Supplemental Digital Content is available in the text.
Keywords: atrial fibrillation, continuous monitoring, critical illness, postoperative, secondary
Objective:
To estimate the incidence of new-onset atrial fibrillation in critically ill patients.
Design:
Prospective cohort.
Setting:
Medical-surgical ICU.
Subjects:
Consecutive patients without a history of atrial fibrillation but with atrial fibrillation risk factors.
Interventions:
Electrocardiogram patch monitor until discharge from hospital or up to 14 days.
Measurements and Main Results:
A total of 249 participants (median age of 71 yr [interquartile range] 64–78 yr; 35% female) completed the study protocol of which 158 (64%) were admitted to ICU for medical illness, 78 (31%) following noncardiac surgery, and 13 (5%) with trauma. Median Acute Physiology and Chronic Health Evaluation II score was 16 (interquartile range, 12–22). Median duration of patch electrocardiogram monitoring, ICU, and hospital lengths of stay were 6 (interquartile range, 3–12), 4 (interquartile range, 2–8), and 11 days (interquartile range, 5–23 d), respectively.
Atrial fibrillation ≥ 30 seconds was detected by the patch in 44 participants (17.7%), and three participants (1.2%) had atrial fibrillation detected clinically after patch removal, resulting in an overall atrial fibrillation incidence of 18.9% (95% CI, 14.2–24.3%).
Total duration of atrial fibrillation ranged from 53 seconds to the entire monitoring time. The proportion of participants with ≥1 episode(s) of ≥6 minute, ≥1 hour, ≥12 hour and ≥24 hour duration was 14.8%, 13.2%, 7.0%, and 5.3%, respectively. The clinical team recognized only 70% of atrial fibrillation cases that were detected by the electrocardiogram patch.
Conclusions:
Among patients admitted to an ICU, the incidence of new-onset atrial fibrillation is approximately one in five, although approximately one-third of cases are not recognized by the clinical team.
Atrial fibrillation (AF), the most common cardiac arrhythmia, is often newly diagnosed in patients hospitalized for another reason (1–3). However, uncertainty surrounds the epidemiology and management of AF detected in this setting. Challenges in managing new-onset AF during acute illness are driven in part by its complex pathophysiology. Acute factors (e.g., inflammation, catecholamine response, ischemia, metabolic disturbances, and volume shifts) and chronic factors (e.g., valvular disease, atrial myopathy, and hypertension) may contribute to arrhythmogenesis (4, 5). Both the arrhythmia and the underlying conditions may result in adverse outcomes over the short and long terms. Although new-onset AF during acute illness has been associated with worse outcomes, including increased length of stay, stroke, and death; no clear management guidelines exist (2, 6–13). AF occurring transiently with stress (AFOTS) refers to the subset of AF that reverts to sinus rhythm before leaving the hospital and for whom the optimal postdischarge management (i.e., anticoagulation and rhythm control) is uncertain (3, 14). The lack of reliable descriptive data further complicates this issue; the published incidence of new onset AF varies markedly, ranging from 1% to 44%, depending on the population, setting, and detection methods (2, 15).
The primary aim of the present study was to use systematic, high-sensitivity continuous electrocardiogram (ECG) monitoring to obtain accurate estimates of the incidence of new-onset AF in critically ill patients with risk factors for AF. The secondary aims were to describe patterns of AF, risk factors for AF onset and AF detection, and the association between AF and clinical outcomes.
MATERIALS AND METHODS
We have previously reported the full design of the AFOTS incidence study (NCT03552588) (16). Briefly, we enrolled consecutive eligible patients admitted to two tertiary Canadian medicosurgical ICUs (16). We included patients without a history of AF who were at least 65 years old or between the ages of 50 and 64 and had one or more Congestive heart failure, Hypertension, Age ≥ 75, Diabetes, and Stroke/transient ischemic attack (TIA) (2 points) risk factors (17). We included patients without a prior documented history of AF who were in AF at admission to the ICU. We excluded patients with known ECG electrode adhesive allergy, those in whom the 14-day monitor was expected to interfere with necessary care, those not expected to survive for at least 12 hours, those with a primary cardiovascular admission diagnosis, those not screened within 12 hours from admission, and those with sleep apnea admitted to the ICU exclusively for postoperative monitoring. Consecutive eligible participants had a 14-day ECG patch monitor (ZIO XT Patch, iRhythm, Chicago, IL) applied at the time of enrollment (18). This single-lead monitor collects heart rate (HR) information continuously and detects AF using a proprietary algorithm, calculating heart rates on a beat-to-beat basis. The local ethics board approved a deferred consent model (Hamilton Integrated Research Ethics Board Project Number 4740); the study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
The ECG patch remained in place for 14 days, until it fell off or had to be removed, or until the participant was discharged from hospital, whichever occurred first. We collected baseline characteristics at admission and outcome information daily. We followed participants to hospital discharge or until 30 days following enrollment.
The primary outcome was the proportion of patients with at least one episode of AF lasting greater than 30 seconds, as detected by the 14-day monitor and confirmed by a blinded arrhythmia specialist. We also captured the following secondary outcomes: 1) the proportion of patients with AF documented by the clinical team, 2) the proportion of patients with greater than or equal to 6 minutes, greater than or equal to 1 hour, and greater than or equal to 24 hours of patch-detected AF, 3) the burden of patch-detected AF(defined as % of the time in AF), 4) the frequency of AF episodes occurring within prespecified HR ranges (> 50, 50–110, > 110 beats/min), and 5) and factors associated with clinical identification of AF. We also captured clinical outcomes including major bleeding (International Society on Thrombosis and Hemostasis definition [19]), stroke, cardiac arrest necessitating cardiopulmonary resuscitation, and death.
As part of their routine clinical care in the ICU, all patients underwent continuous cardiac monitoring using systems that employ the ST segment and arrhythmia monitoring algorithm (Philips Healthcare, Latham, NY). This algorithm performs analysis using R-R interval irregularity, PR interval variability, and P-wave variability for detection of AF. Events are stored in the alarm section of the telemetry interface. After ICU discharge, treating clinicians decided whether patients required ongoing cardiac monitoring (i.e., telemetry versus not). We reviewed nursing and physician sections of participants’ charts on a daily basis for documentation of AF.
This study was designed to enroll 250 participants based on an anticipated AF incidence of 17% and desired precision of CIs of ± 5% half-widths; this was based on the mean weighted incidence in our group’s prior systematic review (15). We present descriptive statistics including means and proportions with 95% CIs around point estimates, and medians with interquartile ranges (IQRs). Continuous variables were compared between the groups with a t test or Wilcoxon rank-sum test. Categorical variables were compared using the chi-square test or Fisher exact test. We created univariate Poisson regression models with robust error variances including AF as the dependent variable and patient, hospital unit, and AF characteristics as independent variables (20).
RESULTS
Study Population
We approached 457 patients, of which 317 were eligible, and after exclusions, we enrolled a total of 260 eligible participants (only three refused consent), among whom 249 had analyzable patch data (Fig. 1). Table 1 displays the baseline characteristics of enrolled analyzable patients. Primary admission diagnosis was medical illness in 158 (64%), noncardiac surgery in 78 (31%), and trauma in 13 (5%). Table 2 displays the characteristics of ICU care and ECG monitoring. Median duration of patch ECG monitoring, ICU, and hospital stay were 6 (IQR, 3–12), 4 (IQR, 2–8), and 11 days (IQR, 5–23 d), respectively. Of 249 participants, 40 (16.1%) wore the patch for 14 days. The patch fell off prematurely in 50 participants (20.0%), was removed for medically necessary reasons (e.g., MRI, prone ventilation, wound care, and planned procedure in area) in 13 participants (5.2%), removed because the patient was being discharged from hospital in 85 participants (34.1%), and removed from 61 participants (24.5%) who expired.
Figure 1.
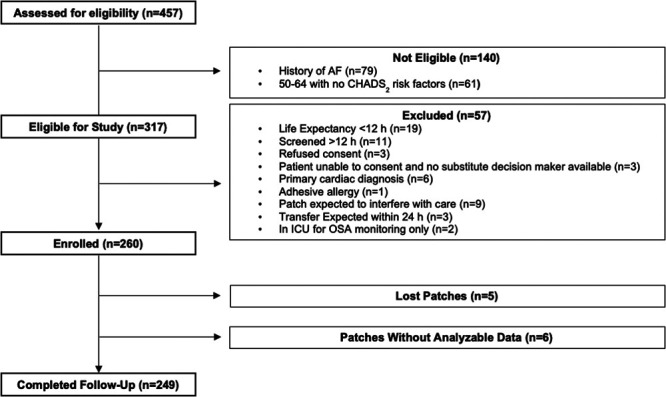
Patient flow in the atrial fibrillation (AF) occurring transiently with stress incidence study. CHADS2 = Congestive heart failure, Hypertension, Age ≥ 75, Diabetes, and Stroke/transient ischemic attack (2 points), OSA = obstructive sleep apnea.
TABLE 1.
Baseline Characteristics of Patients Admitted to the ICU Without a Prior History of Atrial Fibrillation
| Characteristic | Overall, n = 249 | Atrial Fibrillation, n = 47 | No Atrial Fibrillation, n = 202 | pa |
|---|---|---|---|---|
| Age, yr (median [IQR]) | 71.0 (64.0–78.0) | 77.0 (72.0–83.0) | 69.0 (63.0–76.0) | < 0.001 |
| Female sex (n [%]) | 88 (35.3) | 16 (34.0) | 72 (35.6) | 0.8 |
| Congestive heart failure, Hypertension, Age ≥ 75, Diabetes, Stroke/transient ischemic attack (2 points), Vascular Disease, Female Sex Category score (median [IQR]) | 3.0 (2.0–4.0) | 4.0 (3.0–5.0) | 3.0 (2.0–4.0) | 0.002 |
| Acute Physiology and Chronic Health Evaluation II score (median [IQR]) | 16.0 (12.0–22.0) | 18.0 (13.0–24.0) | 16.0 (11.0–21.0) | 0.06 |
| Body mass index (median [IQR]) | 27.2 (23.9–31.8) | 26.8 (23.0–30.8) | 27.5 (24.0–32.0) | 0.3 |
| Primary admission diagnosis (n [%]) | ||||
| Medical illness | 158 (63.5) | 34 (72.3) | 124 (61.4) | 0.2 |
| Infection | 36 (14.5) | 6 (12.8) | 30 (14.9) | |
| Respiratory | 36 (14.5) | 9 (19.1) | 27 (13.4) | |
| Gastrointestinal | 16 (6.4) | 4 (8.5) | 12 (5.9) | |
| Metabolic | 5 (2.0) | 1 (2.1) | 4 (2.0) | |
| Ischemic stroke | 8 (3.2) | 3 (6.3) | 5 (2.5) | |
| Other neurologic | 31 (12.4) | 4 (8.5) | 27 (13.4) | |
| Vascular | 4 (1.6) | 1 (2.1) | 3 (1.5) | |
| Renal | 6 (2.4) | 2 (4.3) | 4 (2.0) | |
| Hematologic | 1 (0.4) | 0 (0.0) | 1 (0.5) | |
| Oncologic | 3 (1.2) | 0 (0.0) | 3 (1.5) | |
| Undifferentiated shock | 12 (4.8) | 4 (8.5) | 8 (4.0) | |
| Noncardiac surgery (n [%]) | 78 (31.3) | 12 (25.5) | 66 (32.7) | 0.3 |
| Neurosurgery | 18 (7.2) | 3 (6.4) | 15 (7.4) | |
| Orthopedic surgery | 15 (6.0) | 2 (4.3) | 13 (6.4) | |
| Vascular surgery | 11 (4.4) | 2 (4.3) | 9 (4.5) | |
| General surgery | 25 (10.0) | 4 (8.5) | 21 (10.4) | |
| Urological surgery | 7 (2.8) | 0 (0.0) | 7 (3.5) | |
| Other surgery | 2 (0.8) | 1 (2.1) | 1 (0.5) | |
| Trauma (n [%]) | 13 (5.2) | 1 (2.1) | 12 (5.9) | 0.5 |
| Surgical | 7 (2.8) | 0 (0.0) | 7 (3.5) | |
| Nonsurgical | 6 (2.4) | 1 (2.1) | 5 (2.5) | |
IQR = interquartile range.
ap is either from a Pearson χ2 test/Fisher exact test, two-sample t test, or Wilcoxon rank-sum test.
TABLE 2.
Characteristics of ICU Care and Electrocardiogram Monitoring
| Characteristic | Overall, n = 249 | AF, n = 47 | No AF, n = 202 | pa |
|---|---|---|---|---|
| Days of invasive ventilation, median (IQR) | 1 (0–3) | 3 (0–9) | 0 (0–3) | 0.001 |
| Days in ICU, median (IQR) | 4 (2–8) | 7 (4–14) | 3. (2–7) | < 0.001 |
| Days in hospital, median (IQR) | 11 (5–23) | 14 (7–30) | 9.5 (5–21) | 0.023 |
| Days on patch monitoring, median (IQR) | 6 (3–12) | 10 (5–13) | 6 (3–11) | 0.001 |
| Days of telemetry monitoring, median (IQR) | 4 (2–9) | 7 (4–13) | 3 (1–9) | 0.001 |
| Peak high-sensitivity troponin (ng/mL), median (IQR) | 32 (11–252) | 69 (21–338) | 28(9–224) | 0.015 |
| Invasive ventilationb, n (%) | 131 (52.6) | 32 (68.1) | 99 (49.0) | 0.018 |
| Dialysisb, n (%) | 15 (6.0) | 7 (14.9) | 8 (4.0) | 0.005 |
| Vasopressors/inotropesb, n (%) | 87 (34.9) | 26 (55.3) | 61 (30.2) | 0.001 |
| Clinical AF detected, n (%) | 33 (13.3) | 33 (70.2) | 0 (0.0) | < 0.001 |
AF = atrial fibrillation, IQR = interquartile range.
ap for categorical variables from χ2 or Fisher exact test is used; p for continuous variables from Wilcoxon rank-sum test.
bAt any point during the study.
% is out of total analyzed.
Atrial Fibrillation Incidence and Characteristics
AF greater than or equal to 30 seconds was detected by the ECG patch in 44 participants (17.7%), and three additional participants (1.2%) had AF detected clinically by 12-lead ECG after patch removal, resulting in an overall AF incidence of 18.9% (95% CI, 14.2–24.3%). Of those participants who developed AF, AF first occurred in the ICU in 44 (93.6%, including those who were in AF on arrival to ICU from another unit), on a step-down unit in 1 (2.1%) and on the ward in 2 (2.1%). The clinical care team recognized and documented 70% of cases of patch-detected AF. Among the 30 participants who had AF documented by the clinical team, eight (26.7%) received rate control alone, chemical cardioversion was attempted in five (16.7%), nine (30.0%) received rate control and attempted chemical cardioversion, one (3.3%) received rate control and attempted chemical and electrical cardioversion, one (3.3%) received rate control and attempted electrical cardioversion, and six (20.0%) received neither. Table A1 (http://links.lww.com/CCX/A475) compares the characteristics between the patients with AF that was and was not recognized by the clinical team. Of the 14 participants in whom the clinical team did not recognize patch-detected AF, two (14.3%) were not on telemetry at the time that AF occurred. Table 3 shows the incidence of AF according to episode length, divided by whether or not AF was detected clinically. Table 4 describes the burden of AF. Total duration of AF ranged from 53 seconds to the entire duration of monitoring time. The proportion of participants with episodes lasting greater than or equal to 6 minutes, greater than or equal to 1 hour, greater than or equal to 12 hours, and greater than or equal to 24 hours was 14.8%, 13.2%, 7.0%, and 5.3%, respectively. Seven patients were in AF, the entire duration of monitoring, with total monitoring durations ranging from 9 hours to 13 days, 16 hours and 38 minutes; four were discharged from hospital in AF and the remaining three died. Longer durations of AF were more likely to be recognized by the clinical team. Table 5 provides descriptive data for heart rates in AF. The median overall HR in AF was 114 beats/min (IQR, 94–134). Three-quarters of patients with AF reached an HR in AF of at least 164 beats/min. When in AF, heart rates were roughly evenly split between the rapid (> 110 beats/min) and controlled rates (50–110 beats/min). Among the prespecified variables tested, all had significant univariable associations with AF: age (per year, relative risk [RR], 1.07; 95% CI, 1.04–1.10; p value from the modified Poisson approach with robust error variances < 0.001), Acute Physiology and Chronic Health Evaluation (APACHE) II score (per 1 point, RR, 1.03; 95% CI, 1.00–1.07; p = 0.034), Congestive heart failure, Hypertension, Age ≥ 75, Diabetes, Stroke/TIA (2 points), Vascular Disease, Female Sex Category (CHA2DS2-VASc) score (per 1 point, RR, 1.26; 95% CI, 1.07–1.47; p = 0.004), use of vasopressors or inotropes at any point during the study (RR, 2.28; 95% CI, 1.36–3.84; p = 0.002), and log(peak high-sensitivity troponin [ng/mL]) (RR, 1.10; 95% CI, 1.01–1.20; p = 0.024).
TABLE 3.
Incidence of Atrial Fibrillation According to Episode Length
| Episode Duration | Overall | AF Incidencea (%) | Patch Detected AF and Clinically Detected AF, n = 28 | Patch Detected AF but No Clinically Detected AF, n = 13 | pb |
|---|---|---|---|---|---|
| ≥ 30 sc | 47 | 18.9 | — | — | — |
| ≥ 6 mina | 36 | 14.8 | 28 | 8 | 0.0006 |
| ≥ 1 hra | 32 | 13.2 | 26 | 6 | 0.0003 |
| ≥ 12 hra | 17 | 7.0 | 15 | 2 | 0.001 |
| ≥ 24 hra | 13 | 5.3 | 12 | 1 | 0.002 |
AF = atrial fibrillation.
aDenominator 243 (excludes three participants with patch-detected AF for whom AF duration data were not available and three patients with clinically detected AF that was captured after patch discontinuation).
bBinomial test exact p values.
cDenominator 249—all patients who completed the study protocol.
TABLE 4.
Burden of Atrial Fibrillation
| Parameter | Overall, n = 41a (Median [IQR]) | Patch Detected AF and Clinically Detected AF, n = 28 (Median [IQR]) | Patch Detected AF and No Clinically Detected AF, n = 13 (Median [IQR]) | pb |
|---|---|---|---|---|
| Burden of atrial fibrillation (%)a | 11.0 (3.0–53.0) | 25.0 (7.0–58.0) | 1.0 (0.1–7.0) | 0.005 |
| #AF episodes | 3.0 (1.0–46.0) | 4.0 (1.5–61.0) | 2.0 (1.0–14.0) | 0.155 |
| Total AF duration (min) | 1,091 (468.0–5,738) | 2,612 (903.5–8,199) | 42.0 (9.0–540.0) | <0.001 |
| Total AF duration (hr) | 18.2 (7.8–95.6) | 43.5 (15.1–136.6) | 0.7 (0.2–9.0) | < 0.001 |
| Duration of longest episode (min) | 633.0 (103.0–2,640) | 1,135 (440.5–5,493) | 17.5 (1.1–467.0) | 0.001 |
| Duration of longest episode (hr) | 10.6 (1.7 - 44.0) | 18.9 (7.3–91.5) | 0.3 (0.0–7.8) | 0.001 |
| Time to first AF (hr) | 24.0 (0.0 - 69.0) | 14.5 (0.0–53.0) | 45.0 (20.0–106.0) | 0.074 |
AF = atrial fibrillation, IQR = interquartile range.
aDenominator includes all patients with patch-detected AF and available duration data. Three participants with patch-detected AF did not have duration data.
bp: from Wilcoxon rank-sum test.
% is out of total analyzed.
TABLE 5.
Heart Rates in Atrial Fibrillation
| Parameter | Overall | Patch Detected AF and Clinically Detected AF | Patch Detected AF and No Clinically Detected AF | p | |||
|---|---|---|---|---|---|---|---|
| n | Median (IQR) | n | Median (IQR) | n | Median (IQR) | ||
| Total analyzed among patch detected AF | 44 | 30 | 14 | ||||
| Minimum heart rate (beats/min) in AFa | 41 | 62 (47–85) | 28 | 60.5 (47–82) | 13 | 70 (46–101) | 0.387 |
| Median heart rate (beats/min) in AFa | 114 (94–134) | 108.5 (88.5–131) | 126 (116–135 | 0.201 | |||
| Maximum heart rate (beats/min) in AFa | 174 (164–194) | 175.5 (166–190 | 170 (152–195 | 0.839 | |||
| % of time in AF > 110 beats/minb | 35 | 51.5 (12–94) | 25 | 48 (11–87) | 10 | 83 (49–99) | 0.125 |
| % of time in AF 51–110 beats/minb | 48.5 (8.5–88) | 52 (13–88) | 16.5 (1–51) | 0.104 | |||
| % of time in AF ≤ 50 beats/minb | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.821 | |||
AF = atrial fibrillation, IQR = interquartile range.
ap: from t test.
bp: from Wilcoxon rank-sum test.
% is out of total analyzed.
Clinical Outcomes
Patients with AF were more likely to die in hospital (38.3% vs 21.3%, p = 0.015). There was no difference between major bleeding (14.9% vs 9.4%, p = 0.27) or nonfatal cardiac arrest requiring cardiopulmonary resuscitation in those with versus those without AF (2.1% vs 1.0%, p = 0.520). None of the study patients experienced an ischemic stroke or systemic embolism following hospital admission.
DISCUSSION
This study provides a practical estimate of the incidence of new-onset AF in critically ill patients, at a rate of nearly one in five (18.9%; 95% CI, 14.2–24.3%). Additionally, new-onset AF was missed by the clinical team in approximately one-third of cases. Finally, AF can be predicted by several common baseline variables and is associated with higher mortality.
Two systematic reviews have identified more than a dozen previous studies that estimated the incidence of new-onset AF, and these found a wide range of estimates (2, 15). We believe the estimate generated by this study is more accurate for several reasons. First, we enrolled a consecutive sample of patients and confirmed the absence of a history of AF, minimizing selection bias. Second, we used a continuous, full-disclosure external monitor with an AF detection algorithm, ensuring capture of all episodes, regardless of length and perceived clinical importance. Third, the study was large enough to estimate the incidence of AF within a practical precision of ±5%.
In addition to estimating the incidence of new-onset AF in the ICU, we have described patterns of AF and risk factors for onset and detection. As expected, longer episodes of AF were more likely to be recognized by the clinical team. Interestingly, patients were tachycardic (HR > 110 beats/min) only about half of the time while in AF; higher heart rates were not associated with a higher likelihood of detection. Participants who had higher baseline risk for AF (i.e., those with older age and higher CHA2DS2-VASc scores) had higher rates of AF in the ICU (21, 22). Patients who were sicker at baseline (i.e., higher APACHE-II score) and with evidence of myocardial injury were at higher risk for AF. The use of vasopressors or inotropes was also associated with higher rates of AF (23).
These results have important implications for conducting and interpreting research addressing AF in the ICU. First, we have the most credible published estimate of the incidence of AF. Our findings can be used by future studies as guide for power calculations. Second, clinicians should raise concerns about important confounding and selection bias in studies that report low incidences of AF (i.e., < 10%) and examine risk factors for AF and/or the association of AF with adverse outcomes. Third, we have demonstrated the importance of continuous monitoring to ensure capture and documentation of all AF episodes. These results are consistent with a smaller study of 66 patients who wore an ECG monitor, in whom 1/3 of AF cases were not noted by the clinical team (24).
This study has important limitations. This study used an AF duration cutoff of 30 seconds although the minimum clinically relevant duration of AF remains unknown (25). This study was designed to evaluate all-comers to the ICU and is not powered to make inferences about any specific subgroups of ICU populations. This study was not designed to assess the association of patient characteristics with the development of AF—all RRs are unadjusted, univariable measures and underlying confounding is likely. Similarly, this study was not designed to assess the association of AF with inhospital outcomes. Finally, the study was also not designed to assess long-term outcomes, although some participants were coenrolled into our ongoing AFOTS follow-up study (26).
CONCLUSIONS
Among patients admitted to an ICU, the incidence of new-onset AF is approximately one in five; one-third of cases are not recognized by the clinical team.
ACKNOWLEDGMENT
We thank for the collaboration and support from the nurses and staff of the ICUs at Hamilton General Hospital and Juravinski Hospital.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccxjournal).
Supported, in part, by peer-reviewed research grants from the Hamilton Health Sciences New Investigator Fund and the Canadian Stroke Prevention Intervention Network (C-SPIN).
Dr. McIntyre is supported by personnel awards from Canadian Stroke Prevention Intervention Network and the Canadian Institutes for Health Research (CIHR). Dr. Spence is supported by a personnel award from CIHR. Dr. Belley-Côté is supported by a personnel award from the McMaster University Department of Medicine. Dr. Whitlock is supported by a mid-career award from the Heart and Stroke Foundation. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. JAMA. 2001; 285:2370–2375 [DOI] [PubMed] [Google Scholar]
- 2.Wetterslev M, Haase N, Hassager C, et al. New-onset atrial fibrillation in adult critically ill patients: A scoping review. Intensive Care Med. 2019; 45:928–938 [DOI] [PubMed] [Google Scholar]
- 3.McIntyre WF, Connolly SJ, Healey JS. Atrial fibrillation occurring transiently with stress. Curr Opin Cardiol. 2018; 33:58–65 [DOI] [PubMed] [Google Scholar]
- 4.Maesen B, Nijs J, Maessen J, et al. Post-operative atrial fibrillation: A maze of mechanisms. Europace. 2012; 14:159–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang EY, Hulme OL, Khurshid S, et al. Initial precipitants and recurrence of atrial fibrillation. Circ Arrhythm Electrophysiol. 2020; 13:e007716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019; 74:104–132 [DOI] [PubMed] [Google Scholar]
- 7.Kirchhof P, Benussi S, Kotecha D, et al. ; ESC Scientific Document Group. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016; 37:2893–2962 [DOI] [PubMed] [Google Scholar]
- 8.Brathwaite D, Weissman C. The new onset of atrial arrhythmias following major noncardiothoracic surgery is associated with increased mortality. Chest. 1998; 114:462–468 [DOI] [PubMed] [Google Scholar]
- 9.Alonso-Coello P, Cook D, Xu SC, et al. ; POISE Investigators. Predictors, prognosis, and management of new clinically important atrial fibrillation after noncardiac surgery: A prospective cohort study. Anesth Analg. 2017; 125:162–169 [DOI] [PubMed] [Google Scholar]
- 10.Bhave PD, Goldman LE, Vittinghoff E, et al. Incidence, predictors, and outcomes associated with postoperative atrial fibrillation after major noncardiac surgery. Am Heart J. 2012; 164:918–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moss TJ, Calland JF, Enfield KB, et al. New-onset atrial fibrillation in the critically ill. Crit Care Med. 2017; 45:790–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaver CM, Chen W, Janz DR, et al. Atrial fibrillation is an independent predictor of mortality in critically ill patients. Crit Care Med. 2015; 43:2104–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernando SM, Mathew R, Hibbert B, et al. New-onset atrial fibrillation and associated outcomes and resource use among critically ill adults-a multicenter retrospective cohort study. Crit Care. 2020; 24:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kochav SM, Reiffel JA. Detection of previously unrecognized (subclinical) atrial fibrillation. Am J Cardiol. 2020; 127:169–175 [DOI] [PubMed] [Google Scholar]
- 15.McIntyre WF, Um KJ, Cheung CC, et al. Atrial fibrillation detected initially during acute medical illness: A systematic review. Eur Heart J Acute Cardiovasc Care. 2019; 8:130–141 [DOI] [PubMed] [Google Scholar]
- 16.McIntyre WF, Lengyel AP, Healey JS, et al. Design and rationale of the atrial fibrillation occurring transiently with stress (AFOTS) incidence study. J Electrocardiol. 2019; 57:95–99 [DOI] [PubMed] [Google Scholar]
- 17.Andrade JG, Verma A, Mitchell LB, et al. ; CCS Atrial Fibrillation Guidelines Committee. 2018 focused update of the Canadian Cardiovascular Society guidelines for the management of atrial fibrillation. Can J Cardiol. 2018; 34:1371–1392 [DOI] [PubMed] [Google Scholar]
- 18.Turakhia MP, Hoang DD, Zimetbaum P, et al. Diagnostic utility of a novel leadless arrhythmia monitoring device. Am J Cardiol. 2013; 112:520–524 [DOI] [PubMed] [Google Scholar]
- 19.Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005; 3:692–694 [DOI] [PubMed] [Google Scholar]
- 20.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004; 159:702–706 [DOI] [PubMed] [Google Scholar]
- 21.Andrade J, Khairy P, Dobrev D, et al. The clinical profile and pathophysiology of atrial fibrillation: Relationships among clinical features, epidemiology, and mechanisms. Circ Res. 2014; 114:1453–1468 [DOI] [PubMed] [Google Scholar]
- 22.Saliba W, Gronich N, Barnett-Griness O, et al. Usefulness of CHADS2 and CHA2DS2-VASc scores in the prediction of new-onset atrial fibrillation: A population-based study. Am J Med. 2016; 129:843–849 [DOI] [PubMed] [Google Scholar]
- 23.McIntyre WF, Um KJ, Alhazzani W, et al. Association of vasopressin plus catecholamine vasopressors vs catecholamines alone with atrial fibrillation in patients with distributive shock: A systematic review and meta-analysis. JAMA. 2018; 319:1889–1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guenancia C, Binquet C, Laurent G, et al. Incidence and predictors of new-onset atrial fibrillation in septic shock patients in a medical ICU: Data from 7-day Holter ECG monitoring. PLoS One. 2015; 10:e0127168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McIntyre WF, Healey JS. Stroke prevention for patients with atrial fibrillation: Beyond the guidelines. J Atr Fibrillation. 2017; 91:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McIntyre WF, Mendoza PA, Belley-Côté EP, et al. Design and rationale of the atrial fibrillation occurring transiently with stress (AFOTS) follow-up cohort study. Clin Cardiol. 2018; 41:1273–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


