finally, in order to explore the long-lasting influence of BD, Petit et al., (2014b) analyzed the alcohol attentional bias twice during a one-year period. The BD group (n = 15) displayed reduced P1 amplitude in the second (T2; Mage = 23.1) relative to the first (T1; Mage = 22.0) evaluation for both alcohol and non-alcohol-related cues, reflecting that the perpetuation of BD over one year may lead to a reduction in the mobilization of attentional resources towards visual information. Furthermore, non-alcoholic stimuli elicited lower P3b amplitude at T2 than in T1 only in the BD group, while P3b amplitudes in response to alcohol-related pictures remained identical. According to the authors, this non-reduction of P3b amplitudes to alcoholic stimuli would reflect the emergence of a bias in the processing of alcohol-related stimuli in youths with a BD pattern.
In the same vein, Ryerson et al. (2017) showed that BD was significantly associated with larger N1 amplitude to alcohol pictures but not to neutral ones, which was suggestive of increased attentional processing of alcohol-related stimuli. Recently, Blanco-Ramos et al., 2019, Lannoy et al., 2020 also explored the attentional bias to alcoholic stimuli during a Go/NoGo task with alcoholic and non-alcoholic beverages. Contrarily to Petit et al. (2012), BDs did not reveal enhanced brain activity to alcohol pictures at the perceptual level (i.e., no differences regarding P1 amplitude).
Altogether, attention studies using neutral stimuli are not conclusive (as one of them observed reduced amplitudes in multiple ERP components including P3b, and the other two studies –conducted by the same research group- reported augmented P3b amplitude in BDs). Regarding the studies using alcohol-related stimuli, those that employed tasks designed to explore alcohol reactivity (4/6; 66.7%), revealed increased brain activity (greater attentional bias) for alcoholic images in BDs. Conversely, the two studies using inhibition tasks did not find significant differences; one explanation for this may be the use of tasks aiming at studying inhibitory processes instead of attentional ones. Additional studies exploring alcohol reactivity are still needed as three studies were conducted by the same group and another one only performed a correlational analysis.
3.6. Emotional processing
Three studies explored the electrophysiological correlates linked to emotional processing in BDs. They used different tasks and all of them revealed alterations in BDs’ brain activity (Huang et al., 2018, Lannoy et al., 2018, Maurage et al., 2009). Huang et al. (2018) used an emotional rating task and analyzed the EEG signal in the time–frequency domain. High-BDs (n = 15; Mage = 23.2) displayed attenuated emotional modulation of event-related theta power and a weaker power in this frequency band to negative and positive pictures, when compared to light-drinkers. Furthermore, light-drinkers, contrary to high-BDs, exhibited higher theta power to emotional relative to neutral images. This was visible in early theta power (peaking at ~250 ms), indicating that BDs may present difficulties in orientation of attention toward stimuli with emotional content, which could negatively influence the evaluation and integration of emotional and cognitive aspects of such stimuli.
In the study of Maurage et al. (2009), the participants were evaluated twice within a nine-month interval throughout an emotional valence judgment task. At the second session, BDs (n = 18; Mage = 18.2) revealed longer P1, N2 and P3b latency than controls (with no differences in terms of amplitude), suggesting that short-term BD, similarly to long-term alcoholism, was associated with a slowed cerebral activity during emotional processing.
Finally, Lannoy et al. (2018) employed an emotional crossmodal task requiring the identification of happiness and anger among three conditions (unimodal, crossmodal congruent, and crossmodal incongruent) and two modalities (visual and/or auditory). ERPs of early perceptual (P1 for visual and N1 for auditory), modality related (N170 for visual and N2 for auditory) and decisional (P3b) processes were analyzed. They found that while non-drinkers had longer N1 latency for happy versus anger voices, the BD group (n = 17; Mage = 20.2) presented no differences for this condition. This might suggest that the adaptive mechanism for processing anger quickly and with lower recruitment of resources seem to be diminished in BDs. Regarding later processes, BDs showed larger P3b amplitude for happy relative to anger faces during congruent trials, while non-drinkers and moderate drinkers did not show such differences. Consequently, BDs seem to benefit from different type of information (i.e. auditory and visual) and requiere more complex cognitive processes to more accurately discriminate emotional content. They also exhibited delayed P3b latency in comparison with moderate and non-drinkers, being suggestive of an impaired capacity to take advantage of crossmodal information. During incongruent trials, BDs displayed larger P3b amplitude than the other two groups, probably reflecting a neurocompensatory mechanism for emotional processing. Additionally, BDs seem to require more resources and take longer to integrate crossmodal information, which was indexed by delayed latency and increased amplitude in two frontal components (peaking between 150 and 260 ms and around 300–500 ms post-stimuli) during the processing of anger in incongruent trials.
Concluding, the studies exploring emotional processing –though scarce- suggest that BDs display difficulties in processing and directing attention toward visual and auditory information with emotional cues (e.g. anger stimuli). Moreover, they seem to recruit more resources to properly process incongruent emotional stimuli (reflected as increased P3b amplitude), as well as to integrate incongruent emotional information (indexed by enhanced and slower frontal activity).
3.7. Working memory
Working memory (WM) refers to the short-term maintenance and manipulation of information (Baddeley et al., 2001). Five studies –three from the same research group- examined the potential effects of BD on WM: four of them using n-back tasks and the fifth one using the Subsequent memory paradigm.
Crego et al. (2009) explored how BD affects brain functioning in college students during a visual identical-pairs continuous performance task, a paradigm similar to the one-back task, where participants are asked to maintain previous stimulus presentations actively in WM in order to detect whether the subsequent stimulus ‘match’ the previous one (Cornblatt et al., 1988, Shalev et al., 2011). In this study, BDs (n = 42; Mage = 18.9) displayed larger N2 amplitude for the matching stimuli in comparison with age-matched controls. Additionally, increased P3(11) amplitude was only observed in the control group for the matching when compared to the non-matching stimuli. The authors argued that the larger N2 amplitude observed in BDs was suggestive of higher allocation of attentional resources in order to be able to perform at successful/adequate levels. Furthermore, the absence of differences in the P3 amplitude between conditions in the BD group suggested a potential deficiency in the electrophysiological differentiation between relevant and irrelevant information.
Similar results were recently reported by Park and Kim (2018) using a modified spatial 2-back task which included congruent, incongruent, and lure conditions. Authors found enhanced P3 amplitude in BD (n = 25; Mage = 22.1) relative to control individuals, which might represent increased cognitive effort towards the classification and updating of information or in the allocation of attentional resources. Moreover, the non-BD group showed larger P3 amplitudes in response to the congruent stimuli compared to the incongruent and lure stimuli whereas the BD group did not differ significantly among the three conditions. Thus, similarly to Crego et al. (2009), results suggested that BD individuals were less efficient in differentiating relevant and irrelevant stimuli due to difficulties in allocating attentional resources for relevant information.
Likewise, Schroder et al. (2019) used an n-back task with three conditions -control (N0), 2-back (N2) and 3-back (N3)- to compare the WM functioning of light-drinkers and BDs (n = 24; Mage = 24.0). To isolate the WM processes, they extracted difference waveforms by subtracting “N2 minus N0” and “N3 minus N0” for the P2/N2 complex, P3 and N400/P600 complex. Overall, young BDs exhibited a higher processing intensity throughout the information-processing stream, reflected as increased P3 and P600(13) amplitude, while light drinkers displayed an increase in early visual attention (i.e. augmented P2 amplitude) in order to obtain a better memory trace (indicated by enlarged N400(12)). Again, and given that BDs performed the task at the same level as light drinkers, this increment in the neural resources can be interpreted as a compensation mechanism to perform at adequate levels in demanding tasks.
Conversely, Crego et al. (2010) observed lower amplitude in the late positive component (LPC(13) or P600) in BDs (n = 42; Mage = 18.9) relative to controls in the match condition of the identical-pairs continuous performance task. In this study, they combined both ERP and exact low-resolution brain electromagnetic tomography analysis (eLORETA). Results revealed that the reduced LPC was associated with a hypoactivation of the right anterior prefrontal cortex. According to the authors, these findings were indicative of functional alterations also in later stages of the cognitive processing stream such as recognition and self-monitoring of the WM process.
Folgueira-Ares et al. (2017) aimed to explore the brain activity during memory encoding using a Subsequent memory paradigm. This paradigm, which use a face–name pairs association task with subsequent memory testing, enables the evaluation of the Difference due to memory effect (Dm), an electrophysiological measure based on the comparison of the brain activity associated with subsequent successful and unsuccessful retrieval (Paller and Wagner, 2002). Contrary to controls, BDs (n = 25; Mage = 20.8) did not reveal a Dm effect, indicating a lack of electrophysiological differences between successful (subsequently remembered) and unsuccessful (subsequently forgotten) memory encoding.
Overall, findings seem to point to a number of electrophysiological anomalies linked to the performance of WM tasks in BDs, namely involvement of higher attentional/cognitive resources (as reflected by increased N2 and P3 amplitude), impaired differentiation between relevant and irrelevant information, as well as anomalous processing during memory encoding. However, results regarding later stages of WM have provided mixed evidence, so future studies will be needed to clarify the effects of BD on this cognitive process.
3.8. Cognitive control
Cognitive control comprises a group of subprocesses, which recruit different regions of the prefrontal cortex (Miller, 2000). The goal-directed action selection, response execution/inhibition, performance monitoring, and reward-based learning are the main constituent processes of cognitive control (Ridderinkhof et al., 2004). Given that performance monitoring involves the adjustment of ongoing behavior in order to optimize subsequent performance (e.g. the execution and inhibition of a sequence of responses), inhibitory control and performance monitoring are interrelated aspects of cognitive control (Chevrier et al., 2007, Verbruggen and Logan, 2008). As such, half of the studies exploring response inhibition in young BDs have also assessed ERP components linked to performance monitoring capacity.
3.8.1. Performance monitoring
Successful goal-directed behavior involves not only correct selection and execution of a response but also the ability to flexibly adjust behavior when performance problems occur or the environment changes (Ullsperger et al., 2014). Performance monitoring, i.e. the ability to concurrently monitor and rapidly evaluate outcome of one’s actions, plays a major role in everyday life by allowing behavioral adaptation in response to changing environment demands (Peterburs et al., 2015).
Potential impairments in performance monitoring associated with BD have been assessed in eight studies, four by the same research group. Seven of these studies pointed to abnormal neural activity in BDs in comparison with their control peers. Two studies used a Stop-Signal task (Smith and Mattick, 2013, Smith et al., 2016) to explore the Error-Related Negativity (ERN(8)) amplitude during successful and failed inhibition trials. Smith and Mattick (2013) compared two groups of females, BDs (n = 13; Mage = 20.0) and non-BDs, and observed lower ERN amplitude among the BD group, which might reflect difficulties in monitoring their ongoing performance. Smith et al. (2016) used the same sample of females and included a new sample of males (n = 21; Mage = 19.8). They also found – although marginally significant - decreased ERN (p = 0.06) in BDs when compared with controls, indicating that the electrophysiological alterations observed in the previous study probably are common markers of BD and not gender specific.
The ERN amplitude was also explored by Kim and Kim (2019) during a modified Flanker task (i.e. with higher level of difficulty) to assess error-monitoring mechanisms in female BDs. In the same line of Smith et al.’s results, female BDs (n = 25; Mage = 21.4) displayed a lower ERN amplitude when compared to non-BDs. This decreased ERN suggests that young females engaged in BD have an impaired capacity to automatically monitor errors, which may lead to difficulties in the adjustment of their internal performance. The authors also analyzed the error positivity (Pe(8)), but no significant effect of group was observed.
In contrast, Lannoy et al. (2017), using a Speeded Go/NoGo task, showed larger ERN amplitude during classical commission errors (false alarms) in comparison with slow hits (correct categorization that is performed beyond the response time) in BDs (n = 20; Mage = 20.3). According to the authors, BDs may have difficulties in judging their own errors as a failed response in NoGo trials (being reflected by the recruitment of greater neural resources), and this impaired insight may in turn compromise the subsequent learning and adjustment processes. Furthermore, they exhibited a delayed Pe latency for slow hits compared to the control group, pointing to a slower error processing, i.e. BDs would need more time to adjust their behavior after a late response. Conversely, Smith et al., (2017a) used an error awareness task, where participants needed to signal the awareness of inhibitory errors on the subsequent trial. They also analyzed the Pe to explore conscious error detection and the ERN to assess early pre-conscious error detection. Despite BDs (n = 25; Mage = 22.2) had committed more inhibitory errors than controls, they exhibited no brain alterations when compared to controls, reflecting preserved error awareness.
Smith et al. (2015) explored the performance monitoring processes by an Eriksen Flanker task. The authors examined the conflict adaptation effect (a marker of well-adapted monitoring of ongoing performance) in RT, errors, and N2 amplitude and they observed a different pattern of results among females and males. While female BDs (n = 15; Mage = 21.0) exhibited larger conflict adaptation for N2 amplitude than the female controls, male BDs (n = 16; Mage = 23.0) showed decreased N2 amplitude and no differences in the conflict adaptation effect in comparison with the male controls. Once increased conflict adaptation is associated with efficient adaptive performance monitoring (Larson et al., 2014), this pattern of results may be suggestive of a compensatory response such that female BDs need to increase ongoing performance monitoring in order to achieve the same behavioral outcome as controls.
Likewise, Blanco-Ramos et al. (2019) analyzed the NoGo-N2 amplitude to assess conflict monitoring in BDs. As mentioned above, they employed a Go/NoGo task with alcohol and non-alcohol related pictures. The task has two conditions: the alcohol condition (Go-Alcohol), in which the Go stimuli were pictures of alcoholic drinks and the NoGo stimuli were pictures of non-alcoholic drinks; and the non-alcohol condition (Go-NoAlcohol) with the non-alcoholic drinks as Go stimuli. BDs (n = 71; age range: 18–19) revealed an enhanced NoGo-N2 amplitude for non-alcoholic than for alcoholic drinks. Considering the conflict monitoring theory, during the Go-Alcohol condition, alcoholic stimuli may turn the prepotent Go response more activated and thus increase the conflict between the prepotent response and its inhibition over NoGo trials (Blanco-Ramos et al., 2019). Consequently, this increased conflict leads BDs to recruit additional neural resources (as indexed by increased NoGo-N2) to successfully inhibit the primed (Go-Alcohol) response.
Performance monitoring is intimately linked to decision making and reward processing (Ullsperger and von Cramon, 2004). Accordingly, some studies exploring performance monitoring used tasks that have decision making and reward sensitivity as underlying cognitive processes. As such, Lannoy et al. (2017) examined the electrophysiological correlates linked to motivational (reward sensitivity) systems during performance monitoring, using the Balloon Analogue Risk Task. In this task participants are asked to pump a balloon in order to obtain a reward. This allowed them to explore the Feedback-Related Negativity (FRN(10)) and P3 waveforms. However, the results revealed intact feedback-related components in BDs (n = 20; Mage = 20.3).
The FRN amplitude was also explored in a recent study of Na et al. (2019) aiming at examining the early stages of feedback processing in female BDs. Specifically, they used a modified version of the original computerized Iowa Gambling Task (IGT) to analyze potential differences in the FRN amplitude between gain and loss feedback (ΔFRN). During IGT, participants were asked two choose one of four cards (two cards resulting in large immediate gains but greater long-term losses and two cards resulting in small immediate gains but reduced long-term losses) in order to earn as much money as possible over the task. The BD group (n = 27; Mage = 21.4) showed lower ΔFRN amplitudes than the non-BD group, revealing a deficit in early feedback evaluation, which may compromise their decision-making abilities and, consequently, lead them to choose more disadvantageous choices. Additionally, they analyzed ΔP3 (i.e. differences in the P3 amplitude between gain and loss feedback) and P3 amplitude to assess the late feedback processing as well as the allocation of attentional resources through top-down mechanisms; however, no group differences were observed in this component.
In sum, the enhanced N2 amplitude appears to support the idea that BDs need to recruit additional resources to compensate for potential neural anomalies/dysfunctions, in this case, during performance monitoring. Regarding ERN, BDs seem to exhibit decreased amplitude as reported by three of the five studies exploring this waveform. This reduced ERN may suggest that BDs have difficulties in automatically identify their errors, which could compromise their capacity to monitor/adapt their performance. However, studies are still required to give additional support to these findings.
3.8.2. Inhibitory control
Inhibitory control is crucial for behavior optimization as it enables the suppression and the control of inappropriate or impulsive responses and actions (Diamond, 2013, Ridderinkhof et al., 2004). This executive function allows people to regulate and adapt their behavior according to the demands of the surrounding environment and to long-term goals (Allom et al., 2016). Inhibitory control is particularly important in the study of BD, once its impairment may contribute to the maintenance of alcohol-seeking behavior (Field et al., 2010, López-Caneda et al., 2014a). In the present review, ten studies exploring inhibitory control were included (with a total of four different tasks) and seven of them reported electrophysiological alterations associated with BD. The studies by López-Caneda et al., 2012, López-Caneda et al., 2014b, and López-Caneda et al. (2017b) employed an equiprobably Go/NoGo task with two Go and two NoGo neutral stimuli. Results of the longitudinal study (López-Caneda et al., 2012) showed that BDs (n = 23; Mage = 18.8) displayed increased NoGo-P3 amplitude, which was associated with a greater activation of the right inferior frontal cortex. This enlarged activity linked to inhibitory control in BDs relative to controls was suggestive of the activation of additional neural mechanisms in order to compensate emerging functional alterations in the regions engaged in inhibition. Using part of the same sample and including an additional group of ex-BDs, López-Caneda et al. (2014b) observed that after maintaining a BD pattern for at least two years, BDs (n = 22; age range: 18–19) displayed significantly larger NoGo-P3 amplitude than controls, whereas ex-BDs (n = 10; age range: 18–19) were in an intermediate position between the two other groups (with no significant differences with respect to controls or BDs), suggesting that cessation of BD may act as a brake on the progression of the neurophysiological impairments related to response inhibition.
Similarly, the two studies of Smith et al.’s mentioned in the previous section (Smith et al., 2016, Smith and Mattick, 2013) also explored the P3 component for the successful and failed inhibition trials. The authors reported that females with a BD pattern showed lower P3 amplitude during failed inhibitions than the control ones, which together with the higher number of failed inhibitions may indicate that females engaged in BD have poorer inhibitory control than female controls (Smith and Mattick, 2013). Additionally, they displayed larger P3 amplitude during successful inhibition when compared with controls, suggesting that BDs need to trigger the inhibition process more strongly than controls to effectively inhibit their responses. Smith et al. (2016) also found a marginally significant successful > failed effect for the P3 amplitude (p = 0.08) in the BD group (i.e. females and males), suggesting that the neural abnormalities associated with inhibition presented in the previous study are common to both sexes. The other two studies conducted by this research group did not show alterations in BDs’ brain activity relative to control individuals (Smith et al., 2015, Smith et al., 2017a).
Two studies used Go/NoGo tasks with alcoholic and non-alcoholic stimuli to explore how alcohol-related stimuli may modulate the inhibition of a prepotent response in BDs (Blanco-Ramos et al., 2019, Lannoy et al., 2020). Blanco-Ramos et al. (2019) analyzed the NoGo-P3 amplitude and the results showed larger amplitude in response to NoGo-NoAlcohol than in NoGo-Alcohol trials only in controls and female BDs. Conversely, male BDs did not show such differences. According to the authors, the absence of differences in male BDs suggests that the motivational value of the alcoholic stimuli may be interfering in their inhibitory processes. Using a similar task, Lannoy et al. (2020) observed that BDs (n = 25; Mage = 20.9) exhibited lower NoGo-N2 amplitude for alcoholic stimuli at left (F3) relative to right (F4) frontal sites, while non/light drinkers showed augmented NoGo-N2 amplitude. According to the authors’ interpretation, this reduced NoGo-N2 suggest that attentional/inhibitory processes of BDs may be impacted when alcohol cues are presented, which could be accounted for by an imbalance between the (underactivated) reflective system and the (overactivated) automatic system.
López-Caneda et al. (2017b) analyzed the Go-P3 and NoGo-P3 components and performed a time–frequency analysis to explore the brain oscillations linked to inhibition. Although BDs (n = 36; Mage = 18.1) did not show alterations in the ERP components, they exhibited reduced theta and beta power in comparison with the control group in the NoGo condition. These results were in line with those reported in studies with abstinent chronic alcoholics (e.g. Colrain et al., 2011, Pandey et al., 2016) and they are suggestive of dysfunctions in the oscillatory activity linked to response inhibition. Recently, Holcomb et al. (2019) has also reported decreased power in theta and early beta frequency bands during NoGo trials in BDs (n = 29; Mage = 23.4) when compared to low drinkers. The attenuated theta power may be suggestive of impaired top-down mechanisms involved in IC processes.
Taken together, BDs seem to present abnormal neural activity related to inhibitory control. More specifically, they show enhanced brain activity to successfully inhibit their responses. This overactivation likely reflects a neurocompensatory mechanism that allow BDs to counteract for an underlying deficit resulting from excessive alcohol drinking. Furthermore, these deficits in inhibitory control seem to be also supported by decreased oscillatory activity, particularly reduced theta power. Finally, additional studies exploring inhibitory control mechanisms with alcoholic and non-alcoholic stimuli are still needed to clarify the results of the two studies conducted so far.
3.9. Other cognitive functions
Some of the studies included in this review investigated the neural adversities of BD through tasks involving time perception, verbal episodic memory and risk-taking reactivity.
Bauer and Ceballos (2014) used a motor time estimation task and the results showed that frequent BDs (n = 42; Mage = 19.4) displayed an augmented (i.e. more negative) Slow Potential amplitude than the infrequent BDs. According to the authors, the enhanced Slow Potential observed in females with frequent episodes of BD seems to reflect a compensatory overactivation of the neural circuit responsible for time estimation (a region involving the right posterior parietal cortex).
Verbal episodic memory was explored by Smith et al. (2017b) during a modified version of the Rey Auditory Verbal Learning Task (RAVLT). They used a visual presentation modality of the words and examined the ERPs in the recall phase (P185), for remembered and not remembered words, and in the recognition phase (N340 and P540). While there were no behavioral differences neither in the recall nor in the recognition phase, BDs (n = 39; Mage_females = 20.0; Mage_males = 19.7) exhibited increased amplitude of the P540 waveform -an index of recollection also known as the classical parietal old/new effect (Rugg and Curran, 2007)- relative to drug-naïve controls, suggesting a greater need for recollection-based recognition during task execution in BDs.
Finally, Kiat and Cheadle (2018) assessed decision-free risk-reactivity as a function of BD frequency levels using the Crocodile Dentist task, a risk-taking game that allows the identification of raw risk-taking reactivity (i.e. isolated from decision making processes). Results revealed larger increase in the amplitude of the Late Positive Potential –a waveform equivalent to the P3 component- as participants transitioned from low- to high-risk levels of BD, suggesting enhanced risk-taking reactivity in individuals who are involved in excessive drinking.
3.10. Alcohol variables and ERPs
Nearby half of the studies of the present review (16/34; 47.1%) assessed whether a number of alcohol use variables were associated with electrophysiological dysfunctions. These variables were mainly related to the age of drinking onset and the quantity and intensity of drinking, such as speed of alcohol consumption, weekly amount of alcohol use, number of BD episodes and AUDIT score (see supplementary material for detailed results).
Overall, the results reported might reveal that different alcohol use variables are associated with some of the neural abnormalities observed in BDs. As such, though correlation-type analyzes should be carefully interpreted, results suggest that early onset of (regular) drinking could increase the vulnerability to display abnormal brain activity (e.g. enhanced P1 and P3 amplitude, slower spontaneous alpha peak frequency; Affan et al., 2018, Blanco-Ramos et al., 2019, López-Caneda et al., 2013, López-Caneda et al., 2014b). Further, increased amounts of alcohol consumption as well as faster and hazardous drinking may enhance the susceptibility to exhibit disrupted neural activation during cognitive functioning and even at rest. Specifically, greater intensity and quantity of drinking was associated with enhanced neural activity during attentional and inhibitory processes (i.e. increased P3 amplitude; López-Caneda et al., 2013, López-Caneda et al., 2014b, Smith and Mattick, 2013), performance monitoring (i.e., larger N2 amplitude; Blanco-Ramos et al., 2019, Smith et al., 2015), attention to alcohol-related information (i.e. higher P1 amplitude; Petit et al., 2012, Petit et al., 2014b) and during resting-state (i.e. greater theta power; Affan et al., 2018). Some studies showed, in turn, that more hazardous drinking was predictive of decreased electrophysiological activity during early pre-conscious error detection processes (as indexed by reduced ERN amplitude; Kim and Kim, 2019, Smith and Mattick, 2013) and during response inhibition (reflected by decreased theta power; Holcomb et al., 2019). Four studies conducted regression analysis with alcohol-related variables and EEG measures (Blanco-Ramos et al., 2019, López-Caneda et al., 2014b, Petit et al., 2014b, Smith et al., 2016). Two of them found that alcohol quantity and drinking intensity as well as age of drinking onset may predict brain abnormalities in response inhibition, namely the recruitment of larger neural resources (i.e. increased NoGo-N2 and NoGo-P3; Blanco-Ramos et al., 2019, López-Caneda et al., 2014b).
3.11. Sex-related effects
The majority of the studies reviewed (23/34; 67.6%) explored differences between males and females. However, only five of them found dissimilar patterns of neural activity between sexes (Blanco-Ramos et al., 2019, Courtney and Polich, 2010, Petit et al., 2013, Smith et al., 2015, Smith et al., 2016).
In the study of Courtney and Polich (2010), during passive viewing, female BDs displayed an augmented power in delta, theta, and slow- and fast-beta bands relative to males, which may be caused by an increased sensitivity to high amounts of alcohol in females than males. Further, as mentioned above, female and male BDs presented an opposite pattern of results in the study of Smith et al. (2015), with females showing more affected EEG signal during performance monitoring. Similarly, Smith et al. (2016) only showed significant differences between BDs and controls in P3 latency in females, who revealed delayed P3 latency for failed relative to successful inhibitions.
Conversely, Petit et al. (2013) found that alcoholic stimuli evoked larger P3 amplitude in comparison with non-alcoholic stimuli only in male BDs. The results suggest that, contrary to females, males have an attentional bias to alcohol-related information. Likewise, as previously reported in the study by Blanco-Ramos et al. (2019), male BDs, unlike to females, revealed alterations in the neural activity associated with inhibitory control processes during a context of predominant alcoholic stimuli.
Overall, only five of the 23 studies exploring sex-related differences revealed a differential response between males and females. In addition, results from these five studies have provide mixed evidence, as three of them reported more pronounced abnormalities in female BDs, while the other two informed of electrophysiological anomalies only in BD males. In conclusion, results do not seem to support specific gender vulnerabilities to the effects of BD, at least at the electrophysiological level.
3.12. Behavioral performance
The impact of BD on the behavioral performance was explored by 29 of the 31 studies (the remaining three studies analyzed the brain activity at rest). Remarkably, only seven of these 29 studies (24.1%) – four from the same research group - showed significant differences between BDs and non/low drinkers during tasks involving inhibitory control, performance monitoring and verbal episodic memory (Kim and Kim, 2019, Lannoy et al., 2020, Na et al., 2019, Smith and Mattick, 2013, Smith et al., 2016, Smith et al., 2017a, Smith et al., 2017b).
According to these studies, BDs exhibited poor response inhibition ability in comparison with controls, being reflected by longer stop-signal reaction times (Smith and Mattick, 2013, Smith et al., 2016) and greater number of commission errors (to NoGo Stimuli) during an error awareness task (Smith et al., 2017a). Also, BDs showed poorer inhibition performance for explicit than implicit processing of alcohol-related information (Lannoy et al., 2020). Female BDs also exhibited more errors and shorter RT than non-BDs in a Flanker task, suggesting an impaired capacity to monitor their errors effectively and, consequently, adjust their performance (Kim and Kim, 2019). In the study of Na et al. (2019), females BDs presented a lower total net score in the IGT when compared to non-BDs, reflecting more disadvantageous choices. While non-BDs performance improved over the task, BDs persistently chose cards with more immediate gains even at greater potential risks. Therefore, according to the authors, young BD females seem to have deficits in decision-making, probably due to difficulties in adjusting their performance according to the goal underlying the task (i.e. maximize their profit) or a higher reward sensitivity. Concerning verbal episodic memory, BDs showed poor delayed recall in comparison with controls (Smith et al., 2017b).
Concluding, 22 out of 29 studies analyzing behavioral performance reported absence of significant differences between BDs and their control peers.
4. Discussion
The present work systematically reviewed the EEG signatures associated with a BD pattern in adolescents and young adults and provided a qualitative synthesis of the literature. Thirty-four studies fulfilled the inclusion criteria, including 28 ERPs studies, two EROs studies, one study examining both ERPs and EROs, and three resting-state EEG studies. Thirteen of the 29 ERP studies (44.8%) pointed to enhanced brain activity (i.e. increased ERP amplitude) in BDs when compared with non/low drinkers. In contrast, six of them (20.7%) found a reduced electrophysiological signal in BDs and ten studies (34.5%) found significant ERP differences between conditions (e.g. alcohol/non-alcohol stimuli, remembered/forgotten items) only in one group1. Finally, only three studies (10.3%) showed no differences in the ERP amplitude between groups nor between conditions (see supplementary Figure S1). Regarding latencies, only four studies out of 29 (13.8%) found significant between-groups differences, with BDs exhibiting delayed latencies. All the EROs studies found reduced oscillatory brain activity in BDs when compared to controls, namely lower theta and beta power. Conversely, the three studies exploring resting-state reported augmented spontaneous EEG signal in young BDs.
4.1. The compensation and continuum hypotheses
Considering the results highlighted in this review, the most solid electrophysiological finding is the augmented P3 amplitude observed in tasks involving attention, working memory and response inhibition, which could constitute an early biomarker for BD. Indeed, eight studies (34.8%) analyzing between-groups differences and three studies exploring between-conditions differences (13.0%) –i.e., a total of 47.8% of the 23 studies examining P3 –reported increased amplitude in this component in the BD group –in contrast to a single study (4.3%) reporting reduced P3 amplitude. This enlarged P3 has generally been interpreted in terms of a neurocompensatory mechanism enabling BDs to perform the tasks on par with their control peers.
Compensation is usually defined as a brain overactivation accompanied by a satisfactory level of functioning, i.e., an excess of neural activity not seen in the comparison group and associated with normal-level performance (Chanraud and Sullivan, 2014). Concordant with this approach, the findings reported here fall in line with the neurocompensation hypothesis, suggesting that BDs need to reallocate neural resources to compensate for an underlying neural deficit, which would allow them to successfully perform the task. In this sense, in the present review 13 studies showed enlarged electrophysiological activity (not only reflected in the P3 component, but also in other waveforms such as VPP, N2 or P600) in BDs when directly compared to control peers. Twelve of these studies compared the behavioral performance between groups and most of them (11/12; 91.7%) did not find significant between-groups differences. Conversely, from the seven studies reporting poor performance in the BD group, in five of them (71.4%) BDs displayed reduced ERPs amplitude relative to non/low drinkers. Collectively, these results suggest that compensation –in the form of increased neural activity/recruitment- may play an important role at least at the initial stages of BD. This proposal is in accordance with several neuroimaging studies which have showed enhanced activity in different brain areas in BDs when compared to controls, with no alterations in task performance (Brumback et al., 2015, Campanella et al., 2013, Correas et al., 2020, Molnar et al., 2018, Squeglia et al., 2011, Suárez-Suárez et al., 2020, Wetherill et al., 2013).
On the other hand, young BDs also seem to exhibit some neural alterations similar to those observed in alcohol-dependent individuals, which brings support to the proposition postulating that BD and alcohol use disorder (AUD) are two successive steps of the same phenomenon –proposal known as the continuum hypothesis (Enoch, 2006, Lannoy et al., 2019). The suggestion that BD and AUD may lead to qualitatively analogous impairments is reinforced by several findings synthesized in the present review. First, resembling the typical cerebral hyperexcitability during resting states observed in alcoholics (Rangaswamy and Porjesz, 2014, Rangaswamy et al., 2002, Rangaswamy et al., 2003, Mumtaz et al., 2017), BDs also exhibited increased brain activity at rest, both in slow waves (primarily in the theta band) and fast waves (beta band) (Affan et al., 2018, Courtney and Polich, 2010, López-Caneda et al., 2017a). Despite the genesis of these anomalies has not been totally clarified yet, it has been suggested that this hyperexcitability may be due to an excitatory-inhibitory disequilibrium of the central nervous system (CNS) (Courtney and Polich, 2010, Porjesz and Begleiter, 2003). Given that alcohol acts as a CNS depressant, mainly reducing the glutamatergic excitatory neurotransmission and increasing the GABAergic inhibitory activity, repetitive heavy alcohol intake leads to adaptive changes (allostasis) resulting in downregulated GABA-mediated inhibition and upregulated glutamate-excitatory function (Abrahao et al., 2017, Roberto and Varodayan, 2017). When alcohol is withdrawn (for instance, during a period of abstinence), a rebound effect known as the kindling effect –and characterized by excessive brain excitability- occurs, which may result in brain damage (Becker, 1998, Stephens and Duka, 2008). Although BD is not associated with withdrawal symptoms as noticeable as those reported in alcoholics, the frequent alternation between excessive drinking episodes and abstinence might induce a kindling process leading to brain hyperexcitability. However, given that this brain overactivation has also been seen prior to the onset of alcohol drinking –e.g., in offspring of alcoholics (Kamarajan, 2019, Rangaswamy et al., 2004)-, the question of whether these impairments are present before the beginning of the excessive alcohol use or result from the neurotoxic effects of this substance still needs to be addressed through additional research.
Secondly, besides the increased resting (tonic) oscillations –mostly in the theta and beta bands- reported in both BDs and AUD individuals, decreased “active” (phasic) oscillations have also been observed in both groups –particularly in the theta band- during emotional/cognitive tasks (Correas et al., 2019, Kamarajan et al., 2004, Pandey et al., 2016, Holcomb et al., 2019, Huang et al., 2018, López-Caneda et al., 2017b). Again, these similarities between BDs and AUDs dovetail with the idea that BD may lead to consequences analogue to those of AUD, a thesis already pointed out by some neuropsychological studies (e.g., Sanhueza et al., 2011). In addition, these findings strengthen the notion that ERO measures –which contains not only brain oscillations not rigidly time‐ and phase-locked to the stimulus, but also nonphase‐locked EEG activity (Pfurtscheller and Da Silva, 1999)- may provide additional information on brain functioning and thereby be more sensitive than the ERPs. However, further research analyzing EROs in the BD population are required to determine whether these anomalies are also mirrored in other cognitive processes (e.g., conflict monitoring, working memory).
Finally, another additional support to the continuum between BD and AUD is the augmented neural reactivity to alcoholic cues reported in both BDs and alcohol-dependent individuals. As such, several EEG studies indicate that the alcohol cue-reactivity typically observed in AUDs (Herrmann et al., 2000, Namkoong et al., 2004) is also present in young heavy and binge drinkers (Bartholow et al., 2007, Herrmann et al., 2001, Petit et al., 2013). Consistent with these findings, neuroimaging data have revealed amplified brain responses to alcohol cues in both BDs (Brumback et al., 2015) and AUDs (Huang et al., 2018, Tapert et al., 2004). This high motivational reactivity to alcohol-related cues deserves special attention in the initial stages of BD –or even before the transition to BD (Dager et al., 2013)-, as it is strongly related to craving (Bollen et al., 2020, Flaudias et al., 2019) and thus may greatly contribute to the perpetuation and/or escalation of alcohol consumption (Field and Eastwood, 2005, Manchery et al., 2017). Indeed, this evidence becomes even more worrying when the impairments in inhibitory control reported by EEG (Smith and Mattick, 2013, Smith et al., 2017a) and neuropsychological (Bø et al., 2016, Czapla et al., 2015) studies in young BDs are taken into consideration. The combination of altered inhibitory control together with augmented reactivity to alcoholic stimuli may constitute a risk factor for the escalation towards alcohol dependence. Accordingly, future studies should strengthen the research on the interplay of inhibition and alcohol bias, for example through still unexplored functions such as memory inhibition (López-Caneda et al., 2019b) or via neurocognitive stimulation (Dormal et al., 2020). Indeed, examining the extent to which BDs or individuals at early stages of alcohol abuse may have difficulties to inhibit alcohol-related information could hold important clinical implications.
Globally, the results reported in this systematic review appear to support both the compensation and the continuum hypothesis (see Fig. 6). Thus, young BDs seem to display a similar profile as that of alcohol-dependent subjects in activities that require low cognitive effort, such as resting states or during visualization of alcohol pictures. Conversely, during tasks demanding a high involvement of cognitive resources, BDs would need to activate additional brain regions to counteract underpinning neural decline and to maintain performance. However, giving that some studies have also showed decreased electrophysiological activity in BDs during performance of cognitive tasks, one question that remains to be addressed is which impairments are compensated for –and for how long- and which ones not.
Fig. 6.
Schematic depiction of the similarities and differences in the electroencephalographic (EEG) profile of binge drinkers (BDs) and alcohol-dependent individuals. Young BDs seem to display a similar profile as that of alcohol-dependent subjects during (A) resting state, and (B) visualization of alcohol-related pictures. Bottom left (C), representation of the brain overactivation observed in BDs during some cognitive tasks –accompanied by a satisfactory level of performance- presumably related to a neurocompensatory mechanism. Bottom right (D), illustration of how BDs’ electrophysiological activity resembles that of alcoholics as the severity of alcohol use is sustained and/or increases over time.
The preliminary findings from studies exploring performance monitoring suggest that BDs exhibit electrophysiological deficits (indexed as reduced ERN/FRN amplitude) during more automatic or even low demanding processes of error/feedback detection instead of during more cognitively demanding tasks. Noteworthy, this abnormal neural activity may underlie difficulties in monitoring internal behavior as most of the studies showing decreased ERN and FRN also reported the commission of more errors and poorer behavioral outcomes in BDs (Kim and Kim, 2019, Na et al., 2019, Smith and Mattick, 2013, Smith et al., 2016). Electrophysiological studies exploring acute alcohol consumption are consistent with these findings, also showing diminished ERN (Bailey et al., 2014, Bartholow et al., 2012, Easdon et al., 2005). Likewise, alcoholics (Kamarajan et al., 2010) and individuals with high family history density of alcohol problems (Fein and Chang, 2008) revealed reduced amplitude in feedback/outcome related negativity components. However, BD studies exploring conflict monitoring processes using experimental paradigms requiring greater cognitive resources –including a speeded Go/NoGo task, a beverage (alcoholic/non-alcoholic) Go/NoGo task, and a Stroop Go/NoGo task (Blanco-Ramos et al., 2019, Lannoy et al., 2017, Smith et al., 2017a) have failed to show alterations in the amplitude of ERN or N2 in BDs when directly compared to controls. Despite further replication is needed, this evidence may suggest that some electrophysiological deficits (e.g., decreased ERN) might remain hidden when a high allocation of resources is required (since BDs would be trying to compensate the underlying neural deficit), whereas in tasks lacking such behavioral difficulty these ERP anomalies would be apparent. Of course, this assumption deserves additional research, particularly because the question as to why other cognitive processes (e.g., working memory, inhibition) do not show this pattern of results remains elusive.
In this sense, besides the cognitive demands, other factors may underlie the apparent discrepancies between some studies, including the task characteristics/complexity (e.g., simple geometric stimuli vs faces processing in the attentional paradigms), or the sample composition/features –as the number of total standard alcoholic drinks per week might range from 7.3 to 42.9 drinks within the various BD profiles. Thus, it is possible that the P3 and other ERP components may vary from decreased to increased amplitude as a function of the consumption severity, showing the high-BDs an electrophysiological profile relatively equivalent to that of alcohol-dependent individuals (i.e. reduced neural activity) –as both displayed similar levels of alcohol consumption. Otherwise, BDs with lower alcohol use would still be able to compensate for the alcohol-related damage, thereby displaying increased neural activity. Accordingly, while BD studies reporting smaller amounts of alcohol consumed per drinking occasion (i.e., from 21 g to 56 g of alcohol) showed augmented brain activity (Crego et al., 2009, López-Caneda et al., 2012, Schroder et al., 2019), other studies where BDs drank larger amounts of alcohol per occasion (i.e., from 81 g to 132 g of alcohol) revealed decreased brain activity (Lannoy et al., 2020, Maurage et al., 2012). However, this proposal clearly deserves further investigation, particularly with studies comparing BDs and AUD patients. Likewise, the absence, not only of a unified criterion to define BD (see below), but also of standardized measures to quantify alcohol use –e.g., no. of drinks per occasion (not reported in 35% of the studies) or no. of drinks per week (not reported in 59% of the studies)- makes it hard to extract reliable conclusions between the levels of alcohol use and the direction (increase vs decrease) of EEG activity.
4.2. Brain functional abnormalities – Cause or consequence of BD?
It is important to note that some of the neurofunctional anomalies referred here might antecede the onset of the BD habits and not be a direct consequence of excessive alcohol use. As such, several neuroimaging studies have found abnormal brain functioning before the beginning of BD (Jones et al., 2016, Norman et al., 2011, Squeglia et al., 2012, Wetherill et al., 2013), reflecting predisposing neural risk markers for excessive alcohol use. However, new anomalies associated with the onset and maintenance of BD seemed in turn to emerge years later (Squeglia et al., 2012, Wetherill et al., 2013). Thus, the pattern of results obtained from fMRI studies appear to point to both directions: 1) to a distinctive neural activity profile prior to the onset of alcohol drinking (i.e., reduced activity in frontoparietal regions), which may represent a predisposition to engage in future BD; and 2) to anomalies in brain function emerging as a consequence of BD, indexed by increased activity in frontoparietal regions once the BD pattern has been initiated.
With regard to ERP studies, reduced P3 amplitude has frequently been observed in offspring of alcoholics as well as in individuals with high risk for developing AUD, both prior to any alcohol exposure (Campanella et al., 2009, Fein and Chang, 2006, Holguín et al., 1999). These findings have led to the hypothesis that the low P3 amplitude may precede (and constitute a risk for) development of AUD, rather than being a consequence of alcohol abuse (Kamarajan, 2019, Perlman et al., 2009, Porjesz et al., 2005). Similarly to neuroimaging studies, both assumptions (cause vs consequence) are not mutually exclusive. Longitudinal evidence available concerning BD, although scarce, seem to point to a worsening and/or emergence of neurophysiological abnormalities with the maintenance or transition to this pattern (López-Caneda et al., 2013, Maurage et al., 2009). Additionally, the fact that the age of drinking onset and the main variables defining BD (quantity, frequency and intensity of alcohol use) are correlated with the amplitude values of several ERP components (Affan et al., 2018, Blanco-Ramos et al., 2019, Holcomb et al., 2019, Kim and Kim, 2019, López-Caneda et al., 2013, López-Caneda et al., 2014b, Petit et al., 2012, Petit et al., 2014b, Smith and Mattick, 2013, Smith et al., 2015), gives additional support the notion that certain alterations in brain function may constitute a consequence of the BD pattern. Of course, this does not rule out the hypothesis that some EEG anomalies may precede –and thus constitute markers of susceptibility for- BD. Unfortunately, to the best of our knowledge only one ERP study has assessed BDs before they started their drinking habits, and no significant electrophysiological differences were observed between groups (future BDs and non-BDs) at baseline (Maurage et al., 2009). Hence, despite that neuroimaging studies and EEG research in high-risk populations suggest that certain neurofunctional anomalies may precede alcohol consumption, the evidence to date does not allow to establish a clear EEG/ERP endophenotype of BD. Future longitudinal studies are thereby needed to disentangle potential risk markers for future BD from consequences of alcohol use.
In a related vein, the lack of longitudinal studies also precludes us to reliably determine the potential impairments caused by the perpetuation of BD or to conclude whether these impairments are cumulative over the years. The recovery after BD clearly deserves further attention, as the only EEG study conducted suggested that the cessation of BD may act as a brake on electrophysiological impairments (López-Caneda et al., 2014b). Additional support for these observations comes from neuropsychological studies (Carbia et al., 2017a, Carbia et al., 2017b, Mota et al., 2013, Winward et al., 2014a, Winward et al., 2014b) and a neuroimaging study (Brumback et al., 2015), demonstrating that abandoning the BD pattern may –at least partially- mitigate some of the alcohol-related dysfunctions. However, studies remain scarce and thus additional research should focus on this important and relatively unexplored topic.
4.3. EEG signatures – The role of gender
The present systematic review also sheds light on the electrophysiological dissimilarities between sexes. As such, most of the studies (78.3%) failed to find significant differences in the EEG profile between male and female BDs. These results are consistent with those reported in a recent systematic review concerning neuropsychological studies in this population, where 70.6% of the studies did not identify sex-related differences (Carbia et al., 2018). Nevertheless, given that most of the studies did not report the drinking characteristics of females and males separately, it is difficult to conclude whether the absence of gender differences is due to the fact of females consuming fewer amounts of alcohol than males –which is common for the BD pattern (Substance Abuse and Mental Health Services Administration (SAMHSA), 2018)- or if, indeed, there are no real differences at the neural level between male and female BDs. Thus, it would be helpful that the studies exploring sex-related differences describe the gender-specific drinking characteristics to better determine the effects of BD on males and females. In conclusion, and even with these limitations, the results suggest that both males and females are equally affected by BD (at least) at the electrophysiological level.
4.4. Methodological considerations
Even though there has been a growing effort in recent years to understand the electrophysiological impact of BD, some methodological aspects represent a challenge to draw strong conclusions on this topic. For instance, most investigations were conducted by a few number of research groups, as 88.2% of the studies were carried out by only five research teams –with the Spanish and Belgian groups accounting for around 55.9% of all the studies. Consequently, there is a need for a broad replication.
Throughout the quality assessment of the papers, we observed that several studies did not clearly consider the consumption of other drugs (including cannabis; 13/34; 38.2%) and the family history of alcoholism (16/34; 47.1%). The lack of control over these variables might significantly impact the results (and therefore their interpretation), as it is known that both are associated with anomalies in electrophysiological activity as well as in cognitive functioning (Crean et al., 2011, Kamarajan, 2019, Park and Schepp, 2015, Smith et al., 2014). Thus, future studies should clearly define the specific type and amount of drug used –if polydrug use is not an exclusion criterion- and include it in the models, as well as to control for the family history of drug abuse.
Additionally, the absence of a consensual BD conceptualization has led to excessive variability in experimental group selection and alcohol consumption evaluation ((Maurage et al., 2020)Maurage et al., 2020). As such, while most of the studies (25/34; 73.5%) have followed the NIAAA’s BD definition for the quantity of alcohol use in a single occasion (5/4 alcoholic drinks), variability increase for speed and frequency of consumption –the latter not specified by the NIAAA. Indeed, some studies did not consider the intensity criterion defined by the NIAAA (“5/4 drinks in about 2 h”) and not all used the same frequency criterion. Half of the studies (50%) considered at least one episode in the past month, but others classified BDs as having at least one episode in the last week (e.g. Petit et al. 2012), in the last two weeks (e.g. Park and Kim, 2018), in the last six months (e.g. Courtney and Polich, 2010) or even did not refer any frequency criterion (e.g. Ryerson et al., 2017) (see Table 2). Consequently, the levels of alcohol use in the BD groups differ greatly across studies, which may have led to different results and might explain some of the discrepancies observed among the studies. These disparities undoubtedly constrain between-studies comparisons as well as the unified interpretation of the findings and place the focus on the need for targeting efforts in the search for a consensus on the definition of BD –considering (at least) the quantity, intensity and frequency criteria.
4.5. Clinical implications
The present synthesis of the EEG signatures associated with BD could help to understand the processes underlying the transition from this pattern of excessive alcohol use to alcohol dependence. In this sense, some EEG correlates might constitute useful biological markers of cognitive and behavioral characteristics in alcohol dependence.
Evidence has shown that the persistence of binge alcohol consumption and/or alcohol abuse may lead to an abnormal bottom-up system (e.g., increased neural reactivity to alcohol-related cues) resulting in automatic action-tendencies to approach alcohol and craving (Herrmann et al., 2001, Schacht et al., 2012, Wiers et al., 2010, Wiers et al., 2015). Furthermore, an impaired top-down cognitive control system –commonly observed in individuals with AUD- could also emerge if the BD pattern is maintained, as young BDs might not be able to compensate for the alcohol-related damage (Lannoy et al., 2019, López-Caneda et al., 2014a). Altogether, this imbalance between the automatic (bottom-up) and reflective (top-down) systems may entail difficulties in the capacity to control alcohol drinking, resulting in the maintenance and/or escalation of the pattern (Peeters et al., 2012).
These findings highlight the importance of the EEG technique for understanding BD and indicate possible routes for prevention and intervention programs aiming at reducing alcohol misuse and mitigating potential detrimental effects on the brain and other organs. Indeed, EEG measures provide critical information that otherwise would not be apparent from psychological or behavioral assessments; for instance, in predicting (and preventing) relapse in alcohol-dependent patients (Campanella et al., 2019, Petit et al., 2015). Importantly, as ERPs have revealed a high sensitivity to change –e.g. along the treatment process (Campanella et al., 2020, Houston and Schlienz, 2018), they also constitute a valuable tool for monitoring interindividual alterations (Jurado-Barba et al., 2020). EEG assessment may also help to detect specific neurocognitive deficits associated with BD and to assist with cognitive stimulation (e.g., via cognitive training or by transcranial electrical stimulation), which might in turn facilitate early recovery before progression of these abnormalities, even in the absence of behavioral alterations (Di Lemma et al., 2020, Dormal et al., 2020).
4.6. Conclusion
Overall, the current literature suggests that BDs exhibit abnormal EEG signal during both cognitive performance and resting-state conditions. The most solid electrophysiological finding is the augmented P3 amplitude observed in tasks involving attention, working memory and response inhibition, which could constitute an early biomarker for BD. Accordingly, the increased neural activity during cognitive performance suggest the recruitment of additional resources to perform the task at adequate/successful levels, which supports the neurocompensation hypothesis. In contrast, BDs’ EEG profile also resembles that of alcohol-dependent individuals in some paradigms, as they exhibited augmented spontaneous EEG signal at rest, increased reactivity to alcoholic cues, and reduced activity during error detection, which provides in turn additional support to the continuum hypothesis –suggesting that BD and alcohol dependence may share several common features.
Furthermore, the evidence collected in this review does not support the assumption that young females are particularly vulnerable to the effects of BD, at least at the electrophysiological level. Forthcoming studies should explore further those cognitive functions that have barely been assessed so far (e.g. verbal episodic memory, short- and long-term memory, emotional processing, and decision making), since results –although limited- point to neural alterations associated with the BD pattern.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The study was conducted at the Psychology Research Centre (PSI/01662), School of Psychology, University of Minho, supported by the Foundation for Science and Technology (FCT) through the Portuguese State Budget [Ref.: UIDB/PSI/01662/2020]. This study was also supported by the project POCI-01-0145-FEDER-028672, funded by FCT and the European Regional Development Fund (FEDER). Eduardo López-Caneda and Alberto Crego were supported by the FCT and the Portuguese Ministry of Science, Technology and Higher Education, within the scope of the Individual Call to Scientific Employment Stimulus (CEECIND/02979/2018), and within the scope of the Transitory Disposition of the Decrete No. 57/2016, of 29th of August, amended by Law No. 57/2017 of 19 July, respectively. Natália Antunes was supported by a fellowship from the FCT (SFRH/BD/146194/2019). Carina Carbia has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement [grant number 754535].
Footnotes
It should be noted that three studies reported both significant differences between groups (two revealing decreased amplitude in BDs and one increased amplitude in this group) and between conditions, and thus they are counted twice.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2020.102537.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Abrahao K.P., Salinas A.G., Lovinger D.M. Alcohol and the brain: neuronal molecular targets, synapses, and circuits. Neuron. 2017;96:1223–1238. doi: 10.1016/j.neuron.2017.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Affan R.O., Huang S., Cruz S.M., Holcomb L.A., Nguyen E., Marinkovic K. High-intensity binge drinking is associated with alterations in spontaneous neural oscillations in young adults. Alcohol. 2018;70:51–60. doi: 10.1016/j.alcohol.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed K.M., Al Dhubaib B. Zotero: A bibliographic assistant to researcher. J. Pharm. Pharmacol. 2011;2:303–305. doi: 10.4103/0976-500X.85940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allom V., Mullan B., Hagger M. Does inhibitory control training improve health behavior? A meta-analysis. Health Psychol. Rev. 2016;10:168–186. doi: 10.1080/17437199.2015.1051078. [DOI] [PubMed] [Google Scholar]
- Baddeley A., Chincotta D., Adlam A. Working memory and the control of action: Evidence from task switching. J. Exp. Psychol. Gen. 2001;130:641. doi: 10.1037//0096-3445.130.4.641. [DOI] [PubMed] [Google Scholar]
- Bailey K., Bartholow B.D., Saults J.S., Lust S.A. Give me just a little more time: Effects of alcohol on the failure and recovery of cognitive control. J. Abnorm. Psychol. 2014;123:152–167. doi: 10.1037/a0035662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer L.O., Ceballos N.A. Neural and genetic correlates of binge drinking among college women. Biol. Psychol. 2014;97:43–48. doi: 10.1016/j.biopsycho.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholow B.D., Henry E.A., Lust S.A. Effects of alcohol sensitivity on P3 event-related potential reactivity to alcohol cues. Psychol. Addict. Behav. 2007;21:555. doi: 10.1037/0893-164X.21.4.555. [DOI] [PubMed] [Google Scholar]
- Bartholow B.D., Henry E.A., Lust S.A., Saults J.S., Wood P.K. Alcohol effects on performance monitoring and adjustment: Affect modulation and impairment of evaluative cognitive control. J. Abnorm. Psychol. 2012;121:173–186. doi: 10.1037/a0023664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S., Tapert S.F. Adolescent brain development and the risk for alcohol and other drug problems. Neuropsychol. Rev. 2010;20:398–413. doi: 10.1007/s11065-010-9146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker H.C. Kindling in alcohol withdrawal. Alcohol Health Res. World. 1998;22:25. [PMC free article] [PubMed] [Google Scholar]
- Bernardin F., Maheut-Bosser A., Paille F. Cognitive impairments in alcohol-dependent subjects. Front. Psychiatry. 2014;5:78. doi: 10.3389/fpsyt.2014.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S.J. Imaging brain development: the adolescent brain. Neuroimage. 2012;61:397–406. doi: 10.1016/j.neuroimage.2011.11.080. [DOI] [PubMed] [Google Scholar]
- Blanco-Ramos J., Cadaveira F., Folgueira-Ares R., Corral M., Rodríguez Holguín S. Electrophysiological correlates of an alcohol-cued go/nogo task: a dual-process approach to binge drinking in university students. Int. J. Environ. Res. Public Health. 2019;16:4550. doi: 10.3390/ijerph16224550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledowski C., Prvulovic D., Goebel R., Zanella F.E., Linden D.E. Attentional systems in target and distractor processing: a combined ERP and fMRI study. Neuroimage. 2004;22:530–540. doi: 10.1016/j.neuroimage.2003.12.034. [DOI] [PubMed] [Google Scholar]
- Boelema S.R., Harakeh Z., Ormel J., Hartman C.A., Vollebergh W.A., van Zandvoort M.J. Executive functioning shows differential maturation from early to late adolescence: Longitudinal findings from a TRAILS study. Neuropsychology. 2014;28:177. doi: 10.1037/neu0000049. [DOI] [PubMed] [Google Scholar]
- Bø R., Billieux J., Landrø N.I. Which facets of impulsivity predict binge drinking? Addict. Behav. Rep. 2016;3:43–47. doi: 10.1016/j.abrep.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen Z., Masson N., Salvaggio S., D’Hondt F., Maurage P. Craving is everything: An eye-tracking exploration of attentional bias in binge drinking. J. Psychopharmacol. 2020;34:636–647. doi: 10.1177/0269881120913131. [DOI] [PubMed] [Google Scholar]
- Bonomo Y.A., Bowes G., Coffey C., Carlin J.B., Patton G.C. Teenage drinking and the onset of alcohol dependence: a cohort study over seven years. Addict. 2004;99:1520–1528. doi: 10.1111/j.1360-0443.2004.00846.x. [DOI] [PubMed] [Google Scholar]
- Brion M., Pitel A.L., D’Hondt F. New Perspectives in the Exploration of Korsakoff’s Syndrome: The Usefulness of Neurophysiological Markers. Front. Psychol. 2016;7:168. doi: 10.3389/fpsyg.2016.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumback T., Squeglia L.M., Jacobus J., Pulido C., Tapert S.F., Brown S.A. Adolescent heavy drinkers' amplified brain responses to alcohol cues decrease over one month of abstinence. Addict. Behav. 2015;46:45–52. doi: 10.1016/j.addbeh.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanella S. Why it is time to develop the use of cognitive event-related potentials in the treatment of psychiatric diseases. Neuropsychiatr. Dis. Treat. 2013;9:1835. doi: 10.2147/NDT.S53687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanella S., Peigneux P., Petit G., Lallemand F., Saeremans M., Noël X., Ward R. Increased cortical activity in binge drinkers during working memory task: a preliminary assessment through a functional magnetic resonance imaging study. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0062260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanella S., Petit G., Maurage P., Kornreich C., Verbanck P., Noël X. Chronic alcoholism: insights from neurophysiology. Clin. Neurophysiol. 2009;39:191–207. doi: 10.1016/j.neucli.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Campanella S., Schroder E., Kajosch H., Hanak C., Veeser J., Amiot M., Kornreich C. Neurophysiological markers of cue reactivity and inhibition subtend a three-month period of complete alcohol abstinence. Clin. Neurophysiol. 2020;131:555–565. doi: 10.1016/j.clinph.2019.10.020. [DOI] [PubMed] [Google Scholar]
- Campanella S., Schroder E., Kajosch H., Noel X., Kornreich C. Why cognitive event-related potentials (ERPs) should have a role in the management of alcohol disorders. Neurosci. Biobehav. Rev. 2019;106:234–244. doi: 10.1016/j.neubiorev.2018.06.016. [DOI] [PubMed] [Google Scholar]
- Carbia C., Cadaveira F., Caamano-Isorna F., Rodriguez-Holguin S., Corral M. Binge drinking during adolescence and young adulthood is associated with deficits in verbal episodic memory. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0171393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbia C., Cadaveira F., López-Caneda E., Caamaño-Isorna F., Holguín S.R., Corral M. Working memory over a six-year period in young binge drinkers. Alcohol. 2017;61:17–23. doi: 10.1016/j.alcohol.2017.01.013. [DOI] [PubMed] [Google Scholar]
- Carbia C., López-Caneda E., Corral M., Cadaveira F. A systematic review of neuropsychological studies involving young binge drinkers. Neurosci. Biobehav. Rev. 2018;90:332–349. doi: 10.1016/j.neubiorev.2018.04.013. [DOI] [PubMed] [Google Scholar]
- Chanraud, S., Sullivan, E.V., 2014. Compensatory recruitment of neural resources in chronic alcoholism. In Handbook of clinical neurology (Vol. 125, pp. 369-380). Elsevier. [DOI] [PubMed]
- Chevrier A.D., Noseworthy M.D., Schachar R. Dissociation of response inhibition and performance monitoring in the stop signal task using event-related fMRI. Hum. Brain Mapp. 2007;28:1347–1358. doi: 10.1002/hbm.20355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colrain I.M., Sullivan E.V., Ford J.M., Mathalon D.H., McPherson S.L., Roach B.J., Pfefferbaum A. Frontally mediated inhibitory processing and white matter microstructure: age and alcoholism effects. Psychopharmacology. 2011;213:669–679. doi: 10.1007/s00213-010-2073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornblatt B.A., Risch N.J., Faris G., Friedman D., Erlenmeyer-Kimling L. The Continuous Performance Test, identical pairs version (CPT-IP): I. New findings about sustained attention in normal families. Psychiatry Res. 1988;26:223–238. doi: 10.1016/0165-1781(88)90076-5. [DOI] [PubMed] [Google Scholar]
- Courtney K.E., Polich J. Binge drinking effects on EEG in young adult humans. Int. J. Environ. Res. Public Health. 2010;7:2325–2336. doi: 10.3390/ijerph7052325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correas A., Cuesta P., Rosen B.Q., Maestu F., Marinkovic K. Compensatory neuroadaptation to binge drinking: Human evidence for allostasis. Addict. Biol. 2020;e12960 doi: 10.1111/adb.12960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correas Á., López-Caneda E., Beaton L., Rodríguez Holguín S., García-Moreno L.M., Antón-Toro L.F., Marinkovic K. Decreased event-related theta power and phase-synchrony in young binge drinkers during target detection: An anatomically-constrained MEG approach. J. Psychopharmacol. 2019;33:335–346. doi: 10.1177/0269881118805498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crean R.D., Crane N.A., Mason B.J. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J. Addiction Medicine. 2011;5:1. doi: 10.1097/ADM.0b013e31820c23fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crego A., Cadaveira F., Parada M., Corral M., Caamaño-Isorna F., Holguín S.R. Increased amplitude of P3 event-related potential in young binge drinkers. Alcohol. 2012;46:415–425. doi: 10.1016/j.alcohol.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Crego A., Holguín S.R., Parada M., Mota N., Corral M., Cadaveira F. Binge drinking affects attentional and visual working memory processing in young university students. Alcohol. Clin. Exp. Res. 2009;33:1870–1879. doi: 10.1111/j.1530-0277.2009.01025.x. [DOI] [PubMed] [Google Scholar]
- Crego A., Rodriguez-Holguín S., Parada M., Mota N., Corral M., Cadaveira F. Reduced anterior prefrontal cortex activation in young binge drinkers during a visual working memory task. Drug Alcohol Depend. 2010;109:45–56. doi: 10.1016/j.drugalcdep.2009.11.020. [DOI] [PubMed] [Google Scholar]
- Crone E.A., Ridderinkhof K.R. The developing brain: from theory to neuroimaging and back. Dev. Cogn. Neurosci. 2011;1:101–109. doi: 10.1016/j.dcn.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A., Brumback T. The burden of binge and heavy drinking on the brain: effects on adolescent and young adult neural structure and function. Front. Psychol. 2017;8:1111. doi: 10.3389/fpsyg.2017.01111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czapla M., Simon J.J., Friederich H.C., Herpertz S.C., Zimmermann P., Loeber S. Is binge drinking in young adults associated with an alcohol-specific impairment of response inhibition? Eur. Addict. Res. 2015;21:105–113. doi: 10.1159/000367939. [DOI] [PubMed] [Google Scholar]
- Dager A.D., Anderson B.M., Stevens M.C., Pulido C., Rosen R., Jiantonio-Kelly R.E., Wood R.M. Influence of alcohol use and family history of alcoholism on neural response to alcohol cues in college drinkers. Alcohol.: Clin. Exp. Res. 2013;37:E161–E171. doi: 10.1111/j.1530-0277.2012.01879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. Executive functions. Annual Rev. of. Psychology. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lemma L.C., Stancak A., Soto V., Fallon N., Field M. Event-related and readiness potentials when preparing to approach and avoid alcohol cues following cue avoidance training in heavy drinkers. Psychopharmacology. 2020;1–16 doi: 10.1007/s00213-020-05462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doallo S., Cadaveira F., Corral M., Mota N., López-Caneda E., Holguín S.R. Larger mid-dorsolateral prefrontal gray matter volume in young binge drinkers revealed by voxel-based morphometry. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0096380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormal V., Lannoy S., Bollen Z., D’Hondt F., Maurage P. Can we boost attention and inhibition in binge drinking? Electrophysiological impact of neurocognitive stimulation. Psychopharmacology. 2020;1–13 doi: 10.1007/s00213-020-05475-2. [DOI] [PubMed] [Google Scholar]
- Dormal V., Lannoy S., Maurage P. Impact of exchange stay on alcohol consumption: longitudinal exploration in a large sample of European students. Alcohol. Clin. Exp. Res. 2019;43:1220–1224. doi: 10.1111/acer.14028. [DOI] [PubMed] [Google Scholar]
- Easdon C., Izenberg A., Armilio M.L., Yu H., Alain C. Alcohol consumption impairs stimulus-and error-related processing during a Go/No-Go Task. Cogn. Brain Res. 2005;25:873–883. doi: 10.1016/j.cogbrainres.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Enoch M.A. Genetic and environmental influences on the development of alcoholism: resilience vs. risk. Ann. N. Y. Acad. Sci. 2006;1094:193–201. doi: 10.1196/annals.1376.019. [DOI] [PubMed] [Google Scholar]
- Eurobarometer, S., 2010. EU Citizens’ Attitudes Towards Alcohol. Brussels: European Commission. Retrieved from http://ec.europa.eu/public_opinion/archives/ebs/ebs_ 331_en.pdf.
- Fein G., Chang M. Visual P300s in long-term abstinent chronic alcoholics. Alcohol.: Clin. Exp. Res. 2006;30:2000–2007. doi: 10.1111/j.1530-0277.2006.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G., Chang M. Smaller feedback ERN amplitudes during the BART are associated with a greater family history density of alcohol problems in treatment-naive alcoholics. Drug Alcohol Depend. 2008;92:141–148. doi: 10.1016/j.drugalcdep.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M., Eastwood B. Experimental manipulation of attentional bias increases the motivation to drink alcohol. Psychopharmacology. 2005;183:350–357. doi: 10.1007/s00213-005-0202-5. [DOI] [PubMed] [Google Scholar]
- Field M., Wiers R.W., Christiansen P., Fillmore M.T., Verster J.C. Acute alcohol effects on inhibitory control and implicit cognition: implications for loss of control over drinking. Alcohol. Clin. Exp. Res. 2010;34:1346–1352. doi: 10.1111/j.1530-0277.2010.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaudias V., Heeren A., Brousse G., Maurage P. Toward a triadic approach to craving in addictive disorders: the metacognitive Hub Model. Harvard Rev. Psychiatry. 2019;27:326–331. doi: 10.1097/HRP.0000000000000225. [DOI] [PubMed] [Google Scholar]
- Folgueira-Ares R., Cadaveira F., Rodríguez Holguín S., López-Caneda E., Crego A., Pazo-Álvarez P. electrophysiological anomalies in Face–name Memory encoding in Young Binge Drinkers. Front. Psychiatry. 2017;8:216. doi: 10.3389/fpsyt.2017.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein J.R., Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology. 2008;45:152–170. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann D., Knoll L.J., Blakemore S.J. Adolescence as a sensitive period of brain development. Trends Cogn. Sci. 2015;19:558–566. doi: 10.1016/j.tics.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Herrmann M.J., Weijers H.G., Wiesbeck G.A., Aranda D., Böning J., Fallgatter A.J. Event-related potentials and cue-reactivity in alcoholism. Alcohol.: Clin. Exp. Res. 2000;24:1724–1729. doi: 10.1111/j.1530-0277.2000.tb01974.x. [DOI] [PubMed] [Google Scholar]
- Herrmann M.J., Weijers H.G., Wiesbeck G.A., Böning J., Fallgatter A.J. Alcohol cue-reactivity in heavy and light social drinkers as revealed by event-related potentials. Alcohol Alcohol. 2001;36:588–593. doi: 10.1093/alcalc/36.6.588. [DOI] [PubMed] [Google Scholar]
- Holcomb L.A., Huang S., Cruz S.M., Marinkovic K. Neural oscillatory dynamics of inhibitory control in young adult binge drinkers. Biol. Psychol. 2019;146 doi: 10.1016/j.biopsycho.2019.107732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holguín, S.R., Porjesz, B., Chorlian, D. B., Polich, J., Begleiter, H., 1999. Visual P3a in male subjects at high risk for alcoholism. Biological Psychiatry. 46, 281–291. Biol. Psychiatry. https://doi.org/10.1016/S0006-3223(98)00247-9. [DOI] [PubMed]
- Howell N.A., Worbe Y., Lange I., Tait R., Irvine M., Banca P., Voon V. Increased ventral striatal volume in college-aged binge drinkers. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0074164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston R.J., Schlienz N.J. Event-related potentials as biomarkers of behavior change mechanisms in substance use disorder treatment. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:30–40. doi: 10.1016/j.bpsc.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Holcomb L.A., Cruz S.M., Marinkovic K. Altered oscillatory brain dynamics of emotional processing in young binge drinkers. Cogn. Affect. Behav. Neurosci. 2018;18:43–57. doi: 10.3758/s13415-017-0551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S.A., Cservenka A., Nagel B.J. Binge drinking impacts dorsal striatal response during decision making in adolescents. Neuroimage. 2016;129:378–388. doi: 10.1016/j.neuroimage.2016.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S.A., Lueras J.M., Nagel B.J. Effects of binge drinking on the developing brain: studies in humans. Alcohol Res. Health. 2018;39:87. [PMC free article] [PubMed] [Google Scholar]
- Jurado-Barba R., Sion A., Martínez-Maldonado A., Domínguez-Centeno I., Prieto-Montalvo J., Navarrete F., Rubio G. Neuropsychophysiological Measures of Alcohol Dependence: Can We Use EEG in the Clinical Assessment? Front. Psychiatry. 2020;11 doi: 10.3389/fpsyt.2020.00676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C. Academic Press; 2019. Brain Electrophysiological Signatures in Human Alcoholism and Risk. Neuroscience of Alcohol; pp. 119–130. [Google Scholar]
- Kamarajan C., Porjesz B., Jones K.A., Choi K., Chorlian D.B., Padmanabhapillai A., Begleiter H. The role of brain oscillations as functional correlates of cognitive systems: a study of frontal inhibitory control in alcoholism. Int. J. Psychophysiol. 2004;51:155–180. doi: 10.1016/j.ijpsycho.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C., Rangaswamy M., Tang Y., Chorlian D.B., Pandey A.K., Roopesh B.N., Porjesz B. Dysfunctional reward processing in male alcoholics: an ERP study during a gambling task. J. Psychiatr. Res. 2010;44:576–590. doi: 10.1016/j.jpsychires.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki F., Constantinidis C. Bottom-up and top-down attention: different processes and overlapping neural systems. Neuroscientist. 2013;20:509–521. doi: 10.1177/1073858413514136. [DOI] [PubMed] [Google Scholar]
- Kiat J.E., Cheadle J.E. Tick–tock goes the croc: a high-density EEG study of risk-reactivity and binge-drinking. Soc. Cogn. Affect. Neurosci. 2018;13:656–663. doi: 10.1093/scan/nsy038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E.H., Kim M.S. An event-related potential study of error-monitoring deficits in female college students who participate in binge drinking. Clin. Psychopharmacol Neurosci. 2019;17:80. doi: 10.9758/cpn.2019.17.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus, L., Guttormsson, U., Leifman, H., Arpa, S., Molinaro, S., Monshouwer, K., et al., 2016. ESPAD Report 2015. Results from the European School Survey Project on Alcohol and Other Drugs. Lisbon: European Monitoring Centre for Drugs and Drug Addiction and the European School Survey Project on Alcohol and Other Drugs. Available online at: http://www.espad.org/sites/espad.org/ files/ESPAD_report_2015.pdf.
- Kvamme T.L., Schmidt C., Strelchuk D., Chang-Webb Y.C., Baek K., Voon V. Sexually dimorphic brain volume interaction in college-aged binge drinkers. Neuroimage Clin. 2015;10:310–317. doi: 10.1016/j.nicl.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannoy S., Billieux J., Dormal V., Maurage P. Behavioral and cerebral impairments associated with binge drinking in youth: A critical review. Psychol. Belg. 2019;59:116. doi: 10.5334/pb.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannoy S., Billieux J., Maurage P. Beyond inhibition: A dual-process perspective to renew the exploration of binge drinking. Front. Hum. Neurosci. 2014;8:405. doi: 10.3389/fnhum.2014.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannoy S., Dormal V., Billieux J., Brion M., D'Hondt F., Maurage P. A dual-process exploration of binge drinking: Evidence through behavioral and electrophysiological findings. Addict. Biol. 2020;25:1–10. doi: 10.1111/adb.12685. [DOI] [PubMed] [Google Scholar]
- Lannoy S., D’hondt F., Dormal V., Billieux J., Maurage P. Electrophysiological correlates of performance monitoring in binge drinking: Impaired error-related but preserved feedback processing. Clin. Neurophysiol. 2017;128:2110–2121. doi: 10.1016/j.clinph.2017.08.005. [DOI] [PubMed] [Google Scholar]
- Lannoy S., D’Hondt F., Dormal V., Blanco M., Brion M., Billieux J., Maurage P. Electrophysiological correlates of emotional crossmodal processing in binge drinking. Cogn. Affect. Behav. Neurosci. 2018;18:1076–1088. doi: 10.3758/s13415-018-0623-3. [DOI] [PubMed] [Google Scholar]
- Larson M.J., Clayson P.E., Clawson A. Making sense of all the conflict: a theoretical review and critique of conflict-related ERPs. Int. J. Psychophysiol. 2014;93:283–297. doi: 10.1016/j.ijpsycho.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Lees B., Mewton L., Stapinski L.A., Squeglia L.M., Rae C.D., Teesson M. Neurobiological and cognitive profile of young binge drinkers: a systematic review and meta-analysis. Neuropsychol. Rev. 2019;1–29 doi: 10.1007/s11065-019-09411-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Caneda E., Cadaveira F., Campanella S. Binge drinking in the adolescent and young brain. Front. Psychol. 2019;9:2724. doi: 10.3389/fpsyg.2018.02724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Caneda E., Cadaveira F., Correas A., Crego A., Maestú F., Rodríguez Holguín S. The brain of binge drinkers at rest: alterations in theta and beta oscillations in first-year college students with a binge drinking pattern. Front. Behav. Neurosci. 2017;11:168. doi: 10.3389/fnbeh.2017.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Caneda E., Cadaveira F., Crego A., Doallo S., Corral M., Gómez-Suárez A., Rodríguez Holguín S. Effects of a persistent binge drinking pattern of alcohol consumption in young people: a follow-up study using event-related potentials. Alcohol and Alcohol. 2013;48:464–471. doi: 10.1093/alcalc/agt046. [DOI] [PubMed] [Google Scholar]
- López-Caneda E., Cadaveira F., Crego A., Gómez-Suárez A., Corral M., Parada M., Rodríguez Holguín S. Hyperactivation of right inferior frontal cortex in young binge drinkers during response inhibition: a follow-up study. Addiction. 2012;107:1796–1808. doi: 10.1111/j.1360-0443.2012.03908.x. [DOI] [PubMed] [Google Scholar]
- López-Caneda E., Crego A., Campos A.D., González-Villar A., Sampaio A. The Think/No-Think Alcohol Task: A New Paradigm for Assessing Memory Suppression in Alcohol-Related Contexts. (2019b) Alcohol. Clin. Exp. Res. 2019;43:36–47. doi: 10.1111/acer.13916. [DOI] [PubMed] [Google Scholar]
- López-Caneda E., Holguín S.R., Cadaveira F., Corral M., Doallo S. Impact of alcohol use on inhibitory control (and vice versa) during adolescence and young adulthood: a review. Alcohol and Alcohol. 2014;49:173–181. doi: 10.1093/alcalc/agt168. [DOI] [PubMed] [Google Scholar]
- López-Caneda E., Holguín S.R., Corral M., Doallo S., Cadaveira F. Evolution of the binge drinking pattern in college students: Neurophysiological correlates. Alcohol. 2014;48:407–418. doi: 10.1016/j.alcohol.2014.01.009. [DOI] [PubMed] [Google Scholar]
- López-Caneda E., Holguín S.R., Correas Á., Carbia C., González-Villar A., Maestú F., Cadaveira F. Binge drinking affects brain oscillations linked to motor inhibition and execution. J. Psychopharmacol. 2017;31:873–882. doi: 10.1177/0269881116689258. [DOI] [PubMed] [Google Scholar]
- Luck S.J. MIT press; 2014. An introduction to the event-related potential technique. [Google Scholar]
- Luna B., Marek S., Larsen B., Tervo-Clemmens B., Chahal R. An integrative model of the maturation of cognitive control. Annu. Rev. Neurosci. 2015;38:151–170. doi: 10.1146/annurev-neuro-071714-034054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchery L., Yarmush D.E., Luehring-Jones P., Erblich J. Attentional bias to alcohol stimuli predicts elevated cue-induced craving in young adult social drinkers. Addict. Behav. 2017;70:14–17. doi: 10.1016/j.addbeh.2017.01.035. [DOI] [PubMed] [Google Scholar]
- Maurage P., Joassin F., Speth A., Modave J., Philippot P., Campanella S. Cerebral effects of binge drinking: respective influences of global alcohol intake and consumption pattern. Clin. Neurophysiol. 2012;123:892–901. doi: 10.1016/j.clinph.2011.09.018. [DOI] [PubMed] [Google Scholar]
- Maurage P., Lannoy S., Mange J., Grynberg D., Beaunieux H., Banovic I., Gierski F., Naassila M. What We Talk About When We Talk About Binge Drinking: Towards an Integrated Conceptualization and Evaluation. Alcohol Alcohol. 2020;55:468–479. doi: 10.1093/alcalc/agaa041. [DOI] [PubMed] [Google Scholar]
- Maurage P., Pesenti M., Philippot P., Joassin F., Campanella S. Latent deleterious effects of binge drinking over a short period of time revealed only by electrophysiological measures. J. Psychiatry Neurosci. 2009;34:111. [PMC free article] [PubMed] [Google Scholar]
- McHugh, M.L., 2012. Interrater reliability: the kappa statistic. Biochem. Med. 22, 276–282. Retrieved from https://hrcak.srce.hr/89395. [PMC free article] [PubMed]
- McCambridge J., McAlaney J., Rowe R. Adult consequences of late adolescent alcohol consumption: a systematic review of cohort studies. PLoS Med. 2011;8 doi: 10.1371/journal.pmed.1000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty C.A., Ebel B.E., Garrison M.M., DiGiuseppe D.L., Christakis D.A., Rivara F.P. Continuity of binge and harmful drinking from late adolescence to early adulthood. Pediatrics. 2004;114:714–719. doi: 10.1542/peds.2003-0864-L. [DOI] [PubMed] [Google Scholar]
- Miller E.K. The prefontral cortex and cognitive control. Nat. Rev. Neurosci. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., Shekelle, P., Stewart, L.A., 2015. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 4. http://dx.doi.org/10. 1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed]
- Molnar S.M., Beaton L.E., Happer J.P., Holcomb L.A., Huang S., Arienzo D., Marinkovic K. Behavioral and brain activity indices of cognitive control deficits in binge drinkers. Brain Sci. 2018;8:9. doi: 10.3390/brainsci8010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota N., Parada M., Crego A., Doallo S., Caamaño-Isorna F., Holguín S.R., Corral M. Binge drinking trajectory and neuropsychological functioning among university students: a longitudinal study. Drug Alcohol Depend. 2013;133:108–114. doi: 10.1016/j.drugalcdep.2013.05.024. [DOI] [PubMed] [Google Scholar]
- Morris L.S., Dowell N.G., Cercignani M., Harrison N.A., Voon V. Binge drinking differentially affects cortical and subcortical microstructure. Addict. Biol. 2018;23:403–411. doi: 10.1111/adb.12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumtaz W., Vuong P.L., Xia L., Malik A.S., Rashid R.B.A. An EEG-based machine learning method to screen alcohol use disorder. Cogn. Neurodyn. 2017;11:161–171. doi: 10.1007/s11571-016-9416-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na E., Jang K.M., Kim M.S. An event-related potential study of decision-making and feedback utilization in female college students who binge drink. Front. Psychol. 2019;10 doi: 10.3389/fpsyg.2019.02606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namkoong K., Lee E., Lee C.H., Lee B.O., An S.K. Increased P3 amplitudes induced by alcohol-related pictures in patients with alcohol dependence. Alcohol.: Clin. Exp. Res. 2004;28:1317–1323. doi: 10.1097/01.ALC.0000139828.78099.69. [DOI] [PubMed] [Google Scholar]
- National Heart, Lung, and Blood Institute (NHLBI), 2014. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. Available at: National Heart, Lung, and Blood Institute, Bethesda, MD. https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/cohort.
- National Institute of Alcohol Abuse and Alcoholism, 2004. NIAAA Council Approves Definition of Binge Drinking. NIAAA Newsletter, 3. Available online at: https://pubs.niaaa.nih.gov/publications/Newsletter/winter2004/Newsletter_Number3.pdf.
- National Institute of Alcohol Abuse and Alcoholism, 2020. College Drinking [Fact Sheet]. Available online at: https://pubs.niaaa.nih.gov/publications/CollegeFactSheet/CollegeFactSheet.pdf.
- Norman A.L., Pulido C., Squeglia L.M., Spadoni A.D., Paulus M.P., Tapert S.F. Neural activation during inhibition predicts initiation of substance use in adolescence. Drug Alcohol Depend. 2011;119:216–223. doi: 10.1016/j.drugalcdep.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paller K.A., Wagner A.D. Observing the transformation of experience into memory. Trends Cogn. Sci. 2002;6:93–102. doi: 10.1016/S1364-6613(00)01845-3. [DOI] [PubMed] [Google Scholar]
- Pandey A.K., Kamarajan C., Manz N., Chorlian D.B., Stimus A., Porjesz B. Delta, theta, and alpha event-related oscillations in alcoholics during Go/NoGo task: Neurocognitive deficits in execution, inhibition, and attention processing. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2016;65:158–171. doi: 10.1016/j.pnpbp.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Kim M.S. An event-related potential study of spatial working memory in binge drinking college students. PLoS ONE. 2018;13 doi: 10.1371/journal.pone.0203696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Schepp K.G. A systematic review of research on children of alcoholics: Their inherent resilience and vulnerability. J. Child Fam. Stud. 2015;24:1222–1231. doi: 10.1007/s10826-014-9930-7. [DOI] [Google Scholar]
- Parsons O.A. Neurocognitive deficits in alcoholics and social drinkers: a continuum? Alcohol. Clin. Exp. Res. 1998;22:954–961. doi: 10.1111/j.1530-0277.1998.tb03895.x. [DOI] [PubMed] [Google Scholar]
- Patrick M.E., Terry-McElrath Y.M., Kloska D.D., Schulenberg J.E. High-intensity drinking among young adults in the United States: Prevalence, frequency, and developmental change. Alcohol. Clin. Exp. Res. 2016;40:1905–1912. doi: 10.1111/acer.13164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters M., Wiers R.W., Monshouwer K., van de Schoot R., Janssen T., Vollebergh W.A. Automatic processes in at-risk adolescents: the role of alcohol-approach tendencies and response inhibition in drinking behavior. Addiction. 2012;107:1939–1946. doi: 10.1111/j.1360-0443.2012.03948.x. [DOI] [PubMed] [Google Scholar]
- Perlman G., Johnson W., Iacono W.G. The heritability of P300 amplitude in 18-year-olds is robust to adolescent alcohol use. Psychophysiology. 2009;46:962–969. doi: 10.1111/j.1469-8986.2009.00850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterburs J., Thürling M., Rustemeier M., Göricke S., Suchan B., Timmann D., Bellebaum C. A cerebellar role in performance monitoring–Evidence from EEG and voxel-based morphometry in patients with cerebellar degenerative disease. Neuropsychologia. 2015;68:139–147. doi: 10.1016/j.neuropsychologia.2015.01.017. [DOI] [PubMed] [Google Scholar]
- Petit G., Cimochowska A., Cevallos C., Cheron G., Kornreich C., Hanak C., Campanella S. Reduced processing of alcohol cues predicts abstinence in recently detoxified alcoholic patients in a three-month follow up period: An ERP study. Behav. Brain Res. 2015;282:84–94. doi: 10.1016/j.bbr.2014.12.057. [DOI] [PubMed] [Google Scholar]
- Petit G., Maurage P., Kornreich C., Verbanck P., Campanella S. Binge drinking in adolescents: a review of neurophysiological and neuroimaging research. Alcohol and Alcohol. 2014;49:198–206. doi: 10.1093/alcalc/agt172. [DOI] [PubMed] [Google Scholar]
- Petit G., Kornreich C., Dan B., Verbanck P., Campanella S. Electrophysiological correlates of alcohol-and non-alcohol-related stimuli processing in binge drinkers: A follow-up study. J. Psychopharmacol. 2014;28:1041–1052. doi: 10.1177/0269881114545663. [DOI] [PubMed] [Google Scholar]
- Petit G., Kornreich C., Maurage P., Noël X., Letesson C., Verbanck P., Campanella S. Early attentional modulation by alcohol-related cues in young binge drinkers: an event-related potentials study. Clin. Neurophysiol. 2012;123:925–936. doi: 10.1016/j.clinph.2011.10.042. [DOI] [PubMed] [Google Scholar]
- Petit G., Kornreich C., Verbanck P., Campanella S. Gender differences in reactivity to alcohol cues in binge drinkers: A preliminary assessment of event-related potentials. Psychiatry Res. 2013;209:494–503. doi: 10.1016/j.psychres.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G., Da Silva F.L. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin. Neurophysiol. 1999;110:1842–1857. doi: 10.1016/S1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Porjesz B., Begleiter H. Alcoholism and human electrophysiology. Alcohol Res Health. 2003;27:153. [PMC free article] [PubMed] [Google Scholar]
- Porjesz B., Rangaswamy M., Kamarajan C., Jones K.A., Padmanabhapillai A., Begleiter H. The utility of neurophysiological markers in the study of alcoholism. Clin. Neurophysiol. 2005;116:993–1018. doi: 10.1016/j.clinph.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M., Jones K.A., Porjesz B., Chorlian D.B., Padmanabhapillai A., Kamarajan C., Schuckit M.A. Delta and theta oscillations as risk markers in adolescent offspring of alcoholics. Int. J. Psychophysiol. 2007;63:3–15. doi: 10.1016/j.ijpsycho.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangaswamy, M., Porjesz, B., 2014. Understanding alcohol use disorders with neuroelectrophysiology. In Handbook of clinical neurology (Vol. 125, pp. 383-414). Elsevier. https://doi.org/10.1016/B978-0-444-62619-6.00023-9. [DOI] [PMC free article] [PubMed]
- Rangaswamy M., Porjesz B., Chorlian D.B., Wang K., Jones K.A., Bauer L.O., Begleiter H. Beta power in the EEG of alcoholics. Biol. Psychiatry. 2002;52:831–842. doi: 10.1016/S0006-3223(02)01362-8. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M., Porjesz B., Chorlian D.B., Wang K., Jones K.A., Kuperman S., Begleiter H. Resting EEG in offspring of male alcoholics: beta frequencies. Int. J. Psychophysiol. 2004;51:239–251. doi: 10.1016/j.ijpsycho.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M., Porjesz B., Chorlian D.B., Choi K., Jones K.A., Wang K., Begleiter H. Theta power in the EEG of alcoholics. Alcohol. Clin. Exp. Res. 2003;27:607–615. doi: 10.1111/j.1530-0277.2003.tb04397.x. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof K.R., Van Den Wildenberg W.P., Segalowitz S.J., Carter C.S. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn. 2004;56:129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Roberto M., Varodayan F.P. Synaptic targets: chronic alcohol actions. Neuropharmacology. 2017;122:85–99. doi: 10.1016/j.neuropharm.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg M.D., Curran T. Event-related potentials and recognition memory. Trends Cogn. Sci. 2007;11:251–257. doi: 10.1016/j.tics.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Ryerson N.C., Neal L.B., Gable P.A. Attenuating the alcohol allure: attentional broadening reduces rapid motivational response to alcohol pictures. Psychopharmacology. 2017;234:1247–1254. doi: 10.1007/s00213-017-4557-1. [DOI] [PubMed] [Google Scholar]
- Sanhueza C., Moreno L.M.G., Expósito J. Weekend alcoholism in youth and neurocognitive aging. Psicothema. 2011;23:209–214. [PubMed] [Google Scholar]
- Schacht J.P., Anton R.F., Myrick H. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict. Biol. 2012;18:121–133. doi: 10.1111/j.1369-1600.2012.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulenberg, J., Johnston, L., O'Malley, P., Bachman, J., Miech, R., Patrick, M., 2019. Monitoring the Future national survey results on drug use, 1975-2018: Volume II, college students and adults ages 19-60.
- Schroder E., Dousset C., Noel X., Kornreich C., Campanella S. Increased neural activity in hazardous drinkers during high workload in a visual working memory task: a preliminary assessment through event-related potentials. Front. Psychiatry. 2019;10:248. doi: 10.3389/fpsyt.2019.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev L., Ben-Simon A., Mevorach C., Cohen Y., Tsal Y. Conjunctive Continuous Performance Task (CCPT)—A pure measure of sustained attention. Neuropsychologia. 2011;49:2584–2591. doi: 10.1016/j.neuropsychologia.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Sousa S.S., Sampaio A., López-Caneda E., Bec C., Gonçalves Ó.F., Crego A. Increased Nucleus Accumbens Volume in College Binge Drinkers-Preliminary Evidence From Manually Segmented MRI Analysis. Front. Psychiatry. 2020;10:1005. doi: 10.3389/fpsyt.2019.01005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.L., De Blasio F.M., Iredale J.M., Matthews A.J., Bruno R., Dwyer M., Mattick R.P. Verbal learning and memory in cannabis and alcohol users: An event-related potential investigation. Front. Psychol. 2017;8:2129. doi: 10.3389/fpsyg.2017.02129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.L., Iredale J.M., Mattick R.P. Sex differences in the relationship between heavy alcohol use, inhibition and performance monitoring: Disconnect between behavioral and brain functional measures. Psychiatry Res. Neuroimaging. 2016;254:103–111. doi: 10.1016/j.pscychresns.2016.06.012. [DOI] [PubMed] [Google Scholar]
- Smith J.L., Mattick R.P. Evidence of deficits in behavioral inhibition and performance monitoring in young female heavy drinkers. Drug Alcohol Depend. 2013;133:398–404. doi: 10.1016/j.drugalcdep.2013.06.020. [DOI] [PubMed] [Google Scholar]
- Smith J.L., Mattick R.P., Jamadar S.D., Iredale J.M. Deficits in behavioral inhibition in substance abuse and addiction: a meta-analysis. Drug Alcohol Depend. 2014;145:1–33. doi: 10.1016/j.drugalcdep.2014.08.009. [DOI] [PubMed] [Google Scholar]
- Smith J.L., Mattick R.P., Sufani C. Female but not male young heavy drinkers display altered performance monitoring. Psychiatry Res. Neuroimaging. 2015;233:424–435. doi: 10.1016/j.pscychresns.2015.07.014. [DOI] [PubMed] [Google Scholar]
- Smith J.L., Mattick R.P., Sufani C. Error detection and behavioral inhibition in young heavy drinkers. Drug Alcohol Depend. 2017;171:20–30. doi: 10.1016/j.drugalcdep.2016.11.016. [DOI] [PubMed] [Google Scholar]
- Squeglia L.M., Schweinsburg A.D., Pulido C., Tapert S.F. Adolescent binge drinking linked to abnormal spatial working memory brain activation: differential gender effects. Alcohol. Clin. Exp. Res. 2011;35:1831–1841. doi: 10.1111/j.1530-0277.2011.01527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia L.M., Sorg S.F., Schweinsburg A.D., Wetherill R.R., Pulido C., Tapert S.F. Binge drinking differentially affects adolescent male and female brain morphometry. Psychopharmacology. 2012;220:529–539. doi: 10.1007/s00213-011-2500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia L.M., Tapert S.F., Sullivan E.V., Jacobus J., Meloy M.J., Rohlfing T., Pfefferbaum A. Brain development in heavy-drinking adolescents. Am. J. Psychiatry. 2015;172:531–542. doi: 10.1176/appi.ajp.2015.14101249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens D.N., Duka T. Cognitive and emotional consequences of binge drinking: role of amygdala and prefrontal cortex. Philos. Trans. R. Soc. Lond., B. Biol. Sci. 2008;363:3169–3179. doi: 10.1098/rstb.2008.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez-Suárez S., Doallo S., Pérez-García J.M., Corral M., Rodríguez Holguín S., Cadaveira F. Response Inhibition and Binge Drinking During Transition to University: An fMRI Study. Front. Psychiatry. 2020;11:535. doi: 10.3389/fpsyt.2020.00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA), 2018. National Survey on Drug Use and Health (NSDUH). Table 2.20B - Binge Alcohol Use in Past Month among Persons Aged 12 or Older, by Age Group and Demographic Characteristics: Percentages, 2017 and 2018. Available at: https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHDetailedTabs2018R2/NSDUHDetTabsSect2pe2018.htm#tab2-1b.
- Tapert S.F., Schweinsburg A.D., Barlett V.C., Brown S.A., Frank L.R., Brown G.G., Meloy M.J. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcohol.: Clin. Exp. Res. 2004;28:1577–1586. doi: 10.1097/01.ALC.0000141812.81234.A6. [DOI] [PubMed] [Google Scholar]
- Ullsperger M., von Cramon D.Y. Decision making, performance and outcome monitoring in frontal cortical areas. Nat. Neurosci. 2004;7:1173–1174. doi: 10.1038/nn1104-1173. [DOI] [PubMed] [Google Scholar]
- Ullsperger M., Danielmeier C., Jocham G. Neurophysiology of performance monitoring and adaptive behavior. Physiol. Rev. 2014;94:35–79. doi: 10.1152/physrev.00041.2012. [DOI] [PubMed] [Google Scholar]
- Verbruggen F., Logan G.D. Response inhibition in the stop-signal paradigm. Trends Cogn. Sci. 2008;12:418–424. doi: 10.1016/j.tics.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veritas Health Innovation, 2016. Covidence systematic review software. Melbourne, Australia. http://www.covidence.org.
- Voon, V., Grodin, E., Mandali, A., Morris, L., Weidacker, K., Kwako, L., ... & Momenan, R., 2020. Addictions NeuroImaging Assessment (ANIA): towards an integrative framework for alcohol use disorder. Neurosci. Biobehav. Rev. https://doi.org/10.1016/j.neubiorev.2020.04.004. [DOI] [PubMed]
- Ward R.J., Lallemand F., De Witte P. Biochemical and neurotransmitter changes implicated in alcohol-induced brain damage in chronic or ‘binge drinking’alcohol abuse. Alcohol Alcohol. 2009;44:128–135. doi: 10.1093/alcalc/agn100. [DOI] [PubMed] [Google Scholar]
- Wetherill R.R., Squeglia L.M., Yang T.T., Tapert S.F. A longitudinal examination of adolescent response inhibition: Neural differences before and after the initiation of heavy drinking. Psychopharmacology. 2013;230:663–671. doi: 10.1007/s00213-013-3198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiers R.W., Rinck M., Kordts R., Houben K., Strack F. Retraining automatic action-tendencies to approach alcohol in hazardous drinkers. Addiction. 2010;105:279–287. doi: 10.1111/j.1360-0443.2009.02775.x. [DOI] [PubMed] [Google Scholar]
- Wiers C.E., Stelzel C., Gladwin T.E., Park S.Q., Pawelczack S., Gawron C.K., Lindenmeyer J. Effects of cognitive bias modification training on neural alcohol cue reactivity in alcohol dependence. Am. J. Psychiatry. 2015;172:335–343. doi: 10.1176/appi.ajp.2014.13111495. [DOI] [PubMed] [Google Scholar]
- Winward J.L., Bekman N.M., Hanson K.L., Lejuez C.W., Brown S.A. Changes in emotional reactivity and distress tolerance among heavy drinking adolescents during sustained abstinence. Alcohol. Clin. Exp. Res. 2014;38:1761–1769. doi: 10.1111/acer.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winward J.L., Hanson K.L., Bekman N.M., Tapert S.F., Brown S.A. Adolescent heavy episodic drinking: neurocognitive functioning during early abstinence. J. Int. Neuropsychol. Soc. 2014;20:218–229. doi: 10.1017/S1355617713001410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; Geneva: 2011. Global status report on alcohol and health. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.