Supplemental Digital Content is available in the text.
Keywords: acute respiratory distress syndrome, extracorporeal membrane oxygenation, isoflurane; ultraprotective ventilation, volatile sedation
Objectives:
Patients on extracorporeal support for severe acute respiratory distress syndrome may require a prolonged period of deep sedation. In these patients, volatile sedation may represent a valid alternative to IV drugs. The aim of our study was to describe the feasibility of volatile sedation in a large cohort of acute respiratory distress syndrome patients undergoing venovenous extracorporeal membrane oxygenation and ultraprotective ventilation.
Design:
Retrospective monocentric study.
Setting:
Adult ICU, ASST Monza, Italy.
Patients:
Adult patients who underwent volatile sedation with isoflurane during venovenous extracorporeal membrane oxygenation between 2009 and 2019.
Interventions:
Isoflurane was delivered via the AnaConDa system. The sedation level, hemodynamics, and laboratory tests were compared between the volatile sedation phase and the IV sedation phases before and after the isoflurane sedation period.
Measurements and Main Results:
About 74 patients (50 yr [43–56 yr]) were included. Median duration of venovenous extracorporeal membrane oxygenation support was 22 days (14–51 d). Volatile sedation started on day 3 (2–6) of extracorporeal membrane oxygenation support, and its median duration was 7 days (4–13 d), ranging from 1 to 38 days. A total of 970 venovenous extracorporeal membrane oxygenation days were analyzed. During the volatile phase, the sedation level was slightly deeper (bispectral index 39 ± 6) compared with the IV phase before and after isoflurane (42 ± 8 and 43 ± 9, respectively, p < 0.001). Requirements of fentanyl and remifentanyl were reduced during the volatile phase. Minor differences in hemodynamics were observed during volatile sedation: mean arterial pressure was lower (75 ± 13 vs 79 ± 14 and 80 ± 15; p < 0.001), whereas cardiac output was higher (8.5 ± 1.9 vs 7.9 ± 1.8 and 8.0 ± 1.8; p = 0.003). Aspartate aminotransferase levels were lower during the volatile sedation phases (p < 0.001), whereas alanine aminotransferase, triglycerides, and creatine phosphokinase were more altered during the IV sedation phase before isoflurane (p < 0.001).
Conclusions:
Volatile sedation represents an alternative to IV agents to achieve long-term deep sedation in critically ill patients on extracorporeal membrane oxygenation undergoing ultraprotective ventilation.
Patients with severe acute respiratory distress syndrome (ARDS) on venovenous extracorporeal membrane oxygenation (V-V ECMO) support often require prolonged periods of controlled mechanical ventilation and deep sedation (1). Achievement of an adequate level of sedation and its maintenance are common challenges for the intensivists (2, 3). Midazolam and propofol are the most commonly used sedative agents, but their use is associated with well-known adverse effects such as accumulation (4, 5), myotoxicity (6), and tachyphylaxis (7, 8). Furthermore, the pharmacokinetics of these IV sedatives may be affected by the extracorporeal circuit (9–11). Volatile anesthetics represent a promising alternative to IV sedation (12). To date, despite their widespread use for general anesthesia, the experience with volatile agents in the intensive care setting is limited (13–18). Rare complications, such as QT prolongation (19) and malignant hyperthermia (20), were reported. The usefulness of inhalational volatile-based sedation was recently highlighted for patients with coronavirus disease 2019 pneumonia and ARDS (21), also because of the shortage in essential IV sedative medications.
Small retrospective studies (22, 23) reported that volatile sedation might be feasible even during low tidal volumes (TVs) ventilation and extracorporeal support. The low minute ventilation of patients on ECMO allows the halogenated gas concentration target to be reached without high infusion rates, thus reducing its economic impact (12).
The aim of the present study was to describe the feasibility of volatile sedation in V-V ECMO patients undergoing ultraprotective low-frequency ventilation.
MATERIALS AND METHODS
We conducted a retrospective single-center study on patients admitted to the ICU of ASST Monza, Italy, from August 2009 to August 2019, complying with the criteria for inclusion in the study. The study was approved by the Institutional Ethics Committee (Comitato Etico ASST Monza, Ref.3434). Written informed consent was waived because of the retrospective design. Adult patients (> 18 yr old) were included if they met the following inclusion criteria: 1) diagnosis of ARDS, 2) V-V ECMO treatment, and 3) sedation with isoflurane for at least 24 hours.
Data Collection
We retrospectively collected data from patient electronic records. Data were gathered once a day at 10 o’clock during the controlled mechanical ventilation period. Only the first run of volatile sedation was included for each patient. Three steps were considered and compared:
1) IV before isoflurane: the IV sedation phase before volatile sedation (up to 7 d before the start of isoflurane sedation).
2) Isoflurane: the volatile sedation phase.
3) IV after isoflurane: the IV sedation phase after volatile sedation (up to 7 days after the end of isoflurane sedation).
Epidemiological and baseline data were collected: age, gender, body mass index, the severity of illness at ICU admission (Simplified Acute Physiology Score, Sequential Organ Failure Assessment [SOFA] score and Pao2/Fio2 ratio), and duration of V-V ECMO support.
To assess the impact of the type of sedation (IV vs volatile), the following data were collected:
1) Sedation level: bispectral index (BIS), Richmond Agitation-Sedation Scale (RASS), sedatives, and opioids dosage.
2) ECMO parameters: blood flow and gas flow.
3) Ventilation settings: TV, respiratory rate, Fio2, and positive end-expiratory pressure.
4) Respiratory parameters: calculated pulmonary shunt.
5) Hemodynamic variables: mean arterial pressure (MAP), heart rate (HR), central venous pressure, mean pulmonary arterial pressure, cardiac output (assessed by thermodilution via a pulmonary artery catheter), vasoactive drugs use and dosage, use of antihypertensive agents, and number of antihypertensive drugs.
6) Laboratory tests: bilirubin, transaminases, creatinine, urea, triglycerides, and creatine phosphokinase (CPK).
Isoflurane Administration: Anesthetic Conserving Device
At our institution, volatile sedation is routinely used during controlled mechanical ventilation in ECMO patients developing tolerance to IV sedative agents. In these patients, the halogenated gas is administered to the patient through the anesthetic conserving device (AnaConDa, Sedana Medical, Danderyd, Sweden), a modified vaporizer incorporated in the breathing circuit.
Liquid isoflurane is delivered from a syringe pump through the anesthetic agent line into the vaporizer. Inside the device, a porous rod converts the anesthetic from the liquid into the gaseous phase. The evaporated gas is then carried with the inspiratory flow from the ventilator to the patient. In our setting, the device is inserted in the inhalation branch of the breathing circuit rather than between the endotracheal tube and the Y-piece (Fig. 1). This configuration reduces the airway dead space (about 100 mL) in patients undergoing ventilation with very low TV and allows the use of active humidification. A gas analyzer samples the gas from a side port and displays the concentration of the volatile agent. Gas scavenging is performed from the expiratory outlet of the ventilator by a standard evacuation system.
Figure 1.
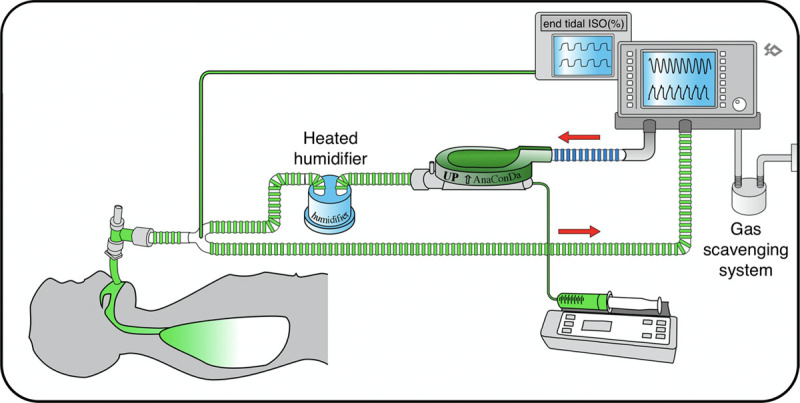
Schematics of connection of the AnaConDa device (AnaConDa, Sedana Medical, Danderyd, Sweden) to the ventilatory circuit. The AnaConDa device is placed on the inspiratory branch of the circuit between the ventilator and the humidifier. This connection is routinely used for all acute respiratory distress syndrome patients requiring volatile sedation.
Sedation Management
Transition from IV to volatile sedation is routinely performed in ECMO patients when the disease severity precludes the possibility of decreasing the sedation plan and switching to assisted mechanical ventilation. Volatile sedation is then maintained until clinical improvement. Depth of sedation is monitored with validated tools: a clinical score (RASS) and BIS. RASS is a 10-point scale, ranging from +4 “combative” to –5 “unarousable,” based on a clinical evaluation of the patient (24). BIS is an electroencephalography (EEG)-derived index, expressed by a number between 0 (isoelectric EEG) and 100 (awake), used to monitor cortical suppression. This index allows to titrate sedative doses to reach the appropriate depth of sedation (25–27).
Statistical Analysis
Numerical data are presented as mean ± sd or median (interquartile range), and categorical data as absolute (relative) frequency. Parametric tests were applied to compare normally distributed variables; otherwise, nonparametric tests were used. Pearson chi-square or the Fisher exact test was used for categorical variables. To analyze the effect of sedation on outcome variables, we used a restricted maximum likelihood method to fit a general linear mixed model. The sedation phase (i.e., IV before isoflurane, isoflurane, and IV after Isoflurane) was considered as the independent factor, whereas patients and the number of days of sedation per phase were considered as random effects. A two-tailed p value of less than 0.05 was considered statistically significant. The JMP 14.0 statistical software (SAS, Cary, NC) was used for statistical analysis.
RESULTS
Over the study period, 74 patients met the study criteria and were included (Fig. S1, Supplemental Digital Content, for patient flowchart, http://links.lww.com/CCX/A474). Baseline characteristics of the study population are reported in Table 1.
TABLE 1.
Baseline Characteristics of the Study Population
| Age, yr | 50 (43–56) |
| Females, n | 26 (35%) |
| Body mass index, kg/m2 | 26 (23–31) |
| Sequential Organ Failure Assessment score | 9 (6–12) |
| Simplified Acute Physiology Score II score | 35 (27–48) |
| Pao2/Fio2, mm Hg | 70 (52–88) |
| Cause of acute respiratory distress syndrome | |
| Viral pneumonia | 26 (30%) |
| Bacterial pneumonia | 25(30%) |
| Autoimmune disorder | 10 (17%) |
| Trauma | 3 (6%) |
| Unknown | 10 (17%) |
Data are expressed as median (interquartile range) or absolute (relative) frequency.
The data from a total of 970 ECMO-days were analyzed. The median duration of V-V ECMO support was 22 days (14–51 d). Before ECMO start, 62 patients (84%) had a Pao2/Fio2 ratio below 100 mm Hg, the others between 100 and 200 mm Hg. Volatile sedation started on day 3 (2–6) of ECMO support, and its median duration was 7 days (4–13 d), ranging from 1 to 38 days. Data for the volatile sedation phase were available for all 74 patients. Eight patients started isoflurane sedation on day 1, whereas 28 patients switched to assisted ventilation right after the volatile sedation phase. Therefore, data for the IV phase before and after isoflurane sedation were analyzed in 66 and 46 patients, respectively. Twenty patients (27%) died during ECMO support, whereas three patients (4%) died in the ICU after discontinuation of the extracorporeal support. Fifty-one patients (69%) were discharged alive from the hospital.
Table 2 shows the sedative setting and sedation level during the three study steps. Compared with IV sedation, volatile sedation was characterized by a statistically significant (but clinically nonrelevant) reduction in BIS values, with lower requirements of opioids. No patient received propofol infusion during volatile sedation, but a minimal proportion of patients required midazolam.
TABLE 2.
Sedation Parameters and Sedative Agents
| Parameter | IV Before Isoflurane (260 d) | Isoflurane (506 d) | IV After Isoflurane (204 d) | p |
|---|---|---|---|---|
| Sedative agents | ||||
| Isoflurane | ||||
| No. of days (%) | 506 (100%) | |||
| Infusion rate, mL/hr | 12.5 ± 4.4 | |||
| End tidal, % | 1.2 ± 0.4 | |||
| Propofol | ||||
| No. of days (%) | 218 (83.8%) | 169 (82.8%) | 0.802 | |
| Dose, mg/kg/hr | 3.97 ± 1.42 | 3.76 ± 1.59 | 0.626 | |
| Midazolam | ||||
| No. of days (%) | 81 (31.1%) | 20 (4.0%)a,b | 58 (28.4%) | < 0.001 |
| Dose, mg/kg/hr | 0.08 ± 0.05 | 0.05 ± 0.04a | 0.06 ± 0.03a | < 0.001 |
| Bispectral index | 42 ± 8 | 43 ± 9 | < 0.001 | |
| Richmond Agitation-Sedation Scale, No. of days (%) | 0.128 | |||
| –5 | 224 (86.2%) | 474 (94.4%) | 174 (85.3%) | |
| –4 | 28 (10.8%) | 28 (5.6%) | 20 (9.8%) | |
| –3 | 4 (1.5%) | 0 | 5 (2.5%) | |
| –2 | 1 (0.4%) | 0 | 4 (2%) | |
| Opioids, No. of days (%) | 250 (96.2%) | 464 (91.7%)a | 194 (95.1%) | 0.036 |
| Fentanyl | ||||
| Dose, µg/kg/hr | 1.63 ± 0.54 | 1.41 ± 0.57a,b | 1.78 ± 0.96 | < 0.001 |
| Remifentanyl | ||||
| Dose, µg/kg/min | 0.14 ± 0.07 | 0.07 ± 0.04a,b | 0.12 ± 0.03 | 0.005 |
| Neuromuscular blocking agents, No. of days (%) | 236 (90.8%) | 459 (90.7%)b | 172 (84.3%)a | 0.011 |
| Cisatracurium | ||||
| Dose, mg/kg/h | 0.18 ± 0.06 | 0.18 ± 0.08a | 0.23 ± 0.04a | 0.003 |
| Rocuronium | ||||
| Dose, mg/kg/hr | 0.69 ± 0.26 | 0.71 ± 0.31b | 0.79 ± 0.36a | < 0.001 |
ap < 0.05 vs IV before isoflurane.
bp < 0.05 vs IV after isoflurane.
Data are expressed as mean ± sd or absolute (relative, % of the step) frequency.
Hemodynamic parameters during the two study phases are presented in Table 3. MAP was slightly lower during volatile sedation than during the IV sedation phase, whereas cardiac output was higher. The requirements of vasoactive drugs were lower during volatile sedation. On the other hand, the use of antihypertensive agents was more frequent during IV sedation.
TABLE 3.
Hemodynamic Parameters and Vasoactive Drugs
| Parameter | IV Before Isoflurane (260 d) | Isoflurane (506 d) | IV After Isoflurane (204 d) | p |
|---|---|---|---|---|
| Mean arterial pressure, mm Hg | 79 ± 14 | 75 ± 13a,b | 80 ± 15 | < 0.001 |
| Heart rate, /min | 92 ± 16 | 99 ± 14a | 101 ± 14a | < 0.001 |
| Central venous pressure, mm Hg | 11 ± 4 | 11 ± 3 | 11 ± 3 | 0.080 |
| Mean pulmonary arterial pressure, mm Hg | 29 ± 6 | 28 ± 6b | 30 ± 6 | 0.027 |
| Cardiac output, L/min | 7.9 ± 1.8 | 8.5 ± 1.9a,b | 8.0 ± 1.8 | < 0.001 |
| Vasoactive drugs, No. of days (%) | 115 (44.2%) | 162 (32%) | 59 (28.9%) | < 0.001 |
| Dopamine, µg/kg/min | 5.7 ± 2.5 | 10.7 ± 8.1 | 12.7 ± 7.4 | 0.790 |
| Norepinephrine, µg/kg/min | 0.12 ± 0.13 | 0.08 ± 0.06a | 0.12 ± 0.17 | 0.001 |
| Dobutamine, µg/kg/min | 5.0 ± 3.1 | 4.1 ± 1.9b | 2.6 ± 1.6a | < 0.001 |
| Antihypertensive agents, No. of days (%) | 8 (3.1%) | 12 (2.4%) | 16 (7.8%) | 0.004 |
ap < 0.05 vs IV before isoflurane.
bp < 0.05 vs IV after isoflurane.
Data are expressed as mean ± sd, median (interquartile range), and absolute (relative, % of the step) frequency.
All patients received ultraprotective ventilation, with a mean TV of 3.7 ± 1.4 mL/kg of ideal body weight and an average respiratory rate of 10 ± 3 breaths/min. Mean TV during the isoflurane phase was 264 ± 79 mL, ranging from 45 to 584 mL. Only minor differences in ventilation and ECMO parameters were detected (see Table S1, Supplemental Digital Content, http://links.lww.com/CCX/A474). During the volatile sedation phase, the Fio2 and the intrapulmonary shunt fraction were lower.
During the isoflurane phase, patients had lower levels of aspartate aminotransferase. Alanine aminotransferase, triglycerides, and creatine phosphokinase were higher during the IV sedation phase before isoflurane, whereas gamma glutamyl transpeptidase progressively increased over time (Table 4).
TABLE 4.
Laboratory Tests
| Parameter | IV Before Isoflurane (260 d) | Isoflurane (506 d) | IV After Isoflurane (204 d) | p |
|---|---|---|---|---|
| Bilirubin, mg/dL | 1.8 ± 2.6 | 1.3 ± 2.2 | 2.3 ± 3.3 | 0.310 |
| Alanine aminotransferase, U/L | 59 ± 76 | 36 ± 32a | 40 ± 30a | < 0.001 |
| Aspartate aminotransferase, U/L | 68 ± 61 | 49 ± 46a,b | 63 ± 63 | < 0.001 |
| Gamma-glutamyl transpeptidase, U/L | 202 ± 189 | 251 ± 273a,b | 335 ± 294a | < 0.001 |
| Creatinine, mg/dL | 1.1 ± 0.9 | 0.9 ± 0.7a | 0.8 ± 0.5a | < 0.001 |
| Urea, mg/dL | 71 ± 42 | 64 ± 37 | 68 ± 35 | 0.114 |
| Triglycerides, mg/dL | 279 ± 160 | 232 ± 140a | 242 ± 123a | < 0.001 |
| Creatine phosphokinase, U/L | 289 ± 590 | 124 ± 423a | 138 ± 638a | < 0.001 |
ap < 0.05 vs IV before isoflurane.
bp < 0.05 vs IV after isoflurane.
Data are presented as mean ± sd.
DISCUSSION
In this retrospective study, we described the feasibility of volatile sedation with isoflurane in ARDS patients undergoing V-V ECMO and ultraprotective ventilation. Sedation with isoflurane allowed to achieve a deep level of sedation (RASS –4 to –5, BIS 39 ± 6), and the depth of sedation proved to be slightly more profound than during IV agent sedation. Sedation with halogenates allowed IV sedation to be interrupted, thus allowing to carry out a proper washout from propofol and midazolam. It is noteworthy that we did not observe a difference between the dosage of propofol before and after the volatile sedation phase, thus excluding a significant reduction of propofol resistance after the halogenated gas cycle. Contrarily, a reduction in midazolam dosage was observed, but we cannot confirm that this finding may support the possibility to use volatile sedation to recover from benzodiazepine tachyphylaxis. A clinically significant reduction in the dose of opiates was recorded, which witness the known opioid-sparing and analgesic properties of halogenated gases. We also observed a reduction in days of treatment and dosages of neuromuscular blocking agents after volatile anesthesia, possibly due to a time-dependent effect. Hemodynamics during volatile anesthesia were not largely affected. During volatile sedation, patients had higher cardiac output and HR and lower pulmonary and systemic pressures, suggesting a mild vasodilation as compared with IV anesthesia. Accordingly, vasoactive drugs during volatile sedation were more frequently employed, but at lower dosages. Similarly, no adverse effect was observed on gas exchange, as shown by stable ventilatory and ECMO setting. Of note, a reduction in intrapulmonary shunt was observed during volatile anesthesia. There were no adverse effects on kidney function during the volatile phase, whereas liver and muscular laboratory tests were more altered during the IV one.
To date, this is by far the largest study on this topic. Previous small case series reported the feasibility of volatile sedation in patients on V-V ECMO (22, 23, 28) in 6, 7, and 1 patients, respectively. Another retrospective trial (29) compared the effect of propofol and isoflurane sedation in 91 surgical patients supported with venovenous or venoarterial ECMO. Isoflurane sedation had no impact on patient outcome, but a lower rate of side effects was reported. The study population was, however, heterogeneous: patients included in the study suffered from various clinical conditions, such as ARDS, trauma, cardiac arrest, and pulmonary embolism. In addition, few data on ventilation setting and respiratory function were provided.
All these studies confirmed the feasibility of sedation with isoflurane and included patients on spontaneous breathing. Anesthesia machines feature variable bypass vaporizers, which allow to maintain a stable concentration of volatile agents inside the breathing circuit, regardless of the patient ventilation. Contrarily, when volatile agents are delivered by anesthetic conserving devices, the delivered inspiratory concentration of halogenated gas is inversely proportional to minute ventilation. In this context, a transient increase of ventilation determines a decrease in the volatile agent concentration, whereas bradypnea may lead to a dramatic increase of the halogenated concentration. This may lead to an unstable sedation plan; therefore, at our institution, volatile sedation is performed only during controlled mechanical ventilation and the study only explored this setting. The originality of our study also relies on the severity of patients (median of Pao2/Fio2 before ECMO: 71 mmHg; average pulmonary shunt: 57%). All patients required ultraprotective ventilation; therefore, the AnaConDa device was placed on the inhalation branch to avoid the increase of airway dead space. This resulted in an increase of halogenated gas consumption: to maintain a mean end-tidal isoflurane concentration of 1.2%, an infusion rate of 12–13 mL/hr was required, significantly higher than other studies (22, 23).
Volatile sedation may be associated with potential benefits. In patients with ARDS, use of sevoflurane may improve oxygenation and decrease the levels of inflammatory markers, compared with midazolam (13). A meta-analysis of trials on mechanically ventilated patients showed that volatile-based sedation is associated with a reduction in time to extubation, with no increase in short-term adverse outcomes (30). In addition, cerebral and cardiac protective effects and improved cognitive functions on awakening have been demonstrated (31). These potential benefits combine with a negligible hemodynamic impact, as previously reported by our group (15). The present study confirmed that ventilation with ultraprotective TV and severe respiratory conditions do not represent a limitation to the use of volatile sedation in the critical patient.
Current evidence suggests to maintain light sedation in all patients receiving mechanical ventilation (32). However, severe ARDS patients in the early phase of the disease often require deep sedation (33) and eventual neuromuscular blockade to permit protective ventilation. In this context, volatile sedation seems to a feasible option to propofol and midazolam. Our study only focused on this controlled mechanical ventilation period, whereas we did not analyze the latter phase. However, we remark that the shift to assisted ventilation with lighter sedation (e.g., dexmedetomidine) is strongly recommended as soon as patients improve.
This study presents some limitations. First, it is a retrospective, single-center study; thus, the generalizability of the results is limited. Second, the duration of the IV and volatile phases was not defined a priori and it was variable in each patient. Finally, we could not gather any information concerning the onset and the offset of the sedative effects of volatile sedation, which in these patients might be affected by pulmonary shunt. This topic should be further explored by prospective studies.
CONCLUSIONS
Long-term volatile sedation is a feasible alternative to IV sedation in ARDS patients on V-V ECMO requiring ultraprotective ventilation. These findings need to be confirmed in larger, prospective studies comparing sedation with IV and volatile agents.
ACKNOWLEDGMENTS
We thank Dario Winterton, MD, for his careful revision of the article, and Mara Favitta, RN, Nicoletta Tosello, RN, and Velia Binda, RN, for their help with data gathering.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccxjournal).
Drs. Grasselli and Giani contributed equally to this work.
Drs. Giani and Grasselli conceived the study, participated in its design, and wrote the article. Drs. Fumagalli, Mariani, and Redaelli performed the data collection. Drs. Giani and Scaravilli performed the statistical analysis. Drs. Scaravilli, Fumagalli, and Mariani contributed to the writing of the article. Drs. Zanella, Patroniti, Pesenti, and Foti participated in the study design. All authors revised the article and approved the final version.
The authors have disclosed that they do not have any potential conflicts of interest.
The study was performed in ASST Monza hospital, Monza, Italy.
REFERENCES
- 1.Shekar K, Gregory SD, Fraser JF. Mechanical circulatory support in the new era: An overview. Crit Care. 2016; 20:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nigoghossian CD, Dzierba AL, Etheridge J, et al. Effect of extracorporeal membrane oxygenation use on sedative requirements in patients with severe acute respiratory distress syndrome. Pharmacotherapy. 2016; 36:607–616 [DOI] [PubMed] [Google Scholar]
- 3.deBacker J, Tamberg E, Munshi L, et al. Sedation practice in extracorporeal membrane oxygenation-treated patients with acute respiratory distress syndrome: A retrospective study. ASAIO J. 2018; 64:544–551 [DOI] [PubMed] [Google Scholar]
- 4.Barrientos-Vega R, Mar Sánchez-Soria M, Morales-García C, et al. Prolonged sedation of critically ill patients with midazolam or propofol: impact on weaning and costs. Crit Care Med. 1997; 25:33–40 [DOI] [PubMed] [Google Scholar]
- 5.Shelly MP, Sultan MA, Bodenham A, et al. Midazolam infusions in critically ill patients. Eur J Anaesthesiol. 1991; 8:21–27 [PubMed] [Google Scholar]
- 6.Hemphill S, McMenamin L, Bellamy MC, et al. Propofol infusion syndrome: A structured literature review and analysis of published case reports. Br J Anaesth. 2019; 122:448–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krajčová A, Waldauf P, Anděl M, et al. Propofol infusion syndrome: A structured review of experimental studies and 153 published case reports. Crit Care. 2015; 19:398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckley PM. Propofol in patients needing long-term sedation in intensive care: An assessment of the development of tolerance. A pilot study. Intensive Care Med. 1997; 23:969–974 [DOI] [PubMed] [Google Scholar]
- 9.Tukacs M. Pharmacokinetics and extracorporeal membrane oxygenation in adults: A literature review. AACN Adv Crit Care. 2018; 29:246–258 [DOI] [PubMed] [Google Scholar]
- 10.Dzierba AL, Abrams D, Brodie D. Medicating patients during extracorporeal membrane oxygenation: The evidence is building. Crit Care. 2017; 21:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhatt-Meht V, Annich G. Sedative clearance during extracorporeal membrane oxygenation. Perfusion. 2005; 20:309–315 [DOI] [PubMed] [Google Scholar]
- 12.Baron R, Binder A, Braune S, et al. ; DAS-Taskforce 2015. Evidence and consensus based guideline for the management of delirium, analgesia, and sedation in intensive care medicine. Revision 2015 (DAS-Guideline 2015) - short version. Ger Med Sci GMS E-J. 2015; 13:Doc19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jabaudon M, Boucher P, Imhoff E, et al. Sevoflurane for sedation in acute respiratory distress syndrome. A randomized controlled pilot study. Am J Respir Crit Care Med. 2017; 195:792–800 [DOI] [PubMed] [Google Scholar]
- 14.Perbet S, Bourdeaux D, Lenoire A, et al. ; PRONOBURN group. Sevoflurane for procedural sedation in critically ill patients: A pharmacokinetic comparative study between burn and non-burn patients. Anaesth Crit Care Pain Med. 2018; 37:551–556 [DOI] [PubMed] [Google Scholar]
- 15.Migliari M, Bellani G, Rona R, et al. Short-term evaluation of sedation with sevoflurane administered by the anesthetic conserving device in critically ill patients. Intensive Care Med. 2009; 35:1240–1246 [DOI] [PubMed] [Google Scholar]
- 16.Bösel J, Purrucker JC, Nowak F, et al. Volatile isoflurane sedation in cerebrovascular intensive care patients using AnaConDa(®): effects on cerebral oxygenation, circulation, and pressure. Intensive Care Med. 2012; 38:1955–1964 [DOI] [PubMed] [Google Scholar]
- 17.Sackey PV, Martling CR, Granath F, et al. Prolonged isoflurane sedation of intensive care unit patients with the Anesthetic Conserving Device. Crit Care Med. 2004; 32:2241–2246 [DOI] [PubMed] [Google Scholar]
- 18.L’her E, Dy L, Pili R, et al. Feasibility and potential cost/benefit of routine isoflurane sedation using an anesthetic-conserving device: A prospective observational study. Respir Care. 2008; 53:1295–1303 [PubMed] [Google Scholar]
- 19.Thiruvenkatarajan V, Osborn KD, Van Wijk RM, et al. Torsade de pointes in a patient with acute prolonged QT syndrome and poorly controlled diabetes during sevoflurane anaesthesia. Anaesth Intensive Care. 2010; 38:555–559 [DOI] [PubMed] [Google Scholar]
- 20.Schuster F, Moegele S, Johannsen S, et al. Malignant hyperthermia in the intensive care setting. Crit Care. 2014; 18:411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jerath A, Panckhurst J, Parotto M, et al. Safety and efficacy of volatile anesthetic agents compared with standard intravenous midazolam/propofol sedation in ventilated critical care patients: A meta-analysis and systematic review of prospective trials. Anesth Analg. 2017; 124:1190–1199 [DOI] [PubMed] [Google Scholar]
- 22.Meiser A, Bomberg H, Lepper PM, et al. Inhaled sedation in patients with acute respiratory distress syndrome undergoing extracorporeal membrane oxygenation. Anesth Analg. 2017; 125:1235–1239 [DOI] [PubMed] [Google Scholar]
- 23.Rand A, Zahn PK, Schildhauer TA, et al. Inhalative sedation with small tidal volumes under venovenous ECMO. J Artif Organs. 2018; 21:201–205 [DOI] [PubMed] [Google Scholar]
- 24.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: Validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002; 166:1338–1344 [DOI] [PubMed] [Google Scholar]
- 25.Yaman F, Ozcan N, Ozcan A, et al. Assesment of correlation between bispectral index and four common sedation scales used in mechanically ventilated patients in ICU. Eur Rev Med Pharmacol Sci. 2012; 16:660–666 [PubMed] [Google Scholar]
- 26.Simmons LE, Riker RR, Prato BS, et al. Assessing sedation during intensive care unit mechanical ventilation with the Bispectral Index and the Sedation-Agitation Scale. Crit Care Med. 1999; 27:1499–1504 [DOI] [PubMed] [Google Scholar]
- 27.Zheng J, Gao Y, Xu X, et al. Correlation of bispectral index and Richmond agitation sedation scale for evaluating sedation depth: a retrospective study. J Thorac Dis. 2018; 10:190–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laufenberg M, Schneider T. [Severe exacerbation of COPD requiring ventilation : Use of vv-ECMO combined with inhalation anesthetics]. Med Klin Intensivmed Notfallmedizin. 2017; 112:352–355 [DOI] [PubMed] [Google Scholar]
- 29.Verkoyen K, Schildhauer TA, Strauch JT, et al. The effects of propofol and isoflurane sedation on the outcomes of surgical patients receiving extracorporeal membrane oxygenation. ASAIO J. 2017; 63:174–178 [DOI] [PubMed] [Google Scholar]
- 30.Kim HY, Lee JE, Kim HY, et al. Volatile sedation in the intensive care unit: A systematic review and meta-analysis. Medicine (Baltimore). 2017; 96:e8976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soukup J, Schärff K, Kubosch K, et al. State of the art: Sedation concepts with volatile anesthetics in critically Ill patients. J Crit Care. 2009; 24:535–544 [DOI] [PubMed] [Google Scholar]
- 32.Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018; 46:e825–e873 [DOI] [PubMed] [Google Scholar]
- 33.Roberts DJ, Haroon B, Hall RI. Sedation for critically ill or injured adults in the intensive care unit: A shifting paradigm. Drugs. 2012; 72:1881–1916 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


