Abstract
To explore the association between bacterial vaginosis (BV) and periodontitis (PD) and to determine whether PD and BV might be linked with systemic serum alterations. We used the National Health and Nutrition Examination Survey 2001–2004, with women aged 18–49 years old and diagnosed with or without BV according to Nugent’s method. PD was defined according to the 2012 case definition. We compared serum counts according to the presence of PD and the presence of BV. Multivariable regression was used to explore and identify relevant variables towards the presence of BV. 961 women fulfilled the inclusion criteria. In women with BV, PD was associated with higher inflammation, characterized by increased white blood cells (p = 0.006) and lymphocyte (p = 0.009) counts. Predictive models presented a statistically significant association between PD and BV [Odds Ratio (OD) = 1.69, 95% Confidence Interval (CI): 1.09–2.61 for periodontitis; OD = 2.37, 95% CI: 1.30–4.29 for severe PD]. Fully adjusted models for age, smoking, body mass index, diabetes mellitus and number of systemic conditions reinforced this association [OD = 1.71, 95% CI: 1.06–2.76 for PD; OD = 2.21, 95% CI: 1.15–4.25 for severe PD]. An association between BV and PD is conceivable. PD was associated with higher systemic markers of inflammation in women with BV. Our data is novel and could serve as a foundation to guide future studies in the confirmation of this association and the underlying mechanisms.
Subject terms: Biomarkers, Diseases, Health care
Introduction
Bacterial vaginosis (BV) is a common vaginal condition with unknown aetiology which represents a relevant shift in the vaginal microbiota. BV is characterized by a thin gray/white and malodorous “fishy” discharge, with high vaginal pH (> 4.5), and vaginal epithelial cells heavily coated with bacteria1–4. The abnormal vaginal discharge is the result of an imbalanced vaginal microbiota with increase of anaerobic microorganisms (e.g., Gardnerella vaginalis, Prevotella Intermedia, Peptostreptococcus, and Bacteriodes spp)1,2,5. Also, BV is associated with reproductive and obstetric sequelae, increasing women’s risk of pelvic inflammatory disease, spontaneous abortion, preterm delivery, low birth weight, and postpartum endometritis2,5–9. Susceptibility to BV is dependent on the host immune response and is multifactorial1,2,10–12. Genetics, environmental and physiologic stressors, hormonal variations based on the reproductive status and use of hormonal contraceptives are relevant factors towards BV1,10.
Periodontitis (PD) is a chronic multifactorial inflammatory condition characterized by inflamed gums and bone destruction caused by a dysbiotic plaque13–15 It is preceded by gum inflammation (gingivitis) and an uncontrolled inflammatory response from the innate and adaptive immune system16. PD has also been associated with several chronic and systemic diseases17–23. A recent review discussed the available evidence and possible mechanisms between female infertility related conditions as a modifiable risk factor towards PD24,25. Particularly, women with BV were associated with a significant diversification of salivary microbiota and higher counts of Prevotella intermedia (a PD-related bacteria) in the supragingival microbiota compared with women without BV26. However, the mechanism through which the oral microbiota suffers dysbiosis in the presence of BV is not determined26.
Data from the Longitudinal Study of Vaginal Flora reported a significant association between PD and BV27, and women with BV were also found to have higher risk of gingivitis28. Comprehensively, bacterial communities from the vagina and the periodontium have higher counts of Fusobacteria nucleatum and Prevotella intermedia27,29–31. Also, the presence of these bacteria may trigger infections in primed women27,32, and may have unpleasant consequences during pregnancy, such as preterm birth and low birth weight2,5–9. Further, some studies also support the notion of haematogenous spread or oral-genital direct transfer27,29,33, however, the relationship between these two pathologies remains poorly understood.
For this reason, we aimed to investigate the likelihood of an association between BV and PD in a representative American cohort of adult women. Secondly, we explored whether this association might lead to systemic alterations measured via blood samples, through complete blood count (white, red and platelet lineages) and C-reactive protein (CRP) levels.
Results
Baseline characteristics
From a total of 21,161 participants in National Health and Nutrition Examination Survey (NHANES) 2001–2002 and 2003–2004, we excluded 10,301 (48.7%) males, 7395 women under 18 years or over 50 years old (34.9%), 301 pregnant women (1.4%) and 38 women who did not perform the pregnancy test or had an invalid result (0.2%). Overall, from 3126 included women, a final sample of 961 completed both BV and periodontal clinical examinations. Table 1 displays the characteristics of the included women participants.
Table 1.
Participants characteristics.
| Non-BV (n = 510) | BV (n = 451) | p valuea | |
|---|---|---|---|
| Age, mean (SD) (years) | 32.4 (10.1) | 32.0 (10.3) | 0.556 |
| Race/ethnicity, n (%) | |||
| Mexican American | 122 (51.1) | 117 (49.0) | 0.000 |
| Non-Hispanics White | 258 (63.1) | 151 (36.9) | |
| Non-Hispanic Black | 148 (63.0) | 87 (37.0) | |
| Other Hispanic | 26 (51.0) | 25 (49.0) | |
| Other race | 10 (50.0) | 10 (50.0) | |
| Education | |||
| < High school | 126 (43.8) | 162 (56.3) | 0.000 |
| High school | 129 (54.0) | 110 (46.0) | |
| > High school | 255 (58.8) | 179 (41.2) | |
| Active smokers, n (%) | 95 (9.9) | 115 (12.0) | 0.007 |
| BMI, mean (SD) (kg/m2) | 27.2 (7.2) | 28.2 (8.3) | 0.017 |
| Marital status | |||
| Single | 163 (47.8) | 178 (52.2) | 0.002 |
| Married/living with a partner | 299 (58.7) | 210 (41.3) | |
| Divorced/separated/widowed | 48 (47.5) | 53 (52.5) | |
| Medical conditions, n (%) | |||
| Diabetes mellitus | 17 (1.8) | 19 (2.0) | 0.325 |
| Other medical conditions | 0.5 (0.8) | 0.6 (0.9) | 0.528 |
| Periodontal health, n (%) | 472 (49.1) | 397 (41.3) | 0.013 |
| Periodontitis staging, n (%) | |||
| Mild (stage I) | 21 (51.2) | 20 (48.8) | |
| Moderate (stage II) | 15 (39.5) | 23 (60.5) | |
| Severe (stage III) | 2 (15.4) | 11 (84.6) | |
| PISA | 11.8 (26.4) | 13.9 (27.8) | 0.012 |
| PESA | 93.5 (232.0) | 87.0 (260.4) | 0.543 |
Significant correlations are identified in bold (p < 0.05).
BV, patients with bacterial vaginosis; n, number; SD, standard deviations; Non-BV, patients without bacterial vaginosis; PESA, periodontal epithelial surface area; PISA, periodontal inflamed surface area; <, higher than; >, less than.
Values are given as mean (standard error) or % (standard error).
aChi-square test for categorical variables, Mann–Whitney test for continuous variables, p < 0.05.
The prevalence of PD in the overall sample was 9.6% (54% in patients with BV and 38% in non-BV women), although women with BV had higher prevalence (p = 0.013). Women with BV had a higher percentage of active smokers (12.0%), higher levels of body mass index (BMI) (28.2 kg/m2) and presenting with higher education levels (56.3%) when compared to participants with no clinical signs of BV. Indeed, the number of medical conditions recorded was similar in both groups. Mean ± Standard deviation (SD) of periodontal inflamed surface area (PISA) and periodontal epithelial surface area (PESA) were 11.8 ± 26.4 and 93.5 ± 232.0 in patients without BV versus 13.9 ± 27.8 and 87.0 ± 260.4 for those with BV, respectively (p = 0.012 for PISA and p = 0.543 for PESA).
Hematologic counts
The complete blood count was used to compare the blood counts of the PD group with the periodontally healthy women, according to the BV diagnosis. Globally, women with PD presented higher counts of white blood cells (WBC) (p = 0.019) and lymphocytes (p = 0.021), and lower counts of monocyte (p = 0.050). Specifically in women without BV, PD was associated with lower counts of monocytes (p = 0.013). Women diagnosed with both BV and PD presented increased counts of WBC (p = 0.006), lymphocytes (p = 0.009) and CRP (p = 0.045) (Table 2).
Table 2.
Hematologic counts according to bacterial vaginosis and periodontal state.
| Variables, mean (SD) | BV (n = 510) | Non-BV (n = 451) | Global (n = 961) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-PD (n = 472) | PD (n = 38) | p valuea | Non-PD (n = 397) | PD (n = 54) | p valuea | Non-PD (n = 869) | PD (n = 92) | p valuea | |
| WBC count (109/L) | 7.2 (2.6) | 8.2 (2.7) | 0.006 | 7.0 (2.5) | 7.1 (2.6) | 0.857 | 7.1 (2.5) | 7.8 (2.7) | 0.019 |
| Monocyte (%) | 7.0 (2.6) | 6.8 (2.3) | 0.578 | 7.0 (2.3) | 6.2 (2.6) | 0.013 | 7.0 (2.5) | 6.6 (2.5) | 0.050 |
| Monocyte (109/L) | 0.5 (0.2) | 0.5 (0.2) | 0.387 | 0.5 (0.2) | 0.5 (0.2) | 0.192 | 0.5 (0.2) | 0.5 (0.2) | 0.993 |
| Segmented neutrophils (%) | 55.8 (15.5) | 55.7 (15.2) | 0.896 | 57.0 (13.7) | 57.0 (17.2) | 0.469 | 56.4 (14.6) | 56.2 (16.0) | 0.660 |
| Segmented neutrophils (109/L) | 4.2 (1.8) | 4.4 (2.1) | 0.086 | 4.2 (1.8) | 4.4 (2.1) | 0.646 | 4.3 (1.9) | 4.6 (2.2) | 0.094 |
| Eosinophils (%) | 2.4 (2.5) | 2.3 (1.8) | 0.810 | 2.3 (2.1) | 1.7 (1.1) | 0.058 | 2.4 (2.3) | 2.1 (1.6) | 0.178 |
| Eosinophils (109/L) | 0.2 (0.2) | 0.1 (0.1) | 0.735 | 0.2 (0.2) | 0.1 (0.1) | 0.266 | 0.2 (0.2) | 0.2 (0.1) | 0.662 |
| Basophils (%) | 0.6 (0.5) | 0.6 (0.4) | 0.988 | 0.6 (0.4) | 0.5 (0.5) | 0.080 | 0.7 (0.4) | 0.3 (0.3) | 0.281 |
| Basophils (109/L) | 0.0 (0.1) | 0.0 (0.1) | 0.075 | 0.0 (0.1) | 0.0 (0.1) | 0.339 | 0.0 (0.1) | 0.0 (0.1) | 0.361 |
| Lymphocyte (%) | 29.6 (10.6) | 30.6 (10.7) | 0.474 | 29.4 (9.2) | 28.8 (10.9) | 0.506 | 29.5 (9.9) | 29.9 (10.7) | 0.850 |
| Lymphocyte (109/L) | 2.1 (0.8) | 2.5 (0.8) | 0.009 | 2.1 (0.7) | 2.1 (0.7) | 0.914 | 2.1 (0.8) | 2.3 (0.8) | 0.021 |
| RBC count (million cells/µL) | 4.3 (1.0) | 4.4 (0.7) | 0.211 | 4.3 (0.9) | 4.3 (1.2) | 0.398 | 4.3 (1.0) | 4.4 (0.9) | 0.613 |
| Hemoglobin (g/dL) | 12.6 (3.1) | 13.0 (2.4) | 0.395 | 12.9 (2.8) | 12.8 (3.6) | 0.501 | 12.8 (2.9) | 12.9 (2.9) | 0.890 |
| Hematocrit (%) | 37.3 (8.9) | 38.7 (6.8) | 0.301 | 38.0 (8.2) | 38.2 (10.5) | 0.642 | 37.7 (8.5) | 38.4 (8.4) | 0.668 |
| MCV (fL) | 83.6 (19.4) | 85.5 (14.4) | 0.488 | 85.0 (17.7) | 83.0 (22.0) | 0.240 | 84.3 (18.5) | 84.5 (17.7) | 0.791 |
| MCH (pg) | 28.3 (6.7) | 28.8 (5.2) | 0.585 | 28.9 (6.2) | 28.0 (7.6) | 0.179 | 28.6 (6.4) | 28.5 (6.2) | 0.617 |
| MCHC (g/dL) | 32.3 (7.1) | 33.0 (4.8) | 0.416 | 32.7 (6.5) | 31.6 (8.1) | 0.100 | 32.5 (6.8) | 32.5 (6.3) | 0.657 |
| RDW (%) | 12.3 (3.0) | 12.8 (2.4) | 0.144 | 12.2 (2.7) | 12.4 (3.8) | 0.575 | 12.2 (2.8) | 12.6 (3.0) | 0.420 |
| Platelet count (109/L) | 278.9 (91.4) | 297.4 (82.7) | 0.183 | 281.6 (90.7) | 292.4 (106.9) | 0.672 | 280.3 (90.9) | 295.4 (92.6) | 0.197 |
| MPV (fL) | 8.0 (1.9) | 8.3 (1.6) | 0.134 | 8.0 (1.8) | 7.6 (2.1) | 0.075 | 8.0 (1.9) | 8.0 (1.8) | 0.903 |
| CRP (mg/dL) | 0.4 (1.1) | 0.7 (1.0) | 0.045 | 0.4 (1.0) | 0.4 (0.6) | 0.939 | 0.4 (1.0) | 0.6 (0.9) | 0.093 |
Significant correlations are identified in bold (p < 0.05).
BV, patients with bacterial vaginosis; CRP, C-reactive protein; Non-BV, patients without bacterial vaginosis; P(−), no periodontitis, P(+), periodontitis; WBC, white blood cells; RBC, red blood cells; MCV, mean cell volume; MCH, mean cell hemoglobin; MCHC, mean cell hemoglobin concentration; RCD, red cell distribution; MPV, mean platelet volume.
aMann–Whitney test for continuous variables, p < 0.05.
Then, we graphically explored the behaviour of CRP levels of women with and without BV according to the levels of PISA (Fig. 1). In BV women, CRP levels tend to increase with the increase of PISA levels. Up to 4.4 levels of PISA, women with BV presented lower levels of CRP than non-BV counterparts.
Figure 1.
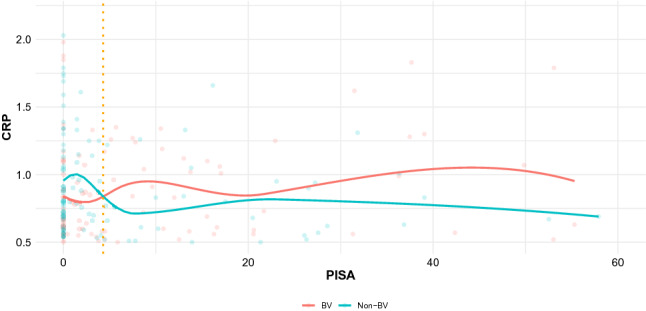
Comparison of C-reactive protein (CRP) (ng/mL) according to Periodontal Inflamed Surface Area (PISA) for women with and without BV.
Predictive models of periodontitis on bacterial vaginosis patients
In the crude model (model 1), the association of PD with BV was statistically significant [odds ratio (OR) = 1.69, 95% confidence interval (CI): 1.09–2.61 for PD; OR = 2.37, 95% CI: 1.30–4.29 for severe PD] (Table 3). Also, the fully adjusted models with age, smoking habits, BMI, diabetes mellitus and number of systemic conditions confirmed a significant association (OR = 1.71, 95% CI: 1.06–2.76 for PD; OR = 2.21, 95% CI: 1.15–4.25 for severe PD) (Table 3).
Table 3.
Odds ratios (OR) and correspondent 95% confidence intervals (CI) towards bacterial vaginosis, according to the periodontal status, calculated within binary logistic regression analyses for different adjustment levels.
| Periodontitis OR (95% CI) | Severe periodontitis OR (95% CI) | |
|---|---|---|
| Model 1 | 1.69 (1.09–2.61)* | 2.37 (1.30–4.29)** |
| Model 2 | 1.76 (1.13–2.74)* | 2.52 (1.37–4.63)** |
| Model 3 | 1.66 (1.04–2.65)* | 2.29 (1.21–4.33)* |
| Model 4 | 1.67 (1.04–2.69)* | 2.14 (1.12–4.09)* |
| Model 5 | 1.68 (1.04–2.70)* | 2.16 (1.13–4.12)* |
| Model 6 | 1.71 (1.06–2.76)* | 2.21 (1.15–4.25)* |
Model 1, unadjusted model; Model 2, includes adjustment for age; Model 3, includes adjustment for age and smoking habits; Model 4, includes adjustment for age, smoking habits and BMI; Model 5, includes adjustment for age, smoking habits, BMI and diabetes mellitus; Model 6, includes adjustment for age, smoking habits, BMI, diabetes mellitus and number of systemic diseases (excluding diabetes). Statistically significant: *p < 0.05; **p < 0.01; ***p < 0.001.
Discussion
We aimed to investigate whether BV and PD were associated in a representative nationwide sample of women Americans. Our results support our initial hypothesis of a possible link between BV and PD. Women with BV had higher prevalence of PD, levels of periodontal inflammation, percentage of active smokers and average BMI. Also, women with BV and PD presented significantly more systemic inflammation than those with healthy periodontium.
Thus so far, this study is the first to explore the likelihood of a systemic association via inflammatory hallmarks between BV and PD. Our findings emphasize that when both BV and PD co-exist, a significant systemic inflammatory load is detected (measured as CRP) and may be dependent on the periodontal inflammation (measured as PISA). Further, BV patients with PD present higher counts of white and lymphocyte cells than BV patients without PD.
Considering the leukocyte data in this study, our results are consistent with the available evidence, where an increase in WBC is expected from an indirect impact of PD34,35. Still, the counts of neutrophils were not a significant parameter in this analysis, despite their strong involvement in the pathogenesis of periodontal disease as they contribute to tissue destruction by releasing toxic products, such as enzymes and cytokines36–38. BV is commonly defined by the absence of neutrophils in vaginal swabs observed by flow cytometry4,39,40, and therefore an impact on circulating neutrophil counts might be unlikely to exist. However, since the periodontal examination was partially employed, this may have influenced these contradictory results.
The reported prevalence of BV in this American population is consistent with the report by Coudray and Madhivanan41 which shows a prevalence of 29.2%. Others, Javed et al.42 and Peebles et al.43 both found BV to be more prevalent in Non-Hispanic Black women with 51.4% and 33.2%, respectively. However, our results demonstrate higher rates in Non-Hispanic White women. Also, our result contrasts with the prevalence of BV in NHANES 2001–200444. A possible explanation for those differences may be due to the data mining when evaluating patients with both BV and PD examinations, that resulted in the exclusion of a substantial percentage of the overall sample (44.4%).
Our results are in accordance with previous literature stating there is a higher number of women who were diagnosed with BV and had smoking habits. In this sense, smoking habits has a significant effect on innate immunity, and it has been shown to have varying effects on markers of cervicovaginal immunity, namely with higher interleukin (IL)-10 levels in cervicovaginal secretions10,45–47. It is also a major modifiable risk factor for PD48–50 and it is hypothesized that smoking may lead to an increase in the prevalence of periodontal pathogens as well as delays the neutrophils recruitment into periodontal tissues, and thus compromise the immune response16,51–56. Further, our results also show that women with BV had higher BMI compared with women without BV, and this result is in contrast with Loken et al.57 result, however this can be explained due to the distinct types of populations and environmental factors.
These results might contribute to increase awareness of obstetricians/gynecologists for the higher risk of women with BV towards the presence of PD and the elevated inflamed burden that these patients may be exposed to.
Further, the coexistence of BV and PD in pregnant women may have severe consequences. Several lines of evidence indicate that infectious processes are associated with preterm delivery, namely overt or subclinical intrauterine infection, lower genital tract infection and distant infections, such as periodontal infection58. On the one hand, periodontal bacteria and/or their pathogenic subproducts may spread into the bloodstream and reach the foeto-placental unit triggering an inflammatory response59. On the other hand, inflammatory cytokines and mediators (such as CRP) due to periodontal inflammation also play a key role, perpetuating this inflammatory response59. Lastly, high levels of maternal CRP in early pregnancy are associated with preterm birth60–62, and PD might be seen as a contributing element for this unpleasant outcome.
Undoubtedly, BV may cause genital mucosal inflammation3, though evidence on local cervicovaginal cytokine levels in BV is still debatable3,63–65. Nevertheless, the consequences of maintained elevated systemic CRP levels in women with BV are unknown given the lack of clinical evidence. On the other hand, PD is a recognized factor towards persistent inflammatory states on a plethora of other chronic diseases, for instance in diabetes, cardiovascular diseases or rheumatoid arthritis17,66–68. Further, the treatment of gum disease is effective in alleviating this inflammatory burden69–71. Therefore, future studies are warranted to unveil whether treating PD in women with BV might mitigate the inflammatory load observed in this study and the added value of doing so.
The present study is an epidemiological representative study of a segment of the American population composed of women in fertile age. A big strength of this study is the size of the data set used, comprising two NHANES waves. BV was assessed using Nugent’s score which is widely regarded as the gold standard for the diagnosis of BV in research and clinical studies72–75, and we employed the most up-to-date periodontal classification76. Also, these results raise awareness on the interplay between oral health and women genital tract health and stress the fact that there is a need for more research to conclude a possible relationship between both diseases as well as to understand their linking mechanisms.
There are, however, some limitations to mention. Due to its novelty, we are not able to compare our results. Also, NHANES waves stated in this paper used a partial index to assess periodontal status which diminishes the sensitivity and specificity77,78, and this may limit the validity of the established association duo to a possible underestimation of PD. Hence future studies shall implement full-mouth periodontal charts to contribute to more consistent results. Furthermore, this is a cohort study that precluded the inference of causality or temporal relationship between the analyzed variables.
Conclusions
Women with BV have higher risk of presenting PD, and therefore an association between BV and PD is possible. Also, women with BV and PD present significantly higher systemic markers of inflammation than those without PD. Future studies are warranted to ascertain further this association as well the mechanisms upon this interplay.
Materials and methods
Study design and participants
We conducted a secondary analysis on data from the NHANES, a stratified multistage national representative survey with civilian noninstitutionalized population in fifty states of the United States of America (USA) and the District of Columbia. All specific details about the sampling, design, medical records and periodontal data-collections are available at www.cdc.gov/nchs/nhanes.htm. Both NHANES 2001–2002 and 2003–2004 were reviewed and approved by the Centers for Disease Control (CDC) and Prevention National Center for Health Statistics Research (NCHS) Ethics Review Board, and all included participants provided written informed consent.
For the purpose of this study, NHANES 2001–2002 and NHANES 2003–2004 data was retrieved with the following inclusion criteria: women between the ages of 18 and 49 years; non-pregnant; who received periodontal examination and the assessment to BV. This secondary study was performed following the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines79.
Bacterial vaginosis assessment
Self-vaginal swabs were collected and then the swab was onto a glass slide to air dry80,81. The smears were processed at Magee-Women’s Hospital (Pittsburgh, Pennsylvania), and the BV score was calculated according to Nugent’s method82. In this sense, scores between 0 and 3 were considered normal vaginal microbiota, scores of 7 or higher were considered positive for BV, whereas scores between 4 and 6 were considered intermediate. For analysis, the outcome was defined as BV confirmed (positive) or not (negative or intermediate).
Periodontal examination
Half-mouth periodontal examination was conducted on randomly assigned quadrants, one upper and one lower, by calibrated examiners as described elsewhere83,84. Probing pocket depth (PPD), clinical attachment loss (CAL) and bleeding on probing (BOP) measurements were performed at three sites per tooth (mesio-buccal, mid-buccal and disto-buccal). The diagnosis and staging of PD was carried out according to the CDC and Prevention-American Academy of Periodontology consensus for epidemiologic studies recommendation85. As such, mild PD was defined as ≥ 2 interproximal sites with CAL ≥ 3 mm and ≥ 2 interproximal sites with PPD ≥ 4 mm (not on the same tooth) or 1 site with PPD ≥ 5 mm. Moderate PD was defined as ≥ 2 interproximal sites with CAL ≥ 4 mm (not on the same tooth) or ≥ 2 interproximal sites with PPD ≥ 5 mm, also not on the same tooth. Severe PD was defined as the presence of ≥ 2 interproximal sites with CAL ≥ 6 mm (not on the same tooth) and ≥ 1 interproximal site with PPD ≥ 5 mm. The sum of mild, moderate and severe PD corresponded to the overall number of PD.
Also, the PISA and the PESA for every tooth in all subjects were estimated in Microsoft Excel spreadsheet. PISA is the area of bleeding of the pocket epithelium (mm2) and PESA is the surface area of the root covered with pocket epithelium (mm2)86,87.
Covariates
Self-reported sociodemographic characteristics regarding age, race or ethnicity (i.e., Mexican American, Non-Hispanics White, Non-Hispanics Black, other Hispanics and Other race) and highest education level (i.e., less than high school, complete high school or similar, higher than high school). Further, in this analysis we collected data about current smokers (i.e. who had smoked ≥ 100 cigarettes during their lifetime and were still smoking). BMI was calculated as weight in kilograms divided by height in meters squared. Marital status was defined as single, married/living with a partner, divorced/separated/widowed. Diabetes mellitus was categorized as yes or no according to the self-reported questionnaire. Medical conditions were assessed as the sum of binary variables (to note the presence of asthma, congestive heart failure, coronary heart disease, angina, stroke, heart attack, emphysema, overweight, bronchitis, liver, thyroid and cancer) as previously done in Leira et al.88.
Blood levels data included WBC count (109/L), monocyte percentage (%), monocyte (109/L), segmented neutrophils percentage (%), segmented neutrophils (109/L), percentage of eosinophils (%), eosinophils (109/L), percentage of basophils (%), basophils (109/L), lymphocyte percentage (%), lymphocyte (109/L), red blood cell (RBC) count (million cells/uL), hemoglobin (g/dL), hematocrit (%), mean cell volume (MCV) (fL), mean cell hemoglobin (MCH) (pg), mean cell hemoglobin concentration (MCHC) (g/dL), percentage of red cell distribution (RCD) width (%), percentage of platelet count (%), mean platelet volume (MPV) (fL), CRP (mg/dL). Overall, the markers expected to present association were WBC, Segmented neutrophils percentage, RBC, Hemoglobin, Hematocrit, MCV, MCH and MCHC according to Botelho89.
We used PISA as a proxy for periodontal (local) inflammation and serum levels of CRP as a proxy for systemic inflammation, respectively88,90.
Data management, test methods and analysis
Data from the NHANES 2001–2002 and 2003–2004 were uploaded through SAS Universal Viewer for Windows and transformed to SPSS version 25.0 for Macintosh (Armonk, New York, IBM Corp.). All periodontal database was transferred to a Microsoft Excel with an appropriate algorithm to assess the periodontal case definition. Descriptive measures are described as mean ± SD for continuous variables, and number of cases (n) and percentage (%) for categorical variables. Mean values explicit comparison was performed by t-Student test when data assumptions for the application of this test were met (normality and homoscedasticity). When those assumptions were not verified, Mann–Whitney was used. For categorical variables we compared baseline variables according to BV patients diagnosed using Chi-square test. Additionally, in order to investigate the trend in CRP levels according to PISA values in patients with and without BV, we explore a graph using scatterplots from ggplot2 package for R (version 4.0), and the tendency was computed and fitted via ‘geom_smooth’. A multivariate stepwise adjusted logistic regression method was used to model the influence of the investigated factors towards the presence of PD in patients with BV. Logistic regression analyses were performed, accounting for PD staging, for all women and as a function of positive diagnosis of BV. Logistic regression analyses calculated the OR and the 95% CI, for different adjustment levels. Model variables were selected among clinical and demographic characteristics. Following the initial crude model (model 1), five progressively adjusted models were calculated (model 2: age; model 3: age and smoking habits; model 4: age, smoking habits and BMI; model 5: age, smoking habits, BMI and diabetes mellitus; model 6: age, smoking habits, BMI, diabetes mellitus and number of systemic diseases excluding diabetes mellitus). A significance level of 5% was set in all inferential analyses.
Supplementary Information
Author contributions
C.E., J.B., J.J.M. and V.M. conceived and planned the investigation. J.B. and V.M. performed the data curation and formal analysis. C.E., J.B. and V.M. were responsible for the methodology and project administration; J.J.M. and V.M. supervised and validated the manuscript. C.E., J.B., J.J.M. and V.M. written the original draft; J.B. and V.M. reviewed the manuscript. C.E., J.B., J.J.M. and V.M. have approved the submitted version.
Funding
This work is financed by national funds through the FCT - Foundation for Science and Technology, I.P., under the project UIDB/04585/2020.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-020-79496-4.
References
- 1.Bautista CT, et al. Bacterial vaginosis: a synthesis of the literature on etiology, prevalence, risk factors, and relationship with chlamydia and gonorrhea infections. Mil. Med. Res. 2016;3:1–10. doi: 10.1186/s40779-016-0074-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradshaw CS, Sobel JD. Current treatment of bacterial vaginosis: limitations and need for innovation. J. Infect. Dis. 2016;214:S14–S20. doi: 10.1093/infdis/jiw159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell C, Marrazzo J. Bacterial vaginosis and the cervicovaginal immune response. Am. J. Reprod. Immunol. 2014;71:555–563. doi: 10.1111/aji.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Onderdonk AB, Delaney ML, Fichorova RN. The Human Microbiome during bacterial vaginosis. Clin. Microbiol. Rev. 2016;29:223–238. doi: 10.1128/CMR.00075-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Machado D, Castro J, Palmeira-de-Oliveira A, Martinez-de-Oliveira J, Cerca N. Bacterial vaginosis biofilms: challenges to current therapies and emerging solutions. Front. Microbiol. 2016;6:1–13. doi: 10.3389/fmicb.2015.01528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leitich H, et al. Bacterial vaginosis as a risk factor for preterm delivery: a meta-analysis. Am. J. Obstet. Gynecol. 2003;189:139–147. doi: 10.1067/mob.2003.339. [DOI] [PubMed] [Google Scholar]
- 7.Guerra B, et al. Pregnancy outcome after early detection of bacterial vaginosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006;128:40–45. doi: 10.1016/j.ejogrb.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 8.Rothman KJ, Funch DP, Alfredson T, Brady J, Dreyer NA. Randomized field trial of vaginal douching, pelvic inflammatory disease and pregnancy. Epidemiology. 2003;14:340–348. [PubMed] [Google Scholar]
- 9.Jacobsson B, Pernevi P, Chidekel L, Platz-Christensen J. Bacterial vaginosis in early pregnancy may predispose for preterm birth and postpartum endometritis. Acta Obstet. Gynecol. Scand. 2002;81:1006–1010. doi: 10.1034/j.1600-0412.2002.811103.x. [DOI] [PubMed] [Google Scholar]
- 10.Murphy K, Mitchell CM. The interplay of host immunity, environment and the risk of bacterial vaginosis and associated reproductive health outcomes. J. Infect. Dis. 2016;214:S29–S35. doi: 10.1093/infdis/jiw140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdullateef RM, Ijaiya MA, Abayomi F, Adeniran AS, Idris H. Bacterial vaginosis: prevalence and associated risk factors among non-pregnant women of reproductive age attending a Nigerian tertiary hospital. Malawi Med. J. 2017;29:290–293. doi: 10.4314/mmj.v29i4.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turovskiy Y, Sutyak Noll K, Chikindas ML. The aetiology of bacterial vaginosis. J. Appl. Microbiol. 2011;110:1105–1128. doi: 10.1111/j.1365-2672.2011.04977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caton JG, et al. A new classification scheme for periodontal and peri-implant diseases and conditions: introduction and key changes from the 1999 classification. J. Periodontol. 2018;89:S1–S8. doi: 10.1002/JPER.18-0157. [DOI] [PubMed] [Google Scholar]
- 14.Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 2010;8:481–490. doi: 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- 15.Papapanou PN, et al. Periodontitis: consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J. Periodontol. 2018;89:S173–S182. doi: 10.1002/JPER.17-0721. [DOI] [PubMed] [Google Scholar]
- 16.Ebersole JL, et al. Periodontal disease immunology: ‘double indemnity’ in protecting the host. Periodontology. 2013;2000(62):163–202. doi: 10.1111/prd.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Preshaw PM, et al. Periodontitis and diabetes: a two-way relationship. Diabetologia. 2012;55:21–31. doi: 10.1007/s00125-011-2342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Botelho J, et al. Stress, salivary cortisol and periodontitis: a systematic review and meta-analysis of observational studies. Arch. Oral Biol. 2018;96:58–65. doi: 10.1016/j.archoralbio.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Polak D, Shapira L. An update on the evidence for pathogenic mechanisms that may link periodontitis and diabetes. J. Clin. Periodontol. 2018;45:150–166. doi: 10.1111/jcpe.12803. [DOI] [PubMed] [Google Scholar]
- 20.Hussain SB, et al. Is there a bidirectional association between rheumatoid arthritis and periodontitis? A systematic review and meta-analysis. Semin. Arthritis Rheum. 2020;50:414–422. doi: 10.1016/j.semarthrit.2020.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Leira Y, et al. Association between periodontitis and ischemic stroke: a systematic review and meta-analysis. Eur. J. Epidemiol. 2017;32:43–53. doi: 10.1007/s10654-016-0170-6. [DOI] [PubMed] [Google Scholar]
- 22.Muñoz Aguilera E, et al. Periodontitis is associated with hypertension: a systematic review and meta-analysis. Cardiovasc. Res. 2020;116:28–39. doi: 10.1093/cvr/cvz201. [DOI] [PubMed] [Google Scholar]
- 23.Lafon A, et al. Periodontal disease and stroke: a meta-analysis of cohort studies. Eur. J. Neurol. 2014;21:1155–1161. doi: 10.1111/ene.12415. [DOI] [PubMed] [Google Scholar]
- 24.Machado V, et al. Validity of the association between periodontitis and female infertility conditions: a concise review. Reproduction. 2020;160:R41–R54. doi: 10.1530/REP-20-0176. [DOI] [PubMed] [Google Scholar]
- 25.Machado V, Escalda C, Proença L, Mendes JJ, Botelho J. Is there a bidirectional association between polycystic ovarian syndrome and periodontitis? A systematic review and meta-analysis. J. Clin. Med. 2020;9:1961. doi: 10.3390/jcm9061961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balle C, et al. Relationship between the oral and vaginal microbiota of South African adolescents with high prevalence of bacterial vaginosis. Microorganisms. 2020;8:1–18. doi: 10.3390/microorganisms8071004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zabor EC, et al. Association between periodontal disease, bacterial vaginosis, and sexual risk behaviours. J. Clin. Periodontol. 2010;37:888–893. doi: 10.1111/j.1600-051X.2010.01593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Persson R, et al. The vaginal microflora in relation to gingivitis. BMC Infect. Dis. 2009;9:1–8. doi: 10.1186/1471-2334-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill GB. Investigating the source of amniotic fluid isolates of fusobacteria. Clin. Infect. Dis. 1993;16:S423–S424. doi: 10.1093/clinids/16.Supplement_4.S423. [DOI] [PubMed] [Google Scholar]
- 30.Larsen JM. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology. 2017;151:363–374. doi: 10.1111/imm.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coyne MJ, et al. A family of anti-Bacteroidales peptide toxins wide-spread in the human gut microbiota. Nat. Commun. 2019;10:25–28. doi: 10.1038/s41467-019-11494-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ren H, Du M. Role of maternal periodontitis in preterm birth. Front. Immunol. 2017;8:1–10. doi: 10.3389/fimmu.2017.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edwards S, Carne C. Oral sex and transmission of non-viral STIs. Sex. Transm. Infect. 1998;74:95–100. doi: 10.1136/sti.74.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stein JM, MacHulla HKG, Smeets R, Lampert F, Reichert S. Human leukocyte antigen polymorphism in chronic and aggressive periodontitis among Caucasians: a meta-analysis. J. Clin. Periodontol. 2008;35:183–192. doi: 10.1111/j.1600-051X.2007.01189.x. [DOI] [PubMed] [Google Scholar]
- 35.Cafferata EA, et al. The therapeutic potential of regulatory T lymphocytes in periodontitis: a systematic review. J. Periodontal Res. 2019;54:207–217. doi: 10.1111/jre.12629. [DOI] [PubMed] [Google Scholar]
- 36.Aboodi GM, Goldberg MB, Glogauer M. Refractory periodontitis population characterized by a hyperactive oral neutrophil phenotype. J. Periodontol. 2011;82:726–733. doi: 10.1902/jop.2010.100508. [DOI] [PubMed] [Google Scholar]
- 37.Seymour GJ, Gemmell E, Reinhardt RA, Eastcott J, Taubman MA. Immunopathogenesis of chronic inflammatory periodontal disease: cellular and molecular mechanisms. J. Periodontal Res. 1993;28:478–486. doi: 10.1111/j.1600-0765.1993.tb02108.x. [DOI] [PubMed] [Google Scholar]
- 38.Kantarci A, Oyaizu K, Van Dyke TE. Neutrophil-mediated tissue injury in periodontal disease pathogenesis: findings from localized aggressive periodontitis. J. Periodontol. 2003;74:66–75. doi: 10.1902/jop.2003.74.1.66. [DOI] [PubMed] [Google Scholar]
- 39.Kalia N, Singh J, Kaur M. Immunopathology of recurrent vulvovaginal infections: new aspects and research directions. Front. Immunol. 2019;10:1–22. doi: 10.3389/fimmu.2019.02034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roselletti E, et al. Apoptosis of vaginal epithelial cells in clinical samples from women with diagnosed bacterial vaginosis. Sci. Rep. 2020;10:1978. doi: 10.1038/s41598-020-58862-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coudray MS, Madhivanan P. Bacterial vaginosis: a brief synopsis of the literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019;245:143–148. doi: 10.1016/j.ejogrb.2019.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Javed A, Parvaiz F, Manzoor S. Bacterial vaginosis: an insight into the prevalence, alternative treatments regimen and it’s associated resistance patterns. Microb. Pathog. 2018;127:21–30. doi: 10.1016/j.micpath.2018.11.046. [DOI] [PubMed] [Google Scholar]
- 43.Peebles K, Velloza J, Balkus JE, McClelland RS, Barnabas RV. High global burden and costs of bacterial vaginosis. Sex. Transm. Dis. 2019;46:304–311. doi: 10.1097/OLQ.0000000000000972. [DOI] [PubMed] [Google Scholar]
- 44.Allsworth JE. Prevalence of bacterial vaginosis. Obstet. Gynecol. 2007;109:114–120. doi: 10.1097/01.AOG.0000247627.84791.91. [DOI] [PubMed] [Google Scholar]
- 45.Lieberman JA, Moscicki A, Sumerel JL, Ma Y, Scott ME. Determination of cytokine protein levels in cervical mucus samples from young women by a multiplex immunoassay method and assessment of correlates. Clin. Vaccine Immunol. 2008;15:49–54. doi: 10.1128/CVI.00216-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scott ME, Ma Y, Farhat S, Shiboski S, Moscicki A-B. Covariates of cervical cytokine mRNA expression by real-time PCR in adolescents and young women: effects of Chlamydia trachomatis infection, hormonal contraception, and smoking. J. Clin. Immunol. 2006;26:222–232. doi: 10.1007/s10875-006-9010-x. [DOI] [PubMed] [Google Scholar]
- 47.Simhan HN, Caritis SN, Hillier SL, Krohn MA. Cervical anti-inflammatory cytokine concentrations among first-trimester pregnant smokers. Am. J. Obstet. Gynecol. 2005;193:1999–2003. doi: 10.1016/j.ajog.2005.04.054. [DOI] [PubMed] [Google Scholar]
- 48.Palmer RM, Wilson RF, Hasan AS, Scott DA. Mechanisms of action of environmental factors: tobacco smoking. J. Clin. Periodontol. 2005;32:180–195. doi: 10.1111/j.1600-051X.2005.00786.x. [DOI] [PubMed] [Google Scholar]
- 49.Bergström J, Ellasson S. Noxious effect of cigarette smoking on periodontal health. J. Periodontal Res. 1987;22:513–517. doi: 10.1111/j.1600-0765.1987.tb02364.x. [DOI] [PubMed] [Google Scholar]
- 50.Johnson GK, Hill M. Cigarette smoking and the periodontal patient. J. Periodontol. 2004;75:196–209. doi: 10.1902/jop.2004.75.2.196. [DOI] [PubMed] [Google Scholar]
- 51.Leite FRM, Nascimento GG, Scheutz F, López R. Effect of smoking on periodontitis: a systematic review and meta-regression. Am. J. Prev. Med. 2018;54:831–841. doi: 10.1016/j.amepre.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 52.Shchipkova AY, Nagaraja HN, Kumar PS. Subgingival Microbial profiles of smokers with periodontitis. J. Dent. Res. 2010;89:1247–1253. doi: 10.1177/0022034510377203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Söder B, Jin LJ, Wickholm S. Granulocyte elastase, matrix metalloproteinase-8 and prostaglandin E 2 in gingival crevicular fluid in matched clinical sites in smokers and non-smokers with persistent periodontitis. J. Clin. Periodontol. 2002;29:384–391. doi: 10.1034/j.1600-051X.2002.290502.x. [DOI] [PubMed] [Google Scholar]
- 54.Nociti FH, Casati MZ, Duarte PM. Current perspective of the impact of smoking on the progression and treatment of periodontitis. Periodontology. 2015;2000(67):187–210. doi: 10.1111/prd.12063. [DOI] [PubMed] [Google Scholar]
- 55.Johannsen A, Susin C, Gustafsson A. Smoking and inflammation: evidence for a synergistic role in chronic disease. Periodontology. 2014;2000(64):111–126. doi: 10.1111/j.1600-0757.2012.00456.x. [DOI] [PubMed] [Google Scholar]
- 56.Buduneli N, Scott DA. Tobacco-induced suppression of the vascular response to dental plaque. Mol. Oral Microbiol. 2018;33:271–282. doi: 10.1111/omi.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lokken EM, et al. A prospective cohort study of the association between body mass index and incident bacterial vaginosis. Sex. Transm. Dis. 2019;46:31–36. doi: 10.1097/OLQ.0000000000000905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Klein LL, Gibbs RS. Infection and preterm birth. Obstet. Gynecol. Clin. N. Am. 2005;32:397–410. doi: 10.1016/j.ogc.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 59.Madianos PN, Bobetsis YA, Offenbacher S. Adverse pregnancy outcomes (APOs) and periodontal disease: pathogenic mechanisms. J. Clin. Periodontol. 2013;40:170–180. doi: 10.1111/jcpe.12082. [DOI] [PubMed] [Google Scholar]
- 60.Goffinet F, et al. Bacterial vaginosis : prevalence and predictive value for premature delivery and neonatal infection in women with preterm labour and intact membranes. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003;108:146–151. doi: 10.1016/S0301-2115(02)00423-2. [DOI] [PubMed] [Google Scholar]
- 61.Pitiphat W, et al. Plasma C-reactive protein in early pregnancy and preterm delivery. Am. J. Epidemiol. 2005;162:1108–1113. doi: 10.1093/aje/kwi323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Torricelli M, et al. Inflammatory and infectious risk factors are associated with the response to tocolysis in patients with preterm labor. J. Matern. Neonatal Med. 2011;24:43–46. doi: 10.3109/14767058.2010.482614. [DOI] [PubMed] [Google Scholar]
- 63.Borgdorff H, et al. Cervicovaginal microbiome dysbiosis is associated with proteome changes related to alterations of the cervicovaginal mucosal barrier. Mucosal Immunol. 2015;9:621–633. doi: 10.1038/mi.2015.86. [DOI] [PubMed] [Google Scholar]
- 64.Reid G. Is bacterial vaginosis a disease ? Appl. Microbiol. Biotechnol. 2017;102:553–558. doi: 10.1007/s00253-017-8659-9. [DOI] [PubMed] [Google Scholar]
- 65.Jespers V, et al. A longitudinal analysis of the vaginal microbiota and vaginal immune mediators in women from sub-Saharan Africa. Sci. Rep. 2017;7:1–13. doi: 10.1038/s41598-017-12198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Konkel JE, Boyle CO, Krishnan S. Distal consequences of oral inflammation. Front. Immunol. 2019;10:1–16. doi: 10.3389/fimmu.2019.01403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Panezai J, et al. Upregulation of circulating inflammatory biomarkers under the influence of periodontal disease in rheumatoid arthritis patients. Cytokine. 2020;131:1–9. doi: 10.1016/j.cyto.2020.155117. [DOI] [PubMed] [Google Scholar]
- 68.Mulhall H, Huck O, Amar S. Porphyromonas gingivalis, a long-range pathogen: systemic impact and therapeutic implications. Microorganisms. 2020;8:869. doi: 10.3390/microorganisms8060869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.D’Aiuto F, et al. Systemic effects of periodontitis treatment in patients with type 2 diabetes: a 12 month, single-centre, investigator-masked, randomised trial. Lancet Diabetes Endocrinol. 2018;6:954–965. doi: 10.1016/S2213-8587(18)30038-X. [DOI] [PubMed] [Google Scholar]
- 70.Tonetti MS, D’Aiuto F, Nibali L. Treatment of periodontitis and endothelial function. Jpn. J. Chest Dis. 2008;67:353. doi: 10.1056/NEJMoa063186. [DOI] [PubMed] [Google Scholar]
- 71.Orlandi M, Graziani F, D’Aiuto F. Periodontal therapy and cardiovascular risk. Periodontology. 2020;2000(83):107–124. doi: 10.1111/prd.12299. [DOI] [PubMed] [Google Scholar]
- 72.Schwebke J. Validity of the vaginal gram stain for the diagnosis of bacterial vaginosis. Obstet. Gynecol. 1996;88:573–576. doi: 10.1016/0029-7844(96)00233-5. [DOI] [PubMed] [Google Scholar]
- 73.Anukm KC, Idemoh C, Olise NA. Evaluation of bacterial vaginosis (BV) using Nugent Scoring System. J. Mol. Biol. Res. 2014;13:25–32. [Google Scholar]
- 74.Marrazzo JM, et al. Bacterial vaginosis: identifying research gaps proceedings of a workshop sponsored by DHHS/NIH/NIAID, November 19–20, 2008. Sex. Transm. Dis. 2010;37:732–744. doi: 10.1097/OLQ.0b013e3181fbbc95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Coleman JS, Gaydos A. Molecular diagnosis of bacterial vaginosis: an update. J. Clin. Microbiol. 2018;56:e00342–e418. doi: 10.1128/JCM.00342-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J. Clin. Periodontol. 2018;45:149–161. doi: 10.1111/jcpe.12945. [DOI] [PubMed] [Google Scholar]
- 77.Botelho J, Machado V, Proença L, Mendes JJ. The 2018 periodontitis case definition improves accuracy performance of full-mouth partial diagnostic protocols. Sci. Rep. 2020 doi: 10.1038/s41598-020-63700-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Machado V, et al. Partial recording protocols performance on the assessment of periodontitis severity and extent. Rev. Port. Estomatol. Med. Dentária e Cir. Maxilofac. 2018;59:145–153. [Google Scholar]
- 79.von Elm E, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int. J. Surg. 2014;12:1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 80.National Health and Nutrition Examination Survey 2001–2002. https://wwwn.cdc.gov/Nchs/Nhanes/limited_access/L34_B_R.htm.
- 81.National Health and Nutrition Examination Survey 2003–2004.
- 82.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J. Clin. Microbiol. 1991;29:297–301. doi: 10.1128/JCM.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dye BA, et al. Overview and quality assurance for the oral health component of the National Health and Nutrition Examination Survey (NHANES), 2003–2004. J. Public Health Dent. 2008;68:218–226. doi: 10.1111/j.1752-7325.2007.00076.x. [DOI] [PubMed] [Google Scholar]
- 84.Dye BA, Thornton-Evans G. A brief history of national surveillance efforts for periodontal disease in the United States. J. Periodontol. 2007;78:1373–1379. doi: 10.1902/jop.2007.060210. [DOI] [PubMed] [Google Scholar]
- 85.Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. Update of the case definitions for population-based surveillance of periodontitis. J. Periodontol. 2012;83:1449–1454. doi: 10.1902/jop.2012.110664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hujoel PP, White BA, García RI, Listgarten MA. The dentogingival epithelial surface area revisited. J. Periodontal Res. 2001;36:48–55. doi: 10.1034/j.1600-0765.2001.00011.x. [DOI] [PubMed] [Google Scholar]
- 87.Nesse W, et al. Periodontal inflamed surface area: quantifying inflammatory burden. J. Clin. Periodontol. 2008;35:668–673. doi: 10.1111/j.1600-051X.2008.01249.x. [DOI] [PubMed] [Google Scholar]
- 88.Leira Y, et al. Periodontitis and systemic markers of neurodegeneration: a case–control study. J. Clin. Periodontol. 2020;47:561–571. doi: 10.1111/jcpe.13267. [DOI] [PubMed] [Google Scholar]
- 89.Botelho J, et al. Periodontitis and circulating blood cell profiles: a systematic review and meta-analysis. Exp. Hematol. 2020;37:732. doi: 10.1016/j.exphem.2020.10.001. [DOI] [PubMed] [Google Scholar]
- 90.Watson J, Salis ID, Hamilton W, Salisbury C. I ’m fishing really: inflammatory marker testing in primary care—a qualitative study. Br. J. Gen. Pract. 2016;1:e200–e206. doi: 10.3399/bjgp16X683857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


