Abstract
Purpose
To characterize the post-marketing reporting of serotonin syndrome (SS) due to drug-drug interactions (DDIs) with linezolid and investigate the relationship with pharmacokinetic/pharmacodynamic (PK/PD) properties of serotonergic agents.
Methods
We queried the worldwide FDA Adverse Event Reporting System to extract SS records due to DDIs where linezolid was reported as suspect. For each serotonergic agent concomitantly reported, proportion of SS reports and mean number of DDIs were calculated and three different “SS reporting zones” were created. Relevant PK (peak concentration, area under plasma concentration curve, volume of distribution (VD), and lipophilicity) and PD (values of binding affinity (Ki) and IC50 for serotonin reuptake transporter (SERT) and 5-HT2A) parameters were extracted for each serotonergic agent, and relevant PK/PD indexes were calculated to assess correlation with mean number of DDIs (PV index).
Results
Six hundred sixty-nine reports of SS mentioning linezolid were found, being linezolid-citalopram (N = 69; 10.3%) the most frequently DDI reported. Citalopram and methadone showed respectively the highest proportion of SS reports (0.28%) and the lowest mean number of DDIs (1.41). Citalopram, escitalopram, and methadone emerged as red (i.e., alert)-zone medications: they exhibited high lipophilicity and large VD (proxies of excellent central nervous system penetration) coupled with high potency. Among PK/PD indexes, a significant correlation with PV index was found for VD/Ki SERT ratio (p = 0.05).
Discussion
Our integrated approach suggests that linezolid is more likely to cause SS when co-administered with citalopram, escitalopram, and methadone, as inferred from their pharmacological properties. Proper management of SS should be tailored on a case-by-case basis.
Electronic supplementary material
The online version of this article (10.1007/s00228-020-02990-1) contains supplementary material, which is available to authorized users.
Keywords: Linezolid, Serotonin syndrome, Drug-drug interactions, Pharmacovigilance-pharmacokinetic/pharmacodynamic approach, Infectious disease “dilemma”
Introduction
Linezolid is an oxazolidinone antibiotic with activity against multidrug-resistant Gram-positive organisms [1], showing lipophilic features, excellent tissue penetration including the central nervous system (CNS), and weak reversible non-selective monoamine oxidase (MAO) inhibitory effects at therapeutic serum concentrations (according to an inhibitory binding affinity constant (Ki) of 56 μM and 0.71 μM, respectively for MAO-A and MAO-B) [2, 3]. MAO is involved in the metabolism of the monoamine neurotransmitters, and its inhibition may potentially lead to excess of serotonin (5-hydroxytriptamine (5-HT)) in the CNS and occurrence of serotonin syndrome (SS), a potential life-threatening condition [4, 5]. Inhibition of the serotonin reuptake transporter (SERT), causing accumulation of serotonin in the synaptic cleft and overstimulation of 5-HT2A receptors, is supposed to be the main mechanism involved in SS [5].
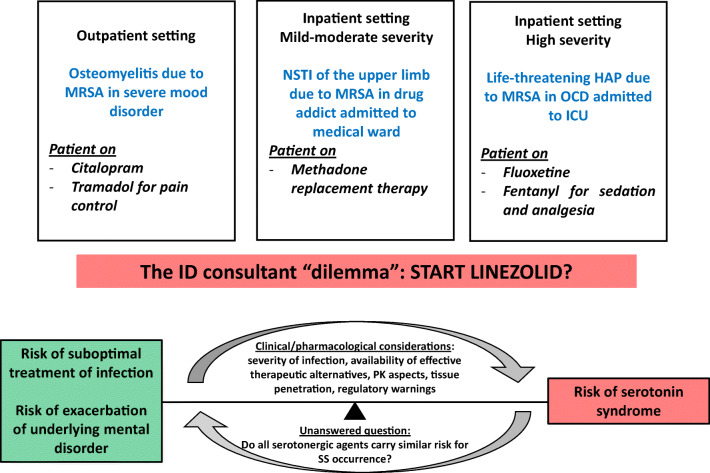
Several drugs may cause serotonin excess through different pathways [6], thus posing concerns when concomitant treatments with linezolid are needed. Although reports describing occurrence of SS due to drug-drug interactions (DDIs) with linezolid were reported [3, 7, 8], and the Food and Drug Administration (FDA) warned against co-administration of linezolid with serotonergic drugs recommending a 2-week washout period in patients already receiving these medications [9], treatment of life-threatening infections may not be delayed, especially if second-line alternatives are not effective or available. However, it remains unclear whether all serotonergic agents carry similar risk of SS, and the infectious disease consultant faces a “dilemma,” with relevant implications on rational drug use in specific clinical scenarios, as exemplified in Fig. 1.
Fig. 1.
Examples of clinical scenarios posing a “dilemma” to the infectious disease consultant regarding the use of linezolid in patients on serotonergic agents (MRSA, methicillin-resistant Staphylococcus aureus; NSTI, necrotizing soft tissue infection; HAP, hospital-acquired pneumonia; OCD, obsessive-compulsive disorder; ICU, intensive care unit; ID, infectious disease; PK, pharmacokinetic; SS, serotonin syndrome)
In order to address this aspect and provide clues to help in decision-making, we investigated the relationship between real-world data, namely, spontaneous reports submitted to the US FDA adverse event reporting system (FAERS) database, and pharmacokinetic/pharmacodynamic (PK/PD) properties of serotonergic agents.
Materials and methods
We carried out a “two-step” approach to investigate whether the reporting pattern of SS induced by linezolid is potentially related to the burden of co-medications resulting in clinically significant DDIs, and relevant PK/PD properties of serotonergic agents, an emerging approach recently proposed to assess the pharmacological basis of adverse events (AEs) observed in a worldwide pharmacovigilance (PV) database [10–12].
First, the FAERS public dashboard database, the US repository of AEs and medication errors comprising more than 18 million reports gathered worldwide, was queried to retrieve SS reports recorded until December 2019, using “serotonin syndrome” as preferred term, to extract only those records where linezolid was reported as suspect. In order to select potential DDIs, co-administered serotonergic agents reported as suspect were identified according to the lists proposed by Bower et al. [6] and Woytowish et al. [13]. Post-marketing studies using the FAERS are effective for continuous monitoring of old medications [14], including the analysis of potential DDIs [15], with noteworthy performance (i.e., the capacity to discriminate true from positive drug-event associations) [16], especially for side effects with low/rare background incidence and a likely drug-attributable component such as SS.
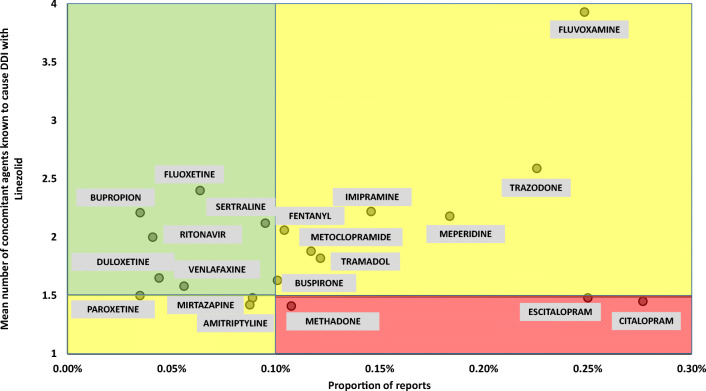
For each serotonergic agent concomitantly reported with linezolid in ≥ 5 cases of SS, two indexes were created: (a) proportion of SS reports, as compared with the overall number of reports, and (b) mean number of DDIs, based on concomitant serotonergic agents recorded in SS reports, being 1 the value corresponding to the single interaction between linezolid and the drug of interest. A scatterplot showing the relationship between the two indexes for each serotonergic agent was created. Threshold values of ≥ 0.1% and ≤ 1.5 were respectively selected for proportion of SS reports (according to 0.09% incidence of SS in a retrospective study including patients exposed to serotonergic medications [17]) and mean number of DDIs (based on the estimation that in almost half of records, no other concomitant serotonergic agent was reported, except for drug of interest), thus identifying three different “SS reporting zones”:
“red (i.e., alert)-zone,” including medications with high proportion of SS reports and low mean number of DDIs
“yellow-zone,” including medications with high proportion of SS reports coupled with high mean number of DDIs or low proportion of SS reports coupled with low mean number of DDIs
“green-zone,” including medications with low proportion of SS reports and high mean number of DDIs
In the second step, physiochemical/PK (peak concentration (Cmax), area under plasma concentration curve (AUC), volume of distribution (VD), and lipophilicity (LogP)) and PD (values of Ki and IC50 (concentration corresponding to 50% inhibition of activity in vitro) for SERT and 5-HT2A) data of each selected serotonergic agents were extracted from MEDLINE/PubMed and DrugBank databases [18] (searches performed on April 22, 2020). For PK parameters, the highest mean value reported for each medication when administered at therapeutic dosage was selected. The following PK/PD indexes were created: Cmax/Ki SERT, Cmax/IC50 SERT, Cmax/Ki 5-HT2A, AUC/Ki SERT, AUC/IC50 SERT, AUC/Ki 5-HT2A, VD/Ki SERT, VD /IC50 SERT, VD/Ki 5-HT2A, LogP/Ki SERT, LogP/IC50 SERT, and LogP/Ki 5-HT2A.
Correlation between PK/PD indexes and mean number of DDIs (PV index) was assessed by calculating Spearman’s rank correlation coefficient, considering significant a p value < 0.05.
Results
Overall, 11,429 reports of SS were found, 669 (5.9%) of which mentioning linezolid as suspect agent. Mean age was 55 ± 26.3 years, with no gender preponderance; 99.1% of reports were serious (i.e., resulting in death, hospitalization, or life-threatening event), and death was reported in 41 cases (18 with citalopram co-administration).
Single DDI was reported in 366 cases (54.7%), being linezolid-citalopram (N = 69; 10.3%) and linezolid-fentanyl (46; 6.9%) the most frequent, while multiple DDIs were reported in 179 cases (26.8%), being linezolid-citalopram-tramadol (23; 3.4%) the predominant (Supplementary Table 1). In 124 reports (18.5%), no concomitant serotonergic medications were recorded. Citalopram (N = 112), fentanyl (N = 100), sertraline (N = 74), escitalopram (N = 65), and tramadol (N = 51) were the most frequently serotonergic agents mentioned as suspect with linezolid in cases of SS.
Citalopram and methadone showed respectively the highest proportion of SS reports (0.277%) and the lowest mean number of DDIs (1.41; Supplementary Table 2). Citalopram, escitalopram, and methadone emerged as red-zone medications (Fig. 2). Additionally, amitriptyline (1.42), mirtazapine (1.48), and paroxetine (1.5) also exhibited a mean number of DDIs ≤ 1.5, although classified as “yellow-zone” agents due to their low proportion of SS reports.
Fig. 2.
Scatterplot showing the relationship between the proportion of SS reports (on x-axis) and the mean number of DDIs (on y-axis) for each serotonergic agent. Threshold values of ≥ 0.1% and ≤ 1.5 were respectively selected for proportion of SS reports and mean number of DDIs, identifying three different SS risk zones (red-zone, high-risk medications; yellow-zone, intermediate-risk medications; green-zone, low-risk medications)
A summary of PK/PD properties of serotonergic medications is shown in Supplementary Table 3. Among antidepressants, venlafaxine and sertraline showed respectively the highest VD and the lowest IC50 for SERT, while methadone exhibited both the highest VD and LogP, as well as the lowest IC50 for SERT among opioids.
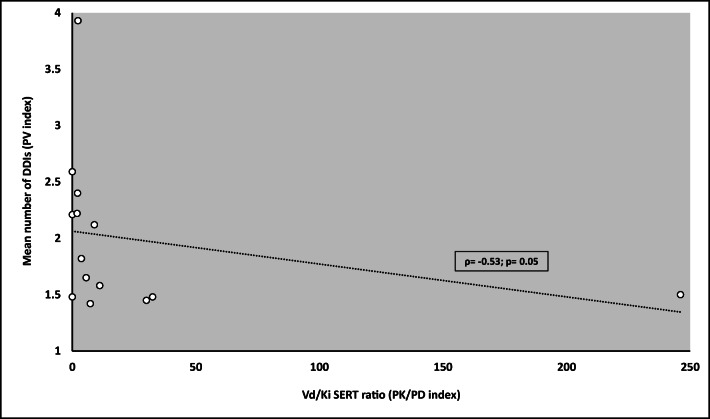
A significant correlation between PK/PD and PV indexes was found for the VD/Ki SERT ratio (ρ = − 0.53; p = 0.05; Fig. 3), while a trend was observed for the VD/IC50 SERT (ρ = − 0.405; p = 0.11), LogP/Ki SERT (ρ = − 0.504; p = 0.07), and LogP/IC50 SERT ratios (ρ = − 0.399; p = 0.09; Table 1). No significant correlation emerged between PK/PD indexes investigating plasma exposure of serotonergic agents (namely, Cmax and AUC) or binding affinity for 5-HT2A and PV index (Table 1).
Fig. 3.
Scatterplot showing the relationship between VD/Ki SERT ratio (PK/PD index; x-axis) and mean number of DDIs (PV index; y-axis). A significant correlation was found (ρ = − 0.53; p = 0.05)
Table 1.
Summary of correlation between PK/PD indexes (independent variable) and PV index (mean number of DDIs; dependent variable)
| PK/PD – PV correlation |
ρ value (95% CI) |
p value | Interpretation |
|---|---|---|---|
|
Cmax/Ki SERT – mean number of DDIs (N = 14) |
− 0.334 (− 0.735–0.238) |
0.24 | No significant correlation |
|
Cmax/IC50 SERT – mean number of DDIs (N = 19) |
− 0.20 (− 0.60–0.28) |
0.41 | No significant correlation |
|
Cmax/Ki 5-HT2A – mean number of DDIs (N = 17) |
0.002 (− 0.479–0.483) |
0.99 | No significant correlation |
|
AUC/Ki SERT – mean number of DDIs (N = 14) |
− 0.374 (− 0.755–0.195) |
0.19 | No significant correlation |
|
AUC/IC50 SERT – mean number of DDIs (N = 18) |
− 0.376 (− 0.717–0.11) |
0.12 | Trend to significant correlation (agents with high exposure on time and high potency on SERT are more likely to cause SS when used individually with linezolid) |
|
AUC/Ki 5-HT2A – mean number of DDIs (N = 17) |
− 0.134 (− 0.577–0.371) |
0.61 | No significant correlation |
|
VD/Ki SERT – mean number of DDIs (N = 14) |
− 0.53 (− 0.828–0.00) |
0.05 | Significant correlation (agents with high VD and high potency on SERT are more likely to cause SS when used individually with linezolid) |
|
VD /IC50 SERT – mean number of DDIs (N = 17) |
− 0.405 (− 0.741–0.094) |
0.11 | Trend to significant correlation (agents with high VD and high potency on SERT are more likely to cause SS when used individually with linezolid) |
|
VD/Ki 5-HT2A – mean number of DDIs (N = 17) |
− 0.163 (− 0.597–0.345) |
0.53 | No significant correlation |
|
LogP/Ki SERT – mean number of DDIs (N = 14) |
− 0.504 (− 0.816–0.037) |
0.07 | Trend to significant correlation (agents with high lipophilicity and high potency on SERT are more likely to cause SS when used individually with linezolid) |
|
LogP/IC50 SERT – mean number of DDIs (N = 19) |
− 0.399 (− 0.722–0.067) |
0.09 | Trend to significant correlation (agents with high lipophilicity and high potency on SERT are more likely to cause SS when used individually with linezolid) |
|
LogP/Ki 5-HT2A – mean number of DDIs (N = 17) |
− 0.123 (− 0.57–0.38) |
0.64 | No significant correlation |
Spearman’s rank correlation coefficient was calculated, and a p value < 0.05 was considered significant
N number of serotonergic medications for which specific PK/PD parameters are retrieved, PK pharmacokinetic, PD pharmacodynamic, PV pharmacovigilance, Cmax peak concentration, AUC area under plasma concentration curve, VD volume of distribution, Ki binding affinity, IC50 concentration corresponding to 50% inhibition of activity in vitro, SERT serotonin reuptake transporter, DDIs drug-drug interactions, SS serotonin syndrome
Discussion
Our real-world study, by investigating the worldwide reporting of SS with linezolid and concomitant serotonergic drugs and analyzing their potential contributing role in the light of relevant PK/PD properties, provides some clues towards safer prescribing. Although previously proposed [10–12], to the best of our knowledge, this is the first study in which this PV-PK/PD approach combined multiple sources of data and also accounted for the contributing role of DDIs.
Citalopram, escitalopram, and methadone emerged as “red-light drugs” according to PV indexes, given the high proportion of reports coupled with their potential to precipitate SS when mainly administered with linezolid. Notably, these findings are closely correlated with PK/PD features, thus confirming the role of SERT inhibition as key molecular pathway involved in SS [5, 11] and suggesting that agents with high binding affinity (low Ki or IC50) for this transporter and large VD or LogP (high lipophilicity to effectively cross the blood-brain barrier) are more likely to precipitate the disease [19]. Consequently, it is expected that agents showing a low mean number of DDIs should exhibit a large VD/Ki SERT ratio (high CNS penetration and high potency), as found in our analysis. This is the case of citalopram, escitalopram, and methadone, which, by virtue of their PK/PD properties, are more likely to cause SS when used individually with linezolid, in contrast with other serotonergic agents, which were reported in SS cases only under polypharmacy regimen [20–22]. Our results are in line with those of recently proposed systematic bioinformatics approach [23], which also indicated SERT inhibition by methadone as a key mechanistic link to SS. Conversely, our findings showed non-significant correlation between Cmax, AUC, and PV indices, as well as 5-HT2A seams to play a minor role in comparison to SERT in the occurrence of SS. Consequently, our analysis of the different proposed PK/PD indexes likely suggested that penetration in the CNS of a specific serotonergic agent, coupled with its potency on SERT inhibition, could be more important than plasma exposure in precipitating SS when co-administered with linezolid.
Additionally, different implications could be suggested for the two “yellow-zones.” Particularly, the one including agents with low proportion of SS reports and low mean number of DDIs, namely, paroxetine, amitriptyline, and mirtazapine, could be considered as a zone exhibiting an intermediate reporting risk between the other “yellow-zone” (including agents with high proportion of SS reports and high mean number of DDIs) and the “red-zone”, due to the ability of these serotonergic medications in precipitating SS mainly when co-administered with linezolid in absence of other serotonergic drugs.
The regulatory recommendation of a reasonable washout period when starting linezolid after serotonergic agents poses a clinical challenge when prompt treatment of serious infections is needed. Given its potent activity against multidrug-resistant Gram-positive pathogens and excellent tissue penetration [24], linezolid represents the first-line agent for management of several deep-seated infections (i.e., pneumonia, meningitis, osteomyelitis) for which potential alternatives (namely, daptomycin, ceftaroline, ceftobiprole) may exhibit PK disadvantages [25, 26].
We put forward four main determinants to be considered in tailored risk-benefit assessment depending on clinical scenario: (1) color zone of the different serotonergic medications according to PV indexes and PK/PD properties, (2) severity of infection, (3) availability of effective therapeutic alternatives, and (4) risk of worsening of underlying mental disorder.
In the setting of an intubated and deeply sedated critical patient requiring treatment of a life-threatening infection, antidepressant therapy could be abruptly withdrawn, and sedation with extemporaneous or long-term administration of fentanyl or meperidine (yellow-zone medications) could be replaced with morphine. Conversely, discontinuing citalopram/escitalopram in a patient affected by severe mood disorder requiring prolonged treatment with linezolid for a spondylodiscitis could be challenging. In this scenario, exacerbation of mental illness and suicide risk could exceed the likelihood of SS occurrence, thus requiring close monitoring while maintaining both treatments. Similarly, linezolid should be promptly initiated under strict monitoring in treatment of severe pneumonia despite concomitant methadone replacement therapy, according to severity of infection and the risk for opiate withdrawal syndrome in case of methadone discontinuation.
We acknowledge the limitations of this study, related to both pharmacovigilance analyses, including FAERS data (e.g., quality of reports, potential existence of remaining duplicates, reporting biases, lack of exposure data, inability in establishing firm causality between drug exposure and occurrence of SS, absence of data to calculate the time to onset from drug administration in the public FAERS dashboard, and limited verification of events through clinical features [27, 28]), and pharmacokinetic-pharmacodynamic assessment. Additionally, absolute incidence of SS cannot be inferred by analyzing FAERS data. Consequently, although we calculated proportion of SS reports for each serotonergic agent, our index was not intended to assess absolute incidence of SS, but it would only provide a relative proportion as a percentage of overall reports for a specific agent. Moreover, SS may be underdiagnosed due to lack of recognition, thus likely increasing the extent of under-reporting, a well-known phenomenon. Furthermore, heterogeneity of PK/PD studies in assessing the different parameters have to be considered. Particularly, the value of VD may be affected by serotonergic agents subjected to high pre-systemic extraction after oral dosage. However, a trend to significant correlation was also found for indexes correlating lipophilicity (LogP) and binding affinity to SERT (Ki or IC50), thus supporting the hypothesis that agents with high lipophilicity and penetration in CNS coupled with high potency on SERT are more likely to precipitate the disease when used individually with linezolid.
In summary, citalopram, escitalopram, and methadone emerged as “red-light medications,” according to their PV indices. High SERT inhibitory potency coupled with large VD and high lipophilicity, proxies for excellent CNS penetration, may explain the role of “red-light drugs” in precipitating SS when individually co-administered with linezolid. Proper management of SS, including drug discontinuation and/or strict monitoring, should be tailored on a case-by-case basis.
Electronic supplementary material
(DOCX 29 kb)
Acknowledgements
Open access funding provided by Alma Mater Studiorum - Università di Bologna within the CRUI-CARE Agreement.
Author’s contributions
MG and ER made substantial contributions to conception and design of the study. MG made substantial contributions to acquisition and analysis of data. MG, ER, and FDP made substantial contributions in interpretation of data. MG was involved in drafting the manuscript. ER and FDP made substantial contributions in revising the manuscript critically for important intellectual content. All authors approved the final version of the manuscript.
Funding
ER and FDP at the University of Bologna are supported by institutional research funds (Ricerca Fondamentale Orientata).
Data availability
Data supporting the findings of this study were derived from the following resource available in the public domain: https://fis.fda.gov/sense/app/d10be6bb-494e-4 cd2-82e4-0135608ddc13/sheet/7a47a261-d58b-4203-a8aa-6d3021737452/state/analysis.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zahedi Bialvaei A, Rahbar M, Yousefi M, Asgharzadeh M, Samadi KH. Linezolid: a promising option in the treatment of Gram-positives. J Antimicrob Chemother. 2017;72(2):354–364. doi: 10.1093/jac/dkw450. [DOI] [PubMed] [Google Scholar]
- 2.Pea F. Intracellular pharmacokinetics of antibacterials and their clinical implications. Clin Pharmacokinet. 2018;57(2):177–189. doi: 10.1007/s40262-017-0572-y. [DOI] [PubMed] [Google Scholar]
- 3.Lawrence KR, Adra M, Gillman PK. Serotonin toxicity associated with the use of linezolid: a review of postmarketing data. Clin Infect Dis. 2006;42(11):1578–1583. doi: 10.1086/503839. [DOI] [PubMed] [Google Scholar]
- 4.Quinn DK, Stern TA. Linezolid and serotonin syndrome. Prim Care Companion J Clin Psychiatry. 2009;11(6):353–356. doi: 10.4088/PCC.09r00853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Francescangeli J, Karamchandani K, Powell M, Bonavia A (2019) The serotonin syndrome: from molecular mechanisms to clinical practice. Int J Mol Sci 20(9) [DOI] [PMC free article] [PubMed]
- 6.Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112–1120. doi: 10.1056/NEJMra041867. [DOI] [PubMed] [Google Scholar]
- 7.Morales-Molina JA, Mateu-de Antonio J, Marín-Casino M, Grau S. Linezolid-associated serotonin syndrome: what we can learn from cases reported so far. J Antimicrob Chemother. 2005;56(6):1176–1178. doi: 10.1093/jac/dki368. [DOI] [PubMed] [Google Scholar]
- 8.Abadie D, Rousseau V, Logerot S, Cottin J, Montastruc JL, Montastruc F. Serotonin syndrome: analysis of cases registered in the French pharmacovigilance database. J Clin Psychopharmacol. 2015;35(4):382–388. doi: 10.1097/JCP.0000000000000344. [DOI] [PubMed] [Google Scholar]
- 9.Food and Drug Administration. FDA drug safety communication: updated information about the drug interaction between linezolid (Zyvox) and serotonergic psychiatric medications. www.fda.gov/Drugs/DrugSafety/ucm276251.htm (accessed Apr 25, 2020).
- 10.Carnovale C, Mazhar F, Arzenton E, Moretti U, Pozzi M, Mosini G, Leoni O, Scatigna M, Clementi E, Radice S. Bullous pemphigoid induced by dipeptidyl peptidase-4 (DPP-4) inhibitors: a pharmacovigilance-pharmacodynamic/pharmacokinetic assessment through an analysis of the vigibase®. Expert Opin Drug Saf. 2019;18(11):1099–1108. doi: 10.1080/14740338.2019.1668373. [DOI] [PubMed] [Google Scholar]
- 11.Rickli A, Liakoni E, Hoener MC, Liechti ME. Opioid-induced inhibition of the human 5-HT and noradrenaline transporters in vitro: link to clinical reports of serotonin syndrome. Br J Pharmacol. 2018;175(3):532–543. doi: 10.1111/bph.14105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montastruc JL, Rousseau V, de Canecaude C, Roussin A, Montastruc F (2020) Role of serotonin and norepinephrine transporters in antidepressant-induced arterial hypertension: a pharmacoepidemiological-pharmacodynamic study. Eur J Clin Pharmacol. 10.1007/s00228-020-02913-0 Online ahead of print. [DOI] [PubMed]
- 13.Woytowish MR, Maynor LM. Clinical relevance of linezolid-associated serotonin toxicity. Ann Pharmacother. 2013;47(3):388–397. doi: 10.1345/aph.1R386. [DOI] [PubMed] [Google Scholar]
- 14.Antonazzo IC, Poluzzi E, Forcesi E, Salvo F, Pariente A, Marchesini G, De Ponti F, Raschi E. Myopathy with DPP-4 inhibitors and statins in the real world: investigating the likelihood of drug-drug interactions through the FDA adverse event reporting system. Acta Diabetol. 2020;57(1):71–80. doi: 10.1007/s00592-019-01378-7. [DOI] [PubMed] [Google Scholar]
- 15.Delafoy C, Dolladille C, Besnier P, Sassier M, Bourgine J, Lelong-Boulouard V, Alexandre J, Guillaume C, Fedrizzi S, Chrétien B. Clinically significant drug-drug interactions between tramadol and CYP3A4 inhibitors: disproportionality analysis in VigiBase® and hypothesis on the underlying mechanism. Eur J Clin Pharmacol. 2020;76(7):1049–1051. doi: 10.1007/s00228-020-02881-5. [DOI] [PubMed] [Google Scholar]
- 16.Harpaz R, DuMouchel W, LePendu P, Bauer-Mehren A, Ryan P, Shah NH. Performance of pharmacovigilance signal-detection algorithms for the FDA adverse event reporting system. Clin Pharmacol Ther. 2013;93(6):539–546. doi: 10.1038/clpt.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen CT, Xie L, Alley S, McCarron RM, Baser O, Wang Z (2017) Epidemiology and economic burden of serotonin syndrome with concomitant use of serotonergic agents: a retrospective study utilizing two large US claims databases. Prim Care Companion CNS Disord 19(6) [DOI] [PubMed]
- 18.The Metabolomics Innovation Centre. DrugBank. https ://www.drugbank.ca. (accessed Apr 22, 2020).
- 19.Begley DJ. The blood-brain barrier: principles for targeting peptides and drugs to the central nervous system. J Pharm Pharmacol. 1996;48(2):136–146. doi: 10.1111/j.2042-7158.1996.tb07112.x. [DOI] [PubMed] [Google Scholar]
- 20.Owens MJ, Knight DL, Nemeroff CB. Second-generation SSRIs: human monoamine transporter binding profile of escitalopram and R-fluoxetine. Biol Psychiatry. 2001;50(5):345–350. doi: 10.1016/S0006-3223(01)01145-3. [DOI] [PubMed] [Google Scholar]
- 21.Baldo BA, Rose MA. The anaesthetist, opioid analgesic drugs, and serotonin toxicity: a mechanistic and clinical review. Br J Anaesth. 2020;124(1):44–62. doi: 10.1016/j.bja.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Cao YJ, Smith PF, Wire MB, Lou Y, Lancaster CT, Causon RC, Bigelow GE, Martinez E, Fuchs EJ, Radebaugh C, McCabe S, Hendrix CW. Pharmacokinetics and pharmacodynamics of methadone enantiomers after coadministration with fosamprenavir-ritonavir in opioid-dependent subjects. Pharmacotherapy. 2008;28(7):863–874. doi: 10.1592/phco.28.7.863. [DOI] [PubMed] [Google Scholar]
- 23.Culbertson VL, Rahman SE, Bosen GC, Caylor ML, Echevarria MM, Xu D. Implications of off-target serotoninergic drug activity: an analysis of serotonin syndrome reports using a systematic bioinformatics approach. Pharmacotherapy. 2018;38(9):888–898. doi: 10.1002/phar.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roger C, Roberts JA, Muller L. Clinical pharmacokinetics and pharmacodynamics of oxazolidinones. Clin Pharmacokinet. 2018;57(5):559–575. doi: 10.1007/s40262-017-0601-x. [DOI] [PubMed] [Google Scholar]
- 25.Thabit AK, Fatani DF, Bamakhrama MS, Barnawi OA, Basudan LO, Alhejaili SF. Antibiotic penetration into bone and joints: an updated review. Int J Infect Dis. 2019;81:128–136. doi: 10.1016/j.ijid.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Nau R, Sörgel F, Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev. 2010;23(4):858–883. doi: 10.1128/CMR.00007-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pozzi M, Carnovale C, Mazhar F, Peeters GGAM, Gentili M, Nobile M, Radice S, Clementi E. Adverse drug reactions related to mood and emotion in pediatric patients treated for attention deficit/hyperactivity disorder: a comparative analysis of the US Food and Drug Administration Adverse Event Reporting System Database. J Clin Psychopharmacol. 2019;39(4):386–392. doi: 10.1097/JCP.0000000000001058. [DOI] [PubMed] [Google Scholar]
- 28.Poluzzi E, Raschi E, Piccinni C, et al. Data mining techniques in pharmacovigilance: analysis of the publicly accessible FDA adverse event reporting system (AERS) In: Karahoca A, et al., editors. Data mining applications in engineering and medicine. Croatia: InTech; 2012. pp. 267–301. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 29 kb)
Data Availability Statement
Data supporting the findings of this study were derived from the following resource available in the public domain: https://fis.fda.gov/sense/app/d10be6bb-494e-4 cd2-82e4-0135608ddc13/sheet/7a47a261-d58b-4203-a8aa-6d3021737452/state/analysis.