Abstract
In recent years, increasing the midsole bending stiffness (MBS) of running shoes by embedding carbon fibre plates in the midsole resulted in many world records set during long-distance running competitions. Although several theories were introduced to unravel the mechanisms behind these performance benefits, no definitive explanation was provided so far. This study aimed to investigate how the function of the gastrocnemius medialis (GM) muscle and Achilles tendon is altered when running in shoes with increased MBS. Here, we provide the first direct evidence that the amount and velocity of GM muscle fascicle shortening is reduced when running with increased MBS. Compared to control, running in the stiffest condition at 90% of speed at lactate threshold resulted in less muscle fascicle shortening (p = 0.006, d = 0.87), slower average shortening velocity (p = 0.002, d = 0.93) and greater estimated Achilles tendon energy return (p ≤ 0.001, d = 0.96), without a significant change in GM fascicle work (p = 0.335, d = 0.40) or GM energy cost (p = 0.569, d = 0.30). The findings of this study suggest that running in stiff shoes allows the ankle plantarflexor muscle–tendon unit to continue to operate on a more favourable position of the muscle’s force–length–velocity relationship by lowering muscle shortening velocity and increasing tendon energy return.
Subject terms: Biophysics, Anatomy
Introduction
In recent years, world records were set at long-distance running competitions over 5 km, 15 km, half-marathon, marathon, and 100 km distances while running in the Nike Vaporfly 4% or Nike Vaporfly Next% shoes (note: subsequently referred to as ‘Vaporfly’). Several studies investigated the effects of running in the Vaporfly shoes on biomechanical and physiological variables1–5. Compared to other footwear, running in the Vaporfly shoes (1) improved running economy by 4% on average1,4,5, (2) decreased the negative work and peak extension (i.e., dorsiflexion) angle at the metatarsophalangeal (MTP) joint2, (3) decreased the positive and negative work, and peak plantarflexion moment and dorsiflexion angle at the ankle joint2, and (4) increased stance times1,4. At the time when the long-distance running world records were set, the Vaporfly shoes possessed features that significantly differentiated them from other competitor’s running footwear. The main features included (1) a single curved carbon fibre plate embedded in the midsole, (2) highly resilient midsole material, and (3) a greater stack height (i.e., midsole thickness)6. The use of carbon fibre plates to increase the longitudinal midsole bending stiffness (MBS) of shoes was first introduced by Stefanyshyn and Nigg7. The MTP joint undergoes substantial extension during the stance phase of running where negative work is performed8. This absorbed energy was hypothesised to be disadvantageous for athletic performance9. One way to inhibit the extension of the MTP joint and therefore reduce the negative work performed was to increase the MBS of shoes7. Stefanyshyn and Fusco10 investigated the effects of MBS on sprinting performance and showed that 87% of all participants performed better when they inserted carbon fibre plates in their own shoes. Furthermore, the level of MBS that resulted in best performance differed between sprinters, suggesting that there is a subject-specific optimum in stiffness, which allows athletes to perform at their best. Roy and Stefanyshyn11 were the first to investigate if increased MBS can be used to improve long-distance running performance. Their findings showed that the mean rate of oxygen consumption () can be lowered when running in a stiffer shoe; however, if MBS was increased too much, rose again, supporting the idea of an optimal level of MBS10.
Although the underlaying mechanisms of improved performance when running in footwear with increased MBS are not well understood, multiple effects were proposed: (1) minimising energy loss at the MTP joint7, (2) storing and returning elastic energy to the athlete12,13, (3) the ‘teeter-totter’ effect14,15, and (4) optimising the force–velocity profile of the ankle plantarflexor muscles16–18. In brief, the principle of minimising energy loss suggests that athletic improvements can be achieved by reducing the energy absorbed at joints due to less eccentric work performed by muscles crossing these joints. The principle of storing and returning elastic energy suggests that elastic strain energy is stored in carbon fibre plates when being bent as the MTP joint undergoes extension. Due to the plate’s hysteresis, a portion of this stored energy is then speculated to be returned to the athlete during the subsequent flexion of the MTP joint. The ‘teeter-totter’ effect suggests that the ground reaction force (GRF) produces a reaction force at the heel due to the curvature of the carbon fibre plate as the centre of pressure travels anteriorly during the stance phase of running. This heel force was speculated to act at the right location, the right time, and with the right frequency19, and therefore potentially improve running economy. The principle of optimising the force–velocity profile of the ankle plantarflexor muscles suggests that the velocity of muscle shortening is reduced due to a change in gear ratio between the external (i.e., distance between GRF and ankle joint centre) and internal (i.e., distance between Achilles tendon (AT) and ankle joint centre) moment arms20. Willwacher et al.16 demonstrated that, compared to a control shoe, running in stiff shoes increased the average GRF moment arm to all lower limb joint centres. The observed effects were greatest at the ankle joint, the major positive work generator in running8. Thus, with a relatively constant internal moment arm, these findings suggest that running in shoes with increased MBS would result in a greater gear ratio. This was speculated to potentially affect the force-generating capacity of the ankle plantarflexor muscles16,17. Takahashi et al.17 showed that the gear ratio was greater and the shortening velocity of the soleus muscle was reduced when walking in shoes with increased MBS. Interestingly, the metabolic cost was increased when walking in the stiff compared to a control condition. Ray and Takahashi21, however, demonstrated that increasing the stiffness of shoes when walking at faster speeds reduced the metabolic cost. This suggested that stiffening elements in shoes had a speed-dependent effect during walking. The fact that metabolic cost increased and altered ankle joint angles were observed in the stiff condition during slow walking is contrary to previously reported findings in running1,2,11, and would therefore suggest that different mechanisms occur between these two tasks. For this reason, Cigoja et al.18 investigated the effects of MBS on force and velocity of the shank muscle–tendon unit (MTU) (i.e., estimate of triceps surae muscle and AT) during running. Compared to control, it was reported that running in a stiff shoe decreased peak shortening velocities of the shank MTU likely due to increased stance times. The authors speculated that these reduced shortening velocities could have implications on the metabolic cost of running and therefore affect long-distance running performance. Any changes in shank MTU shortening velocities due to the use of carbon fibre plates could result in reduced energy cost of the ankle plantarflexor muscles, and thus whole-body metabolic cost. However, due to the methodologies used in their study, Cigoja et al.18 could not address whether the observed differences in shank MTU shortening velocities occurred due to changes at the muscle (i.e., triceps surae) or tendon (i.e., AT).
Thus, the primary purpose of this study was to investigate if the total shortening and the average shortening velocity of the gastrocnemius medialis (GM) muscle is reduced when running in shoes with increased midsole bending stiffness (i.e., Stiff, Stiffer, Stiffest) compared to a control shoe (i.e., Control). It was hypothesised that the total amount of shortening will not differ between footwear conditions because previous studies did not report differences in ankle joint plantarflexion angles2,11,13,22. It was further hypothesised that running in footwear with increased MBS will reduce average GM shortening velocities due to increased stance times. The secondary purpose of this study was to investigate if increased midsole bending stiffness of shoes will affect modelled GM energy cost and AT energy return. It was hypothesised that, compared to control, GM energy cost will decrease with increased MBS due to reduced average GM shortening velocities. We further hypothesised that no difference in AT energy return would be observed between footwear conditions because previous studies suggested that primarily the function of muscles would be affected by changes in MBS of shoes16,17,23.
Results
Energy cost of running
There was no significant effect of MBS on the energy cost of running (Erun; p = 0.298). Overall, two participants had the lowest Erun in Control (i.e., Nike Free Run 2018), three in Stiff (i.e., Control + 1 mm carbon fibre plate), seven in Stiffer (i.e., Control + 1.5 mm carbon fibre plate), and two in Stiffest (i.e., Control + 2 mm carbon fibre plate). There was no difference (p = 0.25, d = 0.13) in Erun between running in the first (4.69 ± 0.29 J kg−1 m−1) and last (4.64 ± 0.25 J kg−1 m−1) condition.
Gastrocnemius medialis muscle and Achilles tendon dynamics
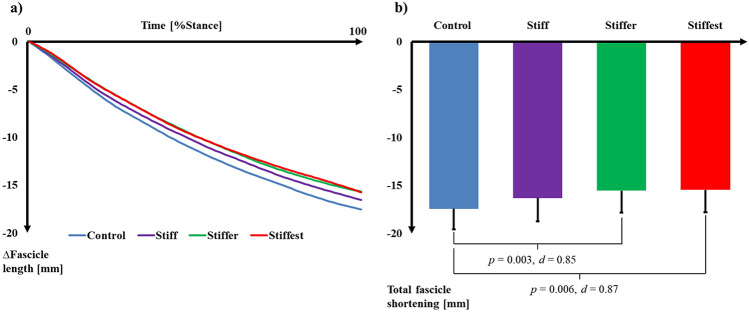
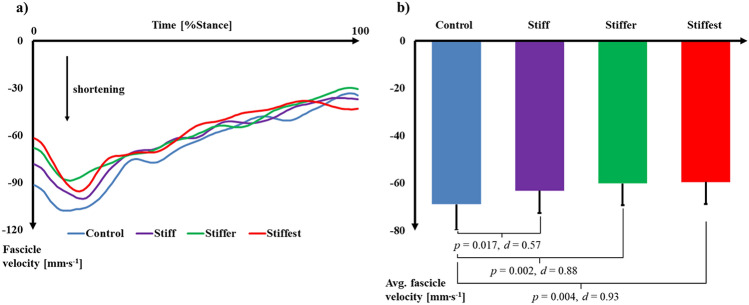
Significant effects of MBS were observed on GM fascicle length at toe-off (p = 0.020), total change of GM fascicle length (p = 0.001), and average fascicle velocity (p = 0.010) during stance. There was no effect of MBS on fascicle length at heel-strike (p = 0.223). After correcting for multiple comparisons (p ≤ 0.017), GM fascicle length at toe-off in Stiff (p = 0.024, d = 0.21), Stiffer (p = 0.021, d = 0.22), and Stiffest (p = 0.021, d = 0.35) was not different compared to Control (Table 1). Pairwise comparisons revealed significant differences in total change of fascicle length between Control and Stiffer (p = 0.003, d = 0.85) and Stiffest (p = 0.006, d = 0.87) (Fig. 1). There were also significant differences in average fascicle velocity between Control and Stiff (p = 0.017, d = 0.57), Stiffer (p = 0.002, d = 0.88), and Stiffest (p = 0.004, d = 0.93) (Fig. 2).
Table 1.
Measured dynamics and modelled energetics (mean ± SD; n = 14) for the gastrocnemius medialis (GM) muscle and Achilles tendon (AT).
| Variable | Control | Stiff | Stiffer | Stiffest |
|---|---|---|---|---|
| Erun (J kg−1 m−1) | 4.85 ± 0.31 | 4.85 ± 0.37 | 4.75 ± 0.36 | 4.85 ± 0.29 |
| GM fascicle length at heel-strike (mm) | 52.0 ± 5.0 | 51.9 ± 5.7 | 51.2 ± 5.4 | 51.7 ± 5.4 |
| GM fascicle length at toe-off (mm) | 34.6 ± 4.9 | 35.6 ± 4.7 | 35.7 ± 4.9 | 36.2 ± 4.7 |
| GM total length change (mm) | − 17.4 ± 2.1 | − 16.3 ± 2.4 | − 15.5 ± 2.3* | − 15.5 ± 2.3* |
| Average GM shortening velocity (mm s−1) | − 69.0 ± 10.9 | − 63.2 ± 9.4* | − 60.1 ± 9.2* | − 59.6 ± 9.2* |
| GM efficiency (%) | 35.5 ± 4.0 | 35.2 ± 4.7 | 35.8 ± 4.5 | 35.2 ± 4.3 |
| GM energy cost (J kg−1) | 0.237 ± 0.043 | 0.226 ± 0.047 | 0.231 ± 0.057 | 0.222 ± 0.054 |
| GM fascicle work (J kg−1) | 0.084 ± 0.015 | 0.078 ± 0.013 | 0.082 ± 0.017 | 0.077 ± 0.017 |
| Peak GM force (N kg−1) | 11.63 ± 1.40 | 11.73 ± 1.29 | 11.94 ± 1.51 | 11.81 ± 1.48 |
| GM force per crossbridge (pN) | 2.55 ± 0.07 | 2.59 ± 0.05 | 2.60 ± 0.05* | 2.61 ± 0.05* |
| In-series sarcomeres (count in 103) | 30.521 ± 3.490 | 31.193 ± 3.812 | 31.019 ± 3.841 | 31.525 ± 3.406 |
| Cycles per crossbridge (count) | 46.01 ± 6.10 | 43.15 ± 5.17 | 41.60 ± 5.59* | 41.22 ± 5.30* |
| AT energy return (J kg−1) | 0.15 ± 0.03 | 0.17 ± 0.04* | 0.18 ± 0.04* | 0.19 ± 0.05* |
| AT force (N kg−1) | 58.96 ± 6.69 | 59.76 ± 6.47 | 60.92 ± 7.68 | 60.38 ± 7.60 |
*Significant (p ≤ 0.017) differences compared to Control.
Figure 1.
(a) Time-normalised, average (n = 14) change in gastrocnemius medialis muscle fascicle length over the stance phase of running relative to the length at heel-strike. (b) Average (± SD; n = 14) total gastrocnemius medialis fascicle shortening over the stance phase of running. p values and Cohen’s d effect sizes denote significant (p ≤ 0.017) differences between conditions.
Figure 2.
(a) Time-normalised, average (n = 14) gastrocnemius medialis muscle fascicle velocity over the stance phase of running. (b) Average (± SD; n = 14) gastrocnemius medialis fascicle velocity over the stance phase of running. p values and Cohen’s d effect sizes denote significant (p ≤ 0.017) differences between conditions.
There was no significant difference between MBS conditions for GM muscle energy cost (p = 0.469). With regards to the estimated GM energy cost during stance, significant effects of MBS were found for estimated average force per crossbridge (p ≤ 0.001), the number of in-series sarcomeres (p = 0.013), and the number of cycles per crossbridge (p = 0.026). Compared to Control, estimated GM force per crossbridge was significantly higher in Stiff (p = 0.010, d = 0.59), Stiffer (p = 0.002, d = 0.83), and Stiffest (p = 0.001, d = 0.96). After correcting for multiple comparisons, the number of in-series sarcomeres did not differ between MBS conditions. The estimated number of crossbridge cycles during stance was significantly lower in Stiffer (p = 0.005 d = 0.75) and Stiffest (p = 0.013, d = 0.84). Achilles tendon energy return was also significantly different between MBS conditions (p ≤ 0.001), where compared to Control more energy was returned in Stiff (p = 0.006, d = 0.64), Stiffer (p ≤ 0.001, d = 0.96), and Stiffest (p ≤ 0.001, d = 1.17). No effects of MBS on peak GM force (p = 0.333), GM fascicle work (p = 0.139) or average AT force (p = 0.143) were observed. Combining the results for GM energy cost and fascicle work, no significant difference (p = 0.184) in GM efficiency was found (Table 1).
There was no significant MBS × %Stance interaction (p = 0.946); however, a significant main effect of both MBS condition (p = 0.046) and %Stance (p ≤ 0.001) was found, suggesting that instantaneous GM muscle energy cost differed significantly across the entire stance phase (Fig. 3). There was a significant difference in instantaneous GM energy cost between MBS conditions during 0–10%Stance (p = 0.003) and 80–90%Stance (p = 0.050).
Figure 3.
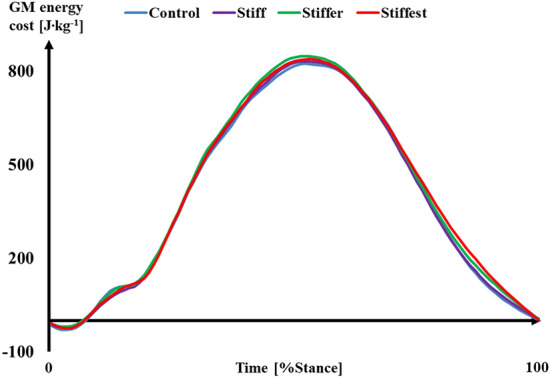
Average (n = 14) gastrocnemius medialis (GM) muscle energy cost over the stance phase of running.
Control vs. Best
When Control was compared to the stiff condition where Erun was lowest (i.e., Best), Erun was significantly (p = 0.04, d = 0.50) improved by 1.97 ± 1.81% (range: -0.79% to 4.71%) in Best. Compared to Control, running in Best resulted in reduced total GM fascicle shortening (Control: − 17.43 ± 2.14 mm, Best: − 15.84 ± 2.18 mm, p = 0.007, d = 0.73), slower average fascicle velocity (Control: − 68.99 ± 10.94 mm s−1, Best: − 61.11 ± 8.95 mm s−1, p = 0.005, d = 0.79), greater AT energy return (Control: 0.15 ± 0.03 J kg−1, Best: 0.18 ± 0.04 J kg−1, p ≤ 0.001, d = 0.96), and increased average AT force (Control: 58.96 ± 6.69 N kg−1, Best: 60.69 ± 6.44 N kg−1, p = 0.035, d = 0.26). Between Control and Best, there were no significant differences in GM energy cost (Control: 0.24 ± 0.04 J kg−1, Best: 0.24 ± 0.06 J kg−1, p = 0.942, d = 0.02) or GM fascicle work (Control: 0.08 ± 0.02 J kg−1, Best: 0.08 ± 0.02 J kg−1, p = 0.928, d = 0.003).
Lower limb kinematics and kinetics
One-way repeated measures ANOVAs and Friedman’s tests revealed significant effects of MBS on stance time (p ≤ 0.001); positive (p ≤ 0.001) and negative (p = 0.009) MTP, positive (p = 0.009) and negative (p = 0.001) ankle, and positive (p = 0.023) and negative (p = 0.013) hip joint work; and maximum MTP (p ≤ 0.001) and ankle (p = 0.021) plantarflexion velocity. Compared to Control, stance times were significantly longer in Stiff (p ≤ 0.001, d = 0.20), Stiffer (p = 0.008, d = 0.21), and Stiffest (p ≤ 0.001, d = 0.31); positive MTP joint work was greater in Stiff (p = 0.017, d = 0.37), Stiffer (p = 0.001, d = 0.88), and Stiffest (p ≤ 0.001, d = 0.94); positive ankle joint work was greater in Stiff (p = 0.008, d = 0.42) and Stiffer (p ≤ 0.001, d = 0.47); positive knee joint work was lower in Stiffest (p = 0.015, d = 0.33); positive hip joint work was greater in Stiffest (p = 0.009, d = 0.30); negative MTP joint work was lower in Stiffest (p = 0.017, d = 0.38); negative ankle joint work was greater in Stiffer (p = 0.004, d = 0.39); negative hip joint work was greater in Stiffest (p = 0.008, d = 0.32); and maximum MTP (p ≤ 0.001, d = 1.24) and ankle (p = 0.017, d = 0.49) plantarflexion velocities were lower in Stiffest. There was no effect of MBS (p = 0.462) on total lower limb joint work (i.e., sum of positive and negative work across all joints) (Supplementary Table S5).
Discussion
The primary purpose of this study was to investigate if the total shortening and the average shortening velocity of the GM muscle is reduced when running in shoes with increased MBS. The hypothesis that total GM fascicle shortening would not be affected by MBS was rejected as running in Stiffer and Stiffest significantly reduced the amount of fascicle shortening compared to Control. The second hypothesis that running in footwear with increased MBS would reduce average GM shortening velocities was accepted. This study provides the first direct evidence that muscle function can be altered by increasing the MBS of running shoes. In fact, the lower GM shortening velocities, in theory, should reduce contractile energy cost as a result of the muscle’s force–velocity relationship24; however, this hypothesis could not be supported here since no significant reductions in the estimated GM muscle energy cost were shown. Although there was a substantial reduction (~ 5%) in estimated GM energy cost when running in Stiffest compared to Control, the differences shown were not significant and of small to medium effect across all conditions. Contrary to our null hypothesis that AT energy return would not be affected by MBS, AT energy return, in fact, was significantly greater in all stiff conditions compared to Control. While GM energy cost was lower in the stiff conditions (Table 1) compared to Control, this difference was not significant. Considerable between-subject variability likely precluded a significant MBS effect on estimated GM energy cost.
Although the GM fascicles shortened less with increased MBS, the sagittal ankle joint angles did not differ between conditions (Supplementary Fig. S4). Therefore, it is likely that the AT performed the outstanding “shortening” of the entire shank MTU resulting in more energy returned. This increased AT energy return when running in stiffer shoes could potentially allow the ankle plantarflexor muscles to continue to operate on a more favourable position of the muscle’s force–length–velocity relationship, reducing the required level of muscle activation to achieve a target force and thus should improve Erun24,25. It is important to consider the potential benefits of running in stiff shoes during runs of longer periods of time than what was measured in this study. It has previously been demonstrated that the Erun progressively increases during a long-distance run26–29. This increase was partially related to the increase in muscle contractile energy cost30 and/or the redistribution of joint work to MTUs surrounding more proximal MTUs (e.g., knee), which are speculated to metabolically be more costly than the triceps surae muscles and AT31. It has been suggested that the tendons of more proximal MTUs are less equipped for elastic energy storage and return31, which would require the muscle in series to perform additional work32. This additional muscle work would require greater active muscle volume, potentially elevating the metabolic cost of running32. Together, running in stiffer shoes may reduce muscle fatigue during long-distance running events, delay the onset of joint work redistribution33, and therefore mitigate progressive increases in Erun normally seen when running in less stiff footwear. Future studies should aim to investigate how in-vivo muscle and tendon dynamics change during a prolonged run in footwear with different MBS.
Based on the findings of Cigoja et al.18, it was expected to find differences in GM shortening velocities towards the end of stance. The highest GM shortening velocities, however, were observed during early stance (~ 0 to 10% of stance). During this period, the generated muscular forces were estimated to be lower compared to later instances of stance due to relatively low GRFs immediately after heel strike. It is therefore speculated that the GM muscle shortened in early stance, while the GRFs are relatively small, to stretch the AT. This “pre-tuning” of the tendon would then allow the AT to stretch even further as the ankle joint undergoes dorsiflexion, and potentially stores elastic energy within the tendon. A portion of this AT elastic energy is speculated to subsequently be returned to the runner.
Although significant effects of MBS on GM shortening and shortening velocity were observed, neither Erun nor GM energy cost differed significantly between stiffness conditions. It is possible that MBS not only affected GM function but also the shortening and/or shortening velocity of other lower limb muscles. Previous studies have shown that MBS can result in a redistribution of lower limb joint work, where more positive work is performed at the MTP joint and less positive work is performed at the knee joint13. Similarly, the findings of this study showed significantly more positive work performed at the MTP and ankle joint and less positive work performed at the knee joint when running in the stiffer conditions (Supplementary Table S5). It is therefore plausible to speculate that not only the function of distal MTUs are affected by MBS but also the MTUs surrounding more proximal joints (e.g., the knee and hip). The MBS may have affected different MTUs in such a manner that “positive” and “negative” effects cancelled each other out so that no differences in whole-body Erun were observed during a 5 min. and 30 s-run. Future studies should attempt to synchronously record muscle fascicle and tendon images of multiple MTUs to investigate if different MTUs are affected differently by the MBS of shoes.
A recently published article investigated the effects of running with differently stiff carbon fibre plates on soleus muscle fascicle dynamics and running economy34. The authors found no systematic differences in soleus fascicle pennation, force, length, velocity, or stride-average soleus active muscle volume between stiffness conditions. Beck et al.34 therefore concluded that running with carbon fibre plates may not improve running economy and long-distance running performance. There were some methodological differences between Beck et al.’s study and ours that need to be considered when comparing the findings. First, the muscle under investigation differed between studies. Although it has been shown that the three triceps surae muscles function similarly during stance (i.e., they consistently shorten throughout ground contact) across a range of running speeds35, Lai et al.35 demonstrated that there can still be differences in fascicle length changes between the soleus and GM muscle. More specifically, the GM muscle shortens to a greater extent than the soleus muscle during running. It is therefore reasonable to speculate that if differences in muscle fascicle shortening and shortening velocity exist when running in various stiffness conditions, then these differences will likely be found at a muscle that undergoes a greater range of length changes. Second, there are considerable morphological differences between the GM and soleus muscle. For example, the GM muscle contains a higher percentage of Type II (or fast-twitch) fibres than the soleus36. The fact that the GM muscle predominantly consists of fast-twitch fibres allows it to generate greater forces during locomotion compared to the soleus37. In-vivo measurements of the GM and soleus forces in an animal model have shown that GM forces increase by a factor of 5 when transitioning from a walking to a running speed37. Peak soleus force increases, however, were negligible. The effects of high running speeds on GM and soleus forces become evidently important when considering that participants in Beck et al.’s study34 were running at a constant speed of 3.5 m s−1; whereas, our participants ran at 90% of individual sLT, which was for only 3 out of 17 participants below 3.5 m s−1.
Upon their release, the Vaporfly shoes varied in many footwear features from conventional running shoes: curved carbon fibre plate, resilient midsole material, high stack height (i.e., midsole thickness), etc. This current study cannot explain how each of these features affected lower limb muscle function and consequently Erun during the most recent world record runs. This study can only address how systematically increasing the MBS of shoes affects GM muscle and AT tendon energetics in running. When Control was compared to Best, a ~ 2% improvement in Erun was observed on average when running in shoes with straight carbon fibre plates. Previous studies reported improvements in Erun of 4% on average when running in the Vaporfly shoes1,3–5. We speculate that similar reductions in total GM shortening and shortening velocity could be observed in Vaporfly compared to ‘conventional’ running footwear. This could potentially explain parts of the 4% metabolic energy savings when running in Vaporfly. The fact that the metabolic savings in Vaporfly shoes were reported to be twice as high as in our study suggests that other mechanisms produced by the Vaporfly shoes contributed to additional metabolic cost savings. One possible characteristic would be the curved design of the carbon fibre plate. Nigg et al.14 suggested that a curved carbon fibre plate could act as a ’teeter-totter’, where a reaction force is created at the heel as the runner’s force is applied at the forefoot and the centre of pressure moves anteriorly, passed the pivot point of the plate. This ’teeter-totter’ mechanism was speculated to be one of the main contributors of improved Erun when running in the Vaporfly shoes and could account for some of the outstanding 2% in metabolic savings of the current study. Although curved plates were speculated to have a different function than the straight plates, the biomechanical findings in this study (e.g., stance times and ankle and MTP joint work, angular velocities, and angles) were comparable to previously reported result when using curved plates1–3. Therefore, it is plausible to speculate that the GM muscle was similarly affected during the recent world record runs. Future studies, however, should investigate how the curvature of carbon fibre plates affects muscle and tendon dynamics during running.
In our study, there were no group effects of MBS on Erun. This is in accordance with some studies34,38,39 but contrary to other studies11,22. More importantly, however, when Control was compared to Best (i.e., plated condition, which resulted in lowest Erun), an average improvement in Erun of 1.97% was observed in the best plated shoe condition. This is in accordance with the findings by Madden et al.38. The authors, at first, demonstrated that there were no differences in running economy between a control and stiff shoe; however, once they split up their runners into responders and non-responders, the responders showed an average improvement in running economy of 2.9% when running in the stiff shoe condition. A 2.9% improvement in running economy would correspond to a performance improvement of 1.93%40 or 2.26%41, which on a 2-h. marathon would correspond to a decrease in running time of ~ 139 or ~ 163 s. Stefanyshyn and Fusco10 showed group improvements in sprinting performance (i.e., in absolute time) of ~ 0.47% when the stiffness of sprinting spikes was increased by inserting straight carbon fibre plates with a stiffness of 47 N·mm−1 in athletes’ own footwear. More importantly, however, the athletes had their individual best performance in different stiffness conditions. When these subject-specific performance responses were taken into account, mean improvements in the best stiffness condition of 1.2% were observed compared to the own shoes. This not only demonstrates that subject-specific performances responses exist when the stiffness of shoes is increased, but also that performance improvements are much greater (i.e., 2.5-fold) when the individual effects are taken into account compared to group differences. Additionally, it shows that although there may11,22 or may not34,38,39 be significant group differences (e.g., Control vs. Stiff vs. Stiffer vs. Stiffest), the true effect of MBS on performance is greatly underestimated if the subject-specific responses are not taken into account. In our study, an average improvement in Erun of 1.97% in Best would correspond to ~ 9440 or ~ 11141 s on a 2-h. marathon. It needs to be acknowledged, however, that a comparison between Control and one of three plated conditions (i.e., Best) increases the probability of obtaining a false positive finding.
There are some limitations and assumptions associated with this study. We used a single GM muscle fascicle as a representation of the entire triceps surae muscle group. It is possible that regional differences in muscle fascicle properties (i.e., distal vs. proximal end of the muscle) exist42. The imaged area of the GM muscle, however, was consistent between footwear conditions within a subject, and therefore it is assumed that regional differences in fascicle properties only minimally affected the interpretation of the results of this study. Further, the force acting through the AT was assumed to be solely generated by the GM muscle. This is not true as other muscles (e.g., gastrocnemius lateralis, soleus, etc.) also contribute to ankle joint plantarflexion. Therefore, the absolute GM force, fascicle work, and energy cost were likely overestimated. In a study of a within-subject design, however, this assumption would not affect the interpretation of the results. Further, it needs to be noted that the AT length changes were not directly assessed during the stance phase, as direct measurements of tendon length changes during running are challenging, and do not fit theoretical expectations43. We calculated tendon length changes from GM muscle fascicle and MTU length changes taking the ankle and knee joint angles into account44. This assumes that the aponeurosis is of equal stiffness as the free tendon, and does not contribute to the storage and return of elastic energy, which may not be true45,46, and could change with the level of muscle activation47. Thus, we have likely underestimated the total amount of energy return by the AT (and aponeurosis), particularly in instances where AT force was high. Lastly, in order to aid in the interpretation of changes in Erun between Control and Best, the smallest worthwhile change (SWC) as measured by the metabolic cart was determined. The SWC for Erun was calculated to be 0.95%. It needs to be noted that not all participants exhibited changes in Erun greater than the SWC. For 9 out of 14 participants, however, the change in Erun between Control and Best was greater than 0.95% (range 1.00–4.71%).
Conclusions
This study is the first to provide direct evidence that gastrocnemius medialis muscle function can be altered when running in shoes with increased midsole bending stiffness. Specifically, when the stiffness of running shoes is increased ‘optimally’, the gastrocnemius medialis shortening velocities were reduced and Achilles tendon energy return was increased. These effects on gastrocnemius medialis muscle and Achilles tendon function could be one of the underlying mechanisms of increased performance when running in shoes with embedded carbon fibre plates.
Materials and methods
Participants
Seventeen trained male runners (mean ± SD; age: 26.6 ± 5.9 years, height: 1.78 ± 0.08 m, mass: 70.7 ± 9.1 kg) visited the laboratory on two separate occasions. Participants were included in this study if they were able to run 10 km in under 44 min., were free from lower limb injuries 6 months prior to testing, and fit a size 9, 10, or 11 US running shoe. This study was approved by the University of Calgary Conjoint Health Research Ethics Board (REB19-1432). All experiments were performed according to the approved protocol and in accordance with the ethical standards of the Declaration of Helsinki. All participants gave written informed consent prior to participating in this study. Where relevant, informed consent was obtained from participants for publication of identifying information/images in an online open-access publication.
Footwear conditions
The control condition (Control) consisted of a Nike Free Run 2018 (Nike Inc., Beaverton, USA) shoe. The stiff conditions were achieved by inserting straight carbon fibre plates of 1 mm (Stiff), 1.5 mm (Stiffer), and 2 mm (Stiffest) thickness between the factory insole and midsole, and along the full length of the shoe. The MBS of the shoes was determined by a 3-point bending test11,13, where the forefoot of the shoe was placed on a custom-made structure with two supporting pins. An indenter was attached to an ElectroPuls E10000 Linear-Torsion testing machine (Instron, Norwood, USA), which applied a compressive force vertically in the area of the MTP joint. The testing machine was set to displace the shoe by 20 mm at a speed of 10 mm s−1. This was repeated ten times for each shoe size and condition. The force and displacement data were filtered using a dual pass 2nd order Butterworth filter with a cut-off frequency of 5 Hz. The slope of the force–displacement curve (i.e., stiffness) was determined for all ten loading cycles. The stiffness values were first averaged between 80 and 90% of each loading curve (i.e., linear portion of the force–displacement curve) and then across all ten cycles. The shoes’ hysteresis (i.e., lost energy) was determined as the difference in mechanical energy that was stored during the loading phase and that was returned during the unloading phase. The lost energy was represented as a percentage of the energy stored during the loading phase. The MBS and hysteresis values were: Control, 1.58 N mm−1 and 35.26%; Stiff, 4.02 N mm−1 and 22.72%; Stiffer, 6.46 N mm−1 and 23.91%; Stiffest, 13.28 N mm−1 and 22.37%. The footwear conditions were weight matched by inserting small masses in the forefoot, midfoot, and rearfoot region of the control shoe. Across all conditions, the masses were 259, 280, and 296 g for the size 9, 10, and 11 US shoes, respectively.
Data collection
On the first visit, individual speed at lactate threshold (sLT) was determined based on methods described elsewhere48 while participants ran in Control on a treadmill (Bertec Corporation, Columbus, USA) with no gradient. In brief, participants first performed a 5-min. warm-up at self-selected speed. Immediately after the warm-up, a fingertip blood sample was taken to determine resting blood lactate concentration (Lactate Pro, Sports Resource Group Inc., Minneapolis, USA). After the warm-up, the treadmill belt speed was increased by 0.8 km h−1 every two minutes, after which blood lactate concentration was measured. This was repeated until blood lactate concentration rose more than 1 mmol from the previous sample. sLT was determined as the speed at the stage preceding the final stage.
On the second visit, participants ran for 5 min. and 30 s at 90% of sLT in each shoe condition, respectively. The order of conditions was randomised, and participants rested for 15 min. between footwear conditions to minimise potential fatigue effects. Subjects were asked to refrain from extensive physical activity for 24 h. before the testing session. Prior to running in each footwear condition, participants ran at 3 m s−1 for 3 min. to get accustomed to running in the shoe conditions and with the measurement equipment. Immediately after the 3-min. familiarisation run, speed was increased to 90% of individual sLT for 5 min. and 30 s. Gastrocnemius medialis (GM) muscle fascicle images of the right leg were acquired using B-mode ultrasound imaging and a flat-shaped, 60 mm-long, linear probe (LV8-5N60-A2, ArtUs, Telemed, Vilnius, Lithuania) with a centre frequency of 8 MHz and a sampling frequency of 154 Hz. The GM muscle was chosen over other triceps surae muscles because Lai et al.35 showed that muscle fascicle length changes during the stance phase of running were greatest at the GM muscle. Indirect calorimetry was used to measure breath-by-breath rate of oxygen uptake ( in ml kg−1 min−1) and carbon dioxide output ( in ml kg−1 min−1) using a metabolic cart (Quark CPET, COSMED srl, Rome, ITA). Prior to each testing session, the metabolic cart was calibrated using room air and a gas mixture of known composition. The flow sensor was calibrated manually with a 3-l syringe. Three-dimensional kinematic and kinetic data of the right lower limb were recorded at 200 and 1000 Hz using eight high-speed video cameras (Vicon Motion Systems Ltd., Oxford, UK) and a force-instrumented treadmill (Bertec Corporation, Columbus, USA), respectively. Retroreflective markers of 12 mm diameter were mounted on anatomical landmarks of the pelvis and right lower limb according to Cigoja et al.13. Kinematic, kinetic, and ultrasound data were synchronously recorded for approximately 20 s starting at the end of minute 5 of each run, which resulted in 28–33 steps of the right lower limb per footwear condition and participant, respectively. The middle 25 steps were identified and used for further analyses. Breath-by-breath metabolic data were recorded for the entire duration of the run.
Data processing and analysis
Ultrasound data
The GM muscle fascicle images (Fig. 4) of the right lower limb were tracked using a semi-automated tracking algorithm49. Manual adjustments were made when the tracking algorithm did not identify the fascicle end points well. Muscle fascicle lengths were smoothened using a dual-pass second order (i.e., zero-lag fourth order) Butterworth filter with a low-pass cut-off frequency of 25 Hz. Muscle fascicle velocity was calculated by deriving fascicle length with respect to time. Fascicle lengths at heel-strike and toe-off, total change of fascicle length and average fascicle velocity over the stance phase of running were identified and used for further comparisons. The ultrasound data for three participants were excluded from any analyses due to insufficient quality of muscle fascicle images.
Figure 4.
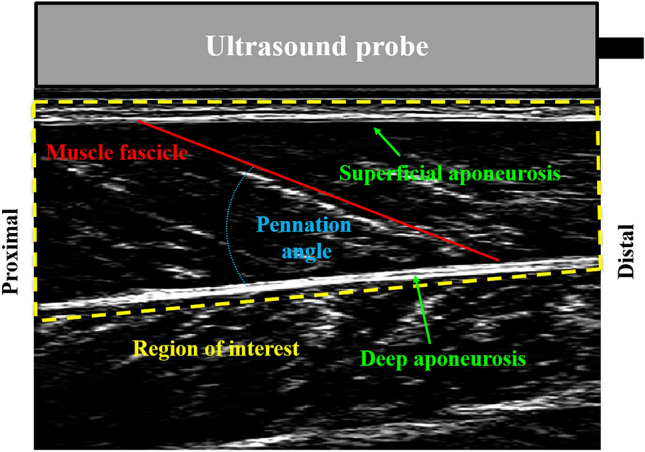
Exemplary image of the gastrocnemius medialis muscle fascicle during running.
Gastrocnemius medialis and Achilles tendon energetics
Mechanical work performed by the GM fascicles was calculated as the integral of fascicle force and fascicle length change over the entire stance phase. Achilles tendon force was estimated based on a previously described musculoskeletal model18,50,51. For this, the sagittal plane ankle joint moment was divided by the AT moment arm. The AT moment arm was estimated by measuring the horizontal distances between the medial and lateral malleoli to the posterior aspect of the calcaneus using digital callipers. The medial and lateral distances were then averaged. The GM force was estimated based on the relative physiological cross-sectional area of all ankle plantarflexor muscles by multiplying the AT force by 0.174652. Then, the scaled AT force was divided by the cosine of the pennation angle of the GM muscle fascicle. Positive fascicle work was considered fascicle shortening; the point during which the fascicles were generating mechanical energy during the stance phase24.
The GM energy cost was calculated during the entire stance phase for each footwear condition according to Fletcher and MacIntosh53. In brief, GM energy cost was approximated from the estimated number of active in-parallel crossbridges required to generate the calculated GM force, the number of crossbridge cycles during the stance phase, and the number of half-sarcomeres in series. To estimate the number of in-parallel crossbridges, the calculated GM force was divided by the estimated force per crossbridge. Because the force per crossbridge decreases with increasing shortening velocity54, the force per crossbridge was scaled to sarcomere shortening velocity at physiological temperatures54, assuming a crossbridge force of 3 pN under near-isometric conditions55. Sarcomere shortening velocity relative to maximal shortening velocity was calculated from the instantaneous fascicle shortening velocity throughout the stance phase, and assuming a maximal shortening velocity of 10.6 Lengthfascicle s−1. The latter was calculated from the assumed maximal shortening velocities of Type I and Type II fibres of 4.4 Lengthfascicle s−1 and 16.8 Lengthfascicle s−1 at physiological temperatures56 and assuming the GM consisted of 50% Type I fibres57. The number of half-sarcomeres in series was estimated as the ratio of fascicle length during stance to half-sarcomere length, assuming an optimal sarcomere length during maximal activation of 2.6 µm at the short side of the plateau region of the sarcomere force–length relationship58. The number of crossbridge cycles was estimated from the amount of shortening within each half-sarcomere, which was estimated from the amount of GM fascicle length change during the stance phase. To accommodate this magnitude of shortening, we assumed the filaments move 10 nm with each crossbridge cycle59. Lastly, we assumed that one adenosine triphosphate (ATP) was consumed for each crossbridge cycle, and that 48 kJ were liberated per mol of ATP60,61. The GM energy cost was only calculated during the stance phase of running because GM force and power has been shown to be greater during stance with minimal to no force and power during swing62. It can therefore be assumed that the energy consumed by the GM muscle is negligible during swing compared to stance.
The shank MTU length was calculated based on methods proposed by Hawkins and Hull63 using the instantaneous ankle and knee joint angles. Instantaneous tendon length was estimated by subtracting the GM fascicle length from the shank MTU length, taking the effect of muscle pennation angle into account64. Achilles tendon power was calculated by multiplying tendon force by tendon lengthening/shortening velocity at each instant during the stance phase. Tendon work was determined by integrating AT power relative to time. Positive tendon work represented the utilization of elastic strain energy that was stored earlier in the stance phase65.
Metabolic data
The Erun (in J kg−1 m−1) was calculated as the breath-by-breath energy expenditure averaged across the final minute of each run, relative to body mass and distance covered within the last minute of the run for each condition, respectively. The Peronnet and Massicotte equation66,67 was used to convert the measured O2 consumption and CO2 production to units of energy (Joule). The best stiff condition was determined as the condition with a carbon fibre plate, which resulted in the lowest Erun. To aid in interpretation of changes in Erun, we calculated the technical error of measurement using the root mean square method68,69. The technical error (TE) of measurement was determined using VO2 averaged across four intervals of 15 s during the final minute of running in the control condition. Then, the SWC, which represents the smallest difference between conditions that can be considered “meaningful”70,71, was calculated as 0.5 × TE72. The SWC in Erun was considered 0.95%.
Kinematic and kinetic data
Raw kinematic and kinetic data of the right lower limb were analysed using a custom written MATLAB code (Version R2019a, the MathWorks Inc., Natick, USA). Force data were downsampled to 200 Hz to match the motion capture data. Kinematic and kinetic data were then smoothed using a dual-pass second order Butterworth filter with a low-pass cut-off frequency of 50 Hz. Cardan angles were calculated for the MTP, ankle, knee, and hip joint with a flexion–extension, abduction–adduction, and internal–external rotation sequence. An inverse dynamics approach was performed to determine internal joint reaction moments. Instantaneous joint power was calculated as the dot product between joint moment and angular velocity for the sagittal, frontal, and transverse plane, respectively; then, joint power was summed across planes. Positive and negative joint work was calculated as the time-integral of positive and negative joint power, respectively.
Statistics
Values are presented as mean ± standard deviation, unless otherwise indicated. Shapiro–Wilk tests were performed to test for normality of all dependent variables. If the Shapiro–Wilk test revealed normal distribution, one-way repeated measures analysis of variance (ANOVA) was used to test effects of MBS on a dependent variable; otherwise, the non-parametric equivalent (i.e., Friedman test) was used. Where the ANOVA or Friedman test revealed significant effects (i.e., p ≤ 0.050) of MBS, multiple pairwise comparisons were performed using two-tailed Student’s paired t test or Wilcoxon’s signed-rank test to test for differences between Control and each stiff condition (i.e., Control vs. Stiff, Control vs. Stiffer, and Control vs. Stiffest). For multiple comparisons, the significance level was set to p ≤ 0.017 (0.050 ÷ 3 comparisons = 0.017). Effect sizes were quantified using Cohen’s d and were considered small, medium, and large for d ≥ 0.2, d ≥ 0.5, and d ≥ 0.8, respectively73. A two-way ANOVA (MBS × %Stance) with repeated measures (MBS) was used to test for differences in GM muscle energy cost across the entire stance phase. When there was no significant interaction but a significant main effect was found, Tukey’s post-hoc test was used to detect significant differences between the MBS conditions.
Supplementary Information
Acknowledgements
This study was financially supported by the International Society of Biomechanics in Sports Student Mini Research Grant awarded to SC.
Author contributions
S.C. conceptualised the study, collected, processed, and analysed the data, and drafted the first version of the manuscript. J.R.F. conceptualised the study, analysed the data, and drafted the first version of the manuscript. M.E. collected the data, interpreted the results, and revised the manuscript. D.J.S. and B.M.N. interpreted the results and revised the manuscript. All authors approved the submitted manuscript and agreed to be accountable to all aspects of the work.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-020-80791-3.
References
- 1.Hoogkamer W, et al. A comparison of the energetic cost of running in marathon racing shoes. Sport. Med. 2018;48:1009–1019. doi: 10.1007/s40279-017-0811-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoogkamer W, Kipp S, Kram R. The biomechanics of competitive male runners in three marathon racing shoes: A randomized crossover study. Sport. Med. 2019;49:133–143. doi: 10.1007/s40279-018-1024-z. [DOI] [PubMed] [Google Scholar]
- 3.Hunter I, et al. Running economy, mechanics, and marathon racing shoes. J. Sports Sci. 2019;37:2367–2373. doi: 10.1080/02640414.2019.1633837. [DOI] [PubMed] [Google Scholar]
- 4.Barnes KR, Kilding AE. A randomized crossover study investigating the running economy of highly-trained male and female distance runners in marathon racing shoes versus track spikes. Sport. Med. 2019;49:331–342. doi: 10.1007/s40279-018-1012-3. [DOI] [PubMed] [Google Scholar]
- 5.Quealy K, Katz J. Nike says its $250 running shoes will make you run much faster. What if that’s actually true? N. Y. Times. 2018;20:20. [Google Scholar]
- 6.Connell, J. L. et al. Stacked cushioning arrangement for sole structure. (2018).
- 7.Stefanyshyn DJ, Nigg BM. Influence of midsole bending stiffness on joint energy and jump height performance. Med. Sci. Sports Exerc. 2000;32:471–476. doi: 10.1097/00005768-200002000-00032. [DOI] [PubMed] [Google Scholar]
- 8.Stefanyshyn DJ, Nigg BM. Mechanical energy contribution of the metatarsophalangeal joint to running and sprinting. J. Biomech. 1997;30:1081–1085. doi: 10.1016/S0021-9290(97)00081-X. [DOI] [PubMed] [Google Scholar]
- 9.Stefanyshyn DJ, Nigg BM. Contribution of the lower extremity joints to mechanical energy in running vertical jumps and running long jumps. J. Sports Sci. 1998;16:177–186. doi: 10.1080/026404198366885. [DOI] [PubMed] [Google Scholar]
- 10.Stefanyshyn DJ, Fusco C. Athletics: Increased shoe bending stiffness increases sprint performance. Sport. Biomech. 2004;3:55–66. doi: 10.1080/14763140408522830. [DOI] [PubMed] [Google Scholar]
- 11.Roy JPR, Stefanyshyn DJ. Shoe midsole longitudinal bending stiffness and running economy, joint energy, and EMG. Med. Sci. Sports Exerc. 2006;38:562–569. doi: 10.1249/01.mss.0000193562.22001.e8. [DOI] [PubMed] [Google Scholar]
- 12.Willwacher S, König M, Potthast W, Brüggemann G. Does specific footwear facilitate energy storage and return at the metatarsophalangeal joint in running? J. Appl. Biomech. 2013;29:583–592. doi: 10.1123/jab.29.5.583. [DOI] [PubMed] [Google Scholar]
- 13.Cigoja S, et al. Does increased midsole bending stiffness of sport shoes redistribute lower limb joint work during running? J. Sci. Med. Sport. 2019;22:20. doi: 10.1016/j.jsams.2019.06.015. [DOI] [PubMed] [Google Scholar]
- 14.Nigg BM, Cigoja S, Nigg SR. Effects of running shoe construction on performance in long distance running. Footwear Sci. 2020 doi: 10.1080/19424280.2020.1778799. [DOI] [Google Scholar]
- 15.Nigg BM, Cigoja S, Nigg SR. Teeter-totter effect: A new mechanism to understand shoe-related improvements in long-distance running. Br. J. Sports Med. 2020;10:1–2. doi: 10.1136/bjsports-2020-102550. [DOI] [PubMed] [Google Scholar]
- 16.Willwacher S, König M, Braunstein B, Goldmann JP, Brüggemann GP. The gearing function of running shoe longitudinal bending stiffness. Gait Posture. 2014;40:386–390. doi: 10.1016/j.gaitpost.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi KZ, Gross MT, Van Werkhoven H, Piazza SJ, Sawicki GS. Adding stiffness to the foot modulates soleus force-velocity behaviour during human walking. Sci. Rep. 2016;6:1–11. doi: 10.1038/s41598-016-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cigoja S, et al. The effects of increased midsole bending stiffness of sport shoes on muscle-tendon unit shortening and shortening velocity: A randomised crossover trial in recreational male runners. Sport. Med. Open. 2020;6:1–11. doi: 10.1186/s40798-019-0218-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nigg BM, Stefanyshyn DJ, Denoth J. Mechanical considerations of work and energy. In: Nigg BM, MacIntosh BR, Mester J, editors. Biomechanics and Biology of Movement. Human Kinetics: Champaign; 2000. pp. 5–18. [Google Scholar]
- 20.Carrier DR, Heglund NC, Earls KD. Variable gearing during locomotion in the human musculoskeletal system. Science (80–) 1994;265:651–653. doi: 10.1126/science.8036513. [DOI] [PubMed] [Google Scholar]
- 21.Ray SF, Takahashi KZ. Gearing up the human ankle-foot system to reduce energy cost of fast walking. Sci. Rep. 2020;10:1–12. doi: 10.1038/s41598-019-56847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh K, Park S. The bending stiffness of shoes is beneficial to running energetics if it does not disturb the natural MTP joint flexion. J. Biomech. 2017;53:127–135. doi: 10.1016/j.jbiomech.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Stefanyshyn DJ, Wannop JW. The influence of forefoot bending stiffness of footwear on athletic injury and performance. Footwear Sci. 2016;8:51–63. doi: 10.1080/19424280.2016.1144652. [DOI] [Google Scholar]
- 24.Fletcher JR, Groves EM, Pfister TR, MacIntosh BR. Can muscle shortening alone, explain the energy cost of muscle contraction in vivo? Eur. J. Appl. Physiol. 2013;113:2313–2322. doi: 10.1007/s00421-013-2665-0. [DOI] [PubMed] [Google Scholar]
- 25.Roberts TJ, Kram R, Weyand PG, Taylor CR. Energetics of bipedal running. I. Metabolic cost of generating force. J. Exp. Biol. 1998;201:2745–2751. doi: 10.1242/jeb.201.19.2745. [DOI] [PubMed] [Google Scholar]
- 26.Brueckner JC, et al. The energy cost of running increases with the distance covered. Eur. J. Appl. Physiol. Occup. Physiol. 1991;62:385–389. doi: 10.1007/BF00626607. [DOI] [PubMed] [Google Scholar]
- 27.Xu F, Montgomery DL. Effect of prolonged exercise at 65 and 80% VO2max on running economy. Int. J. Sports Med. 1995;16:309–315. doi: 10.1055/s-2007-973011. [DOI] [PubMed] [Google Scholar]
- 28.Hunter I, Smith GA. Preferred and optimal stride frequency, stiffness and economy: Changes with fatigue during a 1-h high-intensity run. Eur. J. Appl. Physiol. 2007;100:653–661. doi: 10.1007/s00421-007-0456-1. [DOI] [PubMed] [Google Scholar]
- 29.Petersen K, Hansen CB, Aagaard P, Madsen K. Muscle mechanical characteristics in fatigue and recovery from a marathon race in highly trained runners. Eur. J. Appl. Physiol. 2007;101:385–396. doi: 10.1007/s00421-007-0504-x. [DOI] [PubMed] [Google Scholar]
- 30.Fletcher JR, MacIntosh BR. Changes in Achilles tendon stiffness and energy cost following a prolonged run in trained distance runners. PLoS One. 2018;13:1–17. doi: 10.1371/journal.pone.0202026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanno M, Willwacher S, Epro G, Brüggemann GP. Positive work contribution shifts from distal to proximal joints during a prolonged run. Med. Sci. Sports Exerc. 2018;50:2507–2517. doi: 10.1249/MSS.0000000000001707. [DOI] [PubMed] [Google Scholar]
- 32.Fletcher JR, Macintosh BR. Running economy from a muscle energetics perspective. Front. Physiol. 2017;8:1–15. doi: 10.3389/fphys.2017.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cigoja, S., Fletcher, J. R. & Nigg, B. M. Can increased midsole bending stiffness of sport shoes delay the onset of lower limb joint work redistribution during a prolonged run? In Proceedings to the 38th Conference of the International Society of Biomechanics in Sports (2020).
- 34.Beck ON, Golyski PR, Sawicki GS. Adding carbon fiber to shoe soles does not improve running economy: A muscle-level explanation. Sci. Rep. 2020;10(17154):1–13. doi: 10.1038/s41598-020-74097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai AKM, Lichtwark GA, Schache AG, Pandy MG. Differences in in vivo muscle fascicle and tendinous tissue behavior between the ankle plantarflexors during running. Scand. J. Med. Sci. Sport. 2018;28:1828–1836. doi: 10.1111/sms.13089. [DOI] [PubMed] [Google Scholar]
- 36.Myatt JP, Schilling N, Thorpe SKS. Distribution patterns of fibre types in the triceps surae muscle group of chimpanzees and orangutans. J. Anat. 2011;218:402–412. doi: 10.1111/j.1469-7580.2010.01338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herzog W, Leonard TR. Validation of optimization models that estimate the forces exerted by synergistic muscles. J. Biomech. 1991;24:31–39. doi: 10.1016/0021-9290(91)90375-W. [DOI] [PubMed] [Google Scholar]
- 38.Madden R, Sakaguchi M, Tomaras EK, Wannop JW, Stefanyshyn D. Forefoot bending stiffness, running economy and kinematics during overground running. Footwear Sci. 2016;8:91–98. doi: 10.1080/19424280.2015.1130754. [DOI] [Google Scholar]
- 39.Flores N, Delattre N, Berton E, Rao G. Does an increase in energy return and/or longitudinal bending stiffness shoe features reduce the energetic cost of running? Eur. J. Appl. Physiol. 2019;119:429–439. doi: 10.1007/s00421-018-4038-1. [DOI] [PubMed] [Google Scholar]
- 40.Kipp S, Kram R, Hoogkamer W. Extrapolating metabolic savings in running: Implications for performance predictions. Front. Physiol. 2019;10:1–8. doi: 10.3389/fphys.2019.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoogkamer W, Kipp S, Spiering BA, Kram R. Altered running economy directly translates to altered distance-running performance. Med. Sci. Sports Exerc. 2016;48:2175–2180. doi: 10.1249/MSS.0000000000001012. [DOI] [PubMed] [Google Scholar]
- 42.Moo EK, Herzog W. Sarcomere contractile behaviours are location-dependent in living whole muscle. Can. Soc. Biomech. Sup. 2018;1:20. [Google Scholar]
- 43.Matijevich ES, Branscombe LM, Zelik KE. Ultrasound estimates of Achilles tendon exhibit unexpected shortening during ankle plantarflexion. J. Biomech. 2018;72:200–206. doi: 10.1016/j.jbiomech.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hawkins D, Hull ML. A method for determining lower extremity muscle-tendon lengths during flexion/extension movements. J. Biomech. 1990;23:487–494. doi: 10.1016/0021-9290(90)90304-L. [DOI] [PubMed] [Google Scholar]
- 45.Herzog W. The problem with skeletal muscle series elasticity. BMC Biomed. Eng. 2019;1:1–14. doi: 10.1186/s42490-019-0031-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arampatzis A, Karamanidis K, Morey-Klapsing G, De Monte G, Stafilidis S. Mechanical properties of the triceps surae tendon and aponeurosis in relation to intensity of sport activity. J. Biomech. 2007;40:1946–1952. doi: 10.1016/j.jbiomech.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 47.Lieber RL, Leonard ME, Brown-Maupin CG. Load-strain properties of frog aponeurosis and tendon. Cells Tissues Organs. 2000;166:48–54. doi: 10.1159/000016708. [DOI] [PubMed] [Google Scholar]
- 48.Fletcher JR, Esau SP, Macintosh BR. Economy of running: Beyond the measurement of oxygen uptake. J. Appl. Physiol. 2009;107:1918–1922. doi: 10.1152/japplphysiol.00307.2009. [DOI] [PubMed] [Google Scholar]
- 49.Farris DJ, Lichtwark GA. UltraTrack: Software for semi-automated tracking of muscle fascicles in sequences of B-mode ultrasound images. Comput. Methods Programs Biomed. 2016;128:111–118. doi: 10.1016/j.cmpb.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 50.Scott SH, Winter DA. Internal forces at chronic running injury sites. Med. Sci. Sport. Exerc. 1990;22:357–369. doi: 10.1249/00005768-199006000-00013. [DOI] [PubMed] [Google Scholar]
- 51.Firminger CR, Bruce OL, Wannop JW, Stefanyshyn DJ, Edwards WB. Effect of shoe and surface stiffness on lower limb tendon strain in jumping. Med. Sci. Sport. Exerc. 2019;51:1–9. doi: 10.1249/MSS.0000000000002004. [DOI] [PubMed] [Google Scholar]
- 52.Fukunaga T, et al. Physiological cross-sectional area of human leg muscles based on magnetic resonance imaging. J. Orthop. Res. 1992;10:926–934. doi: 10.1002/jor.1100100623. [DOI] [PubMed] [Google Scholar]
- 53.Fletcher JR, MacIntosh BR. Achilles tendon strain energy in distance running: Consider the muscle energy cost. J. Appl. Physiol. 2015;118:193–199. doi: 10.1152/japplphysiol.00732.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Tombe PP, Keurs HEDJ. Force and velocity of sarcomere shortening in trabeculae from rat heart. Circ. Res. 1990;66:1239–1254. doi: 10.1161/01.RES.66.5.1239. [DOI] [PubMed] [Google Scholar]
- 55.Finer JT, Simmons RM, Spudicht JA. Single myosin molecule mechanics: Piconewton forces and nanometre steps. Nature. 1994;368:113–119. doi: 10.1038/368113a0. [DOI] [PubMed] [Google Scholar]
- 56.Bohm S, Mersmann F, Santuz A, Arampatzis A, Bohm S. The force–length–velocity potential of the human soleus muscle is related to the energetic cost of running. Proc. R. Soc. B. 2019;20:20. doi: 10.1098/rspb.2019.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Edgerton VR, Smith JL. Muscle fibre type populations of human leg muscles. Histochem. J. 1975;7:259–266. doi: 10.1007/BF01003594. [DOI] [PubMed] [Google Scholar]
- 58.Herzog W, Keurs HEDJ. Physiology force–length relation of in-vivo human rectus femoris muscles. Pflügers Arch. 1988;20:642–647. doi: 10.1007/BF00580860. [DOI] [PubMed] [Google Scholar]
- 59.Barclay CJ, Woledge RC, Curtin NA. Inferring crossbridge properties from skeletal muscle energetics. Prog. Biophys. Mol. Biol. 2010;102:53–71. doi: 10.1016/j.pbiomolbio.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 60.Barclay CJ. Energetics of contraction. Compr. Physiol. 2015;5:961–995. doi: 10.1002/cphy.c140038. [DOI] [PubMed] [Google Scholar]
- 61.Homsher E, Kean CJ. Skeletal muscle energetics and metabolism. Annu. Rev. Physiol. 1978;40:93–131. doi: 10.1146/annurev.ph.40.030178.000521. [DOI] [PubMed] [Google Scholar]
- 62.Farris DJ, Sawicki GS. Human medial gastrocnemius force-velocity behavior shifts with locomotion speed and gait. Proc. Natl. Acad. Sci. USA. 2012;109:977–982. doi: 10.1073/pnas.1107972109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hawkins D. A method for determining lower extremity muscle-tendon lengths during flexion/extension movements. J. Biomech. 1990;23:487–494. doi: 10.1016/0021-9290(90)90304-L. [DOI] [PubMed] [Google Scholar]
- 64.Fukunaga T, Kubo K, Kawakami Y, Fukashiro S, Maganaris CN. In vivo behaviour of human muscle tendon during walking. Proc. Biol. Sci. 2001;268:229–233. doi: 10.1098/rspb.2000.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lai A, Schache AG, Lin Y, Pandy MG. Tendon elastic strain energy in the human ankle plantar-flexors and its role with increased running speed. J. Exp. Biol. 2014 doi: 10.1242/jeb.100826. [DOI] [PubMed] [Google Scholar]
- 66.Peronnet F, Massicotte D. Table of nonprotein respiratory quotient: An update. Can. J. Sport Sci. 1991;16:23–29. [PubMed] [Google Scholar]
- 67.Kipp S, Byrnes WC, Kram R. Calculating metabolic energy expenditure across a wide range of exercise intensities: The equation matters. Appl. Physiol. Nutr. Metab. 2018;43:639–642. doi: 10.1139/apnm-2017-0781. [DOI] [PubMed] [Google Scholar]
- 68.Fletcher JR, Asmussen MJ, Nigg SR, MacIntosh BR, Nigg BM. The effect of torsional shoe sole stiffness on knee moment and gross efficiency in cycling. J. Sports Sci. 2019;37:1457–1463. doi: 10.1080/02640414.2019.1565650. [DOI] [PubMed] [Google Scholar]
- 69.Batterham AM, George KP. Reliability in evidence-based clinical practice: A primer for allied health professionals. Phys. Ther. Sport. 2000;1:54–62. doi: 10.1054/ptsp.2000.0010. [DOI] [Google Scholar]
- 70.Saunders PU, Pyne DB, Telford RD, Hawley JA. Reliability and variability of running economy in elite distance runners. Med. Sci. Sport. Exerc. 2004;36:1972–1976. doi: 10.1249/01.MSS.0000145468.17329.9F. [DOI] [PubMed] [Google Scholar]
- 71.Shaw AJ, Ingham SA, Fudge BW, Folland JP. The reliability of runnign economy expressed as oxygen cost and energy cost in trained distance runners. Appl. Physiol. Nutr. Metab. 2013;38:1268–1272. doi: 10.1139/apnm-2013-0055. [DOI] [PubMed] [Google Scholar]
- 72.Hopkins WG. Measures of reliability in sports medicine and science. Sport. Med. 2000;30:1–15. doi: 10.2165/00007256-200030010-00001. [DOI] [PubMed] [Google Scholar]
- 73.Cohen J. Statistical Power Analysis for the Behavioral Sciences. New Jersey: Lawrence Erlbaum Associates; 1988. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.