Abstract
Objective
Sarcoidosis is a systemic granulomatous disease rarely complicated by pulmonary hypertension (PH). The prevalence of PH in sarcoidosis is unclear and has differences between ethnic groups. This study aimed to investigate the prevalence and predictors of PH in a Turkish cohort.
Methods
The study included 55 patients with biopsy-proven sarcoidosis in a single center. All patients underwent detailed transthoracic echocardiography (TTE) to assess the probability of PH as recommended. Right heart catheterization (RHC) was performed for patients with intermediate–high risk of PH. Patients with mean pulmonary artery pressure >20 mm Hg by RHC were defined as PH. Demographic and clinical characteristics, laboratory data, spirometry, 6-min walk test, and TTE were compared between low and intermediate–high risk PH groups.
Results
The probability of PH was low with 47 patients. Eight patients had intermediate–high probability of PH, and two of them refused to undergo RHC. Of six intermediate–high probability patients, three had PH, and all of them had post-precapillary PH. The prevalence of PH in sarcoidosis was 5.5% (3/55). Six-minute walk distance (6 MWD) and diastolic parameters (E/A ratio, E’ wave, and left atrial volume) were significantly lower, and New York Heart association class and N-terminal probrain natriuretic peptide (NT-proBNP) level were higher in intermediate–high risk PH patients compared with low-risk PH patients.
Conclusion
The frequency of PH in sarcoidosis was 5.5% in a Turkish cohort. NT-proBNP, 6 MWD, diastolic function parameters, and myocardial strain parameters can be useful predictors of PH in patients with sarcoidosis, besides known echocardiographic parameters.
Keywords: brain natriuretic peptide, sarcoidosis, 6-minute walk test, pulmonary hypertension
Introduction
Sarcoidosis is a systemic granulomatous disease with unknown etiology. Pulmonary hypertension (PH) is recognized as a complication of usually advanced pulmonary sarcoidosis and associated with increasing mortality. PH in sarcoidosis can occur as consequences of multifactorial conditions, such as interstitial lung disease, granulomatous infiltration, or external compression of the pulmonary artery and myocardial involvement. The exact prevalence of PH in patients with sarcoidosis is unclear and related to disease severity and ethnic differences (1-3). This study aimed to assess the prevalence and characteristics of PH in Turkish patients with sarcoidosis.
Methods
Study population
This single-center study was conducted at Marmara University, İstanbul. The study included 60 patients with biopsy-proven extra-cardiac sarcoidosis. Patients with poor echogenicity, left ventricular systolic dysfunction (ejection fraction <50%), moderate-to-severe left-sided valvular disease, and other etiologies of PH were excluded. One patient had left ventricular dysfunction, one patient had poor echogenicity, one patient had severe valvular disease, and two patients had pulmonary embolism. After application of the exclusion criteria, the study population consisted of the remaining 55 patients with sarcoidosis.
Measurements
Demographic data, including history of systemic hypertension or diabetes, organ involvement, duration of illness, steroid usage, functional capacity using New York Heart Association (NYHA) class, radiological stages (according to chest radiography), and results of pulmonary function testing, were recorded for all patients. Pulmonary function parameters were measured according to the European Respiratory Society recommendations (4). The 6-min walk test (6 MWT) was conducted in accordance with the American Thoracic Society guidelines (5). Pulse oximetry saturations were recorded at the beginning and end of the test. The total walking distances were recorded at the end of the test.
All patients were studied prospectively by transthoracic echocardiography (TTE) to evaluate the probability of PH according to current guidelines (6). Right heart catheterization (RHC) was performed to assess intermediate-to-high probability of PH.
Echocardiography probability of PH
All study patients underwent detailed TTE to estimate the probability of PH based on the guidelines of the available device (Epiq 7, Philips Healthcare, Andover, MA, USA) with 3.5 MHz (S5-1) transducer. Digitally stored images were analyzed offline (Xcelera, Philips). The parameters of left and right heart systolic and diastolic functions were measured based on the recommendations (7, 8). Tricuspid regurgitation velocity and other echocardiographic signs were combined to assess the probability of PH as recommended (6). All patients underwent myocardial strain analysis (9).
Right heart catheterization
Patients with mean pulmonary arterial pressure (mPAP) >20 mm Hg in RHC were defined as PH based on current recommendations (10). Precapillary PH was defined as mPAP >20 mm Hg, pulmonary capillary wedge pressure (PCWP) ≤15 mm Hg, and pulmonary vascular resistance (PVR) ≥3 WU, whereas mPAP >20 mm Hg and PCWP >15 mm Hg were postcapillary PH. Additionally, postcapillary PH with PVR ≥3 WU was interpreted as combined post- and precapillary PH (10). The cardiac index was calculated using the indirect Fick method.
Statistical analysis
SPSS for Windows (version 16.0; SPSS, Chicago, IL, USA) was used to analyze the data. One-sample Kolmogorov–Smirnov test was used to assess the distribution of continuous variables. Normal distributed data were presented as mean±SD, whereas variables not displaying normal distribution were presented as median with interquartile range. Categorical parameters were expressed as frequencies and percentages. The Mann–Whitney U test was used to compare numerical data between low and intermediate–high risk of PH. Categorical parameters were assessed between groups using the χ2 test. Significance was determined at p<0.05.
Results
Figure 1 shows the study flowchart of patient selection. The remaining 55 patients with sarcoidosis were assessed using echocardiography to estimate the risk of PH. Eight of the patients had intermediate–high risk of PH, and RHC was recommended. Two of intermediate–high risk patients refused to undergo RHC. The remaining six patients underwent RHC, and three of them were incompatible with PH. The prevalence of PH was 5.5% (3/55).
Figure 1.
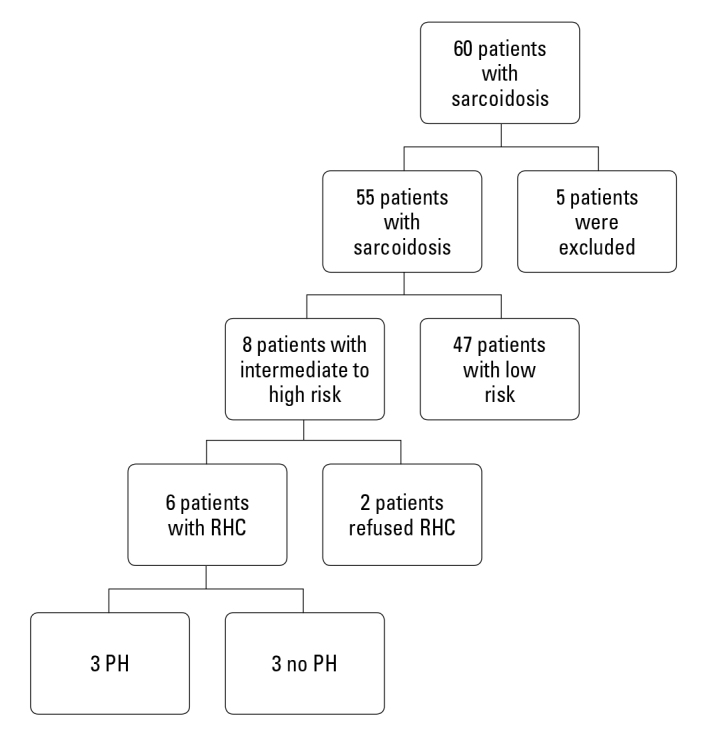
Study flowchart of patient selection and RHC results
PH - pulmonary hypertension; RHC - right heart catheterization
Pulmonary manifestations were present in 96.4% (53/55) of all patients. Steroid and immunomodulatory treatments were administered to 23 (41.8%) and 4 (7.3%) of patients, respectively. Table 1 shows the baseline characteristics of the patients. No differences were found between low and intermediate–high risk PH groups for cardiovascular comorbidities, such as hypertension, diabetes mellitus, or smoking. Patients with intermediate–high risk of PH were older and had higher body mass index (BMI). No differences were found in pulmonary function test parameters between groups. By contrast, patients with intermediate–high risk of PH had worse NYHA class, lower 6 MWD, and higher N-terminal probrain natriuretic peptide (NT-proBNP) levels.
Table 1.
Baseline characteristics of all patients with low and intermediate–high risk of PH
| All patients with sarcoidosis (n=55) | Low risk of PH on echo (n=47) | Intermediate–high risk of PH on echo (n=8) | P-value* | |
|---|---|---|---|---|
| Age (years) | 52.7±10.1 | 51.4±10.7 | 59.9±8.3 | 0.026 |
| Female sex | 45 (81.8%) | 37 (78.7%) | 8 (100%) | 0.315 |
| BMI (kg/m2) | 30.2±5.2 | 29.5±5.0 | 33.8±5.4 | 0.043 |
| Hypertension | 11 (20%) | 9 (19.1%) | 2 (25%) | 0.651 |
| Diabetes mellitus | 11 (20%) | 8 (17.1%) | 3 (37.5%) | 0.332 |
| Smoking | 6 (10.1%) | 6 (12.8%) | - | 0.572 |
| Duration of sarcoidosis | 4.7±4.5 | 4.6±4.6 | 5.6±3.7 | 0.384 |
| Baseline NYHA ≥2 | 17 (30.1%) | 11 (20%) | 6 (75%) | 0.008 |
| Hemoglobin (gr/dL) | 13.2±1.7 | 13.3±1.5 | 12.6±2.6 | 0.442 |
| Creatinine (mg/dL) | 0.7±0.3 | 0.7±0.2 | 0.7±0.3 | 0.502 |
| Uric acid (mg/dL) | 5.3±1.6 | 5.2±1.6 | 5.6±1.9 | 0.833 |
| NT-proBNP (pg/mL) | 74.7 (207) | 63.1 (140) | 450 (1662) | 0.003 |
| Radiologic stage ≥3 | 10 (18.2%) | 7 (14.9%) | 3 (37.5%) | 0.152 |
| FEV1 (%) | 91.8±19.7 | 92.8±17.7 | 86.1±30.1 | 0.441 |
| FVC (%) | 94.2±21.3 | 94.9±18.6 | 89.9±34.4 | 0.402 |
| FEV1/FVC ratio | 1.0±0.1 | 0.98±0.1 | 0.96±0.1 | 0.984 |
| DLCO (%) | 77.2±14.6 | 78.4±14.6 | 70.0±12.5 | 0.172 |
| FVC/DLCO ratio | 1.2±0.3 | 1.23±0.3 | 1.3±0.3 | 0.930 |
| 6 MWD (m) | 426.6±89.1 | 440.9±81.7 | 342.5±89.1 | 0.007 |
| Before test O2 (%) | 96.9±3.3 | 97.8±1.2 | 91.5±6.1 | 0.001 |
| After test O2 (%) | 96.7±4.5 | 97.8±1.7 | 90.1±8.8 | <0.001 |
Comparison of low and intermediate–high risk of PH
6 MWD - six-minute walk distance; BMI - body mass index; DLCO - diffusing lung capacity for carbon monoxide; FEV 1 - forced expiratory volume in 1 s; FVC: forced vital capacity; LV GLS - left ventricular global longitudinal strain; NT-proBNP - N-terminal probrain natriuretic peptide; NYHA - New York Heart Association class; O2 - pulse oxygen saturation before and after the six-minute walk test; PH - pulmonary hypertension
Table 2 shows the echocardiographic parameters of patients. Although no differences were found in left ventricular (LV) ejection fraction between groups, mitral lateral S’ wave was lower in patients with intermediate–high risk of PH. LV diastolic parameters, such as transmitral E/A ratio and mitral lateral E’ wave, were lower in patients with intermediate–high of PH, and left atrial area was higher. However, right ventricular (RV) functional parameters, such as RV fractional area change, were impaired in patients with intermediate–high risk of PH. Right heart size and thickness were higher in patients with intermediate–high risk of PH. No differences were found for other RV functional parameters, such as tricuspid lateral S’ wave or tricuspid annular plane systolic excursion (TAPSE), between groups. Systolic pulmonary artery pressure (SPAP), right atrial pressure (RAP), and tricuspid regurgitation velocity were significantly higher in patients with intermediate–high risk of PH.
Table 2.
Echocardiographic parameters of all patients with low and intermediate risk of PH
| All patients with sarcoidosis (n=55) | Low risk of PH on echo (n=47) | Intermediate–high risk of PH on echo (n=8) | P-value* | |
|---|---|---|---|---|
| LVEDD (mm) | 44.8±4.3 | 44.8±4.1 | 44.7±5.5 | 0.977 |
| LVESD (mm) | 28.4±4.2 | 28.2±3.9 | 29.6±5.3 | 0.547 |
| LV EF (%) | 65.3±6.5 | 65.7±6.1 | 62.9±8.4 | 0.110 |
| RV basal diameter (mm) | 30.2±3.7 | 29.8±3.7 | 32.2±3.0 | 0.040 |
| RV/LV ratio | 0.7±0.1 | 0.7±0.1 | 0.7±0.1 | 0.983 |
| LAA (cm2) | 15.2±3.5 | 14.6±3.1 | 18.6±3.9 | 0.006 |
| RAA (cm2) | 12.9±2.7 | 12.6±2.8 | 14.7±1.9 | 0.019 |
| Septum (mm) | 10.1±1.5 | 9.8±1.4 | 11.1±1.5 | 0.019 |
| LV mass (gr) | 148.0±34.8 | 145.2±33.6 | 164.3±39.7 | 0.231 |
| RV wall thickness (mm) | 4.8±0.9 | 4.7±0.6 | 5.9±1.7 | 0.029 |
| PA diameter (mm) | 20.6±2.4 | 20.3±2.3 | 22.7±1.7 | 0.004 |
| Transmitral E velocity (cm/s) | 0.8±0.2 | 0.8±0.2 | 0.8±0.4 | 0.178 |
| Transmitral E-wave DT (ms) | 200.5±40.4 | 196.7±33.5 | 222.4±67.9 | 0.098 |
| Transmitral E/A ratio | 1.0±0.3 | 1.1±0.3 | 0.9±0.1 | 0.041 |
| Mitral lateral E’ (cm/s) | 11.2±3.7 | 11.7±3.6 | 7.9±2.4 | 0.004 |
| Mitral lateral S’ (cm/s) | 10.8±2.9 | 11.2±2.9 | 8.3±1.9 | 0.009 |
| E/E’ ratio (cm/s) | 7.5±2.9 | 7.0±2.1 | 9.9±5.2 | 0.081 |
| Tricuspid lateral S’ (cm/s) | 12.9±2.3 | 12.8±2.1 | 13.5±3.4 | 0.552 |
| TAPSE (cm) | 22.9±4.0 | 23.0±3.9 | 22.1±5.1 | 0.587 |
| RV FAC (%) | 45.4±8.9 | 46.6±8.5 | 38.6±8.8 | 0.018 |
| RV outflow acceleration time (m/s) | 119.3±27.7 | 122.7±25.1 | 99.5±5.0 | 0.069 |
| Tricuspid regurgitation velocity (m/s) | 2.3±0.4 | 2.2±0.3 | 3.1±0.5 | <0.001 |
| RAP (mm Hg) | 3.2±0.9 | 3.0 | 4.3±2.3 | 0.001 |
| SPAP (mm Hg) | 25.8±9.6 | 22.9±5.1 | 42.8±2.3 | <0.001 |
| VCI collapse <50% | 1 (1.8%) | - | 1 (12.5%) | 0.142 |
| RV outflow midsystolic notch | 2 (3.6%) | - | 2 (25%) | 0.010 |
| PR velocity >2.2 m/s | 1 (1.8%) | - | 1 (12.5%) | 0.142 |
| RV outflow VTI | 18.3±3.6 | 18.5±3.6 | 17.5±3.9 | 0.366 |
| LV GLS (%) | −16.9±3.8 | −17.2±3.7 | −15.1±4.0 | 0.143 |
| RV GLS (%) | −18.9±5.9 | −17.8±4.7 | −14.3±5.3 | 0.086 |
Comparison of low and intermediate–high risk of PH
DT - deceleration time; EF - ejection fraction; FAC - fractional area change; LAA - left atrium area; LV - left ventricular; LVEDD - LV end-diastolic diameter; LVESD - LV end-systolic diameter; LV GLS - left ventricular global longitudinal strain; PY - pulmonary valve regurgitation; RAA - right atrium area; RAP - right atrial pressure estimated by VCI collapse; RV - Right ventricular; RV GLS - RV global longitudinal strain; sPAB - systolic pulmonary artery pressure measured by tricuspid valve regurgitation velocity; TAPSE - tricuspid annular plane systolic excursion; VCI - vena cava inferior; VTI - velocity time integral
Table 3 shows that three of the patients with PH had combined post- and precapillary PH. Two patients with PH had radiologic stage IV. All three patients with PH had low diffusing lung capacity for carbon monoxide (DLCO) level (<65%) and low 6 MWD (<350 m). Without significant systolic and diastolic dysfunction (EF >50% and E/e’ <14), three patients with PH had high NT-proBNP level (>125 pg/ml) and markedly low right and left ventricular global longitudinal strain (RV GLS and LV GLS, respectively).
Table 3.
Characteristics of the three patients with pulmonary hypertension
| PH patients | 1 | 2 | 3 |
|---|---|---|---|
| Echocardiographic probability of PH | High | Intermediate | High |
| Age (years) | 63 | 62 | 67 |
| Sex | Female | Female | Female |
| BMI (kg/m2) | 36.2 | 41.6 | 28.4 |
| Hypertension | + | − | − |
| Radiologic stage | IV | I | IV |
| Duration of sarcoidosis (years) | 4 | 10 | 8 |
| Steroid using | + | + | + |
| NHYA class | IV | II | IV |
| DLCO | 62 | 63 | 61 |
| 6 MWD (m) | 270 | 225 | 306 |
| Desaturation during 6 MWT | + | − | + |
| NT-proBNP (pg/mL) | 314 | 3551 | 2065 |
| EF (%) | 61 | 74 | 66 |
| E/e’ ratio | 7.3 | 7.5 | 10 |
| TAPSE | 21 | 18.9 | 16 |
| FAC (%) | 32.6 | 29.6 | 23.8 |
| RV EF (%) | 30 | 27.9 | 40.7 |
| RV GLS (%) | −13 | −9.8 | −10.3 |
| RA res. (%) | 13 | 24 | 15.6 |
| LV GLS (%) | −17.7 | −13.2 | −13.4 |
| mPAP (mm Hg) | 50 | 33 | 45 |
| PVR (WU) | 6 | 3 | 13 |
| PCWP (mm Hg) | 22 | 21 | 18 |
| DPG (mm Hg) | 10 | 0 | 13 |
| CI (L/min/m2) | 1.9 | 1.7 | 2.03 |
BMI - body mass index; CI - cardiac index measured by RHC; DLCO - diffusing lung capacity for carbon monoxide; DPG - diastolic pulmonary gradient; EF - ejection fraction; LV GLS - left ventricular global longitudinal strain; mPAB - mean pulmonary artery pressure measured by RHC; NYHA - New York Heart Association class; PH - pulmonary hypertension; PVR - pulmonary vascular resistance measured by RHC; RA res -right atrium reservoir function; RV EF - right ventricular ejection fraction; RV GLS - RV global longitudinal strain; sPAB - systolic pulmonary artery pressure measured by tricuspid valve regurgitation velocity
Discussion
This is the first study to evaluate evaluating prospectively and systematically the prevalence of PH in patients with sarcoidosis in a Turkish cohort based on current recommendations (6, 10). This study also demonstrated that several factors might be predictors for PH in patients with sarcoidosis. The prevalence of PH was 5.5%, and none of them had precapillary PH. A recent study from Germany reported that the prevalence of PH was 4.5% (3.6% for precapillary PH) (11). This study involved 111 patients with sarcoidosis, and 10 patients underwent RHC based on echocardiographic findings (SPAP ≥30 mm Hg). Another study by a European Caucasian cohort investigated the prevalence of PH in 399 patients with sarcoidosis using echocardiography signs, similar to our study design, and 28 patients underwent RHC. The estimated prevalence of PH in this cohort was 2.5% (2.3% for precapillary PH) (1). Alhamad et al. investigated the prevalence of PH in Arab patients (2). Echocardiography was used to retrospectively analyze 96 patients with sarcoidosis, and PH was accepted as SPAP ≥40 mm Hg. RHC was not performed. The prevalence 20%, with a predominance of female patients, similar to our study results. Shorr et al. (3) reported that the prevalence of PH was 73.8% in patients with advanced sarcoidosis who were candidates for lung transplantation, of which 65% and 70% were women and African Americans, respectively (3). The PH prevalence of 5.5% was low in our population. This might be influenced by the study design, wherein only intermediate–high probability patients with PH underwent RHC due to ethical considerations. Furthermore, our study population included those with advanced disease (10/55). Moreover, ethnic differences might affect the prevalence of PH.
The mechanisms of PH in sarcoidosis are multifactorial and may develop as a consequence of interstitial lung disease, granulomatous infiltration, or external pulmonary artery compression or myocardial involvement. During the 6th World Symposium on Pulmonary Hypertension, sarcoidosis was classified into group 5 remaining different conditions (10). Our study showed that all three patients had combined post- and precapillary PH. Without clinically overt cardiac involvement (EF >50%), three patients had impaired LV and RV GLS, which might be a consequence of asymptomatic ventricular infiltration as previously studied (12, 13). However, they had a high NT-proBNP level. Increased NT-proBNP levels can occur in LV systolic or diastolic dysfunction. A recent study showed that patients with sarcoidosis also had LV diastolic impairment (14). Impaired diastolic function might be the reason of postcapillary PH in our study population. In a retrospective study, BNP levels were correlated with PH severity as estimated by echocardiography in patients with sarcoidosis (15). Moreover, two patients with PH had advanced disease (stage IV), including interstitial fibrosis, which was known to be the most common cause of PH in sarcoidosis. Elderly is associated with PH based on previous studies (16, 17). Furthermore, obesity causes pulmonary and cardiac conditions that are substrates for PH due to left heart disease (group 2 PH) and PH due to lung disease (group 3 PH) (18, 19). In our study, the patients with intermediate–high risk of PH were significantly older and had higher BMI compared with those with low risk of PH. However, three patients with PH were >60 years, and two of them were morbidly obese (BMI >35 kg/m2). Comorbid conditions such as elderly and obesity can cause PH in patients with sarcoidosis.
Study limitations
First, this study was limited by the sample size of patients with sarcoidosis. Second, all patients did not undergo RHC because of ethical considerations, which might affect the prevalence of PH in the cohort. Third, cardiac magnetic resonance imaging or positron emission tomography was not used to estimate cardiac involvement, which might explain the etiology of PH. Finally, 49% of patients were on immunosuppressive therapy, which might influence the hemodynamic parameters.
Conclusion
The prevalence of PH in patients with sarcoidosis was 5.5% in a Turkish cohort. In addition to recommended echocardiographic parameters to assess the probability of PH, diastolic function parameters, RV and LV GLS, and clinical parameters, such as NT-proBNP and 6 MWD, can be used for early detection of sarcoidosis-related PH.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – D.K.Ö., B.M.; Design – D.K.Ö., B.M.; Supervision – D.K.Ö., B.M., D.K., B.C.; Fundings – D.K.Ö., B.M., D.K., S.S.Ş.; Materials – D.K.Ö., B.M., S.S.Ş.; Data collection and/or processing – D.K.Ö., D.K., B.C.; Analysis and/or interpretation – D.K.Ö., B.M., B.T.; Literature search – D.K.Ö., B.T., S.S.Ş.; Writing – D.K.Ö., B.T.; Critical review – D.K.Ö., B.M., D.K., B.T., S.S.Ş., B.C.
References
- 1.Huitema MP, Bakker ALM, Mager JJ, Rensing BJWM, Smits F, Snijder RJ, et al. Prevalence of pulmonary hypertension in pulmonary sarcoidosis: the first large European prospective study. Eur Respir J. 2019;54:1900897. doi: 10.1183/13993003.00897-2019. [DOI] [PubMed] [Google Scholar]
- 2.Alhamad EH, Idrees MM, Alanezi MO, Alboukai AA, Shaik SA. Sarcoidosis-associated pulmonary hypertension: Clinical features and outcomes in Arab patients. Ann Thorac Med. 2010;5:86–91. doi: 10.4103/1817-1737.62471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shorr AF, Helman DL, Davies DB, Nathan SD. Pulmonary hypertension in advanced sarcoidosis: epidemiology and clinical characteristics. Eur Respir J. 2005;25:783–8. doi: 10.1183/09031936.05.00083404. [DOI] [PubMed] [Google Scholar]
- 4.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 5.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 6.Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Respir J. 2015;46:903–75. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 7.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R, et al. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ ASE/Industry Task Force to standardize deformation imaging. J Am Soc Echocardiogr. 2015;28:183–93. doi: 10.1016/j.echo.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1801913. doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pabst S, Hammerstingl C, Grau N, Kreuz J, Grohe C, Juergens UR, et al. Pulmonary arterial hypertension in patients with sarcoidosis: the Pulsar single center experience. Adv Exp Med Biol. 2013;755:299–305. doi: 10.1007/978-94-007-4546-9_38. [DOI] [PubMed] [Google Scholar]
- 12.Tigen K, Sunbul M, Karaahmet T, Tasar O, Dundar C, Yalcinsoy M, et al. Early Detection of Bi-ventricular and Atrial Mechanical Dysfunction Using Two-Dimensional Speckle Tracking Echocardiography in Patients with Sarcoidosis. Lung. 2015;193:669–75. doi: 10.1007/s00408-015-9748-0. [DOI] [PubMed] [Google Scholar]
- 13.Kaptan Ozen D, Mutlu B, Kocakaya D, Turan B, Sert Sekerci S, Ceyhan B, et al. The effect of global longitudinal strain on impaired six-minute walk test performance in patients with sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2020;37:66–73. doi: 10.36141/svdld.v37i1.8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aydin Kaderli A, Gullulu S, Coskun F, Yilmaz D, Uzaslan E. Impaired left ventricular systolic and diastolic functions in patients with early grade pulmonary sarcoidosis. Eur J Echocardiogr. 2010;11:809–13. doi: 10.1093/ejechocard/jeq070. [DOI] [PubMed] [Google Scholar]
- 15.Mirsaeidi M, Omar HR, Baughman R, Machado R, Sweiss N. The association between BNP, 6MWD test, DLCO% and pulmonary hypertension in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2016;33:317–20. [PubMed] [Google Scholar]
- 16.McArdle JR, Trow TK, Lerz K. Pulmonary hypertension in older adults. Clin Chest Med. 2007;28:717–33. doi: 10.1016/j.ccm.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Berra G, Noble S, Soccal PM, Beghetti M, Lador F. Pulmonary hypertension in the elderly: a different disease? Breathe (Sheff) 2016;12:43–9. doi: 10.1183/20734735.003416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ayinapudi K, Singh T, Motwani A, Le Jemtel TH, Oparil S. Obesity and Pulmonary Hypertension. Curr Hypertens Rep. 2018;20:99. doi: 10.1007/s11906-018-0899-2. [DOI] [PubMed] [Google Scholar]
- 19.Poms AD, Turner M, Farber HW, Meltzer LA, McGoon MD. Comorbid conditions and outcomes in patients with pulmonary arterial hypertension: a REVEAL registry analysis. Chest. 2013;144:169–76. doi: 10.1378/chest.11-3241. [DOI] [PubMed] [Google Scholar]


