Graphical abstract
Keywords: Ultrasound energy, Process intensification, Cellulose valorization
Highlights
-
•
The nitration of microcrystalline cellulose assisted by ultrasound was suitable.
-
•
Ultrasound amplitude, acid mixtures, and reaction time were evaluated using a cup horn system.
Abstract
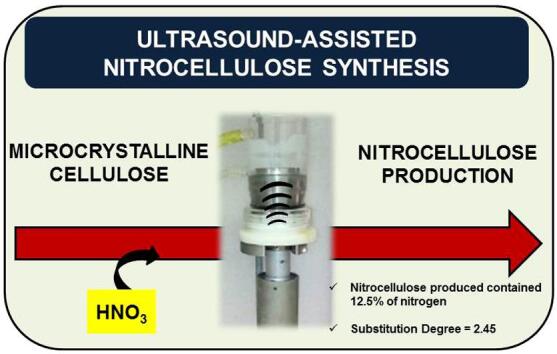
Nitrocellulose is a nitrated cellulose polymer with a broad application in industry. Depending on the nitrogen content, this polymer can be used for manufacturing explosives, varnishes, clothes, and films, being considered a product of high value-added. In this work, the use of ultrasound was investigated for the intensification of nitrocellulose synthesis from microcrystalline cellulose. The ultrasound-assisted nitrocellulose synthesis (UANS) was carried out using several ultrasound systems, such as baths and cup horns, allowing the evaluation of the frequency (from 20 to 130 kHz) and delivered power (from 23 to 134 W dm−3) to the reaction medium. The following parameters were evaluated: acid mixture (H2SO4, H3PO4, CH2O2 or CH3COOH with HNO3, 2 to 14.4 mol L−1), ultrasound amplitude (10 to 70%) and reaction time (5 to 50 min). Better nitrocellulose yield (nitrogen content of 12.5% was obtained from 1 g of microcrystalline cellulose employing a cup horn system operating at 20 kHz, 750 W of nominal power with 60% of amplitude, 25 mL of acid solution (13.6 mL of 18.4 mol L−1 H2SO4 + 9.2 mL of 14.4 mol L−1 HNO3 + 2.2 mL H2O), at 30 °C for 30 min. At silent conditions (mechanical stirring ranging from 100 to 500 rpm), the nitrogen content was lower than 11.8% which demonstrate the ultrasound effects for nitrocellulose synthesis.
l1. Introduction
The sustainable production of feedstocks for the chemical industry has been considered as an important focus in science research [1], [2]. In the same way, it is of great industrial concern to find protocols for lignocellulosic waste valorization, such as biomass and agricultural residues. It has been observed the potential application of cellulose, or hemicellulose fractions from lignocellulosic wastes, as alternative feedstocks for the production of biofuels, synthetic materials, building blocks, and fine chemicals [3], [4]. The development of sustainable protocols is intimately associated with the use of biopolymer materials, which feature high physicochemical and performance characteristics [5], [6]. On this aspect, cellulose is of considerable interest since it has strong potential as a precursor of some polymers [5], [7].
Cellulose is a natural linear polysaccharide composed of glucose units linked by β(1 → 4) glucosidic bonds [8]. Each glucose units has three reactive hydroxyl groups: one primary hydroxyl group on the C6 position, and two secondary hydroxyl groups on the C2 and C3 positions [8]. This natural biopolymer is the most abundant renewable source, being important for many industrial activities [9]. It is used in the paper-making, non-natural textile/fabric, cosmetics, household article, and pharmaceutical industry, which convert the biomass feedstock to high value-added products [1], [5], [7].
Cellulose nitrate cellul, or nitrocellulose, is an important material that has been extensively used in several industrial processes [10]. Changes in nitrogen content and substitution degree of nitrocellulose allow modifications in the physical-chemistry properties which enable the applicability of nitrocellulose in several process [5], [9]. As an example, at low nitrogen content (below than 12.2%), it can be used in cosmetics and plastics, whereas nitrocellulose with high nitrogen content is used in guns and rocket propellants [11]
The nitration of cellulose occurs by electrophilic attack promoted by the nitronium ion over the oxygen of hydroxyl group, resulting in hydrogen proton elimination [5]. The chemical reaction used in industrial scale for nitrocellulose synthesis is a reversible, highly exothermic esterification reaction in which cellulose fibers obtained from wood pulp or cotton react with mixtures of nitric acid, sulfuric acid and water [12]. Several organic solvents, such as tetrachloromethane, methylene chloride, or nitromethane are used to reduce the consumption of nitric acid and stabilize the esterification process [5]. In general, the nitrocellulose synthesis comprises a number of side steps, long reaction time and the use of toxic reagents as tetrachloromethane and nitromethane [12].
The major part of the studies about nitrocellulose synthesis is associated to the development of alternative feedstocks for industrial process. As example, nitration products obtained from Miscanthus (non-wood, perennial grass) have similar quality to basic nitrocellulose types produced for films, membranes among other products [13]. Some authors proposed the nitrocellulose production from bacterial cellulose produced by fermentation using Acetobacter xylinum [14]. Nitrocellulose with a degree of substitution from 1 to 2.85 was obtained, but physical properties and explosive performance were not similar to the product conventionally produced [14]. Despite these studies, no enhancement process for nitrocellulose yield or the reduction of concentrated reagents has been proposed in last years.
Taking into account the technologies that contribute for process intensification, ultrasound energy has been considered a promising alternative to increase the efficiency of extraction, conversion, and lixiviation procedures [15], [16], [17], [18]. In organic synthesis, ultrasound presents several advantages over the conventional synthesis in terms of reaction rates, yields, purity of products and selectivity [19], [20], [21]. Some studies investigated the synergetic effects of ultrasound to depolymerization, and degradation of cellulose derivatives [22], [23], [24]. In many of these processes the ultrasound effects are associated to acoustic cavitation, radical species formation, shock waves and acoustic streaming [25], [26], [27]. However, there is a lack in the literature about the effects of ultrasound on nitrocellulose synthesis from microcrystalline cellulose.
In the present work, the application of ultrasound energy for nitrocellulose synthesis from microcrystalline cellulose was evaluated. Microcrystalline cellulose was sonicated with several acids mixtures, using baths and cup horns systems. The effect of ultrasound was investigated by evaluating the HNO3 concentration, the acid mixture, the ultrasound amplitude, delivered power, and reaction time. The obtained results using ultrasound-assisted nitrocellulose synthesis (UANS) were compared with those obtained under silent conditions (mechanical stirring).
2. Materials and methods
2.1. Instrumentation
The UANS was performed using several ultrasound systems. Ultrasound bath systems were used at frequencies of: (i) 25 or (ii) 45 kHz (100 W, Transsonic TI-H-5 3.5 L, Elma GmbH & Co, Germany); (iii) 37 or (iv) 80 kHz (330 W, Elmasonic P120H 9.0 L or P120H 12.9 L, Elma GmbH & Co) and (v) 35 or (vi) 130 kHz (200 W, Transsonic TI-H-10 8.6 L, Elma GmbH & Co).
Two cup horn type ultrasound systems were evaluated at 20 kHz, coupled to different US generators. One with 130 W nominal power (model VCX 130 PB, Sonics and Materials, INC., USA), and another coupled with 750 W nominal power (model VC 750, Sonics and Materials, INC., USA).
For comparison purposes, nitrocellulose synthesis was driven at silent conditions, using a hotplate with magnetic stirring (model AREX-F20500413, Velp Scientific Srl, Usmate, Italy), operating at 100 to 500 rpm under the same conditions optimized to UANS.
The nitrocellulose solubility in alcohol–ester mixture (1 + 2 ratio) was measured by filtering the insoluble residue, followed by drying and weighing in accordance with the procedures described in MIL-DTL-244B [28]. A viscometer (model SVM 3000, Anton Paar GmbH, Graz, Austria) was used for the determination of kinematic viscosity of reaction solution before and after the ultrasound treatment.
The nitrogen content was quantified by the ferrous ammonium sulfate titration method described in MIL-STD-286C [29]. This titration is based on saponification of nitrocellulose with concentrated H2SO4 and reduction of the produced HNO3 to NO. Further, the NO products are complexed with FeSO4 to form [Fe(NO)]SO4. The degree of substitution (DS) was calculated by the equation 1 [30]:
| (1) |
Infrared spectra of microcrystalline cellulose and nitrocellulose obtained after UANS were recorded by Fourier transform infrared spectroscopy (FTIR, spectrometer model Spectrum One® FTIR, PerkinElmer, Massachusetts, USA). All the obtained spectra ranged between wave numbers 4000 to 500 cm−1 and the baseline was corrected and vector normalized. Spectral resolution was set at 4 cm−1.
2.2. Materials and chemicals
For this study, microcrystalline cellulose was obtained from Sigma-Aldrich (purity of 99%, Germany). Water was purified using a Milli-Q system (Millipore Corp., USA) and it was used to prepare all the reagents and standard solutions. Nitric acid (HNO3, 65%), sulfuric acid (H2SO4, 95%), phosphoric acid (H3PO4, 85%), formic acid (CH2O2, 99%), acetic acid (CH3COOH, 99%) and sodium carbonate (Na2CO3, 99%) were of analytical grade (Merck, Darmstadt, Germany). Nitrogen and argon (purity of 99.999 and 99.5%, respectively, White Martins, Brazil) were used for analytical techniques.
2.3. Methods
The UANS process was carried out in a glass cylindrical reactor with 5 cm of internal diameter and 30 cm height. Temperature was controlled using a circulating water bath (model MCT 110 Plus, Servylab Ltda., Brazil). Microcrystalline cellulose (1 g) was transferred to the reactor with a mixture of concentrated acids in a solid-to-liquid ratio of 1 + 25 (w v−1) [13]. Finally, this mixture was submitted to UANS and several ultrasound systems and acid mixtures were evaluated. After UANS process, solid residues were filtered on a vacuum filter and then stabilized under continuous stirring as follows: H2O, heated for 1 h at 80 °C; 0.3% Na2CO3 solution, heated for 3 h at 80 °C; H2O, heated for 1 h at 80 °C. The obtained products were first dried at room temperature and further at 105 °C.
Preliminary experiments were performed using 30 °C, 40 min and acid solution of 13.6 mL of 18.4 mol L−1 H2SO4 + 9.2 mL of 14.4 mol L−1 HNO3 + 2.2 mL H2O, in all previously described ultrasound systems at 70% of ultrasound amplitude. After choosing selecting the ultrasound system, a univariate design was proposed to optimize the conditions for UANS. The following parameters were evaluated: HNO3 concentration (2 to 14.4 mol L−1), acid mixture (H2SO4, H3PO4, CH2O2 or CH3COOH with HNO3), ultrasound amplitude (10 to 70%) and reaction time (5 to 50 min). The results obtained with UANS were compared with those by conventional synthesis at silent condition (without ultrasound), which was performed using a hotplate with magnetic stirring (100 to 500 rpm) under the same conditions previously optimized for UANS.
3. Results and discussion
3.1. Influence of ultrasound system for UANS
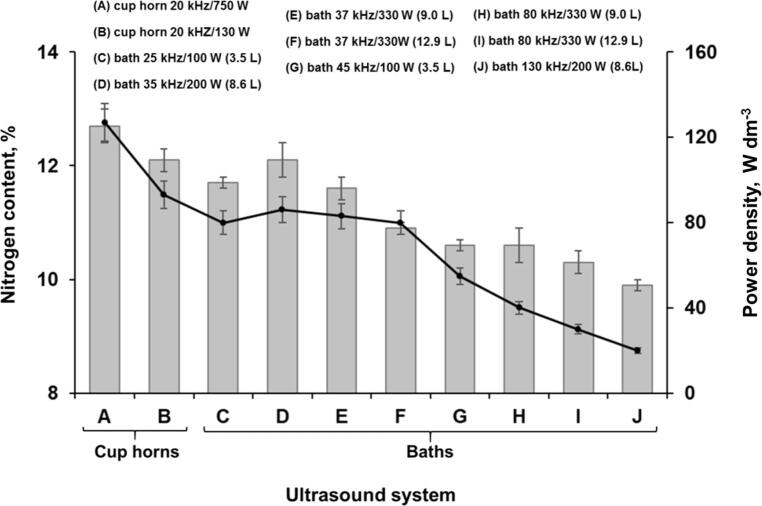
In view of the possible differences related to the delivery of energy among the available ultrasound systems, an evaluation was performed in order to check the influence of the characteristics of these systems on the nitrocellulose synthesis. This evaluation was performed using 1 g of microcrystalline cellulose and 25 mL of acid solution (13.6 mL of 18.4 mol L−1 H2SO4 + 9.2 mL of 14.4 mol L−1 HNO3 + 2.2 mL H2O), which was sonicated during 40 min at 30 °C, with US operating at 70%. The nitrogen content was determined using the ferrous ammonium sulfate titration method described in MIL-STD-286C [29]. The acoustic power delivered to the reaction medium was calculated by calorimetric method, according previous works, [31], [32] and presented as power density (W dm−3). The results obtained for all the evaluated US systems are shown in Fig. 1.
Fig. 1.
Nitrogen content obtained by several ultrasound application systems for UANS (grey bars, left axis), and its correlation with delivered power (black line, right axis). Experimental conditions: 1 g of microcrystalline cellulose and 25 mL of acid solution (13.6 mL of 18.4 mol L−1 H2SO4 + 9.2 mL of 14.4 mol L−1 HNO3 + 2.2 mL H2O), which was sonicated during 40 min at 30 °C, with US amplitude operating at 70% (error bars are the standard deviation, n = 3).
When ultrasound baths systems were evaluated it was observed a trend where delivered energy decreases while increasing the ultrasound frequency, which consequently reduces the nitrogen content of products [33]. It was observed, for the bath systems operating at same nominal power and frequency, that the water volume can affect the power density delivered in the liquid medium, which is in agreement with the previously published studies [34], [35], [36]. The power density and nitrogen content for these systems ranged from 24 to 83 W dm−3 and 9.9 to 12.1%, respectively. By using a cup horn operating at 750 W (134 W dm−3) the nitrogen content was 12.5%, which represents a substitution degree of 2.45. It is important to emphasize that ideally the nitrogen content of a fully nitrated nitrocellulose obtained of each of the three hydroxyl groups (C2, C3 and C6) is approximately 14% [36], [37].
The proposed process allowed to produce nitrocellulose using near to 82% of the hydroxyl groups available for the reaction (substitution degree = 2.45). Based on these results, the cup horn system operating at 20 kHz, with power density of 134 W dm−3 (750 W of nominal power), was selected for the subsequent experiments.
3.2. Influence of acids for UANS of cellulose
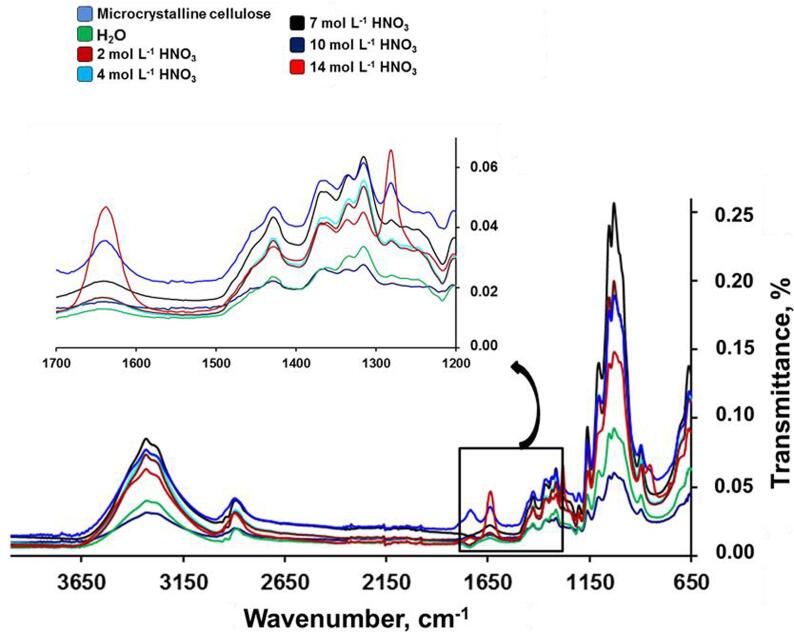
The synthesis of nitrocellulose is conventionally performed using concentrated HNO3. The development of alternative procedures, based on the use of diluted acids, is an important research topic aiming reagents saving and less generation of residues. Thus, HNO3 concentration was investigated a range of 2 to 14.4 mol L−1. In this study, 25 mL of acid solution (13.6 mL of 18.4 mol L−1 H2SO4 + 11.4 mL of diluted HNO3) and 1 g of microcrystalline cellulose were submitted to UANS process. Ultrasound amplitude was set at 70% and the reaction medium was sonicated for 40 min at 30 °C. The obtained products were identified by FTIR using ATR mode. The infrared spectra obtained for all the evaluated HNO3 concentration is presented in Fig. 2.
Fig. 2.
Infrared spectra obtained by several HNO3 concentration for UANS. Experimental conditions: 1 g of microcrystalline cellulose and 25 mL of acid solution (13.6 mL of 18.4 mol L−1 H2SO4 + 11.4 mL of diluted HNO3) for 40 min at 30 °C and 70% of US amplitude.
As presented in Fig. 2, in the region of high frequencies of infrared spectra, two intense peaks were observed at around 2900 and 3500 cm−1 corresponding to the stretching peaks of hydroxyl groups on the glucose rings. In the regions of 1270 and 1650 cm−1 were observed two peaks with medium intensity, corresponding to the different vibrations of the NO2 group. When compared the spectra of experiments performed using several HNO3 concentrations, the intensities related to NO2 groups vibrations increases while increasing the HNO3 concentration, which provides evidence of the influence of HNO3 concentration for the nitration reaction. It is important to emphasize that due to the low nitrogen content in the experiments using diluted HNO3 it was not possible to use the ferrous ammonium sulfate titration method for the determination of nitrogen. In this sense, based on the evidences obtained by evaluating infrared spectra and the low nitrogen content in samples used in the experiments with diluted HNO3 (lower than the limit of quantification of ferrous ammonium sulfate titration method), 14.4 mol L−1 HNO3 was selected for the subsequent experiments.
In view of the increasing demand of methodologies based on the use of milder reaction conditions, the use of 14.4 mol L−1 HNO3 alone is not appropriate for the nitration reaction of cellulose. The main reasons associated to that is the need of a huge amount of reagent, HNO3 present a relatively high vapor pressure, which represent reagent losses by volatility, and the reaction medium becomes very reactive [5]. In this sense, nitrating mixtures containing several auxiliary acids (H2SO4, H3PO4, CH2O2, or CH3COOH) were evaluated. In this study, 25 mL of acid solution (13.6 mL of concentrated auxiliary acid + 9.2 mL of 14.4 mol L−1 HNO3 + 2.2 mL H2O) and 1 g of microcrystalline cellulose was submitted to UANS process (40 min, 30 °C, 70% US amplitude). The obtained results are summarized in Table 1.
Table 1.
Characterization of nitrocellulose produced by UANS from microcrystalline cellulose. Experimental conditions: 1 g of microcrystalline cellulose and 25 mL of acid solution (13.6 mL of concentrated auxiliary acid + 9.2 mL of 14.4 mol L−1 HNO3 + 2.2 mL H2O) for 40 min at 30 °C and 70% of US amplitude.
| Auxiliary acid | Nitrogen content, % | Degree of Substitution | Viscosity of 2% solution in acetone, cP | Solubility in alcohol-ether mixture (1 + 2 ratio), % |
|---|---|---|---|---|
| H2SO4, 95% | 12.5 ± 0.3 | 2.45 ± 0.04 | 17.0 ± 1.2 | 90 ± 7 |
| H3PO4, 85% | 11.1 ± 0.4 | 1.99 ± 0.02 | 15.8 ± 0.9 | 87 ± 13 |
| CH2O2, 99% | 11.5 ± 0.3 | 2.11 ± 0.03 | 8.91 ± 2.65 | 71 ± 9 |
| CH3COOH, 99% | 10.9 ± 0.6 | 1.94 ± 0.05 | 12.6 ± 0.7 | 91 ± 10 |
All the evaluated acids contributed for a nitration reaction with a degree of substitution near or higher than 2. Using H3PO4, CH2O2 or CH3COOH, the nitrogen content was lower than 11.5%, which can be associated with physical–chemical properties of microcrystalline cellulose. Some properties, such as the crystallinity degree, may affects the diffusion of nitrating species (NO2+) into the macromolecule matrix, resulting in a low substitution degree [37].
When H2SO4 was evaluated, high nitrogen content was obtained with a substitution degree of 2.45 (substitution of approximately 82% of hydroxyl groups available for nitration). The products obtained when H2SO4 was used as auxiliary reagent presented high solubility and high values of viscosity, which can be used as evidence about the homogeneous product that were obtained, with a uniform distribution of NO2 groups. Some authors investigated the mechanisms of H2SO4 action in the nitration reaction. This study described that H2SO4 contributes for the HNO3 ionization to NO2+, stabilized the water excess during the reaction, and to work as a swelling agent for cellulose [5].
Based on the presented results it is possible to state that the condition using H2SO4 was most appropriate for the synthesis of nitrocellulose, using microcrystalline cellulose as starting material. Thus, the acid mixture with HNO3 and H2SO4 was selected for subsequent evaluations.
3.3. Evaluation of the US amplitude and reaction time for the nitration of cellulose
The ultrasound energy delivered in the reaction medium, as well the reaction time, are directly related with cavitation and physical effects of ultrasound energy. Then, to investigate how US amplitude may affect the delivered power, and the time that reaction is exposed to US, becomes important aspects to be investigated aiming to understand how the nitration reaction takes place in UANS.
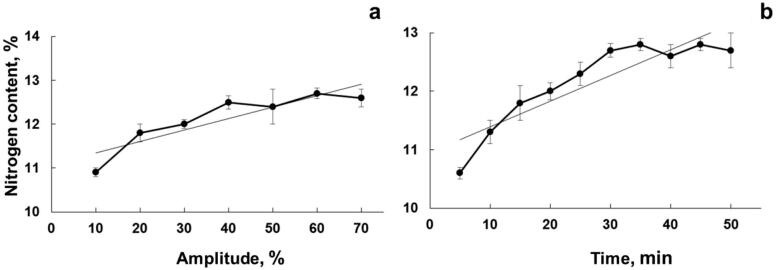
UANS was investigated at different conditions of US amplitude (from 10 to 70%). Reaction time was set at 40 min (30 °C) and UANS was performed using the conditions previously optimized. The obtained results are presented in Fig. 3a.
Fig. 3.
Effect of (a) ultrasound amplitude and (b) reaction time in the UANS process. Experimental conditions: 25 mL of acid solution (13.6 mL of 18.4 mol L−1 H2SO4 + 9.2 mL of 14.4 mol L−1 HNO3 + 2.2 mL H2O); 1 g of microcrystalline cellulose; 30 °C (error bars are the standard deviation, n = 3).
As it was observed, the nitration reaction presented a positive effect while US amplitude was increased. When lower US amplitudes were used (below 50%), less nitrogen content (12.2% or less) were determined from the obtained products. Increasing the amplitude to 60%, the nitrogen content increased to 12.5%, and a sort of a plateau was achieved. Even increasing the US amplitude to 70%, no significant enhancement was observed. Thus, 60% of US amplitude was assumed as a compromise condition (lower energy that provides higher nitrogen content products), being selected for further evaluations of UANS process.
Besides to the US amplitude, the time that ultrasound was used to assist the reaction was also evaluated. The sonication time was ranged from 5 to 50 min (30 °C, 60% of US amplitude), using the experimental conditions previously optimized. As presented in Fig. 3b, after 30 min of UANS the reaction products presented 12.5% nitrogen content. No additional improvement in the nitrogen content was obtained when the reaction system was sonicated for longer times (40 or 50 min). Based on this result, 30 min of reaction time was selected for further evaluations.
After evaluating the conditions associated with the proposed US system, such as amplitude (delivered power) and sonication time, a compromise condition between energy saving and substitution degree was achieved. The optimized condition was 1 g of microcrystalline cellulose, 25 mL of acid solution (13.6 mL of 18.4 mol L−1 H2SO4 + 9.2 mL of 14.4 mol L−1 HNO3 + 2.2 mL H2O), 30 °C, 60% of US amplitude and 30 min of sonication. Considering these conditions, it was possible to achieve a nitrocellulose production with a substitution degree of 2.45 (12.5% of nitrogen content in the obtained product).
3.4. Influence of ultrasound and mechanical stirring
In order to evaluate the effects of ultrasound in the nitration reaction of cellulose, the reaction was performed at silent conditions. The experimental conditions selected in this case were those previously optimized for UANS process (1 g of microcrystalline cellulose, 25 mL of acid solution containing 13.6 mL of 18.4 mol L−1 H2SO4 + 9.2 mL of 14.4 mol L−1 HNO3 + 2.2 mL H2O, at 30 °C for 30 min), but using mechanical stirring (100 to 500 rpm) instead of ultrasound energy. The obtained results for nitrocellulose synthesis at silent conditions can be seen in Fig. 4.
Fig. 4.
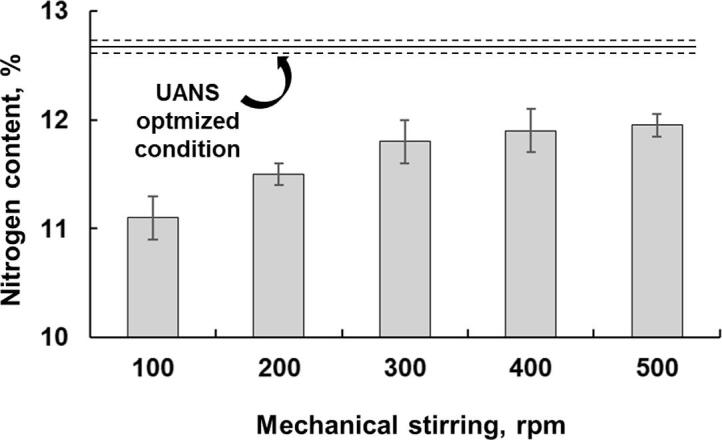
Nitrogen content obtained by silent conditions for nitrocellulose synthesis. Experimental conditions: 1 g of microcrystalline cellulose, 25 mL of acid solution (13.6 mL of 18.4 mol L−1 H2SO4 + 9.2 mL of 14.4 mol L−1 HNO3 + 2.2 mL H2O), at 30 °C for 30 min.
According to the results obtained using silent conditions, the nitrogen content was lower than 11.8%. The nitrogen content presented an increase trend while stirring was also increased, which is in agreement with results obtained by UANS. The rise in the nitrogen content was observed up to 300 rpm. It is important to emphasize that when evaluated a delivered energy in mechanical stirring systems, the power density was lower than 3 W dm−3 for all evaluated conditions. These results can be used to infer that US energy contributed for the nitration reaction of cellulose. Since the US system deliver more energy in the reaction medium, it ensures appropriate conditions for increasing the mass transfer, while promoting an effective contact of nitrate species and OH groups in the cellulose.
3.5. Comparison between the proposed UANS process and other treatments
The UANS process was compared with other nitration protocols, as described in Table 2. As it is observed the proposed process enables a nitrocellulose production with a substitution degree of 2.45. The most part of literature also reported to the use of concentrated acids for the nitration reaction and the use of diluted HNO3 remains a challenge for future works. However, the proposed work was considered a promising alternative for the synthesis of nitrocellulose, from microcrystalline cellulose. High nitrogen content was obtained and it is important to emphasize that physical–chemical properties of reaction products were similar to the products obtained in conventional synthesis.
Table 2.
Comparison between the proposed UANS with other processes.
| Process | Feedstock | Acid solution | Reaction time, min | Temperature, °C | Nitrogen content, % | Substitution degree | Ref. |
|---|---|---|---|---|---|---|---|
| Mechanical Stirring | Miscanthus cellulose | 54.6% of concentrated H2SO4 + 37% of concentrated HNO3 + 6.4% of H2O | 40 | 95 | 11.9 | 2.20 | [13] |
| Fermentation* | Bacterial cellulose | 75% of concentrated H2SO4 + 25% of concentrated HNO3 | 30 | 80 | 13.7 | 2.85 | [14] |
| Mechanical Stirring | Alfa grass fibers | 72% of concentrated H2SO4 + 28% of concentrated HNO3 | 35 | 40 | 12.5 | 2.43 | [37] |
| UANS | Microcrystalline cellulose | 54.6% of concentrated H2SO4 + 37.0% of concentrated HNO3 + 6.4% of H2O | 30 | 30 | 12.5 | 2.45 | This work |
Physical properties and explosive performance were not similar to conventional product.
4. Conclusion
Based on the results obtained in the present study, the proposed UANS process was considered as a promising alternative for nitrocellulose production, given a product with the substitution degree of 2.45. The proposed process is prone to produce nitrocellulose using approximately 82% of the hydroxyl groups available for reaction. It was observed that the cup horn system allowed to get a high substitution degree than those obtained when mechanical stirring (100 to 500 rpm) was evaluated. The main advantages of UANS are: (i) relatively small reaction time, (ii) low temperature, (iii) atmospheric pressure, and (iv) high efficiency of nitrogen substitution.
CRediT authorship contribution statement
Daniel Santos: . : Conceptualization, Methodology, Software, Writing - original draft. Gabrielle D. Iop: Conceptualization, Methodology, Software, Writing - original draft. Cezar A. Bizzi: Formal analysis, Writing - review & editing. Paola A. Mello: Writing - review & editing, Resources. Marcia F. Mesko: Writing - review & editing. Fernanda P. Balbinot: Writing - review & editing. Erico M.M. Flores: Supervision, Project administration, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are grateful to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil, 313786/2019-4), and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS, Brazil, 17/2551-0000960-6 and 17/2551-0000167-7) for supporting this study. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior Brazil (CAPES, Brazil) – Finance code 001.
References
- 1.Gallezot P. Conversion of biomass to selected chemical products. Chem. Soc. Rev. 2012;41(4):1538–1558. doi: 10.1039/C1CS15147A. [DOI] [PubMed] [Google Scholar]
- 2.Centi G., van Santen R.A. 1st ed. Wiley; Weinheim: 2007. Catalysis for Renewables: From Feedstock to Energy Production. [Google Scholar]
- 3.Voss B., Andersen S.I., Taarning E., Christensen C.H. C factors pinpoint resource utilization in chemical industrial processes. ChemSusChem. 2009;2:1152–1162. doi: 10.1002/cssc.200900215. [DOI] [PubMed] [Google Scholar]
- 4.Brown R.C., Brown T.R. 2nd ed. Wiley; Ames: 2014. Biorenewable Resources: Engineering New Products from Agriculture. [Google Scholar]
- 5.Golubev A.E., Kuvshinova S.A., Burmistrov V.A., Koifman O.I. Modern advances in the preparation and modification of cellulose nitrates. Russ. J. Gen. Chem. 2018;88(2):368–381. doi: 10.1134/S1070363218020305. [DOI] [Google Scholar]
- 6.Roy D., Semsarilar M., Guthrie J.T., Perrier S. Cellulose modification by polymer grafting: a review. Chem. Soc. Rev. 2009;38(7):2046. doi: 10.1039/b808639g. [DOI] [PubMed] [Google Scholar]
- 7.Abdul Khalil H.P.S., Davoudpour Y., Islam M.N., Mustapha A., Sudesh K., Dungani R., Jawaid M. Production and modification of nanofibrillated cellulose using various mechanical processes: a review. Carbohydr. Polym. 2014;99:649–665. doi: 10.1016/j.carbpol.2013.08.069. [DOI] [PubMed] [Google Scholar]
- 8.Saxena I.M., Brown R.M. Biosynthesis of cellulose. In: Morohoshi N., Komamine A., editors. Progress in Biotechnology. Elsevier; Netherlands: 2001. pp. 69–76. [Google Scholar]
- 9.D. Klemm, B. Philipp, T. Heinze, U. Heinze, W. Wagenknecht, Comprehensive Cellulose Chemistry. Volume 1: Fundamentals and Analytical Methods, 1st ed., Wiley: Weinheim, Germany, 1998.
- 10.Baranova N.V., Pashina L.A., Necheporenko A.P., Kostochko A.V. Acid and base sites of the surface of cellulose nitrates. Russ. J. Appl. Chem. 2015;88(1):124–128. doi: 10.1134/S1070427215010188. [DOI] [Google Scholar]
- 11.Alinat E., Delaunay N., Archer X., Mallet J.M., Gareil P. A new method for the determination of the nitrogen content of nitrocellulose based on the molar ratio of nitrite-to-nitrate ions released after alkaline hydrolysis. J. Hazard. Mater. 2015;286:92–99. doi: 10.1016/j.jhazmat.2014.12.032. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan F., Simon L., Ioannidis N., Patel S., Ophir Z., Gogos C., Jaffe M., Tirmizi S., Bonnett P., Abbate P. Nitration kinetics of cellulose fibers derived from wood pulp in mixed acids. Ind. Eng. Chem. Res. 2018;57:1883–1893. [Google Scholar]
- 13.Gismatulina Y.A., Budaeva V.V., Sakovich G.V. Nitrocellulose synthesis from miscanthus cellulose. Propellants, Explos., Pyrotech. 2018;43:96–100. [Google Scholar]
- 14.Sun D.P., Ma B., Zhu C.L., Liu C.S., Yang J.Z. Novel nitrocellulose made from bacterial cellulose. J. Energ. Mater. 2010;28:85–97. [Google Scholar]
- 15.Santos D., Silva U.F., Duarte F.A., Bizzi C.A., Flores E.M.M., Mello P.A. Ultrasound-assisted acid hydrolysis of cellulose to chemical building blocks: application to furfural synthesis. Ultrason. Sonochem. 2018;40:81–88. doi: 10.1016/j.ultsonch.2017.04.034. [DOI] [PubMed] [Google Scholar]
- 16.Antes F.G., Diehl L.O., Pereira J.S.F., Guimaraes R.C.L., Guarnieri R.A., Ferreira B.M.S., Dressler V.L., Flores E.M.M. Feasibility of low frequency ultrasound for water removal from crude oil emulsions. Ultrason. Sonochem. 2015;25:70–75. doi: 10.1016/j.ultsonch.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Nunes M.A.G., Mello P.A., Bizzi C.A., Diehl L.O., Moreira E.M., Souza W.F., Gaudino E.C., Cravotto G., Flores E.M.M. Evaluation of nitrogen effect on ultrasound-assisted oxidative desulfurization process. Fuel Process. Technol. 2014;126:521–527. [Google Scholar]
- 18.Cravotto G., Boffa L., Mantegna S., Perego P., Avogadro M., Cintas P. Improved extraction of vegetable oils under high-intensity ultrasound and/or microwaves. Ultrason. Sonochem. 2008;15:898–902. doi: 10.1016/j.ultsonch.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Mason T.J., Lorimer J.P. Wiley-VCH Verlag; Weinheim: 2002. Applied Sonochemistry: The Uses of Power Ultrasound in Chemistry and Processing. [Google Scholar]
- 20.Cravotto G., Cintas P. Power ultrasound in organic synthesis: moving cavitational chemistry from academia to innovative and large-scale applications. Chem. Soc. Rev. 2006;35:180–196. doi: 10.1039/b503848k. [DOI] [PubMed] [Google Scholar]
- 21.Chatel G., Vigier K.O., Jérôme F. Sonochemistry: what potential for conversion of lignocellulosic biomass into platform chemicals? ChemSusChem. 2014;7:2774–2787. doi: 10.1002/cssc.201402289. [DOI] [PubMed] [Google Scholar]
- 22.Kojima Y., Takayasu M., Toma M., Koda S. Degradation of cellulose in NaOH and NaOH/urea aqueous solutions by ultrasonic irradiation. Ultrason. Sonochem. 2019;51:419–423. doi: 10.1016/j.ultsonch.2018.07.030. [DOI] [PubMed] [Google Scholar]
- 23.Udoetok I.A., Wilson L.D., Headley J.V. Ultra-sonication assisted cross-linking of cellulose polymers. Ultrason. Sonochem. 2018;42:567–576. doi: 10.1016/j.ultsonch.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed F., Ayoub Arbab A., Jatoi A.W., Khatri M., Memon N., Khatri Z., Kim I.S. Ultrasonic-assisted deacetylation of cellulose acetate nanofibers: A rapid method to produce cellulose nanofibers. Ultrason. Sonochem. 2017;36:319–325. doi: 10.1016/j.ultsonch.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 25.Ashokkumar M., Cavalieri F., Chemat F., Okitsu K., Sambandam A., Yasui K., Zisu B. 1st ed. Springer; Singapore: 2016. Handbook of Ultrasonics and Sonochemistry. [Google Scholar]
- 26.Kentish S., Ashokkumar M. The physical and chemical effects of ultrassound. In: Feng H., Canovas G.B., Weiss J., editors. Ultrasound Technologies for Food and Bioprocessing. Springer; New York: 2011. [Google Scholar]
- 27.Capote F.P., Castro M.D.L. Elsevier Science; Boston: 2007. Analytical Applications of Ultrasound. [Google Scholar]
- 28.Military Standard MIL-DTL-244B Detail Specification: nitrocellulose; defense quality and standardization office, in, Washington, DC, 1996.
- 29.Military Standard MIL-STD-286C: Propellants, Solid: sampling, examination, and testing; headquarters, department of the army, in, Washington, DC, 2010.
- 30.Ossa M.A.F., López-López M., Torre M., García-Ruiz C. Analytical techniques in the study of highly-nitrated nitrocellulose. TRAC-Trend Anal. Chem. 2011;30:1740–1755. [Google Scholar]
- 31.Koda S., Kimura T., Kondo T., Mitome H. A standard method to calibrate sonochemical efficiency of an individual reaction system. Ultrason. Sonochem. 2003;10:149–156. doi: 10.1016/S1350-4177(03)00084-1. [DOI] [PubMed] [Google Scholar]
- 32.Kimura T., Sakamoto T., Leveque J.-M., Sohmiya H., Fujita M., Ikeda S., Ando T. Standardization of ultrasonic power for sonochemical reaction. Ultrason. Sonochem. 1996;3(3):S157–S161. doi: 10.1016/S1350-4177(96)00021-1. [DOI] [Google Scholar]
- 33.Mason T.J., Cobley A.J., Graves J.E., Morgan D. New evidence for the inverse dependence of mechanical and chemical effects on the frequency of ultrasound. Ultrason. Sonochem. 2011;18:226–230. doi: 10.1016/j.ultsonch.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 34.Diehl L.O., Gatiboni T.L., Mello P.A., Muller E.I., Duarte F.A., Flores E.M.M. Ultrasound-assisted extraction of rare-earth elements from carbonatite rocks. Ultrason. Sonochem. 2018;40:24–29. doi: 10.1016/j.ultsonch.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 35.Pedrotti M.F., Enders M.S.P., Pereira L.S.F., Mesko M.F., Flores E.M.M., Bizzi C.A. Intensification of ultrasonic-assisted crude oil demulsification based on acoustic field distribution data. Ultrason. Sonochem. 2018;40:53–59. doi: 10.1016/j.ultsonch.2017.03.056. [DOI] [PubMed] [Google Scholar]
- 36.Rashwan S.S., Dincer I., Mohany A. Investigation of acoustic and geometric effects on the sonoreactor performance. Ultrason. Sonochem. 2020;68 doi: 10.1016/j.ultsonch.2020.105174. [DOI] [PubMed] [Google Scholar]
- 37.Trache D., Khimeche K., Mezroua A., Benziane M. Physicochemical properties of microcrystalline nitrocellulose from alfa grass fibres and its thermal stability. J. Therm. Anal. Calorim. 2016;124:1485–1496. [Google Scholar]