Graphical abstract
Keywords: PUAE, Intensification, Bio-actives, Pomegranate peel, Antioxidant bioactivities, Pearson correlation
Highlights
-
•
PUAE an emerging and non-destructive, cost and energy effective extraction method.
-
•
Power and duty cycle had most significant effects for influencing PUAE efficacy.
-
•
The optimum conditions were 2.17 S/S ratio at 116 W power; 80% duty cycle for 6 min.
-
•
Significant correlation among responses were established during PUAE.
-
•
At optimal conditions, the extract showed remarkable antioxidant activity.
Abstract
Pomegranate peel (PP) is one of the interesting agri-food by-products because of its abundant bioactive phytochemicals. However, the bioactivity of valuable compounds is affected due to the extraction method used. A pulsed ultrasound-assisted extraction (PUAE) was carried out to intensify the extraction efficacy with reduced power and time. Influence of several process variables viz. peel solids/ solvent ratio, sonication power, duty cycle, and extraction time was studied using empirical quadratic models followed by multicriterial numerical optimization with respect to face-centered composite design. Power-duty cycle combination was found to be most significant (p < 0.05) for process intensification. The optimal process conditions of 2.17 g/100 mL S/S ratio at 116 W power with 80% duty cycle for 6 min resulted into 0.48 g/g yield, 177.54 mg GAE/g total phenolics content, 35.71 mg QE/g total flavonoids, 160.54 mg GAE/g antioxidant capacity, 21.65 mg cyn-3-glc/100 g anthocyanin content with 54.92 browning index in dry pomegranate peel. Significant Pearson correlation analysis was established in all responses with potent phenols and flavonoid relation with highest coefficient (r) 0.931. All response models were significantly validated with regression coefficient (R2) above 0.965. Remarkable antioxidant bioactivities were recorded for the resultant peel extract. Hence, it is strongly recommended that PUAE could be successfully applied for the intensification of the extraction process of bioactive from any peel and or plant systems with minimal process time and power consumption with a green label.
1. Introduction
Pomegranate (Punica granatum L.) belongs to the family Punicaceae is highly cultivated and consumed in several Asian countries and over the world [1]. The industrial processing of pomegranate fruits results in huge amounts of by-products, such as peels and seeds [2]. Though pomegranate peels (PP) are by-products, it constitutes around 78 to 80% of the pomegranate marc (on wet basis), which is also a comparably good source of high-value antioxidants than seeds [1], [3], [4]. PP and its natural extracts contain several primary and secondary metabolites as proteins, fats and oils, dietary fibers, sugars along with functional and nutraceutical ingredients. Including, polyphenolics, flavonoids, condensed and hydrolyzable tannins like penduncalin, punicalagin, and gallic acid, hydroxytyrosol, quercetin, ellagitannins and many others [2]. The natural extract of PP has antimicrobial potential [5] , along with several biological activities such as antioxidant, anti-inflammatory [6] , antihepatotoxic and antigenotoxic activities [7].
Conventionally, natural extracts were obtained using solid–liquid extraction considered as a unit operation using solvent, hydro-distillation, maceration, high-pressure water extraction methods [8], [9], [10]. However, unoptimized extraction process, longer extraction time, use of petroleum solvents, and more importantly, quality loss of thermally sensitive bio-actives are few reasons that inspire researchers to look for novel, cost-effective, safe, and innovative alternatives with durable and green extraction methods executed for food bio-actives [11], [12]. Principally, green extraction is known for the development and design of low energy consuming extraction process with the competence with the use of renewable plant material and alternate solvent, confirm the high safety and quality final extract [12] Despite so many novel extraction techniques such as microwave, pulse electric field, high pressure processing, instant controlled pressure drops, super and sub-critical fluid processing, extrusion, pulsed light and ohmic heating, industry and academician facing problems related to improvement innovation and optimization of processes and procedures and use of non-dedicated instruments [13].
The pulsed ultrasound-assisted extraction (PUAE) is one of the emerging techniques of extraction for bio-actives from PP and many other plant tissues [4], [11]. In comparison with traditional methods, PUAE is creditable for its specificity, sensitivity, economic feasibility, commerciality, green approach, and high yield [13], [14]. PUAE is capable of intensifying the extraction of bioactive molecules through improved mass and momentum transfer due to acoustic cavitation phenomena. The mechanical, physical, and chemical outbreaks implore into bubble cavitation with an interparticle strike, macro turbulence, and collapsing of cavities which breaks the cohesion of a liquid along with accelerated disruption of plant tissues. This cavity collapses producing shocks that imparts mechanical possessions which boost the extraction efficiency due to intensive mixing, disruption of cell walls, particle size reduction, and hot spots. Additionally, a structural alterations of plant tissue results into swelling and solvent permeation into the cells [13]. Besides, PUAE satisfy the six principles green extraction by yielding high value green extract as co-product of renewable PP using alternate solvent with economic, robust, and minimal technical troubles like tip erosion and equipment devaluation [4], [12]. Therefore, PUAE can be termed as a “green and environment-friendly” extraction method.
Several essential variables such as solvent type, concentration, solid: solvent ratio, intensity of sonication power, duty cycle of ultrasonic waves, and extraction time [4], [15] are some of the most critical parameters that affect yield and quality of extractants. However, prolonged extraction, high temperature, and amplitude have shown degradation of certain bio-molecules during sonication [16].
Considering the limitations of the use of synthetic bio-actives in foods and also due to the fact of substantial annual production of pomegranate peels as a by-product from pomegranate processing industry, PUAE extraction of bio-actives has created a lot of interest among researchers. However, meager statistical information available on the interaction effect of PUAE process variables on yield and quality of bio-actives of PP. Therefore, the present work was undertaken to investigate the optimum PUAE process variables viz. type of solvent, concentration of solvent, solid to solvent ratio (S/S), pulsed sonication power, duty cycle, and extraction time using experimental design and numeric optimization method based on desirability [17] which will result in the highest yield of quality bioactive compounds from the pomegranate peel of Indian Bhagava variety.
2. Materials and methods
2.1. Sample, chemicals, and solvents
The fresh pomegranate fruits (Bhagwa verity) were purchased from Sahakari Bhandar, a local fruit market in Mumbai, India. Cleaning, sorting, and washing of fruits was carried out. Fruits were peeled off by removing the arils, PP was dried at 45 °C in a vacuum oven for 36 h. The dried peels were further pulverized into powder using a laboratory grinder. This peel powder was passed through a standard sieve (500-µm mesh) and was kept in a water and airtight high-density polyethylene (HDPE) pouch at −18 °C till further experimentation.
Ethanol, methanol, acetone, HCl, Na2CO3, AlCl3, KCl, potassium acetate, and sodium acetate were procured from SDF Chemicals Mumbai, India. Reagents like DPPH, ABTS, Folin-Ciocalteu’s and high purity standards of gallic acid and quercetin were purchased from HiMedia, Mumbai, India. Sartorius arium® water purification unit, Mumbai used to get distilled water, all further supplementals were of analytical grade.
2.2. Pulsed ultrasound assisted extraction (PUAE) from PP powder
In the present study, Barson-sonifier-450, 20 kHz (Triton Technologies, Mumbai, India) was attached to a probe with a flat tip, used with 1/2′’ tapped horn at different output power intensity levels highest up to 350 W was used for PUAE. In the PUAE, the ultrasounds were pulsed at a fixed pulse duration of one pulse per second. A duty cycle that was denoted by percentage (10–90%) was the width of the pulse span with respect to the pulse duration. The pulse intensity ultrasonic vibrations at the tip of the probe were controlled by setting at any desired extent, in the range 10 to 100% of the total output power (The values of output power intensities were taken from manual provided by Triton Technologies, Mumbai, India).
In order to have PUAE treatment, different solvents such as water, methanol, ethanol, acetone at several concentrations (50, 60, 70, 80, 90, and 100%) were tested for maximum yield along with bioactive content. Among all of them, 50% ethanol was selected for further extraction. The PP powder was mixed with 100 mL ethanol (50%; v/v) with varying S/S ratios (2, 3, 5, 6, 8, and 10 g/100 mL) and subjected for pulsed sonication treatment. During extraction, solvent temperature was maintained by providing ice bath surrounded to the sample to avoid bioactive loss. The extraction sample was then centrifuged at 8000 rpm for 10 min at 4 °C to remove solid peel tissues. The resulting liquid extracts were concentrated to 20 mL using a rotary vacuum evaporator at 65 °C (IKA® RV 10 Rotary evaporator, ThermoFisher Sci., Mumbai, India) to recover the ethanol from the solvent and give green label to the extract. Further dried to solid in a vacuum oven at 45 °C until completely dry without damaging the quality of bio-actives. The results were the average of three replications with up to 5% average relative standard deviation.
2.3. Design of experiments for optimization of PAUE
PUAE processing variables, including S/S ratio (g/100 mL), sonication output power intensity (W), duty cycle (%), and extraction time (mins) were studied as per the face-centered composite design (FCCD). As per the experimental design, the influence of all factors was analyzed at 30 experimental conditions in coded form, including 16 factorials lower (-1) and upper (+1) levels, 8 axial levels (-α and + α), and 6 repetitions of middle level (0) as demonstrated in Table 1. All upper levels and lower levels were selected as per the results obtained from experiments of one variable at a time (OVAT) approach. six responses, i.e., extract yield (g/g), total phenolic content (TPC; mg GAE/g DW), total flavonoids content (TFC; mg QE/g DW), antioxidant capacity (AOX; mg GAEAC/g DW), total anthocyanin content (TAC; mg cyn-3-glc. Eq./g DW), and browning index (BI) were determined for each of the experimental test runs.
Table 1.
FCCD design matrix of the PUAE process parameters and resultant responses.
| Std Run | S/S Ratio (g/100 mL) | Power (W) | Duty Cycle (%) | Time (Min.) | Yield(g/g) | TPC (mg GAE/g DW) | TFC (mg QE/g DW) | AOX (mg GAE/g DW) | TAC (mg Cyn-3-glc eq/100 g DW) | BI |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | 2.00 | 70.00 | 20.00 | 1.00 | 0.33 ± 0.04de | 80.69 ± 3.8lm | 18.51 ± 2.7i | 122.94 ± 7.8bcde | 7.02 ± 1.00 h | 74.86 ± 3.4a |
| 2. | 10.00 | 70.00 | 20.00 | 1.00 | 0.25 ± 0.01e | 75.58 ± 4.2m | 9.31 ± 1.0j | 85.66 ± 6.1e | 7.39 ± 0.05 h | 43.96 ± 2.4fgh |
| 3. | 2.00 | 210.00 | 20.00 | 1.00 | 0.43 ± 0.02abcd | 131.78 ± 6.0efgh | 27.38 ± 1.4defg | 167.36 ± 8.6ab | 15.32 ± 2.43 g | 70.89 ± 4.4ab |
| 4. | 10.00 | 210.00 | 20.00 | 1.00 | 0.35 ± 0.01bcde | 111.46 ± 4.7hijk | 17.56 ± 3.0i | 120.14 ± 7.3cde | 14.24 ± 0.27 g | 43.46 ± 1.8gh |
| 5. | 2.00 | 70.00 | 80.00 | 1.00 | 0.41 ± 0.00abcd | 120.52 ± 6.4hij | 25.97 ± 0.5efgh | 142.56 ± 4.3abcd | 15.70 ± 0.94 g | 73.27 ± 5.2ab |
| 6. | 10.00 | 70.00 | 80.00 | 1.00 | 0.33 ± 0.02de | 106.32 ± 3.1ijk | 17.66 ± 2.9i | 134.13 ± 16.0abcd | 15.01 ± 0.17 g | 42.01 ± 1.4ghi |
| 7. | 2.00 | 210.00 | 80.00 | 1.00 | 0.43 ± 0.08abcd | 126.98 ± 2.4fghi | 28.26 ± 0.3cdefg | 144.31 ± 11.2abcd | 16.67 ± 0.33 fg | 64.11 ± 3.3bcd |
| 8. | 10.00 | 210.00 | 80.00 | 1.00 | 0.36 ± 0.02bcde | 101.27 ± 7.1jkl | 20.86 ± 0.7hi | 129.61 ± 6.4abcde | 17.67 ± 0.28cdefg | 41.27 ± 2.4ghi |
| 9. | 2.00 | 70.00 | 20.00 | 10.00 | 0.37 ± 0.05bcd | 111.49 ± 4.3hijk | 25.73 ± 2.7fgh | 135.98 ± 8.2abcd | 18.23 ± 0.49bcdefg | 66.09 ± 3.4abc |
| 10. | 10.00 | 70.00 | 20.00 | 10.00 | 0.34 ± 0.03cde | 92.60 ± 3.9klm | 20.74 ± 1.9hi | 112.22 ± 8.0de | 17.56 ± 0.07efg | 50.42 ± 2.3efg |
| 11. | 2.00 | 210.00 | 20.00 | 10.00 | 0.42 ± 0.09abcd | 162.05 ± 1.2abcd | 33.80 ± 0.1abc | 172.05 ± 10.5a | 23.85 ± 1.69a | 56.72 ± 3.3cde |
| 12. | 10.00 | 210.00 | 20.00 | 10.00 | 0.40 ± 0.09bcd | 131.34 ± 5.2efgh | 27.95 ± 2.2defg | 150.06 ± 12.1abcd | 22.67 ± 0.05abcd | 41.47 ± 1.5ghi |
| 13. | 2.00 | 70.00 | 80.00 | 10.00 | 0.46 ± 0.01ab | 176.66 ± 0.7ab | 34.99 ± 0.4ab | 167.31 ± 3.1ab | 21.59 ± 0.63abcdef | 53.52 ± 3.3ef |
| 14. | 10.00 | 70.00 | 80.00 | 10.00 | 0.43 ± 0.05abcd | 152.74 ± 1.8bcde | 30.50 ± 0.1bcdef | 163.98 ± 11.1abc | 22.24 ± 0.43abcde | 41.43 ± 2.4ghi |
| 15. | 2.00 | 210.00 | 80.00 | 10.00 | 0.44 ± 0.07abcd | 182.66 ± 0.4a | 37.07 ± 0.0a | 163.55 ± 11.7abc | 22.71 ± 1.39abc | 29.64 ± 1.4jk |
| 16. | 10.00 | 210.00 | 80.00 | 10.00 | 0.38 ± 0.04bcd | 151.88 ± 3.9cde | 32.33 ± 1.4abcd | 161.69 ± 8.4abc | 21.17 ± 0.62abcdef | 20.28 ± 1.2 k |
| 17. | 2.00 | 140.00 | 50.00 | 5.50 | 0.52 ± 0.10a | 171.72 ± 1.0abc | 35.43 ± 1.2ab | 165.93 ± 1.2abc | 23.60 ± 0.34a | 56.04 ± 4.3de |
| 18. | 10.00 | 140.00 | 50.00 | 5.50 | 0.44 ± 0.01abcd | 147.65 ± 0.5def | 30.42 ± 1.9bcdef | 133.35 ± 1.2abcd | 21.24 ± 0.43abcdef | 40.47 ± 3.4hi |
| 19. | 6.00 | 70.00 | 50.00 | 5.50 | 0.41 ± 0.02abcd | 120.18 ± 3.2hij | 27.43 ± 0.4defg | 122.61 ± 2.9bcde | 20.93 ± 0.02abcdef | 50.47 ± 4.2efg |
| 20. | 6.00 | 210.00 | 50.00 | 5.50 | 0.45 ± 0.03abcd | 144.84 ± 1.8defg | 31.24 ± 1.2bcdef | 136.89 ± 2.0abcd | 21.98 ± 0.51abcde | 42.22 ± 3.3ghi |
| 21. | 6.00 | 140.00 | 20.00 | 5.50 | 0.42 ± 0.01abcd | 122.54 ± 2.8ghij | 28.53 ± 0.7cdefg | 129.70 ± 0.7abcde | 21.40 ± 0.57abcdef | 42.14 ± 2.2ghi |
| 22. | 6.00 | 140.00 | 80.00 | 5.50 | 0.45 ± 0.01abc | 152.44 ± 3.6cde | 32.36 ± 1.0abcd | 143.27 ± 0.1abcd | 21.69 ± 0.79abcdef | 30.45 ± 1.5j |
| 23. | 6.00 | 140.00 | 50.00 | 1.00 | 0.37 ± 0.01bcd | 98.71 ± 4.7jklm | 24.43 ± 1.9gh | 114.26 ± 1.3de | 17.66 ± 0.47defg | 41.35 ± 3.4ghi |
| 24. | 6.00 | 140.00 | 50.00 | 10.00 | 0.43 ± 0.04abcd | 146.14 ± 4.1defg | 33.14 ± 1.5abcd | 145.61 ± 0.0abcd | 24.84 ± 0.05a | 33.41 ± 2.3ij |
| 25. | 6.00 | 140.00 | 50.00 | 5.50 | 0.44 ± 0.03abcd | 151.82 ± 3.6cde | 31.52 ± 1.5abcde | 138.04 ± 0.6abcd | 22.77 ± 0.65ab | 48.42 ± 4.2efgh |
| 26. | 6.00 | 140.00 | 50.00 | 5.50 | 0.46 ± 0.02ab | 153.73 ± 1.3bcde | 31.26 ± 1.1bcdef | 137.64 ± 0.1abcd | 23.21 ± 0.28ab | 44.00 ± 2.4fgh |
| 27. | 6.00 | 140.00 | 50.00 | 5.50 | 0.45 ± 0.01abcd | 156.83 ± 0.7bcd | 32.43 ± 0.2abcd | 143.78 ± 2.2abcd | 22.77 ± 0.25ab | 44.56 ± 3.3fgh |
| 28. | 6.00 | 140.00 | 50.00 | 5.50 | 0.45 ± 0.02abcd | 149.80 ± 0.7cdef | 32.36 ± 0.2abcd | 137.28 ± 0.4abcd | 22.14 ± 0.09bcde | 42.61 ± 3.5ghi |
| 29. | 6.00 | 140.00 | 50.00 | 5.50 | 0.45 ± 0.02abcd | 158.27 ± 0.7bcd | 31.93 ± 0.3abcd | 136.79 ± 0.4abcd | 22.51 ± 0.10bcde | 43.50 ± 2.1gh |
| 30. | 6.00 | 140.00 | 50.00 | 5.50 | 0.44 ± 0.01abcd | 153.67 ± 0.8bcde | 31.85 ± 0.1abcd | 141.90 ± 0.5abcd | 23.60 ± 0.02a | 45.91 ± 3.4fgh |
Each parameter was analysed and calculated in five times and values are expressed as mean ± Std. deviation. In the column, the different small letters of alphabets signify that the mean values belong to distinct subsets at 95% confidence interval. TPC: total phenolics content, TFC: total flavonoids content AOX: antioxidant capacity, TAC: total anthocyanin content, and BI: browning index
A quadratic equation was modeled (Eq.1) for all outcomes (Yi; responses) as a result of PUAE processing parameters, which were denoted by x1, x2, x3, and x4.
| (1) |
The proposed equation includes the estimated regression coefficients, βi (i = 1 to 14), along with all PUAE parameters in coded form (x1 to x4). In the quadratic polynomial model, all real values of parameters (X1: S/S ratio; g/100 mL, X2: input power; Watt, X3: duty cycle; %, and X4: time; min) were converted into the coded form using Eq. (2), (3), (4), (5).
| (2) |
| (3) |
| (4) |
| (5) |
2.4. Analytical methods
2.4.1. Determination and calculations of responses of PUAE.
PUAE yield (g/g), was reported as the ratio of the weight of extract obtained per 1 g of PP.
| (6) |
TPC of the extract was assessed with Folin-Ciocalteu assay using gallic acid (0.1–1 mg/mL) as standard [18]. The 1 mL reaction mixture contained an equal amount of diluted extract, 80% methanol, Folin-Ciocalteu reagent (100 µL each), and 700 µL Na2CO3 was mixed well by vortex and incubated in the dark for 20 min at ambient temperature. Immediately after incubation, samples were centrifuged (at 4 °C for 8000 rpm/10 min), and absorbance of decanting was recorded at 735 nm. Further gallic acid equivalent TPC (mg GAE/g DW) was reported.
TFC of the extract was determined with AlCl3 assay, using quercetin (10–100 µg/mL) as standard [19]. In total reaction mixture 0.5 mL diluted sample extract was added to 2.5 mL 60% methanol. After 5 min, 100 µL 10% AlCl3 and 100 µL 1 M potassium acetate solution was added; finally, 5 mL reaction volume was made up using distilled water and was incubated for 5 min. Immediately absorbance was recorded at 510 nm. Further quercetin equivalent total flavonoid content (mg GAE/g DW) was reported.
TAC of the extract was determined with pH difference technique using potassium chloride buffer (pH 1.0) and sodium acetate buffer (pH 4.5), as described by Lee et al. [20]. The test sample was mixed with KCl buffer (pH 1.0) and sodium acetate buffer (pH 4.5) at 1:4 proportion with sufficient dilution to achieve absorption within the linear range of the spectrophotometer at 520 and 700 nm. Absorbance was measured within 10 to 20 min of sample preparation. Results of TAC was demonstrated as mg cyanidin-3-glucoside equivalent per gram of peel (mg Cyn-3-gluc eq./g DW)
| (7) |
Where,
Aeq = (Abs at 520 nm – Abs at 700 nm) for pH 1.0 – (Abs at 520 nm – Abs at 700 nm) for pH 4.5, MW = 449.2 g/mol; DF = dilution factor; ε (molar extinction coefficient in liter per mol per cm) = 26,900
BI as calculated from color characteristics and was measured by using HunterLab colorimeter (Model No. Labscan XE, Hunter Associates Laboratory, Mumbai, Maharashtra India). The liquid extract obtained just after extraction were scanned to measure the L*, a* and b* values of color where L* represents lightness (0) or darkness (1 0 0), a* represents greenness (negative) or redness (positive) and b* represents yellowness (negative) or blueness (positive). The colorimeter was standardized against a black and white tile before use. Browning Index was calculated with the help of Eq. (8), given by Maskan [21] to evaluate the efficacy of PUAE.
| (8) |
Where, the chromaticity coordinate calculated from the XYZ tristimulus values, according to the following formula × = X/(X + Y + Z)
2.4.2. Antioxidant capacity of the extract
Antioxidant capacity of the extract was determined by the method of 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging capacity as described by Sonawane and Arya [22]. Prepared DPPH stock reagent, 80% methanol was diluted to get a working reagent whose absorbance was 1.0 (±0.02) at 517 nm. 350 µL diluted extract sample mixed with 650 µL of working DPPH reagent. The samples were incubated in the dark at ambient temperature, after 30 min of reaction, absorbance was recorded at 517 nm using a UV–Vis spectrophotometer (Jasco, V-730). Standard gallic acid solution (10 mg/mL) in 80% methanol at several concentrations (0.35 to 3.5 µg/mL) was used for a standard curve. Further gallic acid equivalent AOX capacity (mg GAEAC/g DW) was reported.
The ABTS AOX capacity was also determined by standard protocols [23], [24]. The ABTS reagent stock was freshly prepared by mixing 50 mg ABTS and 17 mg potassium persulfate in a test solvent on a magnetic stirrer for 4 h followed by incubation of 12 h in a cool and dark place. The prepared ABTS reagent further diluted to set absorbance 0.7 ± 0.02 at 734 nm to get working ABTS reagent. 350 µL diluted extract sample mixed with 650 µL of working ABTS reagent. The samples were incubated in the dark at ambient temperature. Immediately after 15 min of reaction absorbance was recorded at 734 nm using UV–Vis spectrophotometer (Jasco, V-730). The standard gallic acid curve was linear between 0.35 and 3.5 µg/mL
The DPPH and ABTS radical-scavenging capacity of extract was calculated as a percent inhibition using Eq. (9)
| (9) |
IC-50, i.e., the amount of extract concentration required to scavenge the 50% of free radicals of DPPH and ABTS was calculated by interpolation of the graph of inhibition percentage against extract concentration.
2.5. Optimization of PUAE parameters
The numerical optimization through overall desirability function (D) was adopted from More and Arya [17] to find out the optimum point of all process parameters (X1, X2, X3, and X4). It was carried out to maximize the overall desirability value using Eq. (10).
| (10) |
Equation (10) includes the desirability values (di) of each attribute, including factor and response (d1 to di) with their respective relative importance (r1 to ri) on 1 to 5 scale. The value of D as well as di were ranged between 0 and 1, which reflects the least to highest desirability. The individual desirability (di) was calculated through equations reported by More and Arya [17]. The optimized extraction parameters by the model were validated by experimenting with the closest possible settings for which coded values of factors were further converted to real values using Eq. (2), (3), (4), (5).
2.6. Statistical analysis
Design-Expert version 7.0.0 (Stat-Ease Inc., Minneapolis, MN) was used to design the experimental matrix as well as to evaluate the dataset and for optimization. All experimental variance within the results (n = 5) were analyzed using a one-way ANOVA. The Tukey's HSD tests at p-value: 0.05 and Pearson correlation analysis was executed using SPSS software (IBM SPSS Statistics software version 16.0). All graphs were plotted in Origin 8.5 pro version software.
3. Results and discussion
3.1. Factors screening and levels setting for experimental design by OVAT approach
Screening of various factors and their limits for experimental design were decided through OVAT experimentation. Methanol, ethanol, water, and acetone were varied at concentrations (v/v) from 50, 60, 70, 80, 90, and 100% were used as extraction solvents. The solvent selection was carried out based on the effect of the polarity on the bioactive compound, solvent penetration rate, mass transfer rate, and complexity of the sample matrix, which can remarkably affect the performance of extraction [25].
Several organic solvents were used to identify the bioactive extraction efficacy from PP samples. Table 2 shows influence of solvent type, and concentration (p < 0.05) on TPC, TFC, and AOX capacity. The maximum TPC (185.11 mg GAE/g PP) and TFC (36.59 mg QE/g PP), was observed in 60% and 50% acetone, respectively, followed by ethanol and methanol (Table 2). Further, maximum AOX capacity was recorded with 50% ethanol, followed by acetone and methanol. Water showed minimum extraction as compared to that of the other three solvents under study. The increased extraction yield was found at lowest concentration; furthermore, an increase in percent solvent concentration resulted in decreased TPC, TFC, and AOX capacity of extract (Table 2). Our results are in accordance with Kaderides, Goula, and Adamopoloulos [25] who also observed ethanol: water (1:1) mixture as the most appropriate solvent for the aqueous extraction of phenolic compounds from PP. A possible reason for this could be because of the fact that extraction intensifies with high polarity and reduces with a high molar mass of the solvent [25]. Parallelly lesser molar weight compounds were easily extracted in the solvent with low polarity. This might correspond to the “polarity versus polarity” concept. Moreover, a mixture of solvents can be more efficient for the extraction of phenolics than a single solvent. According to Spigno et al. [27] , more polar medium such as water increases the bio-actives extraction; in this way, both high and low polar molecules along with those of moderately polar molecules get solubilized in solvent-based on the “polarity vs. polarity” principle.
Table 2.
Selection of type of solvent and concentration of solvent.
| Solvent conc. (%) | Methanol |
Ethanol |
Acetone |
||||||
|---|---|---|---|---|---|---|---|---|---|
| TPC | TFC | AOX | TPC | TFC | AOX | TPC | TFC | AOX | |
| (Water) | 107.92 ± 2.36e | 25.86 ± 0.48c | 114.76 ± 0.60d | ||||||
| 50 | 130.41 ± 0.09c | 24.62 ± 0.32c | 122.78 ± 0.52b | 148.23 ± 1.93a | 30.56 ± 0.40a | 139.83 ± 0.78a | 180.91 ± 1.78b | 36.59 ± 0.34a | 133.13 ± 0.30a |
| 60 | 153.15 ± 1.91b | 26.55 ± 0.27b | 120.89 ± 0.20c | 121.13 ± 0.87b | 27.41 ± 0.44b | 128.77 ± 0.50b | 185.11 ± 1.01a | 35.83 ± 0.19b | 129.78 ± 0.55b |
| 70 | 125.26 ± 1.00d | 25.77 ± 0.11c | 112.79 ± 0.40e | 112.34 ± 1.43c | 24.42 ± 0.06c | 120.65 ± 0.34c | 172.07 ± 1.14c | 33.23 ± 0.12c | 119.49 ± 0.73c |
| 80 | 132.92 ± 1.48c | 25.12 ± 0.09c | 111.70 ± 0.62e | 87.66 ± 1.16d | 18.77 ± 0.25d | 95.24 ± 0.27d | 158.99 ± 1.15f | 30.02 ± 0.04d | 107.10 ± 0.46d |
| 90 | 110.33 ± 2.02e | 20.87 ± 0.14d | 102.20 ± 0.27f | 63.15 ± 0.75e | 11.77 ± 0.10f | 74.71 ± 0.76f | 128.86 ± 1.21e | 22.80 ± 0.20e | 76.29 ± 0.58e |
| 100 | 200.28 ± 1.28a | 29.43 ± 0.97a | 171.41 ± 0.90a | 85.72 ± 1.18d | 17.05 ± 0.73e | 92.37 ± 0.40e | 58.28 ± 1.97f | 9.85 ± 0.48f | 43.13 ± 0.59f |
TPC: total phenolics content, TFC: total flavonoids content, AOX: antioxidant activity. Values are expressed as mean ± Std. deviation (n = 5). In the column, the different small letters of alphabets signify that the mean values belong to distinct subsets at 95% confidence interval.
Comparably methanol was found to be more effective extraction solvent for polyphenols than ethanol despite their similar polarities. This behavior might owe to the lesser solubilization of bio-actives in ethanol, presumably because of the existence of the longer ethyl radicals over the methyl radicals existed in methanol, resultantly less solubilization of bio-actives [26]. Most of the foregoing reports showed that the acetone and methanol apparently become appropriate extraction solvents for bio-actives compounds extraction from PP [28]. Besides, an uprising research in the field of green extraction technologies are engrossed on eco-friendly and bio-based effective green solvents which could satisfy the commercial and technical requirements. Moreover, EU (European) environmental policy and legislation for 2010–50 period prioritize and USFDA suggests safe and green edible grade solvents are most suitable for bio-actives extraction purposes [12], [14]. Hence, based on these experimental results 50% ethanol was observed more promising over all other solvent compositions. Even so, to satisfy green extraction principle, water–ethanol (1:1) as extractant solvent was attempted for intensification through pulsed ultrasound treatment for the achievement of maximum yield of bio-actives from PP.
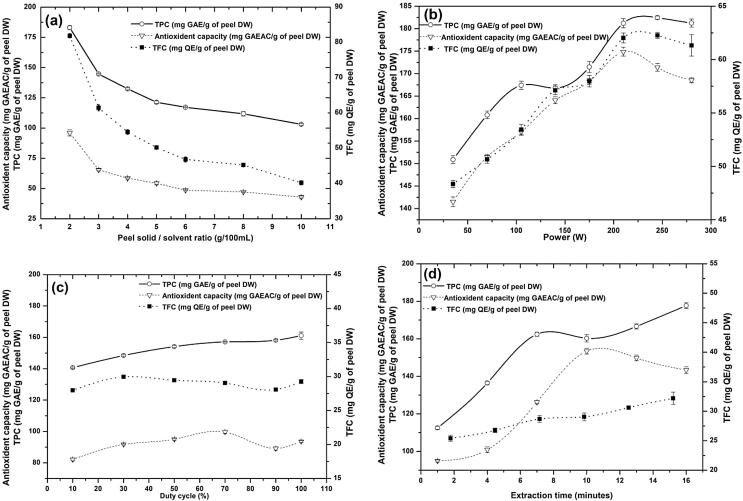
The choice of the ratio of solvent with the plant material is critical in PUAE as it is well evident that that the cavitation phenomenon is strongly influenced by the physical properties of the solvent. Moreover, the S/S ratio can significantly influence the yield and efficacy of the extraction process [13]. Therefore, the suitable range of S/S ratio was also selected based on its effect on TPC, TFC, and AOX capacity. It was studied with trials of seven different ratios (2, 3, 5, 6, 8, and 10 g/100 mL) for 10 min extraction using previously selected 50% ethanol at 105 W power and 50% of duty cycle pulse rate. Results showed that the TPC, TFC, and AOX capacity of extract was significantly decreased (p < 0.05) with the increased contents of solids in solvent (Fig. 1a). Compatible with mass transfer phenomenon, at a higher S/S ratio, the concentration gradient between solute and solvent was considerable, which plays a vital role as a driving force during mass transfer [29]. Nevertheless, a high S/S ratio may cause more solvent consumption for extraction. To avoid this optimum S/S ratio was investigated within range of 2 to 10 g/100 mL in experimental design.
Fig. 1.
Effect of (a) peel solid / solvent ratio, (b) power, (c) duty cycle, and (d) extraction time on total phenols, flavonoids, and antioxidant capacity of extract.
Fig. 1b shows the effect of power on TPC, TFC, and AOX capacity for extraction, which will be used to decide the range of power for polynomial modeling. The effect was evaluated within eight different output power intensities ranging from 35 to 275 with a constant 50% duty cycle for 10 min. All the responses showed a significant increase from 35 to 210 W power. Later it was slightly fluctuating. The responses found at 210 W were 181.20 mg GAE/g of TPC, 62.03 mg QE/g of TFC, and 174.87 mg GAE/g of AOX capacity. This trend was observed by several other researchers viz., Foujdar et al.; Pan et al.; Sharayei et al. [3], [4], [11]. The improvement in extraction of all the three responses might be due to the cavitation, which might have resulted in more tissue damage and elevation of mass transfer resulting in higher extraction of TPC, TFC, and AOX [30]. According to Khadhraoui et al. [31] the bioactive diffusion phenomenon was observed due to ultrasonication using the second Fick’s law. Considering the above facts, a further effect of power was modeled with a range of 70 to 210 W using the quadratic equation.
The upper and lower limit of duty cycle (%) of the pulse rate was also screened by evaluating its effect on bioactive yield. The effect was assessed within six different duty cycles (10, 30, 50, 70, 90, and 100%) for 10 min at 140 W power. Significant rise in TPC from 10 to 100% of the duty cycle was recorded whereas, an increased TFC and AOX capacity of extract were noted to its equilibrium at 70% duty cycle (Fig. 1c). Probable explanation for such behavior is that higher percentage of pulse cycles may cause greater tissue rupturing results in more release of phenolics. Processing at mild temperature showed significant extraction efficiency than the higher temperature, which was further increased at more pulse rate [32], [33]. Moreover, Sicaire et al. [34] describes the degradation of bio-actives at higher cavitation due to formation of hydroperoxide by oxygen and the metallic probe. Therefore, in the present study, further optimum duty cycle was studied within range of 20 to 80% using quadratic polynomial modeling.
The extraction time for quadratic polynomial modeling was decided by studying the yield of bioactive content at six different time spans (1, 4, 7, 10, 13, and 16 min) with 50% ethanol and middle points of all previously analyzed process conditions, i.e., 6 g/100 mL S/S ratio, 140 W power, and 50% duty cycle. A significant rise in TPC at 1 to 7 min (p < 0.05) and later on at 7 min to 16 min recorded. However, at 7 to 10 min time span, an insignificant difference was noted. Theoretically, extraction efficacy was substantially increased with longer extraction time at constant ultrasonic intensity. From Fig. 1d, it can be seen that long extraction time proportionally intensifies the TPC, TFC and AOX. As it happens, a longer time of extraction may improve the more tissue disruption so as more solvent can diffuse in cells and leach out the bio-actives [16]. A similar trend was observed by Foujdar et al.; Pan et al.; Tabaraki et al. [3], [4], [14]. In the case of TFC significant rise between 1 and 3 min was achieved later on; a substantial increase from 10 to 16 min was noted; nonetheless, in between, there was no significant increment in TFC. This might be due to temperature rise owing to longer extraction time [33] that might have affected the antioxidants and consequently showed some insignificant rise in AOX at the middle points of experiments. This might be due to the elevated vapor pressure resulted in the suppression of cavitation at very high temperature [35]. An insignificant rise in the AOX capacity of extract was recorded after 10 min (p < 0.05) of extraction (Fig. 1d). Similar findings were observed by Sood and Gupta [10] during the extraction of TPC and pigments from grape seeds. Considering the facts, the yield of bio-actives as a function of extraction time was modeled within range of 1 to 10 min using polynomial equations.
3.2. Experimental design
The limits of process variables were adopted from previous OVAT experiments and varied as per face centered composite design (FCCD). All FCCD points were used in coded form (Eq. (2), (3), (4), (5)). The lower (-1) and upper (+1) limits for variable were S/S ratio: 2 to 10 (g/100 mL); ultrasound power: 70 to 210 (W); duty cycle: 20 to 80 (%), and extraction time 1 to 10 (minutes). Table 1 illustrates the experimental design matrix, including all 30 experimental points of variables and respective responses.
From equation (1), the quadratic relationship was modeled for each response (Yi) as a result of all process parameters. The interactive behavior of all four variables on all responses was discussed using coefficients of each term (coded) (Table 1). Only the significant interactions were explained with the help of response surfaces.
3.3. Response surface models
The influence of varied PUAE parameters viz. S/S ratio (g/100 mL), power (W), duty cycle (%), and extraction time (minutes) on extract yield (g/g), TPC (mg GAE/g of peel DW), TFC (mg QE/g of peel DW), AOX capacity (mg GAE/g of peel DW), TAC (mg Cyn-3-glc eq./100 g DW), and BI were effectively modeled employing a quadratic equation (Eq. (1)). The coded form of coefficients of all linear, square, and interaction terms in the model has been summarized in Table 3.
Table 3.
The coefficients of the coded terms significant at p < 0.05 in the quadratic imperial model indicating the effect of PUAE process parameters on each response extraction.
| Yield (g/g) | TPC (mg GAE/g DW) | TFC (mg QE/g DW) | AOX (mg GAE/g DW) | TAC (mg Cyn-3-glc eq/100 g DW) | BI | |
|---|---|---|---|---|---|---|
| Intercept | 0.45 | 148.97 | 31.93 | 137.27 | 22.94 | 42.44 |
| x1- S/S Ratio (g/100 mL) | −0.031 | −10.76 | −3.32 | −10.62 | −0.31** | −10.02 |
| x2- Power (W) | 0.018 | 11.53 | 2.53 | 8.80 | 1.70 | −4.78 |
| x3-Duty cycle (%) | 0.022 | 14.00 | 2.81 | 8.57 | 1.49 | −5.22 |
| x4- Time (min.) | 0.021 | 19.68 | 4.79 | 11.75 | 3.79 | −5.68 |
| x1x2 | −1.683E-003** | −2.84* | −0.051** | −0.81** | −0.15** | 0.94** |
| x1x3 | −1.832E-003** | −1.22** | 0.31* | 6.37 | 0.12** | 0.86** |
| x1x4 | 0.012 | −2.44** | 0.92 | 3.54 | −0.15** | 3.75 |
| x2x3 | −0.020 | −10.61 | −1.44 | −10.10 | −1.39 | −2.01 |
| x2x4 | −0.013 | 0.38** | −0.21** | −0.51** | −0.50 | −3.06 |
| x3x4 | 7.998E-004** | 6.93 | 0.42 | 1.98* | −0.98 | −3.58 |
| x1x1 (x12) | 0.025 | 15.75 | 0.96 | 14.33 | −0.62** | 8.21 |
| x2x2 (x22) | −0.025 | −11.42 | −2.63 | −5.56* | −1.58 | 6.30 |
| x3x3 (x32) | −0.016 | −6.44* | −1.52 | 1.18** | −1.49 | −3.75* |
| x4x4 (x42) | −0.052 | −21.50 | −3.19 | −5.37* | −1.79 | −2.66** |
TPC: total phenolics content, TFC: total flavonoids content, AOX: antioxidant capacity TAC: total anthocyanin content, and BI: browning index, x1, x2, x3 and x4 denotes the dimensionless coded values of peel solid to solvent ratio (g/100 mL), power (W), duty cycle (%), time (min), not significant; **p > 0.10; *0.05 < p < 0.1
3.3.1. Linear terms
The changes in yield, TPC, TFC, AOX, and BI of extract were significantly affected by linear terms in the quadratic equation (Eq. (1)) (Table 3). Except for TAC, the corresponding linear terms were insignificant (p > 0.05). The linear term of extraction time (minutes) was most significantly affected towards positive change in almost all the responses except for BI showing negative impact followed by pulsed sonication duty cycle, power, and S/S ratio. The most positive effective factor on extract yield was the duty cycle, followed by extraction time and sonication power. TPC and TFC were found to be significantly influenced by positive linear terms of extraction time next to duty cycle and power (p < 0.05). As long as for AOX and TAC the highest influencing variables were extraction time followed by power and duty cycle, respectively. The positive linear coefficients of sonication power, duty cycle, and extraction time indicate the increase in the yield, TPC, TFC, AOX, and TAC with the increment of factor values. Contrary, the negative terms of S/S ratio described the reduction of the extract yield, TPC, TFC, and AOX with an increase in the S/S ratio when all other terms were constant. The negative linear terms of all four variables signify that the increment of variables reduces the browning index of resultant extract.
3.3.2. Interaction terms
The combined interaction outcomes between all four variables in eq. (1) (x1x2, x1x3, x1x4, x2x3, x2x4, and x3x4) on yield, TPC, TFC, AOX, TAC, and BI was evaluated. The overall combined impact of term x1x2 (S/S ratio-extraction time) on all the responses was insignificant whereas, AOX capacity was found to be the only misfit, to this interaction of S/S ratio-duty cycle (x1x3) contributed significantly (p < 0.05) for intensification of antioxidant capacity. (Table 3). The only interaction term x2x3 showed significantly negative influence in PUAE of all the responses. In the case of x1x4 interaction, which influenced all the responses significantly except for TPC and TAC. Parallel to this; yield, TAC, and BI was significantly reduced due to x2x4 terms. Contrary to this, only yield and AOX was insignificant due to x3x4 interaction. Along with TPC and TFC gave positive effect while AOX and BI showed a negative impact (Table 3).
3.3.3. Square terms
All the square terms of process variables in the developed model significantly contributed to the changes in yield and TFC. Further, only exceptions have been realized in the case of change in TPC and TAC, for which the square term of duty cycle and S/S ratio respectively was not contributing significantly at p < 0.05 (Table 3). Only the square term of S/S ratio had a positive impact on AOX capacity of extract. Whereas BI was positively influenced by square terms of S/S ratio and sonication power at p < 0.05.
3.3.4. ANOVA
While fitting to quadratic model to polynomial equation (Eq. (1)), the coefficient of determination (R2) values for extract yield, TPC, TFC, AOX, TAC, and BI were 0.982; 0.979; 0.995; 0.975; 0.978, and 0.966 respectively and their respective adjusted R2 were 0.964; 0.961; 0.989; 0.952; 0.958 and 0.934 respectively (Table 4). Very closed R2 and adj. R2 values specify the highly desirable fit of the model in the equation. For the model of response, p-values<0.0001 and very high F-values for each model also advised the significance of models for PUAE. For every insignificant Flof value (lack of fit) for all models (0.125; 0.061; 0.161; 0.138; 0.062; 0.103) designating the deviation in the experimental results is not because of noise in the system; apparently, differing PUAE system parameters might be responsible for this (Table 4).
Table 4.
ANOVA data of each model describing the influence of PUAE process parameters on each response extraction.
| Yield (g/g) | TPC (mg GAE/g DW) | TFC (mg QE/g DW) | AOX (mg GAE/g DW) | TAC (mg Cyn-3-glc eq/100 g DW) | BI | |
|---|---|---|---|---|---|---|
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| plof -value | 0.125 | 0.061 | 0.161 | 0.138 | 0.062 | 0.103 |
| F-value | 56.52 | 51.22 | 198.91 | 41.53 | 49.00 | 30.30 |
| Flof -value | 2.90 | 4.31 | 2.51 | 2.74 | 4.25 | 3.25 |
| R2 | 0.982 | 0.979 | 0.995 | 0.975 | 0.978 | 0.966 |
| Adjusted R2 | 0.964 | 0.961 | 0.989 | 0.952 | 0.958 | 0.934 |
TPC: total phenolics content, TFC: total flavonoids content, AOX: antioxidant activity, TAC: total anthocyanin content, BI: browning index, and lof: Lack of fit
In the current study, FCCD was used to plan the experiments. The interactions within all variables influencing the extraction efficacy and quality bio-actives have been inspected using response surface plots and quadratic equation (Eq. (1)), and majorly affecting selective interactions of variables on responses were discussed using response surfaces (Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7).
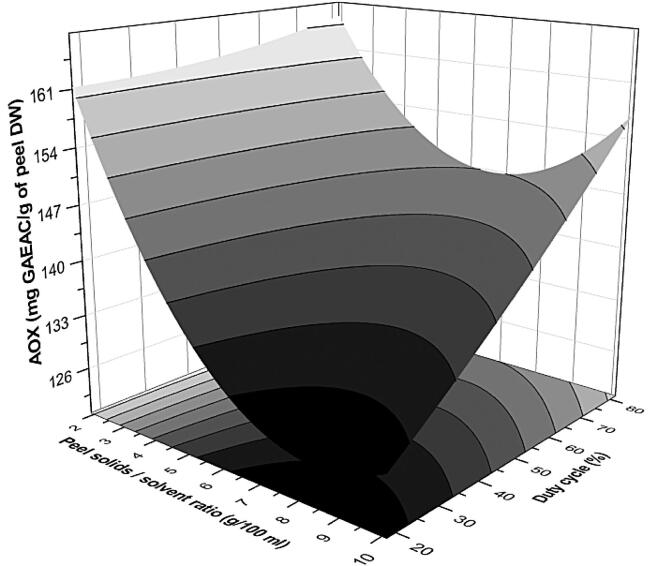
Fig. 2.
Response plots representing the influence of peel S/S ratio-duty cycle interaction on AOX capacity of extract during PUAE from pomegranate peel.
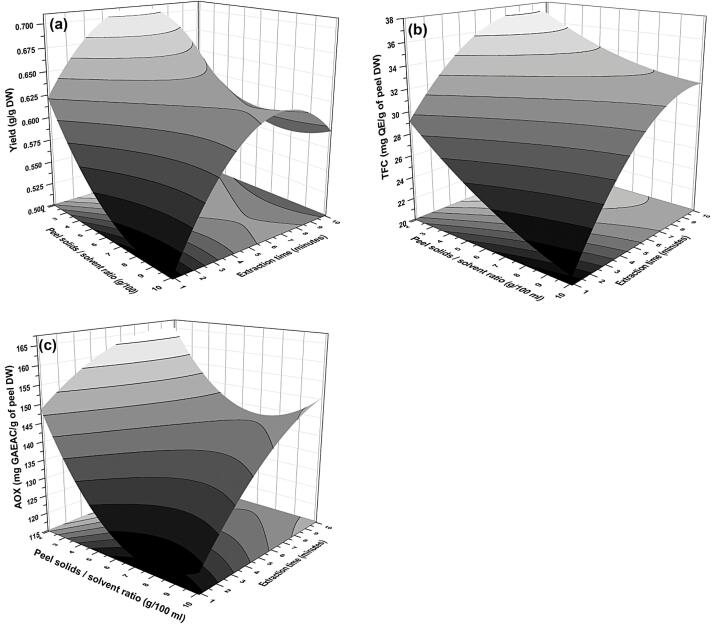
Fig. 3.
Response plots representing the influence of peel S/S ratio-extraction time interaction on (a) yield, (b) TFC, and (c) AOX capacity of extract during PUAE from pomegranate peel.
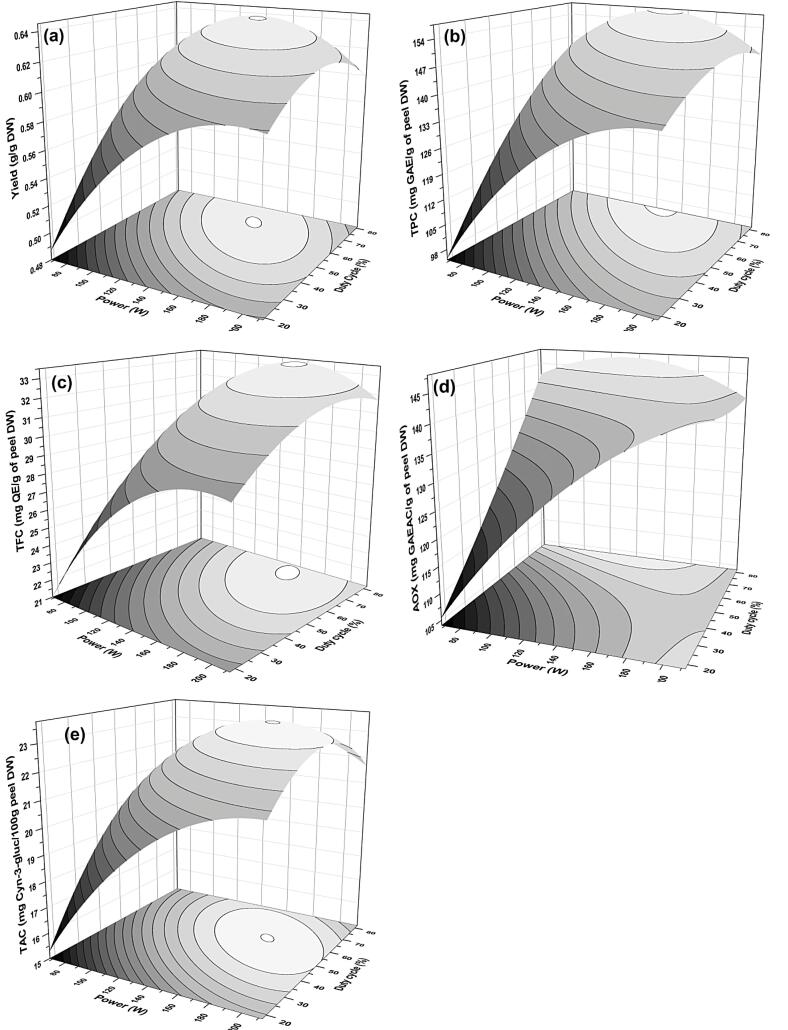
Fig. 4.
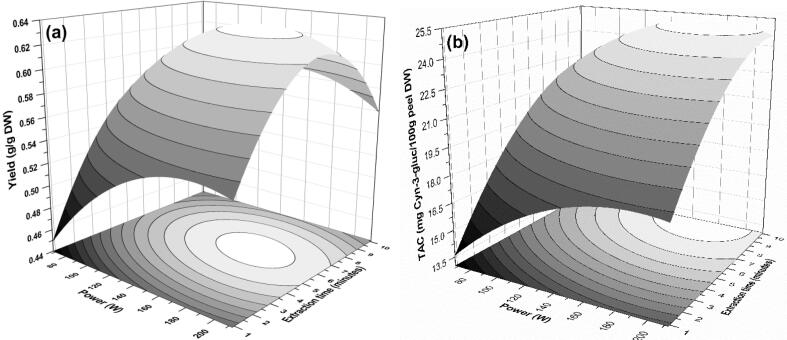
Response plots representing the influence of power-duty cycle interaction on (a) yield, (b) TPC, (c) TFC, (d) AOX, and (e) TAC of extract during PUAE from pomegranate peel.
Fig. 5.
Response plots representing the influence of power-extraction time interaction on (a) yield and (b) TAC of extract during PUAE from pomegranate peel.
Fig. 6.
Response plots representing the influence of power-extraction time interaction on (a) TPC, (b) TFC, and (c) TAC of extract during PUAE from pomegranate peel.
Fig. 7.
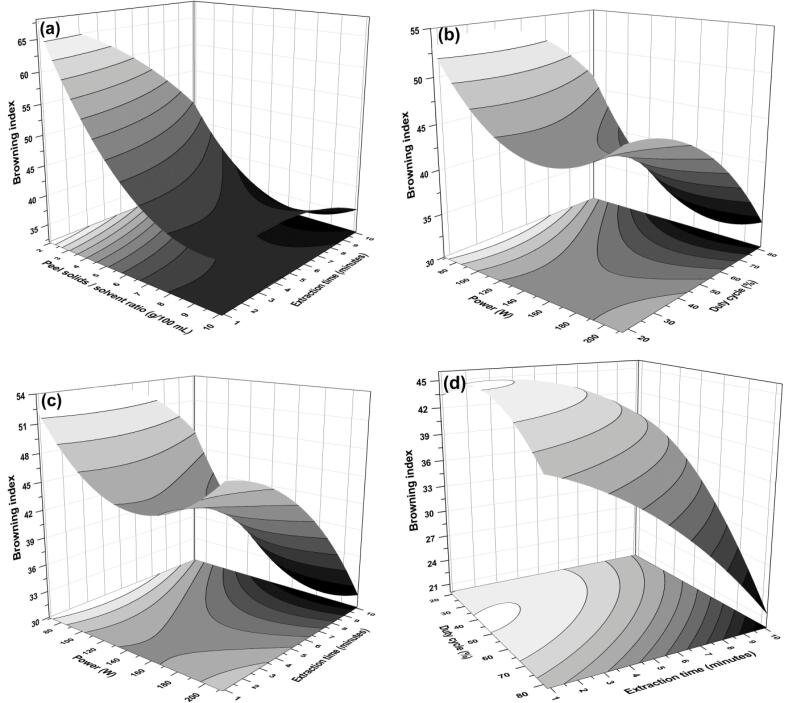
Response plots representing the influence of several interactions viz. (a) S/S ratio-extraction time, (b) power-duty cycle, (c) power-extraction time, and (d) duty cycle-extraction time on browning index of extract during PUAE from pomegranate peel.
3.4. Response surface models:
3.4.1. Effect of S/S ratio-duty cycle (x1x3)
The higher concentration of solids in the solvent showed the lower AOX capacity of extract (Fig. 2). This might be due to the reason that a lower S/S ratio facilitates the diffusion of active ingredients in solvent and thus accelerates mass transfer [36]. A similar phenomenon was observed by Ying et al. [37]. The effect of the duty cycle on AOX can be seen from Fig. 2; with an increase in duty cycle from 20 to 80%, a significant improvement in the AOX capacity of extract was recorded. However, at 100% duty cycle, a decreased AOX was noted.
Probable reasons behind this observation might be due to the pulse inflection of the ultrasound that conquered the generation of bubbles and thus helped to approve the clearance of the cavitation area and intensified the sonochemical reactions. Oppositely continuous duty cycle restricted the cavitation, due to volumetric oscillation, the clusters of degassing bubbles may curtail the propagation of ultrasound by absorbing and scattering the sound waves, which result in the intense expansion and compression of these bubbles and thus limit the sonochemical reaction [36]. The results indicate that the application of pulsed ultrasound with the proper duty cycle might be more efficient over continuous sonication and reduce energy consumption, which is in support of previous findings. Kobus [38] found that pulsed sonication effectively intensified the extraction of bio-actives from dried valerian roots.
3.4.2. Effect of S/S ratio-extraction time (x1x4)
Interaction of S/S ratio to extraction time on all the responses was studied. Among all only yield (g/g), TFC (mg QE/g DW), AOX (mg GAE/g DW) and BI were significantly affected by x1x4 interaction (Table 3). With the reference of the interaction (x1x4), the saddle effect shows the ups and downs in response values of the yield of extract, and TFC (Fig. 3a and b). The yield was observed to be very elevated at 2 g/100 mL S/S ratio and 5 to 7 min of extraction; however, as the extraction time exceeded beyond 7 min, a decreased yield was noted. However, particle collisions and degradation of tissue by cavitation effect promote solvent penetration resulting in enhanced extraction rate [39]. The results are contrary to this relationship could be due to the reduction of the driving force of mass transfer [40] or accountable coefficients (-ve) of the square term of extraction time. Moreover, cavitation intensity also decreases due higher S/S ratio as surface tension and vapor pressure of solvent increase [13]. Evident to this, Xu and Pan [36] shows similar results.
Fig. 3c showed the combined interaction of S/S ratio and time. It was observed that the AOX capacity was significantly (p < 0.05) increased with the increase in extraction time even at a lower S/S ratio. Moreover, the combined saddle effect in Fig. 3c also showed the rise in S/S ratio along with the extraction period, reduced AOX capacity. This could be justified through the certainty of prolonged extraction period with a high S/S ratio that could degrade the natural antioxidants. Sood and Gupta [10] also reported a similar effect of S/S ratio on antioxidants. Nevertheless, the free radicals and hydroperoxide generated due to cavitation can be a probable reason for the lesser antioxidant capacity [34]. This confirmed that the AOX capacity of the extract of PP powder was significantly dependent upon PUAE parameters.
3.4.3. Effect of power-duty cycle (x2x3)
Ultrasonic power intensity has been known for intensification of extraction since, at higher sonic power. The high release of cavitation effect due to increased compression and relaxation cycles of ultrasound waves [11] In the present study the interaction of ultrasonication power and pulse duty cycle (x2x3) was significantly affecting all the responses of PUAE (p < 0.05). The negative interaction coefficient observed from Table 3, revealed that the higher output power at more pulse rate improved the yield, TPC, TFC, and TAC of extract up to equilibrium stationary ridge point. It can be easily reflected from maximum parabolic ellipses of Fig. 4a, b, c, and e. This interaction could be attributed to the impact of simultaneous high output power and duty cycle. Continuous ultrasound radiation through a probe or bath might generate short-term hot spots due to the more violent collapse of bubbles, [16] subsequent chain disruption mechanism in a special order of local erosion, shear forces, sono-poration, fragmentation, capillary effect, and detexturation [31]. This resulting high temperature could upshoot in accumulation and swelling of cellulose, hemicellulose, and complex polysaccharides present in PP due to high power ultrasound [16], [42] , which restricts the mass transfer of bio-actives from peel matrix to solvent. Li et al. [41] findings are in agreement with ours reporting on a strong duty cycle affecting on curcuminoids yield along with Kazemi et al. and Sun et al. [16], [42].
The hyperbolic interaction in Fig. 4d indicated that the duty cycle had a more substantial influence than that of power as a significantly higher AOX was observed at the maximum level of duty cycle even at lower power level. Fig. 4d, interaction signifies that the adequate use of the duty cycle could be advantageous to the continuous mode of ultrasonication with minimum electrical energy utilization [35]. In support of current findings, Kazemi et al. [16] reported the higher power of ultrasonication could impart the dual effect on the AOX capacity of extract. Parallelly, the power of ultrasonication radiation also showed both positive and negative results on the AOX extraction. Sivakumar et al. [43] also explain the effect of the duty cycle as a key role in beetroot pigment extraction and phenolics from strawberries, respectively. Contrary to this, Luque-Garcia and De Castro [44] reported that a duty cycle was insignificantly affecting AOX capacity that might be due to the extraction of other compounds or systems.
3.4.4. Effect of power-extraction time (x2x4)
From the interaction of power-extraction time, at 50% duty cycle, and 6 g/100 mL S/S ratio, the yield of extract was observed in the range of 0.44 to 0.64 g/g of peel powder (Fig. 5a). From Table 3, it was noted that coefficients of interaction x2x4 were negative, conflicting linear term coefficients. In this interaction, maximum yield was at the center point of extraction time while increasing from the center point of power towards the increment. Perhaps this behavior could be attributed due to the multilevel cavitation effect and mechanical vibrations ultrasonic radiations [45]. Even so far, past report [46] described the extended PUAE period coming up with the lower extraction yield. Further, a minimum ultrasonic exposure period could also be essential to initiate the extraction process in PUAE [47].
The synergistic effect in the response plot (Fig. 5b) described maximum retention of TAC at maximum power and extraction time. The negative coefficients probably observed due to positive linear and negative quadratic coefficients of respective terms (Table 3). The longer treatment period extended the residence time of the sample with the extraction medium consequently improving the dissolution of anthocyanins and other bio-actives. Parallel increase in the power of ultrasound intensifies this effect [11]. This interaction also showed better results to Li et al. [48] for TPC, TFC, and pro-anthocyanidins along with Ghafoor et al. [49] also describes treatment time is in agreement with the findings for grape extracts.
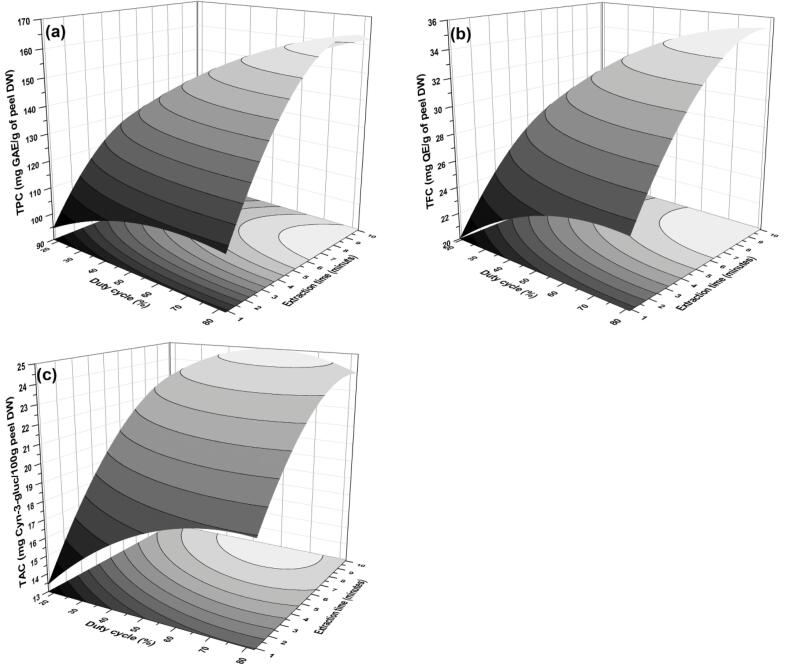
3.4.5. Effect of duty cycle-extraction time (x3x4)
The combined influence of duty cycle and extraction time on extraction efficacy has been deliberated. Among all significantly affected responses (Table 3), the synergistic relationship was observed in case of TPC and TFC (Fig. 6a and b), which means an increase in duty cycle along with extraction time significantly increases the phenolic and flavonoid content of the extract. This relation can be described as the duty cycle that might enhance the TPC and TFC content due to the power of extraction, which is at the middle point along with the respective positive linear coefficient in this interaction [16]. Whereas, parabolic interaction between duty cycle and extraction time describes the more extraction time even at lower duty cycle increases the TAC of extract (Fig. 6c). Probably in PUAE the low duty cycle is enough as it provides better efficacy because of non-steady mass transfer of anthocyanins from PP to extraction medium. Ho et al. [50] describes an akin tendency in case of TPC extraction along with Pan et al. [4].
3.4.6. Effect of process parameters on browning index
The extract color is also an important quality attribute that often reflects the type of process or extraction being used. The most abundant pigment responsible for extract color is anthocyanins [50]. The low BI demonstrates the development of brown color [51]. This might be due to the presence of tannins or enzymatic and non-enzymatic browning. In this study, efforts were made to correlate the qualitative and quantitative efficacy of bio-actives by evaluating the BI during PUAE. Significant interaction effect of S/S ratio-extraction time on the browning index of the extract was observed. Moreover, it can be observed that the interaction has great influence on the minimization of browning (Fig. 7a).
However, positive interaction coefficient owing to the strong positive effect of square term of S/S ratio. The negative coefficient of term of x2x3 and x2x4 (Table 3) with hyperbolic effect in response plot (Fig. 7b and c) signifies the increment in power had vigorous decrement in browning index compare to duty cycle and extraction time. above 60% of duty cycle and 4 min of extraction decreases browning index significantly (p < 0.05). Further, the antagonistic effect observed at higher values of duty cycle along with extraction time. Fig. 7d and negative coefficients in Table 3 reflect the decrement in BI due to x3x4 interaction. From the observed results, it can be described that BI at mild pulsed ultrasound treatment was significantly higher than that of severe extraction conditions. This might be due to lesser physical damage from cavitation, which otherwise would have caused enzymatic browning or more degradation of tannins. Similar trend was observed by Dibanda et al. [52] during study of microwave bleaching of TPC and AOX capacity. Browning also can be imparted due to 1-Methylcyclopropene (1-MCP) is a kind of lipid peroxidation product of the membrane [53] , which can be controlled by potent antioxidants such as phenols and anthocyanins. Evident to that Duan et al. [54] explained that the degradation of phenols and anthocyanins results in increased browning index. Correlation analysis of these observed results could justify that the lower BI indicates the extraction yield of TPC, TFC, and anthocyanins that get intensified by PUAE.
3.5. Pearson correlation analysis
Multivariate Pearson correlation analysis between all the responses performed to assess the relationship among responses during the PUAE of PP can be seen in Table 5. Almost all the responses showed significant positive relationship with each other at p < 0.05. Exceptionally BI showed significant negative relation with TAC. While TPC strongest positive correlation with flavonoid followed by yield, anthocyanin, and antioxidant capacity with Pearson’s correlation coefficients (r) of 0.931, 0.852, 0.827, and 0.788, respectively (Table 5). This reflected flavonoids as majorly contributing compounds for TPC in PP; this finding was in line with Skenderidis et al. [55]. The yield of the extract was found significantly correlating with flavonoid with the second highest coefficients (r: 0.903) followed by phenols (r: 0.852), TAC (r: 0.775), and AOX capacity (r: 682). Similar relationship has been reported by Machado et al. [56]. Although the strongest antioxidant potential correlation was observed with phenolic compounds, flavonoid and anthocyanin also contributed significant positive impact on antioxidation capacity with coefficients (r) 0.772 and 0.584. Kulkarni and Aradhya [57] have successfully described this relation due to gallotannins, cyanidins, hydrolysable tannins, and anthocyanin of PP contributing to the AOX capacity. However, the present work has poor correlation of antioxidants with other responses due to the fact of processing effect on PUAE. The significant positive correlation was observed in anthocyanin and flavonoids (r: 0.878) followed by phenols describing the presence of quercetin, catechin, cyanidin 3-glucoside, gallic acid, ellagic acid, α and β-punicalagin etc. which are also responsible for positive correlation between AOX and TAC [8]. Initially, it was assumed to correlate between browning with improved efficacy and quality of extract by the higher content of TPC and TAC. An insignificant (p < 0.05) negative relation between BI and yield, TPC, TFC, and AOX with correlation coefficients (r) of −0.036, −0.308, −0.228, and 0.014 respectively was recorded. Only an exception was found with anthocyanin content, significantly correlating the BI (r: −0.448). This results eventually describes that high anthocyanin content results in the lower BI of extract. In evidence to this, Orak et al. [58] reported the significant negative correlation between lightness, redness, and yellowness in the peel. Although the other responses were insignificant, however, negative relations may justify the BI as one of the important responses for effective PUAE with high TAC, TPC, and TFC of PP extract.
Table 5.
Pearson correlation analysis between the responses of PUAE of pomegranate peel.
| Yield | TPC | TFC | AOX | TAC | BI | |
|---|---|---|---|---|---|---|
| Yield | 1.000 | |||||
| TPC | 0.852** | 1.000 | ||||
| TFC | 0.903** | 0.931** | 1.000 | |||
| AOX | 0.682** | 0.788** | 0.772** | 1.000 | ||
| TAC | 0.775** | 0.827** | 0.878** | 0.584** | 1.000 | |
| BI | −0.036 | −0.308 | −0.228 | 0.014 | −0.448* | 1.000 |
**. Correlation is significant at the 0.01 level and *. at the 0.05 level (2-tailed).
3.6. Numeric optimization and validation of the model prediction
With existing financial and ecological apprehensions, extraction sector must develop efficient techniques with respect to yields of extract, cleanness to environment, security of operators and space utilization [12]. This tactic is part of intensification of extraction process. Therefore, more precisely, in this work aims the intensification of extraction using pulsed ultrasound for improved yield of PP extract with high quality and purity, along with minimal number of unit operations, time, energy, cost, solvents, and environmental impacts.
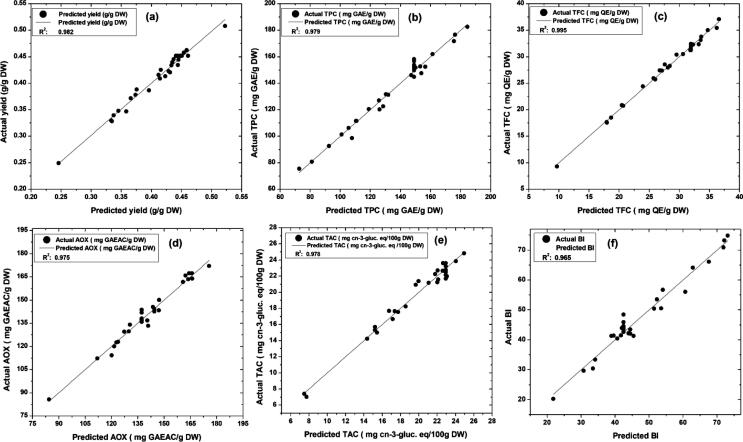
Numerical optimization was implemented through Eq. (10) targeting to get a maximum PUAE yield (g/g) with higher TPC, TFC, AOX, and TAC of the extract with minimum BI (Table 6). The relative importance (ri) was ranged between 1 and 5 (least to most important) was assigned to every variable and response. High extraction yield, TPC, and TFC were aimed by designating maximum importance (ri = 5) next to this AOX, TAC, and BI supposed to be second most important (ri = 4) as these values also signify the quality and efficacy of PUAE. However, variables such as lesser power and extraction time may be required for economic and operation time concern, so power and extraction time also appraised to optimization with 5 and 4 importance, respectively. Whereas, S/S ratio and duty cycle kept in range (ri = 3). The multiple numerical optimizations suggested 2.17 g/100 mL S/S ratio at 115.69 W power and 80% duty cycle for 5.99 min extraction time as the most optimum parameters for efficient (D = 0.760) PUAE of bioactive compounds from dried PP. Model prediction was validated by comparing the response datapoint of the total yield of extract, TPC, TFC, AOX, TAC, and BI; predicted by respective models with the actual experimental results (Fig. 8a-f) and experimenting with proposed conditions of operation parameters by model.
Table 6.
The set of constraints for different factors and responses for optimizing the PUAE conditions.
| CPE parameters | Target | Lower limit (Li) | Upper Limit (Ui) | Relative importance (ri) | Optimized conditions at D |
Actual experimental conditions |
|---|---|---|---|---|---|---|
| D = 0.760 | ||||||
| x1- S/S Ratio (g/100 mL) | In range | 2 | 10 | 3 | 2.17 | 2.20 |
| x2- Power (W) | Minimize | 70 | 210 | 5 | 115.69 | 105.00 |
| x3-Duty Cycle (%) | In range | 20 | 80 | 3 | 80.00 | 80.00 |
| x4- Time (min.) | Minimize | 1 | 10 | 4 | 5.99 | 6.00 |
| Yield (g/g) | Maximize | 0.25 | 0.53 | 5 | 0.511 | 0.48 ± 0.08 |
| TPC (mg GAE/g DW) | Maximize | 75.57 | 182.66 | 5 | 182.66 | 177.54 ± 2.5 |
| TFC (mg QE/g DW) | Maximize | 9.32 | 37.07 | 5 | 36.69 | 35.71 ± 1.3 |
| AOX (mg GAEAC/g DW) | Maximize | 85.66 | 172.07 | 4 | 164.82 | 160.54 ± 3.7 |
| TAC (mg cyn-3-glc eq/100g DW) | Maximize | 7.02 | 24.84 | 4 | 22.51 | 21.65 ± 0.87 |
| BI | Minimize | 20.28 | 74.86 | 4 | 51.88 | 54.92 ± 2.65 |
TPC: total phenolics content, TFC: total flavonoids content, AOX: antioxidant capacity, TAC: total anthocyanin content, BI: browning index, and D: overall desirability value.
Fig. 8.
Correlation of predicted results vs actual results for yield, TPC, TFC, AOX, TAC, and BI of PUAE.
It was noticed that the scattered actual data points of yield, TPC, and TFC were very adjacent to the predicted straight line with very significant determination coefficient (R2: 0.982, 0.979, and 0.995, respectively). On other hand, little distance scattered actual dataset observed in case of AOX, TAC, and BI. The statistically significant R2-value of AOX (0.975), TAC (0.978), and BI (0.965) reflected adequate fitting of the models. Hence overall correlations, of predicted vs actual results represented successful modeling of PUAE from PP. Table 6 describes the results obtained from experiments conducted at optimized conditions. In this case, extract yield was found to be 0.48 g/g followed by 177.54 mg GAE/ g TPC, 35.71 mg QE/g TFC, 160.54 mg GAEAC/g AOX capacity, 21.65 mg cyn-3-glc eq/100 g TAC with 54.92 BI. These results were very close to predicted response values, which indicates the most reliability of the model and extraction efficiency of PUAE. There are several similar reports [17], [59], [60] in support of the current study, which is comparably higher to other reports [11], [14], [61]. Hence, it was proposed that the suggested optimum process parameters in the present work were efficient for the pulsed ultrasound-assisted extraction process.
3.7. Antioxidant capacity of extract.
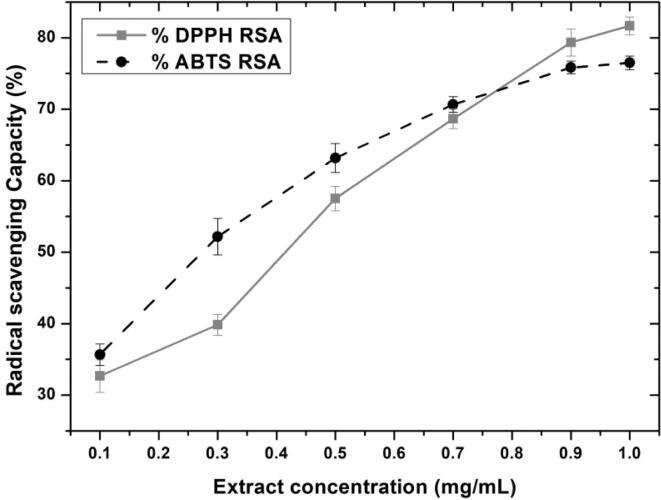
Obtained extract via optimized PUAE parameter was analyzed for free radical scavenging (antioxidation) capacity using DPPH and ABTS reagents. The resultant extract showed a maximum 81.67% and 76.50% radical scavenging ability of DPPH and ABTS, respectively, along with the calculated IC-50 values of 0.365 mg/mL and 0.295 mg/mL respectively (Fig. 9). These results are in accordance with several others [11], [14], [60], [62] showing similarities. Opposing this Kazemi et al [16] , described very contrast results. This could be justified due to other methods of extraction, type of solvent, variety of fruit, etc. Nevertheless, every antioxidant assay only delivers an approximate AOX capacity which depends on its conditions, reagents, and several classes of an antioxidating biomolecule. Hence, several AOX assays assist in verifying the identity and distinguish the different types of antioxidant compounds present in the extract.
Fig. 9.
Antioxidant capacities of extract by DPPH and ABTS reagents.
4. Conclusions
PUAE is an emerging green, energy, and time-efficient extraction process which could be used for the extraction of food bio-actives. The experimentally designed quadratic model was imperative for the study of multivariate interaction, optimization of PUAE conditions, and correlation assessment of factors and responses. Multicriterial numerical optimization suggested 2.17 g/100 mL S/S ratio at 116 W sonication power with 80% duty cycle for efficient extract yield (0.511 g/g peel; DW), TPC (182.66 mg GAE/g peel; DW), TFC (36.69 mg QE/g peel; DW), AOX (164.82 mg GAEAC/g peel; DW), TAC (22.51 mg cyn-3-glc eq/100 g peel; DW), and 51.88 of BI. All PUAE parameters significantly validated from 30 FCCD data points resulting in a regression coefficient (R2) above 0.96. Significant Pearson correlation analysis was established in all responses with strong phenols and flavonoid relation with the highest coefficient (r) 0.931. Hence, it can be concluded that the application of PUAE technique can be effective for extraction bioactive from any food and plant systems with minimal process time and power consumption with the green label.
CRediT authorship contribution statement
Pavankumar R. More: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Writing - original draft. Shalini S. Arya: Conceptualization, Data curation, Funding acquisition, Project administration, Resources, Supervision, Visualization, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by TEQIP-III, Centre of excellence in process intensification with a focus on green technology, Institute of Chemical Technology, Mumbai, India. (Ref: ICT/REG/SSL/2306 Dated: 30th August 2018). We thank TEQIP-III, ICT, Mumbai who supported finance that greatly assisted the research.
References
- 1.Rajha H.N., Abi-Khattar A.M., El Kantar S., Boussetta N., Lebovka N., Maroun R.G., Louka N., Vorobiev E. Comparison of aqueous extraction efficiency and biological activities of polyphenols from pomegranate peels assisted by infrared, ultrasound, pulsed electric fields and high-voltage electrical discharges. Innov. Food Sci. Emerg. Technol. 2019;58 doi: 10.1016/j.ifset.2019.102212. [DOI] [Google Scholar]
- 2.Silva K. Investigation of nutrient content, phytochemical content, antioxidant activity and antibacterial activity of inedible portion of pomegranate (Punica granatum L.), European J. Med. Plants. 2014;4:610–622. doi: 10.9734/ejmp/2014/7561. [DOI] [Google Scholar]
- 3.Foujdar R., Bera M.B., Chopra H.K. Optimization of process variables of probe ultrasonic-assisted extraction of phenolic compounds from the peel of Punica granatum Var. Bhagwa and it’s chemical and bioactivity characterization. J. Food Process. Preserv. 2020;44:1–16. doi: 10.1111/jfpp.14317. [DOI] [Google Scholar]
- 4.Pan Z., Qu W., Ma H., Atungulu G.G., McHugh T.H. Continuous and pulsed ultrasound-assisted extractions of antioxidants from pomegranate peel. Ultrason. Sonochem. 2011;18(5):1249–1257. doi: 10.1016/j.ultsonch.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Devatkal S.K., Jaiswal P., Jha S.N., Bharadwaj R., Viswas K.N. Antibacterial activity of aqueous extract of pomegranate peel against Pseudomonas stutzeri isolated from poultry meat. J. Food Sci. Technol. 2013;50(3):555–560. doi: 10.1007/s13197-011-0351-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jing P.u., Ye T., Shi H., Sheng Y.i., Slavin M., Gao B., Liu L., Yu L.(. Antioxidant properties and phytochemical composition of China-grown pomegranate seeds. Food Chem. 2012;132(3):1457–1464. doi: 10.1016/j.foodchem.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Aqil F., Munagala R., Vadhanam M.V., Kausar H., Jeyabalan J., Schultz D.J., Gupta R.C. Anti-proliferative activity and protection against oxidative DNA damage by punicalagin isolated from pomegranate husk. Food Res. Int. 2012;49(1):345–353. doi: 10.1016/j.foodres.2012.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexandre E.M.C., Araújo P., Duarte M.F., de Freitas V., Pintado M., Saraiva J.A. Experimental design, modeling, and optimization of high-pressure-assisted extraction of bioactive compounds from pomegranate peel. Food Bioprocess Technol. 2017;10(5):886–900. doi: 10.1007/s11947-017-1867-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trigo J.P., Alexandre E.M.C., Oliveira A., Saraiva J.A., Pintado M. Fortification of carrot juice with a high-pressure-obtained pomegranate peel extract: chemical, safety and sensorial aspects. Int. J. Food Sci. Technol. 2020;55(4):1599–1605. doi: 10.1111/ijfs.v55.410.1111/ijfs.14386. [DOI] [Google Scholar]
- 10.Sood A., Gupta M. Extraction process optimization for bioactive compounds in pomegranate peel. Food Biosci. 2015;12:100–106. doi: 10.1016/j.fbio.2015.09.004. [DOI] [Google Scholar]
- 11.Sharayei P., Azarpazhooh E., Zomorodi S., Ramaswamy H.S. Ultrasound assisted extraction of bioactive compounds from pomegranate (Punica granatum L.) peel. LWT - Food Sci. Technol. 2019;101:342–350. doi: 10.1016/j.lwt.2018.11.031. [DOI] [Google Scholar]
- 12.Chemat F., Abert-Vian M., Fabiano-Tixier A.S., Strube J., Uhlenbrock L., Gunjevic V., Cravotto G. Green extraction of natural products. Origins, current status, and future challenges, TrAC - Trends Anal. Chem. 2019;118:248–263. doi: 10.1016/j.trac.2019.05.037. [DOI] [Google Scholar]
- 13.Chemat F., Abert Vian M., Fabiano-Tixier A.-S., Nutrizio M., Režek Jambrak A., Munekata P.E.S., Lorenzo J.M., Barba F.J., Binello A., Cravotto G. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem. 2020;22(8):2325–2353. doi: 10.1039/C9GC03878G. [DOI] [Google Scholar]
- 14.Tabaraki R., Heidarizadi E., Benvidi A. Optimization of ultrasonic-assisted extraction of pomegranate (Punica granatum L.) peel antioxidants by response surface methodology. Sep. Purif. Technol. 2012;98:16–23. doi: 10.1016/j.seppur.2012.06.038. [DOI] [Google Scholar]
- 15.Chemat F., Rombaut N., Sicaire A.G., Meullemiestre A., Fabiano-Tixier A.S., Abert-Vian M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017;34:540–560. doi: 10.1016/j.ultsonch.2016.06.035. [DOI] [PubMed] [Google Scholar]
- 16.Kazemi M., Karim R., Mirhosseini H., Abdul Hamid A. Optimization of pulsed ultrasound-assisted technique for extraction of phenolics from pomegranate peel of Malas variety: Punicalagin and hydroxybenzoic acids. Food Chem. 2016;206:156–166. doi: 10.1016/j.foodchem.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 17.More P.R., Arya S.S. A novel, green cloud point extraction and separation of phenols and flavonoids from pomegranate peel: An optimization study using RCCD. J. Environ. Chem. Eng. 2019;7(5):103306. doi: 10.1016/j.jece:2019.103306. [DOI] [Google Scholar]
- 18.Torres B., Tiwari B.K., Patras A., Wijngaard H.H., Brunton N., Cullen P.J., O’Donnell C.P. Effect of ozone processing on the colour, rheological properties and phenolic content of apple juice. Food Chem. 2011;124(3):721–726. doi: 10.1016/j.foodchem.2010.06.050. [DOI] [Google Scholar]
- 19.Zhishen J., Mengcheng T., Jianming W.u. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64(4):555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]
- 20.Lee J., Durst R.W., Wrolstad R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005;88:1269–1278. doi: 10.1093/jaoac/88.5.1269. [DOI] [PubMed] [Google Scholar]
- 21.Maskan M. Drying, shrinkage and rehydration characteristics of kiwifruits during hot air and microwave drying. J. Food Eng. 2001;48(2):177–182. doi: 10.1016/S0260-8774(00)00155-2. [DOI] [Google Scholar]
- 22.Sonawane S.K., Arya S.S. Citrullus lanatus protein hydrolysate optimization for antioxidant potential. J. Food Meas. Charact. 2017;11(4):1834–1843. doi: 10.1007/s11694-017-9565-7. [DOI] [Google Scholar]
- 23.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26(9-10):1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 24.Bagul M.B., Sonawane S.K., Arya S.S. Bioactive characteristics and optimization of tamarind seed protein hydrolysate for antioxidant-rich food formulations. 3 Biotech. 2018;8:218. doi: 10.1007/s13205-018-1240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaderides K., Goula A.M., Adamopoulos K.G. A process for turning pomegranate peels into a valuable food ingredient using ultrasound-assisted extraction and encapsulation. Innov. Food Sci. Emerg. Technol. 2015;31:204–215. doi: 10.1016/j.ifset.2015.08.006. [DOI] [Google Scholar]
- 26.Boeing J.S., Barizão É.O., e Silva B.C., Montanher P.F., de Cinque Almeida V., Visentainer J.V. Evaluation of solvent effect on the extraction of phenolic compounds and antioxidant capacities from the berries: Application of principal component analysis. Chem. Cent. J. 2014;8(1) doi: 10.1186/s13065-014-0048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spigno G., Tramelli L., De Faveri D.M. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 2007;81(1):200–208. doi: 10.1016/j.jfoodeng.2006.10.021. [DOI] [Google Scholar]
- 28.Viuda-Martos M., Ruiz-Navajas Y., Fernández-López J., Sendra E., Sayas-Barberá E., Pérez-Álvarez J.A. Antioxidant properties of pomegranate (Punica granatum L.) bagasses obtained as co-product in the juice extraction. Food Res. Int. 2011;44(5):1217–1223. doi: 10.1016/j.foodres.2010.10.057. [DOI] [Google Scholar]
- 29.Goula A.M. Ultrasound-assisted extraction of pomegranate seed oil - Kinetic modeling. J. Food Eng. 2013;117(4):492–498. doi: 10.1016/j.jfoodeng.2012.10.009. [DOI] [Google Scholar]
- 30.Jerman T., Trebše P., Mozetič Vodopivec B. Ultrasound-assisted solid liquid extraction (USLE) of olive fruit (Olea europaea) phenolic compounds. Food Chem. 2010;123:175–182. doi: 10.1016/j.foodchem.2010.04.006. [DOI] [Google Scholar]
- 31.Khadhraoui B., Turk M., Fabiano-Tixier A.S., Petitcolas E., Robinet P., Imbert R., El Maâtaoui M., Chemat F. Histo-cytochemistry and scanning electron microscopy for studying spatial and temporal extraction of metabolites induced by ultrasound. Towards chain detexturation mechanism. Ultrason. Sonochem. 2018;42:482–492. doi: 10.1016/j.ultsonch.2017.11.029. [DOI] [PubMed] [Google Scholar]
- 32.Shirsath S.R., Sable S.S., Gaikwad S.G., Sonawane S.H., Saini D.R., Gogate P.R. Intensification of extraction of curcumin from Curcuma amada using ultrasound assisted approach: Effect of different operating parameters. Ultrason. Sonochem. 2017;38:437–445. doi: 10.1016/j.ultsonch.2017.03.040. [DOI] [PubMed] [Google Scholar]
- 33.Salve A.R., Pegu K., Arya S.S. Comparative assessment of high-intensity ultrasound and hydrodynamic cavitation processing on physico-chemical properties and microbial inactivation of peanut milk. Ultrason. Sonochem. 2019;59:104728. doi: 10.1016/j.ultsonch.2019.104728. [DOI] [PubMed] [Google Scholar]
- 34.Sicaire A.G., Vian M.A., Fine F., Carré P., Tostain S., Chemat F. Ultrasound induced green solvent extraction of oil from oleaginous seeds. Ultrason. Sonochem. 2016;31:319–329. doi: 10.1016/j.ultsonch.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 35.Gogate P.R., Wilhelm A.M., Pandit A.B. Some aspects of the design of sonochemical reactors. Ultrason. Sonochem. 2003;10(6):325–330. doi: 10.1016/S1350-4177(03)00103-2. [DOI] [PubMed] [Google Scholar]
- 36.Xu Y., Pan S. Effects of various factors of ultrasonic treatment on the extraction yield of all-trans-lycopene from red grapefruit (Citrus paradise Macf.) Ultrason. Sonochem. 2013;20(4):1026–1032. doi: 10.1016/j.ultsonch.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Ying Z., Han X., Li J. Ultrasound-assisted extraction of polysaccharides from mulberry leaves. Food Chem. 2011;127(3):1273–1279. doi: 10.1016/j.foodchem.2011.01.083. [DOI] [PubMed] [Google Scholar]
- 38.Kobus Z. Dry matter extraction from valerian roots (Valeriana officinalis L.) with the help of pulsed acoustic field. Int. Agrophysics. 2008;22:133–137. [Google Scholar]
- 39.He B., Zhang L.L., Yue X.Y., Liang J., Jiang J., Gao X.L., Yue P.X. Optimization of ultrasound-assisted extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium ashei) wine pomace. Food Chem. 2016;204:70–76. doi: 10.1016/j.foodchem.2016.02.094. [DOI] [PubMed] [Google Scholar]
- 40.Eh A.-S., Teoh S.-G. Novel modified ultrasonication technique for the extraction of lycopene from tomatoes. Ultrason. Sonochem. 2012;19(1):151–159. doi: 10.1016/j.ultsonch.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 41.Li M., Ngadi M.O., Ma Y. Optimisation of pulsed ultrasonic and microwave-assisted extraction for curcuminoids by response surface methodology and kinetic study. Food Chem. 2014;165:29–34. doi: 10.1016/j.foodchem.2014.03.115. [DOI] [PubMed] [Google Scholar]
- 42.Sun Y., Liu D., Chen J., Ye X., Yu D. Effects of different factors of ultrasound treatment on the extraction yield of the all-trans-β-carotene from citrus peels. Ultrason. Sonochem. 2011;18(1):243–249. doi: 10.1016/j.ultsonch.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 43.Sivakumar V., Anna J.L., Vijayeeswarri J., Swaminathan G. Ultrasound assisted enhancement in natural dye extraction from beetroot for industrial applications and natural dyeing of leather. Ultrason. Sonochem. 2009;16(6):782–789. doi: 10.1016/j.ultsonch.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 44.Luque-García J.L., Luque de Castro M.D. Ultrasound-assisted soxhlet extraction: An expeditive approach for solid sample treatment - Application to the extraction of total fat from oleaginous seeds. J. Chromatogr. A. 2004;1034(1-2):237–242. doi: 10.1016/j.chroma.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 45.Wang W., Jung J., Tomasino E., Zhao Y. Optimization of solvent and ultrasound-assisted extraction for different anthocyanin rich fruit and their effects on anthocyanin compositions. LWT - Food Sci. Technol. 2016;72:229–238. doi: 10.1016/j.lwt.2016.04.041. [DOI] [Google Scholar]
- 46.Chen S., Zeng Z., Hu N., Bai B., Wang H., Suo Y. Suo, Simultaneous optimization of the ultrasound-assisted extraction for phenolic compounds content and antioxidant activity of Lycium ruthenicum Murr. fruit using response surface methodology. Food Chem. 2018;242:1–8. doi: 10.1016/j.foodchem.2017.08.105. [DOI] [PubMed] [Google Scholar]
- 47.Paniwnyk L., Cai H., Albu S., Mason T.J., Cole R. The enhancement and scale up of the extraction of anti-oxidants from Rosmarinus officinalis using ultrasound. Ultrason. Sonochem. 2009;16(2):287–292. doi: 10.1016/j.ultsonch.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 48.Li Y., Guo C., Yang J., Wei J., Xu J., Cheng S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006;96(2):254–260. doi: 10.1016/j.foodchem.2005.02.033. [DOI] [Google Scholar]
- 49.Ghafoor K., Choi Y.H., Jeon J.Y., Jo I.H. Optimization of ultrasound-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from grape (Vitis vinifera) seeds. J. Agric. Food Chem. 2009;57(11):4988–4994. doi: 10.1021/jf9001439. [DOI] [PubMed] [Google Scholar]
- 50.Ho S.K., Tan C.P., Thoo Y.Y., Abas F., Ho C.W. Ultrasound-assisted extraction of antioxidants in Misai Kucing (Orthosiphon stamineus) Molecules. 2014;19:12640–12659. doi: 10.3390/molecules190812640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palou E., López-Malo A., Barbosa-Cánovas G.V., Welti-Chanes J., Swanson B.G. Polyphenoloxidase activity and color of blanched and high hydrostatic pressure treated banana puree. J. Food Sci. 1999;64:42–45. doi: 10.1111/j.1365-2621.1999.tb09857.x. [DOI] [Google Scholar]
- 52.Feumba Dibanda R., Panyoo Akdowa E., Rani P. A., Metsatedem Tongwa Q., Mbofung F. C.M. Effect of microwave blanching on antioxidant activity, phenolic compounds and browning behaviour of some fruit peelings. Food Chem. 2020;302:125308. doi: 10.1016/j.foodchem.2019.125308. [DOI] [PubMed] [Google Scholar]
- 53.Zhang L.H., Zhang Y.H., Li L.L., Li Y.X. Effects of 1-MCP on peel browning of pomegranates. Acta Hortic. 2008;774:275–282. doi: 10.17660/ActaHortic.2008.774.36. [DOI] [Google Scholar]
- 54.DUAN X., JIANG Y., SU X., ZHANG Z., SHI J. Antioxidant properties of anthocyanins extracted from litchi (Litchi chinenesis Sonn.) fruit pericarp tissues in relation to their role in the pericarp browning. Food Chem. 2007;101(4):1365–1371. doi: 10.1016/j.foodchem.2005.06.057. [DOI] [Google Scholar]
- 55.Skenderidis P., Mitsagga C., Giavasis I., Petrotos K., Lampakis D., Leontopoulos S., Hadjichristodoulou C., Tsakalof A. The in vitro antimicrobial activity assessment of ultrasound assisted Lycium barbarum fruit extracts and pomegranate fruit peels. J. Food Meas. Charact. 2019;13(3):2017–2031. doi: 10.1007/s11694-019-00123-6. [DOI] [Google Scholar]
- 56.Machado A.P.D.F., Sumere B.R., Mekaru C., Martinez J., Bezerra Rosângela.M.N., Rostagno M.A. Extraction of polyphenols and antioxidants from pomegranate peel using ultrasound: influence of temperature, frequency and operation mode. Int. J. Food Sci. Technol. 2019;54(9):2792–2801. doi: 10.1111/ijfs.v54.910.1111/ijfs.14194. [DOI] [Google Scholar]
- 57.Kulkarni A.P., Aradhya S.M. Chemical changes and antioxidant activity in pomegranate arils during fruit development. Food Chem. 2005;93(2):319–324. doi: 10.1016/j.foodchem.2004.09.029. [DOI] [Google Scholar]
- 58.Orak H.H., Yagar H., Isbilir S.S. Comparison of antioxidant activities of juice, peel, and seed of pomegranate (Punica granatum L.) and inter-relationships with total phenolic, tannin, anthocyanin, and flavonoid contents. Food Sci. Biotechnol. 2012;21(2):373–387. doi: 10.1007/s10068-012-0049-6. [DOI] [Google Scholar]
- 59.Xi J., He L., Yan L. Continuous extraction of phenolic compounds from pomegranate peel using high voltage electrical discharge. Food Chem. 2017;230:354–361. doi: 10.1016/j.foodchem.2017.03.072. [DOI] [PubMed] [Google Scholar]
- 60.Abid M., Yaich H., Cheikhrouhou S., Khemakhem I., Bouaziz M., Attia H., Ayadi M.A. Antioxidant properties and phenolic profile characterization by LC–MS/MS of selected Tunisian pomegranate peels. J. Food Sci. Technol. 2017;54:2890–2901. doi: 10.1007/s13197-017-2727-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Motikar P.D., More P.R., Arya S.S. A novel, green environment-friendly cloud point extraction of polyphenols from pomegranate peels: a comparative assessment with ultrasound and microwave-assisted extraction. Sep. Sci. Technol. 2020;00:1–12. doi: 10.1080/01496395.2020.1746969. [DOI] [Google Scholar]
- 62.Barathikannan K., Venkatadri B., Khusro A., Al-Dhabi N.A., Agastian P., Arasu M.V., Choi H.S., Kim Y.O. Chemical analysis of Punica granatum fruit peel and its in vitro and in vivo biological properties. BMC Complement. Altern. Med. 2016;16:1–10. doi: 10.1186/s12906-016-1237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]