Abstract
Aim
This evidence-based guideline aims to present current and comprehensive recommendations for the diagnosis and management of spontaneous intracerebral haemorrhage (ICH).
Methods
A formal literature search was conducted on MEDLINE (1 January 1990 to 30 June 2019). Data were synthesised using evidence tables. The members of the working group met by teleconference to update and formulate data-based recommendations. The recommendations are graded according to levels of evidence grading algorithm of the Chinese Stroke Association. The guideline draft has been reviewed by Chinese Stroke Association Stroke Council Guideline Writing Committee.
Results
Evidence-based guideline is proposed for the management of patients with ICH. The focus of the guideline is divided into the diagnosis and aetiology of ICH, management of ICH in emergency department, surgical treatment for removal of hematoma, management of complications and prevention of secondary ICH.
Conclusions
This guideline provides a framework for ICH management. Early active and reasonable treatment may improve the clinical outcome of patients.
Keywords: brain, hemorrhage, stroke
Spontaneous intracerebral haemorrhage (ICH) refers to the haemorrhage within the brain parenchyma, characterised with rapid onset, neurological deterioration and poor outcome.1 Cerebral haemorrhage accounts for 23.4% of patients who had stroke in China, of which 46% died or had severe disability within 1 year.2 According to the China’s Health and family planning statistical yearbook 2017, the direct hospitalisation cost of cerebral haemorrhage patients in 2016 was 1.45 billion dollars, which caused heavy health, economic and social burden.3 The American Heart Association/American Stroke Association (AHA/ASA) has presented three versions of the guidelines for the management of spontaneous cerebral haemorrhage,4 and the latest version of the guidelines collected clinical evidence up to 2013. In the past 5 years, the clinical management of cerebral haemorrhage has made new progress. Early active and reasonable treatment may improve the clinical outcome of patients. The Chinese Stroke Association reviewed the research for the past 5 years across the world, making summary and recommendations. This guideline was drafted by relevant experts, applying to the management of patients with ICH (figure 1).
Figure 1.
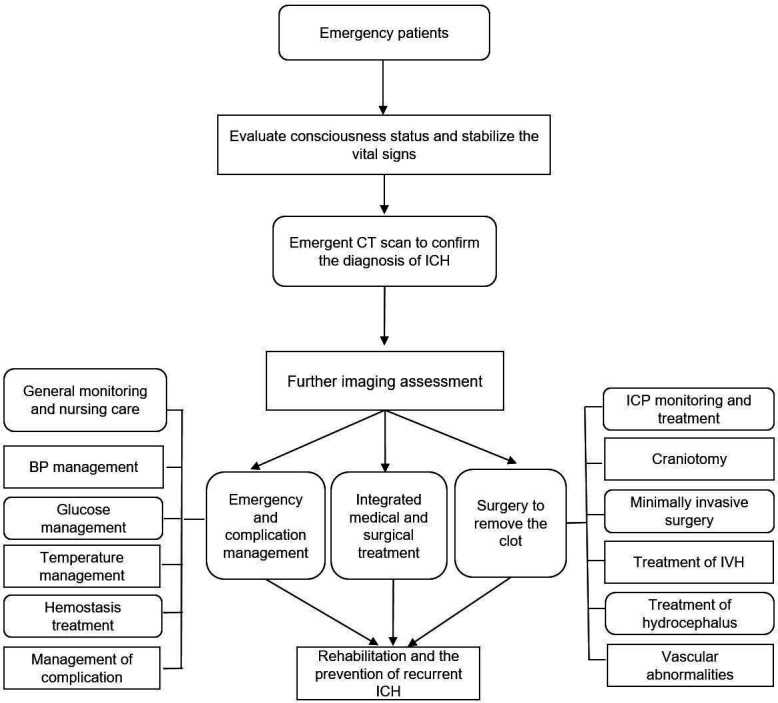
Flowchart of the management of patients with ICH. BP, blood pressure; ICH, intracerebral haemorrhage; IVH, intraventricular haemorrhage.
Recommendations follow the Chinese Stroke Association’s methods of classifying the level of certainty of the treatment effect and the class of evidence,5 which are consistent with the system adopted by the latest AHA/ASA guidelines (as follows).
-
Classification of Recommendations
Class I: there are confirmed evidence or unanimously agreement that the procedure or treatment given is effective.
Class II: there are controversial evidence or disagreement regarding the effectiveness of procedure or treatment.
IIa: some evidence or opinions support its effectiveness.
IIb: there are no good evidence for its effectiveness.
Class III: procedure or treatment is ineffective or harmful in some cases.
-
Level of Evidence
Level of Evidence A: evidence comes from multiple randomised controlled trials (RCTs) or meta-analysis.
Level of Evidence B: evidence comes from single RCT or non-randomised trial.
Level of Evidence C: evidence only comes from experts’ opinions, case studies and so on.
Section 1: diagnosis and aetiology of ICH
Prehospital management and diagnosis
Recommendation
Once diagnosed with ICH, patients should be admitted to a stroke unit or neurointensive care unit based on clinical criteria as appropriate immediately (Class I, Level of evidence A).
Risk score to predict early mortality
Recommendation
Baseline risk score should be part of the initial evaluation of patients with ICH,6 but should not be used as the exclusive judgement of prognosis (Class I, Level of evidence B).
Neuroimaging assessment
Recommendations
Immediate brain imaging (CT or MRI) is recommended to distinguish ischaemic stroke from ICH (Class I, Level of Evidence A).
CTA (CT angiography) and contrast-enhanced CT may be considered to help identify patients at risk of hematoma expansion (Class IIb, Level of Evidence B).
Contrast-enhanced CT, CTA, CTV (CT venography), MRI, MRA (MR angiography), MRV (MR venography) can help confirm the diagnosis of suspected cerebrovascular abnormalities or brain tumours. If CT and MRI results are negative, DSA (digital subtraction angiography) may be performed (Class IIa, Level of Evidence B).
Assessment of the causes of ICH
The causes of ICH should be identified and treated as soon as possible while dealing with the patients’ symptoms (Class I, Level of Evidence B).
Section 2: emergency department (ED) management of ICH
The vital signs of patients in the stroke unit should be continuously monitored (Class I, Level of Evidence A). Comorbidities and other medical conditions should be treated (Class I, Level of Evidence A) (figure 2).
Figure 2.
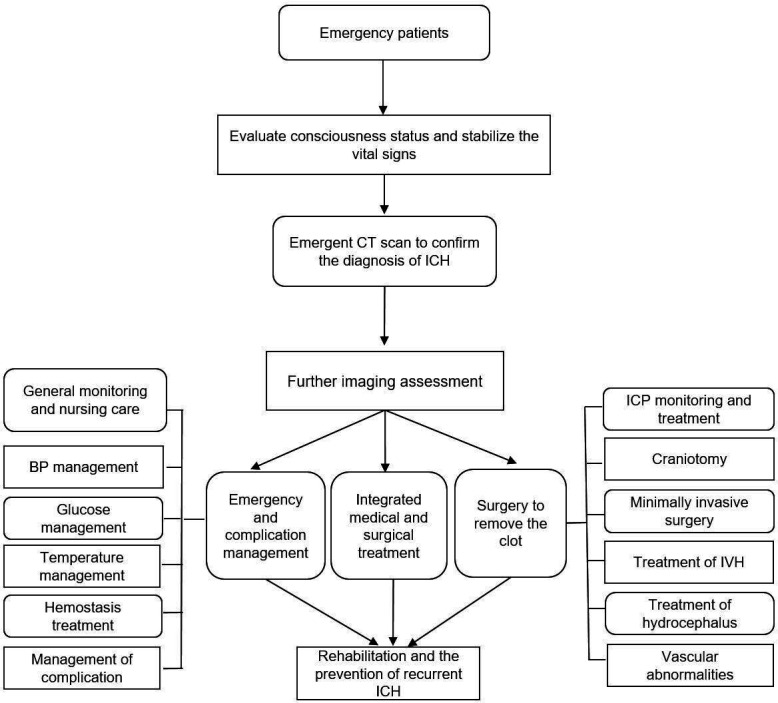
Flowchart of the management of ICH in ED. BP, blood pressure; ED, emergency department; ICH, intracerebral haemorrhage; SBP, systolic blood pressure.
Blood pressure management
Recommendations
For patients with ICH presenting with systolic blood pressure (SBP) up to >150 mm Hg and with no contraindications to acute antihypertensive therapy, it is reasonable to reduce the SBP to less than 140 mm Hg (Class IIa, Level of Evidence A), which may improve functional outcomes of patients (Class IIa, Level of Evidence B).
Aggressive reduction of BP with continuous BP monitoring is reasonable if SBP >220 mm Hg (Class IIa, Level of Evidence C).
BP should be observed closely during BP lowering therapy to avoid excessive BP variability (Class I, Level of Evidence C).
Blood glucose management
Recommendation
Blood glucose levels should be measured and closely monitored to avoid hyperglycaemic and hypoglycaemic (Class I, Level of Evidence B).
Body temperature management
Recommendation
It is reasonable to identify and treat hyperthermia (body temperature >38ºC after ICH (Class IIa, Level of Evidence C).
General haemostasis treatment
Recommendations
Haemostatic drugs in treating patients with ICH with normal coagulation function may inhibit haematoma expansion; however, its clinical efficacy and impact on prognosis is uncertain (Class IIb, Level of Evidence B).
In patients with CT spot sign, rFVIIa (recombinant activated factor VIIa) treatment is not recommended (Class III, Level of Evidence A).
Haemostatic treatment of ICH related to antithrombotic therapy
Recommendations
When ICH occurs after antithrombotic therapy, the drug should be discontinued immediately (Class I, Level of Evidence B).
Infusion of platelets is not recommended for patients with ICH who previously used antiplatelet therapy (Class III, Level of Evidence B).
-
For VKAs (warfarin) associated ICH.
3.1 Intravenous vitamin K should be used (Class I, Level of Evidence A).
3.2 Four-factor prothrombin complex concentrate (4-factor PCC) can be given priorly because PCC has fewer complications than FFP (fresh frozen plasma) and can correct INR (international normalized ratio) faster (Class IIa, Level of Evidence B).
3.3 RFVIIa is not recommended for haemostatic therapy for ICH with normal coagulation function (Class III, Level of Evidence B).
-
For new oral anticoagulant drugs (rivaroxaban, dabigatran or apixaban) related cerebral haemorrhage.
4.1 Activated charcoal might be considered if bleeding occurs within 2 hours of taking new oral anticoagulant drugs (Class IIb, Level of Evidence C).
4.2 Haemodialysis treatment might be considered by patients taking dabigatran (Class IIb, Level of Evidence C), but not by patients taking rivaroxaban and apixaban (Class III, Level of Evidence C).
4.3 FEIBA (factor eight inhibitor bypassing activity), PCCs or rFVIIa might be considered for treatment according to individual conditions. FEIBA or rFVIIa for direct thrombin inhibitor (dabigatran), and PCC for factor Xa inhibitors (rivaroxaban and apixaban) (Class IIb, Level of Evidence C).
4.4 Idarucizumab is recommended for dabigatran reversal (Class I, Level of Evidence A).
For heparin-related ICH, protamine sulfate is recommended (Class I, Level of Evidence C).
For thrombolysis related ICH, treatment with cryoprecipitate of factor VII and tranexamic acid might be considered (Class IIb, Level of Evidence C).
It is reasonable for patients with atrial fibrillation to resuming anticoagulation in 7–8 weeks (Class IIa, Level of Evidence B).
Identification and treatment of hematoma expansion
Recommendations
CTA should be considered to evaluate the risk of hematoma expansion if possible (Class I, Level of Evidence C).
Early intensive BP-lowering treatment at the acute stage of ICH can lower the rate of haematoma growth or have the favourable tendency to do so (Class IIa, Level of Evidence B).
Emergency management of vascular abnormalities related ICH
Recommendation
Surgery can be considered for the life-threatening secondary ICH. While surgically removing the hematoma, the treatment strategy may be weighed against the relative benefits and risks of saving the patient’s life and eliminating the primary cause (Class IIa, Level of Evidence C).
Section 3: surgical treatment for removal of haematoma
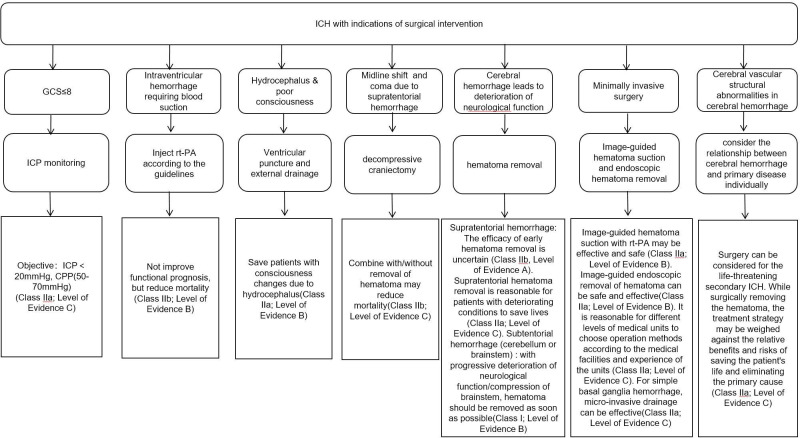
The surgical treatment of patients with ICH is shown in figure 3.
Figure 3.
Flowchart of surgical treatment for intracerebral haemorrhage. CPP, cerebral perfusion pressure; GCS, Glasgow Coma Scale; ICH, intracerebral haemorrhage; ICP, intracranial pressure; rt-PA, recombinant tissue plasminogen activator.
Minimally invasive surgery
Recommendations
Image-guided hematoma suction with recombinant tissue plasminogen activator (rt-PA) may be effective and safe (Class IIa, Level of Evidence B).
Image-guided endoscopic removal of hematoma can be effective and safe (Class IIa, Level of Evidence B).
It is reasonable for different levels of medical units to choose operation methods according to the medical facilities and experience of the units (Class IIa, Level of Evidence C).
In units without image-guided hematoma removal technology, it is reasonable for physicians who have received professional training to consider standard minimally invasive puncture hematoma evacuation for appropriate patients with ICH (Class IIa, Level of Evidence B).
For simple basal ganglia haemorrhage (hematoma volume 25–40 mL), microinvasive drainage can be effective (Class IIa, Level of Evidence C).
Treatment of ventricular haematoma
Recommendations
For patients with intraventricular haemorrhage requiring intraventricular puncture and external drainage, intraventricular injection of rt-PA might reduce mortality, but not improve functional prognosis (Class IIb, Level of Evidence B).
For intraventricular haemorrhage, the efficacy of endoscopy is still uncertain (Class IIb, Level of Evidence B).
Craniotomy operation
Recommendations
For supratentorial ICH, if the patient is in a coma, has significant midline shift or elevated intracranial pressure due to large haematoma, decompressive craniectomy with/without haematoma removal might reduce the mortality (Class IIb, Level of Evidence C). The efficacy of early haematoma removal is uncertain (Class IIb, Level of Evidence A). Supratentorial haematoma removal is reasonable for patients with deteriorating conditions to save lives (Class IIa, Level of Evidence C).
For subtentorial (cerebellum or brainstem) ICH, haematoma removal should be performed as soon as possible in patients with progressive deterioration of neurological function or compression of brainstem and/or hydrocephalus (Class I, Level of Evidence B). External ventricular drainage alone as the initial treatment is not recommended for these patients (Class III, Level of Evidence C).
Section 4: management of complications of ICH
Management of intracranial hypertension
Recommendations
4.1.1.1 Patients with Glasgow Coma Scale ≤8 points can be considered for intracranial pressure monitoring (Class IIa, Level of Evidence C).
4.1.1.2 Osmotic drugs and hyperventilation may be considered in patients with intracranial hypertension (Class IIa, Level of Evidence C).
4.1.1.3 It is reasonable to control intracranial pressure below 20 mm Hg and cerebral perfusion pressure at the range of 50–70 mm Hg (Class IIa, Level of Evidence C).
4.1.2 The position of the head should be appropriately elevated (head of bed elevation to at least 30°) (Class I, Level of Evidence C).
4.1.3 Intravenous infusion of mannitol is recommended to reduce intracranial pressure, with individualised dosage and course of treatment. Cardiac and renal function and electrolyte levels should be closely monitored (Class I, Level of Evidence C).
4.1.4 Usage of furosemide, glycerol fructose and/or albumin may be combined when necessary (Class IIa, Level of Evidence B).
Hydrocephalus
Recommendation
External ventricular drainage is reasonable, especially when the patient’s level of consciousness decreased (Class IIa, Level of Evidence B).
Secondary epilepsy
Recommendations
4.3.1 Patients with seizures should be given antiepileptic drugs (AED) (Class I, Level of Evidence A).
4.3.2 The use of AED for seizure prevention is not recommended (Class III, Level of Evidence B).
4.3.3 Patients with reduced consciousness and imaging findings of cerebral haemorrhage, continuous EEG monitoring is reasonable to determine whether they have epilepsy (Class IIa, Level of Evidence C).
Cardiac complications
Recommendation
ECG and cardiac enzyme measurement is reasonable after cerebral haemorrhage to screen for cardiac complications (Class IIa, Level of Evidence C).
Pulmonary infection and prevention
Recommendation
Swallowing test is recommended before oral feeding to decrease the risk of pneumonia (Class I, Level of Evidence B).
Screening and prevention of deep vein thrombosis (DVT)
Recommendations
Measures should be taken for patients in bed to prevent DVT (Class I, Level of Evidence C).
For patients suspected of DVT, D-dimer and Doppler ultrasonography should be performed (Class I, Level of Evidence C).
Patients should be mobilised as early as possible. Legs should be raised and intravenous infusion of the lower extremities should be avoided as much as possible, especially the limbs with disability (Class I, Level of Evidence C).
Patients who were diagnosed with ICH can be given intermittent pneumatic compression therapy to prevent DVT from the beginning of hospitalisation (Class IIa, Level of Evidence B).
Elastic stockings alone is not recommended (Class III, Level of Evidence A).
For high-risk patients who are prone to DVT (excluding patients with cerebral haemorrhage due to coagulopathy), after confirming that the bleeding has stopped, early anticoagulation (subcutaneous injection of low-dosage low molecular weight heparin or unfractionated heparin) might prevent DVT (Class IIb, Level of Evidence B).
For patients with symptomatic DVT or PE (pulmonary embolism), systemic anticoagulation or placement of IVC (inferior vena cave)filters can be considered (Class IIa, Level of Evidence C). When deciding which treatment to choose, the time from the first bleeding, whether the hematoma is stable, the cause of bleeding, and the patient’s general condition can be considered (Class IIa, Level of Evidence C).
Section 5: prevention of secondary ICH
Prevention of early rebleeding
After cerebral haemorrhage, it is reasonable to detect and treat early rebleeding/haematoma enlargement (Class IIa, Level of Evidence C).
Prevention of late rebleeding
Recommendations
If necessary, anticoagulant therapy may be considered in patients with non-lobar ICH (Class IIa, Level of Evidence B). After warfarin-related spontaneous cerebral haemorrhage, resuming warfarin for long-term anticoagulant for non-valvular atrial fibrillation related ICH is not recommended (Class III, Level of Evidence B).
In patients with ICH with atrial fibrillation, the effectiveness of using dabigatran, rivaroxaban or apixaban to reduce the risk of recurrence is uncertain (Class IIb, Level of Evidence C).
In patients with anticoagulant-related ICH, the best timing to restore oral anticoagulant therapy is uncertain (Class IIb, Level of Evidence B).
If necessary, patients with ICH may consider antiplatelet monotherapy (Class IIa, Level of Evidence B).
The monotherapy of aspirin can be restored within a few days from the onset of ICH, but the best timing is not clear (Class IIa, Level of Evidence B).
Whether the use of statins should be restricted in patients with ICH is uncertain (Class IIb, Level of Evidence C).
All patients with ICH should control blood pressure (Class I, Level of Evidence A).
Blood pressure control measures should be started immediately after ICH onset (Class I recommendation, Level of Evidence A). It is reasonable to target BP <130/80 mm Hg for long-term blood pressure control (Class IIa, Level of Evidence B).
Smoking cessation, avoiding excessive drinking and treatment of obstructive sleep apnoea might be reasonable to reduce the risk of ICH (Class IIb, Level of Evidence B).
Footnotes
YC and SY contributed equally.
Collaborators: Chinese Stroke Association Stroke Council Guideline: Writing Committee Chairmen: Yongjun Wang (yongjunwang@ncrcnd.org.cn, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China). Jizong Zhao (zhaojz205@163.com/zhaojz@public.bta.net.cn, Department of Neurosurgery, Beijing Tiantan Hospital, Capital Medical University, Beijing, China). Vice-Chairmen: Qiang Dong (dong_qiang@fudan.edu.cn, Department of Neurology, Huashan Hospital, Fudan University, Shanghai, China). Anding Xu (tlil@jnu.edu.cn, Department of Neurology and Stroke Center, the First Affiliated Hospital, Jinan University, Guangzhou, China). Members of Academic Committee: Kangning Chen (ckn_640827@126.com, Department of Neurology, The Southwest Hospital, the First Affiliated Hospital of Third Military Medical University, Chongqing, China). Junbo Ge (ge.junbo@zs-hospital.sh.cn, Shanghai Institute of Cardiovascular Diseases, Department of Cardiology, Zhongshan Hospital, Fudan University, Shanghai, China). Li Guo (guoli6@163.com, Department of Neurology, The Second Hospital of Hebei Medical University, Shijiazhuang, China). Li He (heli2003new@126.com, Department of Neurology, West China Hospital, Sichuan University, Chengdu, China). Bo Hu (hubo@hust.edu.cn, Department of Neurology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (HUST), Wuhan, China). Yong Huo (huoyong@263.net.cn, Department of Cardiology, Peking University First Hospital, Beijing, China). Linong Ji (jiln@bjmu.edu.cn, Department of Endocrinology and Metabolism, Peking University People’s Hospital, Medicine at Peking University, Beijing, China). Xunming Ji (robertjixm@hotmail.com/jixunming@vip.163.com, Department of Neurosurgery, Xuanwu Hospital, Capital University of Medicine, Beijing, China), Tielin Li (tielin2013@126.com/tielin.li@tom.com, Zhujiang Hospital of Southern Medical University, Guangzhou, China). Liping Liu (lipingsister@gmail.com, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China). Benyan Luo (luobenyan@zju.edu.cn, Department of Neurology, 1st Affiliated Hospital of Zhejiang University, Hangzhou, China). Zhongrong Miao (zhongrongm@163.com, Department of Interventional Neuroradiology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China). Xiaoyuan Niu (niuxiaoyuan1958@163.com, Department of Neurology, First Hospital of Shanxi Medical University, Taiyuan, China). Bin Peng (pengbin3@hotmail.com, Department of Neurology, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, China). Dingfeng Su (dfsu@smmu.edu.cn, Department of Pharmacology, the Second Military Medical University (SMMU), Shanghai, China). Beisha Tang (bstang7398@163.com, Department of Neurology, Xiangya Hospital, Central South University, Changsha, China). Chen Wang (wangchen-tr2002@163.com, Beijing Tiantan Hospital, Capital Medical University, Beijing, China). Ning Wang (nwang900@yahoo.com, Department of Neurology and Institute of Neurology, First Affiliated Hospital of Fujian Medical University, Fuzhou, China). Shuo Wang (captain9858@vip.sina.com, Department of Neurosurgery, Beijing Tiantan Hospital, Capital Medical University, Beijing, China). Wei Wang (wwang@vip.126.com/wwang@tjh.tjmu.edu.cn, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China). Xin Wang (wang.xin@zs-hospital.sh.cn, Department of Neurology, Zhongshan Hospital, Fudan University, Shanghai, China). Yilong Wang (yilong528@aliyun.com, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China). Shizheng Wu (wushizheng2005@hotmail.com, Qinghai Province People’s Hospital, Xining, China). Peng Xie (xiepeng@cqmu.edu.cn, Chongqing Medical University (CQMU), Chongqing, China). Yuming Xu (13903711125@126.com/xym13903711125@126.com, Department of Neurology, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, China). Yun Xu (xuyun20042001@aliyun.com, Department of Neurology, Drum Tower Hospital, Medical School of Nanjing University, Nanjing, China). Yi Yang (doctoryangyi@163.com/doctor_yangyi@hotmail.com, Department of Neurology, the First Hospital of Jilin University, Changchun, China). Jinsheng Zeng (zengjs@pub.guangzhou.gd.cn, Department of Neurology and Stroke Center, the First Affiliated Hospital of Sun Yat-Sen University, Guangdong, China). Chaodong Zhang (scdzhang@163.com, The First affiliated Hospital of China Medical University, Shenyang, China). Tong Zhang (zt61611@sohu.com, Capital Medical University School of Rehabilitation Medicine, China Rehabilitation Research Center, Beijing, China). Zhuo Zhang (zzhuo005@gmail.com, Beijing Anzhen Hospital, Capital Medical University, Beijing, China). Gang Zhao (zhaogang@fmmu.edu.cn, Department of Neurology, Xijing Hospital, The 4th Military Medical University, Xi’an, China). Xingquan Zhao (zxq@vip.163.com, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China).
Contributors: JZZ designed the framework and protocol and participated in revision. YC drafted the sections of diagnosis and evaluation of causes of ICH. SY and YL drafted the section of emergency department management of ICH. QZ and TY drafted the section of surgical treatment for removal of hematoma. ZS and MZ drafted the section of complications management of ICH. WW drafted the section of recurrence and prevention of secondary ICH. YC and SY reviewed the design and interpretation of all studies, confirmed the level of evidence and classification and revised the manuscript.
Funding: This research received specific funding from Chinese Stroke Association Stroke Council Guideline Writing Committee.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Commissioned; externally peer reviewed.
Contributor Information
Chinese Stroke Association Stroke Council Guideline:
Yongjun Wang, Jizong Zhao, Qiang Dong, Anding Xu, Kangning Chen, Junbo Ge, Li Guo, Li He, Bo Hu, Yong Huo, Linong Ji, Xunming Ji, Tielin Li, Liping Liu, Benyan Luo, Zhongrong Miao, Xiaoyuan Niu, Bin Peng, Dingfeng Su, Beisha Tang, Chen Wang, Ning Wang, Shuo Wang, Wei Wang, Xin Wang, Yilong Wang, Shizheng Wu, Peng Xie, Yuming Xu, Yun Xu, Yi Yang, Jinsheng Zeng, Chaodong Zhang, Tong Zhang, Zhuo Zhang, Gang Zhao, and Xingquan Zhao
References
- 1. van Asch CJ, Luitse MJ, Rinkel GJ, et al. . Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol 2010;9:167–76. 10.1016/S1474-4422(09)70340-0 [DOI] [PubMed] [Google Scholar]
- 2. Wang W, Jiang B, Sun H, et al. . Prevalence, Incidence, and Mortality of Stroke in China: Results from a Nationwide Population-Based Survey of 480 687 Adults. Circulation 2017;135:759–71. 10.1161/CIRCULATIONAHA.116.025250 [DOI] [PubMed] [Google Scholar]
- 3. Health and Family Planning Commission of the people’s Republic of China China’s Health and family planning statistical yearbook 2017. Peking Union Medical College Press, 2017. [Google Scholar]
- 4. Hemphill JC, Greenberg SM, Anderson CS, et al. . Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American heart association/american stroke association. Stroke 2015;46:2032–60. 10.1161/STR.0000000000000069 [DOI] [PubMed] [Google Scholar]
- 5. Dong Y, Guo Z-N, Li Q, et al. . Chinese stroke association guidelines for clinical management of cerebrovascular disorders: Executive summary and 2019 update of clinical management of spontaneous subarachnoid haemorrhage. Stroke Vasc Neurol 2019;4:176–81. 10.1136/svn-2019-000296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hemphill JC, Bonovich DC, Besmertis L, et al. . The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke 2001;32:891–7. 10.1161/01.str.32.4.891 [DOI] [PubMed] [Google Scholar]