Abstract
Background
The treatment, genotyping, and phenotyping of patients with World Health Organization Group 1 pulmonary arterial hypertension (PAH) have evolved dramatically in the last decade.
Research Question
The United States Pulmonary Hypertension Scientific Registry was established as the first US PAH patient registry to investigate genetic information, reproductive histories, and environmental exposure data in a contemporary patient population.
Study Design and Methods
Investigators at 15 US centers enrolled consecutively screened adults diagnosed with Group 1 PAH who had enrolled in the National Biological Sample and Data Repository for PAH (PAH Biobank) within 5 years of a cardiac catheterization demonstrating qualifying hemodynamic criteria. Exposure and reproductive histories were collected by using a structured interview and questionnaire. The biobank provided genetic data.
Results
Between 2015 and 2018, a total of 499 of 979 eligible patients with clinical diagnoses of idiopathic PAH (IPAH) or familial PAH (n = 240 [48%]), associated PAH (APAH; n = 256 [51%]), or pulmonary venoocclusive disease/pulmonary capillary hemangiomatosis (n = 3 [1%]) enrolled. The mean age was 55.8 years, average BMI was 29.2 kg/m2, and 79% were women. Mean duration between symptom onset and diagnostic catheterization was 1.9 years. Sixty-six percent of patients were treated with more than one PAH medication at enrollment. Past use of prescription weight loss drugs (16%), recreational drugs (27%), and oral contraceptive pills (77%) was common. Women often reported miscarriage (37%), although PAH was rarely diagnosed within 6 months of pregnancy (1.9%). Results of genetic testing identified pathogenic or suspected pathogenic variants in 13% of patients, reclassifying 18% of IPAH patients and 5% of APAH patients to heritable PAH.
Interpretation
Patients with Group 1 PAH remain predominately middle-aged women diagnosed with IPAH or APAH. Delays in diagnosis of PAH persist. Treatment with combinations of PAH-targeted medications is more common than in the past. Women often report pregnancy complications, as well as exposure to anorexigens, oral contraceptives, and/or recreational drugs. Results of genetic tests frequently identify unsuspected heritable PAH.
Key Words: estrogens, genetics, pulmonary arterial hypertension, sex
Abbreviations: APAH, associated pulmonary arterial hypertension; ERA, endothelin receptor antagonist; HPAH, heritable pulmonary arterial hypertension; HRT, hormone replacement therapy; IPAH, idiopathic pulmonary arterial hypertension; OCPs, oral contraceptive pills; PAH, pulmonary arterial hypertension; PAP, pulmonary artery pressure; PDE, phosphodiesterase; PDE5i, phosphodiesterase type 5 inhibitor; PPH, primary pulmonary hypertension; RHC, right heart catheterization; USPHSR, United States Pulmonary Hypertension Scientific Registry; WHO, World Health Organization
World Health Organization (WHO) Group 1 pulmonary arterial hypertension (PAH) is a rare disease that narrows small pulmonary arteries, leading to progressive elevation of pulmonary vascular resistance, right ventricular failure, and death. Over the past four decades, investigators have described evolving characteristics of patients diagnosed with PAH and improving survival associated with advances in disease management. In 1987, the first description of a national cohort of patients diagnosed with what then was called primary pulmonary hypertension (PPH)1 reported a mean age at diagnosis of 36 years and a female:male ratio of 1.7:1. The median survival time from diagnosis of PPH was 2.8 years in an era (1981-1991) with no PAH-specific therapy.2 Two decades later, the Registry to Evaluate Early and Long-term PAH Disease Management (REVEAL Registry) reported that patients with PAH diagnosed between 1990 and 2009 were older at diagnosis, more likely to be female, and more likely to be obese than patients from the National Institutes of Health registry.3,4 REVEAL Registry investigators also reported improved survival of patients with PAH in an era (2006-2012) when treatment was available with prostacyclins, endothelin receptor antagonists (ERAs), and phosphodiesterase (PDE) inhibitors.5,6
The treatment of PAH has evolved in the post-REVEAL Registry era,7,8 with regulatory (US Food and Drug Administration) approval of four medications: riociguat,9 macitentan,10 and an oral formulation of treprostinil11 in 2013 and then selexipag12 in 2016.
Discoveries of mutations that are associated with heritable forms of PAH have further characterized patients with PAH and led to an updated clinical classification of pulmonary hypertension, including heritable PAH (HPAH).13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25 These discoveries and establishment of the National Biological Sample and Data Repository for Pulmonary Arterial Hypertension (PAH Biobank) galvanized researchers to organize the United States Pulmonary Hypertension Scientific Registry (USPHSR) as the first registry to include genetic characteristics of patients diagnosed with PAH.
Despite considerable research into understanding how sex hormones may influence the development and progression of PAH, the contribution of estrogens and reproductive factors to the increased susceptibility of women to develop PAH, and potentially their improved survival once diagnosed, remains poorly understood.26 The intersection between hormone exposures and genetics is largely unexplored. USPHSR was designed to provide demographic, physiologic, and genomic data combined with anorexigen and hormone exposure histories, to allow exploration of this relationship and the potential contribution to the disproportionate numbers of women affected by PAH.
The USPHSR investigated a contemporary cohort of 499 adult patients diagnosed with WHO Group 1 PAH to answer the following research questions: What are the demographic and clinical characteristics of this population? What is the reproductive history and rate of endogenous and exogenous sex hormone exposure in these patients? What is the rate of pathogenic or likely pathogenic genetic mutations in this cohort?
Materials and Methods
Design Overview
The USPHSR was designed by an independent steering committee, which oversaw study execution, data analysis, and reporting of the results. The design of the registry has been described in detail elsewhere.27 The registry uses a multicenter prospective cohort design involving 15 diverse, university-affiliated institutions in the United States (e-Appendix 1).
Between 2015 and 2018, a total of 979 consecutive patients with confirmed Group 1 PAH were screened at these 15 centers. Of those screened, 499 individuals met the entry criteria and gave informed consent to participate. These patients were a subset of the 2,853 patients enrolled in the PAH Biobank from a total of 38 sites in the US (www.pahbiobank.org). Patients were offered participation in USPHSR if they met the inclusion criteria described later in this section. The protocol was reviewed and approved by the institutional review board of each participating center (institutional review board committee name and project approval number for all study centers can be viewed in e-Appendix 1).
Settings and Participants
Adult patients (age ≥ 18 years) with newly or previously diagnosed Group 1 PAH were eligible for enrollment if they were within 5 years of their PAH diagnosis and already enrolled in the PAH Biobank. Consistent with REVEAL Registry, the date of diagnosis was defined as the date of the diagnostic right heart catheterization (RHC). The definition of WHO Group 1 PAH and its subclassifications have been well described.28 Enrolling investigators classified subjects based on their clinical assessment. Patients participating in clinical trials were eligible for enrollment. Eligible patients provided written informed consent for the registry and consented to data-sharing for collaborative research deemed by the USPHSR Steering Committee to be of value to the PH community. To maintain patient confidentiality according to the Health Insurance Portability and Accountability Act of 1996, each patient was assigned a unique patient identifier. Patients were classified as newly diagnosed PAH if the diagnostic RHC occurred within 180 days of USPHSR enrollment.
RHC Criteria
RHC had to be performed prior to study entry. Qualifying hemodynamic criteria were based on the contemporary definition at time of study inception: mean pulmonary artery pressure (PAP) ≥ 25 mm Hg at rest, pulmonary capillary wedge pressure or left ventricular end-diastolic pressure ≤ 15 mm Hg, and pulmonary vascular resistance > 3 Wood units.29
Data Collection
At the time of USPHSR enrollment, data were obtained from a patient interview, medical record review, and data stored at the PAH Biobank. Baseline demographic elements, including PAH medications, were collected and entered into electronic case report forms.
Environmental and hormone exposure data were collected by a structured interview using a questionnaire. Study interviewers administered an expanded version of the previously validated Nurses’ Health Study (NHS) I and II questionnaire (http://www.channing.harvard.edu/nhs/index.html).30,31 NHS questionnaires address a person’s exposure to hormones (including oral contraceptive and hormone replacement therapy [HRT] use), menstruation, menopause, pregnancy, abortions, and parity. Exposure to well water, soy products, tobacco smoke, alcohol, drugs, and anorexigens was also collected.
USPHSR investigators accessed biologic data according to protocols established by steering committees of the PAH Biobank and USPHSR. Biologic specimens include DNA, RNA, plasma, serum, and Epstein-Barr virus-immortalized cell lines. Gene panel data in 498 of 499 patients generated by the PAH Biobank included Illumina Omni5 single nucleotide polymorphism data (approximately 4.5 million single nucleotide polymorphisms); coding sequence data for BMPR2, ACVRL1, ENG, CAV1, SMAD9, KCNK3, EIF2AK4, ABCC8, GDF2, KCNA5, SMAD4, and TBX4; and gene dosage data assessing for deletions/duplications for BMPR2, ACVRL1, and ENG by multiplex ligation-dependent probe amplification. Whole-exome sequence data were available for 420 of 499 patients, enabling the assessment of other genes reportedly associated with PAH, including SOX17, BMPR1A/B, KLF2, ATP13A3, and SMAD1.7,24,32
Statistical Analysis
The statistical plan used standards for reporting observational research33 and the checklist provided by the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.34
For the observed characteristics of the study cohort, descriptive statistics were used. In brief, we describe continuous variables using mean, median, SD, and interquartile ranges. To compare continuous variables between two groups (eg, male subjects compared with female subjects), the nonparametric Wilcoxon rank sum test was used. In the event of multiple group comparisons, continuous variables were analyzed by using analysis of variance or Kruskal-Wallis nonparametric tests, as appropriate. For categorical variables, descriptive statistics such as count and percentage were reported, and between-group comparisons were made by using χ2 tests or the Fisher exact test. The level for determination of statistical significance was set at P ≤ .05 unless otherwise noted.
All statistical analyses were performed by using R version 3.6.0 or greater (the R Foundation for Statistical Computing; www.r-project.org).
Results
Demographic Characteristics
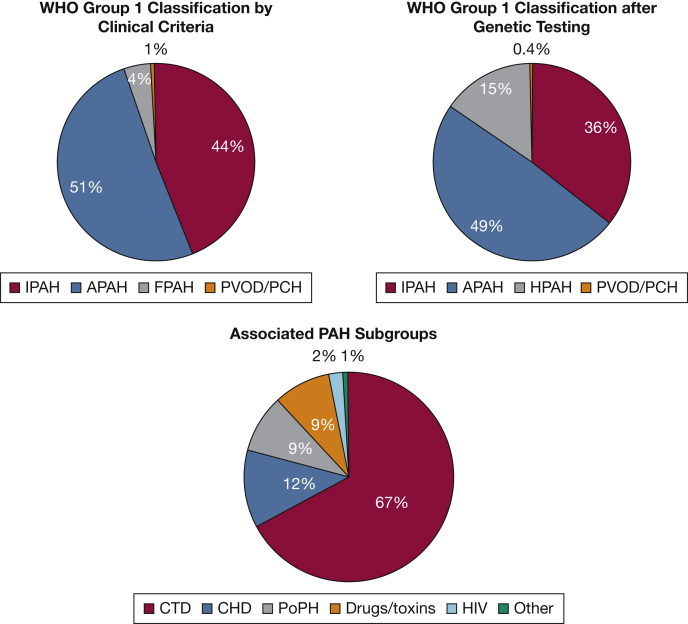
A total of 499 patients diagnosed with WHO Group 1 PAH enrolled in USPHSR. Demographic characteristics are shown in Tables 1 and 2. Participants were predominately female (79%) and white (88%). Forty-eight (9.6%) patients reported Hispanic or Latino ethnicity. The mean ± SD age at enrollment was 55.8 ± 15 years. The mean ± SD BMI was 29.2 ± 7.2 kg/m2. The mean and median time from diagnosis to enrollment in USPHSR was approximately 4 years. Only 7% of patients were diagnosed within 6 months of enrollment. The distribution of Group 1 PAH subgroups is shown in Figure 1. Forty-four percent were classified as idiopathic PAH (IPAH) and 51% as associated PAH (APAH). Further breakdown of the APAH subgroup is shown in Figure 1.
Table 1.
Demographic and Baseline Characteristics of All Subjects Enrolled in USPHSR According to WHO Group 1
| Characteristic | All Patients | IPAH | FPAH | PVOD/PCH | APAH (All Subgroups) | P Valuea |
|---|---|---|---|---|---|---|
| Patients | 499 | 218 (44) | 22 (4) | 3 (1) | 256 (51) | |
| Age at enrollment, y | 55.8 ± 15.0 | 53.4 ± 15.0 | 41.4 ± 12.7 | 50.7 ± 15.4 | 59.2 ± 14.1 | < .0001 |
| Age at diagnosis, y | 51.6 ± 14.9 | 49.1 ± 15.0 | 37.7 ± 11.5 | 46.7 ± 14.6 | 55.0 ± 14.0 | < .0001 |
| Age group | ||||||
| 16-64 y | 386 (77.5) | 182 (83.9) | 22 (100) | 3 (100) | 179 (69.9) | .001 |
| 65-74 y | 90 (18.1) | 26 (12) | 0 | 0 | 64 (25) | |
| ≥ 75 y | 22 (4.4) | 9 (4.1) | 0 | 0 | 13 (5.1) | |
| Female sex | 392 (78.6) | 162 (74.3) | 18 (81.1) | 2 (66.7) | 210 (82) | .04 |
| Race | ||||||
| Asian | 17 (3.4) | 7 (3.2) | 0 | 0 | 10 (3.9) | .63 |
| Biracial | 5 (1) | 4 (1.8) | 0 | 0 | 1 (0.4) | |
| Black | 37 (7.4) | 18 (8.3) | 0 | 0 | 19 (7.4) | |
| Native American/Alaskan | 2 (0.4) | 1 (0.5) | 0 | 0 | 1 (0.4) | |
| Pacific Islander | 1 (0.2) | 0 | 0 | 0 | 1 (0.4) | |
| White | 437 (87.6) | 188 (86.2) | 22 (100) | 3 (100) | 224 (87.5) | |
| Hispanic or Latino ethnicity | 48 (9.6) | 24 (11) | 2 (9.1) | 0 | 22 (8.6) | .38 |
| BMI, kg/m2 | 29.2 ± 7.2 | 30.6 ± 7.3 | 29.6 ± 5.7 | 31.9 ± 10.0 | 27.9 ± 7.0 | < .0001 |
| Time from diagnosis to enrollment, mo | ||||||
| Mean ± SD | 50.8 ± 31.2 | 51.7 ± 30.4 | 45.3 ± 32.5 | 45.8 ± 9.5 | 50.6 ± 32.0 | .56 |
| Median | 47.4 | 49.9 | 33.9 | 44.9 | 46.8 | |
| IQR | 25.5, 73.3 | 26.9, 73.7 | 20.7, 72.4 | 40.8, 50.3 | 24.7, 73.3 | |
| Newly diagnosedb | 34 (6.8) | 13 (6.0) | 2 (9.1) | 0 | 19 (7.4) | .54 |
| Previously diagnosed | 464 (93.2) | 204 (94) | 20 (90.9) | 3 (100) | 237 (92.6) |
Data are presented as No. (%) or mean ± SD unless otherwise indicated. APAH = associated pulmonary arterial hypertension; FPAH = familial pulmonary arterial hypertension; IPAH = idiopathic pulmonary arterial hypertension; IQR = interquartile range; PCH = pulmonary capillary hemangiomatosis; PVOD = pulmonary venoocclusive disease; USPHSR = United States Pulmonary Hypertension Scientific Registry; WHO = World Health Organization.
The P value was obtained from the χ2 or Wilcoxon rank sum test examining the difference in the distribution of the characteristic among patients diagnosed with IPAH vs patients diagnosed with APAH.
Patients classified as newly diagnosed with PAH if the diagnostic right heart catheterization occurred within 180 days of USPHSR enrollment.
Table 2.
Demographic and Baseline Characteristics of Subjects Enrolled in USPHSR With Associated PAH According to Subgroup
| Characteristic | APAH Subgroup |
|||||
|---|---|---|---|---|---|---|
| CTD | CHD | PoPH | Drugs/Toxins | HIV | Othera | |
| Patients | 171 (67) | 30 (12) | 22 (9) | 24 (9) | 5 (2) | 4 (1) |
| Age at enrollment, y | 61.3 ± 13.7 | 52.1 ± 17.4 | 60.8 ± 10.2 | 54.1 ± 12.9 | 50.4 ±5.6 | 54.4 ± 13.6 |
| Age at diagnosis, y | 57.0 ± 13.8 | 48.1 ± 17.1 | 57.5 ± 9.8 | 50.1 ± 11.8 | 46.4 ± 4.4 | 47.8 ± 14.9 |
| Age group | ||||||
| 16-64 y | 110 (64.3) | 22 (73.3) | 17 (77.3) | 21 (87.5) | 5 (100) | 4 (100) |
| 65-74 y | 51 (29.8) | 7 (23.3) | 4 (18.2) | 2 (8.3) | 0 | 0 |
| ≥ 75 y | 10 (5.8) | 1 (3.3) | 1(4.5) | 1 (4.2) | 0 | 0 |
| Female sex | 154 (90.1) | 21 (70.0) | 9 (40.9) | 19 (79.2) | 3 (60.0) | 4 (100.0) |
| Race | ||||||
| Asian | 7 (4.1) | 3 (10) | 0 | 0 | 0 | 0 |
| Biracial | 1 (0.6) | 0 | 0 | 0 | 0 | 0 |
| Black | 15 (8.8) | 0 | 2 (9.1) | 0 | 2 (40) | 0 |
| Native American/Alaskan | 1 (0.6) | 0 | 0 | 0 | 0 | 0 |
| Pacific Islander | 1 (0.6) | 0 | 0 | 0 | 0 | 0 |
| White | 146 (85.4) | 27 (90) | 20 (90.9) | 24 (100) | 3 (60) | 4 (100) |
| BMI, kg/m2 | 27.2 ± 6.6 | 26.9 ± 6.5 | 29.4 ± 7.3 | 31.5 ± 8.6 | 29.2 ± 6.8 | 36.5 ± 6.7 |
| Time from diagnosis to enrollment, mo | ||||||
| Mean ± SD | 52.1 ± 31.8 | 49.0 ± 29.6 | 39.2 ± 31.7 | 47.9 ± 33.7 | 48.2 ± 44.5 | 80.2 ± 18.1 |
| Median | 51.5 | 44.1 | 32.1 | 44.1 | 21.9 | 84.1 |
| IQR | 28.2, 73.4 | 29.6, 66.8 | 19.3, 54.7 | 17.4, 70.7 | 18.9, 86.8 | 69.8, 94.5 |
| Newly diagnosedb | 15 (8.8) | 2 (6.7) | 2 (9.1) | 0 | 0 | 0 |
| Previously diagnosed | 156 (91.2) | 28 (93.3) | 20 (90.9) | 24 (100) | 5 (100) | 4 (100) |
Data are presented as No. (%) or mean ± SD unless otherwise indicated. CHD = congenital heart disease; CTD = connective tissue disease; PAH = pulmonary arterial hypertension; PoPH = portopulmonary hypertension. See Table 1 legend for expansion of other abbreviations.
Includes hereditary hemorrhagic telangiectasia (1), exercise PAH (2), and neurofibromatosis (1).
Patients classified as newly diagnosed with PAH if the diagnostic right heart catheterization occurred within 180 days of USPHSR enrollment.
Figure 1.
WHO Group 1 classification by clinical criteria on enrollment in the National Biological Sample and Data Repository for PAH and change in classification when United States Pulmonary Hypertension Scientific Registry genomic mutational data are included. Individuals previously classified as FPAH without identified mutations are included in the new HPAH classification. Original APAH subgroups are delineated in the lower chart. APAH = associated pulmonary arterial hypertension; CHD = congenital heart disease; CTD = connective tissue disease; FPAH = familial pulmonary arterial hypertension; HPAH = heritable pulmonary arterial hypertension; IPAH = idiopathic pulmonary arterial hypertension; PAH = pulmonary arterial hypertension; PCH = pulmonary capillary hemangiomatosis; PoPH = portopulmonary hypertension; PVOD = pulmonary venoocclusive disease; WHO = World Health Organization.
Patients diagnosed with APAH were older at diagnosis and enrollment (P < .0001) and more commonly female (82% vs 74%; P = .04) compared with those with IPAH. Patients with APAH also had a lower BMI than patients with IPAH (27.9 vs 30.6 kg/m2; P < 0.0001).
Patient Characteristics
Symptoms and Diagnostic Delay
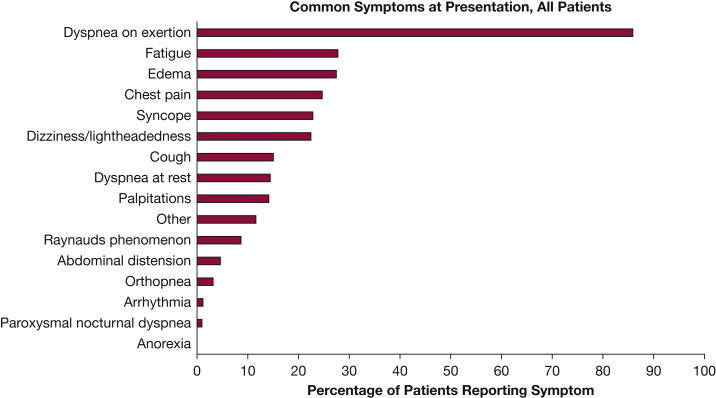
The mean time from symptom onset to diagnostic RHC was 1.9 years. There was no difference in time from symptom onset to diagnosis between those with IPAH and APAH (P = .94) (Table 3). The most common presenting symptom (> 80%) was dyspnea on exertion (Fig 2).
Table 3.
Time From Symptom Onset to Diagnosis According to WHO Group 1 at Enrollment in the PAH Biobank
| Variable | All Patients | IPAH | FPAH | APAH (All Subgroups) | P Valuea |
|---|---|---|---|---|---|
| No. of patients | 462 | 204 | 21 | 237 | |
| Time from symptom onset to diagnosis, y | |||||
| Mean ± SD | 1.9 ± 3.5 | 1.8 ± 3.1 | 1.5 ± 1.7 | 2.0 ± 4.0 | .94 |
| Median | 0.8 | 0.9 | 0.7 | 0.8 | |
| IQR | 0.2, 2.2 | 0.3, 2.0 | 0.4, 2.0 | 0.2, 2.6 |
PAH Biobank = National Biological Sample and Data Repository for PAH. See Table 1 legend for expansion of other abbreviations.
The P value was obtained from the Wilcoxon rank sum test examining the difference in the distribution of the characteristic among patients diagnosed with IPAH vs patients diagnosed with APAH.
Figure 2.
Common symptoms at diagnosis for all United States Pulmonary Hypertension Scientific Registry patients.
Diagnostic Clinical Data
The clinical information for patients at enrollment in the PAH Biobank is shown in Tables 4 and 5. At the time of diagnostic RHC, 225 patients (45%) were functional class III, and 41 (8%) were functional class IV. The mean ± SD 6-min walk distance at diagnosis was 339.5 ± 117.4 m. The average mean PAP was 49.4 mm Hg. There were significant differences in multiple hemodynamic parameters between those with IPAH vs those with APAH, including higher mean PAP (51.7 vs 46.9 mm Hg; P = .0003), higher pulmonary vascular resistance (11.1 vs 9.7 Wood units; P = .01), and lower Fick/thermodilution cardiac index (2.2/2.3 vs 2.4/2.6 L/min/m2; P = .01), respectively.
Table 4.
Diagnostic Clinical Data Including Functional Class, 6MWD, and Right Heart Catheterization Parameters According to WHO Group 1 as Documented on Enrollment in the PAH Biobank
| Characteristic | All Patients | IPAH | FPAH | PVOD/PCH | APAH (All Subgroups) | P Valuea |
|---|---|---|---|---|---|---|
| Functional class | N = 498 | n = 217 | n = 22 | n = 3 | n = 256 | .72 |
| I | 16 (3.2) | 7 (3.2) | 0 | 0 | 9 (3.5) | |
| II | 114 (22.9) | 49 (22.6) | 6 (27.3) | 0 | 59 (23) | |
| III | 225 (45.2) | 97 (44.7) | 5 (22.7) | 1 (33.3) | 122 (47.7) | |
| IV | 41 (8.2) | 18 (8.3) | 5 (22.7) | 1 (33.3) | 17 (6.6) | |
| Indeterminate | 2 (0.4) | 0 | 0 | 0 | 2 (0.8) | |
| Not available | 100 (20.1) | 46 (21.2) | 6 (27.3) | 1 (33.3) | 47 (18.4) | |
| 6MWD, m | 339.5 ± 117.4 | 348.1 ± 122.8 | 411.2 ± 124.1 | 295.5 ± 88.6 | 326.2 ± 109.8 | .09 |
| No. | 381 | 165 | 18 | 3 | 195 | |
| MAP, mm Hg | 93.2 ± 17.4 | 93.5 ± 18.9 | 89.3 ± 12.0 | 94.0 ± NA | 93.4 ± 16.9 | .50 |
| No. | 241 | 97 | 15 | 1 | 128 | |
| Mean PAP, mm Hg | 49.4 ± 13.2 | 51.7 ± 13.5 | 54.8 ± 12.4 | 51.7 ± 14.3 | 46.9 ± 12.5 | .0003 |
| No. | 498 | 217 | 22 | 3 | 256 | |
| PCWP, mm Hg | 10.2 ± 3.8 | 9.9 ± 3.5 | 9.0 ± 3.5 | 13.7 ± 4.9 | 10.4 ± 4.1 | .16 |
| No. | 487 | 214 | 21 | 3 | 249 | |
| Mean RAP, mm Hg | 9.3 ± 5.6 | 9.7 ± 6.0 | 7.5 ± 4.1 | 9.0 ± 4.0 | 9.2 ± 5.3 | .57 |
| No. | 489 | 212 | 22 | 3 | 252 | |
| PVR, Wood units | 10.4 ± 5.8 | 11.1 ± 5.8 | 13.4 ± 6.4 | 6.4 ± 1.6 | 9.7 ± 5.6 | .01 |
| No. | 479 | 210 | 20 | 3 | 246 | |
| Thermodilution cardiac output, L/min | 4.5 ± 1.6 | 4.3 ± 1.4 | 4.2 ± 1.0 | NA | 4.7 ± 1.7 | .04 |
| No. | 291 | 129 | 10 | 0 | 152 | |
| Thermodilution cardiac index, L/min/m2 | 2.4 ± 0.7 | 2.3 ± 0.7 | 2.4 ± 0.7 | NA | 2.6 ± 0.8 | .01 |
| No. | 222 | 101 | 8 | 0 | 113 | |
| Fick cardiac output, L/min | 4.3 ± 1.6 | 4.2 ± 1.5 | 4.0 ± 1.3 | 6.0 ± 0.5 | 4.4 ± 1.6 | .15 |
| No. | 398 | 177 | 19 | 3 | 199 | |
| Fick cardiac index, L/min/m2 | 2.3 ± 0.7 | 2.2 ± 0.7 | 2.0 ± 0.6 | 2.8 ± 0.4 | 2.4 ± 0.8 | .01 |
| No. | 316 | 140 | 16 | 3 | 157 | |
| Svo2, % | 64.0 ± 9.8 | 62.7 ± 8.6 | 64.8 ± 9.9 | 75.4 ± 5.1 | 65.0 ± 10.7 | .05 |
| No. | 289 | 128 | 16 | 2 | 143 |
Data are presented as No. (%) or mean ± SD unless otherwise indicated. 6MWD = 6-min walk distance; MAP = mean arterial pressure; NA = not applicable; PAP = pulmonary artery pressure; PCWP = pulmonary capillary wedge pressure; PVR = pulmonary vascular resistance; RAP = right atrial pressure; Svo2 = mixed venous oxygen saturation. See Table 1 and 3 legends for expansion of other abbreviations.
The P value was obtained from the χ2 or Wilcoxon rank sum test examining the difference in the distribution of the characteristic among patients diagnosed with IPAH vs patients diagnosed with APAH.
Table 5.
Diagnostic Clinical Data Including Functional Class, 6MWD, and Right Heart Catheterization Parameters According to Associated PAH Subgroup as Documented on Enrollment in PAH Biobank
| Characteristic | APAH Subgroup |
|||||
|---|---|---|---|---|---|---|
| CTD | CHD | PoPH | Drugs/Toxins | HIV | Other | |
| Functional class | n = 171 | n = 30 | n = 22 | n = 24 | n = 5 | n = 4 |
| I | 5 (2.9) | 1 (3.3) | 1 (4.5) | 2 (8.3) | 0 | 0 |
| II | 40 (23.4) | 10 (33.3) | 3 (13.6) | 5 (20.8) | 1 (20) | 0 |
| III | 82 (48) | 12 (40) | 11 (50) | 11 (45.8) | 3 (60) | 3 (75) |
| IV | 9 (5.3) | 2 (6.7) | 2 (9.1) | 3 (12.5) | 0 | 1 (25) |
| Indeterminate | 2 (1.2) | 0 | 0 | 0 | 0 | 0 |
| Not available | 33 (19.3) | 5 (16.7) | 5 (22.7) | 3 (12.5) | 1 (20) | 0 |
| 6MWD, m | 318.0 ± 108.0 | 348.8 ± 109.6 | 347.4 ± 121.3 | 326.3 ± 115.3 | 395.9 ± 65.7 | 321.7 ± 155.9 |
| No. | 130 | 24 | 16 | 19 | 3 | 3 |
| MAP, mm Hg | 94.3 ± 17.1 | 83.1 ± 15.6 | 90.6 ± 10.9 | 94.2 ± 21.2 | 98.0 ± 19.5 | 115 ± NA |
| No. | 92 | 9 | 14 | 9 | 3 | 1 |
| Mean PAP, mm Hg | 44.9 ± 12.1 | 52.8 ± 13.7 | 47.4 ± 9.2 | 55.1 ± 11.4 | 45.4 ± 13.0 | 40.5 ± 12.4 |
| No. | 171 | 30 | 22 | 24 | 5 | 4 |
| PCWP , mm Hg | 10.2 ± 4.2 | 11.6 ± 4.3 | 10.5 ± 3.8 | 10.9 ± 3.1 | 11.0 ± 3.5 | 9.5 ± 4.5 |
| No. | 168 | 27 | 21 | 24 | 5 | 4 |
| Mean RAP, mm Hg | 8.9 ± 5.2 | 9.3 ± 4.7 | 8.2 ± 4.7 | 12.3 ± 6.6 | 8.2 ± 2.6 | 10.8 ± 6.2 |
| No. | 167 | 30 | 22 | 24 | 5 | 4 |
| PVR, Wood units | 9.2 ± 5.3 | 11.3 ± 7.0 | 8.0 ± 3.4 | 12.7 ± 5.5 | 8.9 ± 3.7 | 2.0 ± 0.2 |
| No. | 166 | 26 | 21 | 24 | 5 | 2 |
| Thermodilution cardiac output, L/min | 4.6 ± 1.5 | 4.3 ± 1.7 | 5.0 ± 1.6 | 4.7 ± 2.1 | 6.1 ± 3.0 | 9.5 ± 3.6 |
| No. | 106 | 12 | 14 | 14 | 4 | 2 |
| Thermodilution cardiac index , L/min/m2 | 2.5 ± 0.7 | 2.6 ± 0.7 | 2.5 ± 0.8 | 2.5 ± 0.8 | 3.3 ± 1.3 | 4.5 ± 1.4 |
| No. | 78 | 11 | 10 | 9 | 3 | 2 |
| Fick cardiac output, L/min | 4.4 ± 1.6 | 4.6 ± 1.8 | 4.9 ± 1.5 | 3.9 ± 1.2 | 3.9 ± 1.9 | 4.7 ± 4.5 |
| No. | 135 | 23 | 16 | 19 | 4 | 2 |
| Fick cardiac index, L/min/m2 | 2.4 ± 0.8 | 2.3 ± 0.9 | 2.4 ± 0.7 | 2.1 ± 0.4 | 2.3 ± 1.0 | 2.1 ± 1.9 |
| No. | 104 | 21 | 14 | 14 | 2 | 2 |
| Svo2, % | 64.7 ± 10.6 | 67.1 ± 13.5 | 67.2 ± 7.1 | 62.5 ± 12.1 | 68.8 ± 6.1 | 61.5 ± 10.5 |
| No. | 96 | 13 | 12 | 16 | 4 | 2 |
Comorbid Conditions
The most common comorbid conditions were obesity (21%), diabetes (15%), systemic hypertension (37%), hypothyroidism (18%), and clinical depression (11%). The most common connective tissue disease in patients with APAH was scleroderma (66%) (e-Table 1).
Medications
Table 6 depicts the use of PAH-specific medications at the time of enrollment in the PAH Biobank. Most patients (323 of 491 [66%]) were taking multiple PAH-specific medications, including 120 (24%) on a PDE type 5 inhibitor (PDE5i) and ERA. Only one patient was not receiving PAH-specific therapy. Thirty-two patients (6.5%) reported treatment with an oral prostacyclin pathway agent, either treprostinil or selexipag. There were 75 patients (15%) on triple therapy.
Table 6.
PAH-Specific Medications Among Patients at Enrollment in the PAH Biobank
| Variable | Oral Therapies |
Prostacyclin Pathway |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| CCB | ERA | PDE Type 5 Inhibitor | Riociguat | Oral Treprostinil | Selexipag | Inhaled Treprostinil | SC Treprostinil | IV Epoprostenol/Treprostinil | |
| Overall, N = 491 | 34 (6.9) | 264 (53.8) | 366 (74.5) | 11 (2.2) | 21 (4.3) | 11 (2.2) | 45 (9.1) | 23 (4.7) | 101 (20.6) |
| Monotherapy | 10 (2) | 33 (6.7) | 89 (18.1) | 3 (0.6) | 3 (0.6) | ... | 2 (0.4) | 3 (0.6) | 24 (4.8) |
| Combination, oral therapya | 14 (2.6) | 122 (24.8) | 130 (26.5) | 2 (0.4) | 8 (1.6) | 4 (0.8) | 19 (3.9) | 11 (2.2) | 50 (10.2) |
| Combination, prostacyclin analogue | 1 (0.2) | 26 (5.3) | 64 (13) | 1 (0.2) | ... | ... | ... | ... | ... |
| Combination, 3 agentsb | ... | 75 (15.3) | 72 (14.7) | 3 (0.6) | 10 (2) | 7 (1.4) | 24 (4.9) | 7 (1.4) | 26 (5.3) |
| Combination, PAH blinded study drugc | ... | 5 (1) | 9 (1.8) | 1 (0.2) | ... | ... | ... | 1 (0.2) | ... |
| Combination, “other PH” drugd | 1 (0.2) | 3 (0.6) | 2 (0.4) | 1 (0.2) | ... | ... | ... | 1 (0.2) | 1 (0.2) |
Data are presented as No. (%). CCB = calcium channel blocker (amlodipine, diltiazem, other); ERA = endothelin receptor antagonist (ambrisentan, bosentan, macitentan); PDE = phosphodiesterase (sildenafil, tadalafil); SC = subcutaneous. See Table 1 and 3 legends for expansion of other abbreviations.
Combinations with oral therapy, one prostacyclin analogue, three agents, PAH-blinded study drug, and “other PH” drug are mutually exclusive for each medication class.
Oral therapies include CCB, ERA, PDE type 5 inhibitor, and riociguat.
Three agents or triple therapy includes ERA, nitric oxide pathway agent, and prostacyclin analogue; excludes CCB. One patient was on triple combination therapy with inhaled iloprost.
Eighteen patients were listed as being on PAH-blinded study drug; eight of them were listed as being on PAH blinded study drug only.
Four patients were listed as being on combination therapy including an “other” pulmonary hypertension medication that was not further identified. One patient was listed as being on inhaled nitric oxide therapy.
The most common concomitant medications included diuretics (71%), oxygen (34%), warfarin (19%), statin (24%), selective serotonin reuptake inhibitor (22%), aspirin (28%), levothyroxine (21%), and corticosteroids (18%). Interestingly, 97 (19%) patients were treated with a beta-blocker (e-Table 2).
Reproductive History and Sex Hormone Exposures
Results from the questionnaires of female patients (n = 390) are depicted in Table 7. Average menarche age was 12.5 years. Most women (81.1%) reported ever being pregnant, with a mean ± SD number of pregnancies of 2.3 ± 1.8. Only six women (1.9%) reported being pregnant within 6 months of the diagnosis of PAH. Approximately 50% of women breastfed at least one child, with a median duration of total breastfeeding of 10 months. A total of 172 women (44%) were postmenopausal at the time of completing the questionnaire, with an average menopause age of 47 years. Sixty-six patients (17%) had undergone bilateral oophorectomy. More women with APAH had undergone menopause compared with those with IPAH (53.6% vs 35.2%; P < 0.0001). There was an increased rate of ever having a miscarriage in patients with IPAH compared with those with APAH (43.8% vs 33.7%; P = .04).
Table 7.
Reproductive Factors and Endogenous Sex Hormone Exposures in Female USPHSR Subjects According to WHO Group 1 Classification
| Exposure | All Female Patients | IPAH | FPAH | APAHa | P Valueb |
|---|---|---|---|---|---|
| No. of patients | 390 | 162 | 18 | 210 | |
| Ever been pregnant | 314 (81.1) | 128 (80) | 13 (72.2) | 173 (82.8) | .50 |
| Pregnancies | 2.3 ± 1.8 | 2.4 ± 1.9 | 1.8 ± 1.3 | 2.3 ± 1.7 | .86 |
| Pregnant within 6 mo of diagnosis of PAH | 6 (1.9) | 4 (3.1) | 0 | 2 (1.2) | .23 |
| Ever had miscarriage | 115 (36.7) | 56 (43.8) | 1 (7.7) | 58 (33.7) | .04 |
| Ever had abortion | 58 (18.5) | 21 (16.4) | 4 (30.8) | 33 (19.2) | .54 |
| Ever had premature birth | 71 (22.7) | 33 (25.8) | 1 (7.7) | 37 (21.5) | .39 |
| Ever had stillbirth | 13 (4.2) | 4 (3.1) | 1 (7.7) | 8 (4.7) | .50 |
| Ever breastfed children | 158 (50.3) | 69 (53.9) | 10 (76.9) | 79 (45.7) | .16 |
| No. of children breastfedc | 1 (0, 2) | 1 (0, 2) | 1 (1, 2) | 0 (0, 2) | .11 |
| Cumulative time breastfeeding, moc | 10 (4, 19.5) | 12 (4, 23) | 8 (3.5, 17.5) | 10 (4, 17) | .42 |
| Menarche age, y | 12.5 ± 1.6 | 12.5 ± 1.7 | 11.8 ± 1.2 | 12.6 ± 1.5 | .13 |
| Completed natural menopause | 172 (44.2) | 57 (35.2) | 3 (16.7) | 112 (53.6) | < .0001 |
| Menopause age, y | 46.5 ± 7.3 | 45.9 ± 7.5 | 50.7 ± 3.2 | 46.6 ± 7.4 | .64 |
| Oophorectomy | 84 (21.6) | 38 (23.5) | 0 | 46 (22) | .74 |
| Age at removal, y | 42.2 ± 7.8 | 41.1 ± 7.5 | ... | 43.4 ± 8.1 | |
| Bilateral removal | 66 (78.6) | 32 (84.2) | ... | 34 (73.9) |
Data are presented as No. (%) or mean ± SD unless otherwise indicated. See Table 1, Table 2 and 2 legends for expansion of other abbreviations.
PVOD/PCH not included given low number of subjects (n = 2).
The P value was obtained from the χ2 or Wilcoxon rank sum examining the difference in the distribution of the characteristic among patients diagnosed with IPAH vs all patients with APAH.
Values are depicted as median (IQR).
Table 8 depicts exogenous sex hormone exposures in female patients. Few patients had used drugs to induce ovulation (4%), including fertility injections (1%). Most women had taken oral contraceptive pills (OCPs). The most common duration of OCP use was 1 to 5 years. Women diagnosed with IPAH were more likely to have taken OCPs than women diagnosed with APAH (83% vs 71%; P = .01). There was no difference in the duration of OCP use among the two groups (P = .75). Exposure to HRT was less common than exposure to OCPs (75 total female subjects [19%]), with an average exposure duration of 1 to 5 years. There was no difference in use (P = .94) or duration (P = .89) of HRT between women with IPAH vs those with APAH.
Table 8.
Exogenous Sex Hormone Exposures in Female USPHSR Subjects According to WHO Group 1 Classification
| Exposure | All Female Patients | IPAH | FPAH | APAHa | P Valueb |
|---|---|---|---|---|---|
| No. of patients | 390 | 162 | 18 | 210 | |
| Androgen use (current or past) | 3 (0.8) | 2 (1.2) | 0 | 1 (0.5) | .96 |
| Duration of androgen use | |||||
| < 1 y | 1 (33.3) | 0 | 0 | 1 (100) | |
| 1-5 y | 1 (33.3) | 1 (50) | 0 | 0 | |
| > 5 to 10 y | 1 (33.3) | 1 (50) | 0 | 0 | |
| Treatment with DES | 4 (1) | 2 (1.2) | 0 | 2 (1) | .88 |
| Ever used drugs to induce ovulation | 17 (4.4) | 7 (4.3) | 1 (5.6) | 9 (4.3) | .73 |
| Fertility shot use | 5 (1.3) | 2 (1.2) | 0 | 3 (1.4) | .97 |
| Duration of fertility shot use | |||||
| < 1 y | 3 (60) | 1 (50) | 0 | 2 (66.7) | |
| 1-5 y | 1 (20) | 0 | 0 | 1 (33.3) | |
| 5-10 y | 1 (20) | 1 (50) | 0 | 0 | |
| Ever taken OCPs | 299 (76.9) | 135 (83.3) | 16 (88.9) | 148 (70.8) | .01 |
| Duration of OCP use | .75 | ||||
| < 1 y | 39 (13) | 19 (14.1) | 3 (18.8) | 17 (11.5) | |
| 1-5 y | 118 (39.5) | 53 (39.3) | 6 (37.5) | 59 (39.9) | |
| 5-10 y | 62 (20.7) | 28 (20.7) | 4 (25) | 30 (20.3) | |
| 10-15 y | 45 (15.1) | 18 (13.3) | 3 (18.8) | 24 (16.2) | |
| 15-20 y | 14 (4.7) | 8 (5.9) | 0 | 6 (4.1) | |
| > 20 y | 13 (4.3) | 4 (3) | 0 | 9 (6.1) | |
| Unknown | 8 (2.7) | 5 (3.7) | 0 | 3 (2) | |
| Ever taken HRT | 75 (19.3) | 31 (19.1) | 1 (5.6) | 43 (20.6) | .94 |
| Duration of HRT use | .89 | ||||
| < 1 y | 9 (12) | 4 (12.9) | 0 | 5 (11.6) | |
| 1-5 y | 33 (44) | 12 (38.7) | 0 | 21 (48.8) | |
| 5-10 y | 14 (18.7) | 6 (19.4) | 1 (100) | 7 (16.3) | |
| 10-15 y | 5 (6.7) | 2 (6.5) | 0 | 3 (7) | |
| 15-20 y | 6 (8) | 2 (6.5) | 0 | 4 (9.3) | |
| > 20 y | 6 (8) | 4 (12.9) | 0 | 2 (4.7) | |
| Unknown | 2 (2.7) | 1 (3.2) | 0 | 1 (2.3) |
Data are presented as No. (%) unless otherwise indicated. DES = diethylstilbestrol; HRT = hormone replacement therapy; OCPs = oral contraceptive pills. See Table 1 legend for expansion of other abbreviations.
PVOD/PCH not included given low number of subjects (n = 2).
The P value was obtained from the χ2 or Wilcoxon rank sum examining the difference in the distribution of the characteristic among patients diagnosed with IPAH vs all patients with APAH.
Environmental Exposures
Fifty-five patients (11%) reported past use of fenfluramine-phentermine or phentermine, and 84 patients (17%) reported past use of other weight loss medications (e-Table 3). Twenty-two patients (4.5%) reported past use of methamphetamines (e-Table 4). Only 20 patients (4%) were active smokers, but 189 patients (40%) reported a history of smoking. Average pack-years for former smokers was 13. Alcohol use in the last year was reported by 324 patients (66%) with 1.8 ± 1.5 mean drinks daily. Eighty-five (17%) patients reported at least one episode of binge drinking in the last year.
Genomics
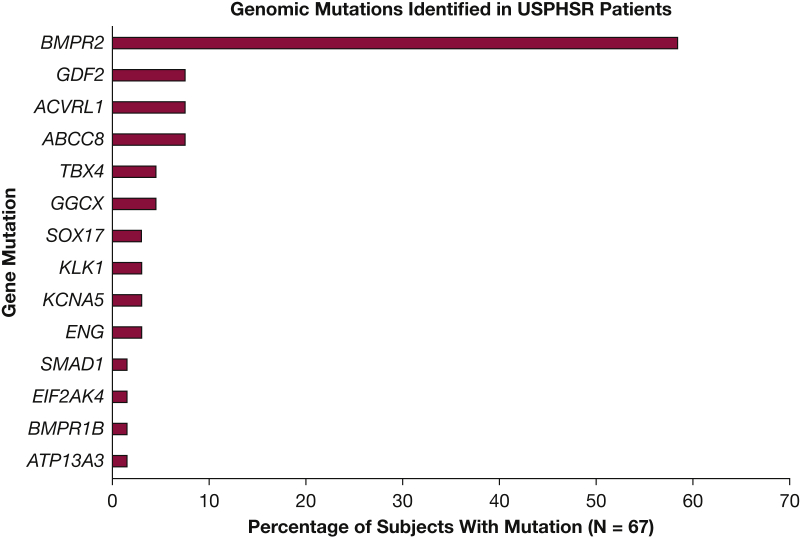
Genomic testing identified 72 pathogenic or likely pathogenic variants in 67 (13.4%) USPHSR patients (Fig 3).32 As depicted, the most commonly identified mutations were in BMPR2 (39 patients [58.2%]). The next most common genes with mutations were ABCC8, ACVRL1, and GDF2 (5 patients each [7.5%]). Five patients had mutations in both BMPR2 and an additional gene. There were also 40 patients with 69 genetic variations of uncertain significance. The clinical data at diagnosis for patients with HPAH, separated according to the most commonly identified genetic mutations, are given in Table 9 and e-Table 5. Most patients identified as HPAH were female (75%) and white (92%).
Figure 3.
Pathogenic and suspected pathogenic variations detected in the USPHSR patient population; 67 unique patients were identified to have mutations in 14 genes. Five patients have mutations in two genes. Tissue kallikrein 1 (KLK1) and gamma glutamyl carboxylase (GGCX) were identified recently as new candidate risk genes for idiopathic pulmonary arterial hypertension with genome-wide significance.32 Established risk genes include BMPR2, ACVRL1, ENG, TBX4, BMPR1B, and EIF2AK4. Newly established risk genes include ABCC8, ATP13A3, GDF2, KCNA5, SOX 17, SMAD1, GGCX, and KLK1. USPHSR = United States Pulmonary Hypertension Scientific Registry.
Table 9.
USPHSR Patients and Their Baseline and Functional Characteristics, HPAH Patients Are Further Grouped by the Most Commonly Identified PAH Risk Genes
| Characteristic | All Patients | HPAHa | ABCC8 | ACVRL1 | BMPR2 | GDF2 | GGCX | TBX4 |
|---|---|---|---|---|---|---|---|---|
| No. of patients | 499 | 76 | 5 | 5 | 39 | 5 | 3 | 3 |
| Age at diagnosis, y | 51.6 ± 17.9 | 43.9 ± 13.5 | 49.9 ± 14.1 | 50.1 ± 14.3 | 41.8 ± 12.6 | 39.3 ± 15.4 | 40.8 ± 15.9 | 54.0 ± 12.2 |
| Female | 392 (78.6) | 57 (75) | 4 (80) | 4 (80) | 30 (76.9) | 3 (60) | 3 (100) | 3 (100) |
| Race | ||||||||
| Asian | 17 (3.4) | 1 (1.3) | ... | ... | ... | 1 (20) | ... | ... |
| Biracial | 5 (1) | 1 (1.3) | ... | ... | ... | ... | ... | ... |
| Black | 37 (7.4) | 4 (5.3) | 2 (40) | ... | 2 (5) | ... | ... | ... |
| Native American/Alaskan | 2 (0.4) | ... | ... | ... | ... | ... | ... | ... |
| Pacific Islander | 1 (0.2) | ... | ... | ... | ... | ... | ... | ... |
| White | 437 (87.6) | 70 (92.1) | 3 (60) | 5 (100) | 37 (95) | 4 (80) | 3 (100) | 3 (100) |
| BMI, kg/m2 | 29.2 ± 7.2 | 31.0 ± 7.2 | 38.4 ± 8.7 | 33.4 ± 5.7 | 28.9 ± 8.0 | 30.5 ± 11.0 | 30.0 ± 8.8 | 32.8 ± 9.2 |
| WHO Group 1 classification (prior to genomic testing) | ||||||||
| IPAH | 218 (44) | 40 (53) | 4 (80) | ... | 25 (64) | 5 (100) | 3 (100) | 1 (50) |
| APAH | 256 (51) | 13 (17) | 1 (20) | 4 (80) | 2 (5) | ... | ... | 1 (50) |
| FPAH | 22 (4) | 22 (29) | ... | 1 (20) | 12 (31) | ... | ... | 1 (50) |
| PVOD | 3 (1) | 1 (1) | ... | ... | ... | ... | ... | ... |
| Functional class at enrollment | ||||||||
| I | 16 (3.2) | ... | ... | ... | ... | ... | ... | ... |
| II | 114 (22.9) | 15 (19.7) | 1 (20) | 1 (20) | 8 (20.5) | 1 (20) | - | 1 (33.3) |
| III | 225 (45.2) | 31 (40.8) | 3 (60) | 3 (60) | 16 (41) | 1 (20) | 1 (33.3) | 2 (66.7) |
| IV | 41 (8.2) | 10 (13.2) | 1 (20) | 1 (20) | 5 (12.8) | 1 (20) | 1 (33.3) | ... |
| Indeterminate | 2 (0.4) | 1 (1.3) | ... | ... | ... | ... | ... | ... |
| Not available | 100 (20.1) | 19 (25.0) | ... | ... | 10 (25.6) | 2 (40) | 1 (33.3) | ... |
| 6MWD at enrollment, m | 339.5 ± 117.4 | 396.8 ± 116.0 | 323.8 ± 133.0 | 370.3 ± 113.2 | 394.4 ± 100.7 | 406.0 ± 54.8 | 408.0 ± 155.6 | 373.0 ± 111.3 |
| No. | 381 | 62 | 5 | 5 | 32 | 3 | 2 | 3 |
Data are presented as No. (%) or mean ± SD unless otherwise indicated. Less common mutations (only found in 1-2 patients) including ATP13A3, BMPR1B, EIF2AK4, ENG, KCNA5, KLK1, SMAD1, and SOX17 are not included. HPAH = heritable pulmonary arterial hypertension. See Table 1 and 4 legends for expansion of other abbreviations.
HPAH includes all those with pathogenic/suspected pathogenic mutations and individuals previously classified as FPAH without identified mutations.
Genetic testing results reclassified 40 of 218 (18%) patients with IPAH and 13 of 256 (5%) patients with APAH as having HPAH (Fig 1, Table 9). Genetic mutations were also found in 13 of 22 (59%) patients previously categorized as having familial PAH based on a family history of disease.
Discussion
The USPHSR is a contemporary multicenter, longitudinal registry of patients diagnosed with WHO Group 1 PAH enrolled in the US PAH Biobank designed to provide and integrate genetics, environmental exposure, and reproductive history data. Past registries of patients diagnosed with PAH in the United States and Europe have not included genomic data, and they provided limited data concerning environmental exposures and reproductive history.1,3,4,35,36
This report describes the baseline characteristics of contemporary patients diagnosed with Group 1 PAH who enrolled in USPHSR.27 Compared with past registries of patients diagnosed with PAH in the United States,1,4 the baseline demographic and clinical characteristics of patients with PAH enrolled in USPHSR from 2013 to 2017 are similar to those of PAH patients who enrolled in REVEAL Registry in 2006 and 2007. The demographic characteristics of the USPHSR cohort also mimic those of the larger PAH Biobank.32 The majority of USPHSR and REVEAL Registry participants were previously diagnosed women who presented with dyspnea and were diagnosed with IPAH or APAH in the fifth or sixth decade of life. In contrast to the National Institutes of Health PPH Registry in the 1980s,1 USPHSR and REVEAL Registry included patients diagnosed with APAH, and patients with IPAH enrolled in these registries were older, more commonly women, and previously diagnosed with PAH.
The USPHSR provides insight into modern treatment of PAH, including the continuing use of PAH monotherapy, the evolving use of combination therapy, and the introduction of newly approved PAH medications. One-third of USPHSR patients remain on monotherapy with a variety of PAH-specific medications. Monotherapy with a PDE5i was most common, whereas monotherapy with newly approved medications, including riociguat, oral treprostinil, and selexipag, was uncommon. Only 10 (2%) USPHSR patients were on calcium channel blocker monotherapy. The proportion of USPHSR patients treated with combination therapy is higher (66%) than the proportion of REVEAL Registry patients treated with combination therapy (50%). Treatment with the combination of an ERA and a PDE5i increased from 12% (REVEAL Registry) to 24% (USPHSR), perhaps reflecting the reported benefit of upfront initiation of an ERA and a PDE5i in treatment-naive PAH patients.37 The proportion of patients with PAH treated with triple combination PAH therapy increased from 8% (REVEAL Registry) to 15% (USPHSR), reflecting both expedient patterns of practice and recent Prostacyclin (PGI 2) Receptor Agonist in Pulmonary Arterial Hypertension (GRIPHON) trial data showing incremental benefit with the addition of selexipag to background dual therapy.38 The practice of using upfront triple therapy awaits evidence from an ongoing clinical trial (Efficacy and Safety of Initial Triple Versus Initial Dual Oral Combination Therapy in Patients With Newly Diagnosed Pulmonary Arterial Hypertension [TRITON]).39
USPHSR is the first US PAH registry to report detailed reproductive histories for women diagnosed with PAH. Overall, a history of miscarriage, premature birth, abortion, or stillbirth was present for many women subsequently diagnosed with PAH, although the diagnosis of PAH was rarely made within 6 months of pregnancy. The overall proportion of women diagnosed with PAH who reported having had a miscarriage was 37%. A higher proportion of women diagnosed with IPAH (44%) than diagnosed with APAH (34%) reported miscarriages (P = .04), with no differences in age at menarche or gravidity. Miscarriage prior to 20 weeks of gestation is a common outcome of pregnancy, occurring in 12% to 15% of recognized pregnancies.40 These USPHSR data suggest that women with undiagnosed PAH may be more likely to experience pregnancy complications, and this finding warrants future investigation with comparisons vs matched healthy control subjects.41, 42, 43
Exposure to exogenous hormones was also common among USPHSR patients. The majority of patients had taken OCPs, and women diagnosed with IPAH were more likely to have taken OCPs than women diagnosed with APAH (83% vs 71%; P = .01). In contrast, most patients with IPAH (81%) and APAH (79%) reported that they had never taken HRT. Controlling for confounders such as age, race, and BMI, as well as comparison of patients with PAH vs matched healthy control subjects, is necessary to explore the possible relationships between exogenous estrogen exposures and the development or severity of PAH.
The association between exposure to anorexigens such as fenfluramine or recreational drugs such as methamphetamine and PAH is well established.28,44,45 Nevertheless, USPHSR data indicate that exposure to these weight loss drugs or recreational drugs remains a common feature of adults diagnosed with PAH in the United States. Fifty-five (11%) patients reported that they had taken fenfluramine-phentermine or phentermine in the past, and 84 (17%) patients reported having taken other weight loss drugs. Similarly, 130 (27%) patients reported prior drug abuse, with 22 (5%) specifically reporting past use of methamphetamine. The rate of exposure to fenfluramine-phentermine or phentermine is higher than that reported for REVEAL Registry patients with PAH (64 of 2,141 [4%]) and the 5% rate of anorexigen exposure reported by the National Institutes of Health PPH Registry.3 Further investigation with USPHSR resources is warranted to examine relationships between obesity, anorexigen exposure, demographic characteristics, and genetic polymorphisms.
USPHSR is the first registry to report detailed genetic data for a multicenter cohort of adults diagnosed with PAH in the United States. We found pathogenic or likely pathogenic mutations in 13% of the cohort, including mutations in 12 of 17 genes reported to cause HPAH by the scientific task force of the 6th World Symposium on Pulmonary Hypertension.7 This finding illustrates the importance of genetic tests with gene panels to examine known causes of HPAH when evaluating patients diagnosed with IPAH. We identified pathogenic BMPR2 mutations more often than mutations in other genes that cause HPAH, similar to reports from investigators in France,46 England,47 and China.48 In contrast, few USPHSR patients had mutations in genes such as TBX4 that are more commonly implicated in pediatric-onset disease.49 Finally, using whole-exome sequence data, we identified potentially deleterious variants in tissue kallikrein 1 (KLK1) and gamma-glutamyl carboxylase (GGCX), two genes with genome-wide significance identified as increasing the risk for HPAH after the 6th World Symposium on Pulmonary Hypertension.32 These findings illustrate the importance of incorporating new genetic knowledge when evaluating patients with IPAH or suspected HPAH.
The reclassification of 40 of 218 (18%) USPHSR patients clinically diagnosed with IPAH and 13 of 256 (5%) USPHSR patients with APAH is an important observation for clinicians. Recognition of a causative gene mutation provides the patient with a clearer understanding of his or her disease, and it provides the opportunity to recognize PAH in members of their family. Existing evidence of poorer survival among patients with pathogenic BMPR2 mutations50 and poor prognosis without response to PAH therapies with bi-allelic EIF2AK4 mutations51 suggest that genetic data may influence risk stratification. There is growing evidence to suggest that mutations in genes such as TBX4 and SOX17 are also associated with a variety of clinical and histopathologic phenotypes of cardiopulmonary disease, which may portend diagnostic and prognostic significance.25,46,52 Further investigation with USPHSR resources is warranted to examine the impact of reclassification of IPAH and APAH to HPAH on their risk assessment and outcome.
Despite its strengths, the current study has several limitations. The generalizability of these findings is limited by numerous factors, including recruitment biases and the limited number of non-white patients enrolled in USPHSR. There are known limitations to the questionnaire used to assess reproductive history and exposures, such as differentiating fenfluramine from phentermine exposure, as there was need to balance the depth and number of questions with the likelihood of completion. Patient responses to the questionnaire are also limited by self-reporting and recall bias. Controlling for confounders, as well as comparison of patients with PAH vs matched healthy control subjects, is necessary in the future to minimize these limitations and further explore the novel observations in this study.
Interpretation
Baseline data from USPHSR provide key observations: (1) WHO Group 1 PAH patients remain predominately middle-aged women diagnosed with IPAH or APAH; (2) delays between symptom onset and diagnosis of PAH are unchanged from prior reports; (3) clinicians increasingly treat PAH with multiple PAH-targeted medications; (4) female patients often took anorexigens, OCPs, and/or recreational drugs, and may be more prone to complications of pregnancy (eg, miscarriage) prior to a diagnosis of PAH; and (5) results of genetic tests frequently identify unsuspected HPAH among patients diagnosed with IPAH and APAH.
As the USPHSR provides the pulmonary hypertension community with useful information to better phenotype patients, the concerning finding of a persistent and substantial time delay from symptom onset to diagnosis in the contemporary PAH population exists. This highlights the continued need for increased clinical awareness, to not only identify patients with PAH earlier but encourage referral of patients to a pulmonary hypertension center for earlier diagnosis and appropriate treatment that may improve outcomes.53,54
This registry also affords investigators the opportunity to ask and answer critical questions pertaining to the interaction of hormonal factors and genetic variability in the pathogenesis of PAH, particularly in women. The goal of USPHSR is to provide insights into genomic differences and common exposures that can lead to a better understanding of the predisposition of women to develop PAH, and lead to the development of personalized strategies to guide prevention, diagnosis, and treatment of this disease.
Acknowledgments
Author contributions: J. B. B. analyzed data, and wrote and edited the manuscript; D. B. B., W. C. N., and C. G. E. conceived the study, designed the analysis, analyzed and interpreted the data, and helped to write and edit the manuscript; E. D. A., R. L. B., W. K. C., H. W. F., and A. E. F. conceived the study, provided study consultation, and edited the manuscript; C. Y. conceived the study, designed the analysis, analyzed and interpreted data, and edited the manuscript; K. F. and A. D. P. conceived the study and provided study consultation; and K. A. L. and M. W. P. acquired, analyzed, and interpreted the genomic data.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: D. B. B. has received university grant monies from Acceleron, Actelion/Johnson & Johnson, Altavant, Arena/United Therapeutics/Lung LLC, Belleraphon, Complexa, Liquidia, National Institutes of Health (NIH), Reata, and USPHSR, has served as a steering committee member, advisory board member, or consultant for Acceleron, Actelion/Johnson & Johnson, Arena/United Therapeutics/Lung LLC, Belleraphon, Complexa, Liquidia, and USPHSR, and has been a longstanding stockholder in Johnson & Johnson. E. D. A. has received NIH grants, not related to this submission, and a CMREF Foundation grant, not related to this submission; H. W. F. is a consultant/member of the Scientific Advisory Board for Actelion (to present; $5,000, Altavant (to present; $4,000), Acceleron (to present $5,000) and United Therapeutics (to present $3,500) and has received additional honoraria from Bayer ($4,000) and Actelion ($2,000). A. E. F. has received funds for participation in Data Safety Monitoring Board (DSMB) for studies undertaken by Actelion which funded part of USPHSR through a 501c3. She has received consulting fees from PhaseBio, and for participation in DSMB with Complexa, and is part of an endpoint adjudication committee for a study funded by United Therapeutics. A. D. P. is Prinicipal, E Squared Trials and Registries Inc. and independent consultant to PhaseBio Pharmaceuticals Inc., Janssen Pharmaceuticals Inc., Altavant Sciences Inc. C. G. E. is employed by Intermountain Healthcare, and Intermountain Healthcare received compensation for C. G. E. service on data safety monitoring boards. C. G. E. has also consulted for the University of California at San Diego and EBSCO. None declared (J. B. B., R. L. B., W. K. C., K. F., K. A. L., M. W. P., C. Y., W. C. N.).
∗USPHSR Investigators Collaborators: Robert Simms, MD, Terry Fortin, MD, Zeenat Safdar, MD, Charles D. Burger, MD, Robert P. Frantz, MD, Nicholas S. Hill, MD, Sophia Airhart, MD, Jean Elwing, MD, Marc Simon, MD, R. James White, MD, PhD, Ivan M. Robbins, MD, and Murali M. Chakinala, MD.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors thank contributors, including the pulmonary hypertension centers that enrolled patients, completed questionnaires, and collected samples used in this study, as well as patients and their families, whose help and participation made this work possible.
Additional information: The e-Appendix and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This work was supported by an unrestricted grant from Actelion Pharmaceuticals US, Inc., donations from Bike PHifty, and the National Heart, Lung, and Blood Institute [Grant HL105333]. Samples and/or data from the National Biological Sample and Data Repository for Pulmonary Arterial Hypertension, which receives government support under an investigator-initiated grant [R24 HL105333] awarded by the National Heart, Lung, and Blood Institute, were used in support of this study. W. C. N., M. W. P., and K. A. L. were funded by Grant HL105333.
Contributor Information
Jessica B. Badlam, Email: Jessica.badlam@uvmhealth.org.
USPHSR Investigators:
Robert Simms, Terry Fortin, Zeenat Safdar, Charles D. Burger, Robert P. Frantz, Nicholas S. Hill, Sophia Airhart, Jean Elwing, Marc Simon, R. James White, Ivan M. Robbins, and Murali M. Chakinala
Supplementary Data
Take-home Points.
Study Question: What are the demographic characteristics, genetics, reproductive histories, and environmental exposures of contemporary patients with WHO Group 1 pulmonary arterial hypertension (PAH)?
Results: Group 1 PAH patients remain predominately middle-aged women diagnosed with idiopathic or associated PAH. Although delays in diagnosis persist, treatment with combinations of PAH-targeted medications are more common than in the past, genetic testing frequently identifies unsuspected heritable PAH, and women with PAH often report pregnancy complications, as well as exposure to anorexigens, oral contraceptives, and/or recreational drugs.
Interpretation: The United States Pulmonary Hypertension Scientific Registry provides novel information on genetics, reproductive histories, and environmental exposures that afford the opportunity to ask and answer critical questions pertaining to the interaction of these factors in the pathogenesis of PAH, particularly in women, and lead to the development of personalized strategies to guide prevention, diagnosis, and treatment of this disease.
References
- 1.Rich S., Dantzker D.R., Ayres S.M. Primary pulmonary hypertension. A national prospective study. Ann Intern Med. 1987;107(2):216–223. doi: 10.7326/0003-4819-107-2-216. [DOI] [PubMed] [Google Scholar]
- 2.D'Alonzo G.E., Barst R.J., Ayres S.M. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115(5):343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 3.Frost A.E., Badesch D.B., Barst R.J. The changing picture of patients with pulmonary arterial hypertension in the United States: how REVEAL differs from historic and non-US contemporary registries. Chest. 2011;139(1):128–137. doi: 10.1378/chest.10-0075. [DOI] [PubMed] [Google Scholar]
- 4.Badesch D.B., Raskob G.E., Elliott C.G. Pulmonary arterial hypertension: baseline characteristics from the REVEAL registry. Chest. 2010;137(2):376–387. doi: 10.1378/chest.09-1140. [DOI] [PubMed] [Google Scholar]
- 5.McGoon M.D., Miller D.P. REVEAL: a contemporary US pulmonary arterial hypertension registry. Eur Respir Rev. 2012;21(123):8–18. doi: 10.1183/09059180.00008211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benza R.L., Miller D.P., Barst R.J., Badesch D.B., Frost A.E., McGoon M.D. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest. 2012;142(2):448–456. doi: 10.1378/chest.11-1460. [DOI] [PubMed] [Google Scholar]
- 7.Morrell N.W., Aldred M.A., Chung W.K. Genetics and genomics of pulmonary arterial hypertension. Eur Respir J. 2019;53(1):1801899. doi: 10.1183/13993003.01899-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galiè N., Channick R.N., Frantz R.P. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019;53(1):1801889. doi: 10.1183/13993003.01889-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghofrani H.A., Galiè N., Grimminger F. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369(4):330–340. doi: 10.1056/NEJMoa1209655. [DOI] [PubMed] [Google Scholar]
- 10.Pulido T., Adzerikho I., Channick R.N. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013;369(9):809–818. doi: 10.1056/NEJMoa1213917. [DOI] [PubMed] [Google Scholar]
- 11.Tapson V.F., Torres F., Kermeen F. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients on background endothelin receptor antagonist and/or phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C study): a randomized controlled trial. Chest. 2012;142(6):1383–1390. doi: 10.1378/chest.11-2212. [DOI] [PubMed] [Google Scholar]
- 12.Sitbon O., Channick R., Chin K.M. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med. 2015;373(26):2522–2533. doi: 10.1056/NEJMoa1503184. [DOI] [PubMed] [Google Scholar]
- 13.Deng Z., Morse J.H., Slager S.L. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Human Genetics. 2000;67(3):737–744. doi: 10.1086/303059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.International PPH Consortium. Lane K.B., Machado R.D. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Nature Genetics. 2000;26(1):81–84. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- 15.Simonneau G., Robbins I.M., Beghetti M. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54(1 suppl):S43–S54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 16.Trembath R.C., Thomson J.R., Machado R.D. Clinical and molecular genetic features of pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia. N Engl J Med. 2001;345(5):325–334. doi: 10.1056/NEJM200108023450503. [DOI] [PubMed] [Google Scholar]
- 17.Harrison R.E., Flanagan J.A., Sankelo M. Molecular and functional analysis identifies ALK-1 as the predominant cause of pulmonary hypertension related to hereditary haemorrhagic telangiectasia. J Med Genetics. 2003;40(12):865–871. doi: 10.1136/jmg.40.12.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shintani M., Yagi H., Nakayama T., Saji T., Matsuoka R. A new nonsense mutation of SMAD8 associated with pulmonary arterial hypertension. J Med Genetics. 2009;46(5):331–337. doi: 10.1136/jmg.2008.062703. [DOI] [PubMed] [Google Scholar]
- 19.Austin E.D., Ma L., LeDuc C. Whole exome sequencing to identify a novel gene (caveolin-1) associated with human pulmonary arterial hypertension. Circ Cardiovasc Genet. 2012;5(3):336–343. doi: 10.1161/CIRCGENETICS.111.961888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma L., Roman-Campos D., Austin E.D. A novel channelopathy in pulmonary arterial hypertension. N Engl J Med. 2013;369(4):351–361. doi: 10.1056/NEJMoa1211097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerstjens-Frederikse W.S., Bongers E.M.H.F., Roofthooft M.T.R. TBX4 mutations (small patella syndrome) are associated with childhood-onset pulmonary arterial hypertension. J Med Genetics. 2013;50(8):500–506. doi: 10.1136/jmedgenet-2012-101152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Best D.H., Sumner K.L., Austin E.D. EIF2AK4 mutations in pulmonary capillary hemangiomatosis. Chest. 2014;145(2):231–236. doi: 10.1378/chest.13-2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eyries M., Montani D., Girerd B. EIF2AK4 mutations cause pulmonary veno-occlusive disease, a recessive form of pulmonary hypertension. Nature Genetics. 2014;46(1):65–69. doi: 10.1038/ng.2844. [DOI] [PubMed] [Google Scholar]
- 24.Graf S., Haimel M., Bleda M. Identification of rare sequence variation underlying heritable pulmonary arterial hypertension. Nat Commun. 2018;9(1):1416. doi: 10.1038/s41467-018-03672-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu N., Welch C.L., Wang J. Rare variants in SOX17 are associated with pulmonary arterial hypertension with congenital heart disease. Genome Med. 2018;10(1):56. doi: 10.1186/s13073-018-0566-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lahm T., Tuder R.M., Petrache I. Progress in solving the sex hormone paradox in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2014;307(1):L7–L26. doi: 10.1152/ajplung.00337.2013. [DOI] [PubMed] [Google Scholar]
- 27.Elliott C.G., Austin E.D., Badesch D. United States Pulmonary Hypertension Scientific Registry (USPHSR): rationale, design, and clinical implications. Pulm Circ. 2019;9(2) doi: 10.1177/2045894019851696. 2045894019851696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simonneau G., Montani D., Celermajer D.S. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):1801913. doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoeper M.M., Bogaard H.J., Condliffe R. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 suppl):D42–D50. doi: 10.1016/j.jacc.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 30.Hennekens C.H., Speizer F.E., Lipnick R.J. A case-control study of oral contraceptive use and breast cancer. J National Cancer Institute. 1984;72(1):39–42. doi: 10.1093/jnci/72.1.39. [DOI] [PubMed] [Google Scholar]
- 31.Lipnick R., Speizer F.E., Bain C. A case-control study of risk indicators among women with premenopausal and early postmenopausal breast cancer. Cancer. 1984;53(4):1020–1024. doi: 10.1002/1097-0142(19840215)53:4<1020::aid-cncr2820530433>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 32.Zhu N., Pauciulo M.W., Welch C.L. Novel risk genes and mechanisms implicated by exome sequencing of 2572 individuals with pulmonary arterial hypertension. Genome Med. 2019;11(1):69. doi: 10.1186/s13073-019-0685-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas L., Peterson E.D. The value of statistical analysis plans in observational research: defining high-quality research from the start. JAMA. 2012;308(8):773–774. doi: 10.1001/jama.2012.9502. [DOI] [PubMed] [Google Scholar]
- 34.von Elm E., Altman D.G., Egger M. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 35.Pittrow D., Ghofrani H.A., Opitz C.F., Huscher D., Hoeper M.M. International, prospective register for the documentation of first-line and maintenance therapy in patients with pulmonary hypertension (CompERA-XL) [in German] Dtsch Med Wochenschr. 2009;134(suppl 5):S173–S175. doi: 10.1055/s-0029-1225318. [DOI] [PubMed] [Google Scholar]
- 36.Humbert M., Sitbon O., Chaouat A. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173(9):1023–1030. doi: 10.1164/rccm.200510-1668OC. [DOI] [PubMed] [Google Scholar]
- 37.Galie N., Barbera J.A., Frost A.E. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med. 2015;373(9):834–844. doi: 10.1056/NEJMoa1413687. [DOI] [PubMed] [Google Scholar]
- 38.Coghlan J.G., Channick R., Chin K. Targeting the prostacyclin pathway with selexipag in patients with pulmonary arterial hypertension receiving double combination therapy: insights from the randomized controlled GRIPHON study. Am J Cardiovasc Drugs. 2018;18(1):37–47. doi: 10.1007/s40256-017-0262-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Institutes of Health Clinical Center. The efficacy and safety of initial triple versus initial dual oral combination therapy in patients with newly diagnosed pulmonary arterial hypertension (TRITON). NCT02558231. ClinicalTrials.gov. Bethesda, MD: National Institutes of Health; 2015. https://clinicaltrials.gov/ct2/show/results/NCT02558231. Updated September 11, 2020.
- 40.Magnus M.C., Wilcox A.J., Morken N.H., Weinberg C.R., Haberg S.E. Role of maternal age and pregnancy history in risk of miscarriage: prospective register based study. BMJ. 2019;364:l869. doi: 10.1136/bmj.l869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown S. Miscarriage and its associations. Semin Reprod Med. 2008;26(5):391–400. doi: 10.1055/s-0028-1087105. [DOI] [PubMed] [Google Scholar]
- 42.Ferre C., Callaghan W., Olson C., Sharma A., Barfield W. Effects of maternal age and age-specific preterm birth rates on overall preterm birth rates—United States, 2007 and 2014. MMWR Morb Mortal Wkly Rep. 2016;65(43):1181–1184. doi: 10.15585/mmwr.mm6543a1. [DOI] [PubMed] [Google Scholar]
- 43.MacDorman M.F., Gregory E.C. Fetal and perinatal mortality: United States, 2013. Natl Vital Stat Rep. 2015;64(8):1–24. [PubMed] [Google Scholar]
- 44.Abenhaim L., Moride Y., Brenot F. Appetite-suppressant drugs and the risk of primary pulmonary hypertension. International Primary Pulmonary Hypertension Study Group. N Engl J Med. 1996;335(9):609–616. doi: 10.1056/NEJM199608293350901. [DOI] [PubMed] [Google Scholar]
- 45.Kramer M.S., Lane D.A. Aminorex, dexfenfluramine, and primary pulmonary hypertension. J Clin Epidemiol. 1998;51(4):361–364. doi: 10.1016/s0895-4356(97)00289-8. [DOI] [PubMed] [Google Scholar]
- 46.Eyries M., Montani D., Nadaud S. Widening the landscape of heritable pulmonary hypertension mutations in paediatric and adult cases. Eur Respir J. 2019;53(3):1801371. doi: 10.1183/13993003.01371-2018. [DOI] [PubMed] [Google Scholar]
- 47.Hadinnapola C., Bleda M., Haimel M. Phenotypic characterization of EIF2AK4 mutation carriers in a large cohort of patients diagnosed clinically with pulmonary arterial hypertension. Circulation. 2017;136(21):2022–2033. doi: 10.1161/CIRCULATIONAHA.117.028351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu D., Liu Q.Q., Eyries M. Molecular genetics and clinical features of Chinese idiopathic and heritable pulmonary arterial hypertension patients. Eur Respir J. 2012;39(3):597–603. doi: 10.1183/09031936.00072911. [DOI] [PubMed] [Google Scholar]
- 49.Zhu N., Gonzaga-Jauregui C., Welch C.L. Exome sequencing in children with pulmonary arterial hypertension demonstrates differences compared with adults. Circ Genom Precis Med. 2018;11(4) doi: 10.1161/CIRCGEN.117.001887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Evans J.D., Girerd B., Montani D. BMPR2 mutations and survival in pulmonary arterial hypertension: an individual participant data meta-analysis. Lancet Respir Med. 2016;4(2):129–137. doi: 10.1016/S2213-2600(15)00544-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Montani D., Girerd B., Jais X. Clinical phenotypes and outcomes of heritable and sporadic pulmonary veno-occlusive disease: a population-based study. Lancet Respir Med. 2017;5(2):125–134. doi: 10.1016/S2213-2600(16)30438-6. [DOI] [PubMed] [Google Scholar]
- 52.Haarman M.G., Kerstjens-Frederikse W.S., Berger R. TBX4 variants and pulmonary diseases: getting out of the ‘box. Curr Opin Pulm Med. 2020;26(3):277–284. doi: 10.1097/MCP.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deaño R.C., Glassner-Kolmin C., Rubenfire M. Referral of patients with pulmonary hypertension diagnoses to tertiary pulmonary hypertension centers: the multicenter RePHerral study. JAMA. 2013;173(10):887–893. doi: 10.1001/jamainternmed.2013.319. [DOI] [PubMed] [Google Scholar]
- 54.Klinger J.R., Elliott C.G., Levine D.J. Therapy for pulmonary arterial hypertension in adults: update of the CHEST Guideline and Expert Panel Report. Chest. 2019;155(3):565–586. doi: 10.1016/j.chest.2018.11.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.