INTRODUCTION:
Hepatocellular carcinoma (HCC) can develop among chronic hepatitis B patients after hepatitis B surface antigen (HBsAg) seroclearance. However, whether HCC risk after HBsAg seroclearance differs between antiviral therapy (AVT)-induced or spontaneous seroclearance cases and ways to identify at-risk populations remain unclear.
METHODS:
A retrospective cohort of 1,200 adult chronic hepatitis B patients who achieved HBsAg seroclearance (median age: 56 years; 824 men; 165 with cirrhosis; 216 AVT-induced cases) were analyzed. The risk of HCC after HBsAg seroclearance and the performance of 6 HCC prediction models were assessed.
RESULTS:
During a median of 4.8 years of follow-up (range: 0.5–17.8 years), HCC developed in 23 patients (1.9%). The HCC incidence rate was higher in the AVT-induced cases than that in the spontaneous cases (3.9% vs 0.9% at 5 years). AVT and cirrhosis were independent factors associated with HCC, with HCC incidence rates of 0.5%, 1.2%, 4.0%, and 10.5% at 5 years for spontaneous/no-cirrhosis, AVT-induced/no-cirrhosis, spontaneous/cirrhosis, and AVT-induced/cirrhosis patients, respectively. Among the 6 predictive HCC models tested, Chinese University-HCC score (0.82) showed the highest C-statistics, which was followed by guide with age, gender, HBV DNA, core promoter mutations and cirrhosis (0.81).
DISCUSSION:
AVT-induced HBsAg seroclearance was associated with higher HCC risk, especially for patients with cirrhosis, indicating that they need careful monitoring for HCC risk. The HCC risk models were able to stratify the HCC risk in patients with HBsAg seroclearance.
INTRODUCTION
Chronic hepatitis B virus (HBV) infection, defined as the persistence of serum hepatitis B surface antigen (HBsAg) for more than 6 months, is a major cause of liver cirrhosis and hepatocellular carcinoma (HCC) worldwide (1,2). During the natural course of chronic HBV infection, some patients show the spontaneous loss or clearance of HBsAg (3–5). The risk of disease progression is very low among chronic hepatitis B (CHB) patients with spontaneous HBsAg seroclearance (6,7) and is considered a state of functional cure (4). HBsAg seroclearance also occurs in CHB patients who received or are receiving antiviral therapy (AVT) (8,9). AVT-induced HBsAg seroclearance is known to be as durable as spontaneous HBsAg seroclearance (10) and is also associated with favorable clinical outcomes (8,11). Therefore, HBsAg seroclearance is considered the ideal endpoint of AVT in HBV guidelines (3,4). However, it is also known that some CHB patients with HBsAg seroclearance still develop HCC (12).
To date, limited information is available to indicate whether AVT-induced HBsAg seroclearance shows the same favorable clinical outcome as spontaneously induced HBsAg seroclearance. In a recent systemic review of 28 studies involving 8,904 CHB patients with HBsAg seroclearance, the incidence of HCC was very low (1.86%), and the risk factors of HCC included cirrhosis, male sex, and age at HBsAg seroclearance (13). However, limited information is available whether the risk of HCC is comparable between patients with AVT-induced and spontaneous-induced HBsAg seroclearance. In addition, because the HCC incidence rate after HBsAg seroclearance is low, risk stratification is needed to guide future follow-up plans, especially regarding HCC surveillance. Yet, there is limited information on the ways to stratify the HCC risk in CHB patients who have achieved HBsAg seroclearance. In this study, we compared the HCC risk between AVT-induced and spontaneously induced HBsAg seroclearance to determine whether AVT-induced HBsAg seroclearance was as safe as spontaneously induced HBsAg seroclearance. We also assessed the performance of several HCC prediction scores developed for CHB patients to determine whether these HCC risk scores could be applied to patients with HBsAg seroclearance.
METHODS
Study design, setting, and participants
This study was a hospital-based, retrospective, multicenter cohort study performed at 3 academic institutions in South Korea. The electronic medical records were screened for patients to include in the study. We included a total of 1,273 adults aged 18 years or older who met all inclusion criteria between January 1, 2000 and December 31, 2016 from each site. The inclusion criteria were as follows: (i) chronic HBV infection, defined by the presence of serum HBsAg for more than 6 months; (ii) no coinfection with hepatitis C virus or HIV; (iii) no history of cancer, including HCC; (iv) no history of liver transplantation or other solid organ transplantation, and (v) patients with documented HBsAg loss. Among them, we excluded 73 patients who met the following exclusion criteria: (i) 1 patient developed HCC within 6 months of HBsAg loss and (ii) 72 patients with follow-up durations less than 6 months from HBsAg loss. Finally, 1,200 chronic HBV monoinfected patients with documented HBsAg loss, without malignancy or transplantation at baseline, and with more than 6 months of follow-up after HBsAg loss were analyzed in this study (Figure 1). The study protocol was reviewed and approved by the Institutional Review Board of each participating site. The requirement for informed consent from the patients was waived because only deidentified data routinely collected during hospital visits were used.
Figure 1.
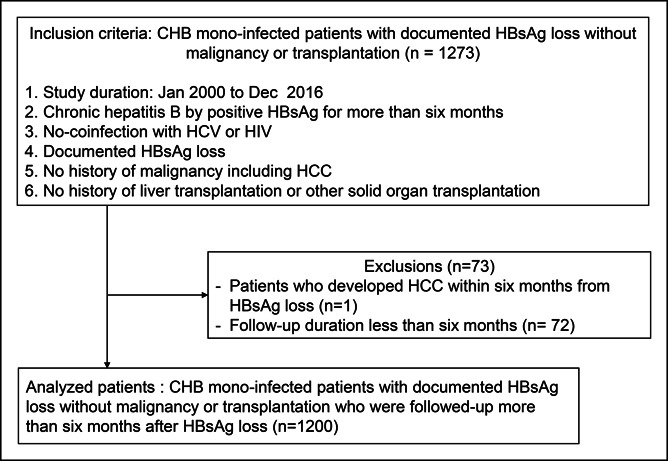
Study participants. A total of 1,200 patients were analyzed in this study. CHB, chronic hepatitis B; HBsAg, hepatitis B surface antigen; HCC, hepatocellular carcinoma; HCV, hepatitis C virus.
Study outcomes, variables, and measurements
The primary outcome was incident HCC during follow-up. HCC was diagnosed according to the regional HCC diagnostic guidelines during the study period (14,15). In brief, HCC was diagnosed either by pathology or clinical criteria showing typical features of HCC in imaging studies. The index date was defined as the first date of documented HBsAg loss. The patients were followed up from the index visit to the date of the diagnosis of HCC or to the last clinic visit (reference date: December 31, 2019).
The variables collected were age, sex, and serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin, bilirubin, and platelet levels measured closest to the index date. Imaging findings on ultrasonography, computed tomography or magnetic resonance imaging, and information on AVT use (interferon, pegylated interferon, lamivudine, adefovir, telbivudine, clevudine, entecavir, and tenofovir) were also collected.
AVT was defined for patients with a history or current use of AVT at HBsAg loss. Liver cirrhosis was defined by radiological evaluation when imaging findings suggestive of cirrhosis, a blunted, nodular liver edge, and/or splenomegaly (16) were identified. For 53 patients (0.4%), imaging studies within a year of the index visit were missing. For them, the fibrosis (FIB)-4 index (age in years × AST level in U/L)/([platelet count × 109/L] × [ALT level in U/L]) was calculated and was used to define cirrhosis (because cirrhosis was defined when the FIB-4 index was >3.25) (17,18). Elevated ALT levels were defined as ALT >34 in men or >30 in women (19). In addition, we also calculated the AST-to-platelet ratio index (20) and the risk estimation for hepatocellular carcinoma in chronic hepatitis B (REACH-B) (21), guide with age, gender, HBV DNA, core promoter mutations and cirrhosis (GAG-HCC) (22), Chinese University-HCC (CU-HCC) (23), platelets, age, gender hepatitis B (PAGE-B) (24), modified PAGE-B (25), and age-male-ALBI-platelets (aMAP) (26) scores using baseline variables.
Statistical analysis
Descriptive statistics for the continuous variables and the categorical variables are presented by median (interquartile range) and frequency (%), respectively. Cox regression models were used to identify the factors associated with incident HCC. AVT, age, sex, liver cirrhosis, and serum AST, ALT, albumin, bilirubin levels, and platelet counts were tested in univariable analysis for HCC development. We included the variables with P values <0.1 on univariable analysis in a multivariable analysis. Age was included in the multivariable analysis, regardless of the P value on univariable analysis. The cumulative HCC incidence rate was estimated using a Kaplan-Meier method, and differences between the groups were compared with the log-rank test.
To assess the prognostic performance of the HCC prediction models in patients with HBsAg loss, the area under the receiver operating characteristic curves (AUROCs) was calculated to predict HCC development at 3 and 5 years, respectively. After calculating the AUROC, one with the highest C-index was used as a reference to test performance of other model. AUROC was compared with test statistical difference between models with the cutoff P value of 0.05. Comparison was performed using the open source statistics software R (version 3.5.1) in conjunction with pROC package (27) that used Delong method (28).
RESULTS
Baseline characteristics
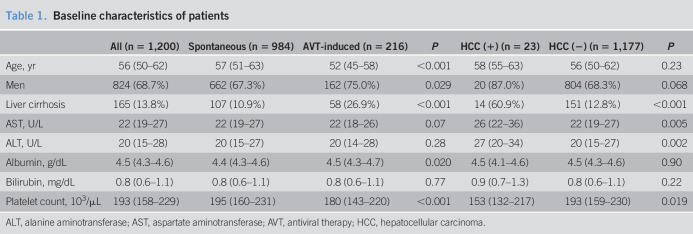
The baseline characteristics at HBsAg loss are summarized in Table 1. The median patient age was 56 years, 68.7% were men, and 13.8% of the analyzed patients had cirrhosis. Of 1,200 patients, 984 patients (82%) were spontaneously induced cases, whereas 216 patients (18%) were AVT-induced cases. Among the 216 AVT-induced cases, the types of AVT used were nucleoside or nucleotide analogs, interferon/pegylated interferon, and both nucleoside or nucleotide analogs and interferon/pegylated interferon for 186 (86.1%), 10 (4.6%), and 17 (7.9%) patients, respectively.
Table 1.
Baseline characteristics of patients
| All (n = 1,200) | Spontaneous (n = 984) | AVT-induced (n = 216) | P | HCC (+) (n = 23) | HCC (−) (n = 1,177) | P | |
| Age, yr | 56 (50–62) | 57 (51–63) | 52 (45–58) | <0.001 | 58 (55–63) | 56 (50–62) | 0.23 |
| Men | 824 (68.7%) | 662 (67.3%) | 162 (75.0%) | 0.029 | 20 (87.0%) | 804 (68.3%) | 0.068 |
| Liver cirrhosis | 165 (13.8%) | 107 (10.9%) | 58 (26.9%) | <0.001 | 14 (60.9%) | 151 (12.8%) | <0.001 |
| AST, U/L | 22 (19–27) | 22 (19–27) | 22 (18–26) | 0.07 | 26 (22–36) | 22 (19–27) | 0.005 |
| ALT, U/L | 20 (15–28) | 20 (15–27) | 20 (14–28) | 0.28 | 27 (20–34) | 20 (15–27) | 0.002 |
| Albumin, g/dL | 4.5 (4.3–4.6) | 4.4 (4.3–4.6) | 4.5 (4.3–4.7) | 0.020 | 4.5 (4.1–4.6) | 4.5 (4.3–4.6) | 0.90 |
| Bilirubin, mg/dL | 0.8 (0.6–1.1) | 0.8 (0.6–1.1) | 0.8 (0.6–1.1) | 0.77 | 0.9 (0.7–1.3) | 0.8 (0.6–1.1) | 0.22 |
| Platelet count, 103/μL | 193 (158–229) | 195 (160–231) | 180 (143–220) | <0.001 | 153 (132–217) | 193 (159–230) | 0.019 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; AVT, antiviral therapy; HCC, hepatocellular carcinoma.
When compared, the AVT-induced cases were younger, comprised more men, had cirrhosis, and showed higher albumin levels and lower platelet counts than those of spontaneous-induced cases (Table 1). When compared between those who developed and those who did not, those who developed HCC comprised more cirrhosis and showed higher AST and ALT levels and lower platelet count (Table 1).
Incidence and risk factors of HCC
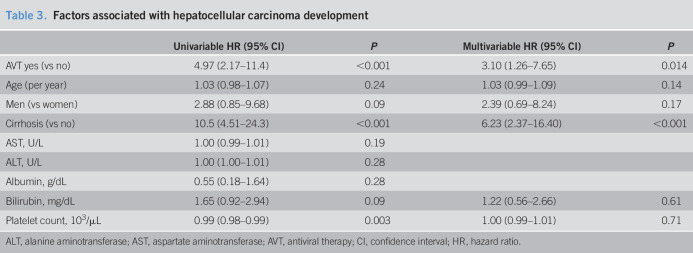
During a median of 4.8 years of follow-up (range: 0.5–17.8 years), HCC developed in 23 patients (1.9%). The cumulative incidence rate of HCC was 1.0%, 1.4%, and 3.2% at 3, 5, and 7 years, respectively. The HCC incidence according to baseline characteristics is summarized in Table 2. The HCC incidence was lower for young adults, women, those without cirrhosis, and those with low FIB-4 or AST-to-platelet ratio index scores. No significant difference was found according to baseline ALT levels. In the multivariable adjusted analysis, AVT (hazard ratio = 3.10, 95% confidence interval: 1.26–7.65) and cirrhosis (hazard ratio = 6.23, 95% confidence interval: 2.37–16.40) were independent factors associated with HCC (Table 3). When stratified according to cirrhosis and AVT use, the 5-year cumulative HCC incidence rate was 0.5%, 1.2%, 4.0%, and 10.5% for patients without cirrhosis/spontaneous cases (n = 877), patients without cirrhosis/AVT-induced cases (n = 158), patients with cirrhosis/spontaneous cases (n = 107), and patients with cirrhosis/AVT-induced cases (n = 58), respectively (Figure 2, P < 0.001).
Table 2.
Risk of hepatocellular carcinoma according to baseline characteristics
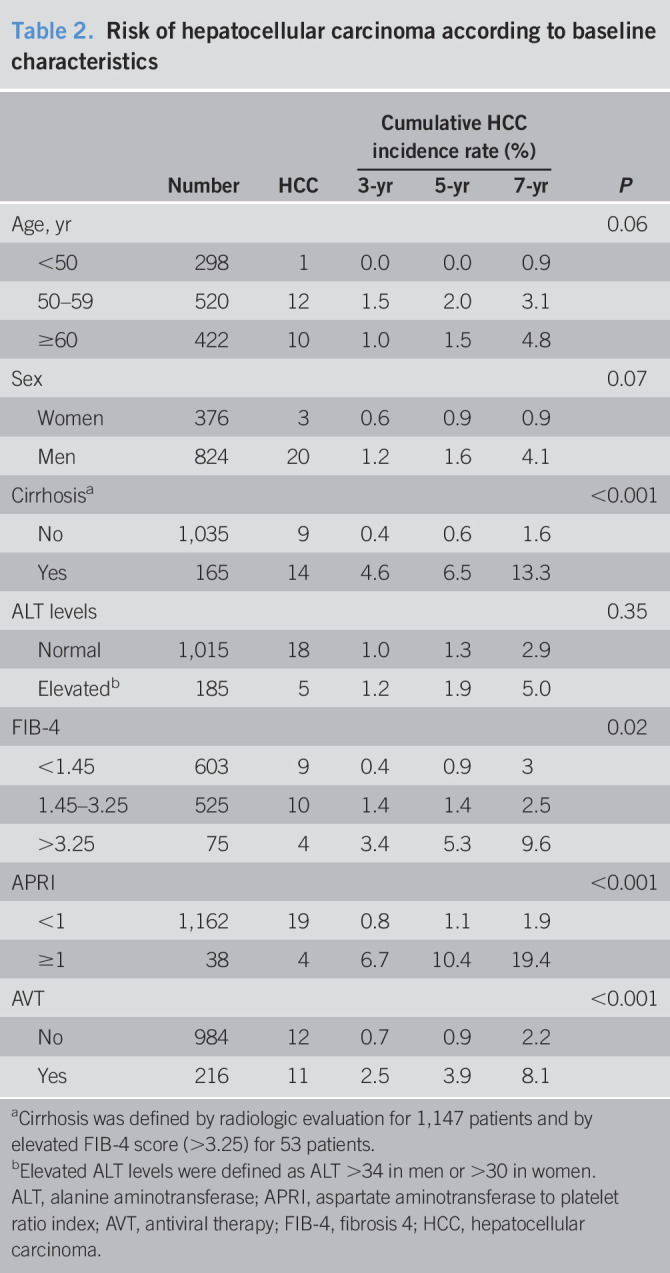
| Number | HCC | Cumulative HCC incidence rate (%) | P | |||
| 3-yr | 5-yr | 7-yr | ||||
| Age, yr | 0.06 | |||||
| <50 | 298 | 1 | 0.0 | 0.0 | 0.9 | |
| 50–59 | 520 | 12 | 1.5 | 2.0 | 3.1 | |
| ≥60 | 422 | 10 | 1.0 | 1.5 | 4.8 | |
| Sex | 0.07 | |||||
| Women | 376 | 3 | 0.6 | 0.9 | 0.9 | |
| Men | 824 | 20 | 1.2 | 1.6 | 4.1 | |
| Cirrhosisa | <0.001 | |||||
| No | 1,035 | 9 | 0.4 | 0.6 | 1.6 | |
| Yes | 165 | 14 | 4.6 | 6.5 | 13.3 | |
| ALT levels | 0.35 | |||||
| Normal | 1,015 | 18 | 1.0 | 1.3 | 2.9 | |
| Elevatedb | 185 | 5 | 1.2 | 1.9 | 5.0 | |
| FIB-4 | 0.02 | |||||
| <1.45 | 603 | 9 | 0.4 | 0.9 | 3 | |
| 1.45–3.25 | 525 | 10 | 1.4 | 1.4 | 2.5 | |
| >3.25 | 75 | 4 | 3.4 | 5.3 | 9.6 | |
| APRI | <0.001 | |||||
| <1 | 1,162 | 19 | 0.8 | 1.1 | 1.9 | |
| ≥1 | 38 | 4 | 6.7 | 10.4 | 19.4 | |
| AVT | <0.001 | |||||
| No | 984 | 12 | 0.7 | 0.9 | 2.2 | |
| Yes | 216 | 11 | 2.5 | 3.9 | 8.1 | |
Cirrhosis was defined by radiologic evaluation for 1,147 patients and by elevated FIB-4 score (>3.25) for 53 patients.
Elevated ALT levels were defined as ALT >34 in men or >30 in women.
ALT, alanine aminotransferase; APRI, aspartate aminotransferase to platelet ratio index; AVT, antiviral therapy; FIB-4, fibrosis 4; HCC, hepatocellular carcinoma.
Table 3.
Factors associated with hepatocellular carcinoma development
ALT, alanine aminotransferase; AST, aspartate aminotransferase; AVT, antiviral therapy; CI, confidence interval; HR, hazard ratio.
Figure 2.
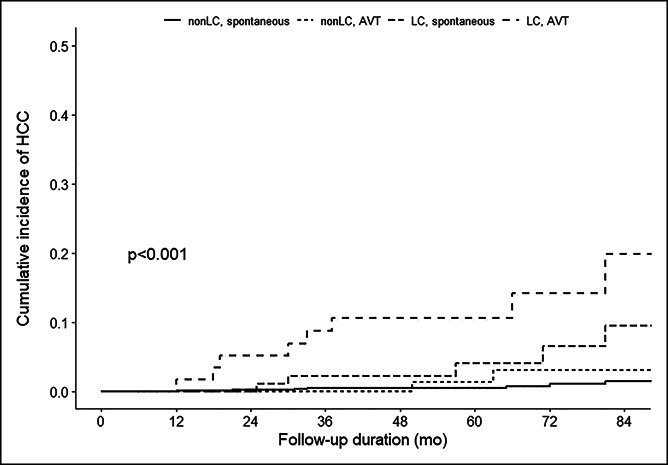
Cumulative incidence of hepatocellular carcinoma according to cirrhosis and antiviral treatment. AVT, antiviral therapy; HCC, hepatocellular carcinoma; LC, liver cirrhosis.
Role of HCC prediction models for patients with HBsAg loss
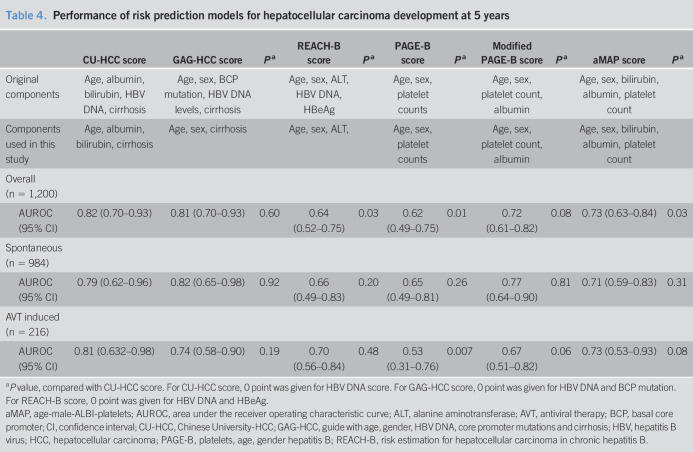
The performance of the HCC prediction models for predicting HCC development at 5 years is summarized in Table 4. Notably, HBV DNA levels, which contribute to the CU-HCC, GAG-HCC, and REACH-B scores, did not contribute to participants with HBsAg loss. Of the 6 models tested, CU-HCC scores showed the highest AUROC in the overall population, which was better than the REACH-B or PAGE-B scores. The GAG-HCC scores showed the highest AUROC in the spontaneously induced cases, and the CU-HCC scores showed the highest AUROC in the AVT-induced cases.
Table 4.
Performance of risk prediction models for hepatocellular carcinoma development at 5 years
| CU-HCC score | GAG-HCC score | Pa | REACH-B score | Pa | PAGE-B score | Pa | Modified PAGE-B score | Pa | aMAP score | Pa | |
| Original components | Age, albumin, bilirubin, HBV DNA, cirrhosis | Age, sex, BCP mutation, HBV DNA levels, cirrhosis | Age, sex, ALT, HBV DNA, HBeAg | Age, sex, platelet counts | Age, sex, platelet count, albumin | Age, sex, bilirubin, albumin, platelet count | |||||
| Components used in this study | Age, albumin, bilirubin, cirrhosis | Age, sex, cirrhosis | Age, sex, ALT, | Age, sex, platelet counts | Age, sex, platelet count, albumin | Age, sex, bilirubin, albumin, platelet count | |||||
| Overall (n = 1,200) | |||||||||||
| AUROC (95% CI) | 0.82 (0.70–0.93) | 0.81 (0.70–0.93) | 0.60 | 0.64 (0.52–0.75) | 0.03 | 0.62 (0.49–0.75) | 0.01 | 0.72 (0.61–0.82) | 0.08 | 0.73 (0.63–0.84) | 0.03 |
| Spontaneous (n = 984) | |||||||||||
| AUROC (95% CI) | 0.79 (0.62–0.96) | 0.82 (0.65–0.98) | 0.92 | 0.66 (0.49–0.83) | 0.20 | 0.65 (0.49–0.81) | 0.26 | 0.77 (0.64–0.90) | 0.81 | 0.71 (0.59–0.83) | 0.31 |
| AVT induced (n = 216) | |||||||||||
| AUROC (95% CI) | 0.81 (0.632–0.98) | 0.74 (0.58–0.90) | 0.19 | 0.70 (0.56–0.84) | 0.48 | 0.53 (0.31–0.76) | 0.007 | 0.67 (0.51–0.82) | 0.06 | 0.73 (0.53–0.93) | 0.08 |
P value, compared with CU-HCC score. For CU-HCC score, 0 point was given for HBV DNA score. For GAG-HCC score, 0 point was given for HBV DNA and BCP mutation. For REACH-B score, 0 point was given for HBV DNA and HBeAg.
aMAP, age-male-ALBI-platelets; AUROC, area under the receiver operating characteristic curve; ALT, alanine aminotransferase; AVT, antiviral therapy; BCP, basal core promoter; CI, confidence interval; CU-HCC, Chinese University-HCC; GAG-HCC, guide with age, gender, HBV DNA, core promoter mutations and cirrhosis; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; PAGE-B, platelets, age, gender hepatitis B; REACH-B, risk estimation for hepatocellular carcinoma in chronic hepatitis B.
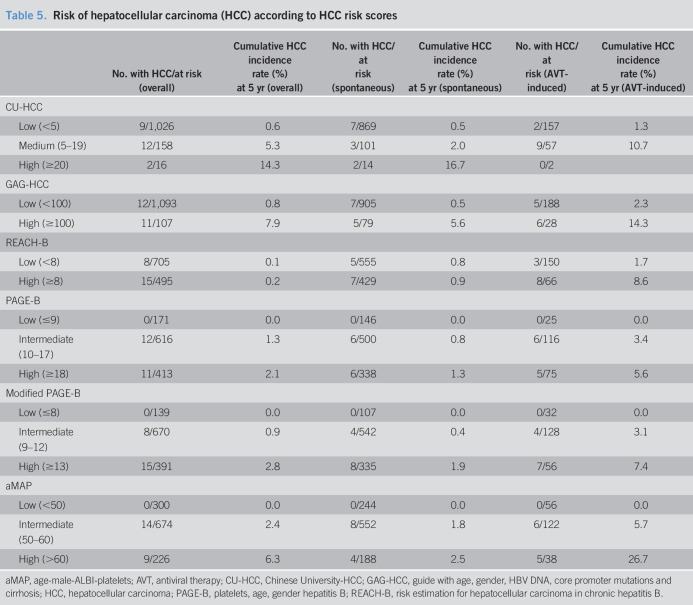
The HCC incidence according to risk scores cutoffs is summarized in Table 5. The HCC incidence was high for those with medium or high CU-HCC scores (5.3% or 14.3% at 5 years, respectively) and those with high GAG-HCC scores (7.9% at 5 years), but the HCC risk was not null for those with low CU-HCC scores (0.6% at 5 years) and those with low GAG-HCC scores (0.8% at 5 years). By contrast, the HCC incidence rate was not high even for high PAGE-B scores (2.1% at 5 years) and high modified PAGE-B scores (2.8% at 5 years), but no HCC developed in patients with low PAGE-B scores (0% at 5 years), low modified PAGE-B scores (0% at 5 years), or low aMAP score (0% at 5 years). When stratified according to AVT use, the HCC incidence rate was higher for AVT-induced cases compared with that of spontaneously induced cases, within the same category of risk scores (Table 5).
Table 5.
Risk of hepatocellular carcinoma (HCC) according to HCC risk scores
| No. with HCC/at risk (overall) | Cumulative HCC incidence rate (%) at 5 yr (overall) | No. with HCC/at risk (spontaneous) | Cumulative HCC incidence rate (%) at 5 yr (spontaneous) | No. with HCC/at risk (AVT-induced) | Cumulative HCC incidence rate (%) at 5 yr (AVT-induced) | |
| CU-HCC | ||||||
| Low (<5) | 9/1,026 | 0.6 | 7/869 | 0.5 | 2/157 | 1.3 |
| Medium (5–19) | 12/158 | 5.3 | 3/101 | 2.0 | 9/57 | 10.7 |
| High (≥20) | 2/16 | 14.3 | 2/14 | 16.7 | 0/2 | |
| GAG-HCC | ||||||
| Low (<100) | 12/1,093 | 0.8 | 7/905 | 0.5 | 5/188 | 2.3 |
| High (≥100) | 11/107 | 7.9 | 5/79 | 5.6 | 6/28 | 14.3 |
| REACH-B | ||||||
| Low (<8) | 8/705 | 0.1 | 5/555 | 0.8 | 3/150 | 1.7 |
| High (≥8) | 15/495 | 0.2 | 7/429 | 0.9 | 8/66 | 8.6 |
| PAGE-B | ||||||
| Low (≤9) | 0/171 | 0.0 | 0/146 | 0.0 | 0/25 | 0.0 |
| Intermediate (10–17) | 12/616 | 1.3 | 6/500 | 0.8 | 6/116 | 3.4 |
| High (≥18) | 11/413 | 2.1 | 6/338 | 1.3 | 5/75 | 5.6 |
| Modified PAGE-B | ||||||
| Low (≤8) | 0/139 | 0.0 | 0/107 | 0.0 | 0/32 | 0.0 |
| Intermediate (9–12) | 8/670 | 0.9 | 4/542 | 0.4 | 4/128 | 3.1 |
| High (≥13) | 15/391 | 2.8 | 8/335 | 1.9 | 7/56 | 7.4 |
| aMAP | ||||||
| Low (<50) | 0/300 | 0.0 | 0/244 | 0.0 | 0/56 | 0.0 |
| Intermediate (50–60) | 14/674 | 2.4 | 8/552 | 1.8 | 6/122 | 5.7 |
| High (>60) | 9/226 | 6.3 | 4/188 | 2.5 | 5/38 | 26.7 |
aMAP, age-male-ALBI-platelets; AVT, antiviral therapy; CU-HCC, Chinese University-HCC; GAG-HCC, guide with age, gender, HBV DNA, core promoter mutations and cirrhosis; HCC, hepatocellular carcinoma; PAGE-B, platelets, age, gender hepatitis B; REACH-B, risk estimation for hepatocellular carcinoma in chronic hepatitis B.
DISCUSSION
In this study, the incidence of HCC among patients with HBsAg seroclearance was low (1.9%), with a cumulative HCC incidence rate of 1.4% at 5 years, during a median follow-up duration of 4.8 years. The patients with AVT-induced seroclearance patients showed a higher risk of HCC compared with the patients with spontaneously induced seroclearance and was an independent risk factor associated with HCC, along with cirrhosis. CU-HCC and GAG-HCC scores, which include cirrhosis as a variable, showed better performance than REACH-B, PAGE-B, modified PAGE-B, or aMAP scores. When the patients were grouped according to the proposed cutoff values, CU-HCC and GAG-HCC showed higher performance in identifying the high-risk group, whereas PAGE-B, modified PAGE-B, and aMAP scores were able to identify patients who were at null risk of developing HCC within 5 years.
In this study, cirrhosis was an independent factor associated with HCC development. Similarly, several studies, including a meta-analysis, have identified the presence of cirrhosis as a key factor associated with future HCC risk in patients with HBsAg seroclearance (12,13,29). Male sex and age at HBsAg seroclearance were also suggested as important risk factors of HCC (29,30). Although age and sex were not independent risk factors in this study, the HCC incidence rate was higher in men compared with that in women and was higher for older patients in this study (Table 2). Notably, the HCC risk was higher for patients with AVT-induced HBsAg seroclearance than that for patients with spontaneously induced (0.9% and 3.9% at 5 years, respectively). AVT is usually considered for patients with persistent inflammation (3–5). Hence, the AVT-induced group might have experienced prolonged inflammation in the liver before HBsAg seroclearance than that by the spontaneous group. Indeed, when the baseline characteristics were compared, the prevalence of cirrhosis was higher in the AVT-induced group (Table 1). HBV DNA persists in the liver and might show very-low-level HBV replication after HBsAg seroclearance (31). Those who achieved spontaneous HBsAg seroclearance through host immune control might have stronger immune control of HBV. In a study by Kim et al. (29), the HCC risk was not higher for patients with AVT-induced HBsAg seroclearance than that for the spontaneously induced group. However, the number of AVT-induced cases was relatively small (n = 105) with shorter follow-up intervals (median 3.2 years) than that in this study. To our knowledge, no other studies have directly compared HCC risk between AVT-induced cases and spontaneously induced cases. Further data are needed to validate our observations. However, our findings indicate that patients with AVT-induced seroclearance warrant more careful attention to increased HCC risk. In addition, when stratified according to cirrhosis, the 5-year cumulative HCC incidence rate was 0.5% and 1.2% for patients with spontaneous- and AVT-induced seroclearance without cirrhosis and 4.0% and 10.5% for patients with spontaneous- and AVT-induced seroclearance with cirrhosis, respectively (Figure 2). This suggests that the ideal AVT endpoint should be HBsAg seroclearance before cirrhosis. The clinical benefit of AVT-induced HBsAg seroclearance might be low when cirrhosis is present.
In this study, the risk of HCC was low (1.4% at 5 years). Consistently, the reported HCC incidence rate after HBsAg seroclearance is (0.86%–3.7%) (29,30,32,33). Hence, risk-stratification is needed to provide individualized care, especially in HCC surveillance. Cirrhosis is a strong risk of HCC in patients with HBsAg seroclearance (12,13,29) and warrants HCC surveillance after HBsAg seroclearance. Yet, HCC was also observed in noncirrhotic patients indicating cirrhosis alone might not be optimal factor to identify individual who need HCC surveillance fort HBsAg seroclearance. In this study, 6 predictive models for HBV-related HCC were assessed. Among these models, CU-HCC showed the highest predictive ability (AUROC 0.82), followed by GAG-HCC (0.81). Liver cirrhosis was the most important predictor for HCC in HBsAg seroclearance patients (13). The integral components of CU-HCC and GAG-HCC include liver cirrhosis, whereas those of REACH-B, PAGE-B, modified PAGE-B, and aMAP do not. CU-HCC and GAG-HCC, which include liver cirrhosis as a variable, showed highest AUROC and observed that HCC risk was high (14.3%–7.9% at 5 years) for those classified as a high risk group. However, although the incidence was low, HCC development was observed for patients classified as a low-risk group by CU-HCC and GAG-HCC (0.6%–0.8% at 5 years). For REACH-B, PAGE-B, and modified PAGE-B score, HCC risk was not very high (0.2%–2.8% at 5 years) for those classified as a high risk group. However, HCC risk was null (0% at 5 years) for those classified as a low-risk group by PAGE-B or modified PAGE-B score. For aMAP scores, HCC risk was comparably high (6.3% at 5 years) for those classified as a high-risk group, and HCC risk was null (0% at 5 years) for those classified as a low-risk group. These different risk models warrant further validation but might be used as an initial step to identify high-risk patient who need HCC surveillance (CU-HCC, GAG-HCC, and aMAP) and to identify very-low-risk patients who might be exempted from HCC surveillance (PAGE-B, modified PAGE-B, and aMAP). Among risk scores, aMAP score was able to triage both high- and very-low-risk patients. Notably, the HCC risk was, especially, higher for patients with AVT-induced seroclearance with higher scores, indicated by HCC risk models (Table 5).
This study had some limitations. The study had the inherent limitations of a retrospective cohort study. Because HBsAg was not systemically evaluated at regular intervals, the age at seroclearance might be earlier than the date of documentation of HBsAg seroclearance in this study. Liver cirrhosis was defined radiologically, which has interobserver and intraobserver variability and was assessed by many radiologists in 3 centers. However, the radiologists were unaware of the study's aim, and the direction of bias was nondifferential. There were 53 patients (0.4%) with missing imaging studies within a year of the index visit, in which we used FIB-4 to define cirrhosis. Hence, more objective measures of fibrosis burden obtained by liver biopsies or fibroscans might provide more accurate information on the future HCC risk. There were several unmeasured factors, including a family history of HCC, alcohol use, smoking, metabolic syndrome, fatty liver, and/or steatohepatitis, which might be associated with HCC development in these patients. The primary outcome incidence was small (HCC, n = 23) and had to include strong alleged risk factors and analyze in the model. Studies with larger sample sizes with more events (primary outcome) are needed. Although HBV genotype study was missing for this study, almost all HBV infections found in South Korea were infected with genotype C HBV (1). Hence, the generalizability to other ethnicities and patients infected with other HBV genotypes warrants validation. HCC surveillance requires medical cost and is not harm free. Hence, additional studies, including cost-effective analysis, are required to find out optimal surveillance strategy for patients with HBsAg seroclearance. The strength of these data was the large sample size, careful definition of the study variables, and relatively long follow-up period.
In conclusion, we found that HCC risk was generally low after HBsAg seroclearance but was not null. AVT was an independent risk factor, along with cirrhosis, and the HCC risk was considerable, especially for cirrhotic patients who achieved HBsAg seroclearance by AVT. This indicates that AVT-induced HBsAg seroclearance might not be as safe as spontaneous HBsAg seroclearance, especially when cirrhosis is present. The HCC risk models showed acceptable performance in patients with HBsAg seroclearance. The CU-HCC and GAG-HCC scores were useful in identifying high-risk patients, whereas PAGE-B and modified PAGE-B scores were useful in identifying very-low-risk patients. These risk scores might be used together to stratify future HCC risk in patients with HBsAg seroclearance.
CONFLICTS OF INTEREST
Guarantor of the article: Dong Hyun Sinn, MD, PhD.
Specific author contributions: Yewan Park, MD, and Jeong-Hoon Lee, MD, PhD, contributed equally to this study. J.-H.L., D.H.S., and J.Y.P.: study design. Y.P. and D.H.S.: drafting of the manuscript. Y.P., J.Y.P., J.-H.L., M.A.K., Y.J.K., J.-H.Y., S.H.A., W.K., G.-Y.G., Y.-H.P., M.S.C., J.H.L., K.C.K., and S.W.P.: data collection. Y.P., D.H.S., and J.H.L.: statistical analysis and interpretation of the data. J.Y.P., J.-H.L., Y.J.K., J.-H.Y., S.H.A., G.-Y.G., Y.-H.P., M.S.C., J.H.L., K.C.K., and S.W.P.: critical revision of the manuscript. D.H.S. and J.Y.P.: study supervision. All authors approved the final submission.
Financial support: None to report.
Potential competing interests: None to report.
Study Highlights.
WHAT IS KNOWN
✓ Loss or seroclearance of hepatitis B surface antigen (HBsAg) can occur spontaneously or by antiviral therapy.
✓ Several hepatocellular carcinoma (HCC) risk models are available to predict HCC risk for chronic hepatitis B patients.
WHAT IS NEW HERE
✓ HCC risk was higher in antiviral therapy-induced HBsAg seroclearance than spontaneous case.
✓ HCC risk models were able to stratify HCC risk in patients with HBsAg seroclearance.
TRANSLATIONAL IMPACT
✓ Patients with antiviral therapy-induced HBsAg seroclearance warrants more close attention for HCC risk.
✓ HCC risk models can be used to stratify HCC risk in patients with HBsAg seroclearance.
Contributor Information
Yewan Park, Email: yewan.park@samsung.com.
Jeong-Hoon Lee, Email: pindra@empal.com.
Minseok Albert Kim, Email: lionjade83@gmail.com.
Yoon Jun Kim, Email: yoonjun@snu.ac.kr.
Jung-Hwan Yoon, Email: yoonjh@snu.ac.kr.
Do Young Kim, Email: DYK1025@yuhs.ac.
Sang Hoon Ahn, Email: ahnsh@yuhs.ac.
Wonseok Kang, Email: wonseok1202.kang@samsung.com.
Geum-Youn Gwak, Email: gy.gwak@samsung.com.
Yong-Han Paik, Email: yh.paik@samsung.com.
Moon Seok Choi, Email: drms.choi@samsung.com.
Joon Hyeok Lee, Email: gijhlee.lee@samsung.com.
Kwang Cheol Koh, Email: kc.koh@samsung.com.
Seung Woon Paik, Email: sw.paik@samsung.com.
REFERENCES
- 1.Cho EJ, Kim SE, Suk KT, et al. Current status and strategies for hepatitis B control in Korea. Clin Mol Hepatol 2017;23:205–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perz JF, Armstrong GL, Farrington LA, et al. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol 2006;45:529–38. [DOI] [PubMed] [Google Scholar]
- 3.European Association for the Study of the Liver. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370–98. [DOI] [PubMed] [Google Scholar]
- 4.Korean Association for the Study of the Liver. KASL clinical practice guidelines for management of chronic hepatitis B. Clin Mol Hepatol 2019;25:93–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67:1560–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J, Yang HI, Lee MH, et al. Incidence and determinants of spontaneous hepatitis B surface antigen seroclearance: A community-based follow-up study. Gastroenterology 2010;139:474–82. [DOI] [PubMed] [Google Scholar]
- 7.Nam SW, Jung JJ, Bae SH, et al. Clinical outcomes of delayed clearance of serum HBsAG in patients with chronic HBV infection. Korean J Intern Med 2007;22:73–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim GA, Lim YS, An J, et al. HBsAg seroclearance after nucleoside analogue therapy in patients with chronic hepatitis B: Clinical outcomes and durability. Gut 2014;63:1325–32. [DOI] [PubMed] [Google Scholar]
- 9.Chi H, Wong D, Peng J, et al. Durability of response after hepatitis B surface antigen seroclearance during nucleos(t)ide analogue treatment in a multiethnic cohort of chronic hepatitis B patients: Results after treatment cessation. Clin Infect Dis 2017;65:680–3. [DOI] [PubMed] [Google Scholar]
- 10.Yip TC, Wong GL, Wong VW, et al. Durability of hepatitis B surface antigen seroclearance in untreated and nucleos(t)ide analogue-treated patients. J Hepatol 2018;68:63–72. [DOI] [PubMed] [Google Scholar]
- 11.Yip TC, Wong GL, Chan HL, et al. HBsAg seroclearance further reduces hepatocellular carcinoma risk after complete viral suppression with nucleos(t)ide analogues. J Hepatol 2019;70:361–70. [DOI] [PubMed] [Google Scholar]
- 12.Liu F, Wang XW, Chen L, et al. Systematic review with meta-analysis: Development of hepatocellular carcinoma in chronic hepatitis B patients with hepatitis B surface antigen seroclearance. Aliment Pharmacol Ther 2016;43:1253–61. [DOI] [PubMed] [Google Scholar]
- 13.Kuang XJ, Jia RR, Huo RR, et al. Systematic review of risk factors of hepatocellular carcinoma after hepatitis B surface antigen seroclearance. J Viral Hepat 2018;25:1026–37. [DOI] [PubMed] [Google Scholar]
- 14.Korean Liver Cancer Study Group, National Cancer Center, Korea. 2014 KLCSG-NCC Korea practice guideline for the management of hepatocellular carcinoma. Gut Liver 2015;9:267–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korean Liver Cancer Study Group, National Cancer Center, Korea. Practice guidelines for management of hepatocellular carcinoma 2009 [in Ko]. Korean J Hepatol 2009;15:391–423. [DOI] [PubMed] [Google Scholar]
- 16.Bonekamp S, Kamel I, Solga S, et al. Can imaging modalities diagnose and stage hepatic fibrosis and cirrhosis accurately?. J Hepatol 2009;50:17–35. [DOI] [PubMed] [Google Scholar]
- 17.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317–25. [DOI] [PubMed] [Google Scholar]
- 18.McPherson S, Stewart SF, Henderson E, et al. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010;59:1265–9. [DOI] [PubMed] [Google Scholar]
- 19.Shim JJ, Kim JW, Oh CH, et al. Serum alanine aminotransferase level and liver-related mortality in patients with chronic hepatitis B: A large national cohort study. Liver Int 2018;38:1751–9. [DOI] [PubMed] [Google Scholar]
- 20.Lin ZH, Xin YN, Dong QJ, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: An updated meta-analysis. Hepatology 2011;53:726–36. [DOI] [PubMed] [Google Scholar]
- 21.Yang HI, Yuen MF, Chan HL, et al. Risk estimation for hepatocellular carcinoma in chronic hepatitis B (REACH-B): Development and validation of a predictive score. Lancet Oncol 2011;12:568–74. [DOI] [PubMed] [Google Scholar]
- 22.Yuen MF, Tanaka Y, Fong DY, et al. Independent risk factors and predictive score for the development of hepatocellular carcinoma in chronic hepatitis B. J Hepatol 2009;50:80–8. [DOI] [PubMed] [Google Scholar]
- 23.Wong VW, Chan SL, Mo F, et al. Clinical scoring system to predict hepatocellular carcinoma in chronic hepatitis B carriers. J Clin Oncol 2010;28:1660–5. [DOI] [PubMed] [Google Scholar]
- 24.Papatheodoridis G, Dalekos G, Sypsa V, et al. PAGE-B predicts the risk of developing hepatocellular carcinoma in Caucasians with chronic hepatitis B on 5-year antiviral therapy. J Hepatol 2016;64:800–6. [DOI] [PubMed] [Google Scholar]
- 25.Kim JH, Kim YD, Lee M, et al. Modified PAGE-B score predicts the risk of hepatocellular carcinoma in Asians with chronic hepatitis B on antiviral therapy. J Hepatol 2018;69:1066–73. [DOI] [PubMed] [Google Scholar]
- 26.Fan R, Papatheodoridis G, Sun J, et al. aMAP risk score predicts hepatocellular carcinoma development in patients with chronic hepatitis. J Hepatol 2020;73:1368–78. [DOI] [PubMed] [Google Scholar]
- 27.Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 2011;12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988;44:837–45. [PubMed] [Google Scholar]
- 29.Kim GA, Lee HC, Kim MJ, et al. Incidence of hepatocellular carcinoma after HBsAg seroclearance in chronic hepatitis B patients: A need for surveillance. J Hepatol 2015;62:1092–9. [DOI] [PubMed] [Google Scholar]
- 30.Yip TC, Chan HL, Wong VW, et al. Impact of age and gender on risk of hepatocellular carcinoma after hepatitis B surface antigen seroclearance. J Hepatol 2017;67:902–8. [DOI] [PubMed] [Google Scholar]
- 31.Loriot MA, Marcellin P, Walker F, et al. Persistence of hepatitis B virus DNA in serum and liver from patients with chronic hepatitis B after loss of HBsAg. J Hepatol 1997;27:251–8. [DOI] [PubMed] [Google Scholar]
- 32.Arase Y, Ikeda K, Suzuki F, et al. Long-term outcome after hepatitis B surface antigen seroclearance in patients with chronic hepatitis B. Am J Med 2006;119:71.e9–16. [DOI] [PubMed] [Google Scholar]
- 33.Simonetti J, Bulkow L, McMahon BJ, et al. Clearance of hepatitis B surface antigen and risk of hepatocellular carcinoma in a cohort chronically infected with hepatitis B virus. Hepatology 2010;51:1531–7. [DOI] [PubMed] [Google Scholar]