Abstract
Aim: Intracerebral hemorrhage (ICH) is one of the most severe complications of thrombolysis. Symptomatic ICHs are associated with adverse outcomes. It has been reported that symptomatic ICHs most commonly occur within the first few hours after the initiation of intravenous thrombolysis. Our aim here was to determine the risk factors for early ICH (within 12 h) after thrombolysis.
Methods: We analyzed patients with acute ischemic stroke who received intravenous alteplase at two hospitals affiliated to Wenzhou Medical University between March 2008 and November 2017. The ICH diagnosis time was defined as the time from the intravenous administration of alteplase to the first detection of hemorrhage on computed tomography. Demographic data, medical history, clinical features, and laboratory examination results were collected. Univariate analysis followed by multivariable logistic regression analysis was performed to determine the predictors of early ICH (within 12 h) after thrombolysis.
Results: Among 197 patients, early ICH (within 12 h) after thrombolysis occurred in 13 patients (6.6%). In the univariate analysis, patients with early ICHs were significantly correlated with prior stroke (P = 0.04). After adjusting for potential confounders in the multivariate analysis, prior stroke (odds ratio [OR]: 5.752, 95% confidence interval [CI]: 1.487–22.248; P = 0.011) and atrial fibrillation (OR: 5.428, 95% CI: 1.427–20.640; P = 0.013) were associated with early ICH.
Conclusions: Prior stroke and atrial fibrillation are independent risk factors for early ICHs (within 12 h) after intravenous thrombolysis with alteplase.
Keywords: Early intracerebral hemorrhage, Intravenous thrombolysis, Acute ischemic stroke
Introduction
Acute ischemic stroke is one of the leading causes of death worldwide, characterized by high morbidity, mortality, and disability rates1). Intravenous thrombolytic treatment with alteplase initiated within 4.5 h after the onset of symptoms is currently the only approved medical therapy available for acute ischemic stroke2). The efficacy and safety of this treatment have been demonstrated by several large studies and meta-analyses3, 4).
Although intravenous alteplase improves clinical outcomes in patients with acute ischemic stroke, the potential complications, especially the risk of bleeding, may offset the benefits of recanalization, thus limiting its use in clinical practice to some extent. Intracerebral hemorrhage (ICH) is one of the most devastating complications of thrombolysis5). Notably, it has been reported in a recent review6) that symptomatic intracerebral hemorrhage (sICH) is associated with poor functional outcomes and increased disability. The definitions of sICH vary across studies, such as the National Institute of Neurological Disorders and Stroke (NINDS) trial, the European Cooperative Acute Stroke Study (ECASS), and the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST)3, 7–9). ICH is clinically diagnosed by performing a computed tomography (CT) scan, but this method does not reveal the precise time when the hemorrhage began. To date, the timing of sICH has not been well described pathologically10). It was reported in one recent review11) that the vast majority of sICHs occurred in 24 h but that approximately 10%–15% occurred after 24 h. In the NINDS trial, all mortal sICH events occurred within 24 h and 80% were within 12 h. Furthermore, a recent study12) indicated that sICH associated with the administration of intravenous alteplase typically occurred within the first 12 h of treatment. The first 12 h after thrombolysis seem to be a crucial time interval for ICH. A multitude of studies13) have reported risk factors for post-thrombolytic ICH or sICH, but few studies have focused on early ICH, particularly in the first 12 h after thrombolysis. In this study, we aimed to identify the risk factors for early ICH (within 12 h) after thrombolysis with alteplase.
Materials and Methods
Subjects
We performed a retrospective analysis of a prospectively included cohort of patients with acute ischemic stroke who received intravenous alteplase at two hospitals affiliated to Wenzhou Medical University between March 2008 and November 2017. The inclusion and exclusion criteria were the same as the ones used in the ECASS III study3). Eligible patients received 0.9 mg of alteplase per kilogram of body weight (with a maximum dose of 90 mg), 10% of which was given as a bolus, followed by delivery of the remaining 90% as a constant infusion over a period of 60 min. CT scans of the brain were performed before treatment with intravenous alteplase started and were repeated 24–36 h later or whenever clinically indicated for patients with worsening stroke symptoms.
Risk Factors
The patients' demographic data, medical history, clinical features, and laboratory examination results were collected. First, we gathered data on pretreatment factors, including demographics (age and sex), medical history (hypertension, diabetes mellitus, hyperlipidemia, atrial fibrillation, prior stroke, smoking, and the use of antithrombotic drugs before stroke), and clinical features (initial systolic and diastolic blood pressure, National Institutes of Health Stroke Scale [NIHSS]14) score on admission, and time from onset to thrombolysis [OTT]). Second, we gathered the results of laboratory examinations, including pretreatment blood glucose levels, international normalized ratio (INR), activated partial thromboplastin time (APTT), and platelet count.
Clinical Assessment
The ICH diagnosis time was defined as the time from the intravenous administration of alteplase to the first detection of hemorrhage on CT. Although the half-life of alteplase is only 4 min, its effects on coagulatory function, such as prolonging prothrombin time and APTT and decreasing fibrinogen, may last more than 24 h15). Currently, studies16–18) suggest that ICH within 36 h after thrombolysis can be ascribed to thrombolytic therapy. Therefore, postthrombolytic ICH within 36 h was considered to be thrombolysis-associated ICH in our study. Early ICH after thrombolysis was defined as ICH with a diagnosis time less than 12 h, whereas an ICH diagnosis time of 12–36 h indicated late ICH. Nonearly ICH included late ICH and no ICH. Among the various definitions of sICH, the ECASS II definition8) of sICH (ICH associated with at least a four-point increment in the NIHSS score) has shown the highest interrater agreement19). Another study11) suggested that the ECASS II definition is the best predictor of unfavorable outcomes and would be the most suitable definition of sICH. We investigated sICH according to the ECASS II definition, except that we reduced the time period from seven days to 36 h after the intravenous administration of alteplase.
Outcomes at three months after stroke onset were assessed on the modified Rankin Scale (mRS)20). A favorable outcome was defined as an mRS score of 0-1, and an unfavorable outcome was defined as an mRS score of 2–6.
Statistical Analysis
All statistical analyses were performed using the software program SPSS Statistics version 20. Data are presented as the mean ± standard deviation for continuous variables and as counts and percentages (%) of subjects for categorical variables. Univariate comparison of two groups was performed with Student's t-test or the Mann–Whitney rank sum test for continuous variables and with Pearson's χ2 test for categorical variables. In order to clarify the associated factors for early ICH, factors significant in the univariate analysis (P < 0.1) and potential confounders (i.e., age, sex, blood glucose, and admission NIHSS score) were entered into multivariate logistic regression models. The results were considered statistically significant when P < 0.05.
Results
A total of 197 patients were included in our study. Among 38 cases of ICH after thrombolysis, early ICH (within 12 h) occurred in 13 patients, with a median ICH diagnosis time of 210 min (range: 20–637 min), and late ICH (12–36 h) occurred in 25 patients, with a median ICH diagnosis time of 1,380 min (range: 780–2,107 min). The number of patients with nonearly ICH was 184, including 25 patients with late ICH and 159 patients with no ICH. The baseline characteristics and outcomes of patients with early ICH, late ICH, no ICH, and nonearly ICH are detailed in Table 1. Patients with early ICH were more likely than those with nonearly ICH to have a previous history of stroke (38.5% versus 13.6%, P = 0.04) and seemed to have a higher proportion of atrial fibrillation (61.5% versus 32.1%, P = 0.06). The other characteristics were similar between the two groups. Moreover, in terms of outcomes, patients with early ICH were less likely to have favorable three-month clinical outcomes compared to those with non-early ICH (P = 0.04). Compared with patients with late ICH, those with early ICH were more likely to have a higher prevalence of atrial fibrillation (61.5% versus 28.0%, P = 0.04) and to be prone to have unfavorable three-month clinical outcomes. Compared with patients with no ICH, those with early ICH had a higher prevalence of prior stroke (38.5% versus 13.8%, P = 0.05), were treated with thrombolytic therapy later, and were prone to have a poor prognosis.
Table 1. Demographic data, medical history, clinical features, laboratory examinations and outcomes of patients with early ICH, late ICH, no ICH and nonearly ICH.
| Early ICH (n = 13) | Late ICH (n = 25) | P1 | No ICH (N = 159) | P2 | Nonearly ICH (n = 184) | P3 | |
|---|---|---|---|---|---|---|---|
| Demographic characteristics | |||||||
| Age | 66.9 ± 9.7 | 65.3 ± 12.1 | 0.68 | 67.0 ± 13.0 | 0.97 | 66.8 ± 12.9 | 0.97 |
| Sex (male), n (%) | 7 (53.8) | 18 (72.0) | 0.30 | 108 (67.9) | 0.46 | 126 (68.5) | 0.43 |
| Stroke risk factors | |||||||
| Hypertension, n (%) | 10 (76.9) | 17 (68.0) | 0.84 | 116 (73.0) | 1.00 | 133 (72.3) | 0.96 |
| Diabetes, n (%) | 4 (30.8) | 5 (20.0) | 0.73 | 50 (31.4) | 1.00 | 55 (29.9) | 1.00 |
| Hyperlipidaemia, n (%) | 4 (30.8) | 6 (24.0) | 0.95 | 60 (37.7) | 0.84 | 66 (35.9) | 0.94 |
| Atrial fibrillation, n (%) | 8 (61.5) | 7 (28.0) | 0.04* | 52 (32.7) | 0.07 | 59 (32.1) | 0.06 |
| Prior stroke, n (%) | 5 (38.5) | 3 (12.0) | 0.13 | 22 (13.8) | 0.05* | 25 (13.6) | 0.04* |
| Current smoking, n (%) | 4 (30.8) | 11 (44.0) | 0.42 | 49 (30.8) | 1.00 | 60 (32.6) | 1.00 |
| Antithrombotic drugs use before stroke, n (%) | 3 (23.1) | 4 (16.0) | 0.67 | 23 (14.5) | 0.66 | 27 (14.7) | 0.68 |
| Clinical features | |||||||
| Initial SBP (mmHg) | 148.7 ± 18.6 | 147.4 ± 17.8 | 0.84 | 152.5 ± 20.8 | 0.52 | 151.8 ± 20.5 | 0.59 |
| Initial DBP (mmHg) | 80.0 ± 14.2 | 85.8 ± 11.1 | 0.17 | 85.1 ± 12.2 | 0.15 | 85.7 ± 12.3 | 0.12 |
| OTT (minutes) | 202.6 ± 33.5 | 206.9 ± 36.1 | 0.72 | 180.9 ± 50.8 | 0.04* | 184.5 ± 49.8 | 0.20 |
| Admission NIHSS score | 13.1 ± 6.0 | 12.6 ± 5.4 | 0.77 | 12.3 ± 6.5 | 0.65 | 12.3 ± 6.3 | 0.66 |
| Laboratory examinations | |||||||
| APTT | 32.2 ± 3.5 | 32.8 ± 3.7 | 0.63 | 32.8 ± 5.2 | 0.68 | 32.8 ± 5.0 | 0.67 |
| INR | 1.00 ± 0.07 | 1.03 ± 0.08 | 0.28 | 1.03 ± 0.10 | 0.38 | 1.03 ± 0.10 | 0.35 |
| Blood glucose (mmol/L) | 8.5 ± 2.5 | 8.3 ± 3.7 | 0.87 | 7.9 ± 3.0 | 0.45 | 7.9 ± 3.1 | 0.51 |
| Platelet count (109/L) | 173.8 ± 45.1 | 179.7 ± 28.9 | 0.75 | 209.1 ± 94.1 | 0.36 | 206.3 ± 90.3 | 0.38 |
| Outcomes (mRS score at 3 months) | 0.03* | 0.02* | 0.04* | ||||
| Favourable outcome (0–1) | 2 (15.4) | 13 (52.0) | 75 (47.2) | 88 (47.8) | |||
| Unfavourable outcome (2–6) | 11 (84.6) | 12 (48.0) | 84 (52.8) | 96 (52.2) |
SBP, systolic blood pressure; DBP, diastolic blood pressure; NIHSS: National Institutes of Health Stroke Scale; OTT, the time from onset to thrombolysis; APTT, activated partial thromboplastin time; INR, international normalized ratio; mRS, modified Rankin Scale.
P1: Early ICH vs Late ICH; P2: Early ICH vs No ICH; P3: Early ICH vs nonearly ICH.
P < 0.05
The multivariable logistic regression analysis for early ICH is shown in Table 2. After adjusting for potential confounders, including age, sex, blood glucose, and admission NIHSS score, prior stroke (odds ratio [OR]: 5.752; 95% confidence interval [CI]: 1.487–22.248; P = 0.011) and atrial fibrillation (OR: 5.428, 95% CI: 1.427–20.640; P = 0.013) were associated with early ICH after thrombolysis.
Table 2. Multivariable logistic regression for early ICH.
| Unadjusted |
||
|---|---|---|
| OR (95% CI) | P value | |
| Prior stroke | 3.995 (1.179–13.534) | 0.026 |
| Atrial fibrillation | 3.404 (1.048–11.062) | 0.042 |
| Adjusted† |
||
| OR (95% CI) | P value | |
| Prior stroke | 5.752 (1.487–22.248) | 0.011* |
| Atrial fibrillation | 5.428 (1.427–20.640) | 0.013* |
| Age | 0.968 (0.918–1.021) | 0.230 |
| Sex | 3.080 (0.869–10.910) | 0.081 |
| Blood glucose | 1.058 (0.897–1.247) | 0.503 |
| admission NIHSS score | 0.969 (0.877–1.070) | 0.532 |
OR, odds ratio; CI, confidence interval.
Adjusted for age, sex, blood glucose, and admission NIHSS score.
P < 0.05
The distribution of the ICH diagnosis time for patients who developed ICH within 36 h after thrombolysis is shown in Table 3. Among 38 cases of ICH after thrombolysis, sICH occurred in 13 patients, with a median ICH diagnosis time of 522 min (range: 78–1,323 min), and asymptomatic ICH occurred in 25 patients, with a median ICH diagnosis time of 1,380 min (range: 20–2,107 min). Compared with patients with nonearly ICH, those with early ICH had an increased probability of developing sICH (P = 0.003). 13 images of first hemorrhage detection on CT of patients with early ICH are shown in Fig. 1.
Table 3. The distribution of ICH diagnosis time for patients with ICH 36 hours after thrombolysis.
| sICH (n = 13), % | asymptomatic ICH (n = 25), % | P value | |
|---|---|---|---|
| < 12 hours (n = 13) | 9 (69.2%) | 4 (30.8%) | 0.003* |
| 0–6 hours | 6 (46.1%) | 3 (23.1%) | |
| 6–12 hours | 3 (23.1%) | 1 (7.7%) | |
| 12–36 hours (n = 25) | 4 (16%) | 21 (84%) | |
| 12–18 hours | 3 (12%) | 2 (8%) | |
| 18–24 hours | 1 (4%) | 10 (40%) | |
| 24–36 hours | 0 | 9 (36%) | |
P < 0.05
Fig. 1.
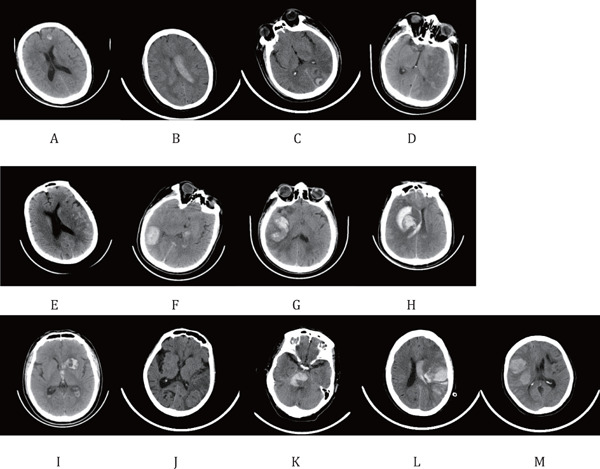
Initial CT detection of hemorrhage in 13 patients with early ICH
Panels (A)–(D) and (E)–(M) show patients with asymptomatic ICH and sICH, respectively.
Discussion
ICH is one of the most feared complications of thrombolysis. However, data regarding risk factors for early ICH (within 12 h) after thrombolysis with alteplase are still scarce.
It was reported in our study that prior stroke and atrial fibrillation are independent risk factors for early ICH after intravenous thrombolysis. Heldner et al.21) also reported that, compared with patients without prior stroke, those with prior stroke after thrombolysis had an increased probability of developing ICH and having adverse outcomes. It was reported in one study22) that blood–brain barrier (BBB) disruption occurred at a mean of 13 h after stroke onset and was associated with increased rates of hemorrhagic transformation. Additionally, reperfusion is considered a risk factor for postthrombolytic ICH once the integrity of the BBB is destroyed in acute ischemic stroke. Therefore, we posited that patients with a history of stroke, given the preexisting abnormality of their BBB integrity and the dysfunction of their vascular basal lamina, may develop BBB disruption in the next stroke more quickly compared to first-time stroke patients and may then be more likely to develop ICH early after reperfusion of acute infarcted tissue. Atrial fibrillation is considered an important risk factor for ICH and sICH after thrombolysis13, 23, 24). Along the same lines, we found that atrial fibrillation played an important role in early ICH. It is acknowledged that patients with embolic stroke are more likely than those with other types of stroke to experience hemorrhagic transformation25). Saposnik et al.26) suggested that the adverse effects of atrial fibrillation were attributable to large areas of low perfusion and low recanalization, resulting in increased infarct volume and ICH severity. We corroborated the former studies and further inferred that ICH-associated atrial fibrillation is prone to occur early after thrombolysis. In addition, patients with early ICH were more likely than those with nonearly ICH to have unfavorable clinical outcomes in three months. This difference may be due to the increased proportion of patients with sICH among patients with early ICH.
Depending on the cohort characteristics and the definition of sICH, the incidence of sICH after the intravenous administration of alteplase differs but generally ranges from 2% to 7%16). The incidence of sICH in our study was approximately 6.6%, which is consistent with prior studies. We also reported that sICH occurred in the early stage after thrombolysis and was especially common within 12 h. Several studies27–29) reported that hemorrhage expansion consistently occurred in approximately 30–40% of patients diagnosed with sICH. However, the currently available treatment for sICH did not effectively reduce the likelihood of in-hospital mortality or hemorrhage expansion. Prompt diagnosis and early treatment may be key variables in improving outcomes for patients with sICH16, 30). Thus, Yaghi et al.30) argued that the frequency of neurological assessments in the first 12 h after the intravenous administration of alteplase should be increased to promote the early detection of sICH. In our study, patients with early ICH tended to develop sICH. Additionally, prior stroke and atrial fibrillation were risk factors for early ICH after intravenous thrombolysis. We recommend an increased frequency of assessment for patients with a history of stroke or atrial fibrillation who are receiving alteplase. Performing CT scans early (even at 12 h) after thrombolysis would have considerable benefits and is vital in the event of severe stroke or coma. Postthrombolytic ICH may cause only slight neurological deterioration in those patients. The time to diagnosis and treatment may be prolonged because clinicians are likely to ignore this subtle neurological deterioration.
Our study had several limitations. First, we defined sICH on the basis of the ECASS II criteria. Adopting other definitions of sICH may affect the grouping and results of our study to some degree. Nevertheless, all definitions of sICH have limitations. No definition achieves an optimal combination of prediction of mortality and outcome and a high interrater agreement rate19). Second, sICH was not diagnosed until CT was performed; the hemorrhage may have occurred well before the time of diagnosis. The time from the initiation of alteplase therapy to sICH diagnosis does not convincingly reflect the actual time to hemorrhage onset. Notwithstanding, as it is difficult to acquire the exact hemorrhage time in clinical practice, most studies use the time from the initiation of the intravenous administration of alteplase to ICH diagnosis as an indicator. Third, the incidence of ICH after thrombolysis is low; consequently, the sample size in this study is relatively small. This limitation should be taken into account in the interpretation of our results. Therefore, further investigations should include large samples and multicenter studies.
Conclusions
In summary, our study demonstrated that prior stroke and atrial fibrillation are independent risk factors for early ICH (within 12 h) after intravenous thrombolysis with alteplase. For patients with prior stroke or atrial fibrillation who are receiving alteplase, the frequency of assessment needs to be increased and the interval from alteplase treatment to CT scanning needs to be shortened. However, this recommendation remains to be tested through larger prospective studies.
Study Funding
This study was funded by the Wenzhou Municipal Sci-Tec Bureau Programs (Grant Y2004A014) and Clinical Scientific Research Fund of the Second Affiliated Hospital of Wenzhou Medical University (SAHoWMU-CR2017-01-212), the National Natural Science Foundation of China (Grant no. 81571114,81771267).
References
- 1). Hacke W, Kaste M, Skyhoj Olsen T, Orgogozo JM, Bogousslavsky J. European Stroke Initiative (EUSI) recommendations for stroke management. The European Stroke Initiative Writing Committee. Eur J Neurol, 2000; 7: 607-623 [DOI] [PubMed] [Google Scholar]
- 2). Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL, American Heart Association Stroke C 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke, 2018; 49: e46-e110 [DOI] [PubMed] [Google Scholar]
- 3). Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D, Investigators E Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med, 2008; 359: 1317-1329 [DOI] [PubMed] [Google Scholar]
- 4). Lees K, Bluhmki E, von Kummer R, Brott T, Toni D, Grotta J, Albers G, Kaste M, Marler J, Hamilton S, Tilley B, Davis S, Donnan G, Hacke W, Allen K, Mau J, Meier D, del Zoppo G, De Silva D, Butcher K, Parsons M, Barber P, Levi C, Bladin C, Byrnes G. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet, 2010; 375: 1695-1703 [DOI] [PubMed] [Google Scholar]
- 5). Derex L, Nighoghossian N. Intracerebral haemorrhage after thrombolysis for acute ischaemic stroke: an update. J Neurol Neurosurg Psychiatry, 2008; 79: 1093-1099 [DOI] [PubMed] [Google Scholar]
- 6). Karaszewski B, Houlden H, Smith EE, Markus HS, Charidimou A, Levi C, Werring DJ. What causes intracerebral bleeding after thrombolysis for acute ischaemic stroke? Recent insights into mechanisms and potential biomarkers. J Neurol Neurosurg Psychiatry, 2015; 86: 1127-1136 [DOI] [PubMed] [Google Scholar]
- 7). National Institute of Neurological D, and Stroke rt PASSG. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med, 1995; 333: 1581-1587 [DOI] [PubMed] [Google Scholar]
- 8). Larrue V, von Kummer RR, Muller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II). Stroke, 2001; 32: 438-441 [DOI] [PubMed] [Google Scholar]
- 9). Wahlgren N, Ahmed N, Eriksson N, Aichner F, Bluhmki E, Davalos A, Erila T, Ford GA, Grond M, Hacke W, Hennerici MG, Kaste M, Kohrmann M, Larrue V, Lees KR, Machnig T, Roine RO, Toni D, Vanhooren G, Safe Implementation of Thrombolysis in Stroke MSI Multivariable analysis of outcome predictors and adjustment of main outcome results to baseline data profile in randomized controlled trials: Safe Implementation of Thrombolysis in Stroke-MOnitoring STudy (SITSMOST). Stroke, 2008; 39: 3316-3322 [DOI] [PubMed] [Google Scholar]
- 10). Yaghi S, Eisenberger A, Willey JZ. Symptomatic intracerebral hemorrhage in acute ischemic stroke after thrombolysis with intravenous recombinant tissue plasminogen activator: a review of natural history and treatment. JAMA neurology, 2014; 71: 1181-1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Strbian D, Sairanen T, Meretoja A, Pitkaniemi J, Putaala J, Salonen O, Silvennoinen H, Kaste M, Tatlisumak T. Patient outcomes from symptomatic intracerebral hemorrhage after stroke thrombolysis. Neurology, 2011; 77: 341-348 [DOI] [PubMed] [Google Scholar]
- 12). Chang A, Llinas EJ, Chen K, Llinas RH, Marsh EB. Shorter Intensive Care Unit Stays? The Majority of Post-Intravenous tPA (Tissue-Type Plasminogen Activator) Symptomatic Hemorrhages Occur Within 12 Hours of Treatment. Stroke, 2018; 49: 1521-1524 [DOI] [PubMed] [Google Scholar]
- 13). Whiteley WN, Slot KB, Fernandes P, Sandercock P, Wardlaw J. Risk factors for intracranial hemorrhage in acute ischemic stroke patients treated with recombinant tissue plasminogen activator: a systematic review and meta-analysis of 55 studies. Stroke, 2012; 43: 2904-2909 [DOI] [PubMed] [Google Scholar]
- 14). Lyden P, Brott T, Tilley B, Welch KM, Mascha EJ, Levine S, Haley EC, Grotta J, Marler J. Improved reliability of the NIH Stroke Scale using video training. NINDS TPA Stroke Study Group. Stroke, 1994; 25: 2220. [DOI] [PubMed] [Google Scholar]
- 15). Matrat A, De Mazancourt P, Derex L, Nighoghossian N, Ffrench P, Rousson R, Hanss M. Characterization of a severe hypofibrinogenemia induced by alteplase in two patients thrombolysed for stroke. Thromb Res, 2013; 131: e45-48 [DOI] [PubMed] [Google Scholar]
- 16). Yaghi S, Willey J, Cucchiara B, Goldstein J, Gonzales N, Khatri P, Kim L, Mayer S, Sheth K, Schwamm L. Treatment and Outcome of Hemorrhagic Transformation After Intravenous Alteplase in Acute Ischemic Stroke: A Scientific Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke, 2017; 48: e343-e361 [DOI] [PubMed] [Google Scholar]
- 17). Khatri P, Wechsler LR, Broderick JP. Intracranial hemorrhage associated with revascularization therapies. Stroke, 2007; 38: 431-440 [DOI] [PubMed] [Google Scholar]
- 18). Seet RC, Rabinstein AA. Symptomatic intracranial hemorrhage following intravenous thrombolysis for acute ischemic stroke: a critical review of case definitions. Cerebrovasc Dis, 2012; 34: 106-114 [DOI] [PubMed] [Google Scholar]
- 19). Gumbinger C, Gruschka P, Bottinger M, Heerlein K, Barrows R, Hacke W, Ringleb P. Improved prediction of poor outcome after thrombolysis using conservative definitions of symptomatic hemorrhage. Stroke, 2012; 43: 240-242 [DOI] [PubMed] [Google Scholar]
- 20). van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke, 1988; 19: 604-607 [DOI] [PubMed] [Google Scholar]
- 21). Heldner MR, Mattle HP, Jung S, Fischer U, Gralla J, Zubler C, El-Koussy M, Schroth G, Arnold M, Mono ML. Thrombolysis in patients with prior stroke within the last 3 months. Eur J Neurol, 2014; 21: 1493-1499 [DOI] [PubMed] [Google Scholar]
- 22). Warach S, Latour LL. Evidence of reperfusion injury, exacerbated by thrombolytic therapy, in human focal brain ischemia using a novel imaging marker of early blood-brain barrier disruption. Stroke, 2004; 35: 2659-2661 [DOI] [PubMed] [Google Scholar]
- 23). Tanne D, Kasner SE, Demchuk AM, Koren-Morag N, Hanson S, Grond M, Levine SR. Markers of increased risk of intracerebral hemorrhage after intravenous recombinant tissue plasminogen activator therapy for acute ischemic stroke in clinical practice: the Multicenter rt-PA Stroke Survey. Circulation, 2002; 105: 1679-1685 [DOI] [PubMed] [Google Scholar]
- 24). Dharmasaroja PA, Muengtaweepongsa S, Pattaraarchachai J, Dharmasaroja P. Intracerebral hemorrhage following intravenous thrombolysis in Thai patients with acute ischemic stroke. J Clin Neurosci, 2012; 19: 799-803 [DOI] [PubMed] [Google Scholar]
- 25). Hart RG, Easton JD. Hemorrhagic infarcts. Stroke, 1986; 17: 586-589 [DOI] [PubMed] [Google Scholar]
- 26). Saposnik G, Gladstone D, Raptis R, Zhou L, Hart RG, Investigators of the Registry of the Canadian Stroke N, and the Stroke Outcomes Research Canada Working G Atrial fibrillation in ischemic stroke: predicting response to thrombolysis and clinical outcomes. Stroke, 2013; 44: 99-104 [DOI] [PubMed] [Google Scholar]
- 27). Alderazi YJ, Barot NV, Peng H, Vahidy FS, Navalkele DD, Sangha N, Misra V, Savitz SI. Clotting factors to treat thrombolysis-related symptomatic intracranial hemorrhage in acute ischemic stroke. J Stroke Cerebrovasc Dis, 2014; 23: e207-214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Mokin M, Kass-Hout T, Kass-Hout O, Zivadinov R, Mehta B. Blood pressure management and evolution of thrombolysis-associated intracerebral hemorrhage in acute ischemic stroke. J Stroke Cerebrovasc Dis, 2012; 21: 852-859 [DOI] [PubMed] [Google Scholar]
- 29). Goldstein JN, Marrero M, Masrur S, Pervez M, Barrocas AM, Abdullah A, Oleinik A, Rosand J, Smith EE, Dzik WH, Schwamm LH. Management of thrombolysis-associated symptomatic intracerebral hemorrhage. Arch Neurol, 2010; 67: 965-969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Yaghi S, Boehme AK, Dibu J, Leon Guerrero CR, Ali S, Martin-Schild S, Sands KA, Noorian AR, Blum CA, Chaudhary S, Schwamm LH, Liebeskind DS, Marshall RS, Willey JZ. Treatment and Outcome of Thrombolysis-Related Hemorrhage: A Multicenter Retrospective Study. JAMA neurology, 2015; 72: 1451-1457 [DOI] [PMC free article] [PubMed] [Google Scholar]


