Abstract
Patients with end-stage renal disease (ESRD) may demonstrate secondary hyperparathyroidism (SHPT), characterized by parathyroid hormone oversecretion in response to electrolyte imbalance (e.g., hypocalcemia and hyperphosphatemia). Moreover, this electrolyte imbalance may affect vocal cord muscle contraction and lead to voice change. Here, we explored the effects of SHPT on the voices of patients with ESRD. We used data of 147,026 patients with ESRD from the registry for catastrophic illness patients, a sub-database of Taiwan National Health Insurance Research Database. We divided these patients into 2 groups based on whether they had hyperparathyroidism (HPT) and compared vocal dysfunction (VD) incidence among them. We also prospectively included 60 ESRD patients with SHPT; 45 of them underwent parathyroidectomy. Preoperatively and postoperatively, voice analysis was used to investigate changes in vocal parameters. In the real-world database analysis, the presence of HPT significantly increased VD incidence in patients with ESRD (p = 0.003): Cox regression analysis results indicated that patients with ESRD had an approximately 1.6-fold increased VD risk (p = 0.003). In the clinical analysis, the “jitter” and “shimmer” factors improved significantly after operation, whereas the aerodynamic factors remained unchanged. In conclusion, SHPT was an independent risk factor for VD in patients with ESRD, mainly affecting their acoustic factors.
Subject terms: Health care, Respiratory tract diseases, Chronic kidney disease
Introduction
Patients with end-stage renal disease (ESRD) may have impaired calcium, phosphorus, and vitamin D metabolism, rendering their kidneys unable to filter out plasma phosphorus produced by the body and to synthesize sufficient vitamin D (particularly calcitriol, the active form of vitamin D)1–3. Without treatment, this condition progresses to hypocalcemia, resulting in excessive intact parathyroid hormone (iPTH) secretion by the parathyroid glands and subsequent hypertrophy of these glands; this condition is called secondary hyperparathyroidism (SHPT)4–6. Moreover, aforementioned abnormal metabolic complications can induce excessive bone mineral loss and extraskeletal calcification, both of which are associated with increased risks of bone fractures, heart disease, and even death7–9.
The clinical symptoms of SHPT include bone pain, malaise, and pruritus10,11. SHPT-associated electrolyte imbalance can lead to neuromuscular manifestations, such as skeletal muscular weakness12,13; in some cases, weakness of the fine muscles of the vocal cords may occur. Based on our clinical observation, we speculate that voice change is an SHPT-associated symptom.
According to our clinical observations, patients with SHPT often complain of hoarseness and vocal symptoms. This observation, not reported thus far, warrants investigation given its significance for clinicians (particularly otolaryngologists) in the expansion of the disease spectrum and consideration of SHPT in the differential diagnosis of vocal dysfunction (VD). The possible causes of VD in patients with ESRD include dehydration of the vocal fold mucosa or weakness of the vocal fold muscle. In patients with ESRD, dehydration is the predominant cause of VD. In this study, video strobe laryngoscopy (VSL) and voice analysis were performed on days between dialysis to minimize dehydration. Electrolyte imbalance, which was reported in patients with ESRD, may affect voice production14.
Therefore, we performed a real-word data analysis using a nationwide database of Taiwan to investigate whether ESRD patients with SHPT have VD risks. Then, we prospectively recruited ESRD patients with SHPT and analyzed their vocal characteristics through VSL and voice analysis before and after parathyroidectomy and investigated vocal motion abnormalities due to SHPT. The aim of this study is to investigate voice changes in patients with SHPT and voice recovery after the surgical procedure. Our findings can help the clinician to evaluate vocal symptoms when diagnosing SHPT.
Methods
Part I–real-world data study
We used data from Taiwan’s National Health Insurance (NHI) Research Database (NHIRD). The NHI program, implemented by Taiwan’s government in 1995, had covered almost all Taiwan residents by 201715–18. Subsequently, Taiwan National Health Research Institutes had constructed NHIRD for research use. NHIRD contains NHI beneficiaries’ medical records, including disease diagnosis at outpatient visits and hospitalizations, drug types and dosages, examination items, operation contents, medical expenditure, area of residence, and income level19. In the NHIRD, diagnosis coding follows the International Classification of Diseases, Ninth Revised Edition, Clinical Modifications (ICD-9-CM)20,21. The NHI system defines chronic kidney failure with long-term renal dialysis as a “catastrophic illness.” The concerned patients are registered in the Registry for Catastrophic Illness Patients (RFCIP)20 after audit and are given catastrophic illness–related treatment at subsidized rates22. However, the audit process is rigorous, with regular dialysis treatment for ≥ 3 months as an eligibility criterion. Therefore, RFCIP data were used for identifying and recruiting chronic renal failure patients requiring kidney dialysis.
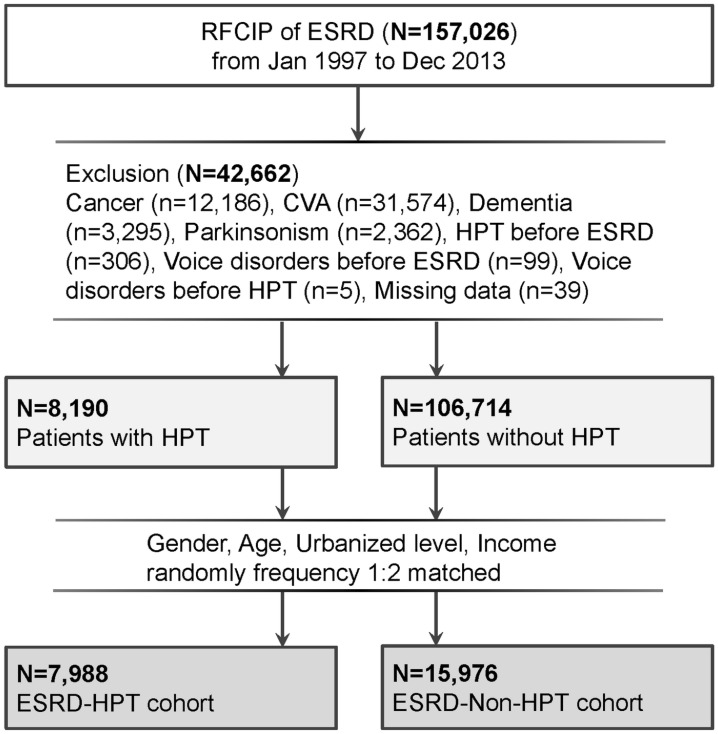
Data of patients with a diagnosis of ESRD between January 1997 and December 2013 were retrieved from the RFCIP by using ESRD-associated ICD-9-CM codes: 585, 586, 403.01, 403.11, 403.91, 404.02, 404.03, 404.12, 404.13, 404.92, and 404.93 (Fig. 1)20. Consequently, data of 147 026 patients with ESRD were extracted from RFCIP after the exclusion of patients with comorbid cancer, cerebrovascular accident, dementia, parkinsonism ESRD, and any voice-related diseases before ESRD and HPT. The index date for the study cohort was the HPT diagnosis date.
Figure 1.
Flow of patient enrolment. RFCIP, Registry for Catastrophic Illness Patients; ESRD, end-stage renal disease; HPT, hyperparathyroidism.
ICD-9-CM codes of HPT were employed to divide the included patients into the ESRD-HPT (study) and ESRD-non-HPT (comparison) cohorts. Then, all ESRD patients with HPT were matched 1:2 by sex, age, urbanization level, and income level with ESRD patients without HPT, who then formed the comparison cohort. An index date matching that of the study cohort was allocated to the comparison cohort.
The main outcome here was VD occurrence (ICD-9-CM 478.5, 478.7, and 784.5). Both cohorts were followed from the index date to VD diagnosis, death, or December, 2013, whichever occurred first.
Comorbidities were defined using the ICD-9-CM codes noted in the claims data: diabetes mellitus (DM; ICD-9-CM 250), hypertension (HTN; ICD-9-CM 401-405), chronic obstructive pulmonary disease (COPD; ICD-9-CM 491, 492, 494, and 496), liver cirrhosis (LC; ICD-9-CM 571.2, 571.5–571.6), asthma (ICD-9-CM 493), chronic rhinosinusitis (CRS; ICD-9-CM 473) and gastroesophageal reflux disease (GERD; ICD-9-CM 530.11 and 530.81)23–26. A comorbidity was included if it appeared in at least 1 inpatient diagnosis or at least 3 outpatient diagnoses.
Part II–clinical study
For the clinical study, we prospectively recruited patients with SHPT undergoing hemodialysis. The exclusion criteria were past history of vocal mucosal lesion (e.g., nodules or polyps) and vocal fold paralysis as well as current diagnoses of other vocal etiologies (e.g., severe asthma) or other systemic diseases. Of the enrolled hemodialysis patients with SHPT, patients with had refractory symptomatic SHPT received total parathyroidectomy and autotransplantation, which were successful.
The vocal functions within 2 weeks before surgery and 3 months after surgery were documented through VSL on a stroboscopy system (Model 9400) from KayPENTAX (Lincoln Park, NJ, USA). VSL and voice analysis were performed on a day between dialysis sessions. For analysis of the laryngeal image, the following data were considered: absence/presence of vocal fold lesion; wave of vocal fold mucosa; pattern of glottal closure and any involvement of supraglottic structures. The reports from laryngeal examination before and after surgery of each patient were taken from two laryngologists. If there was any difference between two reports from two laryngologists, reevaluation was necessary.
We measured acoustic parameters including jitter, shimmer, mean fundamental frequency (F0), and noise-to-harmonics ratio (NHR) by using a Computerized Speech Laboratory (CSL) system (Model 4500; KayPentax, Lincoln Park, NJ, USA)27,28. The participants were asked to phonate the sustained vowel /a/ at their habitual pitch and comfortable loudness, after inhaling deeply. The acoustic parameters of the voice, including fundamental frequency (Hz), jitter (%), shimmer (dB), and noise-harmonic ratio (NHR; dB), were evaluated. The phonation time was 3 s and a segment from the middle of the vowel phonation was analyzed, and bad voice quality data was discarded. Here, F0 is the average of all extracted fundamental frequency values. Shimmer (%) was defined as the average absolute difference between peak amplitudes of consecutive periods divided by the average peak amplitudes during the phonatory segment. Jitter (%) was defined as the average absolute difference between successive periods divided by the average period duration. Moreover, here, the NHR reflected the relative spectral energy contributions of the noise and harmonic components of the acoustic voice signal29.
Moreover, aerodynamic parameters including maximum phonation time (MPT) and durations of the sounds “s” and “z” were analyzed by a speech pathologist using a stopwatch27. Here, the sound “s” is voiceless (i.e., produced without vibration of the vocal folds), whereas the sound “z” is a voiced counterpart of the sound “s”30. The voice of patients who underwent parathyroidectomy was analyzed 1 or 2 weeks before surgery and then 3 months after surgery.
Preoperative and postoperative perceptual evaluation was performed using the GRBAS scoring system (G = grade, R = roughness, B = breathiness, A = asthenia, and S = strain; 0 = normal, 1 = mild, 2 = moderate, and 3 = severe). The GRBAS-sum is the summation of each GRBAS score, and reevaluation was required if a difference of more than 2 points was recorded between the two GRBAS scores. A speech pathologist and an otolaryngologist analyzed the above-mentioned voice parameters in a double-blinded manner. All participants in this study gave written informed consent prior to study begin. The experiment was conducted in line with the relevant guidelines and regulations.
Both parts of this study were approved by the Institutional Review Board of Chang Gung Memorial Hospital (IRB No. 201800401B0).
Statistical analysis
For Part I of this study, we compared the comorbidities and demographic characteristics of the study and comparison cohorts; here, we used Pearson’s chi-square test and an unpaired Student t test to compare categorical and continuous variables, respectively31. Next, we included control variables such as sex, age, income level, urbanization level, and comorbidities as covariates in our univariate model to perform a univariate analysis32. The variables with p < 0.1 in the univariate analysis were included in the multivariate analysis. The cumulative incidence of VD in the 2 cohorts was estimated using a Kaplan–Meier analysis, and then, a two-tailed log-rank test was used determine the relevant differences between the cohorts. We also used multivariable Cox proportional hazard regression models to measure the hazard ratios (HRs) and corresponding 95% CIs for VD. The stability of HRs was also examined using subgroup and sensitivity analyses so as to evaluate whether the comorbidity–SHPT interaction effects on VD were significant.
For Part II of this study, paired t and Wilcoxon signed-rank tests were used to analyze the statistical significance of our parametric and nonparametric measurement data, respectively28. Pearson correlation coefficient analysis was also applied28.
The online version contains supplementary material available at 10.1038/s41598-020-79810-0. All statistical analyses were performed using SAS (version 9.4; SAS Institute, Cary, NC, USA); and significance level was set at p < 0.05.
Results
Part I–real-world data study
After the 1:2 matching of sex, age, urbanization level, and income level, the study and comparison cohorts contained 7988 patients with HPT (mean ± standard deviation [SD] follow-up period: 4.6 ± 3.3 year) and 15,976 patients without HPT (mean ± SD observation period: 4.5 ± 3.3 year), respectively (Table 1). The study cohort had a significantly higher DM, COPD, CRS, and GERD prevalence than did the comparison cohort. VD incidence rate was also significantly higher in the study cohort (p = 0.003; 2.2 vs 1.4 per 1000 person-years). The incidence rate ratio of VD thus was 1.56 (95% CI 1.17–2.09; p = 0.003), and the mean duration from HPT diagnosis to VD development was 2.9 ± 2.3 years.
Table 1.
Demographic Characteristics of the Study and Comparison Cohorts.
| Characteristic | ESRD-HPT | ESRD-Non-HPT | p value | |||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Total | 7798 | 15,976 | ||||
| Sex | 1.000* | |||||
| Male | 3176 | 39.8 | 6352 | 39.8 | ||
| Female | 4812 | 60.2 | 9624 | 60.2 | ||
| Age (y) | 1.000* | |||||
| < 65 | 6148 | 77.0 | 12 296 | 77.0 | ||
| ≥ 65 | 1840 | 23.0 | 3680 | 23.0 | ||
| Urbanized level | 1.000* | |||||
| 1 (City) | 2320 | 29.0 | 4640 | 29.0 | ||
| 2 | 3837 | 48.0 | 7674 | 48.0 | ||
| 3 | 1116 | 14.0 | 2232 | 14.0 | ||
| 4 (Village) | 715 | 9.0 | 1430 | 9.0 | ||
| Income† | 1.000* | |||||
| 0 | 1483 | 18.6 | 2966 | 18.6 | ||
| 1-15 840 | 1259 | 15.8 | 2518 | 15.8 | ||
| 15 841-25 000 | 3769 | 47.2 | 7538 | 47.2 | ||
| ≥ 25 001 | 1477 | 18.5 | 2954 | 18.5 | ||
| Comorbidities | ||||||
| DM | 2729 | 34.2 | 8875 | 55.6 | < .001** | |
| HTN | 7491 | 93.8 | 15 031 | 94.1 | .347** | |
| COPD | 1455 | 18.2 | 2650 | 16.6 | .002** | |
| Liver cirrhosis | 596 | 7.5 | 1300 | 8.1 | .068** | |
| Asthma | 843 | 10.6 | 1628 | 10.2 | .384** | |
| CRS | 324 | 4.1 | 558 | 3.5 | .029** | |
| GERD | 2031 | 25.4 | 3331 | 20.9 | < .001** | |
| Outcome | ||||||
| VD | 82 | 2.2 | 102 | 1.4 | .003** | |
ESRD, end-stage renal disease; HPT, hyperparathyroidism; DM, diabetes mellitus; HTN, hypertension; COPD, chronic obstructive pulmonary disease; LC, liver cirrhosis; CRS, chronic rhinosinusitis; GERD, gastroesophageal reflux disease; VD, vocal dysfunction.
*Pearson chi-squared test; **Student t test; †New Taiwan Dollar, per month.
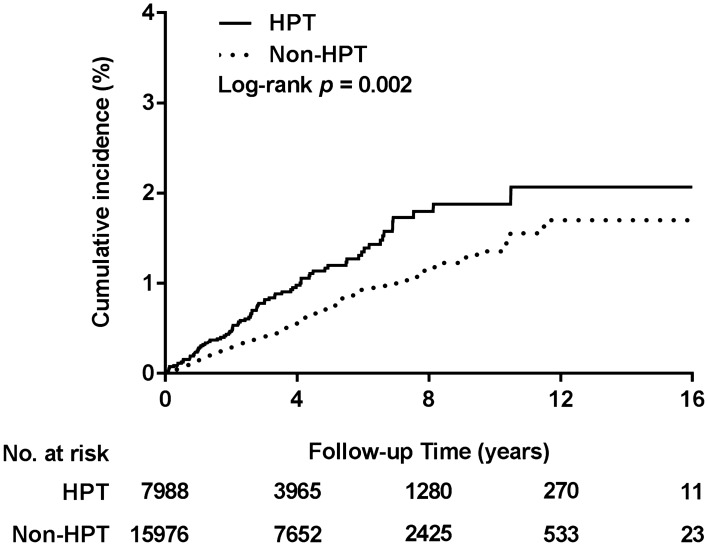
According to the Kaplan–Meier analysis results, the cumulative incidence of VD was significantly higher in the study cohort than in the comparison cohort (log-rank test, p = 0.002; Fig. 2). The results of Cox proportional hazard model analysis (Table 2) for VD risk demonstrated that HPT risk was approximately 1.6 times higher in the study cohort than in the comparison cohort (adjusted HR [95% CI] for the main model: 1.56 [1.17–2.09], adjusted HR [95% CI] for the full model: 1.59 [1.18–2.14]; both p = 0.003). In the sensitivity analyses, by alternately adding one comorbidity in the main model, we noted a considerable, constant risk of VD in the study cohort. Moreover, the subgroup analysis results revealed that interaction effects of HPT with sex, age, and comorbidities were nonsignificant for VD risk.
Figure 2.
Cumulative incidence of voice dysfunction. ESRD, end-stage renal disease; HPT, hyperparathyroidism.
Table 2.
Multivariable Cox Proportional Hazards Regression Model of the Association Between VD and Potential Risk Factors.
| Variables | Adjusted HR (95% CI) | p value |
|---|---|---|
| Multivariable regression analysis | ||
| Non-HPT | Reference | |
| HPT (main model†) | 1.56 (1.17–2.09) | .003 |
| HPT (full model‡) | 1.59 (1.18–2.14) | .003 |
| Sensitivity analysis§ | ||
| Main model + DM | 1.56 (1.16–2.10) | .004 |
| Main model + COPD | 1.57 (1.17–2.10) | .003 |
| Main model + LC | 1.56 (1.17–2.09) | .003 |
| Main model + CRS | 1.56 (1.16–2.08) | .003 |
| Main model + GERD | 1.58 (1.18–2.11) | .002 |
| Subgroup analysis | ||
| Sex | ||
| Female | 1.35 (0.93–1.98) | .116 |
| Male | 1.94 (1.22–3.06) | .005 |
| Age | ||
| < 65 | 1.66 (1.20–2.29) | .002 |
| ≥ 65 | 1.22 (0.62–2.41) | .571 |
| DM | ||
| No | 1.87 (1.25–2.78) | .002 |
| Yes | 1.19 (0.73–1.95) | .476 |
| COPD | ||
| No | 1.68 (1.22–2.29) | .001 |
| Yes | 1.10 (0.50–2.41) | .813 |
| LC | ||
| No | 1.51 (1.12–2.04) | .007 |
| Yes | 2.41 (0.73–7.92) | .147 |
| CRS | ||
| No | 1.67 (1.24–2.24) | .001 |
| Yes | 0.37 (0.08–1.76) | .213 |
| GERD | ||
| No | 1.75 (1.26–2.41) | .001 |
| Yes | 1.02 (0.52–2.00) | .953 |
HR, hazard ratio.
†Main model was adjusted for sex, age, urbanization level, and income level.
‡Full model was adjusted for sex, age, urbanization level, income level, and comorbidities.
§The models were adjusted for covariates in the main model as well as for each additional listed comorbidity.
Part II—clinical study
We enrolled 60 hemodialysis patients with SHPT, comprising 25 men and 35 women (mean ± SD age 52.84 ± 11.52 [range 26–80] years; mean ± SD dialysis duration: 82.3 ± 24.2 [range 32–153] months; Table 3), half of whom complained of hoarseness or other laryngeal symptoms. Of them, 45 received total parathyroidectomy and autotransplantation.
Table 3.
Characteristics of Enrolled Patients with ESRD (N = 60).
| Characteristic | Number |
|---|---|
| Sex | |
| Male (n) | 25 |
| Female (n) | 35 |
| Mean age (range) | 52.96 (26—80) |
| Plasma level | |
| Calcium (mg/dL) | 10.00 ± 1.03 |
| Phosphorous (mg/dL) | 5.33 ± 1.34 |
| iPTH (pg/mL) | 1216.16 ± 767.03 |
| Aerodynamic† | |
| MPT (s) | 13.39 ± 5.68 |
| s (s) | 14.57 ± 7.30 |
| z (s) | 14.80 ± 7.30 |
| Acoustic‡ | |
| F0 (Hz) | 177.99 ± 43.11 |
| Jitter (%) | 1.41 ± 0.84 |
| Shimmer (dB) | 0.38 ± 0.18 |
| NHR | 0.13 ± 0.03 |
iPTH, intact parathyroid hormone; F0, fundamental frequency; MPT, maximum phonation time; s, voiceless “s” produced without vibration of vocal folds; z, voiced “z” produced with vibration of vocal folds; NHR, noise-to-harmonics ratio.
†Aerodynamic parameters with stop watch.
‡Acoustic parameters in a Computerized Speech Laboratory.
In all included 60 patients, the mean ± SD plasma calcium, phosphorus, and iPTH levels were 10.00 ± 1.03 mg/dL, 5.33 ± 1.34 mg/dL, and 1216.16 ± 767.03 pg/mL, respectively. Regarding voice-associated parameters measured through CSL, the mean ± SD values of F0, jitter, shimmer, and NHR were 177.99 ± 43.11 Hz, 1.41% ± 0.84%, 0.38 ± 0.18 dB, and 0.13 ± 0.03, respectively; moreover, the mean ± SD MPT, “s” sound duration, and “z” sound duration were 13.39 ± 5.68, 14.57 ± 7.30, and 14.80 ± 7.30 s, respectively.
Of the 45 patients with SHPT who underwent total parathyroidectomy, 32 patients completed the preoperative and postoperative vocal evaluations through VSL and voice analysis.
There was no significant difference in laryngeal analysis between pre and post operation. No vocal fold lesion and no glottal gap was found among 32 patients before and after operation. The wave of vocal fold mucosa was normal and symmetric among these 32 patients. No supraglottic constriction was found in the study.
Table 4 lists the levels of calcium, phosphorus, and iPTH in these patients, all of which significantly decreased after surgery. In the objective vocal measurements, F0, jitter, and shimmer values demonstrated significant improvements postoperatively (F0: 178.0 ± 42.9 vs 236.5 ± 83.7 Hz, p = 0.01; jitter: 1.41% ± 0.84% vs 0.84% ± 0.35%, p = 0.047; shimmer: 0.38 ± 0.18 vs 0.24 ± 0.09 dB, p = 0.033). However, the preoperative and postoperative differences in perceptual evaluation and aerodynamic parameters, including MPT and duration of “s” and “z” sounds, were nonsignificant.
Table 4.
Changes in Plasma Levels of SHPT Indicators, voice analysis, and Subjective Voice Assessment Results Before and 3 Months After Parathyroidectomy.
| Variables | Preoperative | Postoperative | p value |
|---|---|---|---|
| Plasma levels | |||
| Calcium (mg/dL) | 10.00 ± 1.03 | 8.24 ± 1.44 | .000* |
| Phosphorus (mg/dL) | 5.33 ± 1.34 | 3.72 ± 1.55 | .040* |
| iPTH (pg/mL) | 1216.23 ± 767.05 | 88.73 ± 166.51 | .000* |
| Aerodynamic† | |||
| F0 (Hz) | 178.03 ± 42.91 | 236.55 ± 83.79 | .010* |
| Male | 146.85 ± 34.67 | 167.09 ± 48.07 | .267 |
| Female | 203.92 ± 38.24 | 231.44 ± 36.14 | .178 |
| MPT (s) | 13.39 ± 5.64 | 14.48 ± 3.85 | .922 |
| s (s) | 11.91 ± 5.70 | 13.17 ± 9.84 | .988 |
| z (s) | 14.57 ± 7.25 | 14.93 ± 4.65 | .744 |
| Acoustic‡ | |||
| Jitter (%) | 1.41 ± 0.84 | 0.84 ± 0.35 | .047* |
| Shimmer (dB) | 0.38 ± 0.18 | 0.24 ± 0.09 | .033* |
| NHR | 0.13 ± 0.03 | 0.13 ± 0.03 | .342 |
| Perceptual | |||
| GRBAS Sum | 0.464 ± 0.63 | 0.273 ± 0.47 | .344 |
iPTH, intact parathyroid hormone; F0, fundamental frequency; MPT, maximum phonation time; s, voiceless “s” produced without vibration of vocal folds; z, voiced “z” produced with vibration of vocal folds; NHR, noise-to-harmonics ratio.
†Aerodynamic parameters through video strobe laryngoscopy.
‡Acoustic parameters through Computerized Speech Laboratory.
*Student t test.
Discussion
ESRD-complicated with SHPT can influence the calcium and phosphorus balance, potentially leading to fine vocal muscle weakness and consequent functional vocal disturbances. Here, by using real-world and clinical data, we proved the aforementioned hypothesis and for the first time investigated the influence of HPT in voice change. Our results confirmed that SHPT in patients with ESRD is a predisposing factor for VD, such that ESRD patients with SHPT have an approximately 1.6 times higher VD risk than do ESRD patients without SHPT.
Insufficient water in the body may lead to decrease in the water content of the vocal cords and decrease in the secretion of the respiratory tract. The drying of the vocal cord surface may lead to changes in voice quality. Therefore, patients on hemodialysis often experience transient hoarseness at the end of dialysis33–35.
To clarify whether the VD was due to dialysis-associated dehydration or SHPT, we used a nationwide database and enrolled hemodialysis patients and divided them to cohorts of patients with and without HPT to investigate VD occurrence. Consequently, we could eliminate dialysis-associated VD as the causative mechanism, confirming HPT as a risk factor for VD.
Calcium blocks sodium ion flux through voltage-gated sodium channels. Thus, hypercalcemia results in inhibition of depolarization and impairment of action potential generation in neurons and muscle cells36. Moreover, hypercalcemia can damage the recurrent laryngeal nerve and intrinsic laryngeal muscle, and this can result in dysphonia. These findings support our hypothesis that iPTH influences vocal production.
On the basis of Taiwan Renal Registry Data System, a large-scale study reported that a serum phosphorus level of ≥ 6.5 mg/dL was associated with increased mortality37. However, a significant imbalance of such electrolytes can be life-threatening.
Thus, when hemodialysis patients report changes in their voices, clinicians must include SHPT in the differential diagnosis. If in doubt, plasma phosphorus, calcium and iPTH levels could be estimated. Taken together, these steps could facilitate early detection of SHPT.
In our current clinical study, SHPT was found to significantly increase plasma calcium, phosphate, and iPTH levels before surgery. In the voice analysis after surgery, only the acoustic indices F0, jitter, and shimmer demonstrated significant differences. Studies have reported that abnormal vocal cord lesions typically increase jitter and shimmer. If Jitter exceeds the threshold of 1% and shimmer exceeds the threshold of 0.35 dB, abnormal performance is regarded to have occurred38. In the current study, the mean preoperative jitter was 1.41% (i.e., > 1%), but normalized to 0.84 postoperatively; similarly, the mean preoperative shimmer was 0.38 dB (i.e., > 0.35), but normalized to 0.24 dB postoperatively. Therefore, we speculated that SHPT mainly causes changes in the acoustic indices jitter and shimmer in the voice analysis, with the thresholds identical to those reported previously; this is a clinically significant observation. Jitter and shimmer represent variations in vocal fold vibrations. The change in vibration may result from changes in vocal muscle strength. An improvement (i.e., decrease) in the acoustic parameters produces a more harmonic voice.
This study had several strengths. First, we analyzed a large amount of data from numerous dialysis patients. Second, we verified the real-world evidence regarding HPT as an independent risk factor for VD. We also analyzed the clinical data of ESRD patients with and without SHPT and even evaluated their data before and after parathroidectomy to further verify the relationship between SHPT and voice disorders. Consequently, we provided reference indexes for clinicians when performing vocal examination through VSL and voice analysis.
Our study however has limitations related to real-world data investigations. First, the NHIRD does not provide patient data for clinical symptoms, laboratory data, and VSL results; therefore, we could not determine the VD severity in the included patients. Nevertheless, in our clinical study, we performed clinical examinations to obtain the data unavailable in the NHIRD. Although only ESRD patients with SHPT were enrolled in this study, our research could still provide clinical evidence supporting our hypothesis. In future studies, laryngeal electromyography (LEMG) should be applied to evaluate laryngeal muscle activity.
Based on our current findings, the use of VSL examinations and voice analysis is ideal for analyzing ESRD patients with voice disturbances. Moreover, the abnormal acoustic indices could be used for speculating whether SHPT may be an underlying cause of VD. Nevertheless, future studies should confirm the accuracy and sensitivity of these factors. Moreover, voice changes after nonsurgical treatment of SHPT warrant further research.
In conclusion, by using real-world and clinical patient data, we confirmed that SHPT is a predisposing factor for VD. In addition, the comparative results before and after surgery indicated that the acoustic indices jitter and shimmer in voice analysis might indicate the influence of SHPT on the voice of patients with ESRD. Thus, when a clinician encounters a patient with ESRD complaining of voice disturbances, they must consider including SHPT in the differential diagnosis to facilitate early detection and treatment of the underlying problem.
Supplementary Information
Acknowledgements
This study received support in the form of grants from the Chiayi Branch of Chang Gung Memorial Hospital in Taiwan (project numbers: CMRPG6G0421, CMRPG6G0422, CMRPG6G0423 and CMRPG6J0251). The authors wish to thank the Health Information and Epidemiology Laboratory (CLRPG6G0041) of Chiayi Chang Gung Memorial Hospital for data analysis, statistical analysis, and discussions. The National Health Insurance Research Database used in this study was provided by Taiwan’s National Health Research Institutes.
Author contributions
C.-M.H., G.-H.C., F.-F.C. and M.-S.T. wrote the main manuscript text and tables, Y.-T.T., E.I.H. and M.-Y.Y. prepared Figs. 1–2. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-020-79810-0.
References
- 1.Dusso A, Lopez-Hilker S, Rapp N, Slatopolsky E. Extra-renal production of calcitriol in chronic renal failure. Kidney Int. 1988;34:368–375. doi: 10.1038/ki.1988.190. [DOI] [PubMed] [Google Scholar]
- 2.Sherwood LM. Vitamin D, parathyroid hormone, and renal failure. N. Engl. J. Med. 1987;316:1601–1603. doi: 10.1056/NEJM198706183162511. [DOI] [PubMed] [Google Scholar]
- 3.St John A, Thomas M, Dick I, Young P, Prince RL. Parathyroid function in mild to moderate renal failure: evaluation by oral calcium suppression test. J. Clin. Endocrinol. Metab. 1994;78:1436–1438. doi: 10.1210/jcem.78.6.8200947. [DOI] [PubMed] [Google Scholar]
- 4.Ballinger AE, Palmer SC, Nistor I, Craig JC, Strippoli GF. Calcimimetics for secondary hyperparathyroidism in chronic kidney disease patients. Cochrane Database Syst. Rev. 2014 doi: 10.1002/14651858.CD006254.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Santo RM, et al. The high prevalence of alexithymia in hemodialyzed patients with secondary hyperparathyroidism unsuppressed by medical therapy is cured by parathyroidectomy. J. Ren. Nutr. 2010;20:S64–70. doi: 10.1053/j.jrn.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Mittman N, Desiraju B, Meyer KB, Chattopadhyay J, Avram MM. Treatment of secondary hyperparathyroidism in ESRD: a 2-year, single-center crossover study. Kidney Int. Suppl. 2010 doi: 10.1038/ki.2010.191. [DOI] [PubMed] [Google Scholar]
- 7.Chan GC, Lee PC, Kwan LP, Yip TP, Tang SC. Acute thyroiditis: an under-recognized complication of parathyroidectomy in end-stage renal failure patients with secondary hyperparathyroidism. Nephrology (Carlton) 2017;22(572):2017. doi: 10.1111/nep.12825. [DOI] [PubMed] [Google Scholar]
- 8.Ford EJ, Chandran PK, Graefe HH. Parathyroid calcification as a complication of secondary hyperparathyroidism. Nephron. 1991;57:498–499. doi: 10.1159/000186364. [DOI] [PubMed] [Google Scholar]
- 9.Prodanovic N, Spiric Z, Trninic G, Eric M. Digital clubbing as an unusual complication of the secondary hyperparathyroidism associated with atypical neutrophils: a case report. Eur. Rev. Med. Pharmacol. Sci. 2012;16(Suppl 4):98–102. [PubMed] [Google Scholar]
- 10.Chou FF, Lee CH, Chen JB, Huang SC, Lee CT. Sleep disturbances before and after parathyroidectomy for secondary hyperparathyroidism. Surgery. 2005;137:426–430. doi: 10.1016/j.surg.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Chou FF, Lee CH, Chen JB. General weakness as an indication for parathyroid surgery in patients with secondary hyperparathyroidism. Arch. Surg. 1999;134:1108–1111. doi: 10.1001/archsurg.134.10.1108. [DOI] [PubMed] [Google Scholar]
- 12.Cordellat IM. Hyperparathyroidism: primary or secondary disease? Reumatol. Clin. 2012;8:287–291. doi: 10.1016/j.reuma.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Hajjar K, Hagenacker T. Neuromuscular disorder as initial manifestation of secondary hyperparathyroidism: a case report. Eur. J. Transl. Myol. 2017;27:6100. doi: 10.4081/ejtm.2017.6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sawant A, Garland SJ, House AA, Overend TJ. Morphological, electrophysiological, and metabolic characteristics of skeletal muscle in people with end-stage renal disease: a critical review. Physiother. Can. 2011;63:355–376. doi: 10.3138/ptc.2010-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.NHRI. Sampling method and representativeness of Longitudinal Health Insurance Database (LHID). (Accessed October 2017).
- 16.Chang G-H, et al. Real-world database examining the association between sjögren’s syndrome and chronic rhinosinusitis. J. Clin. Med. 2019;8:155. doi: 10.3390/jcm8020155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai YT, et al. Risk of acute epiglottitis in patients with preexisting diabetes mellitus: a population-based case-control study. PLoS ONE. 2018;13:e0199036. doi: 10.1371/journal.pone.0199036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pasieka JL, Parsons LL. A prospective surgical outcome study assessing the impact of parathyroidectomy on symptoms in patients with secondary and tertiary hyperparathyroidism. Surgery. 2000;128:531–539. doi: 10.1067/msy.2000.108117. [DOI] [PubMed] [Google Scholar]
- 19.Tsai MS, et al. Sleep apnea and risk of vertigo: a nationwide population-based cohort study. The Laryngoscope. 2018;128:763–768. doi: 10.1002/lary.26789. [DOI] [PubMed] [Google Scholar]
- 20.Chang GH, et al. End-stage renal disease: a risk factor of deep neck infection—a nationwide follow-up study in Taiwan. BMC Infect. Dis. 2017;17:424. doi: 10.1186/s12879-017-2531-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang G-H, et al. High risk of deep neck infection in patients with type 1 diabetes mellitus: a nationwide population-based cohort study. J. Clin. Med. 2018;7:385. doi: 10.3390/jcm7110385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arnett FC, et al. The american rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 23.Lin YH, Chang TS, Yao YC, Li YC. Increased risk of chronic sinusitis in adults with gastroesophgeal reflux disease: a nationwide population-based cohort study. Medicine. 2015;94:e1642. doi: 10.1097/MD.0000000000001642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su VY, et al. Chronic obstructive pulmonary disease associated with increased risk of bipolar disorder. Chronic Respir. Dis. 2017;14:151–160. doi: 10.1177/1479972316680846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang GH, et al. Real-world database examining the association between Sjogren's syndrome and chronic rhinosinusitis. J Clin Med. 2019 doi: 10.3390/jcm8020155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu CF, et al. Increased risk of deep neck infection among HIV-infected patients in the era of highly active antiretroviral therapy–a population-based follow-up study. BMC Infect. Dis. 2013;13:183. doi: 10.1186/1471-2334-13-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xue C, Kang J, Hedberg C, Zhang Y, Jiang JJ. Dynamically monitoring vocal fatigue and recovery using aerodynamic, acoustic, and subjective self-rating measurements. J. Voice Offic. J. Voice Found. 2019 doi: 10.1016/j.jvoice.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 28.Tsai MS, et al. Autologous thyroid cartilage graft implantation in medialization laryngoplasty: a modified approach for treating unilateral vocal fold paralysis. Sci. Rep. 2017;7:4790. doi: 10.1038/s41598-017-05024-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehta DD, Deliyski DD, Zeitels SM, Quatieri TF, Hillman RE. Voice production mechanisms following phonosurgical treatment of early glottic cancer. Ann. Otol. Rhinol. Laryngol. 2010;119:1–9. doi: 10.1177/000348941011900101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eckel FC, Boone DR. The S/Z ratio as an indicator of laryngeal pathology. J. Speech Hear Disord. 1981;46:147–149. doi: 10.1044/jshd.4602.147. [DOI] [PubMed] [Google Scholar]
- 31.Tsai MS, et al. Unilateral vocal fold paralysis and risk of pneumonia: a nationwide population-based cohort study. Otolaryngol. Head Neck Surg. 2018;158:896–903. doi: 10.1177/0194599818756285. [DOI] [PubMed] [Google Scholar]
- 32.Chang GH, et al. High risk of deep neck infection in patients with type 1 diabetes mellitus: a nationwide population-based cohort study. J. Clin. Med. 2018 doi: 10.3390/jcm7110385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sagiroglu S, Doganer A. The effect of electrolyte balance on the voice in hemodialysis patients. Eur. Arch. Otorhinolaryngol. 2018;275:2755–2761. doi: 10.1007/s00405-018-5098-x. [DOI] [PubMed] [Google Scholar]
- 34.Kumar RB, Bhat JS. Voice in chronic renal failure. J. Voice. 2010;24:690–693. doi: 10.1016/j.jvoice.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Jung SY, et al. Voice change in end-stage renal disease patients after hemodialysis: correlation of subjective hoarseness and objective acoustic parameters. J. Voice. 2014;28:226–230. doi: 10.1016/j.jvoice.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 36.Koketsu K, Nishi S, Soeda H. Effects of calcium ions on prolonged action potentials and hyperpolarizing responses. Nature. 1963;200:786–787. doi: 10.1038/200786a0. [DOI] [PubMed] [Google Scholar]
- 37.Liu CT, et al. Roles of serum calcium, phosphorus, PTH and ALP on mortality in peritoneal dialysis patients: a nationwide, population-based longitudinal study using TWRDS 2005–2012. Sci. Rep. 2017;7:33. doi: 10.1038/s41598-017-00080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teixeira JP, Oliveira C, Lopes C. Vocal acoustic analysis-jitter, shimmer and HNR parameters. Proc. Technol. 2013;9:1112–1122. doi: 10.1016/j.protcy.2013.12.124. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.