Abstract
Newcastle disease virus is a member of family Paramyxoviridae that infects chicken. Its genome comprises ~15.2 kb negative-sense RNA that encodes six major proteins. The virus encodes various proteins; among all, nucleocapsid (NP) and matrix (M) help in virus replication and its budding from the host cells, respectively. In this study, we investigated the intracellular distribution of NP and M upon expression in the yeast Saccharomyces cerevisiae. We observed nuclear targeting of M, and vacuolar localization of NP was observed in a fraction of yeast cells. Prolonged expression of GFP fused NP or M resulted in altered cell viability and intracellular production of reactive oxygen species in yeast cells. The expression of viral proteins did not alter the morphology and number of the organelles such as nucleus, mitochondria, endoplasmic reticulum, and peroxisomes. However, a significant effect was observed on vacuolar morphology and number in yeast cells. These observations point towards the importance of host cellular reorganization in viral infection. These findings may enable us to understand the conserved pathways affected in eukaryotic cells as a result of viral protein expression.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-020-02624-4.
Keywords: Newcastle disease virus, Nucleoprotein, Matrix protein, Organelles, Yeast
Introduction
The paramyxoviruses are a large group of enveloped RNA viruses. They are extremely important as some of them cause significant human and animal diseases such as human respiratory syncytial virus (HRSV), human parainfluenza virus types 1–4 (HPIV 1–4), Hendra virus, measles virus, mumps virus, Nipah virus, and Newcastle disease virus (NDV). Newcastle disease causes substantial damage to the economy of the poultry industry (Ogali et al. 2018). Its causative agent NDV is a negative-sense, single-stranded, enveloped RNA virus and belongs to the genus Avulavirus under family Paramyxoviridae (Ganar et al. 2014). Its genome size is ~15.2 kb which encodes for six major structural proteins, namely nucleoprotein (NP), phosphoprotein (P), matrix protein (M), fusion protein (F), hemagglutinin-neuraminidase (HN) and RNA-dependent RNA polymerase (L). The ribonucleoprotein (RNP) complex that serves as a template for RNA synthesis comprises RNA, together with NP, P and L proteins (Ganar et al. 2014). The HN protein is involved in the attachment of the virions to the host-cell receptors, while F protein mediates the fusion of the viral envelope with the host cell plasma membrane. NP protein is made up of 489 amino acids (aa) and is reported to form a herring bone-like structure (Ganar et al. 2014). The M protein is the most abundant in the virions and is composed of 364 aa corresponding to a molecular weight of 40 kDa. It surrounds the nucleocapsid and constitutes a bridge between the lipid membrane and the nucleocapsid (Ganar et al. 2014).
Cytopathic effects caused due to NDV infection such as rounding, vacuolation, syncytia formation and cell death have been observed in cultured cells (Ravindra et al. 2009). Effects like nuclear condensation, cytoplasm blebbing and DNA fragmentation due to the induction of p53 and the Bax-dependent apoptotic pathway have also been reported (Ravindra et al. 2009). On the other hand, a role for the autophagic vesicles in NDV replication was reported (Sun et al. 2014). NP and P proteins of NDV were identified as potent endoplasmic reticulum (ER) stress inducers that regulate the transcription of the ER chaperons (Cheng et al. 2016). Prolonged ER stress may trigger autophagy to compensate for the exceeded capacity of proteasome degradation system (Hoyer-Hansen and Jaattela 2007; Li et al. 2008). M protein of NDV was reported to localize to the nucleus at the early stages of infection as also reported for several other paramyxoviruses (Duan et al. 2014; Peeples et al. 1992). A bipartite nuclear localization signal (NLS) and interaction with the host nucleolar phosphoprotein B23 were reported to be necessary for the nuclear localization of the M protein (Duan et al. 2014). A peroxisomal targeting signal 2 (PTS 2) sequence (SVxxxxxQL) at the N terminal (3–11aa ) of NP protein of NDV and other viral proteins was predicted using the PATTINPROT prediction program (Mohan and Atreya 2003). Proteins destined to peroxisomes contain PTS1, a tripeptide motif at its carboxy-terminal, or PTS2 which constitutes a combination of nine amino acid bipartite sequences (consensus [RKS]-[ILVH]-x(5)-[QH]-[LAE]) at its amino-terminus (Gould et al. 1988; Olivier et al. 2000; Swinkels et al. 1991; Terlecky et al. 1996). This targeting is aided by receptors unique to both PTS1 and PTS2 pathways (Lametschwandtner et al. 1998; Subramani 1998; Terlecky et al. 1996). Recent literature also reports the localization of several viral proteins to peroxisomes and alterations in the expression of the peroxisomal proteins due to viral infection (You et al. 2015).
To understand the alterations in host sub-cellular structures upon expression of NDV proteins, we cloned the NP and M protein-coding regions of NDV in a yeast expression vector. The localization and cellular effects caused by these proteins were analyzed in Saccharomyces cerevisiae. S. cerevisiae is a well-studied model for understanding highly conserved cellular pathways. Several earlier studies have used this model system to study localization, interaction and cellular effects caused due to the expression of viral proteins (Zhao 2017).
Materials and methods
Microorganisms and growth conditions
The yeast strains used in this study are listed in Table 1. S. cerevisiae strains were grown in either (i) complete media (YPD) containing 1% yeast extract, 2% bactopeptone, 1% glucose or (ii) selective media (YND) containing 0.17% yeast nitrogen base without amino acids 0.5% ammonium sulfate and 2% glucose. pH was adjusted to 6.0 using KOH. The media was supplemented with amino acids leucine (3 mg/ml), lysine (10 mg/ml), histidine (10 mg/ml), uracil (3 mg/ml) as per the requirements. E. coli strain DH5α was used for cloning purposes and was grown at 37 °C in LB medium (1% tryptone, 0.5% yeast extract, 0.5% NaCl) and was supplemented with 100 µg/ml ampicillin whenever required (Kuravi et al. 2006).
Table 1.
S. cerevisiae strains used in this study
| Strain | Description |
|---|---|
| BY4742 WT | MATα his3∆ leu2∆ lys∆ ura3∆ |
| BY4742 ctt1 | ctt1:: his3∆ leu2∆ lys∆ ura3∆ |
| BY4742 pUG35GFP | BY4742 WT.URA3::PMET25 pUG35GFP |
| BY4742 DsRed-SKL | BY4742 WT.HIS3::PMET25 DsRed.SKL |
| BY4742 NDVNP-GFP | BY4742 WT.URA3::PMET25 NDVNP-GFP |
| BY4742 NDVNP-GFP DsRed-SKL | BY4742 WT.URA3::PMET25NDVNP-GFP.HIS::DsRed-SKL |
| BY4742 NDVM-GFP | BY4742 WT.URA3::PMET25 NDVM-GFP |
| BY4742 pUG36GFP | BY4742 WT.URA3::PMET25 pUG36GFP |
| BY4742 GFP-NDVNP | BY4742 WT.URA3::PMET25 GFP-NDVNP |
Construction of plasmids and yeast strains
Standard recombinant techniques were carried out using plasmids and primers as listed in Tables 2 and 3. The NP open reading frame (ORF) of NDV was amplified from pET28a-NDV-NP plasmid (SK lab collection) using primers pr-SGL001 and pr-SGL002 without the STOP codon. The 1.47 kb XbaI–EcoRI fragment from pET28a-NDV-NP plasmid was inserted between XbaI and EcoRI of pUG35 plasmid to obtain the C-terminal GFP fusion. Similarly, to construct NP fused to GFP at the N-terminus, the NP ORF was amplified from pET28a-NDV-NP plasmid (SK lab collection) using primers pr-SGL003 and pr-SGL004. The 1.47 kb XmaI–EcoRI fragment obtained was inserted between XmaI and EcoRI of pUG36 plasmid. The M ORF of NDV was amplified from pcDNA-NDV-M plasmid (SK lab collection) using primers pr-SGL005 and pr-SGL006 without STOP codon. The 1.09 kb XbaI–EcoRI fragment from pcDNA-NDV-M plasmid was inserted between XbaI and EcoRI of pUG35 plasmid. The obtained plasmids were transformed into S. cerevisiae BY4742 (MATα; his3Δ1; leu2Δ0; lys2Δ0; ura3Δ0) wild type (WT) (Dharmacon) cells using LiAc-PEG method (Gietz et al. 1995). Transformants were selected for uracil auxotrophy. Other plasmids used in the study and their source are listed in Table 2. For visualizing mitochondria, ER and peroxisomes, cells were transformed with plasmids pHS12-mCherry, pSM1959 and pUG34-DsRed-SKL, respectively, and selected for specific auxotrophy as listed in Table 2. ctt1 (Dharmacon) cells were cultured similar to WT cells.
Table 2.
Plasmids used in this study
| Plasmid | Description | Source |
|---|---|---|
| pET28A NP | T7lac; kanR | SK lab collection |
| pCDNA M | T7; ampR | SK lab collection |
| pUG35GFP | PMET25 GFP; ampR Sc-URA3 | From Prof. Johannes Hegemann |
| pUG34 DsRed-SKL | PMET25 DsRed.SKL; ampR; Sc-HIS3 | Kuravi et al. (2006) |
| pUG35 NDVNP GFP | PMET25 NP-GFP; ampR Sc-URA3 | This study |
| pUG36GFP | PMET25 GFP; ampR Sc-URA3 | From Prof. Johannes Hegemann |
| pUG36GFP NDVNP | PMET25 GFP-NP; ampR Sc-URA3 | This study |
| pUG35 NDVM GFP | PMET25 M-GFP; ampR Sc-URA3 | This study |
| pHS12-mCherry | PT7 preCOX4-mCHERRY; ampR Sc-LEU2 | Addgene plasmid 25,444 |
| pSM1959 | Sec63 mRFP; ampR Sc-LEU2 | Addgene plasmid 41,837 (Metzger et al. 2008) |
Table 3.
Primers used in this study
| Primer | Sequence |
|---|---|
| pr-SGL001 | 5′-TGCTCTAGAATGTCTTCCGTATTTGATG-3’ |
| pr-SGL002 | 5′-CGGAATTCATACCCCCAGTCGGTGTCGT-3’ |
| pr-SGL003 | 5′-TCCCCCCGGGATGTCTTCCGTATTTGATG-3’ |
| pr-SGL004 | 5′-CGGAATTCTCAATACCCCCAGTCGGTGT-3’ |
| pr-SGL005 | 5′-TGCTCTAGAATGGACTCATCTAGGACAAT-3’ |
| pr-SGL006 | 5′-CGGAATTCTTTCTTAAAAGGATTGTATTTGGCAAGGG-3’ |
Yeast RNA isolation and RT-PCR
RNA isolation from yeast cells was performed using TRIzol reagent (Invitrogen, USA). Cells were cultured in YND media and were lysed using zirconium beads in the presence of TRIzol. Chloroform was added to the lysed cells and was subsequently centrifuged to obtain three layers. Upper aqueous layer containing RNA was collected and re-extracted using chloroform. To the collected aqueous layer, isopropanol was added and centrifuged to obtain a pellet. The pellet was washed with 70% ethanol and air-dried. The dried pellet was dissolved in RNase-free water. Concentration and purity of RNA were measured using Thermo scientific MultiskanGo UV/Vis microplate spectrophotometer, and quantification was performed using SkanIt software. The obtained RNA was converted into cDNA, and viral genes were amplified using primers prSGL001 and prSGL002 for NP-GFP, prSGL003 and prSGL004 for GFP-NP, and prSGL005 and prSGL006 for M-GFP (Table 3).
Protein expression analysis
Cells corresponding to 3 OD units of each strain were processed by trichloro acetic acid (TCA) method to obtain yeast whole cell extracts for SDS-PAGE (Baerends et al. 2000). Equal volumes of lysates were loaded per lane, and gels were subjected to western blot analysis. The gels were run at a constant voltage of 130 V and transferred onto nitrocellulose membrane using Bio-Rad Trans-Blot® Turbo™. Blots were probed with rabbit polyclonal GFP antibody (BioBharati Life Science, Kolkata, India). Goat anti-rabbit IgG (BioBharati Life Science, Kolkata, India) conjugated to horseradish peroxidase was used as secondary antibody for detection. For actin detection, blots were incubated with anti-β-actin antibody (Invitrogen, USA) overnight at 4 °C. Goat anti-mouse IgG (Invitrogen, USA) conjugated with horseradish peroxidase was used as the secondary antibody.
Microscopy
Wide field fluorescence imaging was performed using the Nikon Ti-S eclipse inverted microscope with a 100× objective/NA–1.30. Yeast cells were grown overnight and sub-cultured further for microscopy experiments. For analyzing the expression of viral proteins, GFP signal was visualized using 465–495 nm excitation filter.
The Leica TCS SP8 confocal laser-scanning microscope (CLSM) was used for imaging cell organelles. HC PL APO CS 63x/1.40 OIL objective was used for image acquisition. GFP signal was visualized by excitation with 488 nm laser. Hoechst (10 µg/ml, Invitrogen) stained cells were visualized by excitation with 405 nm laser. DsRed, mCherry, mRFP and FM4-64 (5 µg/ml, Invitrogen) stained cells were visualized by excitation with 552 nm laser. Z-stack images were acquired to ensure no fluorescent structures were missed. Images were assembled using Adobe Photoshop 7.0. The intensity profiles were plotted using ImageJ software.
Flow cytometry
BD FACS Calibur Flow Cytometer (BD Biosciences) was used to analyze 1 × 105 cells per experiment, and data acquisition and analysis was done by FCS Express 6 software. Cells were analyzed according to fluorescence signal vs forward/side scatter (FSC/SSC) analysis. Propidium iodide (PI) (1 µg/ml, Invitrogen, USA) and dihydroethidium (DHE) (5 µg/ml, Invitrogen, USA) were added to an aliquot of cells and incubated for 30 min prior to the experiment. FL3-H channel was used for the analysis of PI (1 µg/ml) and DHE (5 µg/ml) stained cells.
Statistical analysis
All experiments reported have been repeated at least a minimum of two times. The results are expressed as the mean ± standard deviation. Statistical analyses of the differences between groups were performed using one-way ANOVA (GraphPad Prism version 7.04, GraphPad Software, California, USA). Values of p < 0.05 were considered significant (*).
Results
Cloning and expression of NDV NP and M proteins in S. cerevisiae
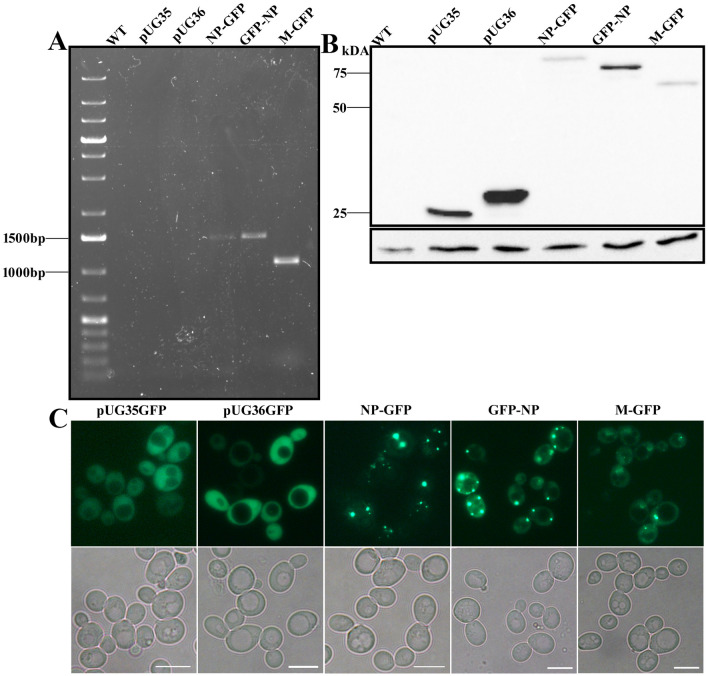
As mentioned earlier, a PTS2 signal at the N-terminus of NP protein was predicted by in silico analysis (Mohan and Atreya 2003). To avoid any discrepancy in localization studies, both N- and C-terminal GFP fusions of NP protein were constructed. An NLS at the N-terminus of M protein was predicted in earlier studies (Coleman and Peeples 1993); hence, a C-terminal GFP fusion was constructed for this study. The expression of the fusion proteins was analyzed in WT cells. In order to confirm the transcription of NDV NP and M gene, whole cell RNA was isolated from plasmid containing cells. The RNA upon DNase treatment was used as a template for reverse transcriptase PCR. cDNA obtained was further amplified using M and NP-specific primers, as depicted in Fig. 1a. Furthermore, the expression of NDV NP and M proteins was confirmed by western blotting using α-GFP antibody (Fig. 1b). The expression of GFP fused NP and M protein of sizes ~80 and ~ 67 kDa, respectively, was observed whereas a band around ~ 25 kDa in lane pUG35 and pUG36 indicates expression of only GFP in empty vector transformed cells (Fig. 1b). The small difference in the size of GFP band observed in the vectors is due to additional buffer nucleotides for fusion with GFP. Subsequently, the expression of GFP-tagged proteins was also confirmed by fluorescence microscopy. Cytosolic distribution of GFP was observed in cells expressing empty vectors, whereas cells expressing NP-GFP and GFP-NP showed discrete GFP puncta which were variable in number per cell. On the other hand, cells expressing M-GFP had a mixed population with a percentage of cells exhibiting cytosolic fluorescence and concentrated GFP fluorescence. This GFP fluorescence was not like the discrete puncta observed in NP expressing cells (Fig. 1c).
Fig. 1.
Expression of GFP-tagged NDV NP and M proteins in yeast. Yeast RNA was isolated and used for RT-PCR to confirm transcription of viral proteins (a). TCA extracts of cell lysates expressing NDV NP-GFP, GFP-NP, M-GFP, pUG35, pUG36 and WT cells without any plasmid were analyzed by western blotting using α-GFP (b). A single band corresponding to the size of the respective proteins is observed. The panel below represents actin used as a loading control. (c) Fluorescence microscopy images of the cells expressing the above-mentioned plasmids. Upper panel represents GFP signal, and lower panel is the bright field image. Scale bar represents 5 µm
NDV M protein is localized to the nucleus and NP is localized to the vacuolar membrane when expressed in yeast cells
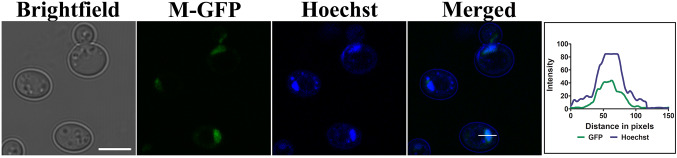
Transient localization of M protein to the nucleus in cell lines has been reported (Duan et al. 2014). To analyze whether the M-GFP fusion protein expressed in yeast cells is functional and localized to the nucleus, M-GFP expressing cells were stained with Hoechst dye that selectively binds to DNA and labels nucleus in yeast cells (Fig. 2). As mentioned above, M-GFP showed both cytosolic and discretely concentrated GFP fluorescence (Fig. 1c). The concentrated GFP fluorescence in the cells colocalized with the Hoechst dye suggesting nuclear localization of M-GFP in these cells. An intensity line profile representing colocalization of blue and green signals is shown in Fig. 2.
Fig. 2.
Subcellular localization of M-GFP in yeast cells. M-GFP expressing cells were incubated with Hoechst (1 μg/μl), a nuclear staining dye for 1 h at 30 °C. Samples were subsequently analyzed by confocal microscopy. Scale bar represents 5 µm. Line drawn in merged panel is analyzed for the colocalization of GFP signal and Hoechst signal (blue). An overlap between the two signals in the intensity profile obtained from the microscopy image (line drawn) is also depicted
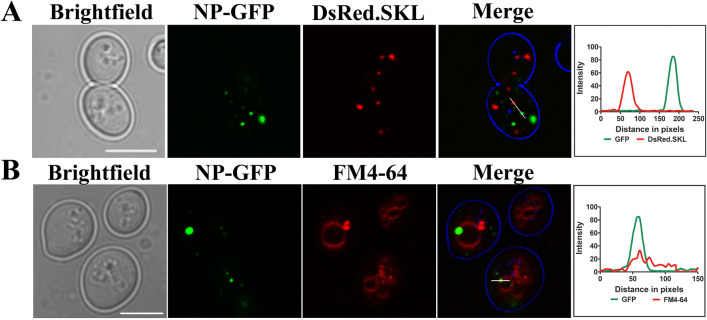
As an N-terminal PTS2 targeting signal for NDV NP protein was reported in the literature (Mohan and Atreya 2003), C-terminal GFP fusion protein was analyzed for its peroxisomal localization. To mark peroxisomes, these cells were further transformed with a plasmid expressing DsRed-SKL that is selectively targeted to peroxisomes. No colocalization with the peroxisomal marker could be detected as depicted in Fig. 3a. However, a fraction of NP-GFP was observed to colocalize with FM4-64 dye that stains vacuole (Fig. 3b). Intensity profile generated using ImageJ depicts colocalization of green and red signals (Fig. 3b).
Fig. 3.
Subcellular localization of NP-GFP in yeast cells. (a) NP-GFP expressing cells co-transformed with DsRed-SKL plasmid to visualize peroxisomes. No colocalization, as also depicted by intensity profile generated by ImageJ is observed. NP-GFP expressing cells were incubated with FM4-64 (1 μg/μl), a vacuolar membrane staining dye for 1 h at 30 °C. Samples were subsequently analyzed by confocal microscopy. It is to be noted that not all NP-GFP puncta colocalize with the vacuolar marker. Lines drawn in merged panel are analyzed for the colocalization of GFP signal and DsRed.SKL or FM4-64 signal (red) in (a) and (b). Scale bar represents 5 µm. An overlap in the intensity profile obtained from the microscopy image is also depicted
Expression of NP and M proteins does not alter the morphology of ER, mitochondria, nucleus and peroxisomes in yeast cells
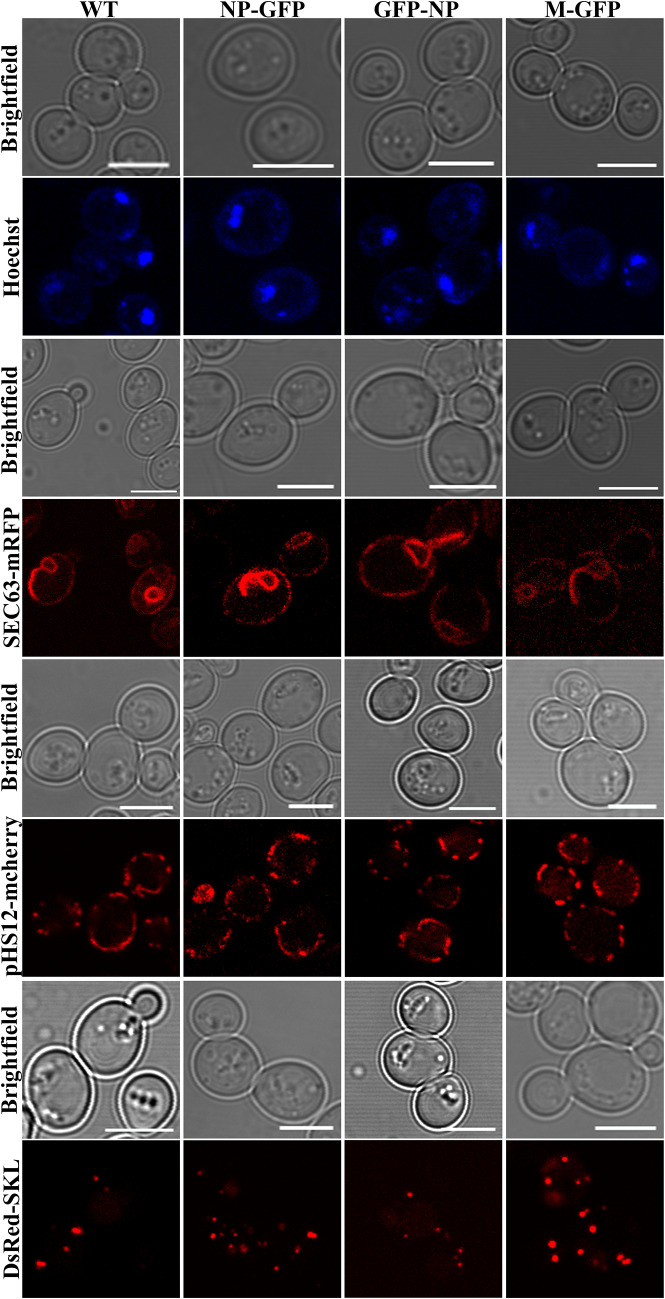
Recent studies have reported altered organelle dynamics and morphology upon viral infection or expression of viral proteins in various host organisms, including S. cerevisiae (Glingston et al. 2019). To analyze whether the expression of NP and M proteins altered organelle morphology, we either co-transformed yeast cells expressing viral proteins with plasmids that selectively mark the organelles or stained the organelle with fluorescent dyes. Sec63-mRFP, pHS12-mCherry and DsRed-SKL were used to mark ER, mitochondria and peroxisomes, respectively. To analyze nuclear morphology, cells expressing the viral proteins were stained with Hoechst dye. The above cells were cultured till they reached exponential growth phase and were analyzed for organelle morphology by confocal microscopy. The morphology of the above organelles in the viral protein expressing cells was observed to be unaltered and was similar as in control cells (Fig. 4). The morphology and number of the organelles in the late stationary phase was also similar to that in the control cells emphasizing no effect of the viral proteins.
Fig. 4.
Effect of expression of NP-GFP, GFP-NP and M-GFP on cell organelles in yeast. Cells expressing the viral proteins were co-transformed with plasmids Sec63-mRFP, pHS12-mCherry, DsRed-SKL to mark endoplasmic reticulum, mitochondria and peroxisomes, respectively. Nucleus was visualized using Hoechst dye (1 μg/μl). The corresponding bright field image is shown in each panel. Scale bar represents 5 µm
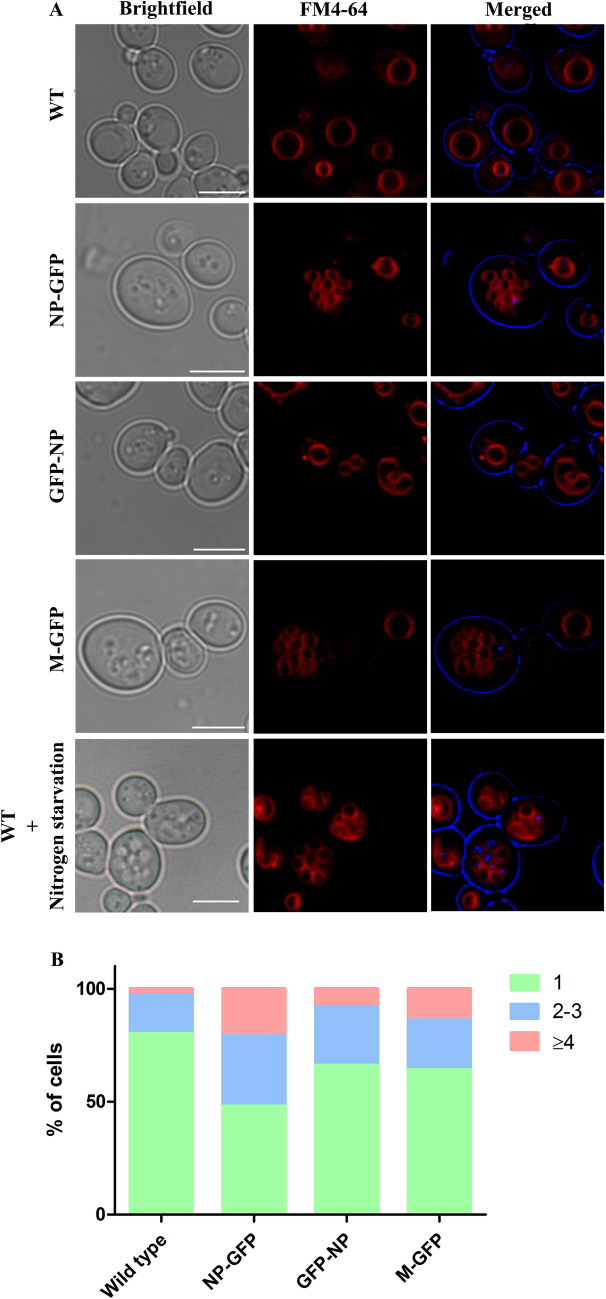
Expression of NP and M proteins alters the morphology and number of vacuoles in yeast cells
We used the lipophilic membrane dye FM4-64 that selectively labels vacuoles in yeast cells to analyze the effect of NP and M proteins. Cells expressing the viral proteins showed an interesting vacuole phenotype. Control cells depicted single large vacuoles in most of the cells (Fig. 5a,b). However, a significant fraction of the viral protein expressing cells showed an increased number of vacuoles (typically between 4–8/cell). Quantitative data showed a significant number of cells with three or more vacuoles in both NP-GFP and M-GFP expressing cells (Fig. 5b). For this, vacuoles in 100 cells from each strain were counted from respective confocal images. A significant increase in the number of vacuoles can be observed in NP and M expressing cells. The percentage of cells with three or more vacuoles was observed to be 34% in NP-GFP expressing cells, 16% in GFP-NP and 20% in M-GFP cells, respectively, as compared to the WT cells (8% cells with 3 or more vacuoles). Interestingly, this phenotype was also observed when WT yeast cells were shifted to nitrogen-starvation growth media which induces autophagy in these cells (Torggler et al. 2017).
Fig. 5.
Effect of expression of NP-GFP, GFP-NP and M-GFP on vacuoles in yeast. WT, NP-GFP, GFP-NP and M-GFP expressing cells were grown in YND media and stained with FM4-64 (1 μg/μl) to mark vacuoles and samples were subsequently analyzed by confocal microscopy. WT cells grown in nitrogen starvation medium are also depicted in the last panel. The corresponding bright field image is shown in each panel. Scale bar represents 5 µm. The graph represents percentage of cells with 1, 2–3 and 4 or > 4 vacuoles
Effect of NP and M on growth, viability and oxidative stress of yeast cells
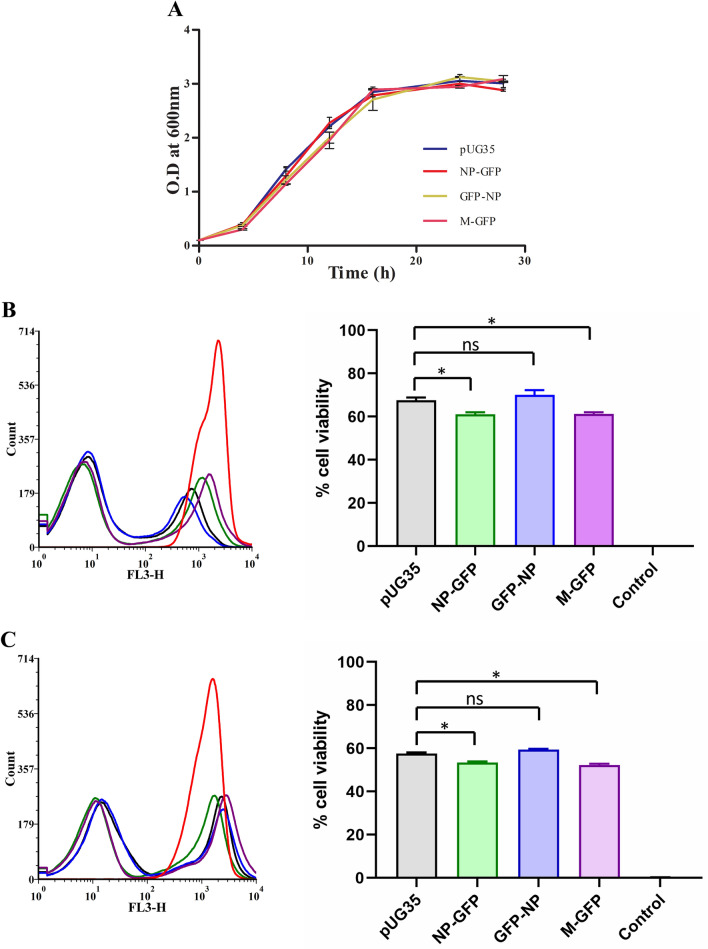
To further understand the effects caused by the expression of NDV NP and M proteins on yeast cells, we analyzed several parameters such as growth kinetics, cell viability, and reactive oxygen species (ROS) accumulation in these cells. The growth rate of NDV NP and M expressing yeast strains was analyzed compared to WT and WT expressing empty vectors. All the strains were grown in YND media and their optical density was measured every 2 h till they reached the stationary phase. Cells expressing viral proteins exhibited similar growth kinetics as observed for cells expressing empty vector (Fig. 6a). (Doubling time in hrs: pUG35—3.14, NP-GFP—3.17, GFP-NP—3.32, M-GFP—2.9).
Fig. 6.
Effect of expression of NP and M proteins on cell growth and viability. The effect of expression of NP and M proteins on yeast growth was assessed by plotting a growth curve. Cell growth was measured spectrophotometrically at OD 600 over the indicated time (a). Cell viability was analyzed quantitatively by flow cytometry of cells cultured for 24 (b) and 48 h (c). Cells expressing the viral proteins and empty vector were stained with PI for the analysis. A sample containing cells killed by heat shock (95 °C, 5 min) was used as a control (PC) for PI staining. Quantitative data obtained from flow cytometry is depicted as histograms and % cell viability as bar graphs. Error bars show the absolute deviations from the mean value. Significance was determined using one-way ANOVA. Values of p > 0.05 were considered nonsignificant (ns), p < 0.05 were considered significant (*)
Effect on cell viability was analyzed using PI which selectively stained dead cells. Quantitative analysis was performed using flow cytometry. To do this, WT cells expressing empty vector, NP and M proteins, respectively, were cultured in YND medium and samples were taken after 24 and 48 h. WT cells incubated at 95 °C for 5 min were taken as a positive control for PI staining. Samples were subsequently incubated with PI for 30 min. Figure 6b,c shows histogram and bar graph representing flow cytometry data obtained from 24 and 48 h sample, respectively. Statistical analysis shows that the cell viability of NP-GFP and M-GFP expressing cells was significantly different (p < 0.05) when compared to empty vector expressing cells in both 24 and 48-h samples (Fig. 6b, c).
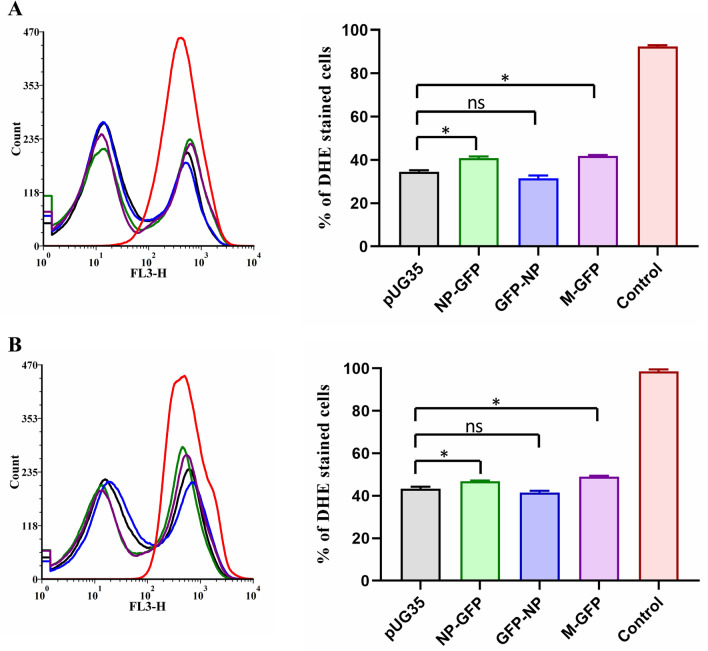
Further, to analyze whether the expression of the viral proteins altered cellular ROS levels, cells cultured for 24 and 48 h were stained with DHE dye. S. cerevisiae cells deleted for the enzyme catalase (ctt1) were taken as a positive control, and ROS accumulation was induced by treating these cells with hydrogen peroxide (Fig. 7). Figure 7a, b shows histogram and bar graph representing flow cytometry data obtained from 24 and 48-h samples, respectively. Statistical analysis shows that the DHE staining of NP-GFP and M-GFP expressing cells was significantly different (p < 0.05) when compared to empty vector expressing cells in both 24 and 48-h samples (Fig. 7a,b).
Fig. 7.
Effect of expression of NP and M proteins on oxidative stress. To assess the oxidative stress, cells cultured for 24 and 48 h were stained with DHE and analyzed by flow cytometry (a, b). ctt1 cells were taken as a control (PC). Quantitative data obtained from flow cytometry are depicted as histograms and % DHE stained cells as bar graphs. Error bars show the absolute deviations from the mean value. Significance was determined using one-way ANOVA. Values of p > 0.05 were considered nonsignificant (ns); those of p < 0.05 were considered significant (*)
Discussion
NDV causes a highly contagious respiratory and neurological disease in many avian species and hence is very important for the agricultural sector (Alexander 2009). Replication of several + RNA viruses such as the brome mosaic virus, tomato bushy stunt virus (plant viruses), flock house virus and nodamuravirus (animal viruses) has been reported in yeast cells (Kushner et al. 2003; Panavas et al. 2005). Intracellular localization of several viral proteins has also been studied extensively in yeast. Interaction of each viral protein with host cellular proteins at defined subcellular sites ensures successful completion of the viral life cycle. However, studies on negative sense RNA viruses in yeast model are limited (Naito et al. 2007). NP proteins of mumps and measles virus which also belong to paramyxoviridae tagged with GFP have been expressed in S. cerevisiae. Interestingly, a C-terminal GFP fusion protein has been reported to be functional in these studies (Slibinskas et al. 2004). Expression of these proteins did not have any effect on cell growth (Čiplys et al. 2011). Yeast has been successfully used for the expression and purification of NDV proteins in earlier studies (Iram et al. 2014; Khulape et al. 2015). To the best of our knowledge, yeast as a host to study molecular details such as effect on host organelles upon expression of NDV proteins has not been explored and hence our work also aims at expanding the repertoire of use of yeast as a model for virus study.
Our goal in this study was to clone, express and carry out functional analysis of NP and M proteins of NDV in yeast. Subcellular localization and effects of these viral proteins on yeast cell growth, ROS accumulation and viability were studied. Our data show that NP-GFP and M-GFP fusions were successfully expressed in S. cerevisiae and this heterologous expression resulted in reduced cell viability and increased ROS accumulation when compared to control cells. The most interesting phenotype observed was the fragmentation of vacuoles upon expression of NP-GFP and M-GFP. The morphology of the vacuole in yeast is responsive to cellular stress and is mediated via an alteration in the equilibrium between fission and fusion processes. Recent literature also shows induction of ER stress upon expression of NDV NP protein (Cheng et al. 2016). From studies in yeast, it is known that ER stress can induce vacuole fragmentation (Stauffer and Powers 2015). On the other hand, several mutants involved in autophagy also exhibit increased fragmentation of vacuoles (Boutouja et al. 2019). Presumably, our observation of vacuole fragmentation upon expression of NP and M proteins could be due to one of these important cellular pathways. Activation of both autophagic and apoptotic pathways upon NDV infection has been reported. This lytic nature of NDV has been extensively studied in cancer, and several studies report its specific oncolytic nature (Zamarin and Palese 2012). However, the mechanism of selective infection is not completely understood. Interestingly, upregulation of host translation by activating PI3K/Akt/mTOR and p38 MAPK/Mnk1 pathways upon NDV infection has also been reported (Zhan et al. 2020). Hence, understanding the cellular effects can also be important to decipher the oncolytic properties of this virus.
Although viral RNA synthesis and replication of paramyxoviruses takes place in the cytoplasm, some of the viral proteins are reported to localize in the nucleus at early stages of virus infection. M protein is one such example studied in various viruses such as Sendai virus, NiV, NDV, MeV and MuV (Audsley et al. 2016). Transient nuclear localization of M during an early infection and in the cytoplasm later was reported for HRSV, NDV and Sendai virus (Ghildyal et al. 2003; Peeples 1988; Peeples et al. 1992; Yoshida et al. 1979). In our study, M protein was observed to localize clearly to the nucleus in a fraction of cells and was also visualized in the cytosol. Our results suggest that the localization of M protein in yeast cells was similar to that observed in mammalian cells. Perhaps the nuclear localization of NDV M protein may alter host nuclear components and modulates the cellular proteins thereby promoting viral RNA replication (Duan et al. 2014). NP-GFP fusion protein, on the other hand, was observed to localize to the vacuolar membrane in a fraction of cells. NP was reported to have a PTS2 signal (Mohan and Atreya 2003) albeit no peroxisomal localization was observed in our studies. However, several proteins with similar behavior, i.e., no localization despite having a PTS2, are reported in the literature (de Hoop and Ab 1992; Subramani 1998).
The association of organelle dysfunction with human diseases caused due to several viruses has been widely reported in recent studies. Studying virus–host interactions and the resulted cellular remodeling will greatly improve our understanding of the replication and pathogenesis of viruses. However, it may always not be possible to use the natural host for such studies and hence alternative host models prove to be valuable in such cases. A more comprehensive understanding of the molecular biology of viruses and their dependence on host organelles is of utmost priority for development of broad-spectrum and specific anti-viral strategies.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Prof. Johannes Hegemann for providing us with pUG35 and pUG36 plasmids. We also thank NIPER Guwahati for their confocal microscopy facility.
Funding
Research in the laboratory of SN is supported by grants from Department of Biotechnology (DBT), Government of India (BT/PR16325/NER/95/117/2015; BT/PR25097/NER/95/1013/2017), and SK is supported by DBT, Government of India (BT/562/NE/U-Excel/2016 and BT/PR24308/NER/95/644/2017).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Sachin Kumar, Email: sachinku@iitg.ac.in.
Shirisha Nagotu, Email: snagotu@iitg.ac.in.
References
- Alexander DJ. Ecology and epidemiology of newcastle disease. In: Capua I, Alexander DJ, editors. Avian influenza and newcastle disease: a field and laboratory manual. Milano: Springer Milan; 2009. pp. 19–26. [Google Scholar]
- Audsley MD, Jans DA, Moseley GW. Roles of nuclear trafficking in infection by cytoplasmic negative-strand RNA viruses: paramyxoviruses and beyond. J Gen Virol. 2016;97:2463–2481. doi: 10.1099/jgv.0.000575. [DOI] [PubMed] [Google Scholar]
- Baerends RJS, Faber KN, Kram AM, Kiel JAKW, van der Klei IJ, Veenhuis M. A stretch of positively charged amino acids at the N terminus of Hansenula polymorpha Pex3p is involved in incorporation of the protein into the peroxisomal membrane. J Biol Chem. 2000;275:9986–9995. doi: 10.1074/jbc.275.14.9986. [DOI] [PubMed] [Google Scholar]
- Boutouja F, Stiehm CM, Reidick C, Mastalski T, Brinkmeier R, Magraoui FE, Platta HW. Vac8 controls vacuolar membrane dynamics during different autophagy pathways in Saccharomyces cerevisiae. Cells. 2019;8:661. doi: 10.3390/cells8070661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JH, Sun YJ, Zhang FQ, Zhang XR, Qiu XS, Yu LP, Wu YT, Ding C. Newcastle disease virus NP and P proteins induce autophagy via the endoplasmic reticulum stress-related unfolded protein response. Sci Rep. 2016;6:24721. doi: 10.1038/srep24721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Čiplys E, Samuel D, Juozapaitis M, Sasnauskas K, Slibinskas R. Overexpression of human virus surface glycoprotein precursors induces cytosolic unfolded protein response in Saccharomyces cerevisiae. Microb Cell Factor. 2011;10:37. doi: 10.1186/1475-2859-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman NA, Peeples ME. The matrix protein of Newcastle disease virus localizes to the nucleus via a bipartite nuclear localization signal. Virology. 1993;195:596–607. doi: 10.1006/viro.1993.1411. [DOI] [PubMed] [Google Scholar]
- de Hoop MJ, Ab G. Import of proteins into peroxisomes and other microbodies. Biochem J. 1992;286(Pt 3):657–669. doi: 10.1042/bj2860657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Z, Chen J, Xu H, Zhu J, Li Q, He L, Liu H, Hu S, Liu X. The nucleolar phosphoprotein B23 targets Newcastle disease virus matrix protein to the nucleoli and facilitates viral replication. Virology. 2014;452–453:212–222. doi: 10.1016/j.virol.2014.01.011. [DOI] [PubMed] [Google Scholar]
- Ganar K, Das M, Sinha S, Kumar S. Newcastle disease virus: current status and our understanding. Virus Res. 2014;184:71–81. doi: 10.1016/j.virusres.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghildyal R, Baulch-Brown C, Mills J, Meanger J. The matrix protein of human respiratory syncytial virus localises to the nucleus of infected cells and inhibits transcription. Arch Virol. 2003;148:1419–1429. doi: 10.1007/s00705-003-0112-y. [DOI] [PubMed] [Google Scholar]
- Gietz RD, Schiestl RH, Willems AR, Woods RA. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast. 1995;11:355–360. doi: 10.1002/yea.320110408. [DOI] [PubMed] [Google Scholar]
- Glingston RS, Deb R, Kumar S, Nagotu S. Organelle dynamics and viral infections: at cross roads. Microbes Infect. 2019;21:20–32. doi: 10.1016/j.micinf.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould SJ, Keller GA, Subramani S. Identification of peroxisomal targeting signals located at the carboxy terminus of four peroxisomal proteins. J Cell Biol. 1988;107:897–905. doi: 10.1083/jcb.107.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer-Hansen M, Jaattela M. Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ. 2007;14:1576–1582. doi: 10.1038/sj.cdd.4402200. [DOI] [PubMed] [Google Scholar]
- Iram N, Shah MS, Ismat F, Habib M, Iqbal M, Hasnain SS, Rahman M. Heterologous expression, characterization and evaluation of the matrix protein from Newcastle disease virus as a target for antiviral therapies. Appl Microbiol Biotechnol. 2014;98:1691–1701. doi: 10.1007/s00253-013-5043-2. [DOI] [PubMed] [Google Scholar]
- Khulape SA, Maity HK, Pathak DC, Mohan CM, Dey S. Antigenic validation of recombinant hemagglutinin-neuraminidase protein of Newcastle disease virus expressed in Saccharomyces cerevisiae. Acta Virol. 2015;59:240–246. doi: 10.4149/av_2015_03_240. [DOI] [PubMed] [Google Scholar]
- Kuravi K, Nagotu S, Krikken AM, Sjollema K, Deckers M, Erdmann R, Veenhuis M, van der Klei IJ. Dynamin-related proteins Vps1p and Dnm1p control peroxisome abundance in Saccharomyces cerevisiae. J Cell Sci. 2006;119:3994–4001. doi: 10.1242/jcs.03166. [DOI] [PubMed] [Google Scholar]
- Kushner DB, Lindenbach BD, Grdzelishvili VZ, Noueiry AO, Paul SM, Ahlquist P. Systematic, genome-wide identification of host genes affecting replication of a positive-strand RNA virus. Proc Natl Acad Sci USA. 2003;100:15764–15769. doi: 10.1073/pnas.2536857100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lametschwandtner G, Brocard C, Fransen M, Van Veldhoven P, Berger J, Hartig A. The difference in recognition of terminal tripeptides as peroxisomal targeting signal 1 between yeast and human is due to different affinities of their receptor Pex5p to the cognate signal and to residues adjacent to it. J Biol Chem. 1998;273:33635–33643. doi: 10.1074/jbc.273.50.33635. [DOI] [PubMed] [Google Scholar]
- Li J, Ni M, Lee B, Barron E, Hinton DR, Lee AS. The unfolded protein response regulator GRP78/BiP is required for endoplasmic reticulum integrity and stress-induced autophagy in mammalian cells. Cell Death Differ. 2008;15:1460–1471. doi: 10.1038/cdd.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger MB, Maurer MJ, Dancy BM, Michaelis S. Degradation of a cytosolic protein requires endoplasmic reticulum-associated degradation machinery. J Biol Chem. 2008;283:32302–32316. doi: 10.1074/jbc.M806424200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan KVK, Atreya CD. Novel organelle-targeting signals in viral proteins. Bioinformatics. 2003;19:10–13. doi: 10.1093/bioinformatics/19.1.10. [DOI] [PubMed] [Google Scholar]
- Naito T, Kiyasu Y, Sugiyama K, Kimura A, Nakano R, Matsukage A, Nagata K. An influenza virus replicon system in yeast identified Tat-SF1 as a stimulatory host factor for viral RNA synthesis. Proc Natl Acad Sci USA. 2007;104:18235–18240. doi: 10.1073/pnas.0705856104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogali IN, Wamuyu LW, Lichoti JK, Mungube EO, Agwanda B, Ommeh SC. Molecular characterization of Newcastle disease virus from backyard poultry farms and live bird markets in Kenya. Int J Microbiol. 2018;2018:2368597. doi: 10.1155/2018/2368597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier LM, Kovacs W, Masuda K, Keller GA, Krisans SK. Identification of peroxisomal targeting signals in cholesterol biosynthetic enzymes. AA-CoA thiolase, hmg-coa synthase, MPPD, and FPP synthase. J Lipid Res. 2000;41:1921–1935. doi: 10.1016/S0022-2275(20)32353-1. [DOI] [PubMed] [Google Scholar]
- Panavas T, Serviene E, Brasher J, Nagy PD. Yeast genome-wide screen reveals dissimilar sets of host genes affecting replication of RNA viruses. Proc Natl Acad Sci USA. 2005;102:7326–7331. doi: 10.1073/pnas.0502604102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeples ME. Newcastle disease virus replication. In: Alexander DJ, editor. Newcastle disease. Boston, MA: Springer US; 1988. pp. 45–78. [Google Scholar]
- Peeples ME, Wang C, Gupta KC, Coleman N. Nuclear entry and nucleolar localization of the Newcastle disease virus (NDV) matrix protein occur early in infection and do not require other NDV proteins. J Virol. 1992;66:3263–3269. doi: 10.1128/JVI.66.5.3263-3269.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindra PV, Tiwari AK, Ratta B, Chaturvedi U, Palia SK, Chauhan RS. Newcastle disease virus-induced cytopathic effect in infected cells is caused by apoptosis. Virus Res. 2009;141:13–20. doi: 10.1016/j.virusres.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Slibinskas R, Samuel D, Gedvilaite A, Staniulis J, Sasnauskas K. Synthesis of the measles virus nucleoprotein in yeast Pichia pastoris and Saccharomyces cerevisiae. J Biotechnol. 2004;107:115–124. doi: 10.1016/j.jbiotec.2003.10.018. [DOI] [PubMed] [Google Scholar]
- Stauffer B, Powers T. Target of rapamycin signaling mediates vacuolar fission caused by endoplasmic reticulum stress in Saccharomyces cerevisiae. Mol Biol Cell. 2015;26:4618–4630. doi: 10.1091/mbc.E15-06-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramani S. Components involved in peroxisome import, biogenesis, proliferation, turnover, and movement. Physiol Rev. 1998;78:171–188. doi: 10.1152/physrev.1998.78.1.171. [DOI] [PubMed] [Google Scholar]
- Sun Y, Yu S, Ding N, Meng C, Meng S, Zhang S, Zhan Y, Qiu X, Tan L, Chen H, Song C, Ding C. Autophagy benefits the replication of Newcastle disease virus in chicken cells and tissues. J Virol. 2014;88:525–537. doi: 10.1128/JVI.01849-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinkels BW, Gould SJ, Bodnar AG, Rachubinski RA, Subramani S. A novel, cleavable peroxisomal targeting signal at the amino-terminus of the rat 3-ketoacyl-CoA thiolase. EMBO J. 1991;10:3255–3262. doi: 10.1002/j.1460-2075.1991.tb04889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terlecky SR, Nuttley WM, Subramani S. The cytosolic and membrane components required for peroxisomal protein import. Experientia. 1996;52:1050–1054. doi: 10.1007/bf01952101. [DOI] [PubMed] [Google Scholar]
- Torggler R, Papinski D, Kraft C. Assays to monitor autophagy in Saccharomyces cerevisiae. Cells. 2017;6:23. doi: 10.3390/cells6030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Nagai Y, Maeno K, Iinuma M, Hamaguchi M, Matsumoto T, Nagayoshi S, Hoshino M. Studies on the role of M protein in virus assembly using a is mutant of HVJ (Sendai virus) Virology. 1979;92:139–154. doi: 10.1016/0042-6822(79)90220-4. [DOI] [PubMed] [Google Scholar]
- You J, Hou S, Malik-Soni N, Xu Z, Kumar A, Rachubinski RA, Frappier L, Hobman TC. Flavivirus infection impairs peroxisome biogenesis and early antiviral signaling. J Virol. 2015;89:12349–12361. doi: 10.1128/jvi.01365-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamarin D, Palese P. Oncolytic Newcastle disease virus for cancer therapy: old challenges and new directions. Future Microbiol. 2012;7:347–367. doi: 10.2217/fmb.12.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y, Yu S, Yang S, Qui X, Meng C, Tan L, Song C, Liao Y, Liu W, Sun Y. Newcastle disease virus infection activates PI3K/Akt/mTOR and p38 MAPK/Mnk1 pathways to benefit viral mRNA translation via interaction of the viral NP protein and host eIF4E. PLoS Pathog. 2020;16:e1008610. doi: 10.1371/journal.ppat.1008610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, 2017 Zhao RY (2017) Yeast for virus research. Microbiol Cell 4:311-330. 10.15698/mic2017.10.592 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.