Abstract
Fruitful progress and change have been accomplished in epilepsy surgery as science and technology advance. Stereotactic electroencephalography (SEEG) was originally developed by Talairach and Bancaud at Hôspital Sainte-Anne in the middle of the 20th century. SEEG has survived, and is now being recognized once again, especially with the development of neurosurgical robots. Many epilepsy centers have already replaced invasive monitoring with subdural electrodes (SDEs) by SEEG with depth electrodes worldwide. SEEG has advantages in terms of complication rates as shown in the previous reports. However, it would be more indispensable to demonstrate how much SEEG has contributed to improving seizure outcomes in epilepsy surgery. Vagus nerve stimulation (VNS) has been an only implantable device since 1990s, and has obtained the autostimulation mode which responds to ictal tachycardia. In addition to VNS, responsive neurostimulator (RNS) joined in the options of palliative treatment for medically refractory epilepsy. RNS is winning popularity in the United States because the device has abilities of both neurostimulation and recording of ambulatory electrocorticography (ECoG). Deep brain stimulation (DBS) has also attained approval as an adjunctive therapy in Europe and the United States. Ablative procedures such as SEEG-guided radiofrequency thermocoagulation (RF-TC) and laser interstitial thermal therapy (LITT) have been developed as less invasive options in epilepsy surgery. There will be more alternatives and tools in this field than ever before. Consequently, we will need to define benefits, indications, and limitations of these new technologies and concepts while adjusting ourselves to a period of fundamental transition in our foreseeable future.
Keywords: epilepsy surgery, SEEG, VNS, RNS, ablative surgery
Introduction
Epilepsy affects 1% of the population, approximately 50–70 million people worldwide. About one-third of epilepsy patients have seizures that are unresponsive to medical treatment with anti-epileptic drugs (AEDs). Children and adults with intractable seizures continue to suffer a serious burden of higher rates of morbidity and mortality. In addition, they could have cognitive and psychiatric impairment.1,2) These patients with medically refractory epilepsy are referred as potential surgical candidates. However, epilepsy surgery is still underutilized, and hence only a small percentage of them could undergo surgical options.2)
In the past decade, remarkable advances have been made in the surgical treatment of epilepsy. Stereotactic electroencephalography (SEEG) is now being recognized once again in North America.3) In addition to vagus nerve stimulation (VNS), responsive neurostimulator (RNS) is now gaining a major position as a new device with a closed-loop system in the United States.4) Deep brain stimulation (DBS) has attained approval as an adjunctive therapy for medically refractory epilepsy in Europe and the United States.5) As less invasive options, ablative procedures such as SEEG-guided radiofrequency thermocoagulation (SEEG-guided RF-TC) and laser interstitial thermal therapy (LITT) have been developed and are expanding the indications in epilepsy surgery.6,7) The advancement of science and technology is too fast. Epilepsy surgery is also making rapid progress. It seems to us that now is the time to change.
Invasive EEG Monitoring with Intracranial Electrodes
Historical view: transition and the legacy
Invasive monitoring with intracranial electrodes is often necessary for seizure focus detection, especially in cases with ambiguity of an epileptogenic area after preoperative non-invasive evaluations or cases without an obvious lesion on a magnetic resonance imaging (MRI).8,9) There are two main technical methods for invasive monitoring in our modern times. One is craniotomy with subdural grids and strips to cover the cerebral cortex harboring a possible seizure focus. If extensive coverage is necessary, a large subdural grid is chosen and enough subdural strips are commonly used. However, large craniotomy is occasionally inevitable in these cases. Depth electrodes are also added with these subdural electrodes (SDEs) at the same time when deeper structures should be additionally investigated9,10) (Fig. 1).
Fig. 1.

A subdural grid electrode was placed to cover the right frontal and temporal cortexes. Six subdural strips around the grid and two depth electrodes through the grid were additionally implanted. A photo from the author’s surgical experience. Consent was obtained from the patient.
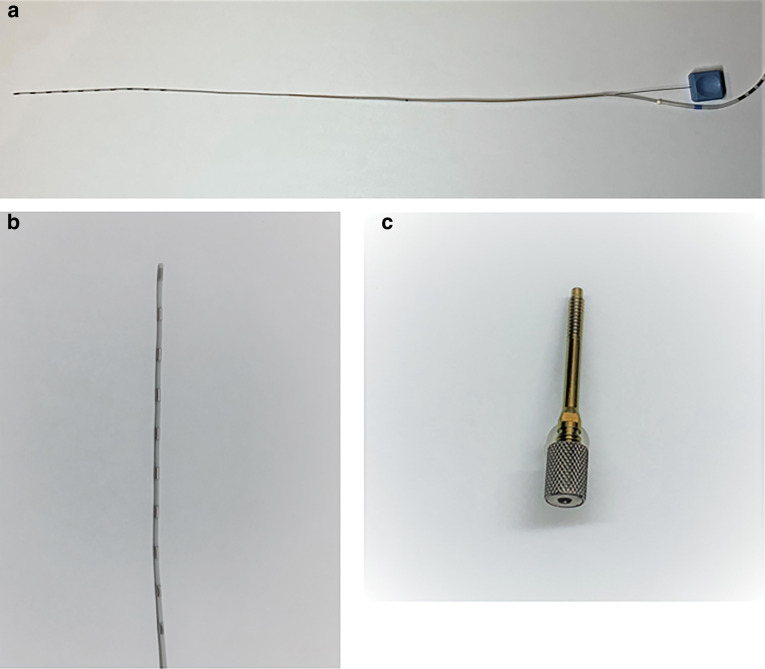
Another method is SEEG, which is more popularized worldwide in the last decade.3,11,12) SEEG is usually performed only with depth electrodes without craniotomy (Figs. 2 and 3). Introduction of robotic assistance is probably one of the main reasons in this efflorescence of SEEG at present (Figs. 4 and 5). It would be timely to look back on the history of invasive monitoring with intracranial electrodes here in this review article.
Fig. 2.
(a) A whole view of a depth electrode with an inner wire stylet. (b) The tip of a depth electrode. (c) An anchor bolt to secure a depth electrode in the skull. Courtesy of Ad-Tech Medical Instrument Corporation, Oak Creek, WI, USA.
Fig. 3.
Three depth electrodes were implanted on each side for an exploratory evaluation of SEEG by conventional frame-based implantation. Courtesy of Dr. Yuichi Kubota, Department of Neurosurgery, Tokyo Women’s Medical University Medical Center East, Tokyo, Japan.
Fig. 4.
ROSA, a neurosurgical robot is useful for frameless implantation of depth electrodes for SEEG. In addition, this robot has been utilized for other neurosurgical procedures such as neuroendoscopy, stereotactic biopsy, pallidotomy, shunt placement, DBS procedures, and stereotactic cyst aspiration. Courtesy of Zimmer Biomet, Warsaw, IN, USA, 2020. DBS: deep brain stimulation, SEEG: stereotactic electroencephalography.
Fig. 5.
A screen of the dedicated software demonstrates trajectories of depth electrodes as preoperative planning. Pictures of neuroimaging such as an MRI and a CT scan can be imported. The gadolinium T1-weighted sequence is indispensable in locating an entry point to avoid hemorrhagic complications. Courtesy of Zimmer Biomet, Warsaw, IN, USA, 2020. CT: computed tomography; MRI: magnetic resonance imaging.
The first case of invasive EEG monitoring was carried out by Wilder Penfield and Herbert Jasper at the Montreal Neurological Institute (MNI) in 1939.13) Jasper had worked with epidural electrocorticography (ECoG) in cats, and then they placed epidural single-contact electrodes through burr holes over both temporal lobes. The invasive monitoring was done with a four-channel ink-writing apparatus only during daytime hours, and showed continuous random delta activity in the left temporal region. The patient ultimately underwent removal of a scar from the posterior part of the left temporal lobe with simultaneous cortical mapping to prevent sequelae of language function in an awake craniotomy. Although the procedure did not work well in terms of seizure freedom, this case really indicated that the concept of EEG-directed surgery was already present at the MNI in the late 1930s.13) After this epidural electrodes, Penfield and Jasper originally reported the technique of subdural strip electrodes.14) However, it did not gain wide acceptance.15)
In the late 1940s, several stereotactic instruments for human were developed by renowned pioneers like Spiegel and Wycis, Hayne and Meyers, William Sweet, Jasper and Hunter, and Jean Talairach.16) In terms of development of SEEG and influence to our present society, Talairach made a great contribution in his life.
SEEG was developed and introduced into practice at Hôspital Sainte-Anne by Talairach and an epileptologist Jean Bancaud.17) Talairach was originally trained as a psychiatrist and then changed his professional career to neurosurgery. Although he dedicated several years to psychosurgery such as bilateral anterior capsulotomy, he turned his attention more to study in stereotactic and epilepsy surgery after a decline in psychosurgery.17)
The original “Sainte-Anne method” consisted of two stages was primarily complicated and time-consuming. In Stage I, after an assessment of seizure semiology and scalp EEG, a patient underwent setting of the stereotactic frame on the head and a series of studies including ventriculography and angiography in the specialized stereotactic operating room. Then all images were developed on semitransparent paper, and the patient’s anatomy was mapped onto the tracings to make a hypothesis-driven SEEG lead placement plan. Depth electrodes were placed according to the plan in Stage II, 15 days after Stage I.17) These electrodes up to 20 were placed orthogonally via the double grid system on the frame through twist drill holes, and their positions were documented on anteroposterior and lateral stereotactic X-rays. Surprisingly, Talairach’s group had established where the vessels were not, avascular zones in which depth electrodes could be passed orthogonally without the risk of vessel injury.18)
The duration for invasive monitoring was short and limited from 6 to 12 hours at Sainte-Anne. Therefore, they often made a final decision of an area and extent of resection only with interictal activity or a single seizure.17) No complications were reported in the first 42 patients in Stage II with this method. The rate of recovery or improvement in 34 patients who underwent open and definitive surgical treatment after Stage II was 79%. These results were praiseworthy with such old-fashioned equipment. The methods and conceptual work by Talairach and Bancaud were created in the 1950s through the 1960s. However, their accomplishment is still alive at this moment and fundamental for most aspects of modern stereotactic and epilepsy surgery.17–19)
Until the beginning of the 1980s, SEEG was the gold standard for workups and evaluations in epilepsy surgery.11) However, since the 1980s, subdural grids have become more popular, especially outside of Europe, because SEEG demands high technical requirements, costs, and expertise.11) In addition, the objective may be equally well realized in many cases from the somewhat simpler technique of subdural strip electrodes.15) Various electrode types concerning different sizes and extents allowed a sufficient coverage of the convexity of the cortex.11) Then SDEs have been widely used as one of the standard techniques to localize seizure foci. On the other hand, new and simplified insertion techniques of SEEG have gained increasing popularity in the past 10 years. Frameless and robot-assisted implantation techniques have progressed and allowed an easier, safe, accurate, and time-saving insertion of SEEG11) (Figs. 4–6).
Fig. 6.

Thirteen depth electrodes were implanted with particular interest on the left temporal lobe by frameless navigation of the ROSA system. The neurosurgical robot reduces the required time and brings convenience for implantation of depth electrodes. Courtesy of Dr. Jorge González-Martínez, Department of Neurosurgery, University of Pittsburgh Medical Center, PA, USA.
SEEG with depth electrodes versus ECoG with SDEs
SDE implantation via a craniotomy has been the principal approach for intracranial EEG monitoring mainly in North America, the United Kingdom, Germany, and Japan. The Talairach SEEG approach has been preserved in France, Italy, and Brazil.20) A paradigm shift toward SEEG from SDE has taken place at many institutes worldwide especially in North America.3) For instance, there was a dramatic change in Ontario, Canada. SEEG has evolved into the principle means of intracranial EEG monitoring, and accounts for 95% of them recently. It took only 5 years to replace SDE with SEEG.12) It seems to be the same trend in Japan, because the Japan Ministry of Health, Labor and Welfare approved reimbursement as insurance coverage for robot-assisted placement of depth electrodes for SEEG early in 2020. However, many groups outside Europe may be unfamiliar with SEEG.21) In addition, there is no high-quality evidence indicating superiority of any one technique over the other for intracranial EEG monitoring.3) The choice between the two depends on the question from scalp EEG recordings and a matter of each institutional preference.12)
The most feared complications related to implantation of depth electrodes for SEEG are intracranial bleeds. The systematic review with a meta-analysis demonstrated safety of SEEG from a large series of 2624 patients and 22085 implanted electrodes.21) The most common complications were hemorrhagic (pooled prevalence 1.0%) or infectious (0.8%). The total of 121 surgical complications (1.3%) and 5 mortalities (0.3%) were identified. Hemorrhagic complications included 26 intracerebral hemorrhages, 11 subdural hematomas, and 3 epidural hematomas. Emergency craniotomies were carried out in 11 patients (0.4%) due to large hematomas out of 2624 SEEG patients. Two patients who had an intracerebral hemorrhage (0.08%) unfortunately died. A total of 28 infections contained 11 cerebral abscesses, 11 superficial wound infections, and 4 cases with meningitis.21)
A large series of 549 SEEG implantations at a single center revealed the true incidence of hemorrhage.22) Postimplant computed tomography (CT) demonstrated that 105 implantations (19.1%) had any type of intracranial hemorrhage. Of these, two patients (0.4%) suffered a permanent neurological deficit and one patient (0.2%) died. Although most of them were small and asymptomatic, the total hemorrhagic rate appeared to be higher than previously reported.22)
A systematic review and meta-analysis were also carried out in published studies regarding 2542 patients with SDE.23) Mean number of electrodes per patient varied from 52 to 95, and the duration of monitoring varied from 5 to 17 days. Infections emerged as the most common group of adverse events with a pooled prevalence estimate of 2.3% for pyogenic neurologic infections, 3% for superficial infection, and 7.1% for asymptomatic positive cerebrospinal fluid cultures. Other major adverse events included intracranial hemorrhage (4.0%), increased intracranial pressure (2.4%), and transient new-onset neurological deficits (4.6%).23)
Another systematic review and meta-analysis collected data from articles regarding SDE published between 1980 and 2012.24) They divided the 32 years into two periods, 1980–1995 and 1996–2012. Neurological deficits (4.3%) and wound infections/meningitis (3.4%) were the most common, followed by hemorrhage/hematoma/cerebrovascular accident (3.2%) for both time periods. Surprisingly, wound infections/meningitis increased from 2.3% to 4.3%, as did hemorrhage/hematoma rising from 1.9% to 4.2%. One explanation for these rising complication rates could be the increasing use of larger or bilateral arrays and grids, which are associated with higher complications rates, compared with depth electrodes.24)
One center in Texas, USA made a well-organized comparison of morbidity and outcomes between SDE and SEEG.20) They performed 260 consecutive intracranial EEG procedures, which consisted 139 cases of SDE and 121 cases of SEEG. Seven symptomatic hemorrhagic sequelae (5.0%) and three infections (2.2%) occurred in the SDE group. However, there was no clinically relevant complications in the SEEG group and a marked difference in complication rates (P = 0.003). These procedures were carried out by the same single surgeon. Interestingly, definitive procedures such as resection or ablative surgery were carried out more in 127 cases (91.4%) of the SDE group than in 90 cases (74.4%) of the SEEG group. In terms of epilepsy outcomes, patients who underwent resection or ablation demonstrated favorable outcomes (Engel class I or II) in 57 of 75 SEEG cases (76.0%) in comparison with 59 of 108 SDE cases (54.6%; P = 0.003) at 1 year. In non-lesional cases, the analysis revealed the same good outcomes in 27 of 39 SEEG cases (69.2%) as compared to 9 of 26 SDE cases (34.6%; P = 0.006).20)
It is widely known that SDE needs invasive monitoring and resections to be done normally within one hospitalization at many epilepsy centers. Surgeons mostly perform resective or definitive surgery after one to several weeks from implantation of SDE because surgeons experienced in SDE design the same craniotomy for both SDE and resective surgery. Another reason is that adhesion between the cortex and the dura gives surgeons much difficulty and challenge in approaching the seizure focus if resective surgery is kept waiting for months. Then, it is suggested that time is limited for delineating the seizure focus in SDE as accurately compared to SEEG.20,25)
Nevertheless, it seems to be very difficult to determine whether invasive monitoring with SEEG or that with SDE would create a better outcome in seizure freedom following surgical resection. A systematic review demonstrated that more patients with SDE (81.6%) underwent resective surgery than SEEG patients (76.9%, P = 0.001).25) In contrast, the analysis revealed SEEG-informed resections were associated with more patients obtaining seizure freedom (61.0%) than SDE-informed resections (56.4%, P = 0.001). However, clinical studies directly comparing these two methods are still necessary to understand differences in seizure outcomes.25)
Pain management for patients is essential in intracranial EEG monitoring. Narcotic use is probably one of the appropriate indicators showing invasiveness of procedures for patients. The SDE group received significantly greater dosages of narcotics (mean, 356 morphine milligram equivalents; MME/patient) compared with those in the SEEG group (mean, 201 MME/patient; P <0.001).20) Another study also revealed that 60% of subdural grid (SDG) patients and 13% of SEEG patients used more than one opioid in the days following electrode removal to discharge from the hospital. The SDG group had a significantly higher MME from implantation through discharge compared with the SEEG group. Patients with the larger SDG implantation required the higher MME in the first 24 hours after implantation.26)
The availability of surgical robots, such as the ROSA (Zimmer Biomet, Warsaw, IN, USA; Figs. 4 and 5) and NeuroMate (Renishaw, Wotton-under-Edge, UK), allows for a combination of accuracy and efficiency in SEEG implantation.27) The use of robots in SEEG implantation may introduce several advantages to the technique. Then robots influenced us to move more toward SEEG instead of SDE. Robots have replaced the need for numerous and time-consuming frame coordinate adjustments. Comparison of the time of implantation between the robot-assisted SEEG and the former and traditional frame-based SEEG series at the same institute revealed a statistical difference. The average time of the frame-based SEEG implantation was 352 minutes, and that of the robot-assisted SEEG was 130 minutes. Then, 222 minutes were cut down by the robotic assistance (P <0.001)28) (Figs. 4–6). Although robots are effectively utilized in SEEG implantations, they are also frequently used in other neurosurgical fields. Robotic assistance has been demonstrated in neuroendoscopy, stereotactic biopsy, pallidotomy, shunt placement, DBS procedures, and stereotactic cyst aspiration.29)
The transition from SDE to SEEG was possibly similar at any epilepsy centers in North America. SEEG was initially used mostly in bilateral cases, deep lesions, or patients with prior epilepsy surgery. After this initial learning period, SEEG became the predominant modality with the robotic technology.20) Then, SDE would be chiefly selected for neocortical epilepsy located around the eloquent cortex such as the motor cortex and the language area.20,30) SEEG probably has more advantages as compared to SDE particularly in terms of complication rates. However, more studies and data regarding outcomes of seizure freedom by definitive procedures following SEEG are obligatory. SEEG would be chosen more frequently as intracranial EEG monitoring since SEEG is obviously less invasive unless intracranial bleeds happen with implantation of depth electrodes. Safety and complications should be fully understood even though it is a small risk because published articles revealed a few mortalities with SEEG implantations. It is really a matter of course to develop and keep wise surgical indications, proper technique with adequate vascular imaging, minimizing of the number of implanted electrodes, and detailed discussions within an epilepsy center and undoubtedly with patients and their families when SEEG is implemented at each institute.21)
Implantable Devices for Medically Refractory Epilepsy
Vagus nerve stimulation
VNS Therapy System (LivaNova USA Inc., Houston, TX, USA) has been approved in more than 70 countries around the world, and more than 100,000 patients have received VNS therapy as of June 20181) (Fig. 7). Ten years have passed in Japan since VNS was approved for an adjunctive and palliative option in the treatment of medically refractory epilepsy. Although VNS is not a definitive procedure, it is indicated for patients who are not candidates amenable to intracranial epilepsy surgery such as focus resection or disconnection surgery. Then VNS has been widely used in Japan and the number of patients treated with VNS increased year by year31) (Fig. 8). Many patients have kept VNS therapy and undergone revisions of a generator for battery depletion. In total of 1462 cases of implantations, there were 853 new implantations (58.3%) and 609 revisions (41.7%) in the last 4 years from 2016 through 2019.
Fig. 7.

AspireSR (Model 106) of the VNS Therapy System responds to ictal tachycardia. The previous systems had only Normal Mode and Magnet Mode functioning as an open-loop system. Ultimately, the system from Model 106 has gained AutoStim Mode as a closed-loop system. Courtesy of LivaNova USA, Inc.
Fig. 8.
The number of the VNS® Therapy Systems implanted each year in Japan since its approval in 2010. A total of 2731 implantation procedures were carried out by the end of 2019. A small decrement was observed in 2017 possibly because several newer AEDs came onto the Japanese market and showed an influence to a certain extent. Courtesy of LivaNova Japan KK. AEDs: anti-epileptic drugs.
The Japan Epilepsy Society established the VNS Qualifying Committee at the time of approval by the ministry. The committee made a guideline regulating implementation of VNS therapy nationwide to prevent unnecessary or unacceptable implantation of this device. The committee also qualifies physicians and surgeons to perform VNS therapy. Neurosurgeons who can carry out implantation of the VNS Therapy System must be certified by both the Japan Neurosurgical Society and the Japan Epilepsy Society. Epileptologists including pediatricians, neurologists, and psychiatrists who can program the setting of VNS parameters must be certified by each specialty society. All of them must attend a short course run by the committee, and then they are qualified for VNS therapy. There are 168 neurosurgeons, 289 pediatricians, 112 neurologists, and 52 psychiatrists with this certificate at the end of 2019. (These data were provided by the LivaNova Japan KK, Tokyo, Japan) Qualified neurosurgeons can also perform programming at their clinic. The rate of increase in the number of implantations seems to be dull in the last several years (Fig. 8). One of the reasons would be that the number of neurologists certified for VNS is not enough. Therefore, more neurologists should join in this treatment to expand VNS therapy. VNS is substantially underutilized in Japan as compared to the United States. Numerous patients with medically refractory epilepsy cannot access this therapeutic option yet in Japan.
In addition to the Normal Mode and the Magnet Mode, the Model 106 (AspireSR) obtained the AutoStim Mode to automatically deliver an additional stimulation, triggered by a cardiac-based seizure-detection algorithm (SDA).32–36) Thence, the closed-loop system has been added to the primary open-loop system. The Model 106 always monitors heart rate, and determines the baseline heart rate as a moving average of the instantaneous heart rate over the previous 5 minutes and the foreground heart rate as a moving average of the most recent 10 seconds.35) When a heart rate increases with a seizure and exceeds a programmed threshold for at least 1 second, the AutoStim Mode begins stimulation. Then heart beat detection (HBD) identifying the R-waves on an electrocardiogram and SDA thresholds should be arranged for each patient. There are 5 HBD settings and 6 SDA thresholds from 20% through 70%.32,33)
Replacement of generators from previous models such as the Model 103 or 105 to the Model 106 is more frequent at present as mentioned above. Seventy percent of patients could have significant additional seizure reduction with the Model 106.37) Another study also revealed that VNS with AutoStim Mode achieved maintenance of prior-established seizure control with markedly less energy consumption and improved seizure control as compared to the previous models.38) Patients who did not respond to the previous models only with the open-loop system may obtain seizure reduction by replacement to the Model 106 even though output currents and duty cycles were reduced.39) However, these additional benefits and the reasons why VNS with autostimulation responding ictal tachycardia works well with less energy consumption, less output current, or less duty cycle are still unclarified. Further studies should be required.
The latest Model 1000 (SenTiva), which is not available in Japan yet, has gained more convenient features as compared to the present Model 106.1) Guided programming, day-night programming, and scheduled programming would be particularly beneficial for physicians and patients. These features may tailor VNS therapy to each patient’s demand.
Responsive neurostimulation
The RNS (RNS System, NeuroPace Inc., Mountain View, CA, USA) is gaining an important position in the treatment of medically refractory epilepsy (Figs. 9 and 10). RNS is not available outside the United States yet in 2020. However, the number of patients who undergo implantation of this device is steadily increasing (Fig. 11). The multicenter, double-blinded, randomized controlled trial of RNS demonstrated improvement in seizure frequency and responder rate over time. There was 44% seizure reduction after 1 year and 66% after 6 years. The responder rate increased from 44% at 1 year to 59% after 6 years of follow-up. Furthermore, patients showed median seizure reductions of 75% after 9 years of treatment.4,36,40–43) A real-world experience from the eight comprehensive epilepsy centers in the United States demonstrated that median seizure reductions were 67% at 1 year, 75% at 2 years, and 82% at ≥3 years in 150 patients treated with RNS. In addition, 35% of patients had a ≥90% seizure reduction, and 18% of patients obtained seizure freedom at the last follow-up. These results exceeded those in the clinical trials.44) Advantages of RNS are not only seizure reduction, and effects in quality of life, mood, and cognition. RNS chronically monitors patients’ brain activity, and reveals unprecedented insight into management and research of epilepsy itself.43)
Fig. 9.

An implantable part of the RNS System is comprised of a neurostimulator and depth and/or subdural cortical leads. The neurostimulator connects one or two leads placed surgically at the seizure focus. Courtesy of NeuroPace, Inc.
Fig. 10.

The cranially seated neurostimulator continually senses electrocorticographic activity, and then provides stimulation when it detects abnormal activity. Detection and stimulation are performed through the two leads, and these parameters are adjusted for optimizing control of seizures. Courtesy of NeuroPace, Inc.
Fig. 11.
Utilization of the RNS System in the United States. The line graph demonstrates the number of cumulative patients treated with the RNS System. Courtesy of NeuroPace, Inc.
It is often critical in patients with mesial temporal lobe epilepsy (MTLE) to determine the side of seizure onset. Then, reduction of medications and sleep deprivation are tried to induce seizures during the limited time of long-term EEG monitoring. However, these strategies may not reflect the patient’s true disease burden.45) In 82 patients who underwent implantation of RNS with bilateral mesial temporal leads for suspected bilateral MTLE, the time to record bilateral temporal onsets was 1st week in 36.2% and 2nd week in 17.4%. Therefore, about a half of patients with bilateral MTLE would be considered unilateral temporal lobe epilepsy within the common 1–2 week window of inpatient scalp EEG monitoring.45,46) Conversely, RNS demonstrated nine patients (11.0%) had only unilateral onsets. These patients were considered bilateral MTLE by inpatient EEG monitoring. Three of them underwent temporal lobectomy, and two patients obtained seizure freedom.45,46) RNS may provide further diagnostic information, because this system is an ambulatory setting and provides a habitual seizure burden. Then RNS may explode our established wisdom in epileptology.
Circadian rhythms of interictal epileptiform activity have been well analyzed by RNS.45,47,48) Rhythms were divided into ultradian (12h), circadian (24h), and multidien (multiday, most commonly 20–30 days in duration) periods. Seizures on ECoG were entrained to these rhythms with the highest risk of seizures when two critical phases were combined and lowest when multidien and circadian rhythms were both anti-phase.48) These findings would be useful to prevent seizures at potentially risky periods by timing and additional dosing of medications.45)
When AEDs are adjusted at a clinic, scalp EEG is often necessary to observe patients’ response. RNS can assess clinical responses to AEDs.49) Significant quantitative changes in ECoG data recorded by RNS were observed in patients who experienced an additional clinical response to a new AED. There was a significant reduction in the detection of epileptiform activity. ECoG data from RNS may provide early prediction of potential benefit with AED changes.45,49)
There are still unresolved issues in RNS. Lead placement and stimulation strategies are the main issues at present.45) Each epilepsy center has its own point of view, and uses RNS with each experience and preference. There is an extraordinarily large number of possible stimulation parameter combinations. Different patients likely require different parameters based on lead location, underlying pathology, EEG patterns, and so on.45) Although RNS has shown efficacy and safety on previous studies,4,40–43) complicated issues such as lead placement and stimulation strategies are now raised for discussion.45) Nonetheless, RNS seems to have potential to lead us to a new world of epilepsy treatment and research.
Deep brain stimulation
DBS for medically refractory epilepsy has been already approved in Europe and the United States. The indication by the US Food and Drug Administration is as follows: “Bilateral stimulation of the anterior nucleus of the thalamus (ANT) for epilepsy is indicated as an adjunctive therapy for reducing the frequency of seizures in individuals 18 years of age or older diagnosed with epilepsy characterized by partial onset seizure with or without secondary generalization that are refractory to three or more anti-epileptic medications.”5) The SANTE trial, a multicenter, double-blind, randomized trial of bilateral stimulation of the ANT for localization-related epilepsy demonstrated the efficacy and safety of ANT-DBS. In all, 110 adult patients with focal seizures including focal to bilateral tonic clonic seizures participated in this trial, which revealed the median seizure reduction from baseline was 40.4% in the stimulated group compared with 14.5% in the control group. The long-term data also showed the median seizure reduction at 1 year was 41% and 69% at 5 years.5) The mechanism of action of ANT-DBS remains unclear. From animal data, however, it is postulated that the disruption of corticothalamic transmission with anterior nucleus stimulation would prevent normal recruitment and synchronization into a generalized seizure.50) Consequently, suitable indications for DBS would be epilepsy with bilateral or widespread ictal onset.51)
Options of neurostimulation and decision-making
As adjunctive and palliative options in epilepsy treatment, all three modalities including VNS, RNS, and DBS will eventually be available in most developed countries.52) However, the choice among them will be an issue, because no comparative trials of these devices have been performed, and published data and clinical experience suggest comparative effectiveness.53) There would be little justification for using RNS or DBS prior to VNS, since less invasive methods would be preferred.52,54) In fact, there are large differences in the use of these devices even among the US epilepsy centers.54) Decision-making would be affected by their own experience or skepticism to these neurostimulation devices. According to the data from the National Association of Epilepsy Centers in 2012, several centers had zero neurostimulation implantations, even though they were level IV epilepsy centers. Some are more ambitious and more aggressive, some less ambitious and less aggressive.52,54) It seems to be impossible to make a standard of choice which would be generally accepted. Overall, long-term outcomes of neurostimulation do not show major differences in seizure control between the approaches chosen.55) At present, the choice should depend on considerations based on thorough preoperative evaluations. If a region of seizure onset is well defined, RNS will be preferred. For patients with multifocal epilepsy or extended regions of epileptogenesis, VNS or ANT-DBS would be a choice. If frontotemporal limbic areas play a role, ANT-DBS would be favored. A well-informed patient’s judgment may critically contribute to the choice of treatment with these devices.55)
Ablative Procedures
SEEG-guided radiofrequency-thermocoagulation
SEEG-guided RT-TC consists of coupling SEEG investigation with RF-TC stereotactic lesioning directly through the recording electrodes.56) It is very convenient because RF-TC lesioning can be performed at the end of the recording, and is indicated when conventional surgical approach to the ictal onset zone is not ideal. A single or multiple lesioning by coagulation should always be performed between contiguous electrode contacts. The power delivered by the generator should be increased until the impedance suddenly changes, which indicates that the thermocoagulation has occurred.57)
A total of 6 retrospective studies of 296 patients revealed the pooled seizure-free and responder rates were 23% and 58%, respectively. The greatest efficacy was observed in patients with periventricular nodular heterotopia and the lowest in patients with normal MRI findings.58) Additionally, a controlled study of temporal lobe epilepsy found that SEEG-guided RF-TC was inferior to anterior temporal lobectomy; none of the patients who underwent SEEG-guided RF-TC became seizure-free at 1 year and only 48% of them were responders.56,59) Although this surgical procedure is remarkably safe, it may be an alternative to resective surgery in a small subset of operable patients.56,60)
Laser interstitial thermal therapy
LITT, also known as stereotactic laser ablation (SLA) or laser thermal ablation, is another type of less invasive surgical technique. LITT is being effectively employed with adults and children with temporal lobe or lesional epilepsies across several US epilepsy centers.61)
On the day of the procedure, patients obtain a CT angiogram to avoid blood vessels like SEEG implantations. Patients undergo placement of an MR-compatible stereotactic head frame after administration of general endotracheal anesthesia. The frame is affixed to the cranium with four cranial pins, and volumetric image series of MRI were acquired. After transportation to an operating room, a stereotactic planning workstation is used to design trajectories. A craniotomy–durotomy is performed with a 3.2-mm twist drill. The laser applicator sheath is placed at the planned entry site and a laser fiber is inserted through the sheath. Patients go back to an MRI scanner to confirm precise placement of the laser probe. Then, ablation is carried out with continuous monitoring of MR thermal imaging, which shows the damage map in near real time.62,63)
LITT is often used for MTLE at present. A multicenter study demonstrated 134 of 231 patients (58.0%) achieved Engel class I outcomes at 1 year postoperatively and 96 of 167 patients (57.5%) achieved Engel I outcomes at 2 years.64) Overall, 48–67% of patients became seizure-free.7,62,65) Patients with initial failure to LITT obtained seizure freedom with subsequent resection surgery.7) In addition to the seizure outcomes, LITT may offer a significantly better cognitive outcome than open resection in many circumstances, presumably because this procedure allows for focal tissue ablation with minimal collateral damage.61) LITT is also far less invasive than open surgery with shorter hospital stay, less pain, and rapid return to normal activities.2) Therefore, LITT could be a major option particularly for patients with MTLE before undergoing resection surgery.
Conclusion
New technologies and concepts in epilepsy surgery are summarized. Expertise and funds are more necessary to implement the advancement. It seems to be a hard task to introduce even one technology such as SEEG. These technologies are rapidly changing, and then it is not straightforward to catch up in a timely manner. Each epilepsy center will be required to invest time and energy in developing equipment. Consequently, it would be unpredictable in the next decade whether we could come up to patients’ expectations. However, efficacy and safety of these new technologies and devices will always be essential for epilepsy patients, and these basics will remain unchanged in our foreseeable future.
Footnotes
Conflicts of Interest Disclosure
The author who is a member of the Japan Neurosurgical Society (JNS) reports no conflict of interest (COI) regarding this article and has made declaration of COI with a self-reported COI disclosure statement form to the JNS Office in the preceding three years.
References
- 1).Wheless JW, Gienapp AJ, Ryvlin P: Vagus nerve stimulation (VNS) therapy update. Epilepsy Behav 88: 2–10, 2018 [DOI] [PubMed] [Google Scholar]
- 2).Kang JY, Sperling MR: Epileptologist’s view: laser interstitial thermal ablation for treatment of temporal lobe epilepsy. Epilepsy Res 142: 149–152, 2018 [DOI] [PubMed] [Google Scholar]
- 3).Wennberg R, Ladino LD, Téllez-Zenteno JF: On the renaissance of stereotactic EEG and its interpretation. Can J Neurol Sci 45: 255–258, 2018 [DOI] [PubMed] [Google Scholar]
- 4).Morrell MJ: RNS System in Epilepsy Study Group: Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology 77: 1295–1304, 2011 [DOI] [PubMed] [Google Scholar]
- 5).Salanova V: Deep brain stimulation for epilepsy. Epilepsy Behav 88: 21–24, 2018 [DOI] [PubMed] [Google Scholar]
- 6).Bourdillon P, Devaux B, Job-Chapron AS, Isnard J: SEEG-guided radiofrequency thermocoagulation. Neurophysiol Clin 48: 59–64, 2018 [DOI] [PubMed] [Google Scholar]
- 7).Petito GT, Wharen RE, Feyissa AM, Grewal SS, Luca JA, Tatum WO: The impact of stereotactic laser ablation at a typical epilepsy center. Epilepsy Behav 78: 37–44, 2018 [DOI] [PubMed] [Google Scholar]
- 8).Olivier A, Boling WW, Tanriverdi T: Techniques in Epilepsy Surgery, The MNI Approach. ed 1 New York: Cambridge University Press, 2012 [Google Scholar]
- 9).Jayakar P, Gotman J, Harvey AS, et al. : Diagnostic utility of invasive EEG for epilepsy surgery: indications, modalities, and techniques. Epilepsia 57: 1735–1747, 2016 [DOI] [PubMed] [Google Scholar]
- 10).Skoch J, Adelson PD, Bhatia S, et al. : Subdural grid and depth electrode monitoring in pediatric patients. Epilepsia 58: 56–65, 2017 [DOI] [PubMed] [Google Scholar]
- 11).Reif PS, Strzelczyk A, Rosenow F: The history of invasive EEG evaluation in epilepsy patients. Seizure 41: 191–195, 2016 [DOI] [PubMed] [Google Scholar]
- 12).Joswig H, Steven DA, Parrent AG, et al. : Intracranial electroencephalographic monitoring: from subdural to depth electrodes. Can J Neurol Sci 45: 336–338, 2018 [DOI] [PubMed] [Google Scholar]
- 13).Almeida AN, Martinez V, Feindel W: The first case of invasive EEG monitoring for the surgical treatment of epilepsy: historical significance and context. Epilepsia 46: 1082–1085, 2005 [DOI] [PubMed] [Google Scholar]
- 14).Penfield W, Jasper H: Epilepsy and the Functional Anatomy of the Human Brain. Boston: Little, Brown & Co, 1954, p 764 [Google Scholar]
- 15).Wyler AR, Ojemann GA, Lettich E, Ward AA: Subdural strip electrodes for localizing epileptogenic foci. J Neurosurg 60: 1195–1200, 1984 [DOI] [PubMed] [Google Scholar]
- 16).Hayne R, Meyers R: An improved model of a human stereotaxic instrument. J Neurosurg 7: 463–466, 1950 [DOI] [PubMed] [Google Scholar]
- 17).Harary M, Cosgrove GR: Jean Talairach: a cerebral cartographer. Neurosurg Focus 47: E12, 2019. doi.org/10.3171/2019.6.FOCUS19320 [DOI] [PubMed] [Google Scholar]
- 18).Kelly PJ: Stereotactic navigation, Jean Talairach, and I. Neurosurgery 54: 454–463, 2004 [DOI] [PubMed] [Google Scholar]
- 19).Kahane P, Landré E, Minotti L, Francione S, Ryvlin P: The Bancaud and Talairach view on the epileptogenic zone: a working hypothesis. Epileptic Disord 8: S16–S26, 2006 [PubMed] [Google Scholar]
- 20).Tandon N, Tong BA, Friedman ER, et al. : Analysis of morbidity and outcomes associated with use of subdural grids vs stereoelectroencephalography in patients with intractable epilepsy. JAMA Neurol 76: 672–681, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Mullin JP, Shriver M, Alomar S, et al. : Is SEEG safe? A systematic review and meta-analysis of stereo-electroencephalography-related complications. Epilepsia 57: 386–401, 2016 [DOI] [PubMed] [Google Scholar]
- 22).McGovern RA, Ruggieri P, Bulacio J, Najim I, Bingaman WE, Gonzalez-Martinez JA: Risk analysis of hemorrhage in stereo-electroencephalography procedures. Epilepsia 60: 571–580, 2019 [DOI] [PubMed] [Google Scholar]
- 23).Arya R, Mangano FT, Horn PS, Holland KD, Rose DF, Glauser TA: Adverse events related to extraoperative invasive EEG monitoring with subdural grid electrodes: A systemic review and meta-analysis. Epilepsia 54: 828–839, 2013 [DOI] [PubMed] [Google Scholar]
- 24).Tebo CC, Evins AI, Christos PJ, Kwon J, Schwartz TH: Evolution of cranial epilepsy surgery complication rates: a 32-year systematic review and meta-analysis. J Neurosurg 120: 1415–1427, 2014 [DOI] [PubMed] [Google Scholar]
- 25).Yan H, Katz JS, Anderson M, et al. : Method of invasive monitoring in epilepsy surgery and seizure freedom and morbidity: A systematic review. Epilepsia 60: 1960–1972, 2019 [DOI] [PubMed] [Google Scholar]
- 26).Wang YC, Grewal SS, Goyal A, et al. : Comparison of narcotic pain control between stereotactic electrocorticography and subdural grid implantation. Epilepsy Behav 103: 106843, 2020. [DOI] [PubMed] [Google Scholar]
- 27).Cardinale F, Cossu M, Castana L, et al. : Stereoelectroencephalography: surgical methodology, safety, and stereotactic application accuracy in 500 procedures. Neurosurgery 72: 353–366, 2013 [DOI] [PubMed] [Google Scholar]
- 28).González-Martínez J, Bulacio J, Thompson S, et al. : Technique, results, and complications related to robot-assisted stereoelectroencephalography. Neurosurgery 78: 169–180, 2016 [DOI] [PubMed] [Google Scholar]
- 29).De Benedictis A, Trezza A, Carai A, et al. : Robot-assisted procedures in pediatric neurosurgery. Neurosurg Focus 42: E7, 2017. doi.org/10.10.3171/2017.2.FOCUS16579 [DOI] [PubMed] [Google Scholar]
- 30).Podkorytova I, Hoes K, Lega B: Stereo-encephalography versus subdural electrodes for seizure localization. Neurosurg Clin N Am 27: 97–109, 2016 [DOI] [PubMed] [Google Scholar]
- 31).Kawai K, Tanaka T, Baba H, et al. : Outcome of vagus nerve stimulation for drug-resistant epilepsy: the first three years of a prospective Japanese registry. Epileptic Disord 19: 327–338, 2017 [DOI] [PubMed] [Google Scholar]
- 32).Boon P, Vonck K, van Rijckevorsel K, et al. : A prospective, multicenter study of cardiac-based seizure detection to activate vagus nerve stimulation. Seizure 32: 52–61, 2015 [DOI] [PubMed] [Google Scholar]
- 33).Schneider UC, Bohlmann K, Vajkoczy P, Straub H-S: Implantation of a new Vagus Nerve Stimulation (VNS) TherapyR generator, AspireSRR: considerations and recommendations during implantation and replacement surgery – comparison to a traditional system. Acta Neurochir (Wien) 157: 721–728, 2015 [DOI] [PubMed] [Google Scholar]
- 34).Fisher RS, Afra P, Macken M, et al. : Automatic vagus nerve stimulation triggered by ictal tachycardia: clinical outcomes and device performance – The U.S. E-37 Trial. Neuromodulation 19: 188–195, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Tahry RE, Hirsch M, Rijckevorsel KV, et al. : Early experiences with tachycardia-triggered vagus nerve stimulation using the AspireSR stimulator. Epileptic Disord 18: 155–162, 2016 [DOI] [PubMed] [Google Scholar]
- 36).Yamamoto T, Inaji M, Maehara T, Kawai K, Doyle WK: New therapeutic modalities using seizure detection devices for medically refractory epilepsy: AspireSR and the RNS System. No Shinkei Geka 46: 247–262, 2018. (Japanese) [DOI] [PubMed] [Google Scholar]
- 37).Hamilton P, Soryal I, Dhahri P, et al. : Clinical outcomes of VNS therapy with AspireSRR (including cardiac-based seizure detection) at a large complex epilepsy and surgery centre. Seizure 58: 120–126, 2018 [DOI] [PubMed] [Google Scholar]
- 38).Kulju T, Haapasalo J, Rainesalo S, Lehtimäki K, Peltola J: Autostimulation in vagus nerve stimulator treatment: modulating neuromodulation. Neuromodulation 22: 630–637, 2019. doi.org/10.10.1111/ner.12897. Epub 2018 Dec 14. [DOI] [PubMed] [Google Scholar]
- 39).Kawaji H, Yamamoto T, Fujimoto A, et al. : Additional seizure reduction by replacement with Vagus Nerve Stimulation Model 106 (AspireSR). Neurosci Lett 716: 134636, 2020. [DOI] [PubMed] [Google Scholar]
- 40).Heck CN, King-Stephens D, Massey AD, et al. : Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS System Pivotal trial. Epilepsia 55: 432–441, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Bergey GK, Morrell MJ, Mizrahi EM, et al. : Long-term treatment with responsive brain stimulation in adults with refractory partial seizures. Neurology 84: 810–817, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Matias CM, Sharan A, Wu C: Responsive neurostimulation for the treatment of epilepsy. Neurosurg Clin N Am 30: 231–242, 2019 [DOI] [PubMed] [Google Scholar]
- 43).Skarpaas TL, Jarosiewicz B, Morell MJ: Brain-responsive neurostimulation for epilepsy (RNS® System). Epilepsy Res 153: 68–70, 2019 [DOI] [PubMed] [Google Scholar]
- 44).Razavi B, Rao VR, Lin C, et al. : Real-world experience with direct brain-responsive neurostimulation for focal onset seizures. Epilepsia 61: 1749–1757, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Geller EB: Responsive neurostimulation: review of clinical trials and insights into focal epilepsy. Epilepsy Behav 88S: 11–20, 2018 [DOI] [PubMed] [Google Scholar]
- 46).King-Stephens D, Mirro E, Weber PB, et al. : Lateralization of mesial temporal lobe epilepsy with chronic ambulatory electrocorticography. Epilepsia 56: 959–967, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47).Anderson CT, Tcheng TK, Sun FT, Morrell MJ: Day-night patterns of epileptiform activity in 65 patients with long-term ambulatory electrocorticography. J Clin Neurophysiol 32: 406–412, 2015 [DOI] [PubMed] [Google Scholar]
- 48).Baud MO, Kleen JK, Mirro EA, et al. : Multi-day rhythms modulate seizure risk in epilepsy. Nat Commun 9: 88, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49).Skarpaas TL, Tcheng TK, Morrell MJ: Clinical and electrocorticographic response to antiepileptic drugs in patients treated with responsive stimulation. Epilepsy Behav 83: 192–200, 2018 [DOI] [PubMed] [Google Scholar]
- 50).Mirski MA, Rossell LA, Terry JB, Fisher RS: Anticonvulsant effect of anterior thalamic high frequency electrical stimulation in the rat. Epilepsy Res 28: 89–100, 1997 [DOI] [PubMed] [Google Scholar]
- 51).Klinger NV, Mittal S: Clinical efficacy of deep brain stimulation for the treatment of medically refractory epilepsy. Clin Neurol Neurosurg 140: 11–25, 2016 [DOI] [PubMed] [Google Scholar]
- 52).Benbadis SR, Geller E, Ryvlin P, et al. : Putting it all together: options for intractable epilepsy. An updated algorithm on the use of epilepsy surgery and neurostimulation. Epilepsy Behav 88S: 33–38, 2018 [DOI] [PubMed] [Google Scholar]
- 53).Dibué-Adjei M, Kamp MA, Vonck K: 30 years of vagus nerve stimulation trials in epilepsy: do we need neuromodulation-specific trial designs? Epilepsy Res 153: 71–75, 2019 [DOI] [PubMed] [Google Scholar]
- 54).Benbadis S, Helmers S, Hirsch L, Sirven J, Vale FL, Wheless J: Yes, neurostimulation has a role in the management of epilepsy. Neurology 83: 845–847, 2014 [DOI] [PubMed] [Google Scholar]
- 55).Schulze-Bonhage A: Long-term outcome in neurostimulation of epilepsy. Epilepsy Behav 91: 25–29, 2019 [DOI] [PubMed] [Google Scholar]
- 56).Bourdillon P, Rheims S, Catenoix H, et al. : Surgical techniques: stereoelectroencephalography-guided radiofrequency-thermocoagulation (SEEG-guided RF-TC). Seizure 77: 64–68, 2020 [DOI] [PubMed] [Google Scholar]
- 57).Bourdillon P, Devaux B, Job-Chapron AS, Isnard J: SEEG-guided radiofrequency thermocoagulation. Neurophysiol Clin 48: 59–64, 2018 [DOI] [PubMed] [Google Scholar]
- 58).Bourdillon P, Cucherat M, Isnard J, et al. : Stereo-electroencephalography-guided radiofrequency thermocoagulation in patients with focal epilepsy: a systematic review and meta-analysis. Epilepsia 59: 2296–2304, 2018 [DOI] [PubMed] [Google Scholar]
- 59).Moles A, Guénot M, Rheims S, et al. : SEEG-guided radiofrequency coagulation (SEEG-guided RF-TC) versus anterior temporal lobectomy (ATL) in temporal lobe epilepsy. J Neurol 265: 1998–2004, 2018 [DOI] [PubMed] [Google Scholar]
- 60).Cossu M, Cardinale F, Casaceli G, et al. : Stereo-EEG-guided radiofrequency thermocoagulations. Epilepsia 58: 66–72, 2017 [DOI] [PubMed] [Google Scholar]
- 61).Drane DL: MRI-guided stereotactic laser ablation for epilepsy surgery: promising preliminary results for cognitive outcome. Epilepsy Res 142: 170–175, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62).Donos C, Breier J, Friedman E, et al. : Laser ablation for mesial temporal lobe epilepsy: surgical and cognitive outcomes with and without mesial temporal sclerosis. Epilepsia 59: 1421–1432, 2018 [DOI] [PubMed] [Google Scholar]
- 63).Willie JT, Laxpati NB, Drane DL, et al. : Real-time magnetic resonance-guided stereotactic laser amygdalohippocampectomy for mesial temporal epilepsy. Neurosurgery 74: 569–585, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64).Wu C, Jermakowicz WJ, Chakravorti S, et al. : Effects of surgical targeting in laser interstitial thermal therapy for mesial temporal lobe epilepsy: a multicenter study of 234 patients. Epilepsia 60: 1171–1183, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65).Grewal SS, Zimmerman RS, Worrell G, et al. : Laser ablation for mesial temporal epilepsy: a multi-site, single institutional series. J Neurosurg 130: 2055–2062, 2019 [DOI] [PubMed] [Google Scholar]