Highlights
-
•
The ultrasound pulses reduce the natural microflora, L. delbrueckii and L. monocytogenes of a raw meat emulsion.
-
•
Each kind of microorganism has a different answer to ultrasound treatments.
-
•
A model of desirability contributes to find the best parameters to apply ultrasound waves to inactivate microorganisms.
Keywords: Non thermal technology, Listeria monocytogenes, Lactobacillus delbrueckii, Inactivation model, Sonication
Abstract
Raw meat emulsions may have natural, spoilage and pathogenic microorganisms due to the origin and characteristics of this food matrix. All of these microorganisms must be minimized during industrial processing to make food consumption safe and meet quality regulations. Therefore, in this research, the effect of probe ultrasound on the inactivation of three kinds of microorganisms in a raw meat emulsion is evaluated. The microorganisms are: natural microflora NAM, Listeria monocytogenes LIS, and Lactobacillus delbrueckii LAC. A high-intensity probe ultrasound system was used, during 1.0, 2.5, 5.0, 7.5 and 10 min, with pulsed waves of 0.0, 10, 20 and 30 seg, and 200, 250, 300, 350 and 400 W of power. The interrelation between time, wave pulse cycle, and power factors was assessed. The results showed a positive linear independence effect in the treatments without wave pulse for each microorganism, and a quadratic interaction with the time and the ultrasound power for the inactivation of the three kinds of microorganisms. Besides, the desirability function for the inactivation reached up to 60% of the microbial population with the probe ultrasound treatment, with 10 min, a 7.56 s wave pulse and 400 W of power. Thus, these results could be useful to decide the incorporation of mild and emerging technologies in a meat industry line process.
1. Introduction
This research work aims to evaluate the influence of treatments of high power ultrasound waves on natural microflora, Listeria monocytogenes and Lactobacillus delbrueckii in a raw meat emulsion. Meat emulsions are elaborated from the raw material of animal origin, like ground meat and fat, water, and with other vegetable and animal source proteins. While the emulsification process occurs, the microbial growth can increase between 103-–104 CFU/g. These microorganisms have been mainly identified as spoilage flora in this kind of meat emulsion, where the most known are lactic acid bacteria (Lactobacillus spp.) and some enterobacteria (Proteus spp. and Pseudomonas spp.) [2], [3]. On the other hand, in these raw emulsions, pathogenic microorganisms such as Listeria monocytogenes and Escherichia coli have been found, and they are frequently used as indicator microorganisms to evaluate the effectiveness of inactivation treatments in quality safety processes [1], [2], [3], [4]. Therefore, according to the regulations for cooked emulsified meat products, the application of thermal processes where the time and temperature depend on the characteristics of each product, is mandatory. Thus, several researchers have suggested that in order to reduce the pathogenic flora in cooked meat products, the internal temperature should reach 72 °C [5], [6], [7]. However, the texture stability of meat products can be affected by increasing cooking times and temperatures, so a mild technology or a hurdle technology before a thermal process could reduce the number of microorganisms before the cooking process.
The positive impact of the ultrasound waves by sonication is widely known. It can contribute to reduce the microbial load in different food systems without altering flavor, color, and nutritional quality [5], [8], [9], [10], [11], [12]. Cavitation phenomena generated by high-intensity ultrasound waves have positively influenced these two aspects, reducing the microbial population and maintaining the physical stability of the products. Power ultrasound has been used to guarantee safe and high-quality food. High power (greater than1 W/cm2 and frequencies between 20 and 500 kHz), ultrasound offers an alternative to traditional food preservation methods and is considered an emerging, green and versatile technology [13], [14]. On the other hand, Barretto, Pollonio, Telis-Romero & da Silva Barretto [15] showed that ultrasound treatments on reconstituted cooked hams produce high flavor scores and sensory acceptance. Regarding the stability of meat emulsions, Cichoski et al. [16], found that ultrasound in pork meat emulsions favored the formation of a stable gel during cooking.
Awad et al. [17], have explained that ultrasound waves are generally considered safe, non-toxic, and environmentally friendly. Thereby, ultrasound could have an advantage over other technologies in the food industry. Yusof & Ashokkumar [18] have described that acoustic cavitations can be beneficially used in food processing applications, which implies the use of lower temperatures and pressure conditions in industrial processes. According to Soria & Villamiel [19], the ultrasound is not a standard technology and, for each application, the time, the intensity, and the ultrasound waves frequency should be considered, along with their effects on the technological and functional properties of food. In general, energy, intensity, and temperature are the main factors that affect the power of ultrasound [20] and, therefore, knowledge of the parameters to apply ultrasound is related to the effects that it could have on food systems.
Guerrero, López-Malo & Alzamora, and Huang et al. [21], [22] have described how the low-frequency high-intensity ultrasound generates strong shear and mechanical forces that induce acoustic cavitation, by the generation and the collapse of large bubbles that cause a high energy release. According to Señorans et al. and Turantas et al. [23], [24], in terms of ultrasound application on different food systems, each case should involve critical factors such as the amplitude, the relationship between exposure time, contact, volume, and the composition of the food. These factors determine the efficiency of the inactivation of microorganisms. Thus, the correct design of experiments is an important strategy to be developed between the researchers and the meat industry, so the conclusive results could be applied to an industrial process. On the other hand, the ultrasound probe in liquid or semi-solid food systems has shown a remarkable impact, as ultrasound waves are directly applied on the food by means of a horn vibrator. If the contact time is of just a few minutes, it will not generate problems related with the contamination with metal particles emitted by the horn [25]. Thereby, in this research, the aim was to evaluate the influence of treatments of high power ultrasound waves on natural microflora, Listeria monocytogenes and Lactobacillus delbrueckii in a raw meat emulsion.
2. Materials and methods
This research was carried out with three groups of microorganisms: a pathogen, Listeria monocytogenes (LIS); a spoilage, Lactobacillus delbrueckii (LAC), and the natural microflora (NAM) present in a raw meat emulsion. LIS and LAC were inoculated into a raw meat emulsion before performing the ultrasound treatments.
2.1. Raw material
A raw meat emulsion (0.5:1) of protein/fat, with the following composition: Total protein: 12.65% (w/w); meat protein: 12.30% (w/w); vegetable protein: 0.40% (w/w); total fat: 25.70% (w/w); moisture: 55.20% (w/w); total carbohydrates: 3.90% (w/w); starch: 3.00% (w/w), 1.96% (w/w) salt, 0.30% (w/w) phosphate, 453.70 ppm ascorbate and a pH of 5.8. It was elaborated under industrial conditions in terms of grinding, mixing and emulsification. The emulsion was aseptically divided into 10 g portions packed in sterile polyethylene bags (Whirl-Pak, Nasco-BB01062) and stored at −10 C until they were used.
2.2. Natural accompanying microflora determination
The concentration of natural microflora (NAM) in the raw meat emulsion was determined and cultures of the samples were performed in triplicate on tryptone soy agar (TSA, Scharlau ref. 01–200) at 37 °C for 24 h. The count of microorganisms was 3.9 log10 CFU/mL ± 0.81. Additionally, the presence of Listeria spp. in the samples was discarded, due to the absence of typical colonies in Palcam agar (Scharlau ref. 01-470), according to the guidelines of the Bacteriological Analytical Manual [26].
2.3. Inocula preparation
The strain of Listeria monocytogenes (LIS) was isolated from the meat food factory, and Lactobacillus delbrueckii (LAC) was isolated from raw materials (cornmeal).The two strains were identified by the 16 s rRNA molecular sequencing (≥99.0% of identity), using a 3730 DNA Analyzer technology (Applied Biosystems®). Bacterial strains were stored in cryovials (CryoBank, Copan) at −70 °C until use. They were activated in 10 mL of tryptic soy broth (TSB, Scharlau ref. 02–200) for 24 h at 37 °C. The overnight cultures were subcultured at 1:10 in TSB, for 24 h, at 37 °C
2.3.1. Inoculation in raw material
10 g of samples of raw meat emulsion were placed in sterile stomacher bags, and each one was inoculated with LIS and LAC, previously adjusting volume until a final concentration of 4.0 at 4.5 log10 CFU/mL was obtained, and NAM was determined in the raw meat emulsion according to the method described in 2.2.
2.4. Ultrasound treatments and microbial inactivation
The treatments were performed with an ultrasound probe (Qsonica, model Q700, Newtown, CT, US) at 20 kHz frequency, with a tip of 320 µm in terms of amplitude and 1.6 mm of diameter, and with an LM35 thermocouple with an integrated circuit. The inoculated raw meat emulsion at 5 °C was aseptically deposited in volumetric vessels, designed in stainless steel, which are 2.0 mm thick, with 2.5 cm of diameter and a 4.0 cm height. The ultrasound probe was inserted up to 2.0 cm into the raw meat emulsion (Fig. 1). The following factors were evaluated: Time of treatment (1.0, 2.5, 5.0, 7.5, 10 min); pulse wave (0.0, 10, 20 and 30 s), and the power (200, 250, 300, 350 and 400 W). Table 1 shows the 100 ultrasound treatments performed in triplicate. In previous essays, to avoid physicochemical changes in the meat emulsion were defined as the sonication conditions temperatures under 15 °C (data not shown). After those treatments, the samples (10 g) were aseptically transferred in a laminar flow cabinet to sterile stomacher bags, and 90 mL of sterile peptone of 0.1% were added before homogenizing for 2 min, using a mechanical homogenizer (Stomacher 400 Circulator; Seward Laboratory Systems, Inc.). For microbiological enumeration, fold series of sample homogenates were prepared and spread in petri dishes, in triplicate: LIS count in Palcam agar (Scharlau ref. 01–470), with 0.1% sodium pyruvate (SP) added; LAC counts were carried out in TSA (Scharlau ref. 01-200), with 5.0% acetic acid and 0.1% SP added, and NAM counts were performed using TSA, with 0.1% SP added. The petri dishes were incubated at 37 °C until the colony viable count did not increase (72 h). The numbers to express the quantities of bacteria were transformed from CFU/mL to log units (Log10).
Fig. 1.
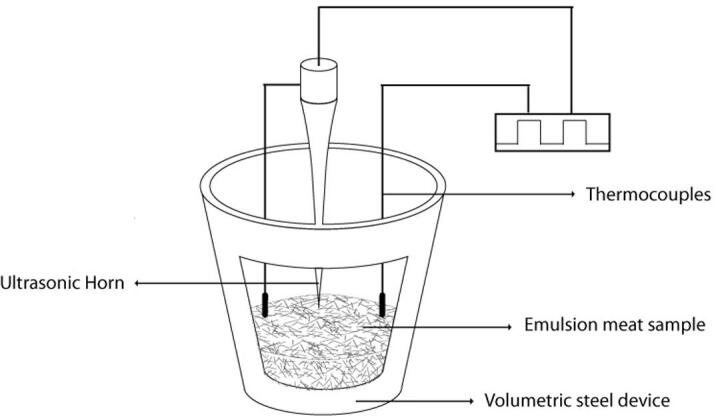
Layout of sonication in emulsion meat samples.
Table 1.
Inactivation percentage for L. monocytogenes (LIS), natural accompanying microflora (NAM) and L. delbruekii (LAC) during ultrasonic treatments in raw meat emulsion.
| RUN | Time (min) | Power (W) | Pulses (s) | Inactivation percentage (%) |
Time (min) | Power (W) | Pulses (s) | Inactivation percentage (%) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LIS | NAM | LAC | RUN | LIS | NAM | LAC | |||||||
| 1 | 1 | 200 | 0 | 0.3 | 0.6 | 0.1 | 51 | 1 | 200 | 20 | 0.8 | 0.6 | 0.9 |
| 2 | 2.5 | 200 | 0 | 0.5 | 0.6 | 1.0 | 52 | 2.5 | 200 | 20 | 0.6 | 0.6 | 0.1 |
| 3 | 5 | 200 | 0 | 0.2 | 0.3 | 0.2 | 53 | 5 | 200 | 20 | 0.3 | 0.6 | 0.4 |
| 4 | 7.5 | 200 | 0 | 0.5 | 0.3 | 0.2 | 54 | 7.5 | 200 | 20 | 0.1 | 3.4 | 0.9 |
| 5 | 10 | 200 | 0 | 0.2 | 0.4 | 1.1 | 55 | 10 | 200 | 20 | 0.4 | 3.9 | 0.7 |
| 6 | 1 | 250 | 0 | 3.0 | 0.1 | 1.0 | 56 | 1 | 250 | 20 | 0.3 | 3.5 | 0.3 |
| 7 | 2.5 | 250 | 0 | 4.2 | 0.2 | 1.0 | 57 | 2.5 | 250 | 20 | 0.3 | 4.0 | 0.8 |
| 8 | 5 | 250 | 0 | 5.7 | 0.3 | 0.6 | 58 | 5 | 250 | 20 | 0.8 | 4.9 | 0.2 |
| 9 | 7.5 | 250 | 0 | 8.5 | 0.6 | 0.5 | 59 | 7.5 | 250 | 20 | 0.7 | 6.7 | 0.2 |
| 10 | 10 | 250 | 0 | 8.2 | 0.6 | 0.3 | 60 | 10 | 250 | 20 | 0.8 | 8.3 | 0.5 |
| 11 | 1 | 300 | 0 | 9.0 | 0.5 | 0.9 | 61 | 1 | 300 | 20 | 2.8 | 6.7 | 0.5 |
| 12 | 2.5 | 300 | 0 | 11.6 | 0.5 | 0.6 | 62 | 2.5 | 300 | 20 | 2.1 | 9.1 | 0.3 |
| 13 | 5 | 300 | 0 | 19.4 | 0.9 | 0.5 | 63 | 5 | 300 | 20 | 2.9 | 10.1 | 0.1 |
| 14 | 7.5 | 300 | 0 | 22.9 | 1.2 | 0.3 | 64 | 7.5 | 300 | 20 | 2.6 | 16.5 | 0.8 |
| 15 | 10 | 300 | 0 | 23.5 | 1.2 | 0.4 | 65 | 10 | 300 | 20 | 2.2 | 20.2 | 0.6 |
| 16 | 1 | 350 | 0 | 23.2 | 9.6 | 5.8 | 66 | 1 | 350 | 20 | 7.6 | 11.8 | 5.6 |
| 17 | 2.5 | 350 | 0 | 26.7 | 16.2 | 9.7 | 67 | 2.5 | 350 | 20 | 8.7 | 14.2 | 10.1 |
| 18 | 5 | 350 | 0 | 37.6 | 25.1 | 12.3 | 68 | 5 | 350 | 20 | 13.8 | 18.6 | 19.3 |
| 19 | 7.5 | 350 | 0 | 49.0 | 28.0 | 23.7 | 69 | 7.5 | 350 | 20 | 18.0 | 25.2 | 23.8 |
| 20 | 10 | 350 | 0 | 49.1 | 34.9 | 26.5 | 70 | 10 | 350 | 20 | 18.1 | 27.4 | 27.3 |
| 21 | 1 | 400 | 0 | 38.3 | 32.2 | 23.7 | 71 | 1 | 400 | 20 | 10.8 | 25.0 | 9.7 |
| 22 | 2.5 | 400 | 0 | 41.6 | 34.1 | 29.6 | 72 | 2.5 | 400 | 20 | 18.3 | 27.4 | 22.1 |
| 23 | 5 | 400 | 0 | 47.5 | 45.7 | 32.0 | 73 | 5 | 400 | 20 | 24.0 | 28.8 | 29.8 |
| 24 | 7.5 | 400 | 0 | 61.3 | 50.2 | 37.9 | 74 | 7.5 | 400 | 20 | 29.0 | 30.5 | 35.4 |
| 25 | 10 | 400 | 0 | 63.3 | 53.4 | 41.3 | 75 | 10 | 400 | 20 | 30.1 | 34.3 | 40.1 |
| 26 | 1 | 200 | 10 | 0.5 | 2.6 | 0.1 | 76 | 1 | 200 | 30 | 0.4 | 0.8 | 0.8 |
| 27 | 2.5 | 200 | 10 | 0.7 | 4.6 | 0.6 | 77 | 2.5 | 200 | 30 | 0.5 | 0.8 | 1.2 |
| 28 | 5 | 200 | 10 | 0.2 | 7.2 | 0.6 | 78 | 5 | 200 | 30 | 0.8 | 0.8 | 0.9 |
| 29 | 7.5 | 200 | 10 | 0.2 | 9.8 | 0.6 | 79 | 7.5 | 200 | 30 | 0.6 | 0.9 | 0.5 |
| 30 | 10 | 200 | 10 | 0.3 | 16.2 | 1.0 | 80 | 10 | 200 | 30 | 0.8 | 0.8 | 0.5 |
| 31 | 1 | 250 | 10 | 1.0 | 5.6 | 0.7 | 81 | 1 | 250 | 30 | 0.4 | 0.3 | 1.0 |
| 32 | 2.5 | 250 | 10 | 0.7 | 10.5 | 0.6 | 82 | 2.5 | 250 | 30 | 0.2 | 4.7 | 1.1 |
| 33 | 5 | 250 | 10 | 0.6 | 12.9 | 0.4 | 83 | 5 | 250 | 30 | 0.8 | 6.9 | 1.5 |
| 34 | 7.5 | 250 | 10 | 3.0 | 21.1 | 0.3 | 84 | 7.5 | 250 | 30 | 0.4 | 11.2 | 1.5 |
| 35 | 10 | 250 | 10 | 3.1 | 24.4 | 0.8 | 85 | 10 | 250 | 30 | 0.9 | 12.7 | 1.6 |
| 36 | 1 | 300 | 10 | 3.4 | 17.9 | 0.9 | 86 | 1 | 300 | 30 | 0.8 | 2.4 | 0.9 |
| 37 | 2.5 | 300 | 10 | 9.0 | 22.3 | 0.6 | 87 | 2.5 | 300 | 30 | 0.9 | 4.6 | 1.1 |
| 38 | 5 | 300 | 10 | 11.3 | 25.3 | 0.9 | 88 | 5 | 300 | 30 | 0.6 | 6.2 | 1.1 |
| 39 | 7.5 | 300 | 10 | 18.7 | 29.2 | 0.7 | 89 | 7.5 | 300 | 30 | 0.8 | 11.1 | 1.3 |
| 40 | 10 | 300 | 10 | 19.0 | 33.4 | 0.6 | 90 | 10 | 300 | 30 | 0.7 | 12.6 | 1.3 |
| 41 | 1 | 350 | 10 | 23.7 | 30.7 | 7.3 | 91 | 1 | 350 | 30 | 5.0 | 5.9 | 1.6 |
| 42 | 2.5 | 350 | 10 | 26.7 | 38.7 | 19.4 | 92 | 2.5 | 350 | 30 | 6.6 | 10.3 | 1.8 |
| 43 | 5 | 350 | 10 | 32.9 | 50.8 | 24.0 | 93 | 5 | 350 | 30 | 8.9 | 15.9 | 1.9 |
| 44 | 7.5 | 350 | 10 | 37.5 | 52.2 | 28.7 | 94 | 7.5 | 350 | 30 | 13.5 | 22.5 | 1.9 |
| 45 | 10 | 350 | 10 | 37.5 | 57.3 | 32.7 | 95 | 10 | 350 | 30 | 13.6 | 23.7 | 2.3 |
| 46 | 1 | 400 | 10 | 28.4 | 39.6 | 18.5 | 96 | 1 | 400 | 30 | 8.6 | 20.8 | 9.1 |
| 47 | 2.5 | 400 | 10 | 29.7 | 43.1 | 27.7 | 97 | 2.5 | 400 | 30 | 16.3 | 25.9 | 14.4 |
| 48 | 5 | 400 | 10 | 33.7 | 48.1 | 31.4 | 98 | 5 | 400 | 30 | 21.5 | 27.9 | 19.3 |
| 49 | 7.5 | 400 | 10 | 40.1 | 57.8 | 43.1 | 99 | 7.5 | 400 | 30 | 25.8 | 30.9 | 20.3 |
| 50 | 10 | 400 | 10 | 40.2 | 66.1 | 47.6 | 100 | 10 | 400 | 30 | 26.1 | 34.0 | 24.5 |
The inactivation percentage (I) was calculated from the count of surviving microorganisms (final concentration after ultrasound treatments), according to Eq. (1):
| (1) |
2.5. Statistical analysis
Statistical analyses were performed using SAS 9.4 for Windows. Response values of second order were estimated from the values of I for each group of microorganisms, according to Eq. (2).
| (2) |
Where Xi corresponds to the values of the factors time, power, and pulses; βi, to the parameters of the main effects and the corresponding interactions; ε to the residuals of the model and Y is the response variable, which for this case corresponds to the average inactivation percentage values.
The models were fixed for each kind of microorganism and were evaluated by the assumptions of the statistical model for the selection of the best transformation method, as follows: For LIS, the Box-Cox transformation with a λ = 0.04 was used; for NAM, square root transformation was applied and for LAC, the data were transformed by the logarithm function. Thus, the significant parameters for the predicted responses (p < 0.05) were selected and the combination of the factors (ultrasonic setting) that maximize the desirability function (inactivation percentage) was estimated [27], [28]. In our study, we defined the percentages above 50% for LIS, NAM, and LAC as desirable inactivation values, because the level of lethality of mild technologies such as ultrasound is not comparable with the effect produced by thermal treatments. A significant reduction in the microbial population of a raw product could mean the optimization of thermal treatments in subsequent phases, with no abuse of temperatures [29].
3. Results and discussion
3.1. Model of microbial inactivation
An independent linear effect (Table 1) was found for each microorganism without a pulse (0 s); the highest inactivation values (I) for LIS and LAC were 63.3% and 53.4%, respectively, at 400 W of power during 10 min. On the other hand, all ultrasound treatments evidenced a positive and significant linear effect of treatment time and potency on inactivation of the three types of microorganisms. Eqs. (3), (4) and (5) show the behavior of each microbial species:
| (3) |
| (4) |
| (5) |
Therefore, the application of the model and the response of the quadratic effect of the pulsed wave showed an optimal value for the inactivation of the NAM populations at less than 10 s. This natural microflora, present in a standard raw meat emulsion, is mainly composed of non-pathogenic lactic acid and coliform bacteria that are Gram-negative, with a less thick peptidoglycan layer their cell wall and, therefore, more sensitive to physical treatments [30], [31]. For LIS, the linear interaction of the model was also found where the shorter pulsed-wave times improve the level of inactivation (Table 2). In general, the most resistant to inactivation microorganism was L. delbrueckii (Gram positive, with a history of thermoresistance), which is associated with a thicker peptidoglycan layer cell wall, thus being more resistant to physical treatments [32].
Table 2.
Estimates for coded data and t-test in a second order response surfaces for inactivation percentage for L. monocytogenes (LIS), natural accompanying microflora (NAM), and L. delbruekii (LAC).
| Term | LIS |
NAM |
LAC |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | Std. Err | t value | Estimate | Std. Err | t value | Estimate | Std. Err | t value | |
| Time | 0.30 | 0.05 | 6.42** | 0.73 | 0.12 | 6.11 ** | 0.19 | 0.07 | 2.81 ** |
| Power | 1.52 | 0.05 | 31.62** | 2.25 | 0.12 | 18.50 ** | 1.52 | 0.07 | 21.60 ** |
| Pulse | −0.64 | 0.05 | −14.09** | −0.13 | 0.12 | −1.10 ns | −0.15 | 0.07 | −2.26 * |
| Time2 | −0.12 | 0.08 | −1.47 ns | −0.12 | 0.21 | −0.56 ns | −0.06 | 0.12 | −0.53 ns |
| TimexPower | 0.27 | 0.07 | 4.15 ** | 0.20 | 0.17 | 1.19 ns | 0.26 | 0.10 | 2.65 * |
| TimexPulse | −0.10 | 0.06 | −1.61 ns | 0.11 | 0.16 | 0.66 ns | 0.01 | 0.09 | −0.05 ns |
| Power2 | 0.26 | 0.08 | 3.18 ** | 0.51 | 0.21 | 2.48 * | 1.00 | 0.12 | 8.44 ** |
| PowerxPulse | −0.43 | 0.06 | −6.68 ** | −0.51 | 0.16 | −3.10 ** | −0.34 | 0.09 | −3.66 ** |
| Pulse2 | 0.13 | 0.08 | 1.69 ** | −1.38 | 0.19 | −7.09 ** | −0.18 | 0.11 | −1.60 ns |
| Adjusted R-square | 92.9% | 81.6% | 85.0% | ||||||
| Coefficient of Variation | 14.0% | 23.0% | 34.0% | ||||||
* p < 0.05, ** p < 0.01, ns p ≥ 0.05.
3.1.1. Effects of wave type and the ultrasound power
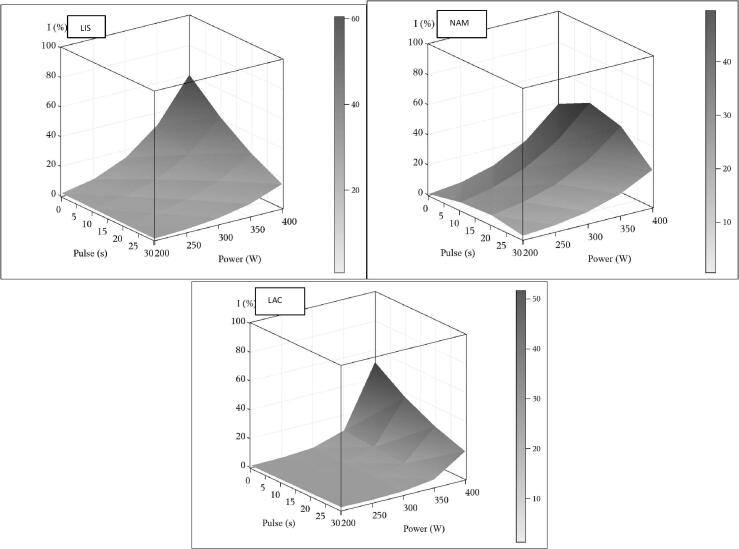
According to the results (Fig. 2), the effect of the ON: OFF pulses shows an antagonistic interaction related to the power intensity for the three kinds of microorganisms. Besides, for the pulsed wave factor, the effect was negative. However, this behavior could be modulated by their interactions. NAM was affected by ultrasonic pulses. The 10 s cycle treatment was the most effective for inactivation to reach up values of 66.1%. Thus, shorter pulse times could stimulate the inactivation effect exerted by the power (W). The ON period in ultrasound is denominated pulse length, while the OFF one is the interval length [33], so the pulsed waves do not have a constant amplitude as a continuous wave and the energy is intermittent in the ON: OFF repetition periods [17], [34]. In the ultrasound pulsed waves treatments, a greater number of bubbles with different amplitudes are generated [25], [35], [36], [37], [38]. Therefore, these amplitude differences could affect the efficacy of microbial inactivation. In general, the ultrasound treatment reduces the microbial load due to the cavitations that damage the biological species by increasing localized temperature and pressure on the product, with shock waves and hydroxyl radicals, which ultimately enhance the biocidal effect [39], [40].
Fig. 2.
Response surfaces wave and power for inactivation percentage (IP%) for L. monocytogenes (LIS), natural accompanying microflora (NAM), and L. delbruekii (LAC). Color scale correspond to standard error of estimations.
3.1.2. Application time and ultrasound power
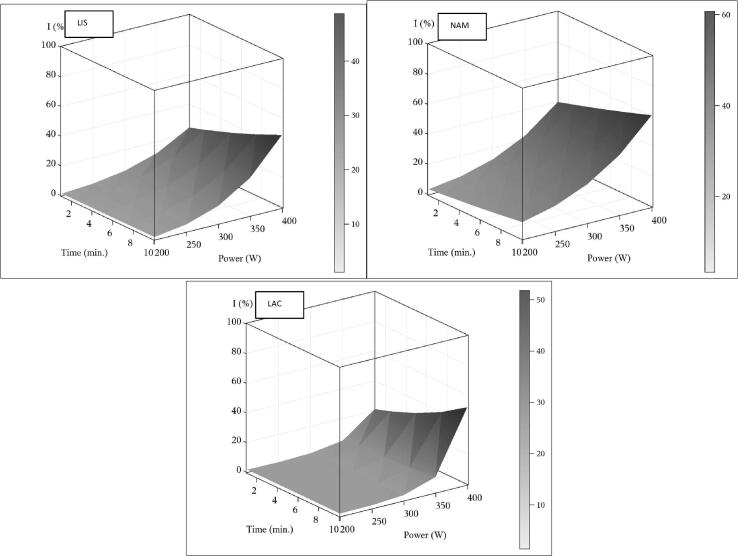
The mathematical model in our study showed that power sonication (W) has a positive effect on inactivation for LAC, LIS, and NAM. Thus, the effect of power is related to the ultrasonic cavitation external force that is proportional to the pressure amplitude of the shock waves generated on the cavitation bubble collapse [12]. Therefore, as a result of the cavitation phenomenon on the surface of bacteria, the pressure generated makes bacteria more vulnerable to sonication treatments [14]. On the other hand, the effect of the sonication time (Fig. 3) on the raw meat emulsion presents synergistic interaction with the power (W), for the LIS and LAC populations, which reached the highest inhibition values at the highest levels of application of these two factors (400 W and 10 min).
Fig. 3.
Response surfaces time and power for inactivation percentage (IP%) for L. monocytogenes (LIS), natural accompanying microflora (NAM), and L. delbruekii (LAC). Color scale correspond to standard error of estimates.
Thus, our model has shown a significant microbial inactivation exerted by ultrasound treatments on non-liquid food matrices that, according to several researchers, have shown significant levels of reduction of microbial load. For example, in strawberries, Gani et al. [40] achieved 2 log reductions, corresponding to 33% of native microflora with ultrasound treatments performed at 60 W and 33 kHz, for 60 min. Alenyorege, Ma & Ayim, [41] achieved reductions up to 3 log of Listeria inocua, with 40 kHz for 10 min in bath ultrasound, in cabbages. In soy sprouts, with the addition of sanitizing agents and ultrasound bath treatments for 10 min, Ngnitcho et al. [42] found 4 log reductions of L. monocytogenes. In semi-skimmed milk reconstituted with 15% of solutes, Gao, Hemar, Lewis & Ashokkumar [11] reached reductions of 33% (2.21 ± 0.03) of Enterobacter aerogenes (gram-negative), with treatments of 50 W during 60 min with ultrasound horn. In Saccharomyces cerevisiae, cellular damage was evidenced with plasmolysis by the ultrasound effect, when a horn was used at 288 W for 10 min [43].
On the other hand, according to Cichoski et al. [16], who found that with high-power ultrasound applications at 25 kHz, 60% amplitude, and 5.5 min of treatment, the temperature increases up to 7 °C and no lipid oxidation is produced. Our model results for this raw emulsion meat showed a positive interaction between power and ultrasound time applications. However, for more than 10 min of treatment, the increase in temperature should be controlled to avoid undesirable chemical and sensory changes.
3.2. Desirability function for simultaneous inactivation of microorganisms
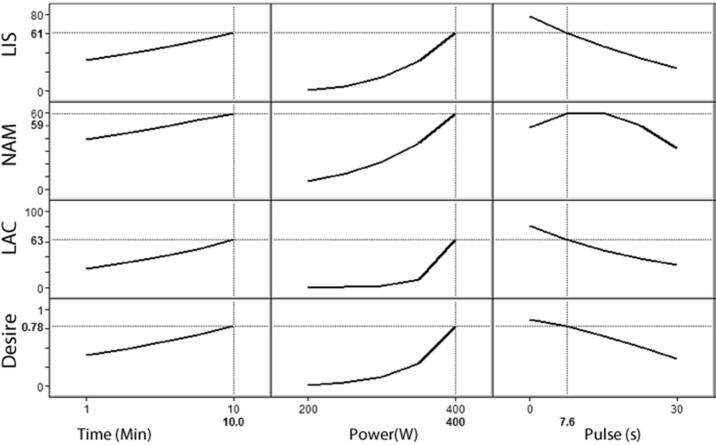
The desirability function allows finding the experimental conditions (factor levels) in order to reach, simultaneously, the optimal value for all of the evaluated variables, including the researcher's priorities during optimization procedures [27], [28]. Based on our definition, the optimal ultrasonic setting to reach the simultaneous reduction of at least 50% of the three microbial populations was achieved in values of 10 min for time, 400 W for power and in a period of 7.56 s for a pulse with the application of the ultrasound probe (Fig. 4). The estimated values in the maximum desirability function (inactivation percentage) for the inactivation of each microorganism in the raw meat emulsion are shown in Table 3. Although for LIS, NAM and LAC the estimated values were above 50%, indicating that the desirability function was maximized using the combination of ultrasonic conditions previously mentioned, a validation of this combination is necessary to consider its potential application on a larger scale.
Fig. 4.
Desirability function for inactivation percentage for L. monocytogenes (LIS), natural accompanying microflora (NAM), and L. delbruekii (LAC). Desirability function was settled for a maximum in all variables.
Table 3.
Estimated values in the maximum desirability function for L. monocytogenes (LIS), natural accompanying microflora (NAM) and L. delbruekii (LAC).
| Response | Estimated Value |
|---|---|
| LIS | 60.82 [60.81,60.83] |
| NAM | 59.49 [59.37,59.61] |
| LAC | 63.16 [60.80,65.52] |
| Desirability overall | 100% |
3.3. Perspective for the incorporation of ultrasound treatment in a line of food processes.
Zinoviadou et al. and Barbav et al. [44], [45] have highlighted the potential of ultrasound as a mild and emerging technology, as it can minimize the use of other treatments, maximize the quality and ensure the safety of food products. According to Inguglia et al. [46], it is possible to propose future developments and commercial applications for the industry. Chemat et al. and Jayasooriya, Bhandari, Torley, & D’Arcy [47], [48], have shown specific examples of the application of ultrasound in meat processing. On the other hand, Jambrak et al. and Al‐Hilphy et al. [49], [50] have described some ultrasound advantages, such as the elimination of the microbial load and lower processing costs. However, they also indicated disadvantages: the production of free radicals, which can negatively impact and damage the quality of the product due to oxidation, and the difficulty to select the appropriate parameters (i.e., pressure, time, temperature, intensity, power, and amplitude). All these parameters must be duly validated, and they are critical to obtain the product desired. Thus, our study has been performed to find parameters such as wave type, time and ultrasound power to be directly applied into a semi-solid raw meat emulsion and, complemented by the design of a transducer system that could be integrated to an industrial process line. This way, the results will contribute to the reduction of the endogenous microbial populations in raw emulsified meat, before a cooking process is applied.
4. Conclusions
The application of barrier technologies through mild technologies is widely known as useful to support the reduction of the growth of microorganisms in raw foods. In this case, the effect of ultrasound waves has been evaluated for the interaction of three kinds of microorganisms in raw emulsified meat. These parameters can support decision-making to adapt ultrasound technology in a continuous industrial process. Therefore, in this study, the effects of microbial inactivation by the effect of ultrasound pulses shown evidence of its possible application to reduce the microbial load before a thermal process (cooking, for example). The application of ultrasound pulses with cycles of 7.7 s and 400 W of power for 10 min in a raw meat emulsion, has represented a microbial reduction of 60% of the natural microflora (L. delbrueckii and L. monocytogenes). Nevertheless, these ultrasound treatments could apply at higher at 10 min to achieve above 60% of desirability on microbial inactivation. However, for industrial applications, the time treatment must be controlled to avoid changes undesirables on the quality of texture and sensory aspects in the meat emulsions.
CRediT authorship contribution statement
C. Aguilar: Data curation, and development of methodology. J. Serna-Jiménez: Performing the experiments. E. Benitez: Application and analysis of statistical techniques. V. Valencia: Resources, provision of study materials. O. Ochoa: Formulation of overarching research goals and aims. L.I. Sotelo: Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Authors thank to Universidad de La Sabana and to the Centro de Investigación y Desarrollo Cárnico, Industria de Alimentos Zenú S.A.S. (Colombia) for the financial support of this study. Project Ing 129-2012.
References
- 1.D. Dave, A.E. Ghaly, Meat spoilage mechanisms and preservation techniques: A critical review, Am. J. Agric. Biol. Sci. 6 (2011) 486–510. https://doi.org/10.3844/ajabssp.2011.486.510.
- 2.A.J. Pellissery, P.G. Vinayamohan, M.A.R. Amalaradjou, K. Venkitanarayanan, Spoilage bacteria and meat quality, in: Meat Quality Analysis: Advanced Evaluation Methods, Techniques, and Technologies, Elsevier, 2019: pp. 307–334. https://doi.org/10.1016/B978-0-12-819233-7.00017-3.
- 3.V. Pothakos, F. Devlieghere, G. Huys, Psychrotrophic lactic acid bacteria (LAB) as a source of fast spoilage occurring on packaged and cold-stored food products, 2014. https://lib.ugent.be/catalog/rug01:002100670 (accessed July 13, 2020).
- 4.Mor-Mur M., Yuste J. Emerging bacterial pathogens in meat and poultry: An overview. Food Bioprocess Technol. 2010;3:24–35. doi: 10.1007/s11947-009-0189-8. [DOI] [Google Scholar]
- 5.Pinton M.B., Correa L.P., Facchi M.M.X., Heck R.T., Leães Y.S.V., Cichoski A.J., Lorenzo J.M., dos Santos M., Pollonio M.A.R., Campagnol P.C.B. Ultrasound: A new approach to reduce phosphate content of meat emulsions. Meat Sci. 2019;152:88–95. doi: 10.1016/j.meatsci.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Šojić B., Pavlić B., Ikonić P., Tomović V., Ikonić B., Zeković Z., Kocić-Tanackov S., Jokanović M., Škaljac S., Ivić M. Coriander essential oil as natural food additive improves quality and safety of cooked pork sausages with different nitrite levels. Meat Sci. 2019;157 doi: 10.1016/j.meatsci.2019.107879. [DOI] [PubMed] [Google Scholar]
- 7.Salejda A.M., Janiewicz U., Korzeniowska M., Kolniak-Ostek J., Krasnowska G. Effect of walnut green husk addition on some quality properties of cooked sausages. LWT - Food Sci. Technol. 2016;65:751–757. doi: 10.1016/j.lwt.2015.08.069. [DOI] [Google Scholar]
- 8.Rastogi N.K. Opportunities and challenges in application of ultrasound in food processing. Crit. Rev. Food Sci. Nutr. 2011;51:705–722. doi: 10.1080/10408391003770583. [DOI] [PubMed] [Google Scholar]
- 9.Horžić D., Jambrak A.R., Belščak-Cvitanović A., Komes D., Lelas V. Comparison of conventional and ultrasound assisted extraction techniques of yellow tea and bioactive composition of obtained extracts. Food Bioprocess Technol. 2012;5:2858–2870. doi: 10.1007/s11947-012-0791-z. [DOI] [Google Scholar]
- 10.Bevilacqua A., Sinigaglia M., Corbo M.R. Ultrasound and antimicrobial compounds: a suitable way to control Fusarium oxysporum in juices. Food Bioprocess Technol. 2013;6:1153–1163. doi: 10.1007/s11947-012-0782-0. [DOI] [Google Scholar]
- 11.Gao S., Lewis G.D., Ashokkumar M., Hemar Y. Inactivation of microorganisms by low-frequency high-power ultrasound: 1. Effect of growth phase and capsule properties of the bacteria. Ultrason. Sonochem. 2014;21:446–453. doi: 10.1016/j.ultsonch.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Krasulya O., Bogush V., Trishina V., Potoroko I., Khmelev S., Sivashanmugam P., Anandan S. Impact of acoustic cavitation on food emulsions. Ultrason. Sonochem. 2016;30:98–102. doi: 10.1016/j.ultsonch.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Chemat F., Ashokkumar M. Preface: Ultrasound in the processing of liquid foods, beverages and alcoholic drinks. Ultrason. Sonochem. 2017;38:753. doi: 10.1016/j.ultsonch.2017.01.041. [DOI] [PubMed] [Google Scholar]
- 14.Alarcon-Rojo A.D., Carrillo-Lopez L.M., Reyes-Villagrana R., Huerta-Jiménez M., Garcia-Galicia I.A. Ultrasound and meat quality: A review. Ultrason. Sonochem. 2019;55:369–382. doi: 10.1016/j.ultsonch.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Barretto T.L., Pollonio M.A.R., Telis-Romero J., da Silva Barretto A.C. Improving sensory acceptance and physicochemical properties by ultrasound application to restructured cooked ham with salt (NaCl) reduction. Meat Sci. 2018;145:55–62. doi: 10.1016/j.meatsci.2018.05.023. [DOI] [PubMed] [Google Scholar]
- 16.Cichoski A.J., Silva M.S., Leães Y.S.V., Brasil C.C.B., de Menezes C.R., Barin J.S., Wagner R., Campagnol P.C.B. Ultrasound: A promising technology to improve the technological quality of meat emulsions. Meat Sci. 2019;148:150–155. doi: 10.1016/j.meatsci.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 17.T.S. Awad, H.A. Moharram, O.E. Shaltout, D. Asker, M.M. Youssef, Applications of ultrasound in analysis, processing and quality control of food: A review, Food Res. Int. 48 (2012) 410–427. https://doi.org/10.1016/j.foodres.20.
- 18.Yusof N.S.M., Ashokkumar M. Ultrasonic transformation of micelle structures: Effect of frequency and power. Ultrason. Sonochem. 2015;24:8–12. doi: 10.1016/j.ultsonch.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Soria A.C., Villamiel M. Effect of ultrasound on the technological properties and bioactivity of food: A review. Trends Food Sci. Technol. 2010;21:323–331. doi: 10.1016/j.tifs.2010.04.003. [DOI] [Google Scholar]
- 20.D. Bates, A. Patist, Industrial applications of high power ultrasonics in the food, beverage and wine industry, in: Case Studies in Novel Food Processing Technologies, Elsevier, 2010: pp. 119–138. https://doi.org/10.1533/9780857090713.2.119.
- 21.Guerrero S., López-Malo A., Alzamora S.M. Effect of ultrasound on the survival of Saccharomyces cerevisiae: Influence of temperature, pH and amplitude. Innov. Food Sci. Emerg. Technol. 2001;2:31–39. doi: 10.1016/S1466-8564(01)00020-0. [DOI] [Google Scholar]
- 22.Huang G., Chen S., Dai C., Sun L., Sun W., Tang Y., Xiong F., He R., Ma H. Effects of ultrasound on microbial growth and enzyme activity. Ultrason. Sonochem. 2017;37:144–149. doi: 10.1016/j.ultsonch.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 23.Señorans F.J., Ibáñez E., Cifuentes A. New trends in food processing. Crit. Rev. Food Sci. Nutr. 2003;43:507–526. doi: 10.1080/10408690390246341. [DOI] [PubMed] [Google Scholar]
- 24.Turantaş F., Kiliç G.B., Kiliç B. Ultrasound in the meat industry: General applications and decontamination efficiency. Int. J. Food Microbiol. 2015;198:59–69. doi: 10.1016/j.ijfoodmicro.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 25.M. Ashokkumar, The characterization of acoustic cavitation bubbles - An overview, in: Ultrason. Sonochem., Elsevier B.V., 2011: pp. 864–872. https://doi.org/10.1016/j.ultsonch.2010.11.016. [DOI] [PubMed]
- 26.A.D. Hitchins, K. Jinneman, Y. Chen, BAM Chapter 10: Detection of Listeria monocytogenes in Foods and Environmental Samples, and Enumeration of Listeria monocytogenes in Foods | FDA, Bacteriological Analytical Manual. (2017). https://www.fda.gov/food/laboratory-methods-food/bam-chapter-10-detection-listeria-monocytogenes-foods-and-environmental-samples-and-enumeration (accessed July 13, 2020).
- 27.Myers Raymond H., Montgomery Douglas C. John Wiley & Sons; 2016. Response Surface Methodology: Process and Product Optimization Using Designed Experiments. [Google Scholar]
- 28.Jabbar S., Abid M., Wu T., Hashim M.M., Saeeduddin M., Hu B., Lei S., Zeng X. Ultrasound-assisted extraction of bioactive compounds and antioxidants from carrot pomace: A response surface approach. J. Food Process. Preserv. 2015;39:1878–1888. doi: 10.1111/jfpp.12425. [DOI] [Google Scholar]
- 29.Aguilar C., Valencia V., Ochoa O., Klotz B. improving food thermal processing: a death-time study on processed meat products. J. Food Process. Preserv. 2013;37:189–197. doi: 10.1111/j.1745-4549.2011.00627.x. [DOI] [Google Scholar]
- 30.N.J. Russell, Bacterial membranes: The effects of chill storage and food processing. An overview, Int. J. Food Microbiol., 2002: 27–34. https://doi.org/10.1016/S0168-1605(02)00176-9. [DOI] [PubMed]
- 31.Mueller E.A., Levin P.A. Bacterial cell wall quality control during environmental stress. MBio. 2020;11:1–15. doi: 10.1128/mBio.02456-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spinks A.T., Dunstan R.H., Harrison T., Coombes P., Kuczera G. Thermal inactivation of water-borne pathogenic and indicator bacteria at sub-boiling temperatures. Water Res. 2006;40:1326–1332. doi: 10.1016/j.watres.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 33.O’Brien W.D. Ultrasound-biophysics mechanisms. Prog. Biophys. Mol. Biol. 2007;93:212–255. doi: 10.1016/j.pbiomolbio.2006.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Juboori R.A., Aravinthan V., Yusaf T. Impact of pulsed ultrasound on bacteria reduction of natural waters. Ultrason. Sonochem. 2015;27:137–147. doi: 10.1016/j.ultsonch.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Delacour C., Lutz C., Kuhn S. Pulsed ultrasound for temperature control and clogging prevention in micro-reactors. Ultrason. Sonochem. 2019;55:67–74. doi: 10.1016/j.ultsonch.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 36.Cheng M., Li F., Han T., Yu A.C.H., Qin P. Effects of ultrasound pulse parameters on cavitation properties of flowing microbubbles under physiologically relevant conditions. Ultrason. Sonochem. 2019;52:512–521. doi: 10.1016/j.ultsonch.2018.12.031. [DOI] [PubMed] [Google Scholar]
- 37.M.A. Cabrera-Trujillo, L.I. Sotelo-Díaz, M.X. Quintanilla-Carvajal, Efecto de la amplitud y pulsación en ultrasonido de sonda a baja frecuencia sobre emulsiones aceite/agua, DYNA (Colombia). 83 (2016) 63–68. https://doi.org/10.15446/dyna.v83n199.56192.
- 38.Yan Z., Fang Q., Huang J., He B., Lin Z. Considerations and guides of the wattmeter method for measuring output acoustical power of Langevin-type transducer systems — II: experiment - PDF Free Download. Ultrasonics. 1997;35:543–546. https://kundoc.com/pdf-considerations-and-guides-of-the-wattmeter-method-for-measuring-output-acoustica.html (accessed July 13, 2020) [Google Scholar]
- 39.M. Abid, S. Jabbar, T. Wu, M.M. Hashim, B. Hu, S. Lei, X. Zhang, X. Zeng, Effect of ultrasound on different quality parameters of apple juice, Ultrason. Sonochem. 20 (2013) 1182–1187. https://doi.org/https://doi.org/10.1016/j.ultsonch.2013.02.010. [DOI] [PubMed]
- 40.Gani A., Baba W.N., Ahmad M., Shah U., Khan A.A., Wani I.A., Masoodi F.A., Gani A. Effect of ultrasound treatment on physico-chemical, nutraceutical and microbial quality of strawberry. LWT - Food Sci. Technol. 2016;66:496–502. doi: 10.1016/j.lwt.2015.10.067. [DOI] [Google Scholar]
- 41.Alenyorege E.A., Ma H., Ayim I. Inactivation kinetics of inoculated Escherichia coli and Listeria innocua in fresh-cut Chinese cabbage using sweeping frequency ultrasound. J. Food Saf. 2019;39 doi: 10.1111/jfs.12696. [DOI] [Google Scholar]
- 42.Ngnitcho P.F.K., Tango C.N., Khan I., Daliri E.B.M., Chellian R., Oh D.H. The applicability of Weibull model for the kinetics inactivation of Listeria monocytogenes and Escherichia coli O157: H7 on soybean sprouts submitted to chemical sanitizers in combination with ultrasound at mild temperatures. LWT - Food Sci. Technol. 2018;91:573–579. doi: 10.1016/j.lwt.2018.01.073. [DOI] [Google Scholar]
- 43.Liu J., Li L., Zhou L., Li B., Xu Z. Effect of ultrasound treatment conditions on Saccharomyces cerevisiae by response surface methodology. Microb. Pathog. 2017;111:497–502. doi: 10.1016/j.micpath.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 44.Zinoviadou K.G., Galanakis C.M., Brnčić M., Grimi N., Boussetta N., Mota M.J., Saraiva J.A., Patras A., Tiwari B., Barba F.J. Fruit juice sonication: Implications on food safety and physicochemical and nutritional properties. Food Res. Int. 2015;77:743–752. doi: 10.1016/j.foodres.2015.05.032. [DOI] [Google Scholar]
- 45.Barba F.J., Koubaa M., do Prado-Silva L., Orlien V., Sant’Ana A. de S. Mild processing applied to the inactivation of the main foodborne bacterial pathogens: A review. Trends Food Sci. Technol. 2017;66:20–35. [Google Scholar]
- 46.Inguglia E.S., Tiwari B.K., Kerry J.P., Burgess C.M. Effects of high intensity ultrasound on the inactivation profiles of Escherichia coli K12 and Listeria innocua with salt and salt replacers. Ultrason. Sonochem. 2018;48:492–498. doi: 10.1016/j.ultsonch.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 47.Chemat F., Zill-E-Huma, Khan M.K. Applications of ultrasound in food technology: processing, preservation and extraction, in. Ultrason. Sonochem., Elsevier B.V. 2011:813–835. doi: 10.1016/j.ultsonch.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 48.Jayasooriya S.D., Bhandari B.R., Torley P., D’Arcy B.R. Effect of high power ultrasound waves on properties of meat: A review. Int. J. Food Prop. 2004;7:301–319. doi: 10.1081/JFP-120030039. [DOI] [Google Scholar]
- 49.Al-Hilphy A.R., Al-Temimi A.B., al Rubaiy H.H.M., Anand U., Delgado-Pando G., Lakhssassi N. Ultrasound applications in poultry meat processing: A systematic review. J. Food Sci. 2020;85:1386–1396. doi: 10.1111/1750-3841.15135. [DOI] [PubMed] [Google Scholar]
- 50.Jambrak A.R., Lelas V., Herceg Z., Badanjak M., Batur V., Muža M. Advantages and disadvantages of high power ultrasound application in the dairy industry. Hrvatska mljekarska udruga. 2009 [Google Scholar]