Graphical abstract
Abbreviations: CMC, carboxymethyl cellulose; MS, maize starch; SA, stearic acid; ST, short-term; MT, moderate-term; LPD, low power density; HPD, high power density; TS, tensile strength; EB, elongation at break; Ra, average roughness; Rmax, maximum roughness height
Keywords: Ultrasonication, Maize starch, Stearic acid, Sodium carboxymethyl cellulose, Power density, Treatment time
Highlights
-
•
-The film-forming components became more compatible in the process of ultrasonication.
-
•
-Smoother and more homogeneous surface of the film were obtained after ultrasound.
-
•
-Suitable ultrasonication could improve the properties of composite film.
Abstract
Ultrasonic treatment can improve the compatibility between a hydrophobic material and a hydrophilic polymer. The light transmittance, crystalline structure, microstructure, surface morphology, moisture barrier, and mechanical properties of a composite film with or without ultrasonication were investigated. Ultrasound increases the film’s light transmittance, resulting in a film that has good transparency. Ultrasonication did not change the crystalline structure of the polymer film, but promoted V-type complex formation. The surface of the film became smooth and homogeneous after the film-form suspension underwent ultrasonic treatment. Compared to the control film, after ultrasonication at 70% amplitude with a duration of 30 min, the average roughness and maximum roughness declined from 212 nm to 17.6 nm and from 768.7 nm to 86.5 nm, respectively. The composite film with ultrasonication exhibited better tensile and moisture barrier properties than the nonsonicated film. However, long-term and strong ultrasonication will destroy the polymer structure to some extent.
1. Introduction
Biodegradable films prepared from natural polymers may replace plastic food packaging, helping to alleviate so-called white pollution composed of plastic waste [1]. The main natural polymers studied thus far have been proteins and polysaccharides. Starch is one of the most important natural biopolymers used in the preparation of biodegradable films and edible coatings [2]. Although starch is biodegradable, inexpensive, edible, abundant, renewable and widespread, polymer films based on starch have several shortcomings, such as high water sensitivity and poor mechanical properties, which severely limit their applications in food packaging. To deal with these problems, the incorporation of other film-forming components, such as hydrophobic agents and reinforcers, has recently drawn more attention from researchers.
Fatty acids, making up one of the most important groups of hydrophobic agents, have been incorporated in biopolymer materials to improve their moisture resistance [3]. According to Schmidt et al. [4], the addition of stearic acid enhances the moisture barrier properties of a cassava starch-based film as compared with the control film. Yang et al. [5] investigated the water vapor permeability of gellan-based film with or without lipids, and found that the water vapor permeability values of gellan film were reduced by the incorporation of lipids, especially when using beeswax. Ma et al. [6] suggested that sodium carboxymethyl cellulose (CMC) would improve the mechanical properties of starch polymer films due to its chemical characteristics, which are similar to those of starch. Previous studies observed that a corn starch-based film with CMC exhibited a higher ultimate tensile strength value than did the CMC-free film [7]. Ghanbarzadeh et al. [8], investigating the influence of the addition of CMC and citric acid to polymer film, found that the incorporation of 20% CMC enhanced the film’s tensile strength by approximately 145% as compared to the starch-based film without CMC. Reinforcing starch-based films with fatty acids or CMC has been investigated extensively. However, not many investigations have been reported on the combined effect of fatty acids and CMC in terms of the physicochemical properties of the polymer film. Furthermore, fatty acids, known to exhibit good hydrophobic properties, are thermodynamically immiscible with hydrophilic starch and CMC. From this point of view, a simple solution casting method will suffer from the incompatibility of film-forming components. According to Slavutsky et al. [9], ultrasonication is an effective method that can improve the compatibility of film-forming components and ensure that they are homogeneously dispersed within polymer matrices.
Dissolving starch and other film-forming components in hot water and then pouring, spraying, or casting on a special film-forming medium is still the main method to prepare the biodegradable starch-based polymer film [10]. In order to form a continuous and homogeneous polymer matrix, the film-forming components should be completely dispersed and dissolved in solvents [11]. Nevertheless, starch and other components are difficult to disperse and dissolve in water, due to their strong intramolecular and intermolecular hydrogen bonding forces and large molecular size [12]. To obtain a more economical and feasible dissolution method, ultrasonic treatment has been applied to film-forming solutions. According to Sujka et al. [13], for ultrasonically treated potato, wheat, corn and rice starch samples, solubility values were higher than for untreated starch samples. Previous reports suggested that ultrasound treatment could facilitate the formation of a homogeneous starch slurry [14]. In view of these findings, it is hypothesized that additional ultrasonication of a film-forming suspension with a hydrophobic component and a hydrophilic component prior to casting may improve the compatibility of the components by generating a homogeneous starch solution. In recent years, many reports on starch modification by ultrasonication treatment have been published. However, studies investigating the effects of different ultrasound power density levels (high power density and low power density) and treatment time (short, moderate, and long) on maize starch (MS)/stearic acid (SA)/CMC-based film have not yet been reported. This research is focused on the use of biodegradable film-forming components (MS, SA, CMC and glycerol), which lead to the preparation of highly environmental friendly polymer film to alleviate the problem of white pollution caused by plastic food packaging. It would be expected that ultrasonically treated MS/SA/CMC composite film would have better mechanical and moisture barrier properties compared to conventional starch-based film due to the presence of hydrophobic carbon chains (SA) and chemical similarity of MS and CMC, which make it satisfactory for some applications such as food packaging.
The aim of this work was to examine the effect of ultrasonic treatment on the physicochemical properties of an MS/SA/CMC composite film under different conditions of ultrasound power density and treatment time, in turn providing some theoretical basis for sonochemistry and its applications in starch modification.
2. Materials and methods
2.1. Materials
MS was purchased from Shandong Dazong Biological Development Co., Ltd. (Shandong, China). SA was obtained from Tianjin Dingshengxin Chemical Industry Co., Ltd. (Tianjin, China). CMC was provided by Yuanye Biotechnology Co., Ltd. (Shanghai, China). Glycerol and ethanol were supplied by Fuyu Fine Chemical Co., Ltd. (Tianjin, China).
2.2. Film casting
Ten grams of MS were mixed with 200 ml of distilled water and glycerol (30% of MS) and CMC (5% of MS) at room temperature. The SA (5% of MS), was dissolved in 30 ml of ethanol, then added to the above starch suspension to prepare the film-forming solution. The film-forming solution was stirred (300 rpm) at 90 °C for 90 min. After the heating procedure, the film-forming solution obtained immediately received ultrasonication for 5 min (short-term: ST), 15 min (moderate-term: MT), and 30 min (long-term: LT), using an ultrasonic processor (model VCX800, Sonics & Materials, Inc., Newtown, USA) equipped with a probe with a diameter of 13 mm. The ultrasound amplitudes were set at 30% (240 W/cm2, low power density: LPD) and 70% (560 W/cm2, high power density: HPD), respectively. The treated film-forming suspension was filtered through gauze; after this, the suspension was poured onto Teflon-coated glass plates. The film-forming suspension was dried at 40 °C, using an oven. The MS/SA/CMC composite film without ultrasonic treatment was used as the control sample. The samples were coded as ST-LPD, ST-HPD, MT-LPD, MT-HPD, LT-LPD and LT-HPD for samples treated with 5 min at 30%, 5 min at 70%, 15 min at 30%, 15 min at 70%, 30 min at 30% and 30 min at 70%, respectively. The pure MS-based film without SA and CMC addition was considered the native sample.
All of the ultrasonically treated film and untreated film were equilibrated at 53% (relative humidity) and 25 °C for 48 h before analysis.
2.3. Light transmittance of the MS-based film
Light transmittance of the ultrasonically treated and untreated films was determined in triplicate as described by Sun et al. [15], using an ultraviolet–visible spectrophotometer. The film sample was cut into a rectangular strip (4 cm × 1 cm). The absorbance spectrum was measured for 400 to 800 nm.
2.4. Mechanical properties of the MS-based film
The tensile strength (TS) and elongation at break (EB) of the ultrasonically treated and untreated films as obtained in a tensile test were determined by an auto tensile tester (XLW, Labthink Instruments Co., Ltd., Jinan, China). The film samples were cut into rectangular strips (120 mm × 20 mm). The initial grip separation was set at 80 mm, and the test speed was 300 mm/min. At least six replicates of each film sample were examined [16].
2.5. Water vapor permeability of the MS-based film
Water vapor permeability values for the ultrasonically treated and untreated films were based on a previous method [17]. The diameter of the test film sample was 64.8 mm. The relative humidity and temperature in the water vapor transmission rate testing equipment were 90% and 38 °C, respectively. All the determinations were obtained in triplicate.
2.6. X-ray diffraction patterns of the composite film
Diffractograms were obtained for the native, control and ultrasonically treated samples with an X-Ray diffractometer (Ultima IV, Rigaku Corporation, Japan) operating at 40 kV and 40 mA with monochromatic Cu-Kα radiation. Diffraction patterns were obtained over the angular region (2θ) of 5-40° with a step size of 0.02° [18], [19], [20].
2.7. Scanning electron microscopy
Micrographs of the starch-based film were observed using a Supra 55 scanning electron microscope (ZEISS, Jena, Germany). The ultrasonically treated and untreated samples were fixed on the test platform by bonding each with conductive carbon tape. Each sample was gold plated and photographed at an accelerating voltage of 20 kV with a magnification of 1000× [16].
2.8. Atomic force microscopy
The morphological surface of each sample of the native, control and ultrasonically treated films was measured with an atomic force microscope (Bruker Multimode8, Madison, USA). The measurement was determined in a ScanAsyst in air mode. The scan size was set to 10 μm × 10 μm.
2.9. Statistical analysis
Effects of ultrasonication on analysed parameters were evaluated by analysis of variance and Duncan’s multiple range test (p < 0.05), using statistical analysis with SPSS 17.0.
3. Results and discussion
3.1. Light transmittance of the composite film
According to Fabra et al. [21], the light transmittance value is correlated with the functional performance of the polymer film, since light transmittance has a direct impact on the appearance of packaging products and on consumer acceptance. Table 1 exhibits the light transmittance values of the native, control and ultrasonically treated films. As displayed in Table 1, the light transmittance values of the MS/SA/CMC composite film without ultrasonication were significantly lower than those for the native sample. This result suggested that the compatibility between film-forming components, especially for SA and CMC, was poor. After ultrasonic treatment, as compared to the untreated polymer film, the light transmittance values of composite films were enhanced by 18.2% to 62.2% (600 nm), indicating that the film-forming components became more compatible through the process of ultrasonication. Previous studies suggested that a better the compatibility of film-forming components yields a higher light transmittance for the polymer film [22]. A similar improvement in the light transmittance values of polymer film has been obtained by other researchers who used ultrasonication to treat a gelatinised MS dispersion [12].
Table 1.
The effect of ultrasonication on light transmittance of the films.
| Film type | Light transmission (%) |
||||
|---|---|---|---|---|---|
| 400-nm wavelength | 500-nm wavelength | 600-nm wavelength | 700-nm wavelength | 800-nm wavelength | |
| Native sample | 45.9 ± 0.99c | 49.0 ± 0.74d | 51.0 ± 1.19e | 52.8 ± 1.23d | 54.5 ± 1.15d |
| Control | 17.3 ± 0.74e | 17.6 ± 0.70f | 18.2 ± 0.83 g | 18.5 ± 0.77f | 18.9 ± 0.88f |
| ST-LPD | 42.0 ± 1.08d | 44.5 ± 1.12e | 47.0 ± 0.66f | 48.4 ± 0.58e | 49.7 ± 0.61e |
| MT-LPD | 49.2 ± 1.00b | 52.5 ± 0.99c | 54.8 ± 0.69 cd | 56.6 ± 1.15c | 58.0 ± 1.35c |
| LT-LPD | 50.2 ± 1.28b | 52.9 ± 1.20c | 55.0 ± 0.96c | 56.4 ± 0.88c | 57.6 ± 0.87c |
| ST-HPD | 47.5 ± 1.05bc | 50.2 ± 1.08 cd | 52.1 ± 1.26de | 53.4 ± 0.64d | 55.2 ± 1.38 cd |
| MT-HPD | 54.3 ± 0.99a | 56.9 ± 0.89b | 58.8 ± 1.02b | 60.0 ± 0.81b | 61.2 ± 0.78b |
| LT-HPD | 57.0 ± 1.06a | 59.9 ± 0.90a | 62.2 ± 1.12a | 63.6 ± 0.99a | 64.7 ± 0.94a |
a – g: Mean values in the same column with different superscripts are significantly different (P<0.05). Data shown in mean ± standard deviation (n = 3).
As indicated in Table 1, increasing the treatment time from 5 to 30 min increases the light transmittance values (600 nm) of both the LPD and HPD samples, from 47.0% and 52.1% to 55.0% and 62.2%, respectively. This finding can be attributed to the full gelatinisation of starch granules and removal of bubbles in the film-forming suspension during ultrasonication, leading to greater film homogeneity being obtained for the MT and LT ultrasonically treated films [12]. Some incompletely gelatinised starch granules (ghosts) were observed in the untreated films, and this can decrease film transparency [23]. With an increase of treatment time from ST to LT, the light transmittance values increased gradually. It is noteworthy that the values obtained for LT-LPD displayed no significant difference from those for the MT-LPD samples, while LT-HPD treatment significantly increased the light transmittance of composite films in comparison with the MT-HPD treated films. The higher the power density, the stronger was the cavitation effect. That is, the cavitation efficiency of HPD-treated samples was greater than for LPD-treated samples.
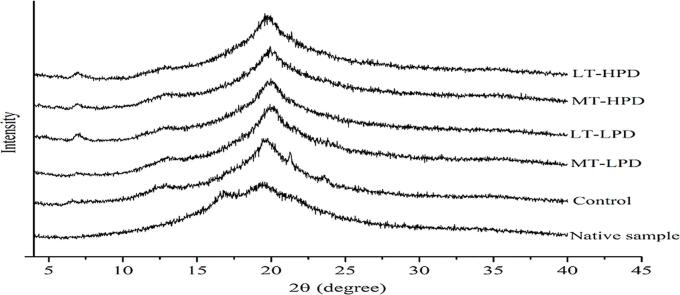
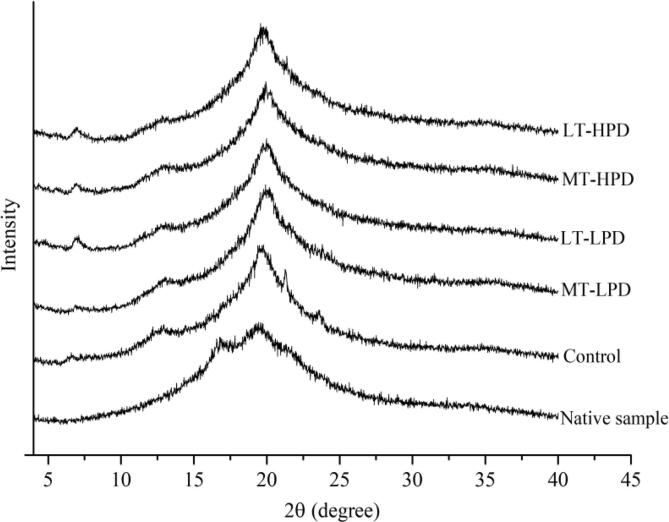
3.2. X-ray diffraction
Fig. 1 shows the crystalline structure of starch-based films formed after ultrasonic treatment with different times and amplitudes. As observed in Fig. 1, the native sample showed a mixture of B- and V-type crystalline patterns, exhibiting two main diffraction peaks at 2θ of 17° (retrogradation of amylose) and 19.7° (inclusion complex formed between amylose and endogenous lipid in starch). After the addition of SA and CMC, the B-type diffraction peak disappeared and the result took on a V-type pattern with three peaks at approximately 2θ of 7.5°, 13°, and 20°. This phenomenon from one point of view suggests that the V-type complex may restrain the retrogradation of the amylose molecules [24]. In addition, two weak diffraction peaks emerged at 2θ of approximately 21° and 24° for the control sample, and these represent the characteristic peaks of SA, indicating an incomplete reaction between amylose and SA due to the poor dispersibility of SA in the film-forming suspension [25]. All the MS/SA/CMC composite films exhibit similar curves, indicating that ultrasonic treatment did not alter the crystalline pattern of the polymer film. Investigation reported by Abral et al. [26] found similar results in a jicama starch-based film. In comparison, the intensity of the V-type diffraction peaks increased slightly, especially for the diffraction peak at 2θ of 7.5°, after the film-forming suspension underwent ultrasonic treatment (data for ST-LPD and ST-HPD treatment are not shown), which suggests that more inclusion complexes were formed in the ultrasonically treated samples. Furthermore, the characteristic diffraction peaks of free SA also were absent for the ultrasonically treated samples. These results indicate that the dispersibility of SA was improved after the film-forming suspension received ultrasonic treatment, which in turn resulted in an increased probability of complexation between the SA and amylose. Previous studies also reported that the film-forming components were homogeneously dispersed within the polymer matrix through the process of ultrasonication [27].
Fig. 1.
X-ray diffraction patterns of ultrasonically treated and untreated films.
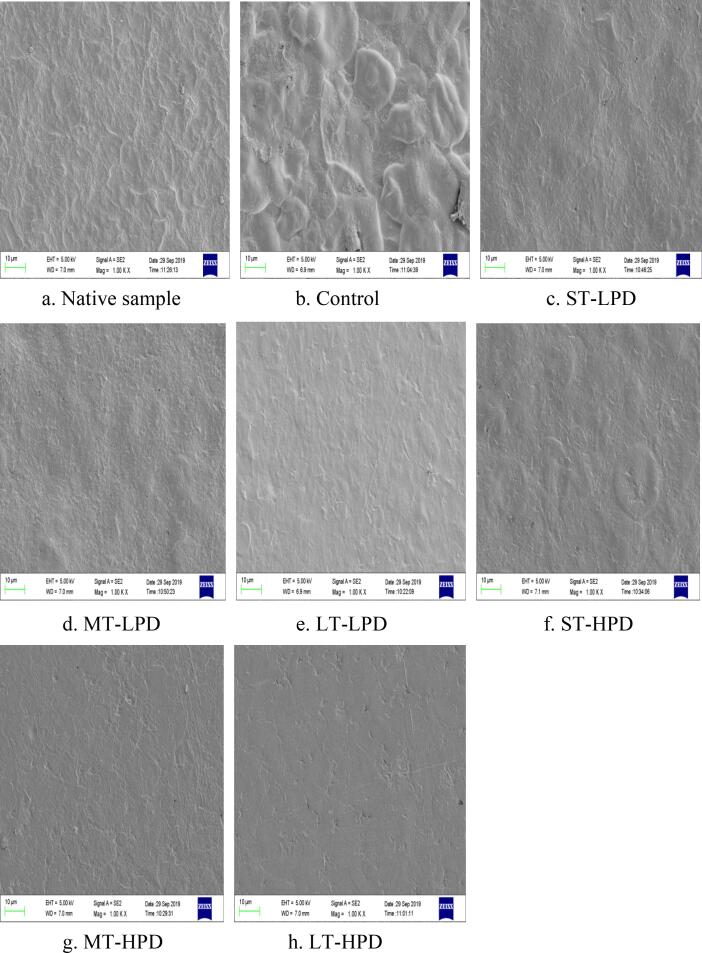
3.3. Microstructure of ultrasonically treated and untreated film
Fig. 2 (a-h) shows scanning electron microscopy graphs of the surface microstructure of the native sample and MS/SA/CMC composite films with or without ultrasonication. As indicated in Fig. 2 (a), the native sample without SA and CMC exhibited a rough surface. This rough surface of this native sample is probably caused by agglomerations of incompletely gelatinised starch and insoluble starch granules (termed ‘ghosts’) on the surface of the polymer matrices [26]. Also, the control film without ultrasonic treatment displayed a rough and irregular surface. This effect is attributed to poor compatibility between SA and MS/CMC. In comparison with the untreated material, the surface of the polymer film became smoother and more homogeneous after the film-forming suspension underwent ultrasonic treatment. This finding can be credited to ultrasonication facilitating adequate blending of the hydrophobic component (SA) and hydrophilic components (MS and CMC), with further improvement in the compatibility between them. Furthermore, the ghosts in the film-forming suspension disintegrate gradually during the process of ultrasonication, thus leading to a homogeneous and compact surface for ultrasonically treated samples, without the irregular structure. Surfaces that were more homogeneous and smoother, as obtained for the films with ultrasonic treatment, present an effect similar to an observation found by Wang et al. [28]. With the increase of treatment time and ultrasonic amplitude, the polymer matrix and its surface became more compact and smoother as indicated in Fig. 2. It is worth noting that some small cavities or cracks appeared on the surface of the LT-HPD sample. According to Suslick [29], high-speed jets of liquids into the surface of film-forming dispersion formed and induced shockwave damage at higher ultrasonication power, leading to the appearance of localised erosion in the film from the LT-HPD conditions. The nonhomogeneous structure suggested there would be negative effects in terms of the tensile and moisture barrier properties of the polymer film, which was confirmed in the results for the tensile test that followed.
Fig. 2.
The surface microstructure of native sample and MS/SA/CMC composite film.
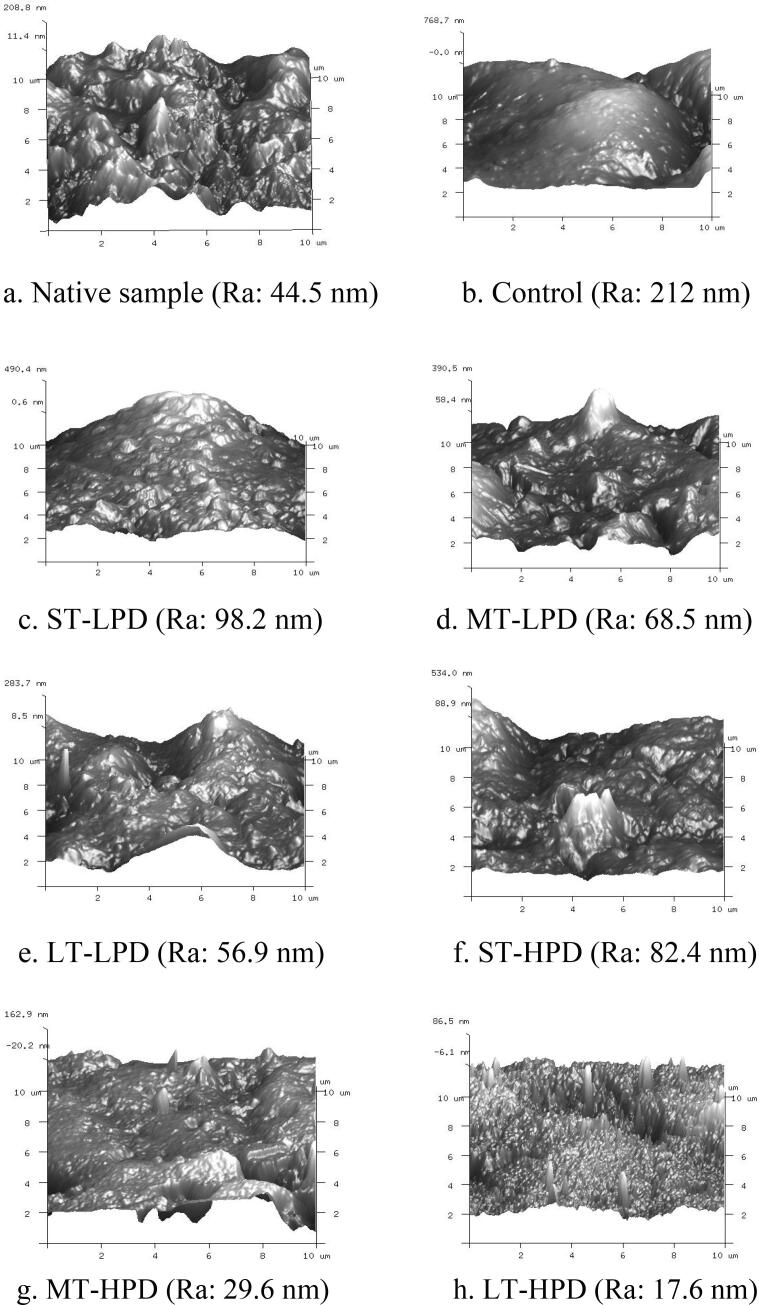
3.4. Surface morphology of MS/SA/CMC composite film
Atomic force microscopy was employed to evaluate the roughness and surface morphology of the polymer materials. In general, the quantitative indexes of surface morphology assessed included average roughness (Ra) and maximum roughness height (Rmax). These data and morphological images of the native sample and MS/SA/CMC composite film with and without ultrasonication are displayed in Fig. 3. Compared with the native sample, after the addition of SA and CMC, the Ra and Rmax values of films were enhanced, changing from 44.5 nm to 212 nm and from 208.8 nm to 768.7 nm, respectively. As shown in Fig. 3 (b), the three-dimensional (3D-) image of the MS/SA/CMC composite film demonstrated a rough structure, indicating that it was difficult to form a cohesive and continuous structure among the film-forming components. Notably, ultrasound treatment led to a significant decrease in Ra and Rmax for the MS/SA/CMC composite film, indicating that the ultrasonication contributed to generating a uniform and continuous polymer matrix with a relative smooth surface, in accordance with the results obtained from scanning electron microscopy measurement. From ST to LT and from LPD to HPD, as the ultrasonic power became stronger and the treatment time longer, the values of Ra and Rmax became lower. The control film without ultrasonication exhibited the highest Ra and Rmax values of 212 nm and 768.7 nm, respectively, which decreased to 17.6 nm and 86.5 nm for the LT-HPD condition. This suggested that the improvement in the flatness of the polymer film was enhanced by the increase of ultrasonic treatment time and amplitude through an acoustic cavitation effect.
Fig. 3.
The surface morphology of MS/SA/CMC composite film.
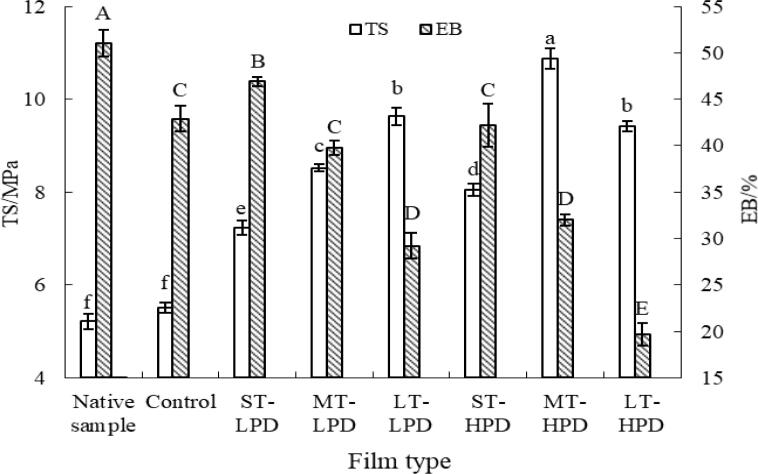
3.5. Mechanical properties
The TS and EB are important for polymer films, as they represent the durability of materials. Fig. 4 displays the TS and EB values of the sonicated and untreated films. Compared to the native sample, after SA and CMC addition, the TS value of the polymer film increased slightly and the EB value decreased. In the presence of SA, the enhancement effect from CMC is probably weakened due to the thermodynamic incompatibility, resulting in incomplete interaction between MS and CMC. As indicated in Fig. 4, the control sample without ultrasound treatment exhibited the lowest TS value among all of the results for the MS/SA/CMC composite films. This suggested that without ultrasonication a less compact polymer structure of MS/SA/CMC composite film formed from the film-forming suspension, due to the poor compatibility of the components. Obviously, the ultrasonic treatment produced a significant increase in the TS value, indicating that the changes in the polymer matrix came from the influence of ultrasonication, generating a more compact and tight structure between film-forming components during the film drying process. That is, the shock waves and mechanical force in the process of ultrasound treatment could allow greater molecular interaction between the film-forming components, yielding a polymer film with higher TS. According to Abral et al. [26], the destruction of ghosts in the film-forming suspension resulted in a more homogeneous matrix of the ultrasonically treated samples, generating more effective reinforcement of the film strength. Another possible cause was the formation of more V-type inclusion complexes during ultrasonication, which may have provided greater strength for the polymer matrix, leading to more effective resistance against an applied external load [17]. Furthermore, this was probably caused by an interfacial interaction between the MS and CMC due to their chemical similarity [7]. A similar finding was obtained by other researchers in a sago starch-based polymer film with ultrasonic treatment, where an increase in the tensile properties was found [27]. As shown in Fig. 4, the ultrasonic amplitude and duration had obvious effects on the mechanical properties of the composite film. The enhancement of TS for the MS/SA/CMC composite film increased with increasing ultrasonic amplitude and treatment time (excluding LT-HPD sample). For instance, when the ultrasonic amplitude and duration were 70% and 15 min (MT-HPD), respectively, the value of TS reached 10.88 MPa, which was 97.5% higher than the value without ultrasonication. Simultaneously, the EB value of the polymer film decreased as the amplitude and time of ultrasonic treatment increased. The decrement of EB values might be attributed to heterogeneous network in the materials (especially for the LT-HPD sample), which would rupture the composite film during stretching and prevent its further elongation. Interestingly, the TS and EB values of the polymer film decreased when ultrasound at the highest treatment time and amplitude (LT-HPD) was applied to film-forming solutions. A previous study suggested that there is a suitable duration of ultrasonication sufficient to obtain a compact and homogeneous polymer matrix; beyond this, the LT and strong ultrasonication can destroy the polymer structure [27]. For the LT-HPD sample, relatively more starch granules were destroyed during the strong ultrasonication procedure, which led to the intermolecular interaction being weakened, and the polymer structure of the composite film being impaired to some extent.
Fig. 4.
Mechanical properties of MS/SA/CMC composite film.
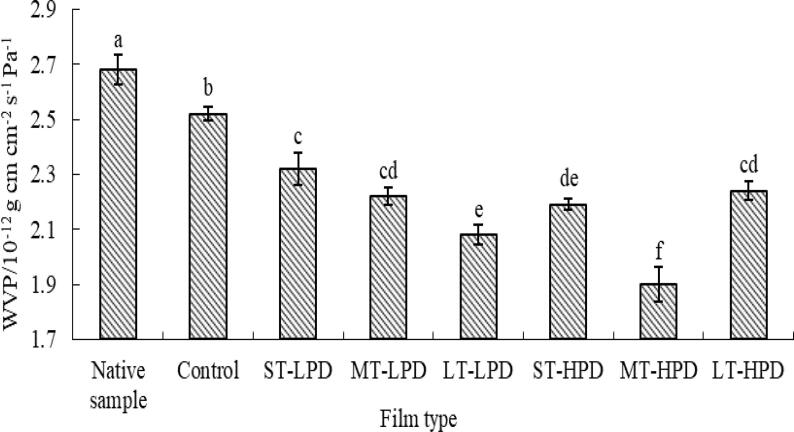
3.6. Water vapor permeability
Water vapor permeability values for the native sample and the MS/SA/CMC composite film with and without ultrasonication are shown in Fig. 5. Compared to the native sample, after the addition of SA and CMC the water vapor permeability value for the polymer film declines from 2.68 × 10−12 to 2.52 × 10−12 g cm cm−2 s−1 Pa−1. This probably results from the presence of the hydrophobic carbon chain of SA in the polymer matrix [30]. In addition, according to Kristo et al. [31], the presence of CMC could induce a tortuous path for water molecules passing through, thus restricting moisture permeation. As observed in Fig. 5, the ultrasonicated MS/SA/CMC composite film exhibited lower values of water vapor permeability than the one not sonicated (control sample), indicating that the ultrasonication improved the moisture resistance of the material. Previous studies suggested that the greater tightness and compactness in the polymer matrices of the ultrasonicated films result in greater resistance to permeation of moisture [27]. Another reason could be that CMC dispersed well in the polymer matrices after the film-forming suspension received ultrasonication, and in turn inhibited the transmission of water molecules [7]. A similar result was found by Cheng et al. [12], who reported that the film formed from ultrasound-treated gelatinised MS suspensions has lower values for water vapor permeability. Meanwhile, effects of ultrasonic amplitude and treatment time show that with higher/longer amplitude/time, water vapor permeability values are lower, except for the LT-HPD sample. This result is likely because the polymer film with strong ultrasonic treatment had less micro- and nanosized porosities, thus lowering the ability of the water molecule to diffuse into porous space, as described by a Fickian diffusion process [32]. The nonhomogeneous structure of the LT-HPD sample as shown in the SEM analysis would impair its barrier against moisture. Therefore, the water vapor permeability value for the LT-HPD sample was higher than that for the MT-HPD sample. This finding suggests that a suitable duration and power of ultrasonication can improve the film’s moisture resistance.
Fig. 5.
Water vapor permeability of MS/SA/CMC composite film.
4. Conclusions
Film-forming suspensions were treated with ultrasound at different amplitudes and durations. The hydrophobic component and hydrophilic component became more compatible in the process of ultrasonication. An ultrasound duration of 15 min at 70% amplitude resulted in the better tensile and moisture barrier properties for the polymer film. The MS/SA/CMC composite film showed excellent properties after ultrasonic treatment, including higher light transmittance, a more homogeneous and smoother surface, desirable tensile properties and moisture resistance. Using ultrasound with a suitable action time and amplitude can improve the properties of polymer film, although an LT-HPD treatment destroys the polymer structure to some extent. The ultrasonic treatment of the film-forming suspension at low concentration levels (starch slurry: 5%) favours the formation of polymer films with excellent properties. Further investigation should explore whether it has the same effect at high levels of concentration.
CRediT authorship contribution statement
Pengfei Liu: Conceptualization, Methodology, Software, Formal analysis, Investigation, Writing - original draft. Wei Gao: Investigation, Methodology, Data curation. Xiaolei Zhang: Investigation. Bin Wang: Investigation. Feixue Zou: Investigation. Bin Yu: Investigation. Lu Lu: . Yishan Fang: Investigation. Zhengzong Wu: Investigation. Chao Yuan: Investigation. Bo Cui: Methodology, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study has been supported by University Student Innovation and Entrepreneurship Training Programs of Shandong Province (S202010431080), Natural Science Foundation of Shandong Province (ZR2018BC064), National Key Research & Development Program in China (Grant No.2019YFD1002704), Key Research and Development Program of Shandong Province (No.2017YYSP024), Innovation Team of Jinan City (2018GXRC004), Natural Science Foundation of China (No.31901645), Shandong major projects of independent innovation (2019JZZY010722), Bohai Sea Granary Science and Technology Demonstration Project (2019BHLC002), Special Project of International Cooperative Research (QLUTGJHZ2018016), Shandong major innovation project of agricultural application technology (SF1405303301) and Special Funds for Taishan Scholars Project.
References
- 1.Parra D.F., Tadini C.C., Lugao A.B. Mechanical properties and water vapor transmission in some blends of cassava starch edible films. Carbohydr. Polym. 2004;58:475–481. [Google Scholar]
- 2.Chiumarelli M., Hubinger M.D. Evaluation of edible films and coatings formulated with cassava starch, glycerol, carnauba wax and stearic acid. Food Hydrocolloid. 2014;38(4):20–27. [Google Scholar]
- 3.Zahedi Y., Ghanbarzadeh B., Sedaghat N. Physical properties of edible emulsified films based on pistachio globulin protein and fatty acids. J. Food Eng. 2010;100:102–108. [Google Scholar]
- 4.Schmidt V.C.R., Porto L.M., Laurindo J.B., Menegalli F.C. Water vapor barrier and mechanical properties of starch films containing stearic acid. Ind. Crop. Prod. 2013;41:227–234. [Google Scholar]
- 5.Yang L., Paulson A.T. Effects of lipids on mechanical and moisture barrier properties of edible gellan film. Food Res. Int. 2000;33:571–578. [Google Scholar]
- 6.Ma X., Chang P.R., Yu J. Properties of biodegradable thermoplastic pea starch/carboxymethyl cellulose and pea starch/microcrystalline cellulose composites. Carbohydr. Polym. 2008;72:369–375. [Google Scholar]
- 7.Ghanbarzadeh B., Almasi H., Entezami A.A. Physical properties of edible modified starch/carboxymethyl cellulose films. Innov. Food Sci. Emerg. 2010;11:697–702. [Google Scholar]
- 8.Ghanbarzadeh B., Almasi H., Entezami A.A. Improving the barrier and mechanical properties of corn starch-based edible films: Effect of citric acid and carboxymethyl cellulose. Ind. Crop. Prod. 2011;33(1):229–235. [Google Scholar]
- 9.Slavutsky A.M., Bertuzzi M.A. Water barrier properties of starch films reinforced with cellulose nanocrystals obtained from sugarcane bagasse. Carbohydr. Polym. 2014;110:53–61. doi: 10.1016/j.carbpol.2014.03.049. [DOI] [PubMed] [Google Scholar]
- 10.Galdeano M.C., Grossmann M.V.E., Mali S., Bello-Perez L.A., Garcia M.A., Zamudio-Flores P.B. Effects of production process and plasticizers on stability of films and sheets of oat starch. Mat. Sci. Eng. C. 2009;29:492–498. [Google Scholar]
- 11.Shamekh S., Myllärinen P., Poutanen K., Forssell P. Film formation properties of potato starch hydrolysates. Starch/Stärke. 2002;54:20–24. [Google Scholar]
- 12.Cheng W.J., Chen J.C., Liu D.H., Ye X.Q., Ke F.S. Impact of ultrasonic treatment on properties of starch film-forming dispersion and the resulting films. Carbohydr. Polym. 2010;81:707–711. [Google Scholar]
- 13.Sujka M., Jamroz J. Ultrasound-treated starch: SEM and TEM imaging, and functional behaviour. Food Hydrocolloid. 2013;31(2):413–419. [Google Scholar]
- 14.Iida Y., Tuziuti T., Yasui K., Towata A., Kozuka T. Control of viscosity in starch and polysaccharide solutions with ultrasound after gelatinization. Innov. Food Sci. Emerg. 2008;9:140–146. [Google Scholar]
- 15.Sun S.L., Liu P.F., Ji N., Hou H.X., Dong H.Z. Effects of low polyhydroxyalkanoate content on the properties of films based on modified starch acquired by extrusion blowing. Food Hydrocolloid. 2017;72:81–89. [Google Scholar]
- 16.Sun S.L., Liu P.F., Ji N., Hou H.X., Dong H.Z. Effects of various cross-linking agents on the physicochemical properties of starch/PHA composite films produced by extrusion blowing. Food Hydrocolloid. 2018;77:964–975. [Google Scholar]
- 17.Liu P.F., Kang X.M., Cui B., Wang R., Wu Z.Z. Effects of glycerides with different molecular structures on the properties of maize starch and its film forming capacity. Ind. Crop. Prod. 2019;129:512–517. [Google Scholar]
- 18.Ning Y.J., Cui B., Yuan C., Zou Y.Y., Liu W.Z., Pan Y. Effects of konjac glucomannan on the rheological, microstructure and digestibility properties of debranched corn starch. Food Hydrocolloid. 2020;100 [Google Scholar]
- 19.Liu P.F., Gao W., Zhang X.L., Wu Z.Z., Yu B., Cui B. Physicochemical properties of pea starch-lauric acid complex modified by maltogenic amylase and pullulanase. Carbohydr. Polym. 2020;242 doi: 10.1016/j.carbpol.2020.116332. [DOI] [PubMed] [Google Scholar]
- 20.Guo L., Li J.H., Gui Y.F., Zhu Y., Cui B. Improving waxy rice starch functionality through branching enzyme and glucoamylase: Role of amylose as a viable substrate. Carbohydr. Polym. 2020;230 doi: 10.1016/j.carbpol.2019.115712. [DOI] [PubMed] [Google Scholar]
- 21.Fabra M.J., Talens P., Chiralt A. Influence of calcium on tensile, optical and water vapour permeability properties of sodium caseinate edible films. J. Food Eng. 2010;96:356–364. [Google Scholar]
- 22.Yan Q.Q., Hou H.X., Guo P., Dong H.Z. Effects of extrusion and glycerol content on properties of oxidized and acetylated corn starch-based films. Carbohydr. Polym. 2012;87:707–712. doi: 10.1016/j.carbpol.2011.08.048. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Hernandez A., Vernon-Carter E.J., Alvarez-Ramirez J. Impact of ghosts on the mechanical, optical, and barrier properties of corn starch films. Starch/Stärke. 2017;69:1–7. [Google Scholar]
- 24.Wang S.J., Li C.L., Copeland L., Niu Q., Wang S. Starch retrogradation: a comprehensive review. Compr. Rev. Food Sci. F. 2015;14(5):568–585. [Google Scholar]
- 25.Lesmes U., Chen S.H., Shener Y., Shimoni E. Effects of long chain fatty acid unsaturation on the structure and controlled release properties of amylose complexes. Food Hydrocolloid. 2009;23:667–675. [Google Scholar]
- 26.Abral H., Anugrah A.S., Hafizulhaq F., Handayani D., Sugiarti E., Muslimin A.N. Effect of nanofibers fraction on properties of the starch based biocomposite prepared in various ultrasonic power. Int. J. Biol. Macromol. 2018;116:1214–1221. doi: 10.1016/j.ijbiomac.2018.05.067. [DOI] [PubMed] [Google Scholar]
- 27.Abral H., Basri A., Muhammad F., Fernando Y., Hafizulhaq F., Mahardika M., Sugiarti E., Sapuan S.M., Ilyas R.A., Stephane I. A simple method for improving the properties of the sago starch films prepared by using ultrasonication treatment. Food Hydrocolloid. 2019;93:276–283. [Google Scholar]
- 28.Wang L.L., Ding J., Fang Y., Pan X., Fan F.J., Li P., Hu Q.H. Effect of ultrasonic power on properties of edible composite films based on rice protein hydrolysates and chitosan. Ultrason. Sonochem. 2020;65 doi: 10.1016/j.ultsonch.2020.105049. [DOI] [PubMed] [Google Scholar]
- 29.Suslick K.S. ChemInform Abstract: Applications of ultrasound to materials chemistry. Cheminform. 1999;29:295–326. [Google Scholar]
- 30.Garcia M.A., Martino M.N., Zaritzky N.E. Lipid addition to improve barrier properties of edible starch-based films and coatings. J. Food Sci. 2000;65:941–944. [Google Scholar]
- 31.Kristo E., Biliaderis C.G. Physical properties of starch nanocrystal reinforced films. Carbohydr. Polym. 2007;29(1):254–259. [Google Scholar]
- 32.Sahari J., Sapuan S.M., Zainudin E.S., Maleque M.A. Thermo-mechanical behaviors of thermoplastic starch derived from sugar palm tree (Arenga pinnata) Carbohydr. Polym. 2013;92:1711–1716. doi: 10.1016/j.carbpol.2012.11.031. [DOI] [PubMed] [Google Scholar]