Abstract
Hypothermia during anesthetic events is a common adverse effect of anesthesia in laboratory animals. In particular, small rodents such as mice is susceptible to hypothermia during anesthetic events. Therefore, the animals will need additional thermal support by external heating devices during and after anesthesia. In general, the time of recovery from anesthesia is typically longer in case of injectable anesthesia rather than inhalant anesthesia. However, the durations of thermal support have been almost limited to 1 hr from administration of anesthesia in general. Our study objectives are two-fold: 1) to compare the levels of hypothermia induced by injectable anesthesia with medetomidine-midazolam-butorphanol (MMB) and inhalant anesthesia with isoflurane (ISO); 2) to find the adequate durations of thermal support for preventing hypothermia induced by their anesthesia in mice. Adult male ICR mice were anesthetized during 40 min without and with the thermal support for 1 (both anesthetic groups), 2, 3, and 5 hr (in MMB group). Without thermal support, the decrease of body temperature in MMB group were more severe than that in ISO group. The durations of thermal support completely prevented hypothermia at 5 hr-support in MMB group and that at 1 hr-support in ISO group. However, the other short durations did not prevent hypothermia at 1, 2 and 3 hr-support in MMB group. These results suggest that the mice should be received thermal support over 5 hr after injection of MMB anesthesia to prevent hypothermia.
Keywords: anesthesia, hypothermia, isoflurane, medetomidine, mice
General anesthesia provide sedation, analgesia, muscle relaxation, immobilization, and unconsciousness for laboratory animals in biomedical research. In addition, general anesthesia is frequently produced using either inhalants, injectables, or both of them. In laboratory rodents, inhalant anesthesia such as isoflurane (ISO) is recommended because of rapid induction and recovery of anesthesia through an anesthetic machine [8, 32]. However, the administration of inhalant anesthesia needs to secure a certain space where sets anesthetic equipment. In addition, this inhalant anesthesia may be unsuitable for the procedures to treat several animals at once and to treat the oral cavity or respiratory tract. The appropriate choice of anesthetic regimen may influence postoperative outcome and experimental data, and result in reducing the use of animals [1, 3, 4, 22, 23]. In these cases, injectable anesthesia will be useful because of easy handling of the administration and no setting of the anesthetic machine.
A part of injectable anesthetics has been the limited use due to no longer commercially available as anesthesia (e.g. pentobarbital sodium) or categorizing as narcotic drugs (e.g. ketamine) in Japan [8, 16]. However, a variety of injectable anesthetics are commercially available. A combination of anesthesia with medetomidine-midazolam-butorphanol (MMB) has been recommended as non-narcotic injectable anesthesia. This combination produces the duration of surgical anesthesia for 40–50 min in mice [16,17,18, 25,26,27, 35]. In addition, its anesthetic effects can be reversed by administration of a selective and specific α2-antagonist, atipamezole [15, 18, 27, 28, 35]. However, several studies have reported that MMB anesthesia has side-effects including bradycardia, hyperglycemia, cataract, exophthalmos, and hypothermia during and after anesthesia [26, 27, 35]. Hypothermia is the one of severe adverse effects during anesthetic events [9]. Small animals such as mice can cause hypothermia easily during and after anesthesia because of their high surface area to body weight ratio [9]. In the other studies, it has been reported that hypothermia during anesthesia produce bradycardia, disturbed circadian rhythm, increased infection, and delayed recovery from anesthesia [5, 9, 10, 12, 20, 24, 30, 32, 34]. To prevent from hypothermia, thermal support has been proposed during and after anesthesia [3, 9, 12, 22, 29, 33]. However, the duration of thermal support has been limited to about 1 hr from administration of anesthesia and there is no report to search the adequate durations for preventing hypothermia induced by MMB anesthesia [3, 10, 12, 22, 27, 29, 33].
This study aimed to investigate the levels of hypothermia induced by MMB and ISO anesthesia, and the appropriate durations of thermal support to prevent the hypothermia in mice.
MATERIALS AND METHODS
Ethical statement
All procedures in this study were took in accordance with the Guidelines for Animal Experimental issued by Japanese Association for Laboratory Animal Science and approved by the provisions of Nippon Veterinary and Life Science University (Approved No. 28S-62, 29K-25, 30K-26, 2019K-14).
Animals
A total of 23 male ICR mice (35–40 g), aged 8 weeks were purchased from Tokyo Laboratory Animal Science Co., Ltd. (Tokyo, Japan). Mice were housed less than 5 animals per group in polycarbonate cages (CL-0104-2; CLEA Japan, Inc., Tokyo, Japan) with sterilized wood-chip bedding (Soft chip; Sankyo Labo Service Corp., Inc., Tokyo, Japan) under controlled conditions: an ambient temperature 23–25°C, the related humidity 40–60%, and the light/dark cycle consisted of a 12 hr/12 hr cycle (lights on at 07:00 and lights off at 19:00). All mice received a commercial diet (EF; Oriental Yeast Co., Ltd., Tokyo, Japan) and water ad libitum. The mice were acclimated to housed condition for a week before the experiment.
Drugs preparation
The doses of drugs were used with as follows: 1) MMB; a combination of 0.3 mg/kg medetomidine hydrochloride (Domitor®; Nippon Zenyaku Kogyo Co., Ltd., Fukushima, Japan), 4.0 mg/kg midazolam (Dormicum®; Astellas Pharma Inc., Tokyo, Japan) and 5.0 mg/kg butorphanol (Vetorphale®; Meiji Seika Pharma Co., Ltd., Tokyo, Japan), and 2) 0.3 mg/kg atipamezole (Antisedan®; Nippon Zenyaku Kogyo Co., Ltd.) [14]. All injectable drugs were diluted with sterilized saline to be total volume of 0.1 ml/10 g body weight and were intraperitoneally injected into mice. Prior to experimentation, the adjusted drugs were kept at 4°C in a refrigerator.
Implantation surgery
A body temperature measuring device (nano tag®; KISSEI COMTEC Co., Ltd., Nagano, Japan) was implanted intraperitoneal cavity of mouse. The device can measure the body temperature of mice persistently. Each mouse was anesthetized with MMB anesthesia at the same dose described above. As soon as the loss of the righting reflex were confirmed, the mice were cut the hair about abdomen and placed in dorsal position on a heating pad (BWT-100A; Bio Research Center Co., Ltd., Nagoya, Japan) controlled at 37°C. The device was implanted into the peritoneal cavity of mice and the surgical site was sutured. Atipamezole 0.3 mg/ kg was injected intraperitoneally to recover from anesthesia. All of the mice were housed individually in polycarbonate cages (CL-0103-2; CLEA Japan, Inc.) and allowed over 2-week periods for recovery from implantation surgery.
Body temperature measurements
Body temperature data of mice were recorded and collected by the device software (nanotag viewer®; KISSEI COMTEC Co., Ltd.). The communication with the device (FeliCa®; SONY Co., Ltd., Tokyo, Japan) was used radio-frequency identification reader (PaSoRi®; SONY Co., Ltd.). Their system allowed mice to measure noninvasive core body temperature continuously. Body temperature of the mice were recorded from 06:00 the day before the experiment to 08:00 on the next day of the experiment. The data acquisition was carried out to restrain the mice at the end of measurement of body temperature.
Experiment 1; The levels of hypothermia induced by anesthesia (without thermal support)
All experiments were conducted at between 13:00 and 18:00. All of the mice were divided with two anesthetic groups as described below.
MMB group: Mice (n=15; 40.10 ± 0.82 g) were treated with MMB anesthesia for 40 min and placed back into their individual home cage. At 40 min later, the mice were antagonized with atipamezole and returned to their home cages again.
ISO group: Mice (n=8; 38.21 ± 0.82 g) were anesthetized with Isoflurane (Mylan Seiyaku Co., Ltd., Tokyo, Japan) during 40 min by using a rodent inhalant anesthetic apparatus (WP-SAA01; LMS Co., Ltd., Tokyo, Japan). An animal was placed in the induction chamber prefilled with 5% concentration at a flow rate of 2 l/min. To confirm loss of the righting reflex, the chamber with the animal was tipped over. After induction of inhalation anesthesia, the mice were rapidly transferred to the nose mask and placed in dorsal recumbency on the polycarbonate cage (CL-0104-2; CLEA Japan, Inc.) with wood chips. Nozzle and nose mask of the apparatus were fixed with a curing tape to the cage previously. The concentration of isoflurane was individually reduced and regulated to 1.5–2% at flow rate of 2 l/min. At 40 min after the induction of inhalation anesthesia, the inhalant anesthetic apparatus was stopped. The mouse was put back in its home cage.
Experiment2; The durations of thermal support for preventing hypothermia induced by anesthesia (with thermal support)
External thermal supports were performed by using heating plate (HP-4530; AS ONE Co., Ltd., Osaka, Japan) and heating pad (BWT-100A; Bio Research Center Co., Ltd.) during anesthesia. A polycarbonate cage with wood chips was placed on heating plate set at 46°C due to maintain 37–38°C on a surface of the warming cage. The warming cage was used during 10 min (in MMB group) and after 40 min (in both anesthetic group) from the onset of anesthesia induction. In the other durations, assuming surgical operative periods (10–40 min after the anesthesia induction), heating pad controlled at 37°C was used for mice during anesthesia. At the end of thermal support periods, mice were returned to their home cages.
MMB group: The mice used in Experiment 1 was assigned randomly four groups. The mice were repeatedly used four times in this experimental trial. At least a week from the first trial, each group of mice was treated with the same protocol again. Each four groups of mice were received thermal support for 1 hr (MMB-1 hr; (n=7; 40.84 ± 0.94 g)), 2 hr (MMB-2 hr; (n=11; 39.32 ± 1.19 g)), 3 hr (MMB-3 hr; (n=16; 41.30 ± 0.68 g)) and 5 hr (MMB-5 hr; (n=11; 39.70 ± 0.95 g)) after injection of anesthesia. Animals were treated with MMB anesthesia and put on the warming cage maintained at 37–38°C. The mice were treated with atipamezole at 40 min after the injection of MMB anesthesia.
ISO group: The mice used in Experiment 1 were received thermal support for only 1 hr (ISO-1 hr; (n=8; 42.40 ± 1.47 g)) after administration of ISO anesthesia. After the induction of inhalation anesthesia, mouse was transferred on heating pad and equipped with the nose mask of inhalant anesthetic apparatus. The mice were received anesthesia during 40 min and thermal support on the warming cage to 60 min from administration of anesthesia.
Statistical analysis
Concerning body temperature data in this study, a normal body temperature (NBT) was defined as a physiologically lowest body temperature of mouse for 24 hr of the day before experiments (07:00–07:00). In addition, the normothermic state of mice was defined as the range of body temperature between the physiologically lowest and highest value on the previous day of the anesthetic experiments. A minimum body temperature (MBT) was defined as the lowest body temperature of mouse during 7 hr from administration of anesthesia. The state of hypothermia was defined as when the value of MBT was lower than the value of NBT described above. Similarly, the state of normothermia was defined as when the value of MBT was same or higher than the value of NBT. All experimental data were analyzed by SAS® University edition (SAS Institute Japan Ltd., Tokyo, Japan) statistically. Results are presented as mean ± SE, and statistically significant considered to be P<0.05. Data were analyzed by student’s t-test in comparison of an intergroup, and by paired t-test in comparison of an intragroup. The frequency of hypothermia was analyzed by Fisher’s exact test in comparison of the two intergroup.
RESULTS
The levels of hypothermia induced by anesthesia (without thermal support)
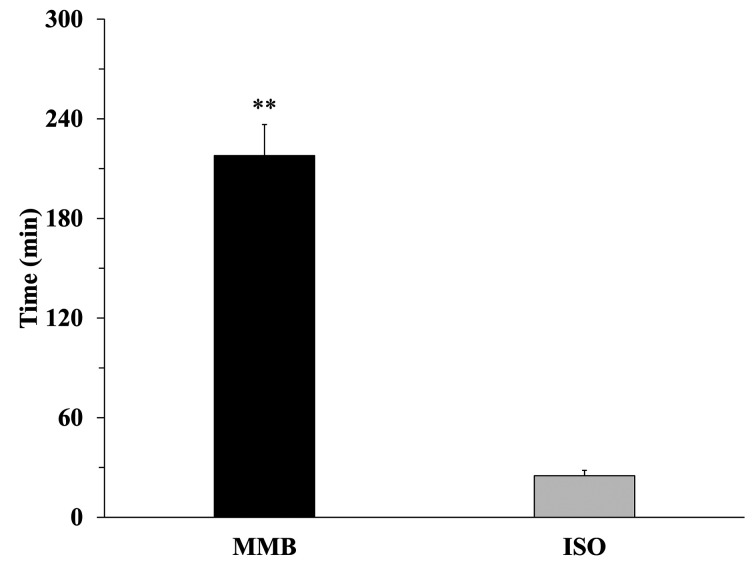
Data of NBT and MBT in MMB and ISO anesthetic groups were shown in Fig. 1. Both anesthesia significantly caused hypothermia in mice (P<0.01). In comparison of each anesthetic groups, MBT in MMB group (28.34 ± 0.23°C) were significantly (P<0.01) lower than that in ISO group (30.95 ± 0.50°C). Data of the recovery time from hypothermia to NBT were shown in Fig. 2. Remarkably, the recovery time in MMB group (217.81 ± 18.78 min) significantly delayed (P<0.01) compared with that in ISO group (25.56 ± 3.27 min).
Fig. 1.
Comparison of body temperature of normal body temperature (NBT: open bar) and minimum body temperature (MBT: closed bar) in both anesthetic groups (MMB; n=15, ISO; n=8). The normal body temperature (NBT) is defined as a lowest body temperature of mice for 24 hr of the day before experiments (07:00–07:00). In addition, the minimum body temperature (MBT) is defined as the lowest body temperature of mice during 6 hr from when the animal was returned to home cage at the end of anesthesia for 40 min. Data are expressed as mean ± SE. Statistically significant considered as P<0.05. Data were analyzed by student’s t-test in comparison of anesthetic intergroup (†: P<0.01), and by paired t-test in comparison of an intragroup (**: P<0.01).
Fig. 2.
The time of recovery from body temperature at 40 min after administration of anesthesia to normal body temperature (NBT) in both anesthetic groups (MMB: n=15; closed bar, ISO: n=8; gray bar). The normal body temperature (NBT) is defined as a lowest body temperature of mice for 24 hr of the day before experiments (07:00–07:00). Data are expressed as mean ± SE. Statistically significant considered as P<0.05. Data were analyzed by student’s t-test (**: P<0.01).
The durations of thermal support for preventing hypothermia induced by anesthesia (with thermal support)
The changes of body temperature from the end of thermal support were shown in Fig. 3. In ISO group, mice maintained the state of normothermia after end of thermal support for 1 hr. In MMB group, the body temperature of mice decreased below NBT within 60 min after the end of thermal support for 1 and 2 hr. However, thermal support for 3 and 5 hr were maintained body temperature of mice within the normothermic range. The individual data of the frequency of hypothermia showed in Table 1. There was significant difference between MMB-3 hr and MMB-5 hr, and the hypothermia mice were observed in MMB-3 hr at a certain frequency but not at all in MMB-5 hr.
Fig. 3.
Changes of body temperature during 60 min from the return to home cage (0 min) in each durations of thermal support (ISO-1 hr: n=8; open circle, MMB-1 hr: n=7; closed circle, MMB-2 hr: n=11; triangle, MMB-3 hr: n=16; square, MMB-5 hr: n=11; cross). Data are expressed as mean ± SE. The gray scale suggested the range of normothermia. The normothermic range was defined as each mean value of body temperature of mouse between lowest (NBT) and highest on a previous day of anesthetic experiments. Statistically significant considered as P<0.05. Data were analyzed with NBT by paired t-test (**: P<0.01, *: P<0.05).
Table 1. The mice with or without hypothermia in each durations of thermal support.
| Agent | Durations of thermal support | Hypothermia |
||
|---|---|---|---|---|
| With | Without | Ratio | ||
| ISO | 1 hr | 0 | 8 | 0.00 |
| MMB | 1 hr | 7 | 0 | 1.00 |
| 2 hr | 9 | 1 | 0.90 | |
| 3 hr | 7 | 9 | 0.44a | |
| 5 hr | 0 | 11 | 0.00b | |
ISO, isoflurane; MMB, medetomidine-midazolam-butorphanol. The number of normothermia or hypothermia in each durations of thermal support. Statistically significant considered as P<0.05. The data of two groups between MMB-3 hr and MMB-5 hr were analyzed by Fisher’s exact test (ab: P<0.05).
DISCUSSION
Our results suggested that 1) the mice under MMB anesthesia showed significantly decreased body temperature and delayed the recovery time to return normothermia compared with ISO anesthesia, and 2) the mice should be received the thermal support for 5 hr in MMB anesthesia and for 1hr in ISO anesthesia.
In the present study, we used an innovative device measuring body temperature of mice. The device used in this study incorporates a consumable battery, memory storage, temperature sensor, and three-axis acceleration sensor. The device enables the continuous measurements of body temperature and locomotor activity without touching mice. Many researchers have used the telemetry system for measuring the consecutive data of body temperature in mice, but it is difficult to get the individual temperature data in group-housing mice by the system. But the device used in this study can measure the individual temperature data under group-housing condition. In addition, the devise does not need to prepare the other specialized devices (e.g. battery charger, data receiver and modulator) and its initial cost is less than the telemetry system. Moreover, the data of body temperature obtained from the device is ideal and independent because the device is implanted to intraperitoneal cavity of the animal. There are the only two reports that the noninvasive data of body temperature in anesthetized mice were analyzed [10, 19].
Hypothermia during anesthesia has been known as one of major adverse effects under anesthesia [2, 9]. In general, hypothermia during anesthesia is induced by the inhibition of central and peripheral nervous system, hypotension and the heat loss of redistribution. Small animals including mice are easily induced to hypothermia during anesthesia because of their high surface area ratio to body weight [9, 11]. Therefore, it also can be induced the delay of recovery from anesthesia, and sometimes the anesthetic death has observed in mice [9]. As the results, hypothermia during anesthesia affects the postoperative outcome, quality of data, and the number of laboratory animals used [3, 4, 22, 33]. It is important to prevent perioperative and postoperative hypothermia [3, 9, 29, 33]. In this study, there were significant differences between normothermia and minimum body temperature after postanesthesia in both anesthetic groups (Fig. 1). In addition, the time of recovery from anesthesia induction to the normothermia delayed significantly in MMB compared with ISO anesthesia (Fig. 2). In our results, the combination of MMB also induced prolonged hypothermia in mice. The combination includes three anesthetic agents of medetomidine, midazolam, and butorphanol [16,17,18, 25, 27, 35]. Medetomidine is an α2-adrenergic agonist with higher selectively than xylazine and produces sedative, hypnotic and analgesic effects for most animals [8, 11]. Midazolam is a benzodiazepine agonist and has sedative and potentiate effects of other drugs such as medetomidine and butorphanol [8, 11, 23]. Butorphanol is used as an analgesic drug in veterinary field [7, 11]. The analgesic effect of butorphanol is also useful to produce postoperative analgesia [14]. In the past reports, the pharmacological functions of an α2-adrenergic receptor subtypes, especially, the role of α2A-adrenergic receptor may provide a clue to the MMB-induced hypothermia observed in this study. The α2A-adrenergic receptor is needed for the sedative, hypothermic and antinociceptive effects induced by dexmedetomidine, which is an enantiomer of medetomidine [13, 21]. The effects of dexmedetomidine were abolished in α2A-receptor knockout or point mutation mouse [13, 21]. Therefore, it seemed that the prolonged hypothermia of mice induced by MMB anesthesia would be caused by medetomidine of α2-adrenergic agonist.
In general anesthesia for mice, inhalation anesthesia has been recommended for surgical procedure currently, because the induction and the regulation of anesthesia can be easy [8, 31, 32]. The anesthetic concentrations of ISO were maintained concentrations of 1.5–2% in present study. It has been suggested that the minimum alveolar concentrations (MAC), which is the concentrations of ED50 for surgery in animals were 1.3–1.4% for mice in ISO anesthesia. The 1.5 times MAC of ISO has been usually used for surgery in the all of animals [8, 31]. The ISO concentration used in the present study was effective and it would work as the control experiment to the injectable anesthesia experiments. As the characteristics of ISO, it is minimal hepatic metabolism in living body of animal because of the low blood gas solubility [6]. These leads both rapid induction and recovery from ISO anesthesia in the animals [6, 8]. Therefore, the results of this study indicated that ISO-induced hypothermia of mice were adequately prevented with thermal support for 1 hr. In addition to hypothermia, an anesthesia affects blood property, respiratory and cardiovascular functions [26, 35]. It has been reported that administration of ISO anesthesia induced the lower decrease of heart rate and the higher respiratory depression than the injection anesthesia although O2 saturation remained more stable under ISO anesthesia [35]. On the other hand, the injection of MMB anesthesia induced the increases of blood glucose, creatinine kinase, inorganic phosphorus and potassium in addition to moderate depression of cardiac and respiratory function [26, 35]. Inhalation anesthesia has many advantages of some physiological parameters affected by anesthesia in mice, but the injectable anesthesia is available without a specific equipment, and this is a great advantage to an inhalation anesthesia.
Previous study has been reported efficacy of several external thermal support units to prevent hypothermia during anesthesia. Taylor suggested that the thermal supports such as heating pad and warm water-blanket prevented isoflurane-induced hypothermia in rodents [33]. Similarly, Caro et al. studied the comparison between water-blanket, warming gel and reflective foil, and clarified that each of thermoregulatory devices kept core body temperature during anesthesia in mice [3]. The durations of thermal support by external heating devices often were terminated within about 1 hr during anesthesia [3, 9, 12, 22, 27, 29, 33]. Moreover, it has been little known adequate periods for thermal support during injectable anesthesia in laboratory rodents. This study indicated the durations of thermal support for an injectable anesthesia in addition to general inhalant anesthesia in mice. In each durations of thermal support, the body temperature of mice maintained normothermia in 1 hr for ISO and 3 hr or 5 hr for MMB anesthesia (Fig. 3). However, the frequency of hypothermia was highly observed in MMB-3 hr, and all of the mice completely maintained normothermia in MMB-5 hr (Table 1). We additionally examined the MMB-4 hr group, and the results were similar to MMB-3 hr, two out of five mice did not maintain normothermia in MMB-4 hr (Data not shown). The levels of anesthetic hypothermia may vary to depend on the strain, sex, age and body weight of mouse, anesthetic durations and postoperative housing-condition (e.g. materials of cage-bedding and number of animal). Fleischmann et al. reported that the body temperature of the mice treated with the anesthetic combination recovered within 2 hr from administration of antagonistic combination to their baseline [10]. In the report, female C57BL/6 mice were treated with the anesthetic combination with 0.5 mg/kg medetomidine, 5.0 mg/kg midazolam and 0.05 mg/kg fentanyl, and its antagonistic combination with 2.5 mg/kg atipamezole, 0.5 mg/kg flumazenil and 1.2 mg/kg naloxone were injected at 50 min after anesthesia [10]. The difference of the doses of medetomidine and atipamezole would result in that the decrease of body temperature was minor influence compared with our results. Fleischmann et al. reported the treatment of 5 times larger amount of antagonist (atipamezole) compared with agonist (medetomidine) recovered to normal body temperature within 2 hr, suggesting that an increased amount of antagonist (atipamezole) might shorten recovery time to normothermia. It needs more investigations of the adequate amounts of antagonist (atipamezole). Moreover, fentanyl has been categorized with a narcotic drug regulated by the law in Japan, and the use of narcotic drugs requires the license for handling. For the anesthetic structure of 0.3 mg/kg medetomidine, 4.0 mg/kg midazolam and 5.0 mg/kg butorphanol, the antagonization at the dose of 0.3 mg/kg atipamezole (same amount of medetomidine) to mice has been widely used in Japan [18, 35]. In the present study, we applied non-narcotic anesthetic (MMB) regimen to mice and noninvasively monitored the body temperature of the mice implanted the measuring device. In addition to thermal supports, it is also important to inject antagonistic drug at the suitable dose to promote the recovery from hypothermia by anesthesia.
In conclusion, this study suggested that the durations of thermal support for MMB anesthesia in mice required 5 hr or over and for ISO did 1 hr from administration of anesthesia. Further study is need to prevent hypothermia by anesthesia, considering the adequate thermal support such as heating devices, comfortable and safety temperature, and thermal support durations.
POTENTIAL CONFLICTS OF INTEREST
The authors have nothing to disclose.
Acknowledgments
We are grateful to all members of the Laboratory of Experimental Animal Science in Nippon Veterinary and Life Sciences University for assistance with our experiments. This work does not achieve from sources outside of this university.
REFERENCES
- 1.Albrecht M., Henke J., Tacke S., Markert M., Guth B.2014. Effects of isoflurane, ketamine-xylazine and a combination of medetomidine, midazolam and fentanyl on physiological variables continuously measured by telemetry in Wistar rats. BMC Vet. Res. 10: 198. doi: 10.1186/s12917-014-0198-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arras M., Autenried P., Rettich A., Spaeni D., Rülicke T.2001. Optimization of intraperitoneal injection anesthesia in mice: drugs, dosages, adverse effects, and anesthesia depth. Comp. Med. 51: 443–456. [PubMed] [Google Scholar]
- 3.Caro A. C., Hankenson F. C., Marx J. O.2013. Comparison of thermoregulatory devices used during anesthesia of C57BL/6 mice and correlations between body temperature and physiologic parameters. J. Am. Assoc. Lab. Anim. Sci. 52: 577–583. [PMC free article] [PubMed] [Google Scholar]
- 4.Devey L., Festing M. F., Wigmore S. J.2008. Effect of temperature control upon a mouse model of partial hepatic ischaemia/reperfusion injury. Lab. Anim. 42: 12–18. doi: 10.1258/la.2007.06009e [DOI] [PubMed] [Google Scholar]
- 5.Dispersyn G., Pain L., Touitou Y.2009. Circadian disruption of body core temperature and rest-activity rhythms after general (propofol) anesthesia in rats. Anesthesiology 110: 1305–1315. doi: 10.1097/ALN.0b013e3181a10225 [DOI] [PubMed] [Google Scholar]
- 6.Eger E. I., 2nd. 1981. Isoflurane: a review. Anesthesiology 55: 559–576. doi: 10.1097/00000542-198111000-00014 [DOI] [PubMed] [Google Scholar]
- 7.Flecknell P. A.2016. Analgesia and post-operative care, p. 182. In: Laboratory Animal Anaesthesia, 4th ed. Elsevier, Waltham. [Google Scholar]
- 8.Flecknell P. A.2016. Basic principles of anaesthesia, pp. 51–66. In: Laboratory Animal Anaesthesia, 4th ed. Elsevier, Waltham. [Google Scholar]
- 9.Flecknell P. A.2016. Managing and monitoring anaesthesia, pp. 92–107. In: Laboratory Animal Anaesthesia, 4th ed., Elsevier, Waltham. [Google Scholar]
- 10.Fleischmann T., Jirkof P., Henke J., Arras M., Cesarovic N.2016. Injection anaesthesia with fentanyl-midazolam-medetomidine in adult female mice: importance of antagonization and perioperative care. Lab. Anim. 50: 264–274. doi: 10.1177/0023677216631458 [DOI] [PubMed] [Google Scholar]
- 11.Gargiulo S., Greco A., Gramanzini M., Esposito S., Affuso A., Brunetti A., Vesce G.2012. Mice anesthesia, analgesia, and care, Part I: anesthetic considerations in preclinical research. ILAR J. 53: E55–E69. doi: 10.1093/ilar.53.1.55 [DOI] [PubMed] [Google Scholar]
- 12.Grahn D. A., Heller M. C., Larkin J. E., Heller H. C.1996. Appropriate thermal manipulations eliminate tremors in rats recovering from halothane anesthesia. J. Appl. Physiol. (1985) 81: 2547–2554. doi: 10.1152/jappl.1996.81.6.2547 [DOI] [PubMed] [Google Scholar]
- 13.Hunter J. C., Fontana D. J., Hedley L. R., Jasper J. R., Lewis R., Link R. E., Secchi R., Sutton J., Eglen R. M.1997. Assessment of the role of alpha2-adrenoceptor subtypes in the antinociceptive, sedative and hypothermic action of dexmedetomidine in transgenic mice. Br. J. Pharmacol. 122: 1339–1344. doi: 10.1038/sj.bjp.0701520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Izer J. M., Whitcomb T. L., Wilson R. P.2014. Atipamezole reverses ketamine-dexmedetomidine anesthesia without altering the antinociceptive effects of butorphanol and buprenorphine in female C57BL/6J mice. J. Am. Assoc. Lab. Anim. Sci. 53: 675–683. [PMC free article] [PubMed] [Google Scholar]
- 15.Janssen C. F., Maiello P., Wright M. J., Jr., Kracinovsky K. B., Newsome J. T.2017. Comparison of Atipamezole with Yohimbine for Antagonism of Xylazine in Mice Anesthetized with Ketamine and Xylazine. J. Am. Assoc. Lab. Anim. Sci. 56: 142–147. [PMC free article] [PubMed] [Google Scholar]
- 16.Kawai S., Takagi Y., Kaneko S., Kurosawa T.2011. Effect of three types of mixed anesthetic agents alternate to ketamine in mice. Exp. Anim. 60: 481–487. doi: 10.1538/expanim.60.481 [DOI] [PubMed] [Google Scholar]
- 17.Kirihara Y., Takechi M., Kurosaki K., Kobayashi Y., Kurosawa T.2013. Anesthetic effects of a mixture of medetomidine, midazolam and butorphanol in two strains of mice. Exp. Anim. 62: 173–180. doi: 10.1538/expanim.62.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirihara Y., Takechi M., Kurosaki K., Kobayashi Y., Saito Y., Takeuchi T.2015. Anesthetic effects of a three-drugs mixture--comparison of administrative routes and antagonistic effects of atipamezole in mice. Exp. Anim. 64: 39–47. doi: 10.1538/expanim.14-0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuroki C., Takahashi Y., Ootsuka Y., Kanmura Y., Kuwaki T.2013. The impact of hypothermia on emergence from isoflurane anesthesia in orexin neuron-ablated mice. Anesth. Analg. 116: 1001–1005. doi: 10.1213/ANE.0b013e31828842f0 [DOI] [PubMed] [Google Scholar]
- 20.Leon L. R., Walker L. D., DuBose D. A., Stephenson L. A.2004. Biotelemetry transmitter implantation in rodents: impact on growth and circadian rhythms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 286: R967–R974. doi: 10.1152/ajpregu.00380.2003 [DOI] [PubMed] [Google Scholar]
- 21.Lähdesmäki J., Sallinen J., MacDonald E., Sirviö J., Scheinin M.2003. Alpha2-adrenergic drug effects on brain monoamines, locomotion, and body temperature are largely abolished in mice lacking the alpha2A-adrenoceptor subtype. Neuropharmacology 44: 882–892. doi: 10.1016/S0028-3908(03)00080-7 [DOI] [PubMed] [Google Scholar]
- 22.Marschner J. A., Schäfer H., Holderied A., Anders H. J.2016. Optimizing Mouse Surgery with Online Rectal Temperature Monitoring and Preoperative Heat Supply. Effects on Post-Ischemic Acute Kidney Injury. PLoS One 11: e0149489. doi: 10.1371/journal.pone.0149489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyer R. E., Fish R. E.2008. Pharmacology of injectable anesthetics, sedatives, and Tranquilizers, pp. 27–83. In: Anesthesia and Analgesia in Laboratory Animals, 2nd ed. (Fish, R. E., Brown, M. J., Danneman, P. J., Meyer, R. E. and Karas, A. Z. eds.), Academic Press, New York. [Google Scholar]
- 24.Mihara T., Kikuchi T., Kamiya Y., Koga M., Uchimoto K., Kurahashi K., Goto T.2012. Day or night administration of ketamine and pentobarbital differentially affect circadian rhythms of pineal melatonin secretion and locomotor activity in rats. Anesth. Analg. 115: 805–813. doi: 10.1213/ANE.0b013e3182632bcb [DOI] [PubMed] [Google Scholar]
- 25.Naganuma Y., Morii K., Saitou T., Hashimoto M., Koyama H.2013. Effect of medetomidine/midazolam/butorphanol in three mouse strains. Exp. Anim. 62 Supplement: S86. [Google Scholar]
- 26.Ochiai Y., Iwano H., Sakamoto T., Hirabayashi M., Kaneko E., Watanabe T., Yamashita K., Yokota H.2016. Blood biochemical changes in mice after administration of a mixture of three anesthetic agents. J. Vet. Med. Sci. 78: 951–956. doi: 10.1292/jvms.15-0474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okamura T.2016. A new injectable anesthetic combination; The characteristics of anesthesia with medetomidine-midazolam-butorphanol. Special issue: Anesthesia and euthanasia in laboratory animals. LABIO 21: 5–9 (in Japanese). [Google Scholar]
- 28.Pertovaara A., Haapalinna A., Sirviö J., Virtanen R.2005. Pharmacological properties, central nervous system effects, and potential therapeutic applications of atipamezole, a selective alpha2-adrenoceptor antagonist. CNS Drug Rev. 11: 273–288. doi: 10.1111/j.1527-3458.2005.tb00047.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schuster C. J., Pang D. S. J.2018. Forced-air pre-warming prevents peri-anaesthetic hypothermia and shortens recovery in adult rats. Lab. Anim. 52: 142–151. doi: 10.1177/0023677217712539 [DOI] [PubMed] [Google Scholar]
- 30.Sheffield C. W., Sessler D. I., Hunt T. K., Scheuenstuhl H.1994. Mild hypothermia during halothane-induced anesthesia decreases resistance to Staphylococcus aureus dermal infection in guinea pigs. Wound Repair Regen. 2: 48–56. doi: 10.1046/j.1524-475X.1994.20108.x [DOI] [PubMed] [Google Scholar]
- 31.Sonner J. M., Gong D., Li J., Eger E. I., 2nd., Laster M. J.1999. Mouse strain modestly influences minimum alveolar anesthetic concentration and convulsivity of inhaled compounds. Anesth. Analg. 89: 1030–1034. [DOI] [PubMed] [Google Scholar]
- 32.Stokes E. L., Flecknell P. A., Richardson C. A.2009. Reported analgesic and anaesthetic administration to rodents undergoing experimental surgical procedures. Lab. Anim. 43: 149–154. doi: 10.1258/la.2008.008020 [DOI] [PubMed] [Google Scholar]
- 33.Taylor D. K.2007. Study of two devices used to maintain normothermia in rats and mice during general anesthesia. J. Am. Assoc. Lab. Anim. Sci. 46: 37–41. [PubMed] [Google Scholar]
- 34.Torossian A., Ruehlmann S., Middeke M., Sessler D. I., Lorenz W., Wulf H. F., Bauhofer A.2004. Mild preseptic hypothermia is detrimental in rats. Crit. Care Med. 32: 1899–1903. doi: 10.1097/01.CCM.0000139608.34486.FD [DOI] [PubMed] [Google Scholar]
- 35.Tsukamoto A., Serizawa K., Sato R., Yamazaki J., Inomata T.2015. Vital signs monitoring during injectable and inhalant anesthesia in mice. Exp. Anim. 64: 57–64. doi: 10.1538/expanim.14-0050 [DOI] [PMC free article] [PubMed] [Google Scholar]