Abstract
Stroke is a major cause of death and disability worldwide. Yet therapeutic strategies available to treat stroke are very limited. There is an urgent need to develop novel therapeutics that can effectively facilitate functional recovery. The injury that results from stroke is known to induce neurogenesis in penumbra of the infarct region. There is considerable interest in harnessing this response for therapeutic purposes. This review summarizes what is currently known about stroke-induced neurogenesis and the factors that have been identified to regulate it. Additionally, some key studies in this field have been highlighted and their implications on future of stroke therapy have been discussed. There is a complex interplay between neuroinflammation and neurogenesis that dictates stroke outcome and possibly recovery. This highlights the need for a better understanding of the neuroinflammatory process and how it affects neurogenesis, as well as the need to identify new mechanisms and potential modulators. Neuroinflammatory processes and their impact on post-stroke repair have therefore also been discussed.
Keywords: Stroke, Neurogenesis, Neuroinflammation, Cytokines, Stroke therapy
Introduction
Stroke is a debilitating disease condition defined as either an interruption of blood supply to the brain due to a clot or embolism, or the rupture of a blood vessel in the brain, which then leads to neurological impairments [1]. It remains the 3rd leading cause of death worldwide, with nearly 15 million people being affected every year [2], while in the USA, it is the number 5 killer, killing nearly 140,000 people every year (https://www.cdc.gov/stroke/facts.htm). Currently, the treatment for ischemic stroke is to administer a thrombolytic agent such as tissue Plasminogen Activator (tPA) or to perform a surgical thrombectomy procedure to mechanically remove the blood clot (thrombus) [3]. However, the optimal time window for these treatments is very small and survivors often exhibit a high degree of morbidity, as well as limited functional recovery [4]. New modes of therapy are therefore urgently needed, especially ones that can be administered after longer periods following stroke onset, that can lead to better functional recovery and reduced morbidity. To this end, post-stroke brain repair processes are of particular research interest. Here, in this review, we discuss stroke-induced neurogenesis as a potential target for therapeutic intervention, as it represents a major repair mechanism that by itself falls short in achieving full recovery in surviving patients, and presumably could be modulated to achieve better outcomes. A detailed understanding of this phenomenon is needed to guide future research and the development of effective intervention strategies.
Neurogenesis in the Post-stroke Brain
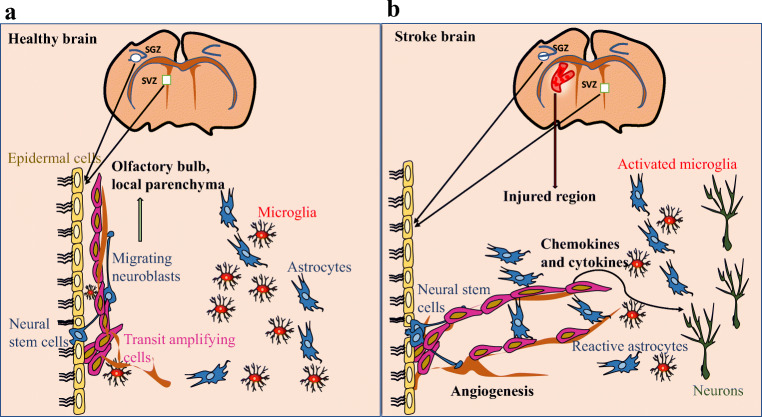
Neurogenesis, or the birth of new neurons, is known to be induced in response to ischemic stroke, in the infarct and surrounding areas. Neural stem cells originating from the sub-ventricular zone (SVZ) and the sub-granular zone of the dentate gyrus are considered to give rise to these new neurons [5–7]. This is thought to be a key process in post-stroke recovery and repair of the damaged brain region [8–10]. In general, this involves the migration of neural stem cells to the infarct and peri-infarct region, followed by their differentiation into functional neurons [11–13]. This process is schematically depicted in Fig. 1, based on information derived from research in rodents and other model systems. An important factor to consider in post-stroke functional recovery is the ultimate survival of these newborn neurons. Several studies have reported their reduced survivability possibly due to their microenvironment lacking trophic factors as well as chronic inflammatory responses [14–16]. Acute neuroinflammation, however, has been reported to promote neurogenesis and may be promoting neuronal survival as well [17, 18]. In addition, age remains a prominent factor affecting neurogenesis. The rate of neurogenesis steadily declines with rising age, with stroke increasing the sharpness of that decline [8, 19, 20]. Notably, Darsalia and collaborators [8] reported that striatal neurogenesis after stroke is similar in young and aged mice, while hippocampal neurogenesis is impaired in aged animals compared with the young animals. This raises the possibility of differential region-specific regulation mechanisms and multiple modulatory opportunities if the mechanisms could be harnessed.
Fig. 1.
Schematic representation of adult neurogenesis in rodents. a Healthy brains: Neural stem cells proliferate from the SVZ and SGZ form neuroblasts that migrate to the olfactory bulb and local parenchyma. b Stroke brains: There is pronounced loss of striatal and cortical neurons, giving rise to increased proliferation of progenitors. The neuroblasts formed before and after the stroke migrate to the site of injury, influenced by chemokines and cytokines secreted by resident and activated microglia, reactive astrocytes, etc. The neuroblasts then differentiate into newborn neurons coupled with angiogenesis at the site of injury, giving rise to mature neurons
Factors Governing Neurogenesis
The process of neurogenesis can generally be categorized into three stages: (1) neural stem cell proliferation, (2) migration of neuroblasts and immature neurons, and (3) differentiation into mature neurons and neurite extension, finally leading to synaptogenesis and stabilization of the synapses. There are a number of molecules that affect one or more of these stages, and they differ between embryonic neurogenesis and adult neurogenesis [21–23]. We will focus on some that have been identified as important for stroke-induced neurogenesis.
Ruan and collaborator in a recent review [24] mention Fibroblast growth factor-2 (FGF-2) [25, 26], Insulin-like Growth Factor-1 (IGF-1) [27, 28], Brain-Derived Neurotrophic Factor (BDNF) [29–31], and Vascular Endothelial Growth Factor (VEGF) [32] as factors that directly affect neural stem cell proliferation, while identifying Stromal-derived factor (SDF-1), Monocyte Chemoattractant Protein (MCP-1), and Matrix metalloproteinases (MMP) 2, 3, and 9 as factors influencing neuroblast migration [33–36]. SDF-1 and MCP-1 are both chemokines that form part of the inflammatory response to the ischemic injury [37, 38], whereas MMPs are matrix metalloproteinases involved in remodeling of the extracellular matrix [35]. Remodeling of the matrix often occurs to allow reparative processes like angiogenesis to take place [39]. In this case, however, the remodeling may be taking place in part to allow the migrating neuroblasts to pass through. In addition, proteolysis of matrix proteins such as perlecan has been implicated in promoting neurogenesis in post-stroke brains [40]. Its c-terminal domain V (DV) is thought to be the active component leading to neurogenesis as well as angiogenesis at the infarct and peri-infarct area [41, 42]. Using neurospheres and fetal cortical neurons in vitro, Trout and colleagues [42] showed that perlecan DV also promoted differentiation into mature neurons as well as neurite extension. In this way, the MMPs may be serving a dual function.
Neuroinflammation and Neurogenesis
For the most part, the dogma is that neuroinflammation and neurogenesis are inversely related. Factors like Sirtuin 7, Glucagon-Like Peptide-1, and Nei like DNA Glycosylase 1 enhance neurogenesis by suppressing neuroinflammation [43–45]. Inhibition of Glycogen Synthase Kinase-3 (GSK-3) has also been shown to increase neurogenesis, while reducing neuroinflammation, implicating Wnt signaling in the process [46–48]. However, depending on the timing, duration, and the profile of cytokines and chemokines released, neurogenesis could be positively impacted [17, 18, 49–51].
An example of how the duration of inflammation can be an important factor is seen by the effect of interleukin-6 (IL-6) on neurogenesis. Short-term treatment of neural stem cells with a hyperactive fusion IL-6 protein induces neurogenesis in vitro [49], while chronic astrocytic IL-6 transgene expression led to reduced neurogenesis in the dentate gyrus of the transgenic mice [20]. Similarly, interleukin 1α (IL-1α) and interleukin-1β (IL-1β) follow an out of phase expression pattern in response to ischemia, where IL-1α expression occurs early following ischemia whereas IL-1β is expressed much later and coincides with reduced IL-1α production, indicating that the two cytokines likely have different roles during the post-stroke neuroinflammatory response [52, 53]. Indeed, IL-1α is reported to be neurogenic [17] and gliogenic [54], while IL-1β is primarily thought to induce neural death and clearance of dead cells and debris [55–57]. One of the ways interleukin-1 (IL-1) potentially regulates neurogenesis is by driving the expression of pentraxin 3 (PTX-3). This protein is a known biomarker for cerebrovascular diseases and plays a key role in maintaining blood-brain barrier (BBB) integrity. While one study identified IL-1 as a driving regulator of PTX-3 [58], a second follow-up study reported the neurogenic ability of PTX-3, inducing IL-1β-dependent proliferation in neurospheres [59]. In the latter study, PTX3 knockout mice also exhibited reduced proliferating stem cell population in the dentate gyrus after MCAO, further supporting the idea that PTX-3 induces neurogenesis.
Among the various cells involved in mediating inflammation in the brain, microglia are of particular interest as a cell type that can infer both neuroprotection and neuronal death. How they affect neurogenesis has been a major focus of many studies. Microglia have been reported to produce trophic factors to guide and support neural stem cell migration and differentiation, but also, on other occasions, produce cytokines that hamper cell survival [60–64]. When microglia in the brain were nearly completely depleted by administration of the Colony-stimulating factor 1 receptor (CSF1R) antagonist PLX3397, the resulting mice exhibited approximately 60% increase in infarct size after MCAO [65], indicating the largely neuroprotective role played by microglia. In a quiescent and ramified state, microglia tend to secrete trophic factors that support surrounding neurons, whereas in a more activated (ameboid) state, they tend to eliminate neurons. The CX3C Receptor 1, expressed by microglia, has been shown to play a key role in the modulation of these characteristics. Its inhibition leads to reduced hippocampal neurogenesis leaving the olfactory bulb unchanged [66, 67].
In addition to microglia, astrocytes have also been shown to influence neurogenesis, particularly reactive astrocytes [68, 69]. Traditionally, they were thought to contribute more to neuronal apoptosis than neuronal survival in the ischemic brain [70]. However, recent studies have informed more about their robust neurogenic properties. In addition to secreting growth and other factors to strengthen synapses, some astrocytes act as neuronal precursors [71–73]. These cells can not only differentiate into mature neurons but are also able to divide asymmetrically to generate a neuron and another precursor [73]. There are ongoing efforts to exploit these properties clinically, especially in conjunction with stem cell–based approaches [74]. Faiz and colleagues (2015) have identified Ascl1 as a gene that can induce the transdifferentiation of astrocytes into neurons [75]. Similarly, Zhang et al. (2018) have implicated IL-17 expression and release from astrocytes in promoting neurogenesis via the NF-κB pathway [76]. Another study reported that the secretion of β-arrestin from astrocytes promoted neurogenesis, while knockout animals displayed reduced proliferation of neural precursor cells [77]. Disrupted in Schizophrenia 1 (DISC-1) is yet another gene expressed by astrocytes known to influence neurogenesis, where a dominant negative mutation is known to cause reduced neurogenesis [78]. Astrocytes, therefore, present an attractive target for stroke therapy, especially when combined with stem cell–based therapeutic approaches and other neurogenesis promoting treatments. If the developmental ability of astrocytes to neurons can be harnessed and modulated, this presents a somewhat renewable pool of neural precursors, which is additional to neuroblasts from the SVZ and the DG. Furthermore, astrocyte-derived growth and support factors can also be clinically targeted to ensure better survival of the post-stroke newborn neurons.
Targeting Neurogenesis in Experimental Stroke
Regarding modulating post-stroke neurogenesis to improve stroke outcome, there are a number of considerations. Selective ablation of post-stroke neurogenesis has been reported to have deleterious effects in stroke recovery [79–81], indicating the potential for manipulating neurogenesis to alter stroke outcome. This is further reinforced by studies showing enhancement of neurogenesis positively affecting stroke outcome [82–85]. In doing these studies, the timing of intervention becomes a key consideration because neurogenesis is a part of the delayed repair process and any intervention targeting an aspect of the neurogenic process needs to be in synchrony for maximum efficacy [86–88]. In addition, it is important to consider the interplay with other repair processes such as angiogenesis [24]. Some recent studies examining post-stroke neurogenesis are listed in Table 1.
Table 1.
Recent studies investigating post-stroke neurogenesis for therapy
| Title | Means of neurogenesis modulation | Model/mice used for study | Study outcome/observations | Citation |
|---|---|---|---|---|
| Aryl Hydrocarbon Receptor Modulates Stroke-Induced Astrogliosis and Neurogenesis in the Adult Mouse Brain | Use of an AHR conditional knockout mouse and the administration of an AHR antagonist called TMF (5 mg/kg/day in 2% DMSO, for 2 days) | Permanent MCAO model: C57/Bl6 mice – Wild-type mice and Nestin-CreERT2/AHR-Flox mice | Both the conditional knockout and treatment with an AHR antagonist led to improved stroke outcome and increased neurogenesis, whereas activation of the AHR led to reduced neurogenesis and increased astrogliosis | Chen WC, Chang LH, Huang SS, Huang YJ, Chih CL, Kuo HC, Lee YH, Lee IH. Aryl hydrocarbon receptor modulates stroke-induced astrogliosis and neurogenesis in the adult mouse brain. J Neuroinflammation. 2019 Oct 12;16(1):187. doi: 10.1186/s12974-019-1572-7. PMID: 31606043; PMCID: PMC6790016. [142] |
| Ergostatrien-7,9(11),22-trien-3β-ol From Antrodia Camphorata Ameliorates Ischemic Stroke Brain Injury via Downregulation of p65NF-κ-B and Caspase 3, and Activation of Akt/GSK3/catenin-associated Neurogenesis | Treatment with crude extract from Antrodia camphorata at 0.3 g/kg or 0.6 g/kg and its active ingredient ergostatrien-7,9(11),22-trien-3β-ol at 60 mg/kg or 120 mg/kg, all given orally | Transient focal MCAO model: Male ICR mice | Both compounds were shown to reduce ischemic brain injury by decreasing p65NF-κB and caspase 3 expression, and they promote neurogenesis (DCX) and neuroprotection (Bcl2) by activating the PI3k/Akt-associated GSK3 inhibition and β-catenin activation | Wang YH, Chern CM, Liou KT, Kuo YH, Shen YC. Ergostatrien-7,9(11),22-trien-3β-ol from Antrodia camphorata ameliorates ischemic stroke brain injury via downregulation of p65NF-κ-B and caspase 3, and activation of Akt/GSK3/catenin-associated neurogenesis. Food Funct. 2019 Aug 1;10(8):4725–4738. doi: 10.1039/c9fo00908f. Epub 2019 Jul 15. PMID: 31304955. [144] |
| Abolition of aberrant neurogenesis ameliorates cognitive impairment after stroke in mice | Exercise, administration of pharmacological agents temozolomide (4 rounds of treatment at 1-week intervals, with each round consisting of 1 injection of TMZ (0.9% in saline containing 10% DMSO, 25 mg/kg, i.p.) per day for 4 consecutive days) and mementine ((0.9% saline, 25 mg/kg, i.p.) once per week over 4 weeks), and conditional genetic deletion of the diphtheria toxin fragment A (DTA) cassette gene, causing apoptosis in NSCs | Permanent MCAO model: C57/Bl6 mice – Wild-type mice and Nestin-CreERT2/NSE-Stop-DTA transgenic mice | Stroke induces hippocampal (SGZ) neurogenesis which seems to cause memory impairments. Enhancement of neurogenesis with mementine and exercise on running wheel led to poorer cognitive performance after stroke. In contrast, reduction of neurogenesis using temozolomide and a transgenic apoptotic induction led to better memory retrieval. | Cuartero MI, de la Parra J, Pérez-Ruiz A, Bravo-Ferrer I, Durán-Laforet V, García-Culebras A, García-Segura JM, Dhaliwal J, Frankland PW, Lizasoain I, Moro MÁ. Abolition of aberrant neurogenesis ameliorates cognitive impairment after stroke in mice. J Clin Invest. 2019 Apr 1;129(4):1536–1550. doi: 10.1172/JCI120412. Epub 2019 Feb 25. PMID: 30676325; PMCID: PMC6436875. [143] |
| MEPO Promotes Neurogenesis and Angiogenesis but Suppresses Gliogenesis in Mice With Acute Ischemic Stroke | Erythropoeitin (EPO) and mutant erythropoeitin (MEPO) administration—5000 U/kg | Transient middle cerebral artery occlusion: Male C57BL/6 mice | EPO and MEPO both increased the regeneration of neurons and blood vessels in peri-infarct region, and suppressed gliogenesis, leading to improved neurological function and neuronal survival rate | Zhang SJ, Wang RL, Zhao HP, Tao Z, Li JC, Ju F, Han ZP, Ma QF, Liu P, Ma SB, Cao GD, Luo YM. MEPO promotes neurogenesis and angiogenesis but suppresses gliogenesis in mice with acute ischemic stroke. Eur J Pharmacol. 2019 Apr 15;849:1–10. doi: 10.1016/j.ejphar.2019.01.066. Epub 2019 Feb 2. PMID: 30716313. [95] |
| Docosanoids Promote Neurogenesis and Angiogenesis, Blood-Brain Barrier Integrity, Penumbra Protection, and Neurobehavioral Recovery After Experimental Ischemic Stroke | Docosahexaenoic acid (DHA) at 5 mg/kg given IV and neuroprotectin D1 (NPD1) at 5 μg/rat treatment, given ICV | Transient middle cerebral artery occlusion: Male Sprague-Dawley Rats | DHA was found to increase neurogenesis in the cortical and subcortical peri-infarct regions. It also led to an activation of NPD1 synthesis in the same regions, which in turn, was found to enhance neurogenesis and axonal regeneration, while reducing BBB permeability, when given exogenously. | Belayev L, Hong SH, Menghani H, Marcell SJ, Obenaus A, Freitas RS, Khoutorova L, Balaszczuk V, Jun B, Oriá RB, Bazan NG. Docosanoids Promote Neurogenesis and Angiogenesis, Blood-Brain Barrier Integrity, Penumbra Protection, and Neurobehavioral Recovery After Experimental Ischemic Stroke. Mol Neurobiol. 2018 Aug;55(8):7090–7106. doi: 10.1007/s12035-018-1136-3. Epub 2018 Jun 1. PMID: 29858774; PMCID: PMC6054805. [92] |
| Photobiomodulation Therapy Promotes Neurogenesis by Improving Post-Stroke Local Microenvironment and Stimulating Neuroprogenitor Cells | Cold white light (808 nm) treatment at the scalp applied for 2 min daily | Photothrombotic stroke model: Male Sprague-Dawley rats | PBM was able to reduce infarct volume and behavioral deficits after stroke, while increasing neurogenesis and synaptogenesis. Reactive gliosis, release of both pro- and anti-inflammatory cytokines, and cytochrome c oxidase were shown to underlie the beneficial effects of PBM. | Yang L, Tucker D, Dong Y, Wu C, Lu Y, Li Y, Zhang J, Liu TC, Zhang Q. Photobiomodulation therapy promotes neurogenesis by improving post-stroke local microenvironment and stimulating neuroprogenitor cells. Exp Neurol. 2018 Jan;299(Pt A):86–96. doi: 10.1016/j.expneurol.2017.10.013. Epub 2017 Oct 19. PMID: 29056360; PMCID: PMC5723531. [146] |
| Pyruvate Kinase M2 Increases Angiogenesis, Neurogenesis, and Functional Recovery Mediated by Upregulation of STAT3 and Focal Adhesion Kinase Activities After Ischemic Stroke in Adult Mice | Recombinant Pyruvate Kinase M2 administration (160 ng/kg), delivered intranasally | Permanent MCAO model: C57/Bl6 mice - Wild type | rPKM2 was neuroprotective after stroke. It also activated STAT3 and promoted angiogenesis via this mechanism. It was also shown to promote NPC migration mediated by Focal Adhesion Kinase expression, in vitro as well as in vivo. Neuronal differentiation and local cerebral blood flow were also shown to be promoted in the ischemic brain by rPKM2. | Chen D, Wei L, Liu ZR, Yang JJ, Gu X, Wei ZZ, Liu LP, Yu SP. Pyruvate Kinase M2 Increases Angiogenesis, Neurogenesis, and Functional Recovery Mediated by Upregulation of STAT3 and Focal Adhesion Kinase Activities After Ischemic Stroke in Adult Mice. Neurotherapeutics. 2018 Jul;15(3):770–784. doi: 10.1007/s13311-018-0635-2. Erratum in: Neurotherapeutics. 2018 Jun 26;: PMID: 29869055; PMCID: PMC6095793. [85] |
| Enriched Housing Promotes Post-Stroke Neurogenesis Through Calpain 1-STAT3/HIF-1α/VEGF Signaling | An enriched housing environment that included social interactions, voluntary and varied physical activity, and introduction of novel objects to provide physical, social, and cognitive stimulation. Additional treatments were Calpain-1 inhibitor PD151746 (0.2 mg/kg dissolved in 1% DMSO), JAK/STAT3 pathway inhibitor AG490 (5 mg/kg), HIF1α inhibitor 2-methoxyestradiol (5 mg/kg), anti-VEGF neutralizing antibody (1 μg/μl), and glycyrrhizin (10 mg/mouse), all given ICV. | Transient middle cerebral artery occlusion: Male C57BL/6 mice | Enriched housing conditions were found to facilitate post-stroke functional recovery, and this was mediated by Calpain-1, which modulated STAT3, HIF1α, VEGF, and HMGB1 levels, to result in increased neurogenesis. | Wu X, Liu S, Hu Z, Zhu G, Zheng G, Wang G. Enriched housing promotes post-stroke neurogenesis through calpain 1-STAT3/HIF-1α/VEGF signaling. Brain Res Bull. 2018 May;139:133–143. doi: 10.1016/j.brainresbull.2018.02.018. Epub 2018 Mar 22. PMID: 29477834. [145] |
| Cannabinoid Type-2 Receptor Drives Neurogenesis and Improves Functional Outcome After Stroke | Administration of cannabinoid type 2 receptor (CB2R) agonist JWH133 (1.5 mg/kg) and the antagonist SR144528 (5 mg/kg), and CB2R genetic deletion. | Permanent MCAO model: C57/Bl6 mice - Wild type and CB2R KO transgenic | Pharmacological inhibition and genetic deletion of CB2R both led to reduced neuronal migration and neurogenesis after stroke. Treatment with the agonist did not have an effect and the antagonist had no effect on neurogenesis in vitro. | Bravo-Ferrer I, Cuartero MI, Zarruk JG, Pradillo JM, Hurtado O, Romera VG, Díaz-Alonso J, García-Segura JM, Guzmán M, Lizasoain I, Galve-Roperh I, Moro MA. Cannabinoid Type-2 Receptor Drives Neurogenesis and Improves Functional Outcome After Stroke. Stroke. 2017 Jan;48(1):204–212. doi: 10.1161/STROKEAHA.116.014793. Epub 2016 Nov 29. PMID: 27899748. [10] |
| MicroRNA Cluster miR-17-92 Cluster in Exosomes Enhance Neuroplasticity and Functional Recovery After Stroke in Rats | Treatment with exosomes containing MicroRNA 17–92 or control MSC-derived exosomes (100 μg total exosome protein, per rats), administered IV | Transient MCAO model: Male Wistar rats. | Treatment with exosomes containing microRNA 17–92 resulted in improved neurological function, enhanced oligodendrogenesis, neurogenesis, and neurite remodeling, and increased neuronal dendrite plasticity in the ischemic boundary zone | Xin H, Katakowski M, Wang F, Qian JY, Liu XS, Ali MM, Buller B, Zhang ZG, Chopp M. MicroRNA cluster miR-17-92 Cluster in Exosomes Enhance Neuroplasticity and Functional Recovery After Stroke in Rats. Stroke. 2017 Mar;48(3):747–753. doi: 10.1161/STROKEAHA.116.015204. Erratum in: Stroke. 2017 May;48(5):e137. PMID: 28232590; PMCID: PMC5330787. [147] |
| Post-stroke Constraint-induced Movement Therapy Increases Functional Recovery, Angiogenesis, and Neurogenesis With Enhanced Expression of HIF-1α and VEGF | Constraint-induced movement therapy using a plaster cast placed on the unimpaired limb after MCAO | Transient MCAO model: Male Sprague-Dawley rats. | Constraint-induced movement therapy resulted in increased lengths of microvessels and showed evidence of enhanced neurogenesis, concomitant with an increase in HIF-1α and VEGF expression and FIH-1 expression | Li C, Zhang B, Zhu Y, Li Y, Liu P, Gao B, Tian S, Du L, Bai Y. Post-stroke Constraint-induced Movement Therapy Increases Functional Recovery, Angiogenesis, and Neurogenesis with Enhanced Expression of HIF-1α and VEGF. Curr Neurovasc Res. 2017;14(4):368–377. doi: 10.2174/1567202614666171128120558. PMID: 29189156. [93] |
In early studies, newborn neurons were detected in gerbil brains after cerebral ischemia, 26 days after ischemic insult and persisted for 7 months [89]. More recent studies, however, showed that in mice and rats, neural stem cell proliferation in the SVZ was significantly enhanced in as early as the first 7–14 days after MCAO [11, 13, 90, 91]. Thored and colleagues reported the presence of neuroblasts from 1 week up to 16 weeks after insult, in the striatum [7]. Similarly, neuroblasts were shown to migrate to the cortex and survive for 35 weeks or more [90]. Therefore, these represent the rodent counterparts for therapeutic windows for treatments aimed at proliferation (7 days and beyond) and migration. These time windows would be different in humans and need to be investigated to determine effective treatment regimes. Lastly, most of these neurons die within 2–5 weeks [15]. It is this critical period that must be targeted if neuroprotective factors or factors promoting neuronal survival are to be administered for therapy, although it is unclear how long that treatment will have to be continued, to ensure the survival of the newborn neurons. It may be important to target the process of synaptogenesis at time periods like this since failure to make connections has been proposed as a mechanism of neuronal death [15].
The process of angiogenesis has been reported to be closely intertwined with the process of neurogenesis after stroke [24, 85, 92–95]. This process is defined as the formation of new blood vessels via the sprouting of preexisting vessels and generally occurs in response to an injury such as cerebral ischemia [96]. It is characterized by proliferation of endothelial cells that then form tube-like structures, ultimately forming the complete blood vessel [97]. Research has shown that endothelial cells secrete a number of neurotrophic factors like VEGF, Angiopoeitin-1, and SDF-1 that facilitate neurogenesis and neuronal differentiation and subsequent survival [33, 98, 99]. Moreover, angiogenic processes precede the neurogenic processes in the infarct area after ischemic insult [100–103]. Research has also shown that modulation of the factors governing neurogenesis also affects angiogenesis [32, 104–108]. These all point to the idea that modulation of neurogenesis can be achieved by modulation of angiogenesis as well as the importance of keeping angiogenic processes in mind while manipulating neurogenesis. A question can be raised about how one could go about targeting neurogenesis specifically without altering angiogenesis. One way to do so may be to delay treatments such that period of angiogenesis is surpassed and mostly neurogenesis is targeted [17], while an alternate method could involve treating with a cocktail with components that would compensate for effects on angiogenesis specifically that might occur as a concurrent effect of one or more of the other components. It might have to be a combination of the two methods or others to achieve maximum targeting efficiency.
Drugs Targeting Neuroinflammation to Alter Neurogenesis
Minocycline is a tetracycline derivative that inhibits microglial activation and has been shown to be neuroprotective following focal cerebral ischemia [109, 110]. It has also been shown to be able to upregulate neurogenesis in multiple models [111–113]. Therefore, this remains the most promising pharmacological agent in this regard. Srivastava and collaborators [114] reported it as safe and efficacious in their clinical trial, which was further supported by the meta-analysis conducted by Malhotra and colleagues [115], of seven randomized clinical trials. It is safe to be administered for sure, but its efficacy still needs some more validation before it is widely accepted for treatment of stroke.
Another study reported that the drug Sildenafil promoted neurogenesis and was able to enhance functional recovery after perinatal/pediatric ischemia in mice [116]. While this is not exactly the same as the hypoxia occurring during ischemic stroke, it is consistent with previous findings that sildenafil promotes neurogenesis after focal cerebral ischemia [117–119]. Engels and collaborators [116] proposed that Sildenafil altered the levels of Wnt signaling pathway members β-catenin and GSK-3, via inhibition of phosphodiesterase type 5, and subsequent increase in cGMP levels. Although they did not find any direct evidence of affected neuroinflammation, GSK-3 and Wnt signaling has been implicated in the regulation of neuroinflammation in several studies [120, 121]. One GSK inhibitor called Tideglusib has been investigated in clinical trials as well, where it was deemed clinically safe but was not effective [122, 123]. Other GSK inhibitors that may be worth exploring include 6-bromoindirubin-30-oxime (BIO) [46, 48] and lithium chloride [47, 124].
Based on several in vitro and in vivo studies investigating the role of IL-1 in stroke, recent studies have considered IL-1 receptor antagonist (IL-1Ra) as an attractive new therapy. Indeed, a small phase 2 clinical trial showed that IL-1Ra is safe in stroke and may be effective [125], and the more recent SCIL-STROKE study confirmed this hypothesis that IL-1Ra may be potent neuroprotective therapy in stroke [126]. IL-1Ra may improve stroke outcome through inhibition of the inflammatory response; however, an interesting recent study found that IL-1Ra administration in rat stroke model potently promotes long-term neurogenesis and functional recovery [127]. This study suggests that, although acute inflammation is an important trigger for post-stroke neurogenesis, a more controlled neuroinflammatory response appears critical for an optimum neurogenic response after stroke. These are only a few of the drugs being tested for efficacy in stroke treatment, via neurogenesis modulation. It is widely recognized that pharmacological modulation of neurogenesis can be a valuable tool to treat stroke and is, therefore, likely to be active area of research focus for the foreseeable future.
Potential of Stem Cell–Based Therapy and Considerations of Challenges
One major observation in ischemia-induced neurogenesis is that only a small quantity of the newborn neurons survive in the peri-infarct area [7, 15]. Therefore, to overcome this, in addition to modulating endogenous neurogenesis, stem cell therapy to treat stroke has also been investigated as a possible alternative or as a potential way to augment the endogenous stroke-induced neurogenesis [128–130].These exogenous stem cells can become the source of some much-needed trophic factors and exert paracrine reparative effects. In turn, this could lead to the microenvironment in the peri-infarct area more supportive of new neuron differentiation and integration into the circuitry.
In one study, transplanting human fetal neural stem cells into the hippocampus 24 h after surgically occluding the middle cerebral artery in mice was reported to have improved behavioral recovery and reduced infarct volume, compared with animals without the transplant [131]. They also noted improved BBB repair and lower number of activated microglia in the transplanted brains, as well as higher abundance of Brain derived neurotrophic factor (BDNF). A subsequent study by the same research group reported the transplant procedure as highly beneficial in combination with tissue plasminogen activator (t-PA) treatment, resulting in lower levels of pro-inflammatory cytokines, tumor necrosis factor (TNF-α) and IL-6, as well as MMP-9 [129]. Taken together, these validate the potential of transplanting fetal neural stem cells as a mode of therapy. Of course, the ethical challenges of obtaining and maintaining such stem cells remain a major limitation of such a process.
The alternative approach to using fetal stem cells is to use inducible pluripotent stem cells (iPSCs) or mesenchymal stem cells (MSCs). Oki et al. [132] used human iPSC-derived neuroepithelial-like stem cells that they transplanted into mice 1 week and 48 h after MCAO. They reported improved forelimb motion recovery, increased VEG-F deposition, and successful differentiation of iPSCs into neurons in the striatum.
Use of MSCs is limited by the observation that most of the systemically transplanted MSCs end up in the lungs and do not make it to the infarct area of the brain [133, 134], where they are actually intended to proliferate and repair the damage. Tobin and colleagues [135] have proposed the use of MSCs that have been activated by Interferon gamma (aMSCs). They reported that both activated and naïve MSCs induced complete behavioral recovery, reduced infarct volumes, and reduced microglial activation and levels of IL-1β, TNF-α, and IL-6 in treated animals, compared with vehicle-treated control stroke animals. However, they propose the activated MSCs are a better treatment option than naïve MSCs because of an increased yield of anti-inflammatory factors from microglia. Interestingly, they did not observe any induction of neurogenesis in the SVZ after MSC treatment.
A phase 1 clinical trial (PISCES) involving the administration of CTX0E03 human neural stem cells via stereotactic ipsilateral putamen injection reported that a dose of up to 20,000 cells is safe and well tolerated in patients [136]. The treatment also resulted in functional improvements and upon further investigation, may very well become a mainstream intervention strategy. Since the study was conducted only on 11 men, it needs to be followed up with the inclusion of female patients and a larger patient population [136].
Another phase 2 clinical trial involving the administration of bone marrow stem cells to stroke patients proved safe in patients, but ineffective in terms of treating stroke [137]. Similar results were obtained in another phase 2 clinical trial where patients were treated with bone marrow derived ALD-401 stem cells [138]. Taken together, these studies indicate that the administration of stem cells is safe. As for effectiveness, there is potential for the stem cells to promote functional recovery in more tightly controlled settings, which was a limitation of all three studies, along with the small population of patients that have been tested.
The process of preconditioning the MSCs and using the resulting media may prove even more effective in stroke treatment [128, 139]. A recent systemic review highlighted the therapeutic potential of extracellular vesicles secreted by various cells like MSCs, macrophages, and neural stem cells, identifying these vesicles as an attractive approach. However, being a recent trend, there is a significant amount of heterogeneity among the results of applications, presumably due to isolation and administration techniques, as well as cell-type of origin [140]. Further work on MSCs preconditioning with various inflammatory mediators found that IL-1α can be used as a key priming stimulus to induce MSCs to produce anti-inflammatory and neurotrophic factors [141], and a further in vivo study in mice demonstrated that conditioned medium of IL-1α-primed MSCs administered peripherally after stroke had beneficial effects on stroke outcome and functional recovery [139]. Further work investigating the efficacy of targeted delivery of IL-1α-primed MSCs in stroke is ongoing.
Conclusion and Future Therapeutic Perspective
Stroke affects millions of people every year. With the world populations steadily rising, the global burden of stroke keeps rising proportionally. As a result, there remains a global and critical need to develop better treatment options. Stroke-induced neurogenesis presents a promising therapeutic target, since it can allow the brain to, essentially, rewire and refresh itself, and heal the damage caused by the ischemia or hemorrhage. However, harnessing neurogenesis remains a challenge because of the intricate interplay of the factors involved, especially ones involved in neuroinflammation. It is now well understood that the two processes much more deeply connected than a simple inverse relationship. Moreover, both processes are interconnected with angiogenesis and together work towards post-stroke brain repair. In order to harness them and improve functional recovery, it is imperative, now more than ever, to characterize the roles played by each immune cell, cytokine, and chemokine, as part of the post-injury microenvironment, taking into special consideration their temporal expression patterns, specific effects on angiogenesis, neurogenesis, neuroprotection, and neuron elimination. All of these need to be considered carefully to craft effective therapeutic cocktails that are to achieve maximum treatment efficiency.
Author Contributions
A.R., N.A., and E.P. performed literature searches and drafted the literature review. A.R., N.A., E.P., and G.J.B. revised and finalized the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
National Institutes of Health Grant number R01NS089515 awarded to G.J.B and R01NS101752 awarded to G.J.B and E.P.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to Participate
This article does not contain any studies with human participants and therefore did not require any consent to participate.
Consent for Publication
All authors consent to the publication of this manuscript. Consent letters can be obtained from the corresponding author upon reasonable request.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abir A. Rahman and Narayanappa Amruta contributed equally to this article.
References
- 1.Ojaghihaghighi S, Vahdati SS, Mikaeilpour A, Ramouz A. Comparison of neurological clinical manifestation in patients with hemorrhagic and ischemic stroke. World J Emerg Med. 2017;8(1):34–38. doi: 10.5847/wjem.j.1920-8642.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jayaraj RL, Azimullah S, Beiram R, Jalal FY, Rosenberg GA. Neuroinflammation: friend and foe for ischemic stroke. J Neuroinflammation. 2019;16(1):142. doi: 10.1186/s12974-019-1516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prabhakaran S, Ruff I, Bernstein RA. Acute stroke intervention: a systematic review. JAMA. 2015;313(14):1451–1462. doi: 10.1001/jama.2015.3058. [DOI] [PubMed] [Google Scholar]
- 4.Dorado L, Millan M, Davalos A. Reperfusion therapies for acute ischemic stroke: an update. Curr Cardiol Rev. 2014;10(4):327–335. doi: 10.2174/1573403x10666140320144637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan L, Zhang CJ, Zhu L, Chen J, Zhang Z, Liu P, Cao X, Meng H, Xu Y. FasL-PDPK1 pathway promotes the cytotoxicity of CD8(+) T cells during ischemic stroke. Transl Stroke Res. 2020;11:747–761. doi: 10.1007/s12975-019-00749-0. [DOI] [PubMed] [Google Scholar]
- 6.Jin K, Wang X, Xie L, Mao XO, Zhu W, Wang Y, Shen J, Mao Y, Banwait S, Greenberg DA. Evidence for stroke-induced neurogenesis in the human brain. Proc Natl Acad Sci U S A. 2006;103:13198–13202. doi: 10.1073/pnas.0603512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, Ekdahl CT, Kokaia Z, Lindvall O. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24(3):739–747. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- 8.Darsalia V, Heldmann U, Lindvall O, Kokaia Z. Stroke-induced neurogenesis in aged brain. Stroke. 2005;36(8):1790–1795. doi: 10.1161/01.STR.0000173151.36031.be. [DOI] [PubMed] [Google Scholar]
- 9.Nygren J, Wieloch T, Pesic J, Brundin P, Deierborg T. Enriched environment attenuates cell genesis in subventricular zone after focal ischemia in mice and decreases migration of newborn cells to the striatum. Stroke. 2006;37:2824–2829. doi: 10.1161/01.STR.0000244769.39952.90. [DOI] [PubMed] [Google Scholar]
- 10.Bravo-Ferrer I, Cuartero MI, Zarruk JG, Pradillo JM, Hurtado O, Romera VG, Díaz-Alonso J, García-Segura JM, Guzmán M, Lizasoain I, Galve-Roperh I, Moro MA. Cannabinoid type-2 receptor drives neurogenesis and improves functional outcome after stroke. Stroke. 2017;48:204–212. doi: 10.1161/STROKEAHA.116.014793. [DOI] [PubMed] [Google Scholar]
- 11.Zhang RL, Zhang ZG, Zhang L, Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001;105:33–41. doi: 10.1016/S0306-4522(01)00117-8. [DOI] [PubMed] [Google Scholar]
- 12.Tonchev AB, Yamashima T, Zhao L, Okano HJ, Okano H. Proliferation of neural and neuronal progenitors after global brain ischemia in young adult macaque monkeys. Mol Cell Neurosci. 2003;23:292–301. doi: 10.1016/S1044-7431(03)00058-7. [DOI] [PubMed] [Google Scholar]
- 13.Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci U S A. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin K, Sun Y, Xie L, Peel A, Mao XO, Batteur S, Greenberg DA. Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Mol Cell Neurosci. 2003;24:171–189. doi: 10.1016/S1044-7431(03)00159-3. [DOI] [PubMed] [Google Scholar]
- 15.Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8(9):963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 16.Zhang R, Zhang Z, Wang L, Wang Y, Gousev A, Zhang L, Ho KL, Morshead C, Chopp M. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Metab. 2004;24(4):441–448. doi: 10.1097/00004647-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Salmeron KE, Maniskas ME, Edwards DN, Wong R, Rajkovic I, Trout A, Rahman AA, Hamilton S, Fraser JF, Pinteaux E, Bix GJ. Interleukin 1 alpha administration is neuroprotective and neuro-restorative following experimental ischemic stroke. J Neuroinflammation. 2019;16(1):222. doi: 10.1186/s12974-019-1599-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiyota T, Ingraham KL, Swan RJ, Jacobsen MT, Andrews SJ, Ikezu T. AAV serotype 2/1-mediated gene delivery of anti-inflammatory interleukin-10 enhances neurogenesis and cognitive function in APPPS1 mice. Gene Ther. 2012;19:724–733. doi: 10.1038/gt.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seki T, Arai Y. Age-related production of new granule cells in the adult dentate gyrus. NeuroReport. 1995;6:2479–2482. doi: 10.1097/00001756-199512150-00010. [DOI] [PubMed] [Google Scholar]
- 20.Valliéres L, Campbell IL, Gage FH, Sawchenko PE. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J Neurosci. 2002;22:486–492. doi: 10.1523/jneurosci.22-02-00486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Temple S. The development of neural stem cells. Nature. 2001;414(6859):112–117. doi: 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- 22.Lois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci U S A. 1993;90(5):2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255(5052):1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 24.Ruan L, Wang B, ZhuGe Q, Jin K. Coupling of neurogenesis and angiogenesis after ischemic stroke. Brain Res. 2015;1623:166–173. doi: 10.1016/j.brainres.2015.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikeda N, Nonoguchi N, Zhao MZ, Watanabe T, Kajimoto Y, Furutama D, Kimura F, Dezawa M, Coffin RS, Otsuki Y, Kuroiwa T, Miyatake SI. Bone marrow stromal cells that enhanced fibroblast growth factor-2 secretion by herpes simplex virus vector improve neurological outcome after transient focal cerebral ischemia in rats. Stroke. 2005;36(12):2725–2730. doi: 10.1161/01.STR.0000190006.88896.d3. [DOI] [PubMed] [Google Scholar]
- 26.Yoshimura S, Takagi Y, Harada J, Teramoto T, Thomas SS, Waeber C, Bakowska JC, Breakefield XO, Moskowitz MA. FGF-2 regulation of neurogenesis in adult hippocampus after brain injury. Proc Natl Acad Sci U S A. 2001;98(10):5874–5879. doi: 10.1073/pnas.101034998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan YP, Sailor KA, Vemuganti R, Dempsey RJ. Insulin-like growth factor-1 is an endogenous mediator of focal ischemia-induced neural progenitor proliferation. Eur J Neurosci. 2006;24(1):45–54. doi: 10.1111/j.1460-9568.2006.04872.x. [DOI] [PubMed] [Google Scholar]
- 28.Zhu W, Fan Y, Hao Q, Shen F, Hashimoto T, Yang GY, Gasmi M, Bartus RT, Young WL, Chen Y. Postischemic IGF-1 gene transfer promotes neurovascular regeneration after experimental stroke. J Cereb Blood Flow Metab. 2009;29(9):1528–1537. doi: 10.1038/jcbfm.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andsberg G, Kokaia Z, Klein RL, Muzyczka N, Lindvall O, Mandel RJ. Neuropathological and behavioral consequences of adeno-associated viral vector-mediated continuous intrastriatal neurotrophin delivery in a focal ischemia model in rats. Neurobiol Dis. 2002;9(2):187–204. doi: 10.1006/nbdi.2001.0456. [DOI] [PubMed] [Google Scholar]
- 30.Kokaia Z, Andsberg G, Yan Q, Lindvall O. Rapid alterations of BDNF protein levels in the rat brain after focal ischemia: evidence for increased synthesis and anterograde axonal transport. Exp Neurol. 1998;154(2):289–301. doi: 10.1006/exnr.1998.6888. [DOI] [PubMed] [Google Scholar]
- 31.Chen J, Zacharek A, Zhang C, Jiang H, Li Y, Roberts C, Lu M, Kapke A, Chopp M. Endothelial nitric oxide synthase regulates brain-derived neurotrophic factor expression and neurogenesis after stroke in mice. J Neurosci. 2005;25(9):2366–2375. doi: 10.1523/JNEUROSCI.5071-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99(18):11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26(50):13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan YP, Sailor KA, Lang BT, Park SW, Vemuganti R, Dempsey RJ. Monocyte chemoattractant protein-1 plays a critical role in neuroblast migration after focal cerebral ischemia. J Cereb Blood Flow Metab. 2007;27(6):1213–1224. doi: 10.1038/sj.jcbfm.9600432. [DOI] [PubMed] [Google Scholar]
- 35.Barkho BZ, Munoz AE, Li X, Li L, Cunningham LA, Zhao X. Endogenous matrix metalloproteinase (MMP)-3 and MMP-9 promote the differentiation and migration of adult neural progenitor cells in response to chemokines. Stem Cells. 2008;26(12):3139–3149. doi: 10.1634/stemcells.2008-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang L, Zhang ZG, Zhang RL, Gregg SR, Hozeska-Solgot A, LeTourneau Y, Wang Y, Chopp M. Matrix metalloproteinase 2 (MMP2) and MMP9 secreted by erythropoietin-activated endothelial cells promote neural progenitor cell migration. J Neurosci. 2006;26(22):5996–6003. doi: 10.1523/JNEUROSCI.5380-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Zhang H, Lin S, Chen X, Yao Y, Mao X, et al. SDF-1/CXCR7 chemokine signaling is induced in the peri-infarct regions in patients with ischemic stroke. Aging Dis. 2018;9(2):287–295. doi: 10.14336/AD.2017.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inose Y, Kato Y, Kitagawa K, Uchiyama S, Shibata N. Activated microglia in ischemic stroke penumbra upregulate MCP-1 and CCR2 expression in response to lysophosphatidylcholine derived from adjacent neurons and astrocytes. Neuropathology. 2015;35(3):209–223. doi: 10.1111/neup.12182. [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Vilas JA, Quesada AR, Medina MA. Hydroxytyrosol targets extracellular matrix remodeling by endothelial cells and inhibits both ex vivo and in vivo angiogenesis. Food Chem. 2017;221:1741–1746. doi: 10.1016/j.foodchem.2016.10.111. [DOI] [PubMed] [Google Scholar]
- 40.Roberts J, Kahle MP, Bix GJ. Perlecan and the blood-brain barrier: beneficial proteolysis? Front Pharmacol. 2012;3:155. doi: 10.3389/fphar.2012.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee B, Clarke D, Al Ahmad A, Kahle M, Parham C, Auckland L, et al. Perlecan domain V is neuroprotective and proangiogenic following ischemic stroke in rodents. J Clin Invest. 2011;121(8):3005–3023. doi: 10.1172/JCI46358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trout AL, Kahle MP, Roberts JM, Marcelo A, de Hoog L, Boychuk JA, et al. Perlecan domain-V enhances neurogenic brain repair after stroke in mice. Transl Stroke Res. 2020. 10.1007/s12975-020-00800-5. [DOI] [PMC free article] [PubMed]
- 43.Bae CS, Song J. The role of glucagon-like peptide 1 (GLP1) in type 3 diabetes: GLP-1 controls insulin resistance, neuroinflammation and neurogenesis in the brain. Int J Mol Sci. 2017;18. 10.3390/ijms18112493. [DOI] [PMC free article] [PubMed]
- 44.Burg N, Bittner S, Ellwardt E. Role of the epigenetic factor Sirt7 in neuroinflammation and neurogenesis. Neurosci Res. 2018;131:1–9. doi: 10.1016/j.neures.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 45.Yang B, Figueroa DM, Hou Y, Babbar M, Baringer SL, Croteau DL, Bohr VA. NEIL1 stimulates neurogenesis and suppresses neuroinflammation after stress. Free Radic Biol Med. 2019;141:47–58. doi: 10.1016/j.freeradbiomed.2019.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang LL, Li J, Gu X, Wei L, Yu SP. Delayed treatment of 6-Bromoindirubin-3′-oxime stimulates neurogenesis and functional recovery after focal ischemic stroke in mice. Int J Dev Neurosci. 2017;57:77–84. doi: 10.1016/j.ijdevneu.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng J, Liu Z, Li W, Tang J, Zhang D, Tang X. Lithium posttreatment confers neuroprotection through glycogen synthase kinase-3β inhibition in intracerebral hemorrhage rats. J Neurosurg. 2017;127:716–724. doi: 10.3171/2016.7.JNS152995. [DOI] [PubMed] [Google Scholar]
- 48.Zhao S, Liu Z, Yu Z, Wu X, Li R, Tang X. BIO alleviates inflammation through inhibition of GSK-3β in a rat model of intracerebral hemorrhage. J Neurosurg. 2019:1–9. 10.3171/2019.4.jns183501. [DOI] [PubMed]
- 49.Islam O, Gong X, Rose-John S, Heese K. Interleukin-6 and neural stem cells: more than gliogenesis. Mol Biol Cell. 2009;20:188–199. doi: 10.1091/mbc.E08-05-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sung PS, Lin PY, Liu CH, Su HC, Tsai KJ. Neuroinflammation and neurogenesis in Alzheimer’s disease and potential therapeutic approaches. Int J Mol Sci. 2020;21. 10.3390/ijms21030701. [DOI] [PMC free article] [PubMed]
- 51.Greco SJ, Rameshwar P. Enhancing effect of IL-1alpha on neurogenesis from adult human mesenchymal stem cells: implication for inflammatory mediators in regenerative medicine. J Immunol. 2007;179(5):3342–3350. doi: 10.4049/jimmunol.179.5.3342. [DOI] [PubMed] [Google Scholar]
- 52.Rider P, Carmi Y, Guttman O, Braiman A, Cohen I, Voronov E, White MR, Dinarello CA, Apte RN. IL-1α and IL-1β recruit different myeloid cells and promote different stages of sterile inflammation. J Immunol. 2011;187:4835–4843. doi: 10.4049/jimmunol.1102048. [DOI] [PubMed] [Google Scholar]
- 53.Luheshi NM, Kovacs KJ, Lopez-Castejon G, Brough D, Denes A. Interleukin-1alpha expression precedes IL-1beta after ischemic brain injury and is localised to areas of focal neuronal loss and penumbral tissues. J Neuroinflammation. 2011;8:186. doi: 10.1186/1742-2094-8-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ajmone-Cat MA, Cacci E, Ragazzoni Y, Minghetti L, Biagioni S. Pro-gliogenic effect of IL-1alpha in the differentiation of embryonic neural precursor cells in vitro. J Neurochem. 2010;113(4):1060–1072. doi: 10.1111/j.1471-4159.2010.06670.x. [DOI] [PubMed] [Google Scholar]
- 55.Wu MD, Montgomery SL, Rivera-Escalera F, Olschowka JA, O’Banion MK. Sustained IL-1beta expression impairs adult hippocampal neurogenesis independent of IL-1 signaling in nestin+ neural precursor cells. Brain Behav Immun. 2013;32:9–18. doi: 10.1016/j.bbi.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang X, Fu S, Wang Y, Yu P, Hu J, Gu W, Xu XM, Lu P. Interleukin-1β mediates proliferation and differentiation of multipotent neural precursor cells through the activation of SAPK/JNK pathway. Mol Cell Neurosci. 2007;36:343–354. doi: 10.1016/j.mcn.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 57.Green HF, Treacy E, Keohane AK, Sullivan AM, O’Keeffe GW, Nolan YM. A role for interleukin-1beta in determining the lineage fate of embryonic rat hippocampal neural precursor cells. Mol Cell Neurosci. 2012;49(3):311–321. doi: 10.1016/j.mcn.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 58.Rodriguez-Grande B, Swana M, Nguyen L, Englezou P, Maysami S, Allan SM, Rothwell NJ, Garlanda C, Denes A, Pinteaux E. The acute-phase protein PTX3 is an essential mediator of glial scar formation and resolution of brain edema after ischemic injury. J Cereb Blood Flow Metab. 2014;34(3):480–488. doi: 10.1038/jcbfm.2013.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rodriguez-Grande B, Varghese L, Molina-Holgado F, Rajkovic O, Garlanda C, Denes A, Pinteaux E. Pentraxin 3 mediates neurogenesis and angiogenesis after cerebral ischaemia. J Neuroinflammation. 2015;12:15. doi: 10.1186/s12974-014-0227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morgan SC, Taylor DL, Pocock JM. Microglia release activators of neuronal proliferation mediated by activation of mitogen-activated protein kinase, phosphatidylinositol-3-kinase/ Akt and delta-Notch signalling cascades. J Neurochem. 2004;90:89–101. doi: 10.1111/j.1471-4159.2004.02461.x. [DOI] [PubMed] [Google Scholar]
- 61.Aarum J, Sandberg K, Haeberlein SL, Persson MA. Migration and differentiation of neural precursor cells can be directed by microglia. Proc Natl Acad Sci U S A. 2003;100(26):15983–15988. doi: 10.1073/pnas.2237050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Diaz-Aparicio I, Paris I, Sierra-Torre V, Plaza-Zabala A, Rodríguez-Iglesias N, Márquez-Ropero M, Beccari S, Huguet P, Abiega O, Alberdi E, Matute C, Bernales I, Schulz A, Otrokocsi L, Sperlagh B, Happonen KE, Lemke G, Maletic-Savatic M, Valero J, Sierra A. Microglia actively remodel adult hippocampal neurogenesis through the phagocytosis secretome. J Neurosci. 2020;40:1453–1482. doi: 10.1523/JNEUROSCI.0993-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sierra A, Encinas JM, Deudero JJP, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7:483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walton NM, Sutter BM, Laywell ED, Levkoff LH, Kearns SM, Marshall GP, 2nd, et al. Microglia instruct subventricular zone neurogenesis. Glia. 2006;54(8):815–825. doi: 10.1002/glia.20419. [DOI] [PubMed] [Google Scholar]
- 65.Szalay G, Martinecz B, Lenart N, Kornyei Z, Orsolits B, Judak L, et al. Microglia protect against brain injury and their selective elimination dysregulates neuronal network activity after stroke. Nat Commun. 2016;7:11499. doi: 10.1038/ncomms11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reshef R, Kreisel T, Beroukhim Kay D, Yirmiya R. Microglia and their CX3CR1 signaling are involved in hippocampal- but not olfactory bulb-related memory and neurogenesis. Brain Behav Immun. 2014. 10.1016/j.bbi.2014.04.009. [DOI] [PubMed]
- 67.Bachstetter AD, Morganti JM, Jernberg J, Schlunk A, Mitchell SH, Brewster KW, Hudson CE, Cole MJ, Harrison JK, Bickford PC, Gemma C. Fractalkine and CX 3CR1 regulate hippocampal neurogenesis in adult and aged rats. Neurobiol Aging. 2011;32:2030–2044. doi: 10.1016/j.neurobiolaging.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mori T, Buffo A, Götz M. The novel roles of glial cells revisited: the contribution of radial glia and astrocytes to neurogenesis. Curr Top Dev Biol. 2005;69:67–99. doi: 10.1016/S0070-2153(05)69004-7. [DOI] [PubMed] [Google Scholar]
- 69.Magnusson JP, Frisén J. Stars from the darkest night: unlocking the neurogenic potential of astrocytes in different brain regions. Development. 2016;143(7):1075–1086. doi: 10.1242/dev.133975. [DOI] [PubMed] [Google Scholar]
- 70.Becerra-Calixto A, Cardona-Gómez GP. The role of astrocytes in neuroprotection after brain stroke: potential in cell therapy. Front Mol Neurosci. 2017;10:88. doi: 10.3389/fnmol.2017.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Götz M, Sirko S, Beckers J, Irmler M. Reactive astrocytes as neural stem or progenitor cells: in vivo lineage, in vitro potential, and genome-wide expression analysis. Glia. 2015;63(8):1452–1468. doi: 10.1002/glia.22850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Laywell ED, Rakic P, Kukekov VG, Holland EC, Steindler DA. Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proc Natl Acad Sci. 2000;97(25):13883–13888. doi: 10.1073/pnas.250471697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seri B, Garcıa-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21(18):7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Corti S, Nizzardo M, Simone C, Falcone M, Donadoni C, Salani S, Rizzo F, Nardini M, Riboldi G, Magri F, Zanetta C, Faravelli I, Bresolin N, Comi GP. Direct reprogramming of human astrocytes into neural stem cells and neurons. Exp Cell Res. 2012;318(13):1528–1541. doi: 10.1016/j.yexcr.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Faiz M, Sachewsky N, Gascón S, Bang KA, Morshead CM, Nagy A. Adult neural stem cells from the subventricular zone give rise to reactive astrocytes in the cortex after stroke. Cell Stem Cell. 2015;17(5):624–634. doi: 10.1016/j.stem.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 76.Zhang Y, Xu D, Qi H, Yuan Y, Liu H, Yao S, et al. Enriched environment promotes post-stroke neurogenesis through NF-κB-mediated secretion of IL-17A from astrocytes. Brain Res. 2018;1687:20–31. doi: 10.1016/j.brainres.2018.02.030. [DOI] [PubMed] [Google Scholar]
- 77.Tao Y, Ma L, Liao Z, Le Q, Yu J, Liu X, et al. Astroglial β-arrestin1-mediated nuclear signaling regulates the expansion of neural precursor cells in adult hippocampus. Sci Rep. 2015;5:15506. doi: 10.1038/srep15506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Terrillion CE, Abazyan B, Yang Z, Crawford J, Shevelkin AV, Jouroukhin Y, Yoo KH, Cho CH, Roychaudhuri R, Snyder SH, Jang MH, Pletnikov MV. DISC1 in astrocytes influences adult neurogenesis and hippocampus-dependent behaviors in mice. Neuropsychopharmacology. 2017;42(11):2242–2251. doi: 10.1038/npp.2017.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jin K, Wang X, Xie L, Mao XO, Greenberg DA. Transgenic ablation of doublecortin-expressing cells suppresses adult neurogenesis and worsens stroke outcome in mice. Proc Natl Acad Sci U S A. 2010;107(17):7993–7998. doi: 10.1073/pnas.1000154107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sun C, Sun H, Wu S, Lee CC, Akamatsu Y, Wang RK, Kernie SG, Liu J. Conditional ablation of neuroprogenitor cells in adult mice impedes recovery of poststroke cognitive function and reduces synaptic connectivity in the perforant pathway. J Neurosci. 2013;33(44):17314–17325. doi: 10.1523/JNEUROSCI.2129-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun F, Wang X, Mao X, Xie L, Jin K. Ablation of neurogenesis attenuates recovery of motor function after focal cerebral ischemia in middle-aged mice. PLoS One. 2012;7(10):e46326. doi: 10.1371/journal.pone.0046326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hao XZ, Yin LK, Tian JQ, Li CC, Feng XY, Yao ZW, Jiang M, Yang YM. Inhibition of Notch1 signaling at the subacute stage of stroke promotes endogenous neurogenesis and motor recovery after stroke. Front Cell Neurosci. 2018;12:245. doi: 10.3389/fncel.2018.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim H, Wei Y, Lee JY, Wu Y, Zheng Y, Moskowitz MA, Chen JW. Myeloperoxidase inhibition increases neurogenesis after ischemic stroke. J Pharmacol Exp Ther. 2016;359(2):262–272. doi: 10.1124/jpet.116.235127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Z, Yuan Y, Zhang Z, Ding K. Inhibition of miRNA-27b enhances neurogenesis via AMPK activation in a mouse ischemic stroke model. FEBS Open Biol. 2019;9(5):859–869. doi: 10.1002/2211-5463.12614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen D, Wei L, Liu ZR, Yang JJ, Gu X, Wei ZZ, Liu LP, Yu SP. Pyruvate kinase M2 increases angiogenesis, neurogenesis, and functional recovery mediated by upregulation of STAT3 and focal adhesion kinase activities after ischemic stroke in adult mice. Neurotherapeutics. 2018;15(3):770–784. doi: 10.1007/s13311-018-0635-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Koh SH, Park HH. Neurogenesis in stroke recovery. Transl Stroke Res. 2017;8(1):3–13. doi: 10.1007/s12975-016-0460-z. [DOI] [PubMed] [Google Scholar]
- 87.Lu J, Manaenko A, Hu Q. Targeting adult neurogenesis for poststroke therapy. Stem Cells Int. 2017;2017:5868632–5868610. doi: 10.1155/2017/5868632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Marques BL, Carvalho GA, Freitas EMM, Chiareli RA, Barbosa TG, Di Araujo AGP, et al. The role of neurogenesis in neurorepair after ischemic stroke. Semin Cell Dev Biol. 2019;95:98–110. doi: 10.1016/j.semcdb.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 89.Liu J, Solway K, Messing RO, Sharp FR. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J Neurosci. 1998;18(19):7768–7778. doi: 10.1523/JNEUROSCI.18-19-07768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kreuzberg M, Kanov E, Timofeev O, Schwaninger M, Monyer H, Khodosevich K. Increased subventricular zone-derived cortical neurogenesis after ischemic lesion. Exp Neurol. 2010;226(1):90–99. doi: 10.1016/j.expneurol.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 91.Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52(6):802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- 92.Belayev L, Hong SH, Menghani H, Marcell SJ, Obenaus A, Freitas RS, Khoutorova L, Balaszczuk V, Jun B, Oriá RB, Bazan NG. Docosanoids promote neurogenesis and angiogenesis, blood-brain barrier integrity, penumbra protection, and neurobehavioral recovery after experimental ischemic stroke. Mol Neurobiol. 2018;55(8):7090–7106. doi: 10.1007/s12035-018-1136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li C, Zhang B, Zhu Y, Li Y, Liu P, Gao B, Tian S, du L, Bai Y. Post-stroke constraint-induced movement therapy increases functional recovery, angiogenesis, and neurogenesis with enhanced expression of HIF-1alpha and VEGF. Curr Neurovasc Res. 2017;14(4):368–377. doi: 10.2174/1567202614666171128120558. [DOI] [PubMed] [Google Scholar]
- 94.Madelaine R, Sloan SA, Huber N, Notwell JH, Leung LC, Skariah G, Halluin C, Paşca SP, Bejerano G, Krasnow MA, Barres BA, Mourrain P. MicroRNA-9 couples brain neurogenesis and angiogenesis. Cell Rep. 2017;20(7):1533–1542. doi: 10.1016/j.celrep.2017.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang SJ, Wang RL, Zhao HP, Tao Z, Li JC, Ju F, Han ZP, Ma QF, Liu P, Ma SB, Cao GD, Luo YM. MEPO promotes neurogenesis and angiogenesis but suppresses gliogenesis in mice with acute ischemic stroke. Eur J Pharmacol. 2019;849:1–10. doi: 10.1016/j.ejphar.2019.01.066. [DOI] [PubMed] [Google Scholar]
- 96.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Risau W. Mechanisms of angiogenesis. Nature. 1997;386(6626):671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 98.Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304(5675):1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 99.Teng H, Zhang ZG, Wang L, Zhang RL, Zhang L, Morris D, Gregg SR, Wu Z, Jiang A, Lu M, Zlokovic BV, Chopp M. Coupling of angiogenesis and neurogenesis in cultured endothelial cells and neural progenitor cells after stroke. J Cereb Blood Flow Metab. 2008;28(4):764–771. doi: 10.1038/sj.jcbfm.9600573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hayashi T, Noshita N, Sugawara T, Chan PH. Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J Cereb Blood Flow Metab. 2003;23(2):166–180. doi: 10.1097/01.WCB.0000041283.53351.CB. [DOI] [PubMed] [Google Scholar]
- 101.Beck H, Acker T, Wiessner C, Allegrini PR, Plate KH. Expression of angiopoietin-1, angiopoietin-2, and tie receptors after middle cerebral artery occlusion in the rat. Am J Pathol. 2000;157(5):1473–1483. doi: 10.1016/S0002-9440(10)64786-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Beck H, Plate KH. Angiogenesis after cerebral ischemia. Acta Neuropathol. 2009;117(5):481–496. doi: 10.1007/s00401-009-0483-6. [DOI] [PubMed] [Google Scholar]
- 103.Marti HJ, Bernaudin M, Bellail A, Schoch H, Euler M, Petit E, et al. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am J Pathol. 2000;156(3):965–976. doi: 10.1016/S0002-9440(10)64964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002;1(2):92–100. doi: 10.1016/s1474-4422(02)00040-6. [DOI] [PubMed] [Google Scholar]
- 105.Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35(7):1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- 106.Shyu WC, Lin SZ, Yang HI, Tzeng YS, Pang CY, Yen PS, Li H. Functional recovery of stroke rats induced by granulocyte colony-stimulating factor-stimulated stem cells. Circulation. 2004;110(13):1847–1854. doi: 10.1161/01.CIR.0000142616.07367.66. [DOI] [PubMed] [Google Scholar]
- 107.Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, Tsukamoto Y, Iso H, Fujimori Y, Stern DM, Naritomi H, Matsuyama T. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114(3):330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, Zhang C, Lu M, Katakowski M, Feldkamp CS, Chopp M. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53(6):743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- 109.Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci U S A. 1999;96(23):13496–13500. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yew WP, Djukic ND, Jayaseelan JSP, Walker FR, Roos KAA, Chataway TK, Muyderman H, Sims NR. Early treatment with minocycline following stroke in rats improves functional recovery and differentially modifies responses of peri-infarct microglia and astrocytes. J Neuroinflammation. 2019;16(1):6. doi: 10.1186/s12974-018-1379-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Giri PK, Lu Y, Lei S, Li W, Zheng J, Lu H, Chen X, Liu Y, Zhang P. Pretreatment with minocycline improves neurogenesis and behavior performance after midazolam exposure in neonatal rats. Neuroreport. 2018;29(3):153–159. doi: 10.1097/WNR.0000000000000937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Inta D, Lang UE, Borgwardt S, Meyer-Lindenberg A, Gass P. Microglia activation and schizophrenia: lessons from the effects of minocycline on postnatal neurogenesis, neuronal survival and synaptic pruning. Schizophr Bull. 2017;43(3):493–496. doi: 10.1093/schbul/sbw088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wadhwa M, Prabhakar A, Ray K, Roy K, Kumari P, Jha PK, Kishore K, Kumar S, Panjwani U. Inhibiting the microglia activation improves the spatial memory and adult neurogenesis in rat hippocampus during 48 h of sleep deprivation. J Neuroinflammation. 2017;14(1):222. doi: 10.1186/s12974-017-0998-z. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 114.Padma Srivastava MV, Bhasin A, Bhatia R, Garg A, Gaikwad S, Prasad K, Singh MB, Tripathi M. Efficacy of minocycline in acute ischemic stroke: a single-blinded, placebo-controlled trial. Neurol India. 2012;60(1):23–28. doi: 10.4103/0028-3886.93584. [DOI] [PubMed] [Google Scholar]
- 115.Malhotra K, Chang JJ, Khunger A, Blacker D, Switzer JA, Goyal N, Hernandez AV, Pasupuleti V, Alexandrov AV, Tsivgoulis G. Minocycline for acute stroke treatment: a systematic review and meta-analysis of randomized clinical trials. J Neurol. 2018;265(8):1871–1879. doi: 10.1007/s00415-018-8935-3. [DOI] [PubMed] [Google Scholar]
- 116.Engels J, Elting N, Braun L, Bendix I, Herz J, Felderhoff-Muser U, et al. Sildenafil enhances quantity of immature neurons and promotes functional recovery in the developing ischemic mouse brain. Dev Neurosci. 2017;39(1–4):287–297. doi: 10.1159/000457832. [DOI] [PubMed] [Google Scholar]
- 117.Zhang R, Wang Y, Zhang L, Zhang Z, Tsang W, Lu M, Zhang L, Chopp M. Sildenafil (Viagra) induces neurogenesis and promotes functional recovery after stroke in rats. Stroke. 2002;33(11):2675–2680. doi: 10.1161/01.str.0000034399.95249.59. [DOI] [PubMed] [Google Scholar]
- 118.Zhang RL, Chopp M, Roberts C, Wei M, Wang X, Liu X, Lu M, Zhang ZG. Sildenafil enhances neurogenesis and oligodendrogenesis in ischemic brain of middle-aged mouse. PLoS One. 2012;7(10):e48141. doi: 10.1371/journal.pone.0048141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang RL, Zhang Z, Zhang L, Wang Y, Zhang C, Chopp M. Delayed treatment with sildenafil enhances neurogenesis and improves functional recovery in aged rats after focal cerebral ischemia. J Neurosci Res. 2006;83(7):1213–1219. doi: 10.1002/jnr.20813. [DOI] [PubMed] [Google Scholar]
- 120.Cheng Y, Pardo M, Armini RS, Martinez A, Mouhsine H, Zagury JF, et al. Stress-induced neuroinflammation is mediated by GSK3-dependent TLR4 signaling that promotes susceptibility to depression-like behavior. Brain Behav Immun. 2016;53:207–222. doi: 10.1016/j.bbi.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vallee A, Lecarpentier Y, Guillevin R, Vallee JN. Effects of cannabidiol interactions with Wnt/beta-catenin pathway and PPARgamma on oxidative stress and neuroinflammation in Alzheimer’s disease. Acta Biochim Biophys Sin Shanghai. 2017;49(10):853–866. doi: 10.1093/abbs/gmx073. [DOI] [PubMed] [Google Scholar]
- 122.Lovestone S, Boada M, Dubois B, Hull M, Rinne JO, Huppertz HJ, et al. A phase II trial of tideglusib in Alzheimer’s disease. J Alzheimers Dis. 2015;45(1):75–88. doi: 10.3233/JAD-141959. [DOI] [PubMed] [Google Scholar]
- 123.Tolosa E, Litvan I, Hoglinger GU, Burn D, Lees A, Andres MV, et al. A phase 2 trial of the GSK-3 inhibitor tideglusib in progressive supranuclear palsy. Mov Disord. 2014;29(4):470–478. doi: 10.1002/mds.25824. [DOI] [PubMed] [Google Scholar]
- 124.Li R, Liu Z, Wu X, Yu Z, Zhao S, Tang X. Lithium chloride promoted hematoma resolution after intracerebral hemorrhage through GSK-3beta-mediated pathways-dependent microglia phagocytosis and M2-phenotype differentiation, angiogenesis and neurogenesis in a rat model. Brain Res Bull. 2019;152:117–127. doi: 10.1016/j.brainresbull.2019.07.019. [DOI] [PubMed] [Google Scholar]
- 125.Emsley HC, Smith CJ, Georgiou RF, Vail A, Hopkins SJ, Rothwell NJ, Tyrrell PJ, Acute Stroke Investigators A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J Neurol Neurosurg Psychiatry. 2005;76(10):1366–1372. doi: 10.1136/jnnp.2004.054882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Smith CJ, Hulme S, Vail A, Heal C, Parry-Jones AR, Scarth S, Hopkins K, Hoadley M, Allan SM, Rothwell NJ, Hopkins SJ, Tyrrell PJ. SCIL-STROKE (Subcutaneous Interleukin-1 Receptor Antagonist in Ischemic Stroke): a randomized controlled phase 2 trial. Stroke. 2018;49(5):1210–1216. doi: 10.1161/STROKEAHA.118.020750. [DOI] [PubMed] [Google Scholar]
- 127.Pradillo JM, Murray KN, Coutts GA, Moraga A, Oroz-Gonjar F, Boutin H, Moro MA, Lizasoain I, Rothwell NJ, Allan SM. Reparative effects of interleukin-1 receptor antagonist in young and aged/co-morbid rodents after cerebral ischemia. Brain Behav Immun. 2017;61:117–126. doi: 10.1016/j.bbi.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bernstock JD, Peruzzotti-Jametti L, Ye D, Gessler FA, Maric D, Vicario N, Lee YJ, Pluchino S, Hallenbeck JM. Neural stem cell transplantation in ischemic stroke: a role for preconditioning and cellular engineering. J Cereb Blood Flow Metab. 2017;37(7):2314–2319. doi: 10.1177/0271678X17700432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Boese AC, Eckert A, Hamblin MH, Lee JP. Human neural stem cells improve early stage stroke outcome in delayed tissue plasminogen activator-treated aged stroke brains. Exp Neurol. 2020;329:113275. doi: 10.1016/j.expneurol.2020.113275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Boese AC, Le QE, Pham D, Hamblin MH, Lee JP. Neural stem cell therapy for subacute and chronic ischemic stroke. Stem Cell Res Ther. 2018;9(1):154. doi: 10.1186/s13287-018-0913-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Huang L, Wong S, Snyder EY, Hamblin MH, Lee JP. Human neural stem cells rapidly ameliorate symptomatic inflammation in early-stage ischemic-reperfusion cerebral injury. Stem Cell Res Ther. 2014;5(6):129. doi: 10.1186/scrt519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Oki K, Tatarishvili J, Wood J, Koch P, Wattananit S, Mine Y, Monni E, Tornero D, Ahlenius H, Ladewig J, Brüstle O, Lindvall O, Kokaia Z. Human-induced pluripotent stem cells form functional neurons and improve recovery after grafting in stroke-damaged brain. Stem Cells. 2012;30(6):1120–1133. doi: 10.1002/stem.1104. [DOI] [PubMed] [Google Scholar]
- 133.Chen J, Li Y, Wang L, Lu M, Zhang X, Chopp M. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci. 2001;189(1–2):49–57. doi: 10.1016/s0022-510x(01)00557-3. [DOI] [PubMed] [Google Scholar]
- 134.Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32(4):1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 135.Tobin MK, Stephen TKL, Lopez KL, Pergande MR, Bartholomew AM, Cologna SM, Lazarov O. Activated mesenchymal stem cells induce recovery following stroke via regulation of inflammation and oligodendrogenesis. J Am Heart Assoc. 2020;9(7):e013583. doi: 10.1161/JAHA.119.013583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kalladka D, Sinden J, Pollock K, Haig C, McLean J, Smith W, McConnachie A, Santosh C, Bath PM, Dunn L, Muir KW. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): a phase 1, first-in-man study. Lancet. 2016;388(10046):787–796. doi: 10.1016/S0140-6736(16)30513-X. [DOI] [PubMed] [Google Scholar]
- 137.Prasad K, Sharma A, Garg A, Mohanty S, Bhatnagar S, Johri S, Singh KK, Nair V, Sarkar RS, Gorthi SP, Hassan KM, Prabhakar S, Marwaha N, Khandelwal N, Misra UK, Kalita J, Nityanand S, InveST Study Group Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke: a multicentric, randomized trial. Stroke. 2014;45(12):3618–3624. doi: 10.1161/STROKEAHA.114.007028. [DOI] [PubMed] [Google Scholar]
- 138.Savitz SI, Yavagal D, Rappard G, Likosky W, Rutledge N, Graffagnino C, Alderazi Y, Elder JA, Chen PR, Budzik RF, Jr, Tarrel R, Huang DY, Hinson JM, Jr, On behalf of the ALD-401 Trial Group A phase 2 randomized, sham-controlled trial of internal carotid artery infusion of autologous bone marrow-derived ALD-401 cells in patients with recent stable ischemic stroke (RECOVER-stroke) Circulation. 2019;139(2):192–205. doi: 10.1161/CIRCULATIONAHA.117.030659. [DOI] [PubMed] [Google Scholar]
- 139.Cunningham CJ, Wong R, Barrington J, Tamburrano S, Pinteaux E, Allan SM. Systemic conditioned medium treatment from interleukin-1 primed mesenchymal stem cells promotes recovery after stroke. Stem Cell Res Ther. 2020;11(1):32. doi: 10.1186/s13287-020-1560-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Thomas JM, Cunningham CJ, Lawrence CB, Pinteaux E, Allan SM. Therapeutic potential of extracellular vesicles in preclinical stroke models: a systematic review and meta-analysis. BMJ Open Sci. 2020;4. [DOI] [PMC free article] [PubMed]
- 141.Redondo-Castro E, Cunningham C, Miller J, Martuscelli L, Aoulad-Ali S, Rothwell NJ, Kielty CM, Allan SM, Pinteaux E. Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro. Stem Cell Res Ther. 2017;8(1):79. doi: 10.1186/s13287-017-0531-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen WC, Chang LH, Huang SS, Huang YJ, Chih CL, Kuo HC, Lee YH, Lee IH. Aryl hydrocarbon receptor modulates stroke-induced astrogliosis and neurogenesis in the adult mouse brain. J Neuroinflammation. 2019;16(1):187. doi: 10.1186/s12974-019-1572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cuartero MI, de la Parra J, Perez-Ruiz A, Bravo-Ferrer I, Duran-Laforet V, Garcia-Culebras A, et al. Abolition of aberrant neurogenesis ameliorates cognitive impairment after stroke in mice. J Clin Invest. 2019;129(4):1536–1550. doi: 10.1172/JCI120412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang YH, Chern CM, Liou KT, Kuo YH, Shen YC. Ergostatrien-7,9(11),22-trien-3beta-ol from Antrodia camphorata ameliorates ischemic stroke brain injury via downregulation of p65NF-kappa-B and caspase 3, and activation of Akt/GSK3/catenin-associated neurogenesis. Food Funct. 2019;10(8):4725–4738. doi: 10.1039/c9fo00908f. [DOI] [PubMed] [Google Scholar]
- 145.Wu X, Liu S, Hu Z, Zhu G, Zheng G, Wang G. Enriched housing promotes post-stroke neurogenesis through calpain 1-STAT3/HIF-1alpha/VEGF signaling. Brain Res Bull. 2018;139:133–143. doi: 10.1016/j.brainresbull.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 146.Yang L, Tucker D, Dong Y, Wu C, Lu Y, Li Y, et al. Photobiomodulation therapy promotes neurogenesis by improving post-stroke local microenvironment and stimulating neuroprogenitor cells. Exp Neurol. 2018;299(Pt A):86–96. doi: 10.1016/j.expneurol.2017.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Xin H, Katakowski M, Wang F, Qian JY, Liu XS, Ali MM, Buller B, Zhang ZG, Chopp M. MicroRNA cluster miR-17-92 cluster in exosomes enhance neuroplasticity and functional recovery after stroke in rats. Stroke. 2017;48(3):747–753. doi: 10.1161/STROKEAHA.116.015204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bravo-Ferrer I, Cuartero MI, Zarruk JG, Pradillo JM, Hurtado O, Romera VG, et al. Cannabinoid type-2 receptor drives neurogenesis and improves functional outcome after stroke. Stroke. 2017;48(1):204–212. doi: 10.1161/STROKEAHA.116.014793. [DOI] [PubMed] [Google Scholar]