Graphical abstract

Keywords: Methicillin-resistant Staphylococcus aureus (MRSA), Mannosylerythritol lipid-A (MEL-A), Ultrasound, Antibacterial, Antibiofilm
Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) is drug-resistant and biofilm-forming pathogenic bacteria with severe morbidity and mortality, and has been continuously detected in food products in recent years. Mannosylerythritol lipids (MELs) are novel biosurfactants and perform antibacterial property against gram-positive bacteria. Ultrasound has been applied into food sterilization as non-thermal techniques and has advantage of maintaining food nutrition and flavor over heat pasteurization. In this work, the synergistic treatment of ultrasound and MEL-A was used to combat planktonic cells and biofilm of MRSA. As a result, the combined treatment has exhibited remarkable antibacterial effect proved by enumeration of viable microbes. Furthermore, flow cytometry, scanning electron microscopy and transmission electron microscopy revealed ultrasound has enhanced the inhibitory effect of MEL-A through exacerbating cell membrane damage. On the other hand, the collaborating working modes to eradicate MRSA biofilm were disturbing cell adhesion to surface by MEL-A and destructing mature biofilm mechanically by ultrasound, reaching to over 90% of clearance rate. The findings of this study illustrated the synergistic antimicrobial mechanism of ultrasound and MEL-A treatments, and offered theoretical basis for their potential applications in food preservation.
1. Introduction
In recent years, food safety problem has posed a severe threat to public health and been received increasing attention worldwide along with frequent outbreaks of foodborne diseases. In fact, foodborne spoilage and pathogenic microorganisms take responsibility for the major food safety challenges [1]. Even worse, owing to abuse and misuse of antibiotics, the escalating tide of drug-resistant strains triggers a tremendous economic burden and seriously menaces human health care. According to the World Health Organization (WHO), over two million people are infected by antibiotic-resistant pathogens, rendering 2300 deaths per year [2]. Due to its serious morbidity and mortality, methicillin-resistant Staphylococcus aureus (MRSA) accounts for plenty of microbial illness and lethal infections terribly. The epidemiology of these infections is quite complicated, also considering the possibility of strain exchanges between humans and animals and vice versa [3]. Lately, MRSA has been continuously detected in a variety of foods, such as beef, poultry meat, pork and milk [4], [5], [6], [7], which has raised concerns about its transmission to humans via handling and/or consumption of food. Except for planktonic cells, the ability of biofilm formation is the advantage of MRSA to resist adverse environment and spread virulence. The structure of biofilm provides shelter and necessary nutrients for the microbes, ensuring the cells’ adaptivity to adverse growth conditions. As a fortress, biofilm allows no access of antibiotics to cells, which increases the resistance of MRSA to different drugs. Due to its high tolerance to antibiotics, the development of new effective anti-MRSA agents or methods is urgently needed.
With the demands for long-time food storage, the different control research of MRSA microbes has come into view and been put applications into the food processing industry for decades, but current studies have shown the artificial or synthetic food preservatives combining physical treatments are possible for pathogenic risk [8]. Thus, natural food preservatives with safety and efficiency are those people dedicated to looking for. Biosurfactants are surface-active biomolecules endowed with diverse biological properties (such as antimicrobial, antiviral, hemolytic and insecticide activities), which are produced by various microorganisms [9]. As a kind of biosurfactants, mannosylerythritol lipids (MELs) have obtained attention due to their environmental compatibility, mild production conditions, structural diversity, self-assembling properties and versatile biochemical functions, and have great applications in food industry, cosmetic, medicine, and other fields [10]. The chemical structures of MELs vary with the different fatty acid chains and the acetyl groups, commonly known as MEL-A, MEL-B, MEL-C, and MEL-D. Among these, MEL-A and MEL-B have shown strong activity against gram-positive bacteria, weak activity against gram-negative bacteria and no activity against fungi [11]. Our previous study demonstrated that MEL-A has potent antimicrobial effect against vegetative cells and spores of Bacillus cereus [12], and functions as dual inhibitory modes against S. aureus through membrane-mediated apoptosis and biofilm disruption [13]. To date, no related reports were focused on MELs acting as anti-MRSA compounds.
Conventional thermal pasteurization and sterilization are the most common techniques generally used to inactivate microorganisms in food products or food processing system, while the demand for new methods that have a reduced impact on the nutritional content and overall food quality is gradually increasing. Ultrasound treatment has the advantage of reduced impact on the overall food quality [3], and has been taken into consideration as an alternative method for food-borne microbe elimination or reduction. The working mechanism was reported that ultrasound can facilitate the transport of membrane-impermeable compounds, such as genetic drugs, peptides and proteins, into cells [14]. Although vast studies have explained that ultrasound alone can significantly decrease bacterial quantity, the synergistic effects of ultrasound and antimicrobial agents, such as antibiotics and essential oils, have attracted more interests [15], [16]. However, there is rare researches on antimicrobial properties of ultrasound and MEL-A cooperation against MRSA. Therefore, the aim of this study is to investigate the synergistic activity of ultrasound and MEL-A against planktonic cells and biofilm of MRSA, and to elucidate its unknown mechanism, which provides a strong basis for further development of MEL-A/ultrasound combination and potential application in food processing industry and other fields.
2. Materials and methods
2.1. Microorganism and reagents
MEL-A was produced by Pseudozyma aphidis DSM 70725 and purified according to the method mentioned by Fan et al. [17]. All the chemicals used in this study were of analytical grade. The bacterial strain methicillin-resistant Staphylococcus aureus CCTCC AB 2015109 MRSA 16558 was obtained from China Center for Type Culture Collection and maintained in slants of Luria-Bertani (LB) agar at 4 °C. One single colony of culture was transferred from the LB slant into 50 mL LB medium, and then the triangular flask was incubated in an orbital shaker (180 rpm, 37 °C) overnight. Cultured cells were harvested by centrifugation at 5000 rpm for 3 min, and the bacterial precipitate was washed twice and resuspended in 0.85% sterile saline solution. The prepared bacterial suspension was diluted to an appropriate concentration (about 109 CFU/mL) for the following antimicrobial experiments.
2.2. Antibacterial assay
2.2.1. Killing kinetics of MEL-A
The kinetics of killing experiments was performed according to Saranya Sugumar et al. with minor modifications [16]. The minimum inhibition concentration (MIC) at 256 μg/mL of MEL-A against MRSA was verified before in our lab (data not shown). 1 mL MEL-A solution was added into 29 mL MRSA bacterial suspension, and the concentration of MEL-A and bacterial density were adjusted to 256 μg/mL and 109 CFU/mL, respectively. Meantime, the inoculated solution without MEL-A was set as the control. For viable counts examination, 1 ml sample was taken from the inoculated solutions every 2 h, and then serially diluted to 10-fold with 0.85% saline and spot plated in triplicate. These plates were incubated at 37 °C for 24 h.
2.2.2. Killing effects of MEL-A and ultrasound
2.2.2.1. Sterilizing treatments
The sterilizing treatment by MEL-A was performed at MIC level (256 μg/mL). In brief, 1 mL MEL-A solution was added 29 mL MRSA bacterial suspension, and the concentration of MEL-A and bacterial density were adjusted to 256 μg/mL and 109 CFU/mL, respectively. Meanwhile, the inoculated solution without MEL-A was set as the control.
The ultrasound treatment was carried out according to the methods reported by Li et al. [18] with some modifications. An ultrasound processor for stationary operation (Scientz-JY92-IIDN; Ningbo Scientz, Zhejiang, China) with a 6 mm diameter ultrasonic probe was used as a treating system in this study. For low-frequency ultrasound (20 kHz), 30 mL of cell suspension with or without MEL-A (256 μg/mL, 109 CFU/mL) was placed in a reaction vessel (a 50 mL centrifuge tube) and was sonicated by submerging the 6-mm-diameter probe (operating immersion depth, 2.0 cm) in the suspension. The experiments were conducted at variable power densities (3.33, 5.00 and 6.67 W/mL) and power intensities (1.85, 2.77 and 3.69 W/cm2), and the operating time was set as 5, 10 and 15 min. The treating process was applied with 2 s on/2 s off pulsed mode. To prevent a lethal thermal effect, and the temperature of the suspension was maintained at 20 °C through immerging in an ice water bath.
The experiments were divided into four groups: Control (without treatment), MEL-A group (256 μg/mL), US group (3.33, 5.00 and 6.67 W/mL) and MEL-A+US group, which means the combination treatment of MEL-A (256 μg/mL) and US (3.33, 5.00 and 6.67 W/mL). All the experimental groups were treating for 5, 10 and 15 min, respectively.
2.2.2.2. Enumeration of viable MRSA
The antibacterial effects against MRSA were evaluated immediately after each treatment by plate counting method. All the samples were serially diluted in 0.85% sterile saline solution. Then, 10 μL of each sample was spot plated onto LB agar dishes. The plates were incubated at 37 °C for 24 h. The experiments for each sample were carried out in triplicate.
2.2.3. Evaluation of intracellular constituent leakage
The leakage of intracellular proteins and nucleic acids was quantitively assayed according to the method of Moghimi et al. with minor modifications [19]. The bacterial suspension of four groups (control, ultrasound treatment, MEL-A treatment, ultrasound and MEL-A treatment) was centrifuged at 8000 rpm for 3 min, and the supernatant was filtrated by 0.22 μm membrane. After that, the filtrate was assayed at the absorption of 260 nm and 280 nm by ultraviolet spectrophotometer (Agilent, USA).
2.2.4. Evaluation of cell membrane integrity
The cell membrane integrity was evaluated by PI staining using flow cytometry according to the previously reported method with minor modifications [20]. The bacterial suspension of four groups (control, ultrasound treatment, MEL-A treatment, ultrasound and MEL-A treatment) was washed by pre-cooling phosphate buffer solution (PBS) twice, and the bacterial precipitate was resuspended by adding 100 μL 1×binding buffer. Then, 10 μL PI staining solution was added into the suspensions and mixed gently. After reacting for 10-15 min in darkness at room temperature, 400 μL 1×binding buffer was added and mixed, and samples were placed on ice and detected by flow cytometry (FACS CantoI1, USA) in one hour.
2.2.5. Observation of bacterial morphology
The morphological changes of MRSA were observed by scanning electron microscope (SEM) and transmission electron microscope (TEM) according to Shi et al. [21]. Briefly, the bacterial cells of four groups (control, ultrasound treatment, MEL-A treatment, ultrasound and MEL-A treatment) were collected and fixed with 2.5% glutaraldehyde at 4 °C overnight. Subsequently, the samples were washed by PBS three times and fixed with 1% OsO4 in PBS for 1-2 h. Then, the samples were further dehydrated through a graded series of ethanol and coated with gold-palladium in Hitachi Model E-1010 ion sputter for 4-5 min and observed in Hitachi Model SU-8010 SEM (Hitachi, Tokyo, Japan). The samples upon TEM observation needed to be soaked in a 1:1 mixture of absolute acetone/embedding solution for 2 h after ethanol dehydration, and then changed to pure embedding solution overnight. Ultrathin sections were stained and observed in Hitachi Model H-7650 TEM (Hitachi, Tokyo, Japan).
2.2.6. Measurement of biofilm eradication by MEL-A
The biofilm eradication effect of MEL-A was assayed using classic crystal violet staining method [22]. Firstly, MRSA was incubated in LB broth overnight. Then, the stationary cells were harvested and washed by 0.85% sterile saline solution. For biofilm growth, MRSA suspension was inoculated in LB broth with 0.25% glucose in 96-well polystyrene microplate, and the cell density was adjusted to 1010 CFU/mL. Subsequently, different concentrations of MEL-A (256, 128, 64 and 32 μg/mL) were added to the bacterial cultures at different time (0 h and 24 h). These microplates were incubated at 37 °C for 48 h. Finally, the microplates were taken out and each well was washed three times by sterile water to remove the planktonic cells. The biofilm fixation was achieved by adding 200 μL of methanol (15 min), and next the supernatants were discarded and air-dried. Then, the biofilm was stained by adding 200 μL of crystal violet solution for 5 min. After that, each well was washed by sterile water to remove extra staining and air-dried at 37 °C. 33% glacial acetic (200 μL) was afterwards added to dissolve the biofilm and incubated at 37 °C for 30 min. Finally, the biofilm biomass was quantified by measuring OD570nm using a microplate reader (Thermo, USA). The biofilm eradication percentage was calculated by the following formula:
Eradication percentage (%)=
M: MEL-A treated; C: Control.
2.2.7. Measurement of biofilm eradication by MEL-A and ultrasound
Ultrasound treatment was employed after biofilm matured and realized by ultrasonic cleaner [23]. The MRSA biofilm formed as described in 2.3.1 and the cultures were refreshed with LB broth containing different concentrations of MEL-A (256, 128, 64 and 32 μg/mL) after 24 h. Then, these microplates were incubated at 37 °C for another 24 h. Finally, the microplates were taken out and immersed in a sterile water-filled ultrasonic bath (JP-040S, Shenzhen, China). The ultrasonic cleaner was operated at 40 kHz with continuous irradiation at 200 W for 10 and 20 min, respectively. The biofilm plate without ultrasonic treatment was set as the control. The water bath kept the temperature constant before and after ultrasonic treatment. The biofilm biomass of all the microplates was detected using crystal violet staining method and the biofilm eradication percentage was calculated as described in 2.3.1.
2.3. Microscopic examination by confocal laser scanning microscope (CLSM)
The biofilm eradication effect of ultrasound and MEL-A was observed intuitively by SYTO® 9 stain and the propidium iodide (PI) doubling staining using confocal laser scanning microscope (CLSM). Specifically, MRSA was inoculated into LB broth (0.25% glucose) with or without MEL-A (256 μg/mL) to form biofilm on petri dishes (Φ60 mm). After culturing at 37 °C for 48 h, the petri dishes were washed with sterile PBS three times to remove planktonic cells. Further, the SYTO® 9 and PI were added to stain live/dead cells of biofilm. Then the samples were incubated for 20-30 min at room temperature and protected from light. The excess stain was discarded and rinsed by sterile water gently. All the samples were observed using a 40 × 0.7NA 3.3 mmWD water objective by confocal laser scanning microscopy (Leica TCS SP8, Germany), and the excitation/emission maxima are approximately 480/500 nm for SYTO® 9 and 490/635 nm for PI.
2.4. Statistical analysis
All the experiments were carried out in triplicate and statistical analysis was carried out by t test using SPSS Statistics 20. Differences between groups were considered to be significant at the level of P < 0.05 in this work.
3. Results
3.1. Antibacterial effect of MEL-A and ultrasound against planktonic cells
3.1.1. Antibacterial effect of MEL-A
The optimal antibacterial concentration of MEL-A was 256 μg/mL against MRSA according to previously study (data not shown). The experiments of killing kinetics demonstrated there was significant decrease in cell quantity after adding MEL-A for several hours. Specifically, the curve of killing kinetics in the Fig. 1A has shown that it would take 2 h at least to realize 1 log reduction of live cells, and the data presented that the antibacterial activity of MEL-A is obviously effective but time-consuming.
Fig. 1.
Time killing investigation of MEL-A against MRSA cells. (A): Killing kinetics of MEL-A. (B) Spot plates of MRSA with MEL-A treatment at different times.
3.1.1.1. Synergistic antibacterial effect of MEL-A and ultrasound
The reduction effect in MRSA cell populations with different treating conditions (ultrasound, MEL-A and MEL-A+US) were investigated, and the results are shown in Table 1. Different ultrasonic power intensities (1.85, 2.77, 3.69 W/cm2) and different treating time (5, 10, 15 min) were applied to compare with single treatment of MEL-A. Obviously, the dead cells of the MEL-A+US group (3.69 W/cm2, 15 min) were much more than other groups, the reduction of which reached to 1.32±0.10 at 15 min (P < 0.05). Notably, the synergetic antibacterial effect of MEL-A and ultrasound was much stronger than any of individual treatment. Besides, there were insignificant differences between these two individual groups, both of which killed less than 0.5 log cells. Interestingly, MEL-A did not function well as an antibacterial compound in such short 15 min. Table 1 demonstrated that the time and power intensity of ultrasound have positive relationship with antibacterial activity. With the time extending and the intensity increasing, the combination of MEL-A and ultrasound showed predominant performance in killing MRSA cells. Overall, the synergetic antibacterial effect of MEL-A and ultrasound is evidently proven to connect to treating time and power intensity. As a result, the optimal killing condition of ultrasound (3.69 W/cm2, 15 min) was used to explore the underlying mechanism of combining ultrasound and MEL-A in the following experiments.
Table 1.
Quantity of dead MRSA cells after US (1.85, 2.77,3.69 W/cm2), MEL-A (256 μg/mL) and MEL-A + US treatment for 5/10/15 min, respectively.
| Time (min) | US (reduction log CFU/mL) | MEL-A (reduction log CFU/mL) | MEL-A+US (reduction log CFU/mL) | ||||
|---|---|---|---|---|---|---|---|
| 1.85 W/cm2 | 2.77 W/cm2 | 3.69 W/cm2 | 1.85 W/cm2 | 2.77 W/cm2 | 3.69 W/cm2 | ||
| 5 | 0.03±0.10a | 0.13±0.10abc | 0.17±0.06abcd | 0.18±0.11abcd | 0.09±0.05ab | 0.34±0.17de | 1.16±0.04f |
| 10 | 0.12±0.09abc | 0.27±0.07bcde | 0.24±0.07bcde | 0.15±0.07abcd | 0.20±0.06abcd | 0.39±0.16e | 1.26±0.13 f |
| 15 | 0.15±0.09abcd | 0.29±0.04cde | 0.28±0.13bcde | 0.25±0.12bcde | 0.30±0.12cde | 0.41±0.05e | 1.32±0.10 f |
Note: Individual data were expressed as mean ± standard error (n=3). Different letters indicate a significant difference cross the table (P < 0.05).
3.1.1.2. Effect on intracellular constituent release
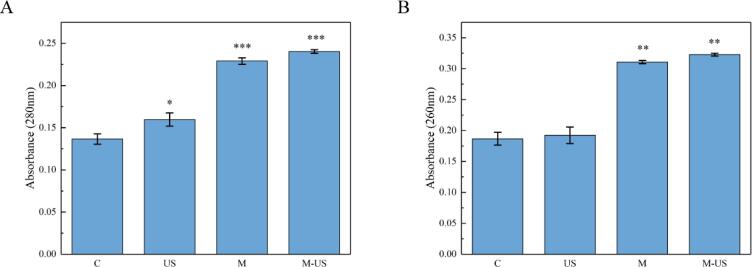
The protein and nucleic acids are essential molecules involved in versatile functions and gene transmission of life [24]. The result of intracellular protein and nucleic acids leakage is presented in Fig. 2. As for protein measurement (Fig. 2A), all of the experimental groups released more protein than the control significantly (P<0.05), which means both treatments made a contribution to intracellular imbalance. In addition, the release of the samples treating by MEL-A and ultrasound simultaneously was higher than those samples exposed to any individual treatment. Analogously, the outcome of nucleic acids showed the same tendency (Fig. 2B), which displayed both treatments have rendered cellular damage and the synergetic operation played a crucial role in destructing cell structure.
Fig. 2.
Leakage of intracellular constituents. (A) Leakage of intracellular proteins. (B) Leakage of intracellular nucleic acids. C: control; US: ultrasound; M: MEL-A; M-US: MEL-A and ultrasound.
3.1.1.3. Effect on cell membrane
To gain a deeper understanding of MEL-A and ultrasound-induced cell injury and death, the integrity of cell membrane was evaluated by PI staining using flow cytometry. Normally, PI has no access to stain cellular DNA with intact membrane, fluorescence intensity represents the quantity of impaired cells [25]. Fig. 3 exhibits the fluorescent cell micrographs of MRSA with different treatments. It was intuitively observed that there were more cells stained by PI in all experimental groups (Fig. 3A-D). Apparently, the combined treatment of MEL-A and ultrasound damaged the largest quantity of MRSA cells. Specially noted, there were 0.25%, 1.5% and 2.67% cells whose membrane was destructed by ultrasound treatment, MEL-A and the combined treatments, respectively, whereas only 0.17% cells of the control group were detected due to autologous death. Further, the present results were consistent with antimicrobial effect by spot assay, suggesting that the synergy effect of MEL-A and ultrasound plays a prominent role in killing MRSA significantly.
Fig. 3.
Fluorescent cell micrographs of MRSA under different treatments. (A) control; (B) ultrasound; (C) MEL-A; (D) MEL-A and ultrasound.
3.1.1.4. Effect on morphology changes
Changes of cell surface induced by ultrasound and MEL-A were examined by SEM (shown in Fig. 4). Untreated MRSA cells were uniform spherical shape with intact cell wall and membrane, appearing as grape-like clusters (Fig. 4A). Exposed to ultrasound for 15 min, it was obviously demonstrated that the shape of MRSA cells has been altered and distorted with wrinkles and gullies distributed on cell surface (Fig. 4B). Meanwhile, some cells were ruptured by MEL-A with visible cracks and pores, and the cell membrane was apparently loss of integrity causing cellular constituents’ leakage (Fig. 4C). Remarkably, more cells lost regular morphological structure in combination treatment of MEL-A and ultrasound, which suffered more severe destruction as shrunken sphere (Fig. 4D), including cytoskeleton collapse, cell membrane disintegration and cell wall damage. Besides, numerous cellular fragments existed in the background of the image after the synergistic antibacterial process.
Fig. 4.
SEM images of MRSA under different treatments. (A) control; (B) ultrasound; (C) MEL-A; (D) MEL-A and ultrasound.
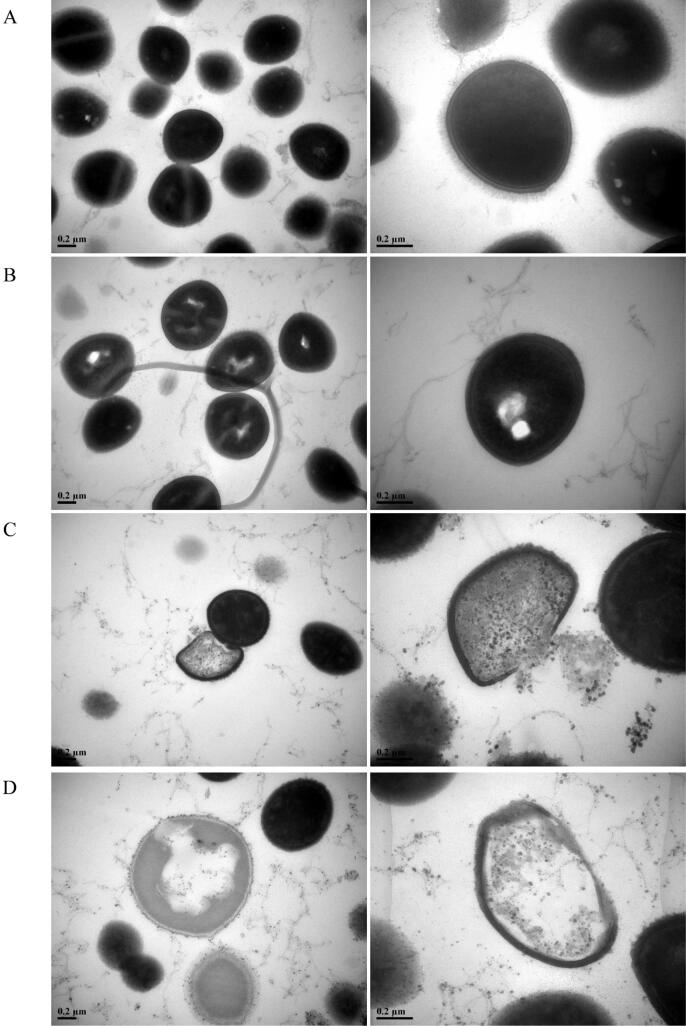
To disclose the interior structure changes of MRSA, TEM was used to take inner images of MRSA upon different treatments. Control sample showed homogeneous electron density in the cytoplasm and presented continuous and smooth cell membranes and walls in spherical shape (shown in Fig. 5A). However, after treatment with ultrasound for 15 min, MRSA cells displayed rough cell walls and blurry cell membranes, and the electron density in the cytoplasm appeared to be heterogeneous, which forming distinct cavities in the interior of cell (Fig. 5B) [26]. Interestingly, obvious cytoplasm release, cell wall and membrane injury emerged inside the cell after exposed to MEL-A, which indicated cell death (Fig. 5C). In combination treatment of MEL- A and ultrasound, MRSA cells were almost in state of ghost due to losing a large proportion of cytoplasm, and the periphery of cell turned vague and invisible indicating the disorganization of cell wall and membrane (Fig. 5D). The findings above further confirmed the disintegrating process of cell interior structure, which were consistent with the findings in SEM examination.
Fig. 5.
TEM images of MRSA under different treatments. (A) control; (B) ultrasound; (C) MEL-A; (D) MEL-A and ultrasound.
3.2. Anti-biofilm effect of MEL-A and ultrasound against MRSA biofilm formation
3.2.1. Effect of MEL-A on biofilm formation and development
The crystal violet assay (Fig. 6) has been used for evaluation of the inhibition of MRSA biofilm formation by MEL-A. The inhibition of the biofilm formation was heightened with the increase of MEL-A concentration, ranging from 32 μg/mL to 256 μg/mL after incubation for 48 h, and 76.1% of biofilm was scavenged by the highest concentration of MEL-A. Interestingly, it did not present the same dose-dependent tendency when adding MEL-A at 24 h, and the biofilm eradication efficacy was significantly lower than the other groups, which was about 50% clearance rate. Previous experiments found that the MRSA cells are able to accomplish high-intensity state, adhere to surface and form biofilm fortress after 24 h. Thereby, the present results imply it was the formation stage that has been disturbed by MEL-A during the whole biofilm process, while mature biofilm can also be destructed by MEL-A to some extent.
Fig. 6.
Eradication effect of MEL-A on MRSA biofilm.
3.2.2. Synergistic effect of MEL-A and ultrasound on biofilm eradication
In order to explore if there is any supplementary antibiofilm effect of ultrasound on MEL-A when treating mature biofilm, the MRSA biofilm biomass was detected in presence of MEL-A or not (adding at 24 h), and the results was shown in Fig. 7. Ultrasound evidently promoted biofilm destruction and detachment, helping improve the eradication percentage from 50% to over 90% significantly. Moreover, the current data revealed that different ultrasound time has played a key role in biofilm-scavenging process. On the other hand, it seems that there was no apparent dose-effect relationship between MEL-A concentration and eradication efficacy when ultrasonic time reached to 20 min. Nevertheless, compared to the control group (less than 60%), MEL-A can disintegrate more than 90% MRSA biofilm under the same ultrasonic intensity and time. Therefore, the potential antibiofilm capacity was produced by the combination of ultrasound and MEL-A, the single treatment of whichever would reduce the eradication effect heavily.
Fig. 7.
Eradication effect of MEL-A and ultrasound on MRSA biofilm.
3.2.2.1. Microscopic analysis by CLSM
The biofilm cell distribution in two-dimensional surface and three-dimensional structure was exhibited in Fig. 8 by fluorescent staining. Firstly, there was a bright green fluorescence of large area emitted by viable bacteria, indicating that the biofilm has grown prosperously in the control group. Comparatively, CLFM images of ultrasound and MEL-A treatment have shown less green fluorescent points, and the whole biofilm structure appeared sparse and sporadic, which suggested that the biofilm was decomposed and hard to form rigid three-dimensional structure after exposed to ultrasound or MEL-A. More strikingly, the cooperation treatment of ultrasound and MEL-A has caused the most severe biofilm reduction, due to the minimum quantity of fluorescent points emerged on CLFM images. In conclusion, these direct visual evidences kept in accordance with previous findings, which proved the eminent synergic effect of ultrasound and MEL-A against MRSA biofilm formation.
Fig. 8.
Confocal laser scanning microscopic images of MRSA biofilm. C: control; US: ultrasound; M: MEL-A; M-US: MEL-A and ultrasound.
4. Discussion
Biosurfactants are extracellular amphiphilic compounds mainly produced by a variety of microbes and have aroused widespread interest due to their unique properties including structural diversity, higher biodegradability, lower toxicity, higher foaming ability, higher selectivity and specific activity at extreme conditions and mild production conditions when compared to the chemical surfactants [10]. Importantly, it has been proven that several kinds of biosurfactants possess antibacterial property against planktonic cells and biofilm. For example, conventional rhamnolipids and sophorolipids have been reported to show strong antibacterial activity against Bacillus subtilis and Staphylococcus aureus [27], [28]. All these characteristics allow biosurfactants to be used as additives and versatile ingredients for the processing of foods. The antimicrobial property has enabled aggregation of greater value to products and the avoidance of contamination both during and after processing [29]. As a kind of biosurfactant, MELs have been reported to show strong antibacterial activity against pathogenic gram-positive bacteria, especially MEL-A and MEL-B [10]. Our previous researches also revealed that MEL-A has significantly inhibited the growth of Bacillus cereus [12] and Staphylococcus aureus [13]. Expect for its intrinsic capacity, MELs have been a key part of nanoparticles as emulsifier, exhibiting potent inhibitive effect on pathogens [30], [31], [32]. However, the abuse of antibiotics resulted in the emergence of methicillin-resistant Staphylococcus aureus (MRSA), which can survive in severe environment and convey a novel challenge to anti-infective treatments [33]. Though MEL-A has restrained the proliferation of Staphylococcus aureus with great potency and efficacy in previous report [13], it seems not to be as the same powerful when facing the ‘superbug’ MRSA. The time-killing curves of Fig. 1 verified the antibacterial capacity of MEL-A (256 μg/mL) against MRSA evidently, but it needed several hours to achieve the desired effect (>1 log CFU/mL). Due to its serious pathogenicity, rapid sterilization or inhibition must become the essential ability of antimicrobial agents or methods.
In recent times, ultrasonication has been widely used as a non-thermal technique to inactivate microbes in food industry, especially in dairy industry. It has been proved effective for the destruction of Escherichia coli, Pseudomonas fluorescens and Listeria monocytogenes with no detrimental effect on the total protein or casein content of pasteurized milk [34]. Therefore, ultrasound has the advantage of nutrition preservation, environmental friendliness and less cost, compared to conventional thermal sterilization methods. Application of ultrasound in food preservation can be divided into two main categories depending upon its area of utilization: directly related to food and indirectly related to food. On one hand, food can be great culture medium for microorganisms, and putting the food for ultrasonication processing helps inactivate pathogenic bacteria through cavitation effect in food. On the other hand, one of the major industrial applications of ultrasound is in surface cleaning and it has proved to be an extremely efficient technology [35]. Therefore, ultrasound can perform microbe-killing efficacy during the process of facilities cleaning, which provide a sterile environment before food storage. To date, plenty of studies have explored the microbial inhibition of ultrasonic processing since the investigation of ultrasound as a potential antimicrobial method began in the 1960s [36]. For instance, Li et al. has found that the unrecoverable damage of Escherichia coli and Staphylococcus aureus by ultrasound was multi-targeted inactivation involved in the cell wall, cytoplasmic membrane, and inner structure [18]. The underlying mechanism of the bactericidal activity upon ultrasound is identified to be acoustic cavitation, which could generate mechanical forces such as shock waves, shear waves and microjets to hurt microorganisms [37]. All these mechanical effects are reported to physically damage, weaken or tear the outer layer of different organisms [38]. However, gram-positive bacteria seemed to be more resistant to cavitation in comparison to gram-negative due to a thicker, more rigid and robust properties of their cell wall [39], [40]. In this work, MRSA has shown a deformed shape after ultrasonic treatment which is similar to other findings [41], whereas the inactivation rate wasn’t as high as expected.
Thereby, the physical and chemical approaches have been united together to combat against MRSA urgently. Remarkably, the synergistic treatment of ultrasound and MEL-A has enhanced the killing effect on planktonic MRSA cells more than MEL-A alone, which achieved cell quantity reduction (>1 log CFU/mL) in 15 min. It has been decades that the combination therapy of ultrasound and antibiotics was applied to kill various bacteria, since Pitt et al. confirmed significant bacterial reduction by the combination of low frequency ultrasound and gentamicin against planktonic cultures of Pseudomonas aeruginosa and E. coli in 1994 [42]. Besides, it has been discovered that ultrasound is able to enhance the inhibitory effect of other antimicrobial agents. Guo et al. elucidated that sonoporation could be the promising candidate to trigger a synergistic effect with thyme essential oil nano emulsion (TEON) and produce a more effective antibacterial efficacy against E. coli [43]. As a kind of food colorant, the inactivation rate of erythrosin B against Listeria innocua also could be significantly increased when exposed to ultrasound [44]. Aiming for more visual evidences, microscopic techniques have been frequently applied into the observation of morphology changes induced by ultrasound, mostly focusing on cell wall and membrane. In this study, SEM and TEM micrographs have confirmed that the synergistic treatment of ultrasound and MEL-A has rendered cell membrane ruptures, cell wall damage, empty cell envelopes and cell fragments (Fig. 4D and Fig. 5D). Our previous work has determined that the inhibitory mode of MELs against S. aureus mainly through membrane-mediated apoptosis [13]. Also, membrane damage and cell shrinkage of MRSA were observed by MEL-A interference (Fig. 4C and Fig. 5C). Apparently, ultrasound has exacerbated the cellular destruction by MEL-A in both quality and quantity. Similarly, Rapoport et al. found that 80 kHz and 0.8-2.4 W/cm2 ultrasound enhanced the permeability of cell membranes of P. aeruginosa and E. coli towards macromolecular compounds [45]. Ayan et al. noted that partial destruction or disintegration of cell walls was observed in some bacteria using the electron micrographs [46]. To sum up, the underlying mechanism behind synergetic modes may be concluded as that stable cavitation of ultrasound contributed to the alteration in bacterial cell membrane including enhanced permeability, which allows antimicrobial agents can penetrate into bacteria cells easily [47]. The mechanical effect of cavitating bubbles produced by ultrasound has created pores in the cell membrane, which significantly prompted the MEL-A’s entrance to cell interior, leading to more potent sterilization against MRSA, which explains the results of cellular constituent leakage and cell membrane integrity after combined treatment (Fig. 9).
Fig. 9.
Diagram of the postulated synergistic antibacterial and antibiofilm mechanism of ultrasound and MEL-A against MRSA
The ability of pathogenic bacteria to form biofilm is often connected with drug resistance, and it is the extracellular polymeric substance (EPS) as fortress structure that makes antibiotics difficult to get through, compared to planktonic (free-floating) cells [48]. Biofilm formation is a dynamic and complicated process which involves a series of procedures including attachment, development, maturation and detachment. Fig. 6 has disclosed that it was the formation stage that is much easier to be intervened by MEL-A rather than mature biofilm, indicating MEL-A may have influence on adhesion to material surfaces and large-scale aggregation from single cell to community. The present findings are similar to our previous research on anti-biofilm effect against non-MRSA. In addition, rhamnolipids has also exhibited disruption of S. aureus biofilms [49]. Moreover, the working mode is attributed to the special surface-active properties of biosurfactants, which could modify the wettability and charge of the surface and then affect the interaction of bacteria with the surface [50]. On the other hand, ultrasound has been spreading applications against bacterial biofilm into medical field, especially in dental clinical practice over the last 10 years [51]. Nevertheless, this effect on biofilm eradication is related to a simple mechanical destruction of the biofilm extracellular matrix due to the effect of ultrasonic cavitation or to the direct contact of the transducer with the biofilm without any interaction with the adhesive mechanism [51]. Hence, the physical method is generally combined with chemical agents to resolve pathogenic biofilm, such as antibiotics. The influence of ultrasonic power density and waveform in enhancing the action of the antibiotics against biofilms of P. aeruginosa was also reported [52]. It is generally assumed that the stable cavitation of ultrasound created numerous pathways on extracellular matrix of bacterial biofilms, and thus facilitated the antibiotics to enter into the biofilms quickly. In this study, the cooperation of ultrasound and MEL-A has achieved marvelous outcomes and improved the biofilm clearance rate to more than 90% (Fig. 7), which was intuitively demonstrated by confocal laser scanning microscope (Fig. 8). Altogether, the reason behind the phenomenon is shown in Fig. 9 and speculated as following: firstly, MEL-A has disturbed cell adhesion to surface in the earlier attachment stage; subsequently, ultrasound has done mechanical destruction to mature biofilm matrix, promoted the whole cell community detachment and allowed MEL-A get entrance into single cell. Therefore, there is great potential to put applications of this reinforced double-antibiofilm approaches into food processing and medical devices sterilization, which utilize MEL-A to inhibit the biofilm formation and ultrasound to eradicate the residual mature biofilm.
In conclusion, the combination of ultrasound and MEL-A has made an impressive advance in sterilization of drug-resistant bacteria, and different microbial life models including solitary life of planktonic cells and community life of biofilm were threatened by this collaborating treatment. Based on the known industrial sterilization approaches, this novel cooperation working mode-synergetic treatment of ultrasound and MEL-A, which have been confirmed the feasibility and efficiency through a series of experiments, will have great potential applications in food industry and other fields.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This study was financially supported by National Key Research and Development Program of China (2018YFC1200100), Public Projects of Zhejiang Province (LGF18C200003) and Nature Science Foundation of Zhejiang Province (LR13C200002), China.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2020.105452.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Zhang Y., Liu X., Wang Y., Jiang P., Quek S. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control. 2016;59:282–289. [Google Scholar]
- 2.Robertson C.A., Evans D.H., Abrahamse H. Photodynamic therapy (PDT): a short review on cellular mechanisms and cancer research applications for PDT. J. Photochem. Photobiol. B. 2009;96:1–8. doi: 10.1016/j.jphotobiol.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Parisi A., Caruso M., Normanno G., Latorre L., Sottili R., Miccolupo A., Fraccalvieri R., Santagada G. Prevalence, antimicrobial susceptibility and molecular typing of Methicillin-Resistant Staphylococcus aureus (MRSA) in bulk tank milk from southern Italy. Food Microbiology. 2016;58:36–42. doi: 10.1016/j.fm.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Tenhagen B.-V.-B.-A., Käsbohrer A., Alt K., Kraushaar B., Guerra B., Schroeter A., Fetsch A. Methicillin-resistant Staphylococcus aureus in cattle food chains –Prevalence, diversity, and antimicrobial resistance in Germany. J. Anim. Sci. 2014;92:2741–2751. doi: 10.2527/jas.2014-7665. [DOI] [PubMed] [Google Scholar]
- 5.de Boer E., Zwartkruis-Nahuis J.T., Wit B., Huijsdens X.W., de Neeling A.J., Bosch T., van Oosterom R.A., Vila A., Heuvelink A.E. Prevalence of methicillin-resistant Staphylococcus aureus in meat. Int. J. Food Microbiol. 2009;134:52–56. doi: 10.1016/j.ijfoodmicro.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 6.A.M. O'Brien, B.M. Hanson, S.A. Farina, J.Y. Wu, J.E. Simmering, S.E. Wardyn, B.M. Forshey, M.E. Kulick, D.B. Wallinga, T.C. Smith, MRSA in conventional and alternative retail pork products, PloS One, 7 (2012) e30092. [DOI] [PMC free article] [PubMed]
- 7.Normanno G., Corrente M., La Salandra G., Dambrosio A., Quaglia N.C., Parisi A., Greco G., Bellacicco A.L., Virgilio S., Celano G.V. Methicillin-resistant Staphylococcus aureus (MRSA) in foods of animal origin product in Italy. Int. J. Food Microbiol. 2007;117:219–222. doi: 10.1016/j.ijfoodmicro.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Silva M., Lidon F. Food preservatives - An overview on applications and side effects. Emir. J. Food Agric. 2016;28(6):366. doi: 10.9755/ejfa.2016-04-351. [DOI] [Google Scholar]
- 9.Ines M., Dhouha G. Glycolipid biosurfactants: Potential related biomedical and biotechnological applications. Carbohydr. Res. 2015;416:59–69. doi: 10.1016/j.carres.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 10.Arutchelvi J.I., Bhaduri S., Uppara P.V., Doble M. Mannosylerythritol lipids: a review. J. Ind. Microbiol. Biotechnol. 2008;35(12):1559–1570. doi: 10.1007/s10295-008-0460-4. [DOI] [PubMed] [Google Scholar]
- 11.Kitamoto D., Yanagishita H., Shinbo T., Nakane T., Kamisawa C., Nakahara T. Surface active properties and antimicrobial activities of mannosylerythritol lipids as biosurfactants produced by Candida antarctica. J. Biotechnol. 1993;29:91–96. doi: 10.1016/0168-1656(93)90042-L. [DOI] [Google Scholar]
- 12.Shu Q., Niu Y., Zhao W., Chen Q. Antibacterial activity of mannosylerythritol lipids against vegetative cells and spores of Bacillus cereus. Food Control. 2019;106 [Google Scholar]
- 13.Shu Q., Wei T., Lu H., Niu Y., Chen Q. Mannosylerythritol lipids: dual inhibitory modes against Staphylococcus aureus through membrane-mediated apoptosis and biofilm disruption. Appl. Microbiol. Biotechnol. 2020;104:5053–5064. doi: 10.1007/s00253-020-10561-8. [DOI] [PubMed] [Google Scholar]
- 14.De Temmerman M.L., Dewitte H., Vandenbroucke R.E., Lucas B., Libert C., Demeester J., De Smedt S.C., Lentacker I., Rejman J. mRNA-Lipoplex loaded microbubble contrast agents for ultrasound-assisted transfection of dendritic cells. Biomaterials. 2011;32:9128–9135. doi: 10.1016/j.biomaterials.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 15.Cai Y., Wang J., Liu X., Wang R., Xia L. A review of the combination therapy of low frequency ultrasound with antibiotics. Biomed Res. Int. 2017;2017:2317846. doi: 10.1155/2017/2317846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugumar S., Ghosh V., Nirmala M.J., Mukherjee A., Chandrasekaran N. Ultrasonic emulsification of eucalyptus oil nanoemulsion: antibacterial activity against Staphylococcus aureus and wound healing activity in Wistar rats. Ultrason. Sonochem. 2014;21:1044–1049. doi: 10.1016/j.ultsonch.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 17.Fan L., Li H., Niu Y., Chen Q. Characterization and inducing melanoma cell apoptosis activity of mannosylerythritol lipids-A produced from Pseudozyma aphidis. PloS One. 2016;11 doi: 10.1371/journal.pone.0148198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J., Ahn J., Liu D., Chen S., Ye X., Ding T. Evaluation of ultrasound-induced damage to Escherichia coli and Staphylococcus aureus by flow cytometry and transmission electron microscopy. Appl. Environ. Microbiol. 2016;82:1828–1837. doi: 10.1128/AEM.03080-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moghimi R., Ghaderi L., Rafati H., Aliahmadi A., McClements D.J. Superior antibacterial activity of nanoemulsion of Thymus daenensis essential oil against E-coli. Food Chem. 2016;194:410–415. doi: 10.1016/j.foodchem.2015.07.139. [DOI] [PubMed] [Google Scholar]
- 20.Ning Y., Yan A., Yang K., Wang Z., Li X., Jia Y. Antibacterial activity of phenyllactic acid against Listeria monocytogenes and Escherichia coli by dual mechanisms. Food Chem. 2017;228:533–540. doi: 10.1016/j.foodchem.2017.01.112. [DOI] [PubMed] [Google Scholar]
- 21.Shi Y., Bian L., Zhu Y., Zhang R., Shao S., Wu Y., Chen Y., Dang Y., Ding Y., Sun H. Multifunctional alkyl ferulate esters as potential food additives: Antibacterial activity and mode of action against Listeria monocytogenes and its application on American sturgeon caviar preservation. Food Control. 2019;96:390–402. doi: 10.1016/j.foodcont.2018.09.030. [DOI] [Google Scholar]
- 22.Qiu Y., Wu Y., Lu B., Zhu G., Gong T., Wang R., Peng Q., Li Y. Inhibition of methicillin-resistant Staphylococcus aureus (MRSA) biofilm by cationic poly (D, L-lactide-co-glycolide) nanoparticles. Biofouling. 2020;36(2):159–168. doi: 10.1080/08927014.2020.1740687. [DOI] [PubMed] [Google Scholar]
- 23.Liu X.u., Wang J., Weng C.-X., Wang R., Cai Y. Low-frequency ultrasound enhances bactericidal activity of antimicrobial agents against Klebsiella pneumoniae biofilm. Biomed Res. Int. 2020;2020:1–6. doi: 10.1155/2020/5916260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Devi K.P., Nisha S.A., Sakthivel R., Pandian S.K. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J. Ethnopharmacol. 2010;130(1):107–115. doi: 10.1016/j.jep.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 25.Han Y., Sun Z., Chen W. Antimicrobial susceptibility and antibacterial mechanism of Limonene against Listeria monocytogenes. Molecules. 2019;25 doi: 10.3390/molecules25010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J., Ding T., Liao X., Chen S., Ye X., Liu D. Synergetic effects of ultrasound and slightly acidic electrolyzed water against Staphylococcus aureus evaluated by flow cytometry and electron microscopy. Ultrason. Sonochem. 2017;38:711–719. doi: 10.1016/j.ultsonch.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 27.Velmurugan M., Baskaran A., Kumar S., Sureka I., Emelda E., Sathiyamurthy K. Screening, stability and antibacterial potential of rhamnolipids from Pseudomonas sp., isolated from hydrocarbon contaminated soil. J. Appl. Pharmaceut. Sci. 2015;5:26–33. [Google Scholar]
- 28.Díaz De Rienzo M.A., Banat I.M., Dolman B., Winterburn J., Martin P.J. Sophorolipid biosurfactants: Possible uses as antibacterial and antibiofilm agent. New Biotechnol. 2015;32(6):720–726. doi: 10.1016/j.nbt.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Ribeiro B.G., Guerra J.M.C., Sarubbo L.A. Biosurfactants: Production and application prospects in the food industry. Biotechnol. Progress. 2020;36(5) doi: 10.1002/btpr.3030. [DOI] [PubMed] [Google Scholar]
- 30.Wu J., Shu Q., Niu Y., Jiao Y., Chen Q. Preparation, characterization, and antibacterial effects of chitosan nanoparticles embedded with essential oils synthesized in an ionic liquid containing system. J. Agric. Food Chem. 2018;66(27):7006–7014. doi: 10.1021/acs.jafc.8b01428. [DOI] [PubMed] [Google Scholar]
- 31.Bakur A., Elshaarani T., Niu Y., Chen Q. Comparative study of antidiabetic, bactericidal, and antitumor activities of MEL@AgNPs, MEL@ZnONPs, and Ag–ZnO/MEL/GA nanocomposites prepared by using MEL and gum arabic. RSC Adv. 2019;9(17):9745–9754. doi: 10.1039/C9RA00344D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bakur A., Niu Y., Kuang H., Chen Q. Synthesis of gold nanoparticles derived from mannosylerythritol lipid and evaluation of their bioactivities. AMB Expr. 2019;9(1) doi: 10.1186/s13568-019-0785-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Purrello S.M., Garau J., Giamarellos E., Mazzei T., Pea F., Soriano A., Stefani S. Methicillin-resistant Staphylococcus aureus infections: A review of the currently available treatment options. J. Global Antimicrob. Resist. 2016;7:178–186. doi: 10.1016/j.jgar.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 34.Cameron M., McMaster L.D., Britz T.J. Impact of ultrasound on dairy spoilage microbes and milk components. Dairy Sci Technol. 2009;89(1):83–98. doi: 10.1051/dst/2008037. [DOI] [Google Scholar]
- 35.Chemat F., Zill-e-Huma, Khan M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011;18(4):813–835. doi: 10.1016/j.ultsonch.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 36.Earnshaw R.G., Appleyard J., Hurst R.M. Understanding physical inactivation processes: combined preservation opportunities using heat, ultrasound and pressure. Int. J. Food Microbiol. 1995;28(2):197–219. doi: 10.1016/0168-1605(95)00057-7. [DOI] [PubMed] [Google Scholar]
- 37.Ashokkumar M. The characterization of acoustic cavitation bubbles – An overview. Ultrason. Sonochem. 2011;18(4):864–872. doi: 10.1016/j.ultsonch.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 38.Zupanc M., Pandur Žiga, Stepišnik Perdih T., Stopar D., Petkovšek M., Dular M. Effects of cavitation on different microorganisms: The current understanding of the mechanisms taking place behind the phenomenon. A review and proposals for further research. Ultrason. Sonochem. 2019;57:147–165. doi: 10.1016/j.ultsonch.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 39.Liao X., Li J., Suo Y., Chen S., Ye X., Liu D., Ding T. Multiple action sites of ultrasound on Escherichia coli and Staphylococcus aureus. Food Sci. Human Welln. 2018;7(1):102–109. doi: 10.1016/j.fshw.2018.01.002. [DOI] [Google Scholar]
- 40.Cameron M., McMaster L.D., Britz T.J. Electron microscopic analysis of dairy microbes inactivated by ultrasound. Ultrason. Sonochem. 2008;15(6):960–964. doi: 10.1016/j.ultsonch.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 41.Gao S., Hemar Y., Ashokkumar M., Paturel S., Lewis G.D. Inactivation of bacteria and yeast using high-frequency ultrasound treatment. Water Res. 2014;60:93–104. doi: 10.1016/j.watres.2014.04.038. [DOI] [PubMed] [Google Scholar]
- 42.Pitt W.G., McBride M.O., Lunceford J.K., Roper R.J., Sagers R.D. Ultrasonic enhancement of antibiotic action on gram-negative bacteria. Antimicrob. Agents Chemother. 1994;38(11):2577–2582. doi: 10.1128/AAC.38.11.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo M., Zhang L., He Q., Arabi S.A., Zhao H., Chen W., Ye X., Liu D. Synergistic antibacterial effects of ultrasound and thyme essential oils nanoemulsion against Escherichia coli O157:H7. Ultrason. Sonochem. 2020;66:104988. doi: 10.1016/j.ultsonch.2020.104988. [DOI] [PubMed] [Google Scholar]
- 44.Bastarrachea L.J., Walsh M., Wrenn S.P., Tikekar R.V. Enhanced antimicrobial effect of ultrasound by the food colorant Erythrosin B. Food Res. Int. 2017;100:344–351. doi: 10.1016/j.foodres.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 45.Rapoport N., Smirnov A.I., Timoshin A., Pratt A.M., Pitt W.G. Factors affecting the permeability of Pseudomonas aeruginosa cell walls toward lipophilic compounds: effects of ultrasound and cell age. Arch. Biochem. Biophys. 1997;344(1):114–124. doi: 10.1006/abbi.1997.0176. [DOI] [PubMed] [Google Scholar]
- 46.Ayan I., Aslan G., Comelekoglu U., Yilmaz N., Colak M. The effect of low-intensity pulsed sound waves delivered by the Exogen device on Staphylococcus aureus morphology and genetics. Acta Orthop. Traumatol. Turc. 2008;42:272–277. doi: 10.3944/aott.2008.272. [DOI] [PubMed] [Google Scholar]
- 47.Yu H., Chen S., Cao P. Synergistic bactericidal effects and mechanisms of low intensity ultrasound and antibiotics against bacteria: A review. Ultrason. Sonochem. 2012;19(3):377–382. doi: 10.1016/j.ultsonch.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 48.Del Pozo J.L. Biofilm-related disease. Expert Rev. Anti Infect. Ther. 2018;16:51–65. doi: 10.1080/14787210.2018.1417036. [DOI] [PubMed] [Google Scholar]
- 49.Ss E.S., Carvalho J.W.P., Aires C.P., Nitschke M. Disruption of Staphylococcus aureus biofilms using rhamnolipid biosurfactants. J. Dairy Sci. 2017;100:7864–7873. doi: 10.3168/jds.2017-13012. [DOI] [PubMed] [Google Scholar]
- 50.Surekha A.G.B., Satputea K., Banatb I.M., Sangshettic J.N., Patild R.H., Gaded W.N. Multiple roles of biosurfactants in biofilms. Curr. Pharm. Des. 2016;22:1429–1448. doi: 10.2174/1381612822666160120152704. [DOI] [PubMed] [Google Scholar]
- 51.Erriu M., Blus C., Szmukler-Moncler S., Buogo S., Levi R., Barbato G., Madonnaripa D., Denotti G., Piras V., Orrù G. Microbial biofilm modulation by ultrasound: Current concepts and controversies. Ultrason. Sonochem. 2014;21(1):15–22. doi: 10.1016/j.ultsonch.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 52.Qian Z., Sagers R.D., Pitt W.G. The role of insonation intensity in acoustic-enhanced antibiotic treatment of bacterial biofilms. Colloids Surf., B. 1997;9(5):239–245. doi: 10.1016/S0927-7765(97)00029-5. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.