Abstract
Aims: Profiling of lipoproteins can predict risk of cardiovascular disease; gel permeation high-performance liquid chromatography (HPLC) improves prediction accuracy by providing detailed data for specific lipoprotein subclasses. This study applied HPLC to examine the effects of evolocumab, which effectively treats hyperlipidemia and mixed dyslipidemia, on lipoprotein subclasses, specifically the number and size of lipoprotein particles.
Methods: This post-hoc analysis used patient blood samples from YUKAWA-2, a phase 3 trial evaluating the efficacy of evolocumab in Japanese adult patients with hyperlipidemia or mixed dyslipidemia and at high risk for cardiovascular disease. We used HPLC to assess observed values and percent change from baseline in cholesterol and triglyceride (TG) concentrations, number of particles in lipoprotein subclasses to week 12, and mean observed values and mean percent change from baseline in variables to weeks 10 and 12. HPLC was also compared with conventional methods in assessing low-density lipoprotein (LDL) cholesterol (LDL-C) values.
Results: Data for all 404 patients were analyzed. Evolocumab significantly decreased cholesterol and TG concentrations, and total particle count, in very low-density lipoprotein (VLDL) and LDL subclasses. Particle size increased slightly in LDL, high-density lipoprotein (HDL), and VLDL, but data varied widely. At very low L-DLC, HPLC measurements were higher than those from conventional methods.
Conclusion: This research used HPLC to assess the effects of evolocumab in 20 lipid subclasses. By lowering lipid content and improving the lipid profile, evolocumab may reduce atherogenicity. This reduction is better quantified by HPLC than by conventional methods in the very low LDL-C range.
Keywords: Evolocumab, Gel permeation high-performance liquid chromatography, Lipoprotein, Lipoprotein subclass, Residual risk
Introduction
The benefits of statins are well established for reducing low-density lipoprotein (LDL) cholesterol (LDL-C) and preventing the development and recurrence of cardiovascular events. However, even intensive statin therapy can only reduce cardiovascular events by about 30% in patients1). Further reduction of cardiovascular events will require better management of residual risk factors such as postprandial dyslipidemia, remnant lipoproteins, number and size of small dense LDL (sdLDL) particles, and low levels in high-density lipoprotein cholesterol (HDL-C).
Lipoprotein profiles related to the risk factors of cardiovascular disease are not limited to lipid quantities, but also apply to lipid qualities such as subclass characteristics and specifically the ratio of lipoproteins within each subclass2). This information has therapeutic implications, and lipoprotein profiles are being increasingly used to determine patients' risk of developing cardiovascular disease2–5); sdLDL, very low-density lipoprotein (VLDL), and remnant lipoproteins are considered to be associated with coronary artery disease6, 7) and peripheral artery disease (PAD)2). Detailed lipoprotein analysis with quantification of the changes in lipoprotein profiles may provide vital information for the clinician in the management of dyslipidemia and the prevention of cardiovascular disease.
In recent years, monoclonal antibodies that inhibit protein convertase subtilisin/kexin type 9 (PCSK9) have become available for clinical use and are recommended in guidelines for the treatment of familial hypercholesterolemia (FH)8) and for the secondary prevention of coronary artery disease in FH and high-risk non-FH patients9). One of those PCSK9 inhibitors, evolocumab, has markedly reduced LDL-C levels by approximately 60% and reduced the incidence rate of cardiovascular events by 15% when added to statin therapy10, 11). These effects were investigated in the YUKAWA-2 study, a phase 3 clinical trial designed to evaluate the efficacy and safety of evolocumab for 12 weeks in Japanese patients at high risk for cardiovascular events due to hyperlipidemia or mixed dyslipidemia12). The study demonstrated the potent LDL-C-lowering effects of evolocumab in Japanese patients, with LDL-C levels reduced by approximately 67% to 76% in the evolocumab group. However, the results did not include information on which specific lipoprotein particles or particle subclasses were affected. For example, did evolocumab decrease sdLDL, which is more atherogenic, or did it decrease large buoyant LDL (lbLDL), which is less atherogenic? Further research into the effects of evolocumab on specific lipoprotein subclasses could greatly benefit treatment practices at the clinical level. However, we need more accurate tools to assess the true risks and benefits of this potentially promising drug.
The results in the YUKAWA-2 study were based on ultracentrifugation for measuring LDL-C < 40 mg/dL (1.0 mmol/L) or triglyceride (TG) > 400 mg/dL (4.5 mmol/L); in all other cases, the Friedewald method was used. However, some patients in the evolocumab group showed post-treatment LDL-C values below the limits of detection by ultracentrifugation, raising the question of whether LDL particles might be eliminated by PCSK9 monoclonal antibody inhibitors. To predict the long-term safety of evolocumab, we must be able to verify whether LDL-C was absent from the blood of those patients, which requires the use of more sensitive technology to reassess post-treatment LDL-C values.
Several techniques are available for lipoprotein analysis. The traditional method of ultracentrifugation is time-consuming and thus limited in clinical application. For calculating LDL-C, the Friedewald method is commonly used, but it requires fasting blood samples in which TG levels are ≤ 400 mg/dL13) and reliability has not been verified for extremely low LDL-C values. In addition, little information appears to be available on the reliability of the direct measurement method at extremely low LDL-C levels. In particular, now that PCSK9 monoclonal antibody inhibitors are in clinical use, new assay methods which provide detailed lipoprotein measurement are needed to assess LDL-C at lower concentrations. Also, when determining the quality of LDL to evaluate its atherogenic effects, information is needed not only on cholesterol and TG content of LDL but also on particle size (diameter) and the number of particles. Proton nuclear magnetic resonance spectroscopy (NMR) measures particle size quickly and conveniently14), but NMR measurement is based on resonance signals, which makes an accurate assessment of lipoprotein cholesterol levels problematic15–18). In contrast, gel permeation high-performance liquid chromatography (HPLC) analyzes lipoproteins by dividing lipoprotein particles into 20 subclasses based on particle size, directly determines cholesterol and TG levels for each subclass, and calculates the number of each lipoprotein particle in detail19–21). This contributes to more accurate lipoprotein profiles, which support the assessment of potential residual risk22, 23).
We analyzed the 20 lipoprotein subclasses obtained by HPLC, using blood samples collected and stored during the YUKAWA-2 study. We also investigated differences between the LDL-C values obtained by HPLC analysis and LDL-C values that were calculated using the Friedewald estimation method or measured by ultracentrifugation in the YUKAWA-2 study. In previous research, the effects of evolocumab on lipoprotein particle concentrations have been analyzed by NMR spectroscopy in the phase 3, 52-week DESCARTES (Durable Effect of PCSK9 Antibody Compared with Placebo Study) trial11). However, to our knowledge, our study is the first to use HPLC in assessing the more detailed and precise effects of evolocumab on lipoproteins.
Aim
We conducted this post-hoc sub-analysis to better evaluate the efficacy of evolocumab in improving the lipoprotein profile in Japanese subjects who had hyperlipidemia or mixed dyslipidemia and high cardiovascular risk, using data from the YUKAWA-2 study. Our goal was to more precisely evaluate the lipoproteins related to cardiovascular event risk.
Methods
Study Design
The YUKAWA-2 study design and results have been reported by Kiyosue et al. (2016)12). Briefly, the YUKAWA-2 study was designed as a 12-week phase 3 clinical study to evaluate the efficacy and safety of evolocumab subcutaneous injections at doses of 140 mg once every 2 weeks and 420 mg monthly in patients who were also under treatment with atorvastatin 5 mg/day or 20 mg/day. The YUKAWA-2 study (NCT01953328) was approved by the Institutional Research Ethics Committee (IRB) of each study site and was conducted in accordance with the Ethical Guidelines for Medical and Health Research Involving Human Subjects. Written informed consent was obtained from each subject.
This study presents a post-hoc analysis of data from the YUKAWA-2 study, so no trial registration was filed, and no new informed consent was required. This study evaluated the efficacy of evolocumab in the target population in greater depth by analyzing changes in the 20 lipoprotein subclasses obtained by HPLC, using blood samples collected during the YUKAWA-2 study. This study also compared the differences in LDL-C values as determined by HPLC to those calculated using the Friedewald method or measured by ultracentrifugation.
Study Population
In the YUKAWA-2 study, a total of 409 Japanese patients were randomized at 52 study centers. They were 20 to 85 years of age, had been diagnosed with hyperlipidemia or mixed dyslipidemia, and were at high cardiovascular risk according to the Japan Atherosclerosis Society criteria24). Patients were required to receive a stable dose of an approved statin for at least 4 weeks before the study, to have no changes in lipid-lowering therapy during that time, and to have documented LDL-C ≥ 100 mg/dL (2.6 mmol/L) and fasting TG ≤ 400 mg/dL (4.5 mmol/L). Patients with type 1 diabetes, newly diagnosed (< 3 months) or poorly controlled (hemoglobin A1c [HbA1c] > 8.5%) type 2 diabetes (T2DM), or newly diagnosed impaired glucose tolerance (< 3 months) were excluded from participation. All other exclusion criteria are available in the supplementary material from the YUKAWA-2 study12).
After entering screening, eligible subjects were randomized 1:1 to either of two atorvastatin dose cohorts: 5 mg/day (non-intensive therapy) or 20 mg/day (intensive therapy). For randomization, stratification factors had three levels: (1) previous diagnosis of familial hypercholesterolemia heterozygotes (HeFH), (2) no HeFH diagnosis and treatment with intensive lipid-lowering therapy, and (3) no HeFH diagnosis and treatment with non-intensive lipid-lowering therapy. After completion of the 4-week lipid-stabilizing period, the eligible subjects in each statin cohort were then randomized at a 1:1:1:1 ratio into 1 of 4 groups for the 12-week double-blind period: subcutaneous evolocumab 140 mg every 2 weeks or 420 mg every month or subcutaneous placebo every 2 weeks or every month. Stratification was by the same factors as for the lipid stabilization period described above.
For the YUKAWA-2 study, the co-primary endpoints were the percent change in LDL-C from baseline to week 12 and mean percent change in LDL-C from baseline to week 10 and week 12. For all analyses related to LDL-C, unless specified otherwise, a reflexive method was used, where the calculated LDL-C concentration was used in the analysis unless that calculated LDL-C was < 40 mg/dL or triglycerides were > 400 mg/dL, in which case ultracentrifugation LDL-C was determined and utilized.
In the YUKAWA-2 study, 404 patients were identified to be eligible and were randomized to evolocumab or placebo within their atorvastatin cohort. Of the 404 subjects randomized to study drugs, all patients received statin treatment and at least 1 dose of study drug. The 404 patients were included in the full analysis set (FAS) population used for analyses of efficacy and safety endpoints. All of those 404 patients were included in the present study.
Endpoints
Co-primary endpoints were established for this study. One primary endpoint was the percent change from baseline in total cholesterol and total TG concentrations and the number of particles in lipoprotein subclasses at week 12, and the other was the mean percent change of those factors from baseline at weeks 10 and 12. Measurements were obtained by HPLC for the 4 major lipoproteins and the 20 lipoprotein subclasses. In an additional exploratory investigation, cholesterol concentration, TG concentration, and the number of particles were determined for each of the 20 lipoprotein subclasses at baseline and week 12, and those mean values were calculated for week 10 and week 12. Subgroup analysis on those values and percent change from baseline to week 12 was then conducted, and the means of those values and percent change were calculated, using presence or absence of T2DM at baseline. In addition, we used four HPLC subclasses (major classes) to assess the percent change in particle size for LDL, HDL, and VLDL from baseline to week 12 and the mean percent change from baseline to week 10 and week 12.
Procedure
Lipoprotein measurement by HPLC was performed at Skylight Biotech, Inc. (100-4 Sunada, Iijima, Akita-shi, Akita, 011-0911 JAPAN). The method of measurement has been described in detail21). The HPLC particle count was calculated from particle concentration and radius, assuming that the particles were spherical.
The method applied an analytical algorithm, using Gaussian approximation, to the 20 subclass groups (group [G] 1–20) obtained by HPLC subfractionation, considering subclass G1–G2 as chylomicron, subclass G3–G7 as VLDL, subclass G8–G13 as LDL, and subclass G14–G20 as HDL, with VLDL remnant in subclass G7–G8 and sdLDL in subclass G11–G13, respectively19–21).
Statistical Analysis
Statistical analyses were performed on the FAS of the YUKAWA-2 study in the double-blind treatment period, which included all subjects randomized to study drugs who had received at least 1 dose of study drug. The YUKAWA-2 study involved two randomization steps. For the first step in the lipid stabilization period, patients were randomized 1:1 to one of two atorvastatin doses. In the second step, patients receiving each atorvastatin dose were randomized 1:1:1:1 to one of the following treatment groups: subcutaneous evolocumab 140 mg every 2 weeks or 420 mg every month, or subcutaneous placebo every 2 weeks or every month. In the present study, unless specified otherwise, those eight groups were consolidated into two groups (the evolocumab group and the placebo group) for evaluation and were not considered separately by atorvastatin dose cohort and dose frequency combination. Unless specified otherwise, no imputation was performed for missing data.
Since some data were missing on VLDL particle size assessed by HPLC, the average size of VLDL particles was calculated as the mean of the size in each cholesterol fraction, weighted by the applicable values as shown below25):
VLDL particle size = [(64 nm × G3 concentration) + (53.6 nm × G4 concentration) + (44.5 nm × G5 concentration) + (36.8 nm × G6 concentration) + (31.3 nm × G7 concentration)] / VLDL concentration.
To describe observed calculated LDL-C, ultracentrifugation LDL-C, and HPLC LDL-C visually, scatter plots for these parameters were shown. Due to the post-hoc explanatory nature of the study, all statistical analyses were descriptive, and no statistical testing was performed. Statistical analysis was conducted by Amgen Astellas BioPharma K.K., using SAS version 9.2 or later.
Results
Patient Characteristics
As previously reported for the YUKAWA-2 study12), the 404 study subjects were 39.6% women and 60.4% men. Age (mean ± standard deviation [SD]) was 61.3 ± 10.3 years, and 57.2% of the subjects were younger than 65 years of age. At baseline, roughly half of the patients had been diagnosed with T2DM and a quarter with metabolic syndrome.
Original LDL-C values from the YUKAWA-2 study were 102.6 ± 28.1 mg/dL (2.65 ± 0.73 mmol/L) in the placebo group and 108.5 ± 35.4 mg/dL (2.80 ± 0.92 mmol/L) in the evolocumab group. In the present study, we used HPLC to assess baseline blood samples from the YUKAWA-2 study; the resulting LDL-C values were 91.1 ± 21.7 mg/dL (2.36 ± 0.56 mmol/L) in the placebo group and 94.6 ± 26.4 mg/dL (2.45 ± 0.68 mmol/L) in the evolocumab group (Table 1).
Table 1. Patient characteristics at baseline.
| Variables | Placebo (n = 202) | Evolocumab (n = 202) |
|---|---|---|
| Female, n (%) | 79 (39.1) | 81 (40.1) |
| Age, years | 60.8 ± 10.1 | 61.8 ± 10.6 |
| Age group ≥ 65 years, n (%) | 81 (40.1) | 92 (45.5) |
| Lipid profile | ||
| LDL-C, mg/dL | 102.6 ± 28.1 | 108.5 ± 35.4 |
| TG, mg/dL | 136.4 ± 75.8 | 141.0 ± 96.9 |
| CM-C, mg/dL§ | 1.76 ± 2.61 | 1.72 ± 2.39 |
| VLDL-C, mg/dL§ | 34.79 ± 13.19 | 36.43 ± 14.95 |
| LDL-C, mg/dL§ | 91.12 ± 21.72 | 94.60 ± 26.44 |
| HDL-C, mg/dL§ | 52.30 ± 11.25 | 52.14 ± 11.78 |
Data are expressed as n (%) or mean ± standard deviation.
Abbreviations: LDL-C, low-density lipoprotein cholesterol; TG, triglyceride; CM-C, chylomicron cholesterol; VLDL-C, very low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol.
Samples were assessed by high-performance liquid chromatography (HPLC).
Absolute Value and Percent Change from Baseline in the Concentration of Cholesterol and TG, and in the Number of Particles, for the 20 Lipoprotein Subclasses
The concentration of cholesterol and TG and the number of particles were reduced in the subclasses corresponding to VLDL, LDL, and chylomicrons, although the data relating to chylomicrons varied widely when lipoproteins were divided into 20 subclasses by HPLC. In accordance with results from the YUKAWA-2 study, the level of LDL-C concentration was reduced by approximately 70% in the evolocumab group in comparison to the placebo group. Specifically, cholesterol was reduced in subclasses G7–8, which correspond to VLDL remnants; in G8–10, which correspond to lbLDL; and in G11–13, which correspond to sdLDL (Fig. 1, Supplemental Table 1). Reduced TG concentration was noted within the evolocumab group in the lipoprotein subclasses corresponding to VLDL and LDL, particularly in subclasses G3–5, which correspond to VLDL-1, and also in G7–11 (Supplemental Fig. 1, Supplemental Table 2). The evolocumab group also showed a reduced number of lipoprotein particles in subclasses G7–G13. Lower particle numbers were seen in the subclasses corresponding to chylomicron (Fig. 2, Supplemental Table 3). These findings were nearly identical to the results for another co-primary endpoint (the mean percent change from baseline at weeks 10 and 12) (Supplemental Table 1–3).
Fig. 1.
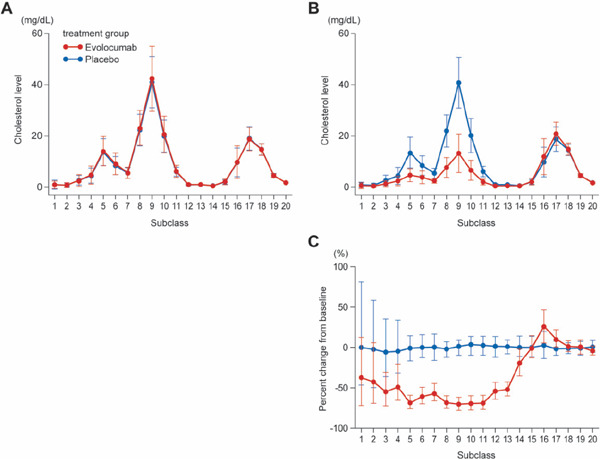
Absolute value and percent change from baseline for concentration of cholesterol in 20 lipoprotein subclasses
A, value at baseline; B, value at week 12; C, percent change between baseline and week 12.
In A and B, points indicate mean ± SD; C, median and 1st and 3rd quartiles (Q1, Q3).
Supplemental Table 1. Absolute value and percent change from baseline for concentration of cholesterol in 20 subclasses.
|
A. Baseline | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sub class 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
| Measured value, mg/dL | ||||||||||||||||||||
| Placebo | 0.98 ± 1.78 | 0.78 ± 0.88 | 2.68 ± 1.87 | 4.53 ± 2.95 | 13.67 ± 5.24 | 8.41 ± 3.54 | 5.50 ± 2.08 | 22.28 ± 6.15 | 40.98 ± 10.03 | 19.89 ± 6.25 | 6.02 ± 1.90 | 0.97 ± 0.37 | 0.99 ± 0.23 | 0.57 ± 0.20 | 2.03 ± 0.93 | 9.65 ± 5.60 | 19.01 ± 4.57 | 14.75 ± 2.23 | 4.56 ± 0.76 | 1.73 ± 0.25 |
| Evolocumab | 0.94 ± 1.43 | 0.79 ± 0.98 | 2.72 ± 2.21 | 4.73 ± 3.54 | 14.25 ± 5.82 | 9.12 ± 4.21 | 5.61 ± 2.20 | 23.11 ± 6.88 | 42.75 ± 12.35 | 20.55 ± 7.16 | 6.17 ± 2.06 | 1.01 ± 0.37 | 1.02 ± 0.24 | 0.57 ± 0.20 | 2.13 ± 1.19 | 9.75 ± 6.36 | 18.72 ± 4.52 | 14.70 ± 2.26 | 4.53 ± 0.68 | 1.75 ± 0.27 |
|
B. Week 12 | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sub class 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
| Measured value, mg/dL | ||||||||||||||||||||
| Placebo | 0.89 ± 1.08 | 0.75 ± 0.81 | 2.62 ± 2.03 | 4.43 ± 3.29 | 13.50 ± 6.03 | 8.45 ± 3.83 | 5.43 ± 1.95 | 21.94 ± 6.29 | 40.78 ± 9.91 | 20.15 ± 6.67 | 6.11 ± 2.05 | 0.98 ± 0.39 | 0.99 ± 0.24 | 0.57 ± 0.19 | 2.04 ± 0.97 | 9.76 ± 5.79 | 18.76 ± 4.78 | 14.51 ± 2.15 | 4.51 ± 0.68 | 1.74 ± 0.25 |
| Evolocumab | 0.59 ± 1.18 | 0.44 ± 0.62 | 1.30 ± 1.27 | 2.43 ± 1.87 | 4.68 ± 2.54 | 3.84 ± 2.48 | 2.41 ± 1.05 | 7.68 ± 3.88 | 13.22 ± 7.50 | 6.59 ± 3.88 | 2.02 ± 1.14 | 0.46 ± 0.20 | 0.50 ± 0.17 | 0.47 ± 0.21 | 2.20 ± 1.33 | 11.92 ± 7.02 | 20.81 ± 4.80 | 14.83 ± 2.36 | 4.53 ± 0.74 | 1.68 ± 0.27 |
| Change from baseline, % | ||||||||||||||||||||
| Placebo | 0.00 (−46.15, 81.05) | −2.30 (−50.00, 58.33) | −5.82 (−36.07, 35.29) | −4.75 (−31.59, 33.87) | −0.91 (−16.57, 14.48) | 0.00 (−12.61, 15.80) | 0.33 (−16.02, 16.57) | −1.94 (−12.14, 6.95) | 1.35 (−9.77, 8.97) | 3.65 (−9.74, 13.59) | 2.60 (−9.68, 13.79) | 1.41 (−11.11, 13.56) | 1.03 (−7.92, 9.20) | 0.00 (−10.98, 14.06) | 0.00 (−11.57, 13.89) | 2.58 (−13.75, 19.62) | −1.71 (−9.75, 7.83) | −1.25 (−7.62, 5.80) | −0.89 (−9.53, 7.58) | 0.59 (−6.08, 8.74) |
| Evolocumab | −37.33 (−71.93, 12.50) | −42.68 (−68.89, 5.88) | −54.86 (−72.31, −30.70) | −49.03 (−65.28, −20.63) | −68.55 (−75.87, −59.23) | −60.77 (−69.73, −49.30) | −57.28 (−65.98, −44.58) | −68.38 (−75.35, −59.75) | −70.24 (−77.91, −61.76) | −69.43 (−77.42, −59.73) | −68.92 (−76.65, −59.27) | −54.10 (−64.00, −43.17) | −52.05 (−60.00, −42.67) | −19.35 (−34.78, −1.72) | −0.58 (−12.74, 14.57) | 26.40 (5.53, 46.28) | 10.40 (0.42, 21.95) | 1.15 (−5.20, 8.37) | 0.65 (−8.29, 9.31) | −4.27 (−9.40, 1.80) |
|
C. Mean of weeks 10 and 12 | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sub class 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
| Measured value, mg/dL | ||||||||||||||||||||
| Placebo | 0.94 ± 0.94 | 0.78 ± 0.70 | 2.65 ± 1.75 | 4.46 ± 2.88 | 13.46 ± 5.15 | 8.40 ± 3.52 | 5.41 ± 1.87 | 21.64 ± 5.94 | 40.02 ± 9.35 | 19.80 ± 6.33 | 6.01 ± 1.94 | 0.97 ± 0.38 | 0.98 ± 0.23 | 0.56 ± 0.19 | 2.02 ± 0.94 | 9.58 ± 5.50 | 18.56 ± 4.48 | 14.45 ± 2.06 | 4.50 ± 0.64 | 1.73 ± 0.23 |
| Evolocumab | 0.59 ± 0.95 | 0.43 ± 0.50 | 1.26 ± 1.05 | 2.33 ± 1.55 | 4.42 ± 2.37 | 3.68 ± 2.36 | 2.33 ± 1.02 | 7.15 ± 3.59 | 11.88 ± 6.71 | 5.85 ± 3.43 | 1.80 ± 0.99 | 0.43 ± 0.17 | 0.47 ± 0.16 | 0.46 ± 0.20 | 2.18 ± 1.32 | 11.80 ± 6.83 | 20.59 ± 4.56 | 14.57 ± 2.09 | 4.43 ± 0.66 | 1.64 ± 0.25 |
| Change from baseline, % | ||||||||||||||||||||
| Placebo | 17.31 (−35.00, 104.29) | 6.15 (−36.41, 78.40) | 1.72 (−29.08, 44.38) | 1.16 (−30.25, 38.26) | −0.53 (−15.27, 15.39) | 0.55 (−13.03, 15.95) | −0.22 (−16.04, 15.30) | −3.48 (−11.80, 5.81) | −1.49 (−10.15, 6.22) | 0.59 (−10.22, 10.79) | 0.37 (−10.39, 10.18) | 1.65 (−9.21, 12.75) | −0.48 (−8.25, 7.87) | 0.00 (−11.48, 10.58) | −1.14 (−9.87, 9.85) | 1.66 (−12.38, 14.24) | −2.39 (−10.16, 6.23) | −1.71 (−7.34, 3.94) | −2.33 (−7.90, 5.87) | 0.32 (−6.39, 6.03) |
| Evolocumab | −27.38 (−59.72, 25.00) | −37.75 (−62.16, 10.81) | −53.09 (−67.74, −32.38) | −48.70 (−64.30, −22.77) | −70.01 (−76.35, −62.35) | −62.36 (−70.10, −52.70) | −59.73 (−66.84, −48.15) | −70.93 (−77.00, −63.33) | −74.29 (−79.71, −67.00) | −73.07 (−78.70, −65.19) | −71.94 (−78.27, −64.17) | −57.73 (−66.67, −47.12) | −54.22 (−62.50, −45.57) | −22.22 (−33.78, −7.35) | −0.99 (−12.34, 12.23) | 27.85 (8.08, 46.67) | 10.15 (0.76, 18.62) | −0.65 (−6.51, 5.78) | −2.16 (−8.56, 6.48) | −6.71 (−10.98, −0.25) |
Data are expressed as mean ± SD or median (interquartile range).
Supplemental Fig. 1.
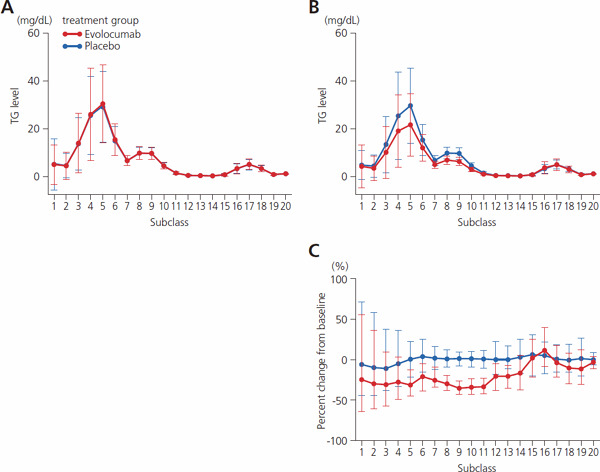
Absolute value and percent change from baseline for concentration of TG in 20 lipoprotein subclasses
A, baseline values; B, values at week 12; C, change from baseline to week 12. In A and B, values indicate mean ± SD; C, median and 1st and 3rd quartiles (Q1, Q3). Abbreviation: TG, triglyceride.
Supplemental Table 2. Absolute value and percent change for concentration of TG in 20 subclasses.
|
A. Baseline | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sub class 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
| Measured value, mg/dL | ||||||||||||||||||||
| Placebo | 5.35 ± 10.46 | 4.53 ± 5.17 | 13.64 ± 10.89 | 25.50 ± 16.28 | 29.48 ± 14.35 | 14.85 ± 6.06 | 6.61 ± 2.12 | 9.75 ± 2.54 | 9.61 ± 2.41 | 4.42 ± 1.27 | 1.42 ± 0.45 | 0.43 ± 0.28 | 0.34 ± 0.18 | 0.25 ± 0.15 | 0.69 ± 0.46 | 3.21 ± 1.95 | 5.04 ± 2.14 | 3.26 ± 1.28 | 0.81 ± 0.42 | 1.14 ± 0.27 |
| Evolocumab | 4.98 ± 8.29 | 4.43 ± 5.61 | 13.56 ± 12.82 | 25.95 ± 19.27 | 30.38 ± 16.36 | 15.41 ± 6.64 | 6.59 ± 2.09 | 9.75 ± 2.66 | 9.66 ± 2.65 | 4.44 ± 1.39 | 1.41 ± 0.48 | 0.44 ± 0.25 | 0.35 ± 0.16 | 0.25 ± 0.15 | 0.75 ± 0.60 | 3.30 ± 2.22 | 5.05 ± 2.20 | 3.32 ± 1.38 | 0.84 ± 0.45 | 1.15 ± 0.25 |
|
B. Week 12 | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sub class 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
| Measured value, mg/dL | ||||||||||||||||||||
| Placebo | 4.75 ± 5.99 | 4.36 ± 4.65 | 13.36 ± 11.76 | 25.32 ± 18.29 | 29.60 ± 15.72 | 15.25 ± 6.42 | 6.68 ± 2.02 | 9.77 ± 2.41 | 9.63 ± 2.45 | 4.46 ± 1.36 | 1.42 ± 0.48 | 0.43 ± 0.22 | 0.34 ± 0.14 | 0.25 ± 0.12 | 0.69 ± 0.40 | 3.21 ± 1.82 | 4.94 ± 1.87 | 3.22 ± 1.19 | 0.81 ± 0.39 | 1.13 ± 0.20 |
| Evolocumab | 4.19 ± 9.12 | 3.41 ± 5.14 | 10.09 ± 10.83 | 19.02 ± 15.15 | 21.59 ± 12.98 | 11.95 ± 5.52 | 4.98 ± 1.51 | 6.86 ± 1.75 | 6.23 ± 1.70 | 2.91 ± 0.92 | 0.93 ± 0.33 | 0.34 ± 0.23 | 0.27 ± 0.15 | 0.21 ± 0.16 | 0.76 ± 0.62 | 3.68 ± 2.51 | 4.91 ± 2.44 | 2.96 ± 1.34 | 0.73 ± 0.41 | 1.09 ± 0.25 |
| Change from baseline, % | ||||||||||||||||||||
| Placebo | −5.98 (−44.44, 71.43) | −9.83 (−44.50, 58.17) | −10.97 (−38.34, 37.45) | −5.10 (−33.18, 36.20) | 0.53 (−21.55, 22.80) | 3.77 (−15.04, 25.40) | 1.84 (−12.15, 16.48) | 0.89 (−9.44, 12.43) | 1.30 (−7.83, 7.62) | 1.16 (−8.93, 10.20) | 0.74 (−10.81, 11.76) | 0.00 (−18.00, 22.22) | 0.00 (−11.54, 17.02) | 3.03 (−17.24, 25.00) | 6.19 (−20.00, 31.48) | 5.08 (−17.21, 21.57) | 0.67 (−15.14, 18.21) | −0.60 (−15.95, 17.43) | 1.33 (−20.00, 26.32) | 0.00 (−6.72, 8.41) |
| Evolocumab | −24.76 (−64.03, 55.23) | −29.86 (−60.53, 36.29) | −30.90 (−57.02, 9.54) | −27.75 (−49.10, 3.12) | −31.39 (−44.96, −12.79) | −20.99 (−39.05, −5.20) | −25.53 (−34.10, −9.85) | −29.88 (−37.96, −19.29) | −35.38 (−42.98, −26.23) | −34.13 (−43.22, −23.86) | −33.57 (−42.15, −22.73) | −20.51 (−38.10, −5.26) | −20.59 (−35.29, −7.41) | −16.67 (−37.50, 5.56) | 1.96 (−21.54, 25.00) | 11.44 (−8.65, 39.56) | −3.83 (−21.81, 17.34) | −10.33 (−29.73, 7.92) | −11.48 (−30.77, 12.36) | −3.19 (−10.95, 3.85) |
|
C. Mean of weeks 10 and 12 | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sub class 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
| Measured value, mg/dL | ||||||||||||||||||||
| Placebo | 4.88 ± 5.22 | 4.39 ± 3.99 | 13.17 ± 9.94 | 24.84 ± 15.34 | 29.27 ± 13.49 | 15.16 ± 5.80 | 6.60 ± 1.91 | 9.63 ± 2.37 | 9.45 ± 2.38 | 4.38 ± 1.28 | 1.41 ± 0.44 | 0.43 ± 0.20 | 0.34 ± 0.13 | 0.25 ± 0.11 | 0.69 ± 0.38 | 3.17 ± 1.75 | 4.87 ± 1.77 | 3.19 ± 1.09 | 0.80 ± 0.35 | 1.13 ± 0.19 |
| Evolocumab | 4.26 ± 8.10 | 3.34 ± 4.38 | 9.66 ± 9.04 | 18.05 ± 12.67 | 20.58 ± 11.15 | 11.59 ± 4.89 | 4.88 ± 1.43 | 6.67 ± 1.64 | 5.95 ± 1.52 | 2.76 ± 0.80 | 0.89 ± 0.28 | 0.34 ± 0.21 | 0.27 ± 0.13 | 0.21 ± 0.14 | 0.76 ± 0.56 | 3.66 ± 2.28 | 4.90 ± 2.10 | 2.94 ± 1.18 | 0.72 ± 0.37 | 1.08 ± 0.23 |
| Change from baseline, % | ||||||||||||||||||||
| Placebo | 10.29 (−38.51, 102.94) | 0.39 (−30.95, 69.89) | −4.95 (−32.80, 39.36) | −4.37 (−27.27, 32.13) | −1.00 (−18.78, 23.26) | 1.52 (−12.33, 20.60) | 0.32 (−11.02, 14.42) | −0.29 (−10.18, 8.97) | −1.05 (−9.36, 5.57) | −0.79 (−9.83, 8.07) | −0.84 (−10.00, 8.82) | 2.44 (−10.83, 21.67) | 1.85 (−8.93, 15.63) | 2.63 (−14.71, 27.50) | 5.41 (−15.07, 28.70) | 1.66 (−12.93, 21.29) | −1.12 (−14.31, 16.71) | −1.35 (−16.72, 16.80) | 2.83 (−17.91, 23.44) | 0.40 (−7.02, 7.43) |
| Evolocumab | −6.87 (−52.78, 72.73) | −19.64 (−50.22, 39.73) | −26.58 (−53.56, 10.25) | −28.66 (−48.38, −2.66) | −32.83 (−47.32, −15.53) | −24.32 (−38.62, −10.35) | −24.02 (−36.66, −14.52) | −30.78 (−38.42, −23.25) | −38.12 (−43.78, −29.16) | −36.88 (−44.61, −27.72) | −34.95 (−43.50, −26.50) | −21.43 (−35.71, −5.56) | −20.59 (−34.48, −8.70) | −14.71 (−34.38, 3.33) | 4.23 (−16.67, 30.65) | 14.21 (−6.91, 43.40) | −2.63 (−19.43, 20.54) | −10.28 (−26.46, 8.12) | −11.43 (−28.95, 12.50) | −3.40 (−11.56, 3.76) |
Data are expressed as mean ± SD or median (interquartile range). Abbreviation: TG, triglyceride.
Fig. 2.
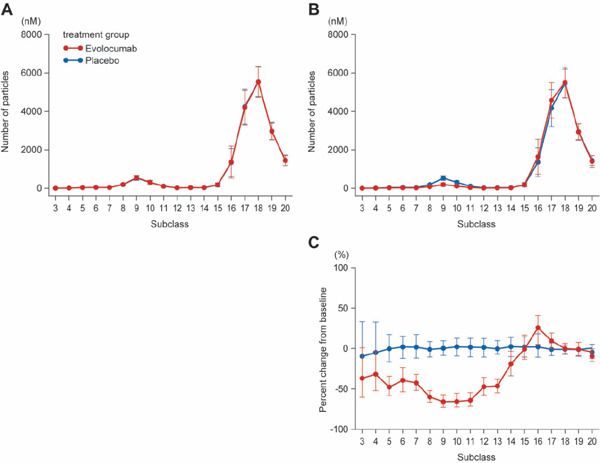
Absolute value and percent change from baseline for number of particles in 20 lipoprotein subclasses
A, value at baseline; B, value at week 12; C, percent change between baseline and week 12.
In A and B, points indicate mean ± SD; C, median and 1st and 3rd quartiles (Q1, Q3).
Supplemental Table 3. Absolute value and percent change for particle size in 20 subclasses.
|
A. Baseline | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sub class 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
| Measured value, nM | ||||||||||||||||||
| Placebo | 3.87 ± 2.94 | 12.71 ± 7.96 | 37.50 ± 14.24 | 45.27 ± 16.12 | 40.96 ± 13.08 | 190.43 ± 45.65 | 530.31 ± 121.46 | 302.87 ± 89.74 | 105.83 ± 31.47 | 24.47 ± 9.35 | 36.31 ± 8.46 | 33.70 ± 10.84 | 171.72 ± 76.06 | 1344.83 ± 722.57 | 4251.06 ± 907.49 | 5547.59 ± 777.31 | 2977.38 ± 457.00 | 1435.47 ± 268.65 |
| Evolocumab | 3.86 ± 3.48 | 13.00 ± 9.53 | 38.84 ± 16.54 | 47.99 ± 18.68 | 41.39 ± 13.64 | 196.07 ± 51.42 | 550.65 ± 148.49 | 311.85 ± 102.70 | 107.98 ± 34.19 | 25.42 ± 9.42 | 37.30 ± 8.57 | 33.74 ± 10.88 | 181.34 ± 100.18 | 1363.02 ± 832.96 | 4195.35 ± 898.01 | 5540.57 ± 803.46 | 2965.98 ± 429.02 | 1453.44 ± 274.55 |
|
B. Week 12 | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sub class 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |||
| Measured value, nM | ||||||||||||||||||||
| Placebo | 3.79 ± 3.17 | 12.57 ± 8.97 | 37.38 ± 15.58 | 46.02 ± 16.86 | 40.85 ± 11.81 | 188.22 ± 46.13 | 528.18 ± 120.62 | 306.64 ± 96.71 | 107.17 ± 34.54 | 24.66 ± 9.70 | 36.37 ± 8.87 | 33.64 ± 9.93 | 172.76 ± 78.59 | 1358.35 ± 750.01 | 4191.19 ± 949.54 | 5457.31 ± 746.88 | 2947.62 ± 420.19 | 1445.52 ± 252.88 | ||
| Evolocumab | 2.62 ± 2.75 | 8.88 ± 6.96 | 21.18 ± 11.68 | 28.89 ± 12.94 | 23.45 ± 7.62 | 80.20 ± 30.74 | 192.96 ± 92.84 | 112.22 ± 57.02 | 39.85 ± 19.35 | 13.36 ± 5.65 | 20.20 ± 6.29 | 27.83 ± 11.25 | 187.14 ± 111.36 | 1641.56 ± 911.73 | 4579.64 ± 931.47 | 5511.89 ± 792.98 | 2928.78 ± 431.67 | 1394.76 ± 296.75 | ||
| Change from baseline, % | ||||||||||||||||||||
| Placebo | −9.66 (−34.68, 33.04) | −5.03 (−31.26, 32.53) | −0.38 (−16.78, 17.36) | 1.97 (−12.21, 15.25) | 1.70 (−11.96, 17.28) | −1.11 (−10.52, 8.66) | 0.26 (−8.45, 9.58) | 2.12 (−9.22, 12.72) | 1.68 (−10.36, 12.65) | 1.35 (−10.58, 12.41) | −0.38 (−7.01, 9.79) | 2.30 (−10.07, 13.75) | 1.87 (−10.58, 14.60) | 2.04 (−10.78, 18.13) | −1.09 (−8.75, 6.91) | −1.01 (−7.11, 4.35) | −1.26 (−8.87, 7.63) | 0.55 (−7.21, 10.25) | ||
| Evolocumab | −36.93 (−60.44, 0.88) | −31.96 (−52.18, −3.63) | −47.87 (−58.18, −34.39) | −39.44 (−53.57, −23.64) | −42.65 (−51.84, −32.00) | −60.10 (−66.54, −52.20) | −65.99 (−72.91, −57.38) | −65.69 (−72.44, −55.41) | −64.23 (−71.45, −54.62) | −47.45 (−57.60, −35.92) | −46.51 (−54.89, −38.21) | −19.15 (−33.98, −3.34) | −0.86 (−12.64, 16.62) | 25.73 (5.00, 41.48) | 9.41 (0.19, 19.23) | −0.08 (−6.33, 6.39) | −0.95 (−9.67, 7.52) | −4.38 (−11.15, 4.61) | ||
|
C. Mean of weeks 10 and 12 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sub class 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
| Measured value, nM | ||||||||||||||||||
| Placebo | 3.76 ± 2.69 | 12.40 ± 7.59 | 37.10 ± 13.62 | 45.74 ± 15.89 | 40.55 ± 11.37 | 185.63 ± 43.92 | 518.31 ± 114.33 | 301.27 ± 91.83 | 105.52 ± 32.62 | 24.61 ± 9.34 | 36.04 ± 8.42 | 33.39 ± 9.50 | 171.04 ± 76.47 | 1333.85 ± 715.05 | 4144.13 ± 892.49 | 5430.76 ± 715.39 | 2936.72 ± 393.33 | 1434.67 ± 234.14 |
| Evolocumab | 2.52 ± 2.29 | 8.44 ± 5.80 | 20.14 ± 10.10 | 27.91 ± 11.70 | 22.88 ± 7.36 | 75.87 ± 28.87 | 175.86 ± 83.77 | 101.19 ± 50.67 | 36.11 ± 16.93 | 12.62 ± 5.07 | 19.29 ± 5.79 | 27.32 ± 10.61 | 185.48 ± 109.08 | 1625.46 ± 884.02 | 4535.65 ± 883.84 | 5419.80 ± 719.77 | 2866.17 ± 403.34 | 1357.59 ± 268.80 |
| Change from baseline, % | ||||||||||||||||||
| Placebo | −2.82 (−32.21, 37.97) | −1.91 (−27.13, 31.85) | −2.73 (−16.32, 18.46) | 2.40 (−13.01, 15.80) | −0.06 (−11.97, 12.37) | −2.57 (−11.22, 6.46) | −1.42 (−9.73, 5.89) | 0.43 (−9.87, 9.72) | −0.68 (−10.61, 9.58) | 0.69 (−9.71, 12.44) | −0.13 (−7.56, 7.52) | 0.10 (−8.04, 10.89) | −0.18 (−9.59, 10.37) | 0.63 (−10.23, 13.77) | −2.08 (−9.14, 4.97) | −2.18 (−7.24, 3.78) | −2.26 (−7.39, 6.05) | 0.32 (−7.27, 7.02) |
| Evolocumab | −33.66 (−56.87, −2.70) | −32.80 (−52.85, −7.31) | −49.21 (−59.29, −37.82) | −42.26 (−52.41, −31.61) | −44.43 (−52.40, −35.06) | −62.40 (−68.02, −55.71) | −69.63 (−75.10, −62.59) | −69.07 (−74.54, −60.74) | −67.45 (−73.44, −59.98) | −50.34 (−59.81, −39.57) | −48.08 (−57.80, −40.56) | −20.51 (−32.75, −6.95) | 0.41 (−12.07, 14.50) | 23.22 (7.32, 43.57) | 8.01 (0.87, 15.82) | −1.74 (−7.15, 4.23) | −3.54 (−10.44, 5.50) | −7.95 (−12.80, 1.15) |
Data are expressed as mean ± SD or median (interquartile range).
Subgroup Analysis of Absolute Value and Percent Change from Baseline in Cholesterol Concentration, TG Concentration, and Number of Particles in the 20 Lipoprotein Subclasses, using Presence or Absence of Type 2 Diabetes at Baseline
There were no notable differences in patient characteristics between the placebo and evolocumab groups, regardless of whether patients had T2DM (Supplemental Table 4). Responses to evolocumab were comparable between T2DM and non-diabetic patients, and findings for cholesterol (Supplemental Fig. 2, Supplemental Table 5) and TG concentrations (Supplemental Fig. 3, Supplemental Table 6) and lipid particle size (Supplemental Fig. 4, Supplemental Table 7) were similar for the T2DM group, the non-diabetes group, and the overall subject population. These findings were nearly identical to the results for another co-primary endpoint (the mean percent change from baseline at weeks 10 and 12) (Supplemental Table 5–7).
Supplemental Table 4. Patient baseline characteristics by presence/absence of diabetes mellitus.
| Patients with diabetes |
Patients without diabetes |
|||
|---|---|---|---|---|
| Placebo (n = 103) | Evolocumab (n = 94) | Placebo (n = 99) | Evolocumab (n = 108) | |
| CM-C, mg/dL | 1.89 ± 2.47 | 1.69 ± 2.32 | 1.62 ± 2.76 | 1.75 ± 2.46 |
| HDL-C, mg/dL | 49.56 ± 8.61 | 52.92 ± 11.21 | 55.12 ± 12.88 | 51.43 ± 12.27 |
| LDL-C, mg/dL | 90.37 ± 21.26 | 91.98 ± 21.47 | 91.90 ± 22.27 | 96.96 ± 30.14 |
| VLDL-C, mg/dL | 34.72 ± 12.17 | 36.24 ± 12.79 | 34.86 ± 14.23 | 36.60 ± 16.72 |
Abbreviations: CM-C, chylomicron cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; VLDL-C, very low-density lipoprotein cholesterol. Data are expressed as mean ± SD. Samples were assessed by high-performance liquid chromatography (HPLC).
Supplemental Fig. 2.
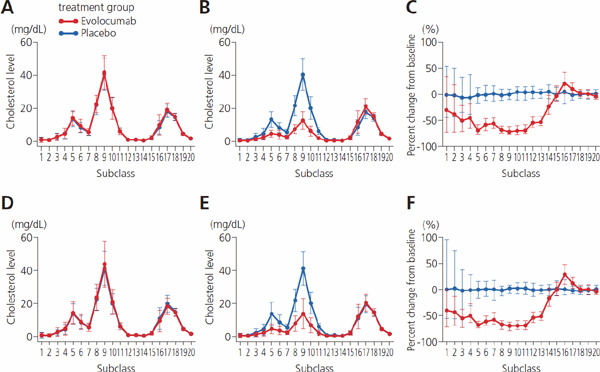
Absolute value and percent change from baseline for concentration of cholesterol in 20 lipoprotein subclasses by presence/absence of diabetes mellitus
A, value at baseline in patients with diabetes; B, value at week 12 with diabetes; C, change from baseline to week 12 with diabetes; D, baseline in patients without diabetes; E, week 12 without diabetes; F, change from baseline to week 12 without diabetes. In A, B, D, and E, values indicate mean ± SD; C and F, median and 1st and 3rd quartiles (Q1, Q3).
Supplemental Table 5. Absolute value and percent change for concentration of cholesterol in 20 subclasses by presence/absence of diabetes mellitus.
|
A. Baseline | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sub class 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
| DM | ||||||||||||||||||||
| Measured value, mg/dL | ||||||||||||||||||||
| Placebo | 1.05 ± 1.62 | 0.84 ± 0.89 | 2.83 ± 1.99 | 4.70 ± 3.03 | 13.51 ± 4.50 | 8.19 ± 3.02 | 5.50 ± 1.96 | 21.93 ± 5.70 | 40.60 ± 9.74 | 19.87 ± 6.53 | 6.03 ± 2.02 | 0.96 ± 0.36 | 0.98 ± 0.24 | 0.54 ± 0.19 | 1.85 ± 0.65 | 8.36 ± 4.17 | 18.11 ± 3.77 | 14.52 ± 2.14 | 4.49 ± 0.79 | 1.70 ± 0.25 |
| Evolocumab | 0.93 ± 1.42 | 0.77 ± 0.93 | 2.64 ± 2.01 | 4.60 ± 3.22 | 14.16 ± 4.47 | 9.30 ± 3.85 | 5.55 ± 1.90 | 22.46 ± 5.19 | 41.54 ± 10.15 | 19.99 ± 6.71 | 5.99 ± 1.85 | 0.99 ± 0.40 | 1.01 ± 0.21 | 0.58 ± 0.20 | 2.15 ± 1.17 | 9.90 ± 6.27 | 19.13 ± 4.11 | 14.90 ± 2.24 | 4.52 ± 0.69 | 1.74 ± 0.28 |
| Non-DM | ||||||||||||||||||||
| Measured value, mg/dL | ||||||||||||||||||||
| Placebo | 0.91 ± 1.93 | 0.72 ± 0.86 | 2.53 ± 1.73 | 4.36 ± 2.87 | 13.84 ± 5.93 | 8.63 ± 4.01 | 5.50 ± 2.22 | 22.63 ± 6.60 | 41.36 ± 10.35 | 19.92 ± 5.98 | 6.02 ± 1.78 | 0.97 ± 0.37 | 1.00 ± 0.22 | 0.60 ± 0.22 | 2.21 ± 1.12 | 10.97 ± 6.53 | 19.95 ± 5.12 | 15.00 ± 2.32 | 4.63 ± 0.73 | 1.76 ± 0.25 |
| Evolocumab | 0.95 ± 1.44 | 0.81 ± 1.03 | 2.79 ± 2.38 | 4.84 ± 3.82 | 14.34 ± 6.84 | 8.97 ± 4.52 | 5.67 ± 2.46 | 23.69 ± 8.09 | 43.84 ± 14.00 | 21.05 ± 7.53 | 6.33 ± 2.23 | 1.02 ± 0.35 | 1.03 ± 0.26 | 0.56 ± 0.20 | 2.11 ± 1.21 | 9.61 ± 6.48 | 18.36 ± 4.86 | 14.51 ± 2.28 | 4.53 ± 0.68 | 1.75 ± 0.26 |
|
B. Week 12 | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sub class 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
| DM | ||||||||||||||||||||
| Measured value, mg/dL | ||||||||||||||||||||
| Placebo | 0.92 ± 1.12 | 0.77 ± 0.82 | 2.68 ± 2.04 | 4.49 ± 3.30 | 13.22 ± 4.62 | 8.20 ± 3.00 | 5.45 ± 1.80 | 21.63 ± 5.96 | 40.28 ± 9.74 | 20.10 ± 6.77 | 6.13 ± 2.15 | 1.00 ± 0.40 | 0.99 ± 0.26 | 0.55 ± 0.18 | 1.82 ± 0.70 | 8.39 ± 4.66 | 17.93 ± 4.36 | 14.30 ± 2.15 | 4.46 ± 0.70 | 1.71 ± 0.25 |
| Evolocumab | 0.48 ± 0.60 | 0.40 ± 0.41 | 1.26 ± 1.02 | 2.45 ± 1.66 | 4.61 ± 2.11 | 4.03 ± 2.58 | 2.42 ± 0.92 | 7.39 ± 2.84 | 12.57 ± 5.22 | 6.28 ± 3.05 | 1.92 ± 0.84 | 0.45 ± 0.18 | 0.49 ± 0.14 | 0.46 ± 0.21 | 2.19 ± 1.28 | 11.72 ± 6.68 | 21.12 ± 4.55 | 15.15 ± 2.29 | 4.55 ± 0.71 | 1.67 ± 0.26 |
| Change from baseline, % | ||||||||||||||||||||
| Placebo | −1.51 (−44.73, 53.85) | −2.38 (−43.33, 50.00) | −6.67 (−34.48, 32.33) | −6.86 (−37.70, 37.35) | −1.93 (−16.99, 12.28) | −1.25 (−12.18, 16.83) | 0.92 (−12.99, 16.07) | −2.14 (−12.13, 9.96) | −0.51 (−10.35, 9.38) | 4.14 (−11.02, 14.24) | 3.61 (−11.19, 13.87) | 3.82 (−9.09, 15.38) | 2.68 (−8.05, 9.48) | 4.65 (−9.09, 18.33) | −1.10 (−11.92, 13.16) | 4.94 (−18.05, 22.97) | −1.66 (−11.55, 9.33) | −0.52 (−7.57, 5.80) | 0.21 (−9.28, 8.25) | 1.16 (−6.63, 9.20) |
| Evolocumab | −30.20 (−72.73, 33.33) | −38.23 (−72.73, 18.00) | −50.68 (−71.98, −21.19) | −45.51 (−65.63, −17.56) | −69.77 (−76.44, −58.55) | −58.39 (−70.37, −46.29) | −56.01 (−66.01, −45.15) | −68.87 (−74.80, −60.61) | −72.27 (−77.09, −61.76) | −70.42 (−77.42, −59.81) | −70.02 (−76.10, −58.52) | −54.64 (−64.13, −43.17) | −53.55 (−60.38, −43.01) | −23.58 (−37.93, −2.50) | −3.57 (−13.73, 16.11) | 22.53 (0.91, 41.60) | 9.95 (0.13, 21.87) | 1.89 (−4.45, 9.12) | 0.71 (−7.58, 9.11) | −4.36 (−9.38, 1.75) |
| Non-DM | ||||||||||||||||||||
| Measured value, mg/dL | ||||||||||||||||||||
| Placebo | 0.86 ± 1.03 | 0.73 ± 0.81 | 2.57 ± 2.03 | 4.37 ± 3.30 | 13.77 ± 7.19 | 8.70 ± 4.51 | 5.41 ± 2.09 | 22.24 ± 6.63 | 41.27 ± 10.10 | 20.20 ± 6.59 | 6.09 ± 1.97 | 0.96 ± 0.38 | 1.00 ± 0.23 | 0.59 ± 0.20 | 2.26 ± 1.14 | 11.16 ± 6.48 | 19.61 ± 5.05 | 14.72 ± 2.14 | 4.56 ± 0.67 | 1.76 ± 0.25 |
| Evolocumab | 0.69 ± 1.52 | 0.47 ± 0.77 | 1.34 ± 1.46 | 2.41 ± 2.04 | 4.74 ± 2.89 | 3.68 ± 2.38 | 2.40 ± 1.16 | 7.93 ± 4.62 | 13.79 ± 9.05 | 6.86 ± 4.50 | 2.11 ± 1.35 | 0.47 ± 0.21 | 0.51 ± 0.19 | 0.47 ± 0.21 | 2.22 ± 1.38 | 12.11 ± 7.33 | 20.53 ± 5.02 | 14.55 ± 2.39 | 4.50 ± 0.76 | 1.68 ± 0.29 |
| Change from baseline, % | ||||||||||||||||||||
| Placebo | 0.00 (−55.56, 95.57) | 2.17 (−55.88, 73.91) | −2.15 (−37.19, 37.44) | −1.13 (−31.50, 28.66) | −0.23 (−16.57, 15.29) | 1.13 (−12.80, 15.61) | 0.29 (−19.83, 17.86) | −1.62 (−12.32, 6.10) | 2.18 (−8.19, 8.05) | 2.98 (−8.68, 13.09) | 2.20 (−9.11, 13.28) | −1.30 (−12.50, 11.76) | −1.08 (−7.92, 8.60) | 0.00 (−11.94, 9.46) | 1.59 (−11.01, 16.76) | 0.48 (−9.93, 18.61) | −2.04 (−8.76, 6.67) | −2.62 (−9.30, 5.82) | −2.00 (−9.65, 7.27) | 0.55 (−5.71, 8.44) |
| Evolocumab | −40.00 (−71.43, 0.00) | −43.49 (−68.62, −12.88) | −55.11 (−72.31, −37.56) | −50.46 (−63.25, −27.32) | −67.92 (−73.75, −60.63) | −61.25 (−67.91, −50.60) | −57.93 (−65.96, −44.08) | −67.26 (−76.24, −59.38) | −69.51 (−79.48, −62.19) | −69.34 (−76.72, −59.73) | −68.81 (−76.81, −59.32) | −53.95 (−63.64, −43.18) | −51.47 (−59.13, −42.53) | −16.67 (−32.50, 1.72) | −0.41 (−12.37, 13.55) | 29.88 (10.28, 47.98) | 11.70 (2.54, 23.59) | 0.15 (−5.50, 8.09) | 0.65 (−9.77, 9.31) | −4.06 (−9.42, 1.80) |
|
C. Mean of weeks 10 and 12 | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sub class 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
| DM | ||||||||||||||||||||
| Measured value, mg/dL | ||||||||||||||||||||
| Placebo | 0.97 ± 0.93 | 0.81 ± 0.71 | 2.71 ± 1.78 | 4.55 ± 2.94 | 13.15 ± 4.14 | 8.16 ± 2.93 | 5.41 ± 1.71 | 21.22 ± 5.37 | 39.38 ± 8.95 | 19.68 ± 6.45 | 6.00 ± 2.05 | 0.99 ± 0.40 | 0.97 ± 0.25 | 0.54 ± 0.17 | 1.81 ± 0.66 | 8.31 ± 4.33 | 17.78 ± 4.08 | 14.26 ± 2.11 | 4.45 ± 0.69 | 1.70 ± 0.24 |
| Evolocumab | 0.51 ± 0.57 | 0.40 ± 0.36 | 1.21 ± 0.83 | 2.30 ± 1.33 | 4.31 ± 1.73 | 3.87 ± 2.43 | 2.33 ± 0.88 | 6.87 ± 2.63 | 11.22 ± 4.53 | 5.53 ± 2.50 | 1.70 ± 0.70 | 0.41 ± 0.16 | 0.46 ± 0.14 | 0.46 ± 0.20 | 2.18 ± 1.31 | 11.69 ± 6.53 | 20.83 ± 4.21 | 14.84 ± 2.11 | 4.46 ± 0.65 | 1.63 ± 0.25 |
| Change from baseline, % | ||||||||||||||||||||
| Placebo | 16.99 (−30.98, 101.99) | 7.39 (−33.24, 81.88) | 0.69 (−25.97, 42.70) | −0.67 (−31.27, 43.09) | −1.17 (−16.60, 13.36) | −2.40 (−12.97, 16.46) | −0.38 (−14.55, 13.26) | −3.51 (−11.54, 5.81) | −3.03 (−11.00, 7.03) | −0.31 (−10.75, 8.89) | −0.51 (−10.41, 9.57) | 1.56 (−8.28, 13.92) | −0.46 (−6.31, 7.15) | 0.37 (−11.29, 14.58) | −2.55 (−11.96, 8.26) | 3.16 (−15.84, 15.97) | −1.50 (−9.94, 7.09) | −1.12 (−7.26, 4.30) | −1.43 (−7.39, 7.20) | 0.77 (−6.60, 6.10) |
| Evolocumab | −19.97 (−60.61, 37.04) | −28.32 (−62.50, 14.71) | −49.88 (−67.74, −30.22) | −45.90 (−63.96, −22.43) | −71.07 (−76.53, −61.81) | −61.85 (−69.19, −50.76) | −58.64 (−66.02, −47.69) | −70.66 (−76.46, −63.37) | −74.16 (−80.05, −66.48) | −72.09 (−79.63, −66.43) | −71.30 (−79.26, −65.50) | −56.97 (−67.50, −46.09) | −54.80 (−62.74, −45.68) | −23.63 (−35.42, −8.77) | −0.59 (−10.90, 10.42) | 25.00 (4.18, 44.70) | 10.16 (−0.05, 17.76) | 0.01 (−5.24, 6.03) | −1.58 (−8.30, 6.36) | −6.71 (−10.37, −0.89) |
| Non-DM | ||||||||||||||||||||
| Measured value, mg/dL | ||||||||||||||||||||
| Placebo | 0.90 ± 0.95 | 0.75 ± 0.70 | 2.58 ± 1.74 | 4.37 ± 2.84 | 13.78 ± 6.01 | 8.65 ± 4.03 | 5.40 ± 2.04 | 22.05 ± 6.46 | 40.66 ± 9.74 | 19.92 ± 6.23 | 6.02 ± 1.83 | 0.96 ± 0.36 | 0.99 ± 0.22 | 0.58 ± 0.19 | 2.23 ± 1.12 | 10.86 ± 6.24 | 19.36 ± 4.74 | 14.64 ± 2.00 | 4.55 ± 0.59 | 1.75 ± 0.22 |
| Evolocumab | 0.67 ± 1.20 | 0.46 ± 0.60 | 1.30 ± 1.21 | 2.34 ± 1.74 | 4.51 ± 2.82 | 3.52 ± 2.30 | 2.34 ± 1.13 | 7.40 ± 4.27 | 12.46 ± 8.15 | 6.14 ± 4.07 | 1.89 ± 1.18 | 0.44 ± 0.19 | 0.48 ± 0.17 | 0.45 ± 0.20 | 2.19 ± 1.33 | 11.89 ± 7.12 | 20.37 ± 4.85 | 14.32 ± 2.06 | 4.40 ± 0.68 | 1.64 ± 0.26 |
| Change from baseline, % | ||||||||||||||||||||
| Placebo | 22.64 (−39.13, 104.29) | 2.27 (−41.47, 77.29) | 13.33 (−35.31, 48.94) | 3.11 (−28.63, 36.40) | −0.10 (−14.46, 15.41) | 0.75 (−13.56, 15.40) | 0.24 (−16.19, 17.15) | −2.84 (−13.17, 6.40) | −0.96 (−9.13, 5.27) | 1.55 (−9.76, 11.85) | 1.92 (−10.39, 11.15) | 1.65 (−9.88, 10.89) | −0.48 (−8.82, 8.10) | −0.56 (−11.49, 8.93) | 1.18 (−8.33, 11.25) | 0.68 (−10.72, 12.11) | −3.94 (−10.25, 5.80) | −2.16 (−8.11, 2.90) | −2.61 (−7.99, 5.52) | 0.00 (−5.59, 6.03) |
| Evolocumab | −33.33 (−58.77, 6.06) | −40.88 (−61.71, 6.72) | −53.61 (−66.27, −35.87) | −50.93 (−64.71, −23.45) | −69.31 (−75.71, −63.07) | −63.61 (−70.54, −54.63) | −60.08 (−67.14, −49.02) | −71.73 (−77.12, −63.15) | −74.46 (−78.86, −67.32) | −73.52 (−78.58, −64.32) | −72.32 (−77.71, −63.64) | −57.79 (−65.45, −48.19) | −53.49 (−61.80, −45.57) | −21.11 (−32.26, −7.14) | −2.06 (−12.63, 12.23) | 28.87 (9.79, 47.77) | 10.05 (2.18, 20.06) | −1.54 (−7.28, 4.26) | −2.83 (−9.12, 6.71) | −6.71 (−11.54, 0.00) |
Abbreviation: DM, diabetes mellitus. Data are expressed as mean ± SD or median (interquartile range).
Supplemental Fig. 3.
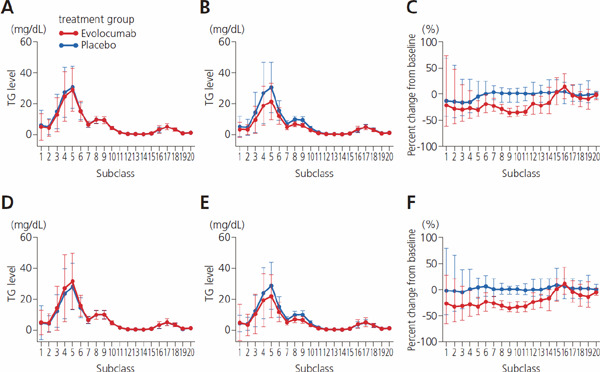
Absolute value and percent change from baseline for concentration of TG in 20 lipoprotein subclasses by presence/absence of diabetes mellitus
A, value at baseline in patients with diabetes; B, value at week 12 with diabetes; C, change from baseline to week 12 with diabetes; D, baseline in patients without diabetes; E, week 12 without diabetes; F, change from baseline to week 12 without diabetes. In A, B, D, and E, values indicate mean ± SD; C and F, median and 1st and 3rd quartiles (Q1, Q3). Abbreviation: TG, triglyceride.
Supplemental Table 6. Absolute value and percent change for concentration of TG in 20 subclasses by presence/absence of diabetes mellitus.
|
A. Baseline | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sub class 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
| DM | ||||||||||||||||||||
| Measured value, mg/dL | ||||||||||||||||||||
| Placebo | 6.01 ± 9.86 | 5.02 ± 5.25 | 14.89 ± 11.19 | 27.41 ± 16.28 | 30.75 ± 13.64 | 15.23 ± 5.62 | 6.77 ± 1.98 | 9.72 ± 2.37 | 9.29 ± 2.16 | 4.21 ± 1.11 | 1.36 ± 0.40 | 0.44 ± 0.23 | 0.34 ± 0.15 | 0.25 ± 0.14 | 0.66 ± 0.45 | 3.02 ± 1.96 | 5.04 ± 2.14 | 3.32 ± 1.26 | 0.83 ± 0.39 | 1.15 ± 0.21 |
| Evolocumab | 4.92 ± 8.81 | 4.22 ± 5.23 | 12.82 ± 10.92 | 24.57 ± 15.94 | 29.10 ± 14.08 | 15.12 ± 6.55 | 6.52 ± 2.04 | 9.55 ± 2.39 | 9.25 ± 2.26 | 4.18 ± 1.21 | 1.33 ± 0.42 | 0.42 ± 0.24 | 0.34 ± 0.16 | 0.24 ± 0.15 | 0.74 ± 0.66 | 3.32 ± 2.37 | 4.99 ± 2.10 | 3.25 ± 1.28 | 0.80 ± 0.40 | 1.14 ± 0.23 |
| Non-DM | ||||||||||||||||||||
| Measured value, mg/dL | ||||||||||||||||||||
| Placebo | 4.66 ± 11.05 | 4.02 ± 5.08 | 12.34 ± 10.49 | 23.53 ± 16.13 | 28.17 ± 15.00 | 14.45 ± 6.50 | 6.45 ± 2.25 | 9.78 ± 2.71 | 9.95 ± 2.61 | 4.63 ± 1.38 | 1.48 ± 0.50 | 0.42 ± 0.32 | 0.34 ± 0.20 | 0.24 ± 0.17 | 0.72 ± 0.48 | 3.41 ± 1.93 | 5.04 ± 2.14 | 3.20 ± 1.30 | 0.80 ± 0.45 | 1.12 ± 0.31 |
| Evolocumab | 5.03 ± 7.84 | 4.63 ± 5.95 | 14.22 ± 14.35 | 27.19 ± 21.85 | 31.54 ± 18.16 | 15.67 ± 6.75 | 6.66 ± 2.14 | 9.94 ± 2.87 | 10.03 ± 2.92 | 4.68 ± 1.50 | 1.49 ± 0.52 | 0.46 ± 0.26 | 0.36 ± 0.16 | 0.26 ± 0.16 | 0.75 ± 0.54 | 3.29 ± 2.09 | 5.10 ± 2.29 | 3.39 ± 1.46 | 0.87 ± 0.49 | 1.15 ± 0.26 |
|
B. Week 12 | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sub class 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
| DM | ||||||||||||||||||||
| Measured value, mg/dL | ||||||||||||||||||||
| Placebo | 5.17 ± 6.62 | 4.71 ± 5.14 | 14.33 ± 13.07 | 26.82 ± 19.90 | 30.50 ± 16.20 | 15.50 ± 6.31 | 6.83 ± 2.01 | 9.75 ± 2.31 | 9.34 ± 2.17 | 4.29 ± 1.22 | 1.38 ± 0.45 | 0.44 ± 0.24 | 0.35 ± 0.15 | 0.26 ± 0.14 | 0.64 ± 0.33 | 2.93 ± 1.62 | 4.91 ± 1.90 | 3.27 ± 1.24 | 0.84 ± 0.41 | 1.14 ± 0.21 |
| Evolocumab | 3.43 ± 4.50 | 3.14 ± 3.26 | 9.81 ± 8.27 | 18.70 ± 12.55 | 21.31 ± 11.84 | 11.99 ± 5.20 | 4.98 ± 1.34 | 6.76 ± 1.44 | 5.96 ± 1.31 | 2.71 ± 0.69 | 0.87 ± 0.23 | 0.32 ± 0.15 | 0.26 ± 0.09 | 0.19 ± 0.09 | 0.69 ± 0.46 | 3.46 ± 1.98 | 4.74 ± 1.83 | 2.88 ± 1.05 | 0.69 ± 0.30 | 1.08 ± 0.17 |
| Change from baseline, % | ||||||||||||||||||||
| Placebo | −12.82 (−42.15, 68.78) | −14.29 (−45.16, 55.33) | −15.96 (−41.46, 28.97) | −15.15 (−35.78, 28.78) | −4.77 (−26.49, 25.40) | 0.47 (−17.61, 25.40) | 3.56 (−10.71, 15.70) | 1.55 (−8.58, 12.60) | 1.35 (−7.53, 10.78) | 1.61 (−8.92, 10.92) | 1.00 (−12.77, 13.28) | 0.00 (−20.00, 22.73) | 3.13 (−14.89, 17.02) | 2.63 (−21.15, 27.78) | 4.65 (−20.73, 28.13) | 4.58 (−17.81, 22.70) | −1.24 (−13.92, 16.87) | −3.23 (−13.60, 18.33) | −1.41 (−16.67, 25.93) | 0.00 (−7.92, 6.12) |
| Evolocumab | −21.49 (−60.78, 74.06) | −28.17 (−56.39, 47.86) | −29.68 (−51.66, 17.93) | −27.08 (−46.46, 6.31) | −30.31 (−43.19, −12.10) | −18.28 (−35.76, −1.47) | −22.53 (−32.58, −8.06) | −28.63 (−37.27, −18.56) | −34.93 (−43.43, −27.28) | −35.03 (−41.64, −24.01) | −33.89 (−40.56, −22.73) | −18.38 (−38.46, −2.44) | −22.05 (−36.11, −4.76) | −16.67 (−37.04, 13.33) | 3.31 (−21.09, 31.11) | 14.24 (−8.24, 39.25) | −2.16 (−19.79, 18.07) | −7.81 (−24.70, 5.29) | −9.29 (−29.41, 9.59) | −1.98 (−10.95, 4.00) |
| Non-DM | ||||||||||||||||||||
| Measured value, mg/dL | ||||||||||||||||||||
| Placebo | 4.33 ± 5.28 | 4.01 ± 4.09 | 12.38 ± 10.25 | 23.81 ± 16.48 | 28.70 ± 15.25 | 15.01 ± 6.55 | 6.54 ± 2.04 | 9.78 ± 2.52 | 9.93 ± 2.68 | 4.62 ± 1.48 | 1.47 ± 0.50 | 0.41 ± 0.20 | 0.34 ± 0.13 | 0.24 ± 0.10 | 0.75 ± 0.45 | 3.50 ± 1.97 | 4.98 ± 1.84 | 3.17 ± 1.15 | 0.78 ± 0.37 | 1.12 ± 0.19 |
| Evolocumab | 4.87 ± 11.79 | 3.66 ± 6.37 | 10.35 ± 12.73 | 19.31 ± 17.20 | 21.83 ± 13.96 | 11.91 ± 5.82 | 4.98 ± 1.66 | 6.94 ± 2.00 | 6.47 ± 1.96 | 3.08 ± 1.05 | 0.99 ± 0.39 | 0.37 ± 0.29 | 0.29 ± 0.18 | 0.23 ± 0.21 | 0.83 ± 0.74 | 3.88 ± 2.90 | 5.06 ± 2.88 | 3.04 ± 1.55 | 0.77 ± 0.49 | 1.10 ± 0.30 |
| Change from baseline, % | ||||||||||||||||||||
| Placebo | −2.15 (−48.84, 79.48) | −2.68 (−41.31, 66.46) | −5.16 (−36.39, 37.78) | 0.89 (−30.19, 38.33) | 4.35 (−16.03, 22.43) | 6.30 (−12.92, 26.64) | 0.89 (−13.79, 21.10) | 0.57 (−9.45, 12.43) | 1.03 (−9.07, 6.12) | 0.81 (−8.95, 7.76) | −1.34 (−10.34, 10.90) | 0.00 (−12.50, 19.18) | 0.00 (−10.87, 20.00) | 4.29 (−11.76, 20.00) | 9.27 (−19.75, 40.35) | 6.19 (−17.21, 21.34) | 2.22 (−16.04, 20.25) | 2.52 (−17.00, 16.99) | 2.16 (−24.55, 27.50) | 0.36 (−5.43, 10.53) |
| Evolocumab | −26.65 (−65.40, 27.27) | −32.74 (−61.19, 20.00) | −31.38 (−58.99, 6.57) | −28.49 (−51.68, −1.93) | −32.22 (−48.60, −15.11) | −23.33 (−41.63, −6.28) | −26.09 (−34.67, −12.32) | −30.88 (−38.42, −20.99) | −35.59 (−42.53, −23.65) | −33.10 (−43.22, −23.22) | −32.23 (−42.57, −22.86) | −23.81 (−37.84, −6.25) | −20.00 (−34.88, −8.70) | −16.67 (−40.00, 0.00) | 1.69 (−21.74, 21.21) | 11.27 (−8.65, 42.34) | −3.97 (−23.68, 16.42) | −10.89 (−31.79, 10.08) | −13.33 (−34.15, 14.71) | −5.31 (−10.91, 2.63) |
|
C. Mean of weeks 10 and 12 | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sub class 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
| DM | ||||||||||||||||||||
| Measured value, mg/dL | ||||||||||||||||||||
| Placebo | 5.20 ± 5.22 | 4.70 ± 4.18 | 14.08 ± 10.75 | 26.20 ± 16.51 | 30.05 ± 13.82 | 15.44 ± 5.96 | 6.73 ± 1.33 | 9.62 ± 2.31 | 9.18 ± 2.17 | 4.24 ± 1.18 | 1.37 ± 0.42 | 0.45 ± 0.21 | 0.34 ± 0.13 | 0.26 ± 0.12 | 0.64 ± 0.32 | 2.94 ± 1.62 | 4.85 ± 1.84 | 3.23 ± 1.13 | 0.82 ± 0.37 | 1.13 ± 0.19 |
| Evolocumab | 3.59 ± 4.26 | 3.07 ± 2.82 | 9.25 ± 6.74 | 17.43 ± 10.10 | 19.97 ± 9.80 | 11.52 ± 4.61 | 4.88 ± 1.33 | 6.58 ± 1.42 | 5.71 ± 1.23 | 2.58 ± 0.64 | 0.83 ± 0.21 | 0.31 ± 0.13 | 0.25 ± 0.08 | 0.20 ± 0.09 | 0.71 ± 0.46 | 3.50 ± 1.91 | 4.74 ± 1.61 | 2.87 ± 0.94 | 0.69 ± 0.27 | 1.07 ± 0.16 |
| Change from baseline, % | ||||||||||||||||||||
| Placebo | 7.86 (−36.34, 103.47) | −0.40 (−31.11, 71.75) | −11.91 (−32.63, 32.38) | −10.05 (−26.53, 17.27) | −6.74 (−19.74, 15.40) | −1.39 (−12.58, 18.21) | 0.20 (−9.53, 10.22) | 0.56 (−9.49, 7.28) | −0.26 (−7.60, 6.81) | 0.45 (−7.67, 8.17) | 0.00 (−8.74, 7.21) | 0.64 (−8.90, 20.14) | 2.09 (−8.88, 14.58) | 2.08 (−15.45, 24.75) | 4.52 (−13.28, 22.79) | −0.69 (−12.72, 21.55) | −1.66 (−14.28, 17.79) | −2.91 (−13.87, 13.93) | 0.25 (−15.87, 21.85) | −0.51 (−7.53, 7.08) |
| Evolocumab | −4.72 (−54.08, 93.51) | −10.99 (−50.00, 38.58) | −24.52 (−47.85, 9.42) | −25.29 (−45.54, −3.54) | −31.02 (−43.98, −15.53) | −23.19 (−32.61, −10.30) | −22.54 (−34.82, −11.74) | −29.43 (−39.05, −20.33) | −38.41 (−44.34, −28.29) | −37.09 (−45.07, −27.06) | −35.52 (−42.75, −26.32) | −19.88 (−36.62, −2.17) | −16.42 (−34.48, −8.70) | −11.08 (−33.33, 7.35) | 5.66 (−17.90, 33.33) | 13.45 (−7.09, 41.08) | −3.52 (−17.89, 18.79) | −10.91 (−22.29, 8.12) | −10.65 (−26.74, 10.11) | −2.17 (−11.11, 3.50) |
| Non-DM | ||||||||||||||||||||
| Measured value, mg/dL | ||||||||||||||||||||
| Placebo | 4.56 ± 5.22 | 4.07 ± 3.78 | 12.26 ± 9.01 | 23.46 ± 14.00 | 28.48 ± 13.17 | 14.87 ± 5.65 | 6.47 ± 1.89 | 9.64 ± 2.44 | 9.71 ± 2.56 | 4.53 ± 1.37 | 1.45 ± 0.46 | 0.42 ± 0.19 | 0.34 ± 0.12 | 0.24 ± 0.10 | 0.74 ± 0.43 | 3.41 ± 1.85 | 4.89 ± 1.70 | 3.14 ± 1.06 | 0.78 ± 0.34 | 1.12 ± 0.18 |
| Evolocumab | 4.85 ± 10.38 | 3.58 ± 5.40 | 10.03 ± 10.70 | 18.60 ± 14.61 | 21.12 ± 12.25 | 11.65 ± 5.15 | 4.89 ± 1.52 | 6.74 ± 1.82 | 6.17 ± 1.71 | 2.91 ± 0.89 | 0.94 ± 0.33 | 0.36 ± 0.26 | 0.28 ± 0.16 | 0.22 ± 0.18 | 0.80 ± 0.64 | 3.80 ± 2.58 | 5.03 ± 2.45 | 3.01 ± 1.35 | 0.76 ± 0.44 | 1.09 ± 0.27 |
| Change from baseline, % | ||||||||||||||||||||
| Placebo | 10.86 (−39.29, 101.52) | 4.52 (−30.95, 69.89) | 2.90 (−34.08, 48.00) | 2.52 (−30.19, 39.37) | 2.93 (−17.99, 28.42) | 2.78 (−12.33, 23.35) | 0.56 (−13.34, 16.25) | −1.74 (−11.98, 13.15) | −1.23 (−10.43, 5.13) | −1.98 (−11.89, 8.07) | −1.61 (−12.03, 10.78) | 2.86 (−11.21, 24.14) | 1.75 (−9.68, 17.86) | 3.33 (−12.50, 28.13) | 9.30 (−15.14, 32.64) | 2.55 (−12.93, 21.29) | −1.12 (−16.01, 16.19) | −0.75 (−17.13, 19.53) | 4.37 (−21.92, 33.17) | 1.87 (−5.91, 8.72) |
| Evolocumab | −7.08 (−50.92, 70.64) | −22.11 (−51.59, 39.73) | −30.39 (−53.72, 11.14) | −31.52 (−49.95, −0.89) | −36.45 (−48.22, −16.55) | −26.66 (−41.45, −11.37) | −25.53 (−36.81, −15.27) | −31.96 (−37.78, −25.00) | −38.12 (−43.61, −30.11) | −36.66 (−43.93, −29.54) | −34.74 (−43.79, −29.23) | −24.55 (−35.56, −7.69) | −21.15 (−33.33, −8.70) | −15.91 (−34.38, 0.00) | 0.00 (−14.47, 29.22) | 14.59 (−4.76, 43.51) | −1.90 (−21.30, 21.20) | −10.03 (−28.06, 8.08) | −12.80 (−30.77, 12.50) | −4.46 (−12.21, 4.13) |
Abbreviations: TG, triglyceride; DM, diabetes mellitus. Data are expressed as mean ± SD or median (interquartile range).
Supplemental Fig. 4.
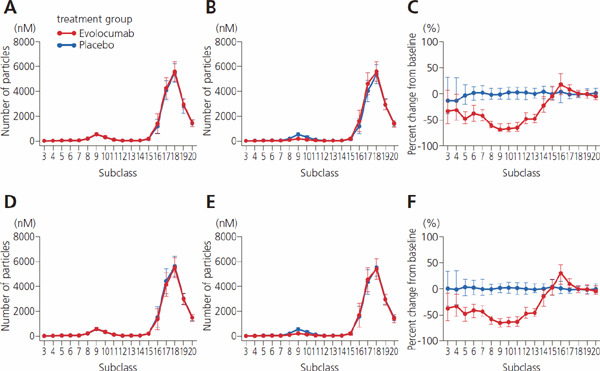
Absolute value and percent change from baseline for number of particles in 20 lipoprotein subclasses by presence/absence of diabetes mellitus
A, value at baseline in patients with diabetes; B, value at week 12 with diabetes; C, change from baseline to week 12 with diabetes; D, baseline in patients without diabetes; E, week 12 without diabetes; F, change from baseline to week 12 without diabetes. In A, B, D, and E, values indicate mean ± SD; C and F, median and 1st and 3rd quartiles (Q1, Q3).
Supplemental Table 7. Absolute value and percent change for particle size in 20 subclasses by presence/absence of diabetes mellitus.
|
A. Baseline | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sub class 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
| DM | ||||||||||||||||||
| Measured value, nM | ||||||||||||||||||
| Placebo | 4.19 ± 3.05 | 13.55 ± 8.04 | 38.23 ± 13.47 | 45.32 ± 15.13 | 41.35 ± 12.26 | 187.97 ± 42.70 | 523.83 ± 116.26 | 300.81 ± 92.55 | 105.37 ± 33.07 | 24.49 ± 8.77 | 35.97 ± 8.61 | 32.56 ± 10.08 | 158.14 ± 56.94 | 1182.07 ± 563.61 | 4076.83 ± 769.97 | 5480.36 ± 755.65 | 2940.53 ± 466.21 | 1401.30 ± 271.89 |
| Evolocumab | 3.68 ± 3.00 | 12.39 ± 8.01 | 37.80 ± 13.75 | 47.98 ± 17.93 | 40.92 ± 12.21 | 190.87 ± 39.11 | 534.10 ± 122.54 | 302.18 ± 96.23 | 104.41 ± 30.61 | 24.82 ± 9.64 | 36.75 ± 7.51 | 34.07 ± 10.73 | 182.76 ± 99.03 | 1381.58 ± 823.70 | 4266.91 ± 818.21 | 5593.25 ± 798.69 | 2949.88 ± 415.11 | 1449.89 ± 287.56 |
| Non-DM | ||||||||||||||||||
| Measured value, mg/dL | ||||||||||||||||||
| Placebo | 3.54 ± 2.79 | 11.84 ± 7.82 | 36.74 ± 15.04 | 45.21 ± 17.16 | 40.55 ± 13.92 | 192.98 ± 48.60 | 536.99 ± 126.86 | 305.00 ± 87.17 | 106.31 ± 29.89 | 24.45 ± 9.96 | 36.66 ± 8.33 | 34.88 ± 11.52 | 185.71 ± 89.89 | 1512.68 ± 826.09 | 4430.74 ± 1002.95 | 5616.93 ± 797.03 | 3015.39 ± 446.55 | 1470.70 ± 262.02 |
| Evolocumab | 4.03 ± 3.87 | 13.55 ± 10.73 | 39.77 ± 18.72 | 48.00 ± 19.41 | 41.81 ± 14.86 | 200.75 ± 60.22 | 565.56 ± 167.71 | 320.57 ± 107.93 | 111.19 ± 36.98 | 25.96 ± 9.23 | 37.81 ± 9.44 | 33.44 ± 11.06 | 180.05 ± 101.68 | 1346.29 ± 844.98 | 4130.88 ± 963.79 | 5493.11 ± 808.75 | 2980.48 ± 442.74 | 1456.65 ± 263.70 |
|
B. Week 12 | ||||||||||||||||||
| Sub class 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DM | ||||||||||||||||||
| Measured value, nM | ||||||||||||||||||
| Placebo | 4.02 ± 3.47 | 13.19 ± 9.65 | 37.71 ± 15.04 | 45.77 ± 15.68 | 41.32 ± 10.96 | 186.14 ± 43.13 | 520.53 ± 116.21 | 304.61 ± 96.98 | 107.09 ± 35.63 | 25.26 ± 10.00 | 36.25 ± 9.38 | 33.18 ± 10.01 | 155.05 ± 57.29 | 1178.62 ± 607.35 | 4026.43 ± 873.29 | 5397.20 ± 770.86 | 2929.25 ± 438.73 | 1415.72 ± 249.40 |
| Evolocumab | 2.55 ± 2.12 | 8.77 ± 5.82 | 20.90 ± 10.52 | 29.45 ± 12.62 | 23.50 ± 6.61 | 77.91 ± 22.66 | 183.80 ± 65.08 | 106.50 ± 44.92 | 37.69 ± 14.33 | 12.79 ± 4.73 | 19.56 ± 5.09 | 27.28 ± 10.16 | 182.90 ± 104.32 | 1601.74 ± 864.66 | 4618.65 ± 896.90 | 5600.32 ± 795.47 | 2928.71 ± 423.75 | 1391.88 ± 273.16 |
| Change from baseline, % | ||||||||||||||||||
| Placebo | −13.33 (−38.67, 31.91) | −13.53 (−32.94, 30.35) | −2.85 (−20.02, 17.36 | 1.82 (−12.59, 14.47) | 2.20 (−11.56, 16.15) | −1.59 (−10.52, 10.28) | −1.15 (−10.40, 10.03) | 2.64 (−10.69, 12.72) | 2.82 (−10.36, 12.76) | 2.46 (−11.77, 13.06) | 0.00 (−7.99, 10.36) | 4.03 (−10.07, 15.27) | −0.41 (−10.58, 12.74) | 3.78 (−17.26, 20.45) | −0.88 (−9.75, 7.29) | −1.12 (−7.05, 4.35) | −0.38 (−9.13, 9.38) | 1.41 (−7.40, 10.67) |
| Evolocumab | −33.49 (−57.69, 6.96) | −31.22 (−50.26, −0.87) | −47.87 (−57.20, −34.39) | −37.93 (−52.80, −21.98) | −41.96 (−51.82, −31.07) | −60.43 (−65.19, −53.30) | −68.33 (−72.90, −57.64) | −66.54 (−72.86, −56.30) | −64.86 (−71.97, −55.07) | −48.20 (−57.76, −36.27) | −47.87 (−55.61, −38.84) | −22.38 (−35.32, −5.65) | −4.48 (−12.42, 14.74) | 18.19 (−1.22, 38.78) | 8.26 (−1.39, 17.87) | 0.61 (−5.25, 6.95) | −0.44 (−8.46, 6.87) | −4.84 (−11.45, 2.43) |
| Non-DM | ||||||||||||||||||
| Measured value, mg/dL | ||||||||||||||||||
| Placebo | 3.56 ± 2.83 | 11.95 ± 8.23 | 37.05 ± 16.18 | 46.27 ± 18.06 | 40.38 ± 12.64 | 190.32 ± 49.12 | 535.91 ± 125.08 | 308.69 ± 96.91 | 107.25 ± 33.58 | 24.04 ± 9.40 | 36.50 ± 8.37 | 34.10 ± 9.87 | 190.66 ± 92.32 | 1539.99 ± 835.43 | 4357.70 ± 998.02 | 5518.06 ± 720.85 | 2966.18 ± 402.08 | 1475.64 ± 254.11 |
| Evolocumab | 2.69 ± 3.22 | 8.98 ± 7.86 | 21.43 ± 12.67 | 28.40 ± 13.26 | 23.41 ± 8.45 | 82.24 ± 36.47 | 201.11 ± 111.65 | 117.32 ± 65.76 | 41.77 ± 22.82 | 13.87 ± 6.33 | 20.76 ± 7.18 | 28.31 ± 12.18 | 190.92 ± 117.66 | 1677.04 ± 954.60 | 4544.89 ± 964.35 | 5433.09 ± 786.30 | 2928.84 ± 440.71 | 1397.33 ± 317.63 |
| Change from baseline, % | ||||||||||||||||||
| Placebo | 0.39 (−32.08, 33.81) | −1.20 (−30.05, 35.25) | 3.10 (−14.08, 18.36) | 2.16 (−11.30, 16.93) | −0.52 (−12.64, 18.28) | −0.90 (−11.12, 8.16) | 1.61 (−7.53, 9.54) | 1.94 (−8.55, 12.74) | 1.42 (−10.95, 12.08) | −0.14 (−10.32, 11.75) | −1.30 (−6.88, 9.79) | 0.03 (−10.35, 9.90) | 3.52 (−12.13, 17.89) | 0.87 (−8.90, 15.12) | −1.71 (−8.28, 6.76) | −0.98 (−7.36, 4.84) | −1.79 (−8.87, 6.06) | −0.52 (−7.21, 9.21) |
| Evolocumab | −37.44 (−61.81, −8.10) | −33.81 (−53.44, −10.43) | −47.80 (−62.31, −35.31) | −41.44 (−56.17, −28.96) | −43.57 (−52.37, −32.70) | −58.53 (−67.71, −52.06) | −65.36 (−72.91, −57.38) | −63.89 (−72.03, −55.41) | −63.45 (−71.06, −54.60) | −47.40 (−57.04, −35.82) | −45.90 (−52.91, −38.00) | −14.43 (−33.47, −1.43) | 2.71 (−12.64, 18.17) | 30.20 (8.80, 45.76) | 9.58 (1.51, 19.23) | −0.26 (−7.21, 6.01) | −0.98 (−11.21, 7.82) | −4.18 (−10.02, 5.29) |
|
C. Mean of weeks 10 and 12 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sub class 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
| DM | ||||||||||||||||||
| Measured value, nM | ||||||||||||||||||
| Placebo | 3.97 ± 2.89 | 12.99 ± 8.12 | 37.30 ± 13.23 | 45.56 ± 15.45 | 40.91 ± 10.50 | 182.80 ± 39.25 | 509.30 ± 107.36 | 298.45 ± 92.59 | 104.96 ± 34.11 | 25.11 ± 9.64 | 35.74 ± 8.83 | 32.68 ± 9.27 | 154.14 ± 54.11 | 1170.31 ± 570.60 | 3990.64 ± 823.72 | 5377.22 ± 742.80 | 2915.11 ± 420.86 | 1405.45 ± 243.42 |
| Evolocumab | 2.41 ± 1.72 | 8.19 ± 4.66 | 19.57 ± 8.61 | 28.27 ± 11.33 | 22.84 ± 6.55 | 73.60 ± 21.56 | 166.79 ± 57.73 | 95.40 ± 37.65 | 34.02 ± 12.23 | 12.10 ± 4.18 | 18.72 ± 4.94 | 27.32 ± 10.03 | 183.05 ± 106.87 | 1601.94 ± 843.94 | 4563.74 ± 823.49 | 5495.60 ± 737.16 | 2871.66 ± 391.40 | 1356.90 ± 261.14 |
| Change from baseline, % | ||||||||||||||||||
| Placebo | −5.59 (−32.52, 36.32) | −8.64 (−27.34, 19.48) | −4.42 (−17.04, 14.65) | 2.49 (−13.32, 14.48) | −0.57 (−11.59, 11.15) | −2.86 (−11.18, 6.90) | −2.66 (−9.90, 6.18) | −0.39 (−9.66, 9.41) | −1.33 (−10.12, 9.56) | 1.57 (−7.23, 12.47) | −0.19 (−7.29, 6.93) | 0.51 (−7.92, 12.87) | −2.42 (−10.60, 9.61) | 1.25 (−12.64, 16.87) | −0.82 (−8.90, 6.02) | −2.23 (−6.45, 3.94) | −1.50 (−7.70, 6.23) | 0.98 (−5.81, 7.54) |
| Evolocumab | −31.15 (−56.47, −3.10) | −30.45 (−51.00, −8.11) | −47.94 (−57.29, −38.15) | −40.55 (−50.05, −30.23) | −43.70 (−52.19, −33.81) | −62.13 (−68.18, −55.74) | −70.35 (−75.76, −61.81) | −68.26 (−74.77, −62.40) | −67.12 (−74.24, −61.12) | −50.35 (−59.87, −39.57) | −48.50 (−58.19, −41.23) | −22.51 (−32.87, −9.07) | 0.02 (−12.66, 11.91) | 20.28 (2.73, 34.68) | 7.04 (0.04, 14.95) | −1.51 (−6.16, 4.09) | −3.01 (−8.95, 4.76) | −7.68 (−12.34, −0.17) |
| Non-DM | ||||||||||||||||||
| Measured value, mg/dL | ||||||||||||||||||
| Placebo | 3.54 ± 2.47 | 11.81 ± 7.01 | 36.90 ± 14.06 | 45.92 ± 16.41 | 40.18 ± 12.23 | 188.48 ± 48.22 | 527.42 ± 120.85 | 304.12 ± 91.46 | 106.09 ± 31.22 | 24.10 ± 9.04 | 36.33 ± 8.03 | 34.11 ± 9.72 | 188.11 ± 90.96 | 1499.10 ± 805.92 | 4299.24 ± 935.90 | 5484.87 ± 686.25 | 2958.55 ± 364.34 | 1464.19 ± 221.75 |
| Evolocumab | 2.61 ± 2.70 | 8.66 ± 6.67 | 20.64 ± 11.28 | 27.59 ± 12.06 | 22.90 ± 8.05 | 77.90 ± 34.07 | 183.93 ± 101.12 | 106.34 ± 59.66 | 37.97 ± 20.10 | 13.09 ± 5.73 | 19.80 ± 6.44 | 27.32 ± 11.15 | 187.65 ± 111.49 | 1646.41 ± 921.96 | 4510.62 ± 937.74 | 5352.26 ± 700.67 | 2861.28 ± 415.57 | 1358.20 ± 276.74 |
| Change from baseline, % | ||||||||||||||||||
| Placebo | 0.72 (−30.95, 40.83) | 6.89 (−26.92, 36.16) | 1.45 (−14.77, 21.65) | 1.62 (−8.84, 17.03) | 1.63 (−13.67, 15.53) | −2.23 (−11.71, 6.45) | −0.89 (−8.43, 5.19) | 1.36 (−9.93, 9.98) | 1.63 (−10.79, 10.72) | 0.35 (−10.29, 12.44) | 0.62 (−8.28, 7.59) | −0.09 (−8.12, 8.55) | 1.99 (−8.71, 12.59) | 0.40 (−8.20, 9.80) | −2.37 (−9.41, 2.57) | −2.18 (−7.83, 3.47) | −2.44 (−6.76, 6.05) | −0.27 (−7.71, 6.87) |
| Evolocumab | −35.81 (−56.93, −2.09) | −35.19 (−53.10, −7.31) | −50.45 (−59.96, −37.50) | −44.47 (−53.72, −32.99) | −45.31 (−52.86, −35.96) | −63.49 (−67.94, −55.26) | −69.26 (−74.30, −63.03) | −69.19 (−73.42, −60.48) | −67.78 (−72.93, −59.48) | −48.93 (−58.68, −39.70) | −47.95 (−56.95, −40.48) | −18.48 (−31.23, −6.03) | 0.79 (−11.20, 15.31) | 25.61 (10.32, 44.19) | 8.96 (1.91, 18.36) | −2.17 (−8.21, 4.23) | −3.71 (−11.32, 5.81) | −8.25 (−13.56, 2.59) |
Abbreviation: DM, diabetes mellitus. Data are expressed as mean ± SD or median (interquartile range).
Absolute Value and Percent Change from Baseline in Particle Size of LDL, HDL, and VLDL
Median particle size remained unchanged in the placebo group and increased numerically in the evolocumab group for LDL (Fig. 3, Supplemental Table 8), HDL (Supplemental Fig. 5, Supplemental Table 9), and weighted VLDL (Supplemental Fig. 6, Supplemental Table 10) particles, but the data varied widely for all three lipoproteins, and the distribution of particle sizes in the placebo group overlapped the distribution in the evolocumab group. As a result, there were no clear differences in particle size distribution between the two groups for any of the three lipoproteins.
Fig. 3.
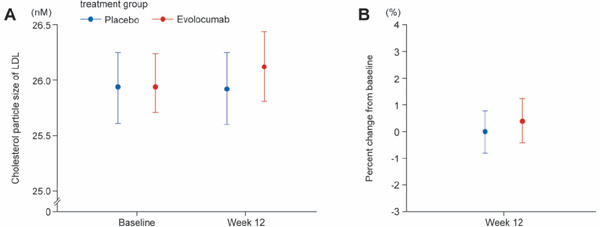
Absolute value and percent change from baseline for particle size of LDL
A, value at baseline and at week 12; B, percent change between baseline and week 12.
Values indicate median and 1st and 3rd quartiles (Q1, Q3).
Abbreviation: LDL, low-density lipoprotein.
Supplemental Table 8. Distribution of LDL particle sizes (absolute values).
| Baseline | Week 12 | |
|---|---|---|
| Cholesterol particle size of LDL, nm | ||
| Placebo | 25.94 (25.61, 26.25) | 25.92 (25.60, 26.25) |
| Evolocumab | 25.94 (25.71, 26.24) | 26.12 (25.81, 26.44) |
| Change from baseline, % | ||
| Placebo | 0.00 (−0.81, 0.78) | |
| Evolocumab | 0.39 (−0.42, 1.24) | |
Abbreviation: LDL, low-density lipoprotein. Data are expressed as median (1st quartile, 3rd quartile).
Supplemental Fig. 5.
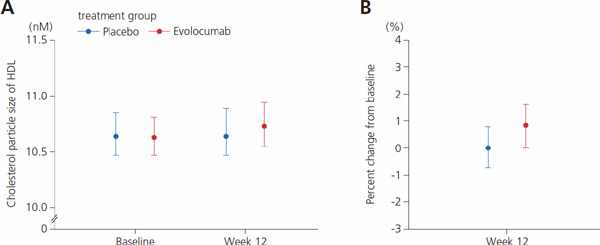
Distribution of HDL particle sizes (absolute values)
Values indicate median and 1st and 3rd quartiles (Q1, Q3). Abbreviation: HDL, high-density lipoprotein.
Supplemental Table 9. Distribution of HDL particle sizes (absolute values).
| Baseline | Week 12 | |
|---|---|---|
| Cholesterol particle size of HDL, nm | ||
| Placebo | 10.64 (10.47, 10.85) | 10.64 (10.47, 10.89) |
| Evolocumab | 10.63 (10.47, 10.81) | 10.73 (10.55, 10.94) |
| Change from baseline, % | ||
| Placebo | 0.00 (−0.74, 0.78) | |
| Evolocumab | 0.84 (0.00, 1.62) | |
Abbreviation: HDL, high-density lipoprotein. Data are expressed as median (1st quartile, 3rd quartile).
Supplemental Fig. 6.
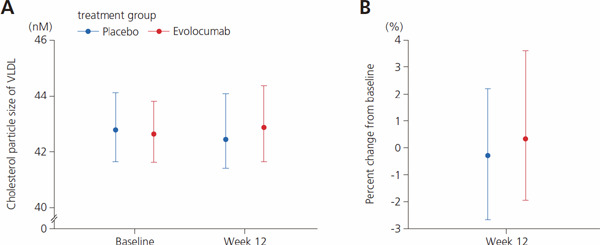
Distribution of VLDL particle sizes (absolute values)
Values indicate median and 1st and 3rd quartiles (Q1, Q3). Abbreviation: VLDL, very low-density lipoprotein.
Supplemental Table 10. Distribution of VLDL particle sizes (absolute values).
| Baseline | Week 12 | |
|---|---|---|
| Cholesterol particle size of VLDL, nm | ||
| Placebo | 42.79 (41.64, 44.12) | 42.45 (41.42, 44.09) |
| Evolocumab | 42.64 (41.63, 43.81) | 42.87 (41.65, 44.37) |
| Change from baseline, % | ||
| Placebo | −0.29 (−2.66, 2.20) | |
| Evolocumab | 0.34 (−1.95, 3.58) | |
Abbreviation: VLDL, very low-density lipoprotein. Data are expressed as median (1st quartile, 3rd quartile).
Accuracy in Monitoring Lower LDL-C Concentrations using the Friedewald Method, Ultracentrifugation, and HLPC
A scatter plot showed an approximately linear relationship between the values calculated using the Friedewald method and those determined by HLPC. However, the HPLC values tended to be higher than the calculated values in patients with low LDL-C (approximately 40 mg/dL or less). Similar results were found when the values determined by ultracentrifugation were plotted against the values determined by HLPC, although the difference was less substantial than the difference between the calculated and HLPC values (Fig. 4). When the distribution of cholesterol levels in each of the 20 subclasses was examined in patients whose LDL-C values were originally calculated to be 0 mg/dL using the Friedewald calculation method, HPLC successfully detected substantial levels of LDL subclasses (G9), indicating greater sensitivity for HPLC, especially in the low range of LDL-C (Supplemental Fig. 7).
Fig. 4.
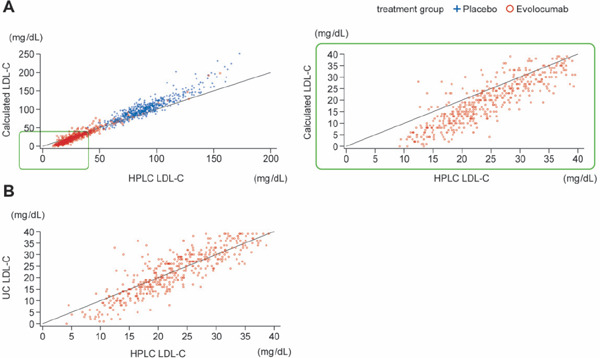
Accuracy in monitoring low LDL-C concentrations using high-performance liquid chromatography (HPLC)
Fig. 4A consists of two graphs. Left: scatter plot of calculated LDL-C and HPLC LDL-C values for all post-baseline scheduled visits by placebo or evolocumab treatment group. Patient population is the full analysis set. The lower left corner segment, outlined in green, represents blood samples having calculated LDL-C values less than 40 mg/dL and HPLC LDL-C values less than 40 mg/dL. That segment is expanded in the scatter plot on the right.
Fig. 4B consists of a scatter plot of UC LDL-C and HPLC LDL-C values for all post-baseline scheduled visits by placebo or evolocumab treatment group and represents blood samples having UC LDL-C values less than 40 mg/dL and HPLC LDL-C values less than 40 mg/dL. Patient population is the full analysis set.
All plots were based on observed data. Missing values were not imputed.
Abbreviations: LDL-C, low-density lipoprotein cholesterol; UC, ultracentrifugation.
Supplemental Fig. 7.
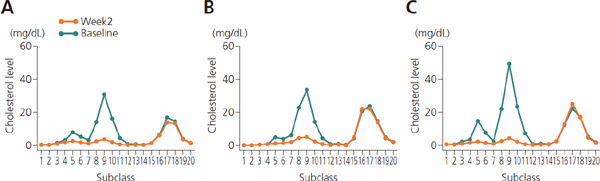
Absolute value for concentration of cholesterol in 20 high-performance liquid chromatography (HPLC) subclasses: Patients for whom calculated LDL-C value was 0
A, B and C represent 3 patients in the group treated with a combination of atorvastatin 20 mg (daily) and evolocumab 420 mg (monthly). For all 3 patients, calculated LDL-C was 0 mg/dL at week 2. Abbreviation: LDL-C, low-density lipoprotein cholesterol.
Discussion
In the Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER) study, a randomized, double-blind, placebo-controlled, multinational clinical trial conducted in 49 countries, evolocumab reduced LDL-C by approximately 60% and significantly reduced the risk of cardiovascular events by 15%10). In contrast, the YUKAWA-2 study in Japanese subjects showed that evolocumab reduced LDL-C by approximately 60% to 70%12), possibly indicating that evolocumab has greater effects specifically in the YUKAWA-2 study population. By analyzing the effects of evolocumab on lipoprotein profiles, based on blood samples from participants in the YUKAWA-2 study, we hoped to further evaluate the detailed lipoprotein measures of the PCSK9 monoclonal antibody inhibitor, evolocumab. Also, gel permeation HPLC provides more concise and accurate measurement of lipoproteins than do conventional assays. The present study used HPLC to provide new findings concerning lipoprotein analyses.
We found that evolocumab reduced cholesterol content, TG content, and the number of lipoprotein particles in some lipoprotein subclasses. Cholesterol concentration was particularly reduced in subclasses G5–11, which correspond to VLDL, intermediate density lipoprotein (IDL), lbLDL, and sdLDL. We consider this reduction to be related to the reduction in LDL-C observed in the YUKAWA-2 study. This result indicates that evolocumab reduced the number of LDL particles contained in LDL subclasses and that this reduction occurred within lbLDL and sdLDL subclasses. Trends in TG concentration were similar to those for LDL. If TG-rich lipoproteins are not metabolized properly and remain in the blood, they can become remnant lipoproteins and promote atherosclerotic progression in the carotid, cerebral, coronary, and peripheral arteries22).
Lipoproteins that are usually detected as remnant lipoprotein subclasses are known to vary depending on the type of dyslipidemia (β-VLDL, chylomicron remnant [CMR], VLDL remnant, or IDL)20). The pathogenic mechanisms of atherosclerosis involve the relatively easy uptake of remnant lipoproteins by the macrophages, which leads to increases in the formation of foam cells2), in platelet aggregation22), in vasospasm, and in other physiological activities related to atherogenicity. The evolocumab-induced reduction in TG content is thought to encourage a decrease in atherogenic remnant lipoproteins. This reduction was not limited to the cholesterol and TG content per lipoprotein particle but also affected the number of particles in the subclasses corresponding to VLDL and LDL, contributing to the ability of evolocumab to improve lipoprotein profiles in patients with hyperlipidemia or mixed dyslipidemia.
Although the number of particles in subclasses 14 to 20, corresponding to HDL, did not change substantially, median values were 15% to 30% higher than placebo in subclasses 16 and 17, and we noted an increase in the subclass that included the relatively large HDL particle, HDL2, although less increase was noted in the smaller HDL3 subclass. A correlation has been reported between increased HDL2 cholesterol and decreased cardiovascular risk23); extreme increases in HDL-C or HDL2-C are considered by some researchers to be atherogenic26), although this finding is controversial27). Further studies are needed to elucidate both the mechanism by which HDL2 increases and the clinical implications of this increase.
Characteristic lipoprotein profiles in T2DM patients typically include high levels of VLDL, remnant TG, and sdLDL and low levels of HDL-C. However, the baseline data for the YUKAWA-2 study showed no major differences in lipoprotein profiles between patients with T2DM and those without diabetes. This may be because the T2DM patients included in the YUKAWA-2 study had relatively mild T2DM; that study excluded patients whose diabetes was newly diagnosed and/or poorly controlled (HbA1c > 8.5%). The present study showed similar results for both diabetic and non-diabetic groups, confirming that evolocumab effectively reduces atherogenic lipoproteins in most patient populations.
In this study, using gel permeation HPLC, we found that evolocumab may slightly increase median LDL and HDL particle sizes, which is considered beneficial for reduction of cardiovascular risk. While large LDL particles have a higher affinity to LDL receptors and tend to be metabolized normally after binding to the receptors, small LDL particles have lower affinity to LDL receptors and thus tend to remain in the blood longer and to be denatured7). These small LDL particles easily infiltrate the intravascular space through the vessel wall and become material for oxidized LDL, which is very atherogenic. Recent studies have shown that an increased proportion of small LDL particles is a risk factor for developing angina pectoris7, 28) or PAD2), even if total LDL-C level in the blood remains unchanged. Given these findings, we can assume that evolocumab improves the fractional catabolic rate of LDL, not only by inhibiting the degradation of LDL receptors and thus increasing their number but also by enhancing the affinity of LDL for those receptors.
We speculate that the potential increase in LDL particle size is related to the mechanism of action of evolocumab, which inhibits PCSK9, increases the number of LDL receptors on hepatocytes, and thus promotes hepatocellular uptake of LDL before the catabolic process of downsizing LDL particles can occur29). Another possible mechanism would be through reduction of blood TG concentration. sdLDL is thought to be most closely related to blood TG levels, and a reduced concentration of TG-rich VLDL may lead to decreases in the cholesteryl ester transfer protein (CETP)-mediated exchange reaction between TG and cholesterol (i.e., reduced transfer of cholesterol esters from LDL to VLDL). This decrease would reduce the loss of LDL density. In the present study, we found that the number of VLDL particles was reduced by the effects of evolocumab, probably because this PCSK9 monoclonal antibody inhibitor reduces the synthesis and secretion of VLDL in the liver30). This phenomenon has been reported in statins31, 32). The present study focused on the effects on lipid subclasses of evolocumab, but it is also important to evaluate the effects of other PCSK9 inhibitors and the responses of LDL receptors on these drugs, including evolocumab; we hope that those important issues can be addressed in future research.
The Friedewald equation is based on the assumption that most serum TG is contained in VLDL and that the ratio of VLDL cholesterol to TG is approximately 1:5 13). For this reason, the use of this equation to calculate LDL-C values in hypertriglyceridemia patients is highly limited. The Friedewald-estimated values for LDL-C level also appear to be lower than actual values at lower levels of LDL-C, particularly < 70 mg/dL33). In contrast, gel permeation HPLC is not affected by hypertriglyceridemia, because lipoprotein particles are first separated into subclasses by diameter through gel permeation, and then cholesterol content is assessed by a system that is sensitive to cholesterol but unaffected by other types of lipids. In this study, even when LDL-C values were calculated to be zero by the Friedewald equation, LDL peaks were apparent on HPLC analysis. This suggests that HPLC can accurately measure even very low cholesterol concentrations. Fig. 4 shows that LDL-C values were higher when using gel permeation HPLC than when applying the Friedewald formula with very low LDL-C (< 40 mg/dL) and were lower for gel permeation HPLC than for the Friedewald calculations with relatively high LDL-C (≥ 40mg/dL). The Friedewald formula is a precise but originally non-rigorous method to calculate LDL values, and it is not surprising that those calculated values did not match our actual in vivo measured values. In the present study, there were large discrepancies between the values obtained by these two methods, particularly in the low range. This finding is of particular importance because of the current increase in the clinical use of PCSK9 monoclonal antibody inhibitors. In the YUKAWA-2 study, when LDL-C was < 40mg/dL, measurements were taken by ultracentrifugation. In this study, we found smaller discrepancies between gel permeation HPLC and ultracentrifugation than between gel permeation HPLC and calculations using the Friedewald formula. However, in the lower-value range, the LDL-C values from HPLC were higher than those from ultracentrifugation. This may indicate that complete sample recovery is not feasible with ultracentrifugation because lipoproteins adhere to the centrifuge tube walls, a limitation of ultracentrifugation. For this reason, we consider gel permeation HPLC to be superior for analyses of very low levels of cholesterol. This method of HPLC measurement is not standard for routine clinical practice, but it provides highly accurate measurements for assessing the effects of therapeutic interventions on changes in lipoproteins and lipids, as described above. Such high-precision assessments, in turn, can be useful in evaluating atherogenicity.
This study has limitations. First, it is exploratory because it was based on post-hoc analysis of specimens from the YUKAWA-2 study. Although our findings indicated favorable effects of evolocumab on lipoproteins, these effects must be confirmed by other prospective studies in the future. Second, this study incorporated non-intensive (atorvastatin 5 mg/day) and intensive (atorvastatin 20 mg/day) statin use, representative of standard practice in Japan, which is more conservative than in other global regions. Third, while the study population of approximately 400 subjects was not particularly small for this type of study, the application of gel permeation HPLC meant that our data analysis relied on an analytical algorithm using a Gaussian approximation. When peak values were not obtained, the data were deemed missing. For this reason, studies with larger numbers of subjects are needed.
Conclusion
The present study assessed the effects of evolocumab, as seen in 20 lipid subclasses. Results suggested that evolocumab may reduce atherogenicity by lowering lipids and improving the lipid profile and indicated that HPLC offers more accurate quantification than the Friedewald formula or ultracentrifugation in the lower range of LDL-C.
Acknowledgments
Medical writing support was provided by EDIT, Inc. (Tokyo, Japan), funded by Amgen Astellas Bio-Pharma K.K.
Author Contributions
DM contributed to data interpretation and discussion and to writing the manuscript. AK, AH, AL, and KM contributed to data interpretation and discussion. JS contributed to data analysis, data interpretation and discussion. SY contributed to study planning and design, data interpretation, and discussion, and supervised all steps of the study and publication. All authors contributed to critical review and final approval of the manuscript.
Data Sharing Statement
Qualified researchers may request data from Amgen clinical studies. Complete details are available at https://wwwext.amgen.com/science/clinical-trials/clinical-data-transparency-practices.
Conflicts of Interest
DM received honoraria from Ono Pharmaceutical Co., Ltd., Skylight Biotech, Inc., Pfizer Japan Inc., Amgen Astellas BioPharma K.K., and Sanofi K.K.; research grants from Otsuka Pharmaceutical Co., Ltd., Shionogi & Co., Ltd., National Institutes of Biomedical Innovation, Health and Nutrition, Hayashibara Co., Ltd., Teijin Pharma Limited, and Kaken Pharmaceutical Co., Ltd.; honoraria and research grants from Kowa Company, Ltd., Nippon Boehringer Ingelheim Co., Ltd., Bayer Yakuhin, Ltd., MSD K.K., Hitachi Chemical Diagnostics Systems Co., Ltd., Takeda Pharmaceutical Company Limited, Sanwa Kagaku Kenkyusho Co., Ltd., Astellas Pharma Inc., Daiichi Sankyo Company, Limited, Mochida Pharmaceutical Co., Ltd., AstraZeneca K.K., Kowa Pharmaceutical Co., Ltd., and Kissei Pharmaceutical Co., Ltd.; AK received honoraria from Ono Pharmaceutical Co., Ltd.; AH received honoraria from Daiichi Sankyo Company, Limited, Bayer Yakuhin, Ltd., Toa Eiyo Ltd., Nippon Boehringer Ingelheim Co., Ltd., Amgen Astellas BioPharma K.K., Sanofi K.K., Astellas Pharma Inc., Sumitomo Dainippon Pharma Co., Ltd., Bristol-Myers Squibb Company, and AstraZeneca K.K.; courses endowed by Boston Scientific Japan K.K., Otsuka Pharmaceutical Co., Ltd., Fukuda Denshi Co., Ltd., Abbott Medical Japan Co., Ltd., Medtronic Japan Co., Ltd., and Japan Lifeline Co., Ltd.,; JS and KM are employees of Amgen Astellas BioPharma K.K.; JAGL is an employee of and stockholder in Amgen Inc.; SY received honoraria from Amgen Astellas BioPharma K.K., Kowa Pharmaceutical Co. Ltd., Kowa Company, Ltd., and Sanofi K.K.; research grants from Ono Pharmaceutical Co., Ltd., Hitachi Chemical Diagnostics Systems Co., Ltd., Takeda Pharmaceutical Company Limited, Mitsubishi Tanabe Pharma Corporation, and Rohto Pharmaceutical Co., Ltd.; courses endowed by Izumisano City, and Kaizuka City; research grants and scholarship grants from Astellas Pharma Inc., and Nippon Boehringer Ingelheim Co., Ltd.; honoraria, research grants and scholarship grants from MSD K.K. and Bayer Yakuhin, Ltd.
Notice of Grant Support
This study was funded by Amgen Inc.
References
- 1). Cannon CP, Steinberg BA, Murphy SA, Mega JL, Braunwald E: Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol, 2006; 48: 438-445 [DOI] [PubMed] [Google Scholar]
- 2). Aday AW, Lawler PR, Cook NR, Ridker PM, Mora S, Pradhan AD: Lipoprotein particle profiles, standard lipids, and peripheral artery disease incidence. Circulation, 2018; 138: 2330-2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Muñoz D, Smith SC, Virani SS, Williams KA, Yeboah J, Ziaeian B: 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol, 2019; 74: e177-e232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Castelli WP, Garrison RJ, Wilson PW, Abbott RD, Kalousdian S, Kannel WB: Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. JAMA, 1986; 256: 2835-2838 [PubMed] [Google Scholar]
- 5). Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Ž, Riccardi G, Taskinen MR, Tokgozoglu L, Verschuren WM, Vlachopoulos C, Wood DA, Zamorano JL: 2016 ESC/EAS guidelines for the management of dyslipidaemias: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis, 2016; 253: 281-344 [DOI] [PubMed] [Google Scholar]
- 6). Gardner CD, Fortmann SP, Krauss RM: Association of small low-density lipoprotein particles with the incidence of coronary artery disease in men and women. JAMA, 1996; 276: 875-881 [PubMed] [Google Scholar]
- 7). Koba S, Hirano T, Ito Y, Tsunoda F, Yokota Y, Ban Y, Iso Y, Suzuki H, Katagiri T: Significance of small dense low-density lipoprotein-cholesterol concentrations in relation to the severity of coronary heart diseases. Atherosclerosis, 2006; 189: 206-214 [DOI] [PubMed] [Google Scholar]
- 8). Harada-Shiba M, Arai H, Ishigaki Y, Ishibashi S, Okamura T, Ogura M, Dobashi K, Nohara A, Bujo H, Miyauchi K, Yamashita S, Yokote K, Working Group by Japan Atherosclerosis Society for Making Guidance of Familial Hypercholesterolemia : Guidelines for diagnosis and treatment of familial hypercholesterolemia 2017. J Atheroscler Thromb, 2018; 25: 751-770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Nohara A, Ohmura H, Okazaki H, Ogura M, Kitagawa K, Koseki M, Sato K, Tsukamoto K, Yamashita S, on behalf of the Japan Atherosclerosis Society Working Group on Statement for Appropriate Use of PCSK9 Inhibitors : Statement for appropriate clinical use of PCSK9 inhibitors. J Atheroscler Thromb, 2018; 25: 747-750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR: Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med, 2017; 376: 1713-1722 [DOI] [PubMed] [Google Scholar]
- 11). Toth PP, Sattar N, Blom DJ, Martin SS, Jones SR, Monsalvo ML, Elliott M, Davis M, Somaratne R, Preiss D: Effect of evolocumab on lipoprotein particles. Am J Cardiol, 2018; 121: 308-314 [DOI] [PubMed] [Google Scholar]
- 12). Kiyosue A, Honarpour N, Kurtz C, Xue A, Wasserman SM, Hirayama A: A phase 3 study of evolocumab (AMG 145) in statin-treated Japanese patients at high cardiovascular risk. Am J Cardiol, 2016; 117: 40-47 [DOI] [PubMed] [Google Scholar]
- 13). Friedewald WT, Levy RI, Fredrickson DS: Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem, 1972; 18: 499-502 [PubMed] [Google Scholar]
- 14). Otvos JD, Jeyarajah EJ, Bennett DW, Krauss RM: Development of a proton nuclear magnetic resonance spectroscopic method for determining plasma lipoprotein concentrations and subspecies distributions from a single, rapid measurement. Clin Chem, 1992; 38: 1632-1638 [PubMed] [Google Scholar]
- 15). Otvos JD, Jeyarajah EJ, Bennett DW: Quantification of plasma lipoproteins by proton nuclear magnetic resonance spectroscopy. Clin Chem, 1991; 37: 377-386 [PubMed] [Google Scholar]
- 16). Jeyarajah EJ, Cromwell WC, Otvos JD: Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med, 2006; 26: 847-870 [DOI] [PubMed] [Google Scholar]
- 17). Ala-Korpela M, Korhonen A, Keisala J, Hörkkö S, Korpi P, Ingman LP, Jokisaari J, Savolainen MJ, Kesäniemi YA: 1H NMR-based absolute quantitation of human lipoproteins and their lipid contents directly from plasma. J Lipid Res, 1994; 35: 2292-2304 [PubMed] [Google Scholar]
- 18). Ala-Korpela M, Lankinen N, Salminen A, Suna T, Soininen P, Laatikainen R., Ingman P, Jauhiainen M, Taskinen MR, Héberger K, Kaski K: The inherent accuracy of 1H NMR spectroscopy to quantify plasma lipoproteins is subclass dependent. Atherosclerosis, 2007; 190; 352-358 [DOI] [PubMed] [Google Scholar]
- 19). Okazaki M, Usui S, Hosaki S: Analysis of plasma lipoproteins by gel permeation chromatography. In: Handbook of Lipoprotein Testing. 2nd Ed, ed by Rifai N, Warnick GR, Dominiczak MH. pp647-669, American Association for Clinical Chemistry, USA, 2000 [Google Scholar]
- 20). Okazaki M, Usui S, Ishigami M, Sakai N, Nakamura T, Matsuzawa Y, Yamashita S: Identification of unique lipoprotein subclasses for visceral obesity by component analysis of cholesterol profile in high-performance liquid chromatography. Arterioscler Thromb Vasc Biol, 2005; 25: 578-584 [DOI] [PubMed] [Google Scholar]
- 21). Okazaki M, Yamashita S: Recent advances in analytical methods on lipoprotein subclasses: calculation of particle numbers from lipid levels by gel permeation HPLC using “spherical particle model”. J Oleo Sci, 2016; 65: 265-282 [DOI] [PubMed] [Google Scholar]
- 22). Masuda D, Yamashita S: Postprandial hyperlipidemia and remnant lipoproteins. J Atheroscler Thromb, 2017; 24: 95-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Superko HR, Pendyala L, Williams PT, Momary KM, King SB, 3rd, Garrett BC: High-density lipoprotein subclasses and their relationship to cardiovascular disease. J Clin Lipidol, 2012; 6: 496-523 [DOI] [PubMed] [Google Scholar]
- 24). Kinoshita M, Yokote K, Arai H, Iida M, Ishigaki Y, Ishibashi S, Umemoto S, Egusa G, Ohmura H, Okamura T, Kihara S, Koba S, Saito I, Shoji T, Daida H, Tsukamoto K, Deguchi J, Dohi S, Dobashi K, Hamaguchi H, Hara M, Hiro T, Biro S, Fujioka Y, Maruyama C, Miyamoto Y, Murakami Y, Yokode M, Yoshida H, Rakugi H, Wakatsuki A, Yamashita S, Committee for Epidemiology and Clinical Management of Atherosclerosis : Japan Atherosclerosis Society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases 2017. J Atheroscler Thromb, 2018; 25: 846-984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Lin C, Rajalahti T, Mjøs SA, Kvalheim OM: Predictive associations between serum fatty acids and lipoproteins in healthy non-obese Norwegians: implications for cardiovascular health. Metabolomics, 2016; 12: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Ishigami M, Yamashita S, Sakai N, Arai T, Hirano K, Hiraoka H, Kameda-Takemura K, Matsuzawa Y: Large and cholesteryl ester-rich high-density lipoproteins in cholesteryl ester transfer protein (CETP) deficiency can not protect macrophages from cholesterol accumulation induced by acetylated low-density lipoproteins. J Biochem, 1994; 116: 257-262 [DOI] [PubMed] [Google Scholar]
- 27). Martin SS, Khokhar AA, May HT, Kulkarni KR, Blaha MJ, Joshi PH, Toth PP, Muhlestein JB, Anderson JL, Knight S, Li Y, Spertus JA, Jones SR; Lipoprotein Investigators Collaborative (LIC): HDL cholesterol subclasses, myocardial infarction, and mortality in secondary prevention: the Lipoprotein Investigators Collaborative. Eur Heart J, 2015; 36:22-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Koba S, Yokota Y, Hirano T, Ito Y, Ban Y, Tsunoda F, Sato T, Shoji M, Suzuki H, Geshi E, Kobayashi Y, Katagiri T: Small LDL-cholesterol is superior to LDL-cholesterol for determining severe coronary atherosclerosis. J Atheroscler Thromb, 2008; 15: 250-260 [DOI] [PubMed] [Google Scholar]
- 29). Norata GD, Tibolla G, Catapano AL: Targeting PCSK9 for hypercholesterolemia. Annu Rev Pharmacol Toxicol, 2014; 54: 273-293 [DOI] [PubMed] [Google Scholar]
- 30). Hollstein T, Vogt A, Grenkowitz T, Stojakovic T, März W, Laufs U, Bölükbasi B, Steinhagen-Thiessen E, Scharnagl H, Kassner U: Treatment with PCSK9 inhibitors reduces atherogenic VLDL remnants in a real-world study. Vascul Pharmacol, 2019; 116: 8-15 [DOI] [PubMed] [Google Scholar]
- 31). Yanagita T, Hara E, Yotsumoto H, Rahman SM, Han S-Y, Cha J-Y, Yamamoto K: NK-104, a potent new 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, enhances posttranslational catabolism of apolipoprotein B-100 and inhibits secretion of apolipoprotein B-100 and triacylglycerols from HepG2 cells. Current Therapeutic Research, 1999; 60: 423-434 [Google Scholar]
- 32). Chapman MJ, Caslake M, Packard C, McTaggart F: New dimension of statin action on apoB atherogenicity. Clin Cardiol, 2003; 26 (1 Suppl 1): I7-I10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Martin SS, Blaha MJ, Elshazly MB, Brinton EA, Toth PP, McEvoy JW, Joshi PH, Kulkarni KR, Mize PD, Kwiterovich PO, Defilippis AP, Blumenthal RS, Jones SR: Friedewald-estimated versus directly measured low-density lipoprotein cholesterol and treatment implications. J Am Coll Cardiol, 2013; 62: 732-739 [DOI] [PubMed] [Google Scholar]


