Abstract
Objective
To evaluate the prognosis values of systemic immune–inflammation index (SII) in non-chronic cerebral venous sinus thrombosis (CVST).
Methods
patients with CVST, admitted to the First Affiliated Hospital of Zhengzhou University, were retrospectively identified from January 2013 to December 2018. We selected patients in acute/subacute phase from database. Functional outcomes of patients were evaluated with the modified Rankin Scale (mRS)—mRS 3–6 as poor outcomes and mRS 6 as death. The overall survival time was defined as the date of onset to the date of death or last follow-up date. Survival analysis was described by the Kaplan-Meier curve and Cox regression analysis. Multivariate logistic regression analysis assessed the relationship between SII and poor functional outcome. The area under the Receiver Operating Curve curve (AUC) was estimated to evaluate the ability of SII in prediction.
Results
A total of 270 patients were included and their duration of follow-up was 22 months (6–66 months), of whom 31 patients had poor outcomes and 24 patients dead. Cox regression analysis showed that SII (HR=1.304, 95% CI: 1.101 to 1.703, p=0.001) was a predictor of death in non-chronic CVST. Patients with higher SII presented lower survival rates (p=0.003). The AUC of SII was 0.792 (95% CI: 0.695 to 0.888, p=0.040) with a sensitivity of 69.6% and specificity of 80.1%. Subgroups analysis demonstrated that SII was an important predictor of poor outcomes in male (OR=1.303, 95% CI: 1.102 to 1.501, p=0.011) and pregnancy/puerperium female (OR=1.407, 95% CI: 1.204 to 1.703, p=0.034).
Conclusions
SII was a potential predictor in the poor prognosis of patients with acute/subacute CVST, especially in male and pregnancy/puerperium female.
Keywords: inflammation, stroke, vein
Highlights.
Systemic immune–inflammation index (SII) was first analysed in cerebral venous sinus thrombosis (CVST) patients.
SII may be significantly related to the poor prognosis of patients with CVST whose time from onset to addition was less than 30 days.
Introduction
Cerebral venous sinus thrombosis (CVST), is a rare type of stroke, accounting for 0.5%–1% of all strokes.1 The disease is more likely to occur in young people, in particular women more regularly.2 CVST has variable pathogenic factors and complex clinical symptoms, which lack specificity and tend to be misdiagnosed.3 Therefore, it is very significant to find some biomarkers to help us predict the prognosis in the early phase.
Some factors, such as D-dimer, C-reactive protein, red cell distribution width and mean platelet volume, provided potential values regarding the presence of patients with CVST,4 5 but due to the ethnic and quantitative differences of the subjects, the results were not consistent and reliable in some researches. Although the CVST prognostic score based on the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT-RS)6 and Cerebral Venous Thrombosis grading scale (CVT-GS)7 score have been used in prognosis of Patients with CVST, some studies indicated that the scales were not proper for Chinese patients.8 Thus, it is necessary to find some more stable and representative parameters to predict the prognosis.
Systemic immune–inflammation index (SII) is a novel inflammatory index to comprehensively reflect the balance of host immune and inflammatory status.9 It was defined as follows: platelet (/L)×neutrophil (/L)/lymphocyte (/L). Up to now, SII has been reported to be associated with survival in patients with various cancers,9–11 such as gastric cancer and prostate cancer. However, there were no reports concerned with the roles of SII in patients with CVST. Inflammation plays an important role in the risk of CVST and the inflammation response activated by the brain lesion is regarded as a fatal response that provokes secondary brain injury.12 Also, due to a lack of understanding about the mechanism of the early phase, people are unable to judge the prognosis of acute/subacute patients with CVST as soon as possible. Compared with platelet to lymphocyte ratio (PLR)13 and neutrophil to lymphocyte ratio (NLR)14 which have been identified in the prognosis of patients with CVST, SII is more stable and representative in reflecting the balance of host immune and inflammatory status because it combines one more factor than them. Therefore, we aim to analyse the association between SII and prognosis in non-chronic patients with CVST whose time from onset to addition was less than 30 days.
Methods
Patient selection
Patients included in the retrospective cohort study were from the database of the Henan CVST Registry in the First Affiliated Hospital of Zhengzhou University (Henan, China). All patients diagnosed with CVST from January 2013 through December 2018 were identified. We selected acute/subacute patients from our database. Inclusion criteria were as follows: (1) meeting the diagnostic criteria for CVST and patients with direct or indirect signs of CVST in the MRI; (2) presence of filling defect or obstruction of cerebral sinus in the magnetic resonance venogram, digital subtraction angiography or operation searching; (3) exhibiting clinical features such as vomiting, visual disturbances, focal neurologic deficit, seizure and other typical symptoms; (4) acute and subacute patients whose time from onset to admission was less than 30 days,15 16 or named non-chronic patients; (5) an initial blood sample for laboratory testing 12 hours of admission. Exclusion criteria were as follows: (1) patients with other unrelated serious brain lesions, serious lung disease or heart disease; (2) patients with undesirable follow-up, including refusal or loss to follow-up; (3) patients younger than 18 years old; (4) patients without complete clinical data and (5) chronic patients whose time from onset to addition was more than 30 days.
Data collection
Clinical data such as age, gender, clinical presentation, laboratory and imaging tests were collected. The inter-rater reliability for involvement of intracranial venous sinus between two investigators was assessed in some cases.
Evaluation of prognosis
We evaluated the modified Rankin Scale (mRS) to determine the patients’ functional outcomes: mRS 0–2 as good outcomes, mRS 3–6 as poor outcomes and death was defined as mRS score of 6. Follow-up information was recorded by telephone interview. Telephone interviewers were not involved in the registry and were blinded to the baseline data. The overall survival time was defined as the date of onset to the date of death from any cause, or to the last follow-up date.
Statistical analysis
All statistical analyses were performed using SPSS V.21.0 software. Continuous variables were expressed as mean±SD or median, which were analysed by independent Student’s t-test or Mann-Whitney test as appropriate. Categorical variables were presented as numbers which were analysed using χ2 test or Fisher exact test. Multivariate logistic regression analysis was used to analysis the association between factors and prognosis. And the association between SII and the survival outcome was explored by Cox regression analysis. The area under the Receiver Operating Curve (AUC) was estimated to evaluate the ability of the SII in predicting clinical prognosis. Survival curves were described by the Kaplan-Meier analysis and compared with Log-rank test. Two-tailed p<0.05 were considered significant.
Results
We included 297 patients who confirmed acute and subacute CVST admitted during the study period from database. We excluded eight patients because of incomplete clinical data, four patients because they were lost to follow-up and 15 patients because they were younger than 18 years. A total of 270 patients were enrolled into this study.
The duration of follow-up was 22 months (6–66 months) and 31 patients were defined as poor prognosis, including 24 patients dead. The baseline clinical data of two groups are shown in table 1. SII (1151.71±1085.54 vs 2893.31±3680.81, p<0.001) was significantly higher in the poor outcome group. And it was higher in the acute and subacute patients than chronic patients (online supplementary table 1). Older patients were identified more frequently in the poor outcome group than good outcome group (33.36±12.49 vs 42.00±14.84, p=0.002). Also, coma was more common among patients with poor outcome (p<0.001). As for laboratory parameters, lymphocyte count reached statistical significance (p<0.001). Additionally, gender (p=0.044) was also regarded as a risk factor. Straight sinus was involved in the poor outcomes group (p=0.011).
Table 1.
Demographic and clinical characteristics of the two outcomes groups in acute/subacute patients with CVST
| Variable | Total | Good (239, 89.0%) | Poor (31, 11.0 %) | P value |
| Age | 34.50±13.12 | 33.36±12.49 | 42.00±14.84 | 0.002* |
| Gender | 270 | 239 | 31 | 0.044* |
| Female | 156 | 134 | 22 | |
| Male | 114 | 105 | 9 | |
| Malignancy | 4 | 3 | 1 | 0.575 |
| Infection | 57 | 48 | 9 | 0.511 |
| Pregnancy or puerperium | 65 | 53 | 12 | 0.518 |
| Intracerebral haemorrhage | 62 | 53 | 9 | 0.674 |
| Coma | 72 | 50 | 22 | <0.001* |
| Mental disorder | 15 | 10 | 5 | 0.303 |
| Lymphocyte | 1.67±0.84 | 1.74±0.85 | 1.19±0.62 | <0.001* |
| Granulocyte | 7.27±4.73 | 7.04±4.63 | 8.79±5.15 | 0.066 |
| Platelet | 244.12±124.21 | 243.15±122.08 | 250.54±139.23 | 0.768 |
| PLR | 179.47±129.72 | 159.61±90.38 | 339.32±257.80 | <0.001* |
| NLR | 6.81±7.90 | 5.50±5.15 | 17.05±15.94 | <0.001* |
| SII | 1380.86±1763.22 | 1151.71±1085.54 | 2893.31±3680.81 | <0.001* |
| Left sigmoid sinus | 70 | 47 | 23 | 0.629 |
| Right sigmoid sinus | 75 | 57 | 18 | 0.218 |
| Left transverse sinus | 98 | 63 | 35 | 0.147 |
| Right transverse sinus | 97 | 73 | 24 | 0.188 |
| Straight sinus | 21 | 14 | 7 | 0.011* |
| Superior sagittal sinus | 159 | 119 | 40 | 0.243 |
| Inferior sagittal sinus | 25 | 13 | 12 | 0.061 |
| Torcular | 28 | 18 | 10 | 0.510 |
| Deep cerebral venous | 8 | 6 | 2 | 0.284 |
*Statistically significant.
CVST, cerebral venous sinus thrombosis; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; SII, systemic immune–inflammation index.
svn-2020-000362supp001.pdf (91.1KB, pdf)
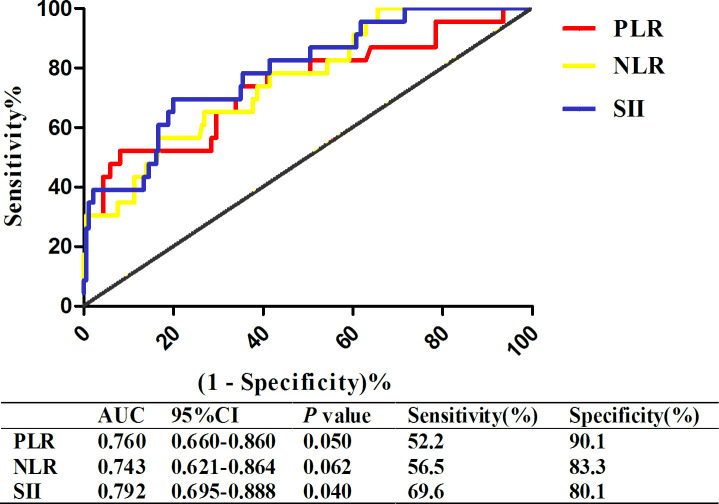
In figure 1, we indicated that the discriminatory capability of SII using AUC was 0.792 (95% CI: 0.695 to 0.888, p=0.040) for patients and using a cut-off level of 1525.04. And it predicted the presence of poor outcome with a sensitivity of 69.6% and specificity of 80.1%. Because SII was defined as platelet (/L)×neutrophil (/L)/lymphocyte (/L), we also compared it with other two related prognostic factors, including PLR and NLR. The results demonstrated that SII was the relatively good prognostic factor of these patients whose time from onset to addition was less than 30 days, but not in patients whose time from onset to addition was more than 30 days (online supplementary figure 1). Subgroup analysis demonstrated that SII was an important predictor of poor outcome in male (OR=1.303, 95% CI: 1.102 to 1.501, p=0.011) and pregnancy/puerperium female (OR=1.407, 95% CI: 1.204 to 1.703, p=0.034), after adjusting for age, coma and straight sinus (table 2).
Figure 1.
ROC curve of SII, PLR and NLR in acute/subacute Patients with CVST. CVST, cerebral venous sinus thrombosis; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; ROC, Receiver Operating Curve; SII, systemic immune–inflammation index.
Table 2.
Multivariable logistic regression analysis in different subgroups with acute/subacute patients with CVST
| Groups | Variable | OR | 95% CI | P value |
| Subgroup 1 | Age | 1.055 | 1.027 to 1.083 | <0.001* |
| SII | 1.303 | 1.102 to 1.501 | 0.011* | |
| Coma | 2.726 | 1.547 to 3.963 | 0.026* | |
| Straight sinus | 2.485 | 1.142 to 5.409 | 0.022* | |
| Subgroup 2 | Age | 1.100 | 1.027 to 1.177 | 0.006* |
| SII | 1.104 | 0.998 to 1.209 | 0.251 | |
| Coma | 2.667 | 1.196 to 5.209 | 0.001* | |
| Straight sinus | 5.104 | 1.998 to 12.209 | 0.009* | |
| Subgroup 3 | Age | 1.216 | 0.862 to 1.715 | 0.266 |
| SII | 1.407 | 1.204 to 1.703 | 0.034* | |
| Coma | 1.101 | 0.997 to 1.501 | 0.061 | |
| Straight sinus | 5.412 | 0.769 to 11.436 | 0.120 | |
| Subgroup 4 | Age | 1.064 | 1.016 to 1.115 | 0.009* |
| SII | 1.207 | 0.986 to 1.505 | 0.119 | |
| Coma | 4.753 | 2.082 to 6.834 | 0.003* | |
| Straight sinus | 4.086 | 1.031 to 8.696 | 0.046* |
*Statistically significant. Subgroup analysis was carried out in male (subgroup 1), female (subgroup 2), pregnancy/puerperium (subgroup 3) and non-pregnancy/puerperium (subgroup 4) Patients with CVST.
CVST, cerebral venous sinus thrombosis; SII, systemic immune–inflammation index.
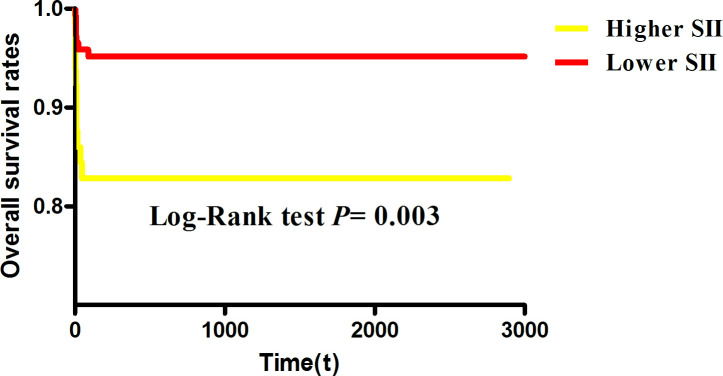
We used Kaplan-Meier analysis method to assess SIIin figure 2. According to the threshold values of SII, we divided the patients into two groups, higher SII group and lower SII group. Patients with higher SII had higher mortality than lower SII (p=0.003). In addition, Cox regression analysis showed that SII (HR=1.304, 95% CI: 1.101 to 1.703, p=0.001) was also a significant predictor in death of non-chronic patients with CVST (figure 3).
Figure 2.
Kaplan-Meier analysis for acute/subacute patients with CVST with higher/lower SII. CVST, cerebral venous sinus thrombosis; SII, systemic immune–inflammation index.
Figure 3.
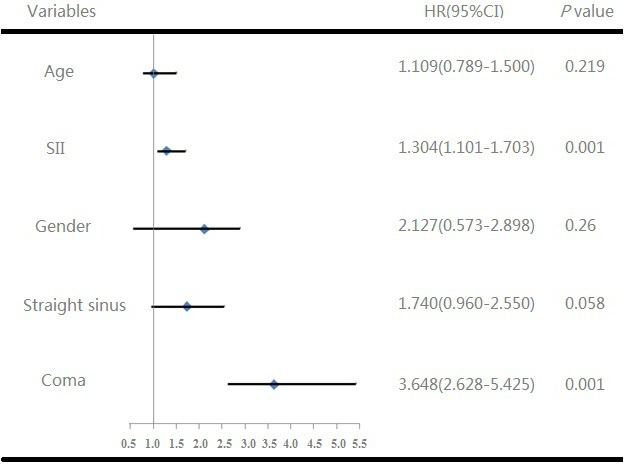
Cox regression analysis of overall survival in acute/subacute patients with CVST. CVST, cerebral venous sinus thrombosis; SII, systemic immune–inflammation index.
Discussion
Our study mainly investigated the association between SII and the prognosis with patients with CVST, and tried to find new factors for prognosis. The major finding was higher SII at admission independently and strongly related to the poor prognosis of non-chronic Patients with CVST, especially in male and pregnancy/puerperium female.
The basis of cerebral sinus venous thrombosis can be linked to Virchow’s triad, which includes injury to the vessel walls, a hypercoagulable state and stasis.17 Recognised predisposing risk factors for thrombosis are known to include inflammation and infection.18 In human studies, the predictive values of PLR and NLR have been analysed in patients with CVST,13 19 20 but phase studies were not carried out. And our studies have also proved that lymphocyte to monocyte ratio, as an inflammation factor, was a potential predictor of poor prognosis in patients with CVST.14 As for SII, it combined the platelet, neutrophil and lymphocyte count, reflecting the inflammation and thrombosis. Therefore, SII was more stable and representative, compared with these factors above.
Recently, it has emerged as a powerful prognostic index in various malignant diseases, including renal cell cancer,21 gastric cancer22 and prostate cancer.23 Evidence is accumulating that neutrophils, apart from their well-known inflammatory function, also promote intravascular thrombus formation. Neutrophils have been shown to be an essential source of tissue factor in the early phase of thrombus formation.24 25 Brain ischaemia is followed by the activation of the immune system in a sterile inflammatory reaction, resulting in the change of cell adhesion molecules, cytokines, as well as infiltration of leukocytes in the ischaemic tissue. Lymphocytes, as an important subtype of the leucocyte family, are also involved in inflammation. Previous studies have demonstrated the association between the low lymphocyte count and increased cardiovascular disease.26 Furthermore, trials have shown an increased association between platelets and development of vascular events.27 28 Platelets are involved in the early stage of atherosclerosis-associated chronic vascular pathology and plaque rupture. Platelets inside the atherosclerotic plaque ensure replication of leukocytes via direct receptor–ligand interactions and increase the leucocyte activity.29 Because these factors play an important role in the early stage of related pathophysiological changes, we used this combination index and have confirmed that SII can predictive prognosis in non-chronic patients with CVST. And we found that patients with higher SII showed poorer prognosis conditions in non-chronic phase. However, we still require more randomised controlled trials to clarify the potential benefits.
As we all know, CVST may occur at any age and in both sexes, but it has a three to one female preponderance.30 It is more common in women of childbearing age,31 because of the use of oral contraceptives (80% of cases) and pregnancy/puerperium (5%–20% of cases).32 In our subgroup analysis, we aimed to explore the association between SII and outcomes in different genders. The results showed that SII was more effective in prognosis of CVST with male and pregnancy/puerperium female. This is an important finding, because previous studies have reported gender-specific pathophysiological differences in CVST, based on the fact that men more often have a chronic onset of symptoms.30 But in our study, we collected the patients whose time from onset to addition was less than 30 days, which was a good addition to the previous facts. Furthermore, SII can be used to inducate the systemic immune and inflammation, so we will suggest a new and different hypothesis that the mechanism of CVST in male and special female is more involved in inflammatory aspects than other groups.
In addition, the previously validated risk score derived from the ISCVT study and Cerebral Venous Thrombosis Portuguese Collaborative Study (VENOPORT) research has been used in prognosis with CVST,6 including gender, mental disorder, coma, venous thrombosis, intracerebral haemorrhage and malignancy. Whereas, more and more evidences showed that ISCVT-RS scores have some limitations. A Chinese retrospective study found that the accuracy is not ideal, and the AUC is only 0.65 (95% CI: 0.53 to 0.77, p<0.01),8 which may be due to the heterogeneity of the research population.33 And we believe that this scale needs to be further improved, especially for the Chinese population. Our findings about SII might provide a way or clue to explore.
Our study was a single-centre study and selection bias was unavoidable. Additional well-designed and larger prospective cohort multicenter studies are required to evaluate this association. In addition, heterogeneity of the research population could not be ignored. It is required to evaluate potential values by further evidence.
Conclusion
Our findings suggested that SII was a potential predictor in the poor prognosis of patients with acute/subacute CVST, especially in male and pregnancy/puerperium female.
Footnotes
Contributors: SL is the first author. BS, YX and ZX provided funding and designed the study. YT, HL and JZ collected the data. YG, LZ, RZ and HF were involved in data cleaning, follow-up and verification. KL revised the article. All authors have read and approved the final manuscript.
Funding: This work was supported by the Science and Technology Department of the Henan Province grant number (No.152102310058), the National Natural Science Foundation of China grant number (Nos. 81530037).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The retrospective cohort study was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information. All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1. Sader N, de Lotbinière-Bassett M, Tso MK, et al. Management of venous sinus thrombosis. Neurosurg Clin N Am 2018;29:585–94. 10.1016/j.nec.2018.06.011 [DOI] [PubMed] [Google Scholar]
- 2. Devasagayam S, Wyatt B, Leyden J, et al. Cerebral venous sinus thrombosis incidence is higher than previously thought: a retrospective population-based study. Stroke 2016;47:2180–2. 10.1161/STROKEAHA.116.013617 [DOI] [PubMed] [Google Scholar]
- 3. Coutinho JM, Zuurbier SM, Aramideh M, et al. The incidence of cerebral venous thrombosis: a cross-sectional study. Stroke 2012;43:3375–7. 10.1161/STROKEAHA.112.671453 [DOI] [PubMed] [Google Scholar]
- 4. Meng R, Wang X, Hussain M, et al. Evaluation of plasma D-dimer plus fibrinogen in predicting acute CVST. Int J Stroke 2014;9:166–73. 10.1111/ijs.12034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Talbot K, Wright M, Keeling D. Normal D-dimer levels do not exclude the diagnosis of cerebral venous sinus thrombosis. J Neurol 2002;249:1603–4. 10.1007/s00415-002-0893-z [DOI] [PubMed] [Google Scholar]
- 6. Ferro JM, Bacelar-Nicolau H, Rodrigues T, et al. Risk score to predict the outcome of patients with cerebral vein and dural sinus thrombosis. Cerebrovasc Dis 2009;28:39–44. 10.1159/000215942 [DOI] [PubMed] [Google Scholar]
- 7. Barboza MA, Chiquete E, Arauz A, et al. A practical score for prediction of outcome after cerebral venous thrombosis. Front Neurol 2018;9:882. 10.3389/fneur.2018.00882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li S, Liu K, Zhang R, et al. Lower lymphocyte to monocyte ratio is a potential predictor of poor outcome in patients with cerebral venous sinus thrombosis. Stroke Vasc Neurol 2019;4:148-153. 10.1136/svn-2018-000180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang B-L, Tian L, Gao X-H, et al. Dynamic change of the systemic immune inflammation index predicts the prognosis of patients with hepatocellular carcinoma after curative resection. Clin Chem Lab Med 2016;54:1963–9. 10.1515/cclm-2015-1191 [DOI] [PubMed] [Google Scholar]
- 10. Dolan RD, McSorley ST, Park JH, et al. The prognostic value of systemic inflammation in patients undergoing surgery for colon cancer: comparison of composite ratios and cumulative scores. Br J Cancer 2018;119:40–51. 10.1038/s41416-018-0095-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cannon NA, Meyer J, Iyengar P, et al. Neutrophil-Lymphocyte and platelet-lymphocyte ratios as prognostic factors after stereotactic radiation therapy for early-stage non-small-cell lung cancer. J Thorac Oncol 2015;10:280–5. 10.1097/JTO.0000000000000399 [DOI] [PubMed] [Google Scholar]
- 12. Vidale S, Consoli A, Arnaboldi M, et al. Postischemic inflammation in acute stroke. J Clin Neurol 2017;13:1–9. 10.3988/jcn.2017.13.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang HF, Pu CQ, Yin X, et al. D-Dimers (DD) in CVST. Int J Neurosci 2017;127:524–30. 10.1080/00207454.2016.1207172 [DOI] [PubMed] [Google Scholar]
- 14. de la Vega Muns G, Quencer R, Ezuddin NS, et al. Utility of Hounsfield unit and hematocrit values in the diagnosis of acute venous sinus thrombosis in unenhanced brain CTS in the pediatric population. Pediatr Radiol 2019;49:234–9. 10.1007/s00247-018-4273-y [DOI] [PubMed] [Google Scholar]
- 15. Akboga YE, Bektas H, Anlar O. Usefulness of platelet to lymphocyte and neutrophil to lymphocyte ratios in predicting the presence of cerebral venous sinus thrombosis and in-hospital major adverse cerebral events. J Neurol Sci 2017;380:226–9. 10.1016/j.jns.2017.07.036 [DOI] [PubMed] [Google Scholar]
- 16. Artoni A, Abbattista M, Bucciarelli P, et al. Platelet to lymphocyte ratio and neutrophil to lymphocyte ratio as risk factors for venous thrombosis. Clin Appl Thromb Hemost 2018;24:808–14. 10.1177/1076029617733039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Siddiqi SA, Nishat S, Kanwar D, et al. Cerebral venous sinus thrombosis: association with primary varicella zoster virus infection. J Stroke Cerebrovasc Dis 2012;21:917.e1–917.e4. 10.1016/j.jstrokecerebrovasdis.2012.04.013 [DOI] [PubMed] [Google Scholar]
- 18. Goeijenbier M, van Wissen M, van de Weg C, et al. Review: viral infections and mechanisms of thrombosis and bleeding. J Med Virol 2012;84:1680–96. 10.1002/jmv.23354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. İdil Soylu A, Arıkan Cortcu S, Uzunkaya F, et al. The correlation of the platelet-to-lymphocyte ratio with the severity of stenosis and stroke in patients with carotid arterial disease. Vascular 2017;25:299–306. 10.1177/1708538116673770 [DOI] [PubMed] [Google Scholar]
- 20. Deser SB, Yucel SM, Demirag MK, et al. The association between platelet/lymphocyte ratio, neutrophil/lymphocyte ratio, and carotid artery stenosis and stroke following carotid endarterectomy. Vascular 2019;1708538119847390. [DOI] [PubMed] [Google Scholar]
- 21. Yang R, Chang Q, Meng X, et al. Prognostic value of systemic immune-inflammation index in cancer: a meta-analysis. J Cancer 2018;9:3295–302. 10.7150/jca.25691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang K, Diao F, Ye Z, et al. Prognostic value of systemic immune-inflammation index in patients with gastric cancer. Chin J Cancer 2017;36:75. 10.1186/s40880-017-0243-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lolli C, Caffo O, Scarpi E, et al. Systemic immune-inflammation index predicts the clinical outcome in patients with mcrpc treated with abiraterone. Front Pharmacol 2016;7:376. 10.3389/fphar.2016.00376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Darbousset R, Thomas GM, Mezouar S, et al. Tissue factor-positive neutrophils bind to injured endothelial wall and initiate thrombus formation. Blood 2012;120:2133–43. 10.1182/blood-2012-06-437772 [DOI] [PubMed] [Google Scholar]
- 25. von Brühl M-L, Stark K, Steinhart A, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med 2012;209:819–35. 10.1084/jem.20112322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Horne BD, Anderson JL, John JM, et al. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol 2005;45:1638–43. 10.1016/j.jacc.2005.02.054 [DOI] [PubMed] [Google Scholar]
- 27. Oz II, Yucel M, Bilici M, et al. Is mean platelet volume a reliable marker to predict ischemic stroke in the follow-up of patients with carotid stenosis? J Stroke Cerebrovasc Dis 2016;25:404–9. 10.1016/j.jstrokecerebrovasdis.2015.10.012 [DOI] [PubMed] [Google Scholar]
- 28. Arévalo-Lorido JC, Carretero-Gómez J, Álvarez-Oliva A, et al. Mean platelet volume in acute phase of ischemic stroke, as predictor of mortality and functional outcome after 1 year. J Stroke Cerebrovasc Dis 2013;22:297–303. 10.1016/j.jstrokecerebrovasdis.2011.09.009 [DOI] [PubMed] [Google Scholar]
- 29. Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest 2005;115:3378–84. 10.1172/JCI27196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Coutinho JM, Ferro JM, Canhão P, et al. Cerebral venous and sinus thrombosis in women. Stroke 2009;40:2356–61. 10.1161/STROKEAHA.108.543884 [DOI] [PubMed] [Google Scholar]
- 31. Abbattista M, Capecchi M, Martinelli I. Pregnancy after cerebral venous thrombosis. Thromb Res 2019;181 Suppl 1:S15–18. 10.1016/S0049-3848(19)30360-3 [DOI] [PubMed] [Google Scholar]
- 32. Bousser MG, Crassard I, thrombosis Cvenous. Pregnancy and oral contraceptives. Thromb Res 2012;130:S19–22. [DOI] [PubMed] [Google Scholar]
- 33. Koopman K, Uyttenboogaart M, Vroomen PC, et al. Development and validation of a predictive outcome score of cerebral venous thrombosis. J Neurol Sci 2009;276:66–8. 10.1016/j.jns.2008.08.033 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
svn-2020-000362supp001.pdf (91.1KB, pdf)