Abstract
Objectives
To investigate the influence of pregnancy on patients with neuromyelitis optica spectrum disorder (NMOSD) and to identify risk factors that predict pregnancy-related attack.
Methods
From January 2015 to April 2019, 418 female patients with NMOSD were registered at Huashan Hospital. We retrospectively reviewed their medical records and identified 110 patients with 136 informative pregnancies, of whom 83 were aquaporin-4 antibody (AQP4-ab)-positive and 21 were myelin oligodendrocyte glycoprotein-antibody-positive. Pregnancy-related attack was defined as an attack that occurred during pregnancy or within 1 year after delivery/abortion. We compared annualised relapse rate (ARR) during 12 months before pregnancy with that during every trimester of pregnancy and after delivery/abortion. Multivariate analyses were used to explore the independent risk factors involved and a nomogram was generated for the prediction of pregnancy-related attack. Thirty-five female patients from 3 other centres formed an external cohort to validate this nomogram.
Results
ARR increased significantly during the first trimester after delivery (p<0.001) or abortion (p=0.019) compared with that before pregnancy. Independent risk factors predicting pregnancy-related attack included age at delivery/abortion (20–26.5, p=0.018; 26.5–33, p=0.001), AQP4-ab titre (≥1:100, p=0.049) and inadequate treatment during pregnancy and postpartum period (p=0.004). The concordance index of nomogram was 0.87 and 0.77 using bootstrap resampling in internal and external validation.
Conclusions
The first trimester post partum is a high-risk period for NMOSD recurrence. Patients with younger age, higher AQP4-ab titre and inadequate treatment are at higher risk for pregnancy-related attack.
Introduction
Neuromyelitis optica spectrum disorders (NMOSD) reflect categories of auto-antibody-induced inflammatory diseases of the central nervous system (CNS)—predominantly involving optic nerves, spinal cord, and brainstem—leading to blindness and paralysis.1 The presence of pathogenic aquaporin-4 antibody (AQP4-ab) in serum is highly specific for NMOSD and found in most patients with NMOSD.2 Serum myelin oligodendrocyte glycoprotein antibody (MOG-ab) has also been recently detected in a portion of patients with AQP4-ab-negative NMOSD.3
NMOSD principally affects women, many of whom develop active disease during childbearing years. Clinical and experimental studies have illustrated that AQP4 is expressed on human and animal placenta, and have associated AQP4-mediated placental inflammation with fetal death.4 5 Unlike MS, a lack of reduction in relapses during pregnancy is observed in NMOSD.6 7 Previous studies have demonstrated that the annualised relapse rate (ARR) of patients with NMOSD increased specifically during the first 3 months post partum,8–12 correlating with high rates of miscarriage.10 Consequently, disability worsening during and after pregnancy has been reported.6 11–14 NMOSD is considered to be the most common type of CNS inflammatory demyelinating disease in China, whereas multiple sclerosis (MS) is relatively rare. However, information regarding pregnancy-related attack in Chinese patients with NMOSD is still lacking.
In the present study, we aimed to investigate the influence of pregnancy on Chinese patients with NMOSD and to identify independent risk factors that predict pregnancy-related attack.
Methods
Cohort and data collection
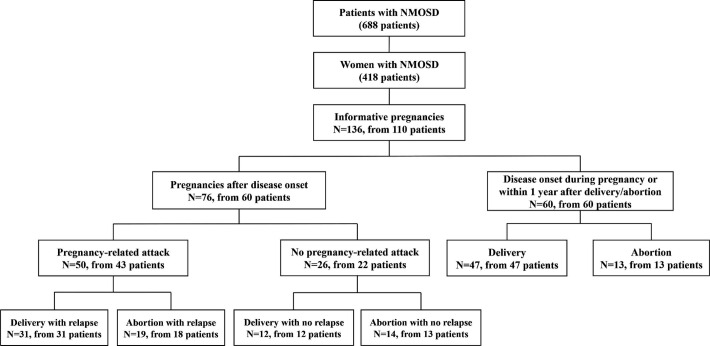
This is a retrospective data collection study. From January 2015 to April 2019, 688 consecutive patients with NMOSD were registered and followed up at Shanghai Huashan Hospital. Among them, 418 patients were female and their medical records were retrospectively reviewed. Altogether, 110 female patients with 136 pregnancies were collected and included in the study (figure 1). The inclusion criteria were: (1) female; (2) fulfil the diagnostic criteria of NMOSD established by the International Panel in 20151; (3) with informative pregnancies.
Figure 1.
Informative pregnancies from 110 female patients with NMOSD. N, number of pregnancies; NMOSD, neuromyelitis optica spectrum disorder.
The demographics and clinical data of the 110 female patients with informative pregnancies were collected. Among them, there were 76 pregnancies conceived after disease onset of 60 patients and 60 pregnancies of 60 patients with disease onset during pregnancy or within 1 year after delivery/abortion. Relapses were defined as new or worsening neurological symptoms lasting longer than 24 hours without other aetiology. Pregnancy-related attack was defined as an attack that occurred during pregnancy or within 1 year of delivery/abortion.7 Pregnancy complications and outcomes were collected.
For patients exhibiting pregnancy-related attacks after disease onset, the number of attacks was recorded during 12–0 months before pregnancy (BP); during the first (DP1), second (DP2) and third (DP3) trimesters of pregnancy and during 0–3 (PP1), 4–6 (PP2) and 7–12 months (PP3+4) after delivery/abortion. We compared the ARR and Expanded Disability Status Scale (EDSS) score during the baseline period of 12 months before pregnancy with that during every trimester of pregnancy and after delivery/abortion.
To identify the risk factors for pregnancy-related attacks, 60 patients with 76 pregnancies after disease onset from Huashan Hospital were included in analysis as the primary cohort. To further verify the discrimination of the risk factors, 35 female patients with 44 pregnancies after disease onset from the Third Affiliated Hospital of Sun Yat-sen University (n=31), Sir Run Run Shaw Hospital (n=7) and the First Affiliated Hospital of Wenzhou Medical University (n=6) were included as the external validation cohort.
All patients at Huashan Hospital and patients in the validation cohort from other three centres had undergone serum AQP4-ab and MOG-ab detection using the same fixed cell-based indirect immune-fluorescence test as part of a routine diagnostic approach. HEK293 cells transfected with either full-length human MOG or the M1 isoform of AQP4 were employed.
Statistical analysis
We performed statistical analyses with SPSS V.22.0 (SPSS, Chicago, Illinois, USA), and the figures were generated with GraphPad Prism 6 (GraphPad Software, La Jolla, California, USA). Continuous variables were expressed as means±1 SD or medians with ranges. The χ2 or Fisher’s exact test was used to compare the discrete variables, while a Student’s t-test or Mann-Whitney U test was employed to compare the quantitative data of the AQP4 and MOG cohort. A paired t-test or Wilcoxon signed-rank test was used to compare ARR and EDSS score during each pregnancy-related period with those before pregnancy, and participants with NMOSD served as their own controls for this comparison.
The association between maternal variables and counts of pregnancy-related attacks was evaluated using univariate Poisson regression, while the association between maternal variables and whether pregnancy-related attacks occurred was determined using univariate logistic regression. Based on clinical reasoning or previous reports,7–9 13 15 variables that were possible to associate with pregnancy-related attack were selected, including age at disease onset, age at delivery/abortion, time interval from disease onset to pregnancy, time interval from last attack to pregnancy, treatment during pregnancy and after delivery/abortion, AQP4-ab titre, relapse within 1 year before pregnancy, ARR before pregnancy, EDSS score before pregnancy and concomitant auto-antibodies. Variables related to a significant change (p<0.1) in the OR defined by univariate analysis were further analysed using multivariate Poisson or logistic regression.
Treatments during pregnancy and after delivery/abortion were classified as inadequate and adequate treatment. Inadequate treatment referred to (1) no treatment at all, (2) usage of low-dose oral prednisone (≤10 mg/day) as single therapy. Adequate treatment was defined as (1) usage of relatively higher dose oral prednisone (>10 mg/day), (2) usage of immunosuppressant (azathioprine 100 mg/day or tacrolimus 3 mg/day) combined with or without oral steroid, (3) a dose of rituximab (375 mg/m2) within 6 months before conception and shortly after delivery.
The first available AQP4-ab titre which was detected in a remission status was included in the univariate and multivariate analysis of potential risk factors.
We enhanced the predictive model of pregnancy-related attack with risk factors depicted in a nomogram using the rms package in R, V.3.6.2 (http://www.r-project.org/).16 Nomogram was generated according to the probability of the occurrence of pregnancy-related attack using multivariate logistic regression in the primary cohort and further validated in the external cohort. We employed the Concordance index (C-index) to then measure the performance of the nomogram, that is, a larger C-index referred to a higher degree of accuracy.17 Bootstrap with 1000 resampling was generated for the calibration curve and C-index in the primary and validation cohort as internal and external validation.18 Statistical significance was set at p<0.05.
Data availability
Anonymised data not exhibited in our study will be made available on request from any qualified investigator.
Results
Cohort study
Among the 110 female patients with 136 informative pregnancies, 83 were AQP4-ab-positive and 21 were MOG-ab-positive, no patients were positive for both antibodies. Of the 110 patients, 60 patients had disease onset during pregnancy or within 1 year after delivery/abortion (10 and 50 patients, respectively), with 42 AQP-4-ab-positive, 16 MOG-ab-positive and 2 seronegative subsets. Sixty patients underwent 76 pregnancies after disease onset; of these, 50 pregnancies from 43 patients had pregnancy-related attacks, with 31 patients having undergone 31 deliveries and 18 patients who underwent 19 abortions. Twenty-six pregnancies from 22 patients had no pregnancy-related attacks, with 12 patients underwent 12 deliveries and 13 patients underwent 14 abortions (figure 1).
The pregnancy-related characteristics of patients with different antibody subsets
The pregnancy-related characteristics of patients with AQP4-ab and MOG-ab are shown in table 1. There were 83 AQP4-ab-positive patients who experienced 108 informative pregnancies, with 126 pregnancy-related attacks, and their median AQP4-ab titre was 1:32 (range, 1:10–1:3200). Of these, the attacks during the 0–3 months after delivery/abortion were the most common, with 51 counts (40.5%). The pregnancy outcomes included 60 term deliveries, 5 premature deliveries, 39 elective abortions, 4 spontaneous abortions and 3 neonatal malformations—including undeveloped external ear, dacryocyst obstruction and scoliosis. We observed no pre-eclampsia during pregnancy. Of the 50 AQP4-ab-positive patients who underwent 66 pregnancies after disease onset, the mean adjusted ARR was 0.33 (95% CI 0.26 to 0.41) during 12–0 months before pregnancy and 0.69 (95% CI 0.61 to 0.78) during 0–12 months after pregnancy.
Table 1.
The pregnancy-related characteristics of patients with NMOSD with AQP4-ab and MOG-ab
| AQP4 cohort | MOG cohort | |
| Number of patients/number of patients with pregnancies after disease onset | 83/50 | 21/5 |
| Number of informative pregnancies/number of pregnancies after disease onset | 108/66 | 21/5 |
| Number of total pregnancy-related attacks | 126 | 28 |
| Age at disease onset, year, mean±SD | 25.8±6.3 | 26.7±5.9 |
| Age at delivery/abortion, year, mean±SD | 28.4±4.6 | 28.3±3.6 |
| AQP4-ab or MOG-ab titre, median (range) | 1:32 (1:10–1:3200) | 1:32 (1:10–1:320) |
| Number of pregnancy-related attacks (%) | ||
| DP1 | 13 (10.3) | 2 (7.1) |
| DP2 | 9 (7.1) | 1 (3.6) |
| DP3 | 4 (3.2) | 0 (0) |
| PP1 | 51 (40.5) | 11 (39.3) |
| PP2 | 25 (19.8) | 5 (17.9) |
| PP3+4 | 24 (19.0) | 9 (32.1) |
| Number of different pregnancy outcomes or complications (%) | ||
| Term delivery | 60 (55.6) | 18 (85.7) |
| Premature delivery | 5 (4.6) | 1 (4.8) |
| Elective abortion | 39 (36.1) | 2 (9.5) |
| Spontaneous abortion | 4 (3.7) | 0 (0) |
| Neonatal malformation | 3 (2.8) | 0 (0) |
| Pre-eclampsia | 0 (0) | 0 (0) |
| BP-ARR mean (95% CI) | 0.33 (0.19 to 0.47) | 0.40 (0.26 to 0.54) |
| Adjusted BP-ARR* mean (95% CI) | 0.33 (0.26 to 0.41) | – |
| PP-ARR mean (95% CI) | 0.65 (0.46 to 0.84) | 0.60 (0.38 to 0.82) |
| Adjusted PP-ARR* mean (95% CI) | 0.69 (0.61 to 0.78) | – |
*ARR was adjusted using a Poisson regression for treatment variables (inadequate or adequate treatment) during 12–0 months prepregnancy and during 0–12 months after delivery/abortion.
AQP4-ab, aquaporin-4 antibody; ARR, annualised relapse rate; BP, before pregnancy; DP, during pregnancy; MOG-ab, myelin oligodendrocyte glycoprotein antibody; NMOSD, neuromyelitis optica spectrum disorder; PP, postpartum period.;
There were 21 MOG-ab-positive patients experiencing 21 informative pregnancies, with 28 pregnancy-related attacks. Of these, the attacks during the first trimester post partum were also the most common, with 11 counts (39.3%). The pregnancy outcomes of the MOG subgroup included 18 term deliveries, 1 premature delivery and 2 elective abortions; no spontaneous abortion, neonatal malformations or pre-eclampsia were observed. The pregnancy outcomes of the remaining six seronegative patients were six term deliveries and one elective abortion.
Altogether there were 42 elective and 4 spontaneous abortions of the entire cohort. The reasons for elective abortions were NMOSD attack during pregnancy (n=14), unplanned pregnancy (n=13), concern over medication side effects (n=8), embryonic demise (n=3), abnormal prenatal screening (n=2), advanced age considered not appropriate for delivery (n=1) and poor physical condition (n=1). Spontaneous abortions were only seen in AQP4-ab-positive patients, and were considered to be induced by over fatigue (n=3) or of unknown reason (n=1).
ARR and EDSS score in each phase of pregnancies after disease onset
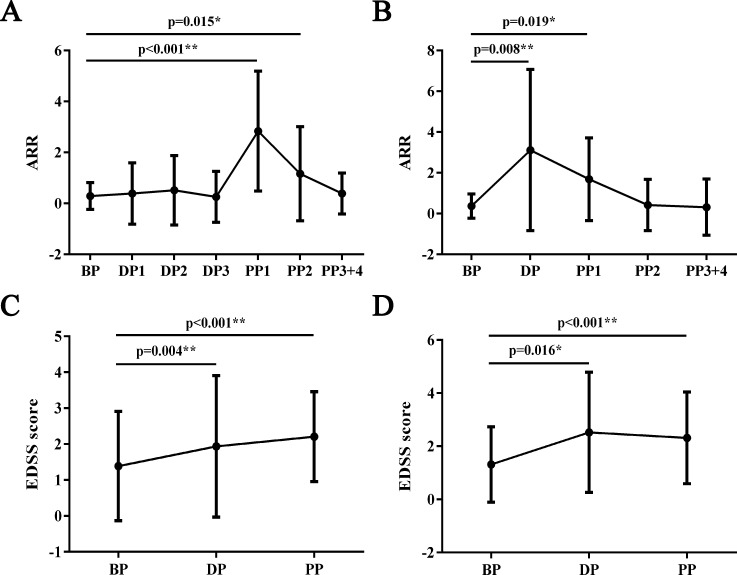
The demographics and clinical characteristics of patients with NMOSD who underwent pregnancies after disease onset in the primary and validation cohort are listed in table 2. The mean ARR and EDSS scores of each period from the 31 deliveries and 19 abortions in the primary cohort are shown in figure 2A–D. In the delivery group, ARR arose significantly during the first and second trimesters post partum compared with prepregnancy period (p<0.001 and p=0.015, respectively), while the ARR during other phases did not differ. The EDSS score increased significantly during pregnancy and within 1 year after delivery compared with that before pregnancy (p=0.004 and p<0.001, respectively). The ARR increased significantly during pregnancy and the first trimester after abortion compared with that before pregnancy (p=0.008 and p=0.019, respectively). Similarly, the EDSS score increased significantly during pregnancy and within 1 year after abortion compared with that before pregnancy (p=0.016 and p<0.001, respectively).
Table 2.
The demographics and clinical characteristics of patients with NMOSD with pregnancies after disease onset
| Primary cohort | Validation cohort | |
| Number of patients | 60 | 35 |
| Number of AQP4-ab/MOG-ab positivity | 50/5 | 33/2 |
| Number of pregnancies after disease onset | 76 | 44 |
| Time interval from disease onset to first relapse, m, median (range) | 15.0 (0–231.0) | 12.0 (0–108.0) |
| Age at disease onset, year, mean±SD | 23.8±6.3 | 23.5±5.4 |
| Age at delivery/abortion, year, mean±SD | 28.7±4.4 | 29.6±4.2 |
| Total number of pregnancy-related attacks | 69 | 44 |
| Type of pregnancy-related attack, n (%) | ||
| Optic neuritis | 31 (44.9) | 19 (45.5) |
| Acute myelitis | 35 (50.7) | 28 (63.6) |
| Area postrema syndrome | 4 (5.8) | 2 (4.5) |
| Acute brainstem syndrome | 4 (5.8) | 0 (0) |
| Concomitant auto-antibodies, n (%) | 26 (43.3) | 15 (42.9) |
| ANA | 22 (36.7) | 14 (40.0) |
| ENA-ab | 12 (20.0) | 5 (14.3) |
| dsDNA-ab | 1 (1.7) | 2 (5.7) |
| ANCA | 0 (0) | 0 (0) |
| ACA | 1 (1.7) | 0 (0) |
| TPO-ab and TG-ab | 13 (21.7) | 1 (2.9) |
| Treatment variables, n (%) | ||
| Adequate treatment* | 10 (13.2) | 13 (29.5) |
| Inadequate treatment† | 66 (86.8) | 31 (70.5) |
*Adequate treatment was defined as (1) usage of relatively higher dose oral prednisone (>10 mg/day), (2) usage of immunosuppressant (azathioprine 100 mg/day or tacrolimus 3 mg/day) combined with or without oral steroid, (3) a dose of rituximab (375 mg/m2) within 6 months before conception and shortly after delivery.
†Inadequate treatment referred to (1) no treatment at all, (2) usage of low-dose oral prednisone (≤10 mg/day) as single therapy.
ACA, anticardiolipin antibody; ANA, antinuclear antibody; ANCA, antineutrophil cytoplasmic antibody; AQP4-ab, aquaporin-4 antibody; dsDNA-ab, double-stranded DNA antibody; ENA-ab, extractable nuclear antigen antibody; MOG-ab, myelin oligodendrocyte glycoprotein antibody; NMOSD, neuromyelitis optica spectrum disorder; TG-ab, thyroglobulin antibody; TPO-ab, thyroid peroxidase antibody.
Figure 2.
ARRs and EDSS scores before, during and after pregnancy. (A) Mean ARRs for each phase of delivery. (B) Mean ARRs for each phase of abortion. (C) Mean EDSS scores for each phase of delivery. (D) Mean EDSS scores for each phase of abortion. A paired t-test or Wilcoxon signed-rank test was used to compare the ARRs and EDSS scores for each pregnancy-related period with those during BP. ARR, annualised relapse rate; BP, 12–0 months before pregnancy; DP, the period during pregnancy; DP1, 0–3 months during pregnancy; DP2, 3–6 months during pregnancy; DP3, 6–9 months during pregnancy; EDSS, Expanded Disability Status Scale; PP, 0–12 months of the postpartum period; PP1, 0–3 months of the postpartum period; PP2, 3–6 months of the postpartum period; PP3+4, 6–12 months of the postpartum period.
Risk factors and the prediction model of pregnancy-related attack
Fifty pregnancies with pregnancy-related attacks and 26 pregnancies without pregnancy-related attacks were included in the analyses of risk factors. Using univariate Poisson regression (table 3), we identified increased age at disease onset as being associated with a decreased OR for counts of pregnancy-related attacks (p=0.045); and age (20–26.5, 26.5–33) at delivery/abortion, inadequate treatment during pregnancy and after delivery/abortion, and high AQP4-ab titre (≥1:100) were associated with an increased OR of counts of pregnancy-related attacks (p=0.008, 0.009, 0.011 and 0.004, respectively). We observed no association between counts of pregnancy-related attacks and time interval from disease onset to pregnancy, time interval from last attack to pregnancy, relapse within 1 year before pregnancy, ARR before pregnancy, EDSS score before pregnancy or counts (≥1, ≥2) of concomitant autoimmune antibodies. Using multivariate Poisson regression, we further defined that age (20–26.5, 26.5–33) at delivery/abortion, inadequate treatment during pregnancy and after delivery/abortion and high AQP4-ab titre (≥1:100) were independently associated with an increased OR of counts of pregnancy-related attacks (p=0.028, 0.016, 0.014 and 0.036, respectively).
Table 3.
Risk factors of pregnancy-related attack using univariate and multivariate Poisson regression
| Variable (number of pregnancies) |
Univariate analysis | Multivariate analysis | ||
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age at disease onset (76) | 0.97 (0.94–1.00) | 0.045* | — | 0.787 |
| Age at delivery/abortion | ||||
| 33–40 (12) | 1.00 | 1.00 | ||
| 26.5–33 (39) | 12.62 (1.89–84.10) | 0.009** | 10.17 (1.55–66.62) | 0.016* |
| 20–26.5 (25) | 12.96 (1.93–87.01) | 0.008** | 8.45 (1.26–56.71) | 0.028* |
| Time interval from disease onset to pregnancy (76) | 1.01 (0.96–1.07) | 0.573 | — | — |
| Time interval from last attack to pregnancy (76) | 1.00 (1.00–1.01) | 0.253 | — | — |
| Treatment during pregnancy and after delivery/abortion | ||||
| Adequate treatment† (10) | 1.00 | 1.00 | ||
| Inadequate treatment‡ (66) | 5.07 (1.44–17.89) | 0.011* | 4.32 (1.34–13.94) | 0.014* |
| AQP4-ab titre | ||||
| <1:100 or negative (45) | 1.00 | 1.00 | ||
| ≥1:100 (31) | 1.89 (1.22–2.91) | 0.004** | 1.56 (1.03–2.37) | 0.036* |
| Relapse within 1 year before pregnancy | ||||
| No (58) | 1.00 | |||
| Yes (18) | 0.75 (0.39–1.42) | 0.376 | — | — |
| ARR before pregnancy (76) | 0.70 (0.41–1.20) | 0.198 | — | — |
| EDSS score before pregnancy (76) | 0.99 (0.82–1.20) | 0.952 | — | — |
| Counts of concomitant auto-antibodies§ | ||||
| <1 (42) | 1.00 | |||
| ≥1 (34) | 1.13 (0.72–1.79) | 0.593 | — | — |
| Counts of concomitant auto-antibodies§ | ||||
| <2 (51) | 1.00 | |||
| ≥2 (25) | 1.16 (0.71–1.90) | 0.558 | — | — |
*P<0.05; **p<0.01.
†Adequate treatment was defined as (1) usage of relatively higher dose oral prednisone (>10 mg/day), (2) usage of immunosuppressant (azathioprine 100 mg/day or tacrolimus 3 mg/day) combined with or without oral steroid, (3) a dose of rituximab (375 mg/m2) within 6 months before conception and shortly after delivery.
‡Inadequate treatment referred to (1) no treatment at all, (2) usage of low-dose oral prednisone (≤10 mg/day) as single therapy.
§Including antinuclear antibody, extractable nuclear antigen antibody, double-stranded DNA antibody, antineutrophil cytoplasmic antibody, anticardiolipin antibody, thyroid peroxidase antibody and thyroglobulin antibody.
AQP4-ab, aquaporin-4 antibody; ARR, annualised relapse rate; EDSS, Expanded Disability Status Scale.
Using univariate logistic regression (table 4), we identified increased age at disease onset as being associated with a decreased OR of the occurrence of pregnancy-related attacks (p=0.027). Age (20–26.5, 26.5–33) at delivery/abortion, inadequate treatment during pregnancy and after delivery/abortion and high AQP4-ab titre (≥1:100) were also associated with an increased OR of the occurrence of pregnancy-related attacks (p=0.002, 0.001, 0.005 and 0.008, respectively). Multivariate logistic regression further demonstrated that age (20–26.5, 26.5–33) at delivery/abortion, inadequate treatment during pregnancy and after delivery/abortion and high AQP4-ab titre (≥1:100) were independently associated with an increased OR of the occurrence of pregnancy-related attacks (p=0.018, 0.001, 0.004 and 0.049, respectively).
Table 4.
Risk factors of pregnancy-related attack using univariate and multivariate logistic regression
| Variable (number of pregnancies) |
Univariate analysis | Multivariate analysis | ||
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age at disease onset (76) | 0.90 (0.83–0.99) | 0.027* | — | 0.799 |
| Age at delivery/abortion | ||||
| 33–40 (12) | 1.00 | 1.00 | ||
| 26.5–33 (39) | 36.67 (4.15–323.86) | 0.001** | 43.61 (4.26–446.91) | 0.001** |
| 20–26.5 (25) | 34.83 (3.70–328.33) | 0.002** | 17.32 (1.64–182.83) | 0.018* |
| Time interval from disease onset to pregnancy (76) | 1.04 (0.90–1.19) | 0.598 | — | — |
| Time interval from last attack to pregnancy (76) | 1.01 (0.99–1.03) | 0.289 | — | — |
| Treatment during pregnancy and after delivery/abortion | ||||
| Adequate treatment† (10) | 1.00 | 1.00 | ||
| Inadequate treatment‡ (66) | 10.67 (2.07–55.07) | 0.005** | 18.45 (2.60–131.19) | 0.004** |
| AQP4-ab titre | ||||
| <1:100 or negative (45) | 1.00 | 1.00 | ||
| ≥1:100 (31) | 4.55 (1.48–13.97) | 0.008** | 4.20 (1.01–17.50) | 0.049* |
| Relapse within 1 year before pregnancy | ||||
| No (58) | 1.00 | |||
| Yes (18) | 0.56 (0.19–1.66) | 0.298 | — | — |
| ARR before pregnancy (76) | 0.57 (0.17–1.95) | 0.373 | — | — |
| EDSS score before pregnancy (76) | 0.96 (0.68–1.34) | 0.808 | — | — |
| Counts of concomitant auto-antibodies§ | ||||
| <1 (42) | 1.00 | |||
| ≥1 (34) | 1.48 (0.56–3.88) | 0.429 | — | — |
| Counts of concomitant auto-antibodies§ | ||||
| <2 (51) | 1.00 | |||
| ≥2 (25) | 1.16 (0.42–3.21) | 0.776 | — | — |
*P< 0.05; **p< 0.01.
†Adequate treatment was defined as (1) usage of relatively higher dose oral prednisone (>10 mg/day), (2) usage of immunosuppressant (azathioprine 100 mg/day or tacrolimus 3 mg/day) combined with or without oral steroid, (3) a dose of rituximab (375 mg/m2) within 6 months before conception and shortly after delivery.
‡Inadequate treatment referred to (1) no treatment at all, (2) usage of low-dose oral prednisone (≤10 mg/day) as single therapy.
§including antinuclear antibody, extractable nuclear antigen antibody, double-stranded DNA antibody, antineutrophil cytoplasmic antibody anticardiolipin antibody, thyroid peroxidase antibody and thyroglobulin antibody.
AQP4-ab, aquaporin-4 antibody; ARR, annualised relapse rate; EDSS, Expanded Disability Status Scale.
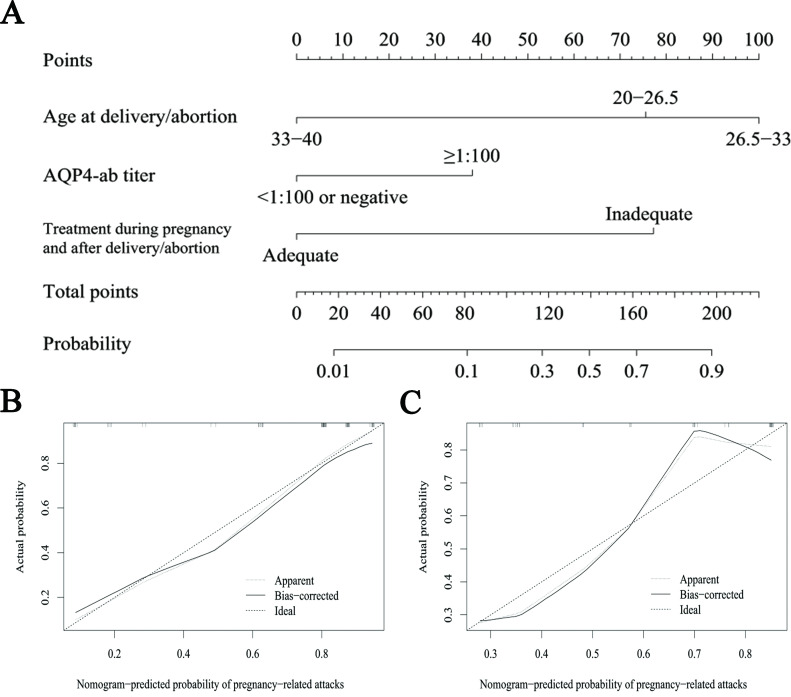
We further created a predictive model for pregnancy-related attacks using a nomogram according to whether pregnancy-related attacks occurred (figure 3A). In the primary cohort, the C-index of this nomogram was 0.86 (95% CI 0.77 to 0.95) and 0.87 with bootstrap resampling, the mean overoptimism value was 0.0570. In the external validation cohort, the C-index was 0.77 (95% CI 0.63 to 0.92) and 0.77 with bootstrap resampling, and the mean overoptimism value was −0.0093. The calibration curve exhibited good agreement between the predicted and actual probability of pregnancy-related attacks in the primary and validation cohorts (figure 3B and C).
Figure 3.
The prediction model of pregnancy-related attacks. (A) Nomogram for predicting the probability of pregnancy-related attacks. An individual patient’s value was based on each variable axis of the nomogram, and the number of points obtained for each variable value was determined with a line drawn upward. The sum of the points is located at the total points axis, which corresponds to the probability of pregnancy-related attacks in downward-pointing line. (B) The calibration curve for predicting the probability of pregnancy-related attacks in the primary cohort. (C) The calibration curve for predicting the probability of pregnancy-related attacks in the validation cohort. AQP4-ab, aquaporin-4 antibody.
The comparison of pregnancy-related attack between patients with AQP4-ab and MOG-ab
There were 58 patients enrolled in this study section, including 42 AQP4-ab-positive and 16 MOG-ab-positive patients (table 5). In the AQP4 cohort, the age at disease onset and age at delivery/abortion were 27.9 (20.6–42.8) and 27.9 (20.1–42.7) years, respectively. The median number of pregnancy-related attack was 1 (1–4), and attacks during the 0–3 months post partum were most common, with 25 counts (39.7%). In the MOG cohort, the age at disease onset and age at delivery/abortion were 28.3 (22.9–38.0) and 28.3 (23.0–37.2) years, respectively. The median number of pregnancy-related attack was 1 (1–3), and attacks during the 0–3 and 6–12 months post partum were most common, with 9 counts (37.5%), respectively. Compared with the AQP4 cohort, the MOG cohort exhibited less concomitant auto-antibodies (p=0.004), more optic neuritis (p<0.001) and less myelitis (p=0.022). The EDSS score in the exacerbation phase of a pregnancy-related attack did not differ between the AQP4 cohort (4, 3–9) and the MOG cohort (4, 3–7.5), while the EDSS score in the remission phase differed significantly between the AQP4 cohort (2, 0–9) and the MOG cohort (1, 0–7.5) (p=0.003).
Table 5.
The comparison of pregnancy-related attack between patients with NMOSD with AQP4-ab and MOG-ab
| AQP4 cohort | MOG cohort | P value | |
| Number of patients | 42 | 16 | — |
| Age at disease onset, year, median (range) | 27.9 (20.6–42.8) | 28.3 (22.9–38.0) | 0.727 |
| Age at delivery/abortion, year, median (range) | 27.9 (20.1–42.7) | 28.3 (23.0–37.2) | 0.793 |
| Number of pregnancy-related attacks, median (range) | 1 (1–4) | 1 (1–3) | 0.944 |
| Number of pregnancy-related attacks, n (%) | |||
| DP1 | 4 (6.3) | 2 (8.3) | 0.666 |
| DP2 | 2 (3.2) | 0 (0) | 1.000 |
| DP3 | 2 (3.2) | 0 (0) | 1.000 |
| PP1 | 25 (39.7) | 9 (37.5) | 0.852 |
| PP2 | 15 (23.8) | 4 (16.7) | 0.471 |
| PP3+4 | 15 (23.8) | 9 (37.5) | 0.202 |
| Type of pregnancy-related attacks, n (%) | |||
| Optic neuritis | 24 (38.1) | 19 (79.2) | <0.001** |
| Acute myelitis | 33 (52.4) | 6 (25.0) | 0.022* |
| Area postrema syndrome | 16 (25.4) | 1 (4.2) | 0.054 |
| Acute brainstem syndrome | 3 (4.8) | 0 (0) | 0.558 |
| Patient with concomitant auto-antibodies, n (%) | 23 (54.8) | 2 (12.5) | 0.004** |
| ANA | 20 (47.6) | 1 (6.3) | 0.003** |
| ENA-ab | 13 (31.0) | 1 (6.3) | 0.105 |
| dsDNA-ab | 2 (4.8) | 0 (0) | 1.000 |
| ANCA | 0 (0) | 0 (0) | — |
| ACA | 1 (2.4) | 0 (0) | 1.000 |
| TPO-ab and TG-ab | 12 (28.6) | 2 (12.5) | 0.350 |
| EDSS score in exacerbation phase, median (range) | 4 (3–9) | 4 (3–7.5) | 0.428 |
| EDSS score in remission phase, median (range) | 2 (0–9) | 1 (0–7.5) | 0.003** |
*P< 0.05, **p< 0.01.
ACA, anticardiolipin antibody; ANA, antinuclear antibody; ANCA, antineutrophil cytoplasmic antibody; AQP4-ab, aquaporin-4 antibody; DP, during pregnancy; dsDNA-ab, double-stranded DNA antibody; EDSS, Expanded Disability Status Scale; ENA-ab, extractable nuclear antigen antibody; MOG-ab, myelin oligodendrocyte glycoprotein antibody; NMOSD, neuromyelitis optica spectrum disorder; PP, postpartum period; TG-ab, thyroglobulin antibody; TPO-ab, thyroid peroxidase antibody.
Discussion
Through a series of studies, investigators had explored the effect of pregnancy on the frequency of NMOSD relapse6–12 and evaluated pregnancy complications and outcomes.7–10 13–15 19 Several researchers tried to identify the risk factors for a pregnancy-related attack7 13 15 and another one investigated the effect of NMOSD on miscarriage and pre-eclampsia in AQP4-ab-positive patients.10 Compared with the aforementioned studies, ours entailed the largest cohort to explicitly summarise the pregnancy-related characteristics of NMOSD with different antibody subsets in China, in which we systematically explored the risk factors of pregnancy-related attacks and generated a predictive model.
We initially investigated the pregnancy-related characteristics of patients with AQP4-ab, as this pathogenic antibody was found in the majority of patients with NMOSD. We observed that the attacks between 0 and 3 months after delivery/abortion were the most common with 51 counts (40.5%), consistent with previous reports on the prevalent phase of pregnancy-related attacks in patients with AQP4-ab.7 9 10 This phenomenon was similarly observed in MOG-ab-positive patients with 11 counts (39.3%). We also calculated that the relapse risk during the first-year post partum was approximately 2.1 and 1.5 times higher than that during 1-year prepregnancy in AQP4-ab-positive and MOG-ab-positive patients undergoing pregnancy after disease onset.
The rate of spontaneous abortion was 3.7% in the AQP4-ab-positive subgroup and 2.9% in the entire cohort, lower than that previously reported in other ethnic group (12.9%),10 but similar to that (2.8%) in the general Chinese pregnant population.20 Pre-eclampsia was not observed in the study cohort. Moreover, we observed some neonatal malformations that might be related to maternal NMOSD, including undeveloped external ear, dacryocyst obstruction and scoliosis, which was not reported before. In many autoimmune conditions, complications and adverse pregnancy outcomes could be induced by maternal IgG auto-antibodies through transplacental passage, resulting in many neonatal diseases, such as neonatal lupus syndrome, myasthenia gravis and Grave’s disease.21 Similarly, AQP4-ab could be transferred from NMOSD mothers to neonates, disappearing a few months later, while the relationship between malformations and AQP4-ab remains unknown.7 No spontaneous abortion, neonatal malformations or pre-eclampsia were observed in MOG-ab-positive subgroup.
In patients with NMOSD with pregnancy-related attacks we found that the ARR elevated significantly during the first (p<0.001) and second (p=0.015) trimesters after delivery, as well as the EDSS score during pregnancy (p=0.004) and 1 year after delivery (p<0.001) compared with the same index before pregnancy. These results were consistent with previous observations.6 8–14 In the abortion group, ARR increased significantly during pregnancy and the first trimester after abortion (p=0.008 and 0.019, respectively), while the EDSS score increased significantly during pregnancy and 1 year after abortion (p=0.016 and p<0.001, respectively). Attacks during pregnancy were not uncommon and often led to miscarriage.10 We speculated that higher ARRs during pregnancy originated from the short pregnancy period before abortion and that subsequent therapy with high-dose methylprednisolone led to a declining ARR after pregnancy. In contrast to MS, the reduction of disease activity during pregnancy was not observed in NMOSD.6 7
Pregnancy is known to play a modulatory role in the course of autoimmune diseases. During pregnancy, the fetoplacental unit secrete cytokines, responsible for an increased level of oestrogen, and leading to a relative immune tolerance to maintain a successful pregnancy. However, this increases Th2-mediated immune responses and stimulates humoral immunity and the production of auto-antibodies.22 23 Theoretically, this change may exacerbate auto-antibody medicated diseases during pregnancy. Nevertheless, the current study and previous investigations found that pregnancy-related attack was the most common after delivery.8–12 This discrepancy between expectations and clinical observations is poorly understood. It therefore seems possible that other underlying pathophysiological pathways responsible for NMOSD activity after pregnancy may exist.
Through multivariate Poisson and logistic regressions, our study revealed that the independent risk factors for pregnancy-related attacks included delivery/abortion age, AQP4-ab titre and treatment variables. Age (20–26.5, p=0.018; 26.5–33, p=0.001) at delivery/abortion, inadequate treatment during pregnancy and postpartum period (p=0.004) and high AQP4-ab titre (≥1:100, p=0.049) were significantly associated with an increased OR of the occurrence of pregnancy-related attacks. The results indicated that patients with younger age at delivery/abortion, higher AQP4-ab titre were at higher risk for pregnancy-related attack and maintenance therapy with appropriate immunosuppressant or sufficient dose of oral steroid should be performed during pregnancy and postpartum period.24 Previous studies also explored the risk factors for pregnancy-related attacks.7 13 15 Shimizu et al observed that an increased risk of pregnancy-related attack was associated with having a relapse during 1 year before pregnancy and discontinued or low-dose immunosuppressive therapy.7 Huang et al reported that negative AQP4-ab, concomitance with autoimmune diseases/antibodies, and no treatment in remission were risk factors for pregnancy-related recurrence.13 Recently, Kim et al showed that discontinuation of oral immunosuppressant in order to become pregnant appeared to increase the risk of pregnancy-related attack while pregnancy-related attack was negatively associated with pregnancy after initiation of rituximab.15 There are some discrepancies among the results of these studies, which may originate from the different features of the study cohorts including sample size, ethnicity or baseline disease activity. Similar to previous investigation,9 we did not observe significant correlation between the prepregnancy ARR and the risk of pregnancy-related attack.
Because of the retrospective nature, the timings of AQP4-ab detection vary among individuals, which constitutes a major limitation of our study. To minimise the possible impact of antibody titre fluctuation over time on the study results, we used the earliest available AQP4-ab titres detected in a remission status in the univariate and multivariate analyses of potential risk factors. This AQP4-ab titre, though not perfect, might reflect the primordial face of individual autoimmune conditions and was least affected by immunosuppressant treatments in a real-world setting. Our results indicated that physicians might need to pay enough attention to the risk of pregnancy-related attack in patients with higher AQP4-ab titre.
We further provided a nomogram to predict the probability of pregnancy-related attack. Previous studies showed that nomograms were superior to conventional staging systems for prognosis prediction of some cancers.25 26 In our nomogram generated from the primary cohort, the calibration curve demonstrated good agreement, while the C-index (0.87 and 0.77) exhibited good discrimination using bootstrap resampling in internal and external validation. This could provide an efficient reference when counselling patients with NMOSD about pregnancy issues.
We also demonstrated that compared with the AQP4 cohort, the pregnancy-related attack in MOG cohort exhibited more optic neuritis, less acute myelitis and lower EDSS scores in the remission phase. These features appear to be consistent with previous reports on the comparison of clinical manifestations and outcomes between the two disease entities.3 27 Limited by the relative rarity of MOG-ab positivity, we had insufficient number of patients with MOG-ab manifesting the onset of disease before pregnancy.
To conclude, our study showed that pregnancy-related NMOSD attacks were most common during 0–3 months after delivery/abortion and we identified independent risk factors for pregnancy-related attacks. The primary limitation of our study included its retrospective nature, small number of MOG subsets. Besides, AQP4-ab titres were not detected at fixed time points after NMOSD onset. Future prospective studies with larger sample size and protocol defined timing of antibody detection are therefore warranted to confirm and extend our findings.
Footnotes
Contributors: LW designed and conceptualised the study, interpreted and analysed the data and drafted and revised the manuscript for intellectual content. LZ, JZ, WH and XC played a major role in the acquisition of data and revised the manuscript for intellectual content. CL and MW revised the manuscript for intellectual content. WL, JX, XL, LC and WQ played a major role in the acquisition of data of the validation cohort and contributed on data interpretation. WQ, JL and CZ revised the manuscript for intellectual content. CQ designed and conceptualised the study, interpreted and analysed the data and revised the manuscript for intellectual content. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Funding: This research was supported by the National Natural Science Foundation of China (grant no. 81771296, 81801196), the Shanghai Municipal Science and Technology Major Project (grant no. 2018SHZDZX01) and ZHANGJIANG LAB, and the National Key Research and Development Program of China (grant no. 2016YFC0901504).
Competing interests: LW, LZ, JZ, WH, XC, CL, MW, WL, JX, XL, LC, WQ, JL, CZ and CQ are sponsor of the current study and report no disclosures.
Patient consent for publication: Not required.
Ethics approval: The study was approved by the Medical Ethics Committee of Huashan Hospital, Fudan University (HIRB-2020007). Written informed consent was obtained from each patient.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request. Anonymised data not exhibited in our study will be made available on request from any qualified investigator.
References
- 1. Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015;85:177–89. 10.1212/WNL.0000000000001729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Papadopoulos MC, Verkman AS. Aquaporin 4 and neuromyelitis optica. Lancet Neurol 2012;11:535–44. 10.1016/S1474-4422(12)70133-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kitley J, Waters P, Woodhall M, et al. Neuromyelitis optica spectrum disorders with aquaporin-4 and myelin-oligodendrocyte glycoprotein antibodies: a comparative study. JAMA Neurol 2014;71:276–83. 10.1001/jamaneurol.2013.5857 [DOI] [PubMed] [Google Scholar]
- 4. Reuss R, Rommer PS, Brück W, et al. A woman with acute myelopathy in pregnancy: case outcome. BMJ 2009;339:b4026. 10.1136/bmj.b4026 [DOI] [PubMed] [Google Scholar]
- 5. Saadoun S, Waters P, Leite MI, et al. Neuromyelitis optica IgG causes placental inflammation and fetal death. J Immunol 2013;191:2999–3005. 10.4049/jimmunol.1301483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bourre B, Marignier R, Zéphir H, et al. Neuromyelitis optica and pregnancy. Neurology 2012;78:875–9. 10.1212/WNL.0b013e31824c466f [DOI] [PubMed] [Google Scholar]
- 7. Shimizu Y, Fujihara K, Ohashi T, et al. Pregnancy-Related relapse risk factors in women with anti-AQP4 antibody positivity and neuromyelitis optica spectrum disorder. Mult Scler 2016;22:1413–20. 10.1177/1352458515583376 [DOI] [PubMed] [Google Scholar]
- 8. Klawiter EC, Bove R, Elsone L, et al. High risk of postpartum relapses in neuromyelitis optica spectrum disorder. Neurology 2017;89:2238–44. 10.1212/WNL.0000000000004681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim W, Kim S-H, Nakashima I, et al. Influence of pregnancy on neuromyelitis optica spectrum disorder. Neurology 2012;78:1264–7. 10.1212/WNL.0b013e318250d812 [DOI] [PubMed] [Google Scholar]
- 10. Nour MM, Nakashima I, Coutinho E, et al. Pregnancy outcomes in aquaporin-4-positive neuromyelitis optica spectrum disorder. Neurology 2016;86:79–87. 10.1212/WNL.0000000000002208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fragoso YD, Adoni T, Bichuetti DB, et al. Neuromyelitis optica and pregnancy. J Neurol 2013;260:2614–9. 10.1007/s00415-013-7031-y [DOI] [PubMed] [Google Scholar]
- 12. Shi B, Zhao M, Geng T, et al. Effectiveness and safety of immunosuppressive therapy in neuromyelitis optica spectrum disorder during pregnancy. J Neurol Sci 2017;377:72–6. 10.1016/j.jns.2017.03.051 [DOI] [PubMed] [Google Scholar]
- 13. Huang Y, Wang Y, Zhou Y, et al. Pregnancy in neuromyelitis optica spectrum disorder: a multicenter study from South China. J Neurol Sci 2017;372:152–6. 10.1016/j.jns.2016.11.054 [DOI] [PubMed] [Google Scholar]
- 14. Wuebbolt D, Nguyen V, D'Souza R, et al. Devic syndrome and pregnancy: a case series. Obstet Med 2018;11:171–7. 10.1177/1753495X18758868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim S-H, Huh S-Y, Jang H, et al. Outcome of pregnancies after onset of the neuromyelitis optica spectrum disorder. Eur J Neurol 2020;27:1546–55. 10.1111/ene.14274 [DOI] [PubMed] [Google Scholar]
- 16. Frank E, Harrell J. Rms: regression modeling strategies. R package version 5.1-4. Available: http://CRAN.Rproject.org/package=rms [Accessed 3 Mar 2020].
- 17. Han K, Song K, Choi BW. How to develop, validate, and compare clinical prediction models involving radiological parameters: study design and statistical methods. Korean J Radiol 2016;17:339–50. 10.3348/kjr.2016.17.3.339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol 2013;31:1188–95. 10.1200/JCO.2012.41.5984 [DOI] [PubMed] [Google Scholar]
- 19. Delgado-García G, Chávez Z, Rivas-Alonso V, et al. Obstetric outcomes in a Mexican cohort of patients with AQP4-antibody-seropositive neuromyelitis optica. Mult Scler Relat Disord 2018;25:268–70. 10.1016/j.msard.2018.08.015 [DOI] [PubMed] [Google Scholar]
- 20. Wei Y, Xu Q, Yang H, et al. Preconception diabetes mellitus and adverse pregnancy outcomes in over 6.4 million women: a population-based cohort study in China. PLoS Med 2019;16:e1002926. 10.1371/journal.pmed.1002926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Borchers AT, Naguwa SM, Keen CL, et al. The implications of autoimmunity and pregnancy. J Autoimmun 2010;34:J287–99. 10.1016/j.jaut.2009.11.015 [DOI] [PubMed] [Google Scholar]
- 22. Wegmann TG, Lin H, Guilbert L, et al. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a Th2 phenomenon? Immunol Today 1993;14:353–6. 10.1016/0167-5699(93)90235-D [DOI] [PubMed] [Google Scholar]
- 23. Lucchinetti CF, Mandler RN, McGavern D, et al. A role for humoral mechanisms in the pathogenesis of Devic's neuromyelitis optica. Brain 2002;125:1450–61. 10.1093/brain/awf151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Borisow N, Hellwig K, Paul F. Neuromyelitis optica spectrum disorders and pregnancy: relapse-preventive measures and personalized treatment strategies. Epma J 2018;9:249–56. 10.1007/s13167-018-0143-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sternberg CN. Are nomograms better than currently available stage groupings for bladder cancer? J Clin Oncol 2006;24:3819–20. 10.1200/JCO.2006.07.1290 [DOI] [PubMed] [Google Scholar]
- 26. Touijer K, Scardino PT, staging Nfor. Prognosis, and predicting treatment outcomes. Cancer 2009;115:3107–11. [DOI] [PubMed] [Google Scholar]
- 27. Sato DK, Callegaro D, Lana-Peixoto MA, et al. Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology 2014;82:474–81. 10.1212/WNL.0000000000000101 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymised data not exhibited in our study will be made available on request from any qualified investigator.